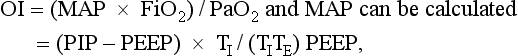Pulmonology
Differential Diagnosis of Neonatal Pulmonary Disorders
1. Although apnea in premature infants is often caused by the degree of immaturity (so-called apnea of prematurity), what are other causes of apnea in this population?
TABLE 18-1
CAUSES OF APNEA IN PREMATURE INFANTS
| SYSTEM | PERTURBATION |
| Central nervous | Intracranial hemorrhage, hypoxic-ischemic encephalopathy, seizures, congenital anomalies, maternal drugs, drugs used to treat the infant |
| Respiratory | Pneumonia, airway obstruction with lesions, anatomic obstruction (e.g., pharynx or tongue blocking airway), upper airway collapse (e.g., tracheal or laryngomalacia), severe respiratory distress syndrome, atelectasis |
| Infectious | Septicemia or meningitis caused by bacterial, fungal, or viral agents |
| Gastrointestinal | Necrotizing enterocolitis, gastroesophageal reflux, positive result to Valsalva maneuver during bowel movements |
| Metabolic | Hypoglycemia, hypocalcemia, hyponatremia or hypernatremia, inborn errors of metabolism, increased or decreased ambient temperature, hypothermia |
| Cardiovascular | Hypotension, congestive heart failure, hypovolemia, patent ductus arteriosus |
| Hematologic | Anemia |
2. Is apnea of prematurity correlated with an increased incidence of sudden infant death syndrome (SIDS)?
No. Although apnea is often believed to be a provocative factor for SIDS, this relationship has never been causally established. It appears that premature infants with apnea of prematurity are no more likely to die as a result of SIDS than those of comparable gestational age who do not have apnea of prematurity. Premature infants do, however, have a higher SIDS rate than do term infants, suggesting that immaturity of respiratory control may be a component of SIDS. Furthermore, several studies have indicated that unless respiratory patterns of premature infants are recorded, apnea will not be detected because the respiratory abnormalities in these babies are very difficult to see clinically. 1
3. Of all newborn infants who die as a result of culture-proven bacteremia, what proportion has pneumonia?
4. What are the most common radiographic features of group B streptococcal (GBS) pneumonia in premature infants? In term infants?
5. In a neonate who is breathing normally, is a low partial pressure of oxygen (arterial PO2) and normal partial pressure of carbon dioxide (PaCO2) more consistent with cyanotic heart disease or severe lung disease?
Neonates who have low oxygen saturations or arterial oxygen levels (arterial PO2), normal carbon dioxide levels (PaCO2), and no signs of respiratory distress usually have cyanotic congenital heart disease. Low arterial PO2 and a rising PaCO2 in a neonate with labored breathing (e.g., grunting, retractions, tachypnea) suggest intrinsic lung disease and its attendant intrapulmonary shunt. A high level of PaCO2 in association with severe retractions, normal arterial PO2 in minimal oxygen support, and signs of gas trapping on chest radiograph is most consistent with upper airway obstruction. Therefore if an infant is easy to oxygenate and impossible to ventilate, think airway; if the infant is easy to ventilate and impossible to oxygenate, think heart; and if both oxygenation and ventilation are problems, think lung disease.
Neonatal Resuscitation
8. What are the approximate endotracheal (ET) tube sizes that would be appropriate for premature infants of varying birth weights?
 2.5-mm internal diameter (ID) for infants weighing <1000 g
2.5-mm internal diameter (ID) for infants weighing <1000 g
 3.0-mm ID for infants 1000 to 2000 g
3.0-mm ID for infants 1000 to 2000 g
9. Before radiographic verification, how far should an ET tube be inserted to be in the appropriate position for infants of varying birth weight?
The “tip-to-lip” rule for placement is the distance from the ET tube tip to the centimeter marking on the tube itself ( Fig. 18-1). Good approximations are as follows:
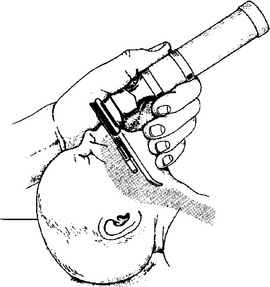
Figure 18-1 Appropriate position for ET tube insertion. (From Goldsmith JP, Karotkin EH, editors. Assisted ventilation of the neonate. 3rd ed. Philadelphia: Saunders; 1996. p. 108.)
 6 to 7 cm for a child of 1000-g birth weight
6 to 7 cm for a child of 1000-g birth weight
 7 to 8 cm for a child of 2000-g birth weight
7 to 8 cm for a child of 2000-g birth weight
10. Does the vigorous neonate born with thick meconium amniotic fluid need to have the trachea suctioned to remove meconium that may have been aspirated?
No. Compared with expectant management, intubation and suctioning of the apparently vigorous meconium-stained infant does not result in a decreased incidence of meconium aspiration syndrome (MAS) or other respiratory disorders. In addition, it may provoke airway injury, especially if the child is active and moving after delivery. 34
11. Which of the following is currently recommended by the neonatal resuscitation program: (A) calcium chloride for asystole, (B) atropine for bradycardia, (C) epinephrine for heart rate below 60 bpm, (D) 5% albumin for hypovolemia?
Two meta-analyses of several randomized controlled trials comparing neonatal resuscitation initiated with room air versus 100% oxygen showed increased survival when resuscitation was initiated with room air. 567
15. Name the initial steps in neonatal resuscitation.
1. Thermal management: The infant should be dried and kept warm.
2. The airway should be assessed and cleared of fluid and birth debris if there are signs of obstruction. Neonatal Resuscitation Textbook recommends “suctioning immediately following birth (including suctioning with a bulb syringe) should be reserved for babies who have obvious obstruction to spontaneous breathing or who require positive-pressure ventilation.”
3. The baby should receive tactile stimulation. The baby’s bottom should not be spanked; gentle stroking and rubbing of the skin of the legs and buttocks should suffice. The thorax should not be rubbed because it may interrupt a respiratory effort.
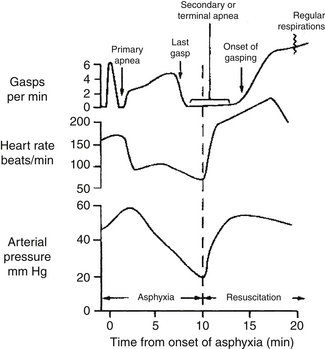
A regular sequence of events occurs when an infant becomes hypoxemic and acidemic. Initially, gasping respiratory efforts increase in depth and frequency for up to 3 minutes, followed by approximately 1 minute of primary apnea ( Fig. 18-2). If oxygen (along with stimulation) is provided during the apneic period, respiratory function spontaneously returns. If asphyxia continues, gasping then resumes for a variable period of time, terminating with the “last gasp” and is followed by secondary apnea. During secondary apnea the only way to restore respiratory function is with positive pressure ventilation and high concentrations of oxygen. Thus a linear relationship exists between the duration of asphyxia and the recovery of respiratory function after resuscitation. The longer that artificial ventilation is delayed after the last gasp, the longer it will take to resuscitate the infant. Clinically, however, the two conditions are indistinguishable, although an infant’s cyanosis will become progressively worse over time.
The first breath of an infant has been measured in the delivery room and is reported to be between −30 and −140 cm H2O. These pressures are needed to overcome the substantial fluid and elastic forces present in the airway at the time of delivery. As surfactant is deposited, however, subsequent breaths rapidly decrease to −4 to −10 cm H2O. If surfactant is decreased, as is the case in RDS, the baby must continue to exert the original very high effort to continue to inflate the lung. With limited energy reserves this effort soon deteriorates, and respiratory failure ensues.
Studies support both routes of intubation for newborn infants. The oral intubation school argues that because neonates are obligate nose breathers, they will demonstrate increased work of breathing and atelectasis after removal of nasotracheal tubes. On the other hand, nasal intubation proponents assert that orotracheal intubation results in grooving of the palate with subsequent orthodontic problems. Nasal tubes, however, have been associated with injury to the nasal cartilage. Therefore operator skill and institutional tradition are primary considerations in this clinical decision. After extubation, however, there does appear to be a higher incidence of atelectasis with nasal ET tubes. 73
Transitional Physiology and the Asphyxiated Fetus
19. Asphyxia is a condition of impaired gas exchange best characterized by what blood gas abnormalities: (A) hypoxemia, (B) hypercapnia, or (C) metabolic acidosis?
20. A child is depressed and requires vigorous resuscitation in the delivery room. Subsequently, he demonstrates labile oxygenation and right-to-left shunting. A heart murmur is auscultated. What is the most likely anatomic or physiologic basis for the murmur?
21. In the American Academy of Pediatrics–American College of Obstetricians and Gynecologists’ guidelines regarding intrapartum asphyxia as a cause of brain injury, what criteria must be present?
 Profound metabolic or mixed acidemia (pH <7) on an umbilical cord arterial blood sample
Profound metabolic or mixed acidemia (pH <7) on an umbilical cord arterial blood sample
 Early onset of neonatal encephalopathy
Early onset of neonatal encephalopathy
 Cerebral palsy (CP) of the spastic quadriplegic or dyskinetic type and no evidence of other potential causes for neonatal encephalopathy, such as trauma, coagulation or genetic disorders, and infectious conditions
Cerebral palsy (CP) of the spastic quadriplegic or dyskinetic type and no evidence of other potential causes for neonatal encephalopathy, such as trauma, coagulation or genetic disorders, and infectious conditions
 Exclusion of other identifiable etiologies, such as trauma, coagulation disorders, infectious conditions, and genetic disorders 9
Exclusion of other identifiable etiologies, such as trauma, coagulation disorders, infectious conditions, and genetic disorders 9
 Of children who develop CP, 73% have 5-minute Apgar scores of 7 to 10.
Of children who develop CP, 73% have 5-minute Apgar scores of 7 to 10.
 A child with a 1-minute Apgar score of 0 to 3 but a 10-minute Apgar score of 4 or higher has a 1% chance of subsequently developing CP.
A child with a 1-minute Apgar score of 0 to 3 but a 10-minute Apgar score of 4 or higher has a 1% chance of subsequently developing CP.
 Of children with a 15-minute Apgar score of 0 to 3, 53% die and 38% of survivors will subsequently develop CP.
Of children with a 15-minute Apgar score of 0 to 3, 53% die and 38% of survivors will subsequently develop CP.
 Of children with a 20-minute Apgar score of 0 to 3, 59% die, and 57% of survivors will subsequently develop CP. 10
Of children with a 20-minute Apgar score of 0 to 3, 59% die, and 57% of survivors will subsequently develop CP. 10
23. True or false? Mental retardation or seizures that are not associated with CP are not likely to be caused by asphyxia or other intrapartum events.
True. The etiology of mental retardation and seizures is not known in most cases.
24. In 1862 William John Little concluded that “spastic rigidity” (i.e., CP) was exclusively caused by perinatal events. This led to the general belief for the next 100 years that CP was a preventable disorder caused by obstetric events. What was Dr. Little’s medical specialty?
25. What prominent neurologist in 1897 came to the conclusion that most cases of CP were not caused by intrapartum events?
26. True or false? Electronic fetal monitoring of the fetal heart rate has resulted in decreased deaths and a decrease in the incidence of CP.
If arterial blood gases were taken from a fetus, the PaO2 (arterial PO2) would be in the range of 25 to 35 mm Hg. Although seemingly low, the strong affinity of fetal hemoglobin for oxygen results in a highly saturated blood that is sufficient to meet the metabolic needs of the fetus. There is, however, little additional room for the PO2 to decrease, and the fetus whose oxygen level begins to decrease even a small amount may develop problems quickly.
The outcome of infants with asphyxia depends on several factors:
31. If Apgar scores are not useful in predicting long-term outcome, why do we even bother recording them?
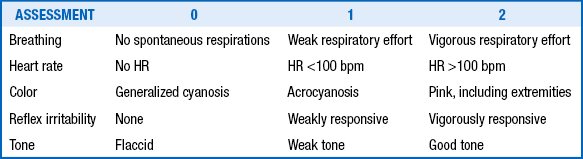
Apgar scores are useful for assessing and describing the condition of neonates after birth and their subsequent transition to an extrauterine state ( Table 18-2). The Apgar scores are generally obtained and totaled at 1 minute and 5 minutes after birth; however, scores should be recorded for longer periods (at 10, 15, and even 20 minutes) if they are low (until the score is ≥7). Low Apgar scores are useful in identifying neonates who are depressed, and the change in score at 1 minute, 5 minutes, and subsequent time intervals is often helpful in assessing the efficacy of resuscitation. Low Apgar scores (<3) that persist beyond 5 minutes have a better correlation with a poor long-term outcome than Apgar scores at 1 minute. 11
The mortality among severely asphyxiated infants is high and can vary from 50% to 75%. Among survivors, long-term neurodevelopmental sequelae are common and occur in approximately one third of infants. Currently, there are no dependable predictors of long-term outcome. The presence and extent of neurologic abnormalities in the early postasphyxial phase and the persistence of abnormal neurologic findings at the time of discharge are the simplest and most effective predictors of long-term outcome. One measure of the severity of early neurologic dysfunction is the clinical staging system developed by Sarnat. Infants with Sarnat stage I encephalopathy are the ones who have mild asphyxia and recover without any significant neurologic sequelae. However, among infants with Sarnat stages II and III encephalopathy, the incidence of long-term neurodevelopmental handicaps can range anywhere from 50% to 100%. In one study of infants who had no detectable heart rate at birth and at 1 minute of age, two thirds died before discharge and 33% of the survivors had severe neurologic handicaps ( Table 18-3).
TABLE 18-3
SARNAT CLASSIFICATION OF POSTANOXIC ENCEPHALOPATHY
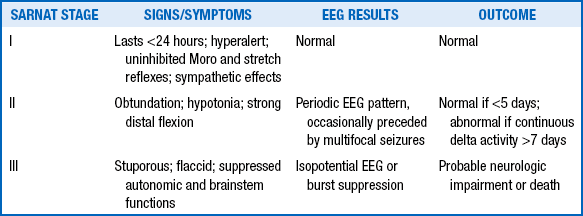
From Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol 1976;33:696–705; and Jain L, Ferre C, Vidyasagar D, et al. Cardiopulmonary resuscitation of apparently stillborn infants. J Pediatr 1991;118:778–82.
34. What are the differences in the pattern of neurologic injury after hypoxic-ischemic insult in preterm and term infants?
35. Asphyxiated infants who are successfully resuscitated often show signs of injury to multiple organ systems. What other organs are involved? Is the injury permanent?
In asphyxiated infants who have been successfully resuscitated, the central nervous system (CNS) is the most frequently involved site (72%), followed by the kidneys (62%), heart (29%), intestines (29%), and lungs (26%). Multiple organ involvement occurs even as an asphyxiated fetus or neonate tries to redistribute blood to vital organs as a part of the “diving reflex.” Fortunately, injury to these organs (except the CNS) is not permanent, and complete recovery of function can be expected in most infants who survive. However, the presence of multiorgan failure can seriously jeopardize chances of survival in the immediate postnatal period. 12
 Acute renal failure (either acute tubular necrosis or acute cortical necrosis)
Acute renal failure (either acute tubular necrosis or acute cortical necrosis)
 Asphyxiated bladder syndrome (bladder muscle injury)
Asphyxiated bladder syndrome (bladder muscle injury)
 Syndrome of inappropriate antidiuretic hormone secretion (SIADH)
Syndrome of inappropriate antidiuretic hormone secretion (SIADH)
37. Prolonged resuscitation in the delivery room often makes resuscitated infants very cold. Is hypothermia harmful to these infants?
Until recently, the presence of hypothermia in resuscitated infants was thought to correlate with poor survival and a higher occurrence of complications. However, recent studies have shown that selective cooling of the brain in infants suspected of having severe hypoxic-ischemic brain damage can improve long-term outcome. Multicenter trials from nurseries around the world have been very promising in this regard. It appears, however, that the primary beneficiary is a child with mild to moderate perinatal asphyxia. Infants with more severe forms of injury do not appear to benefit as much. In addition to improving neurodevelopmental outcomes, cooling reduces mortality risk. Analysis of data from all 10 trials that reported mortality rates showed that infants treated with prolonged moderate hypothermia were less likely to die than those who received normal care. A total of 169 (26%) of the 660 infants treated with therapeutic hypothermia died, compared with 217 (33%) of the 660 infants who received standard care (relative risk 0.78, 95% CI 0.66 to 0.93, P=0.005), with a number needed to treat of 14 (95% CI 8 to 47). 1314
The outcome of depressed infants is usually determined by the degree of resuscitative efforts that are necessary. In one study infants who required chest compressions and epinephrine had the worst outcome, with up to 56% dying in the neonatal period and 21% having an intracranial hemorrhage. Other complications noted in recipients of chest compressions included seizures (18%), respiratory distress (68%), and pneumothorax (24%). 15
Very-low-birth-weight (VLBW) infants have the greatest need for resuscitation at birth, with up to two thirds of infants weighing less than 1500 g requiring some form of resuscitation. The morbidity and mortality rates in VLBW infants requiring cardiopulmonary resuscitation are inversely related to their birth weight. Recent data indicate that VLBW infants do better if they are delivered and cared for at tertiary care centers. The speed of an in-house response team to the delivery room for resuscitation unquestionably is a great advantage of the tertiary care center compared with a community hospital.
40. What are the absolute indications for initiating positive pressure ventilation through a bag-and-mask apparatus in a newborn?
 Need for prolonged bag-and-mask ventilation
Need for prolonged bag-and-mask ventilation
 Prolonged chest compressions (>1 minute)
Prolonged chest compressions (>1 minute)
 Ineffective bag-and-mask ventilation
Ineffective bag-and-mask ventilation
 Congenital diaphragmatic hernia (do not insufflate the bowel with bag-and-mask ventilation, if possible)
Congenital diaphragmatic hernia (do not insufflate the bowel with bag-and-mask ventilation, if possible)
43. What causes persistent bradycardia or cyanosis in an infant who is receiving bag-and-mask ventilation?
 Improper mask size or fit (the mask should fit snugly from the bridge of the baby’s nose to the base of the chin)
Improper mask size or fit (the mask should fit snugly from the bridge of the baby’s nose to the base of the chin)
 Poor seal of mask over the baby’s face
Poor seal of mask over the baby’s face
 Improper positioning of the infant (remember to place the baby in the “sniffing” position, with the neck slightly extended and the chin up)
Improper positioning of the infant (remember to place the baby in the “sniffing” position, with the neck slightly extended and the chin up)
 Airway obstruction or need for suctioning
Airway obstruction or need for suctioning
 Ineffective manual ventilation (remember to watch for that chest rise and use just enough positive pressure ventilation—about 15 to 20 cm H2O pressure for an average term infant—to see good chest rise)
Ineffective manual ventilation (remember to watch for that chest rise and use just enough positive pressure ventilation—about 15 to 20 cm H2O pressure for an average term infant—to see good chest rise)
 Always check the equipment to ensure proper functioning (e.g., laryngoscope blade bulb works, suction is on, 100% free-flow oxygen is turned on, tape for ET tube is available).
Always check the equipment to ensure proper functioning (e.g., laryngoscope blade bulb works, suction is on, 100% free-flow oxygen is turned on, tape for ET tube is available).
 Be sure that the warmer bed is flattened and not at an angle; the latter position of the bed will distort airway landmarks.
Be sure that the warmer bed is flattened and not at an angle; the latter position of the bed will distort airway landmarks.
 Choose the appropriately sized ET tube.
Choose the appropriately sized ET tube.
 Position the baby with the neck slightly extended and the chin up (use a roll under the shoulders to achieve proper extension if necessary). Do not hyperextend the neck ( Fig. 18-3).
Position the baby with the neck slightly extended and the chin up (use a roll under the shoulders to achieve proper extension if necessary). Do not hyperextend the neck ( Fig. 18-3).
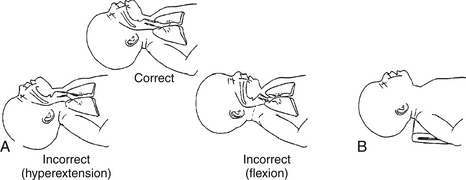
Figure 18-3 A, Correct and incorrect head positions for resuscitation. B, Optimal shoulder roll use for maintaining correct head position. (From Goldmsith JP, Karotkin EH, editors. Assisted ventilation of the neonate. 4th ed. Philadelphia: Saunders; 2003. p. 68.)
 Make sure the hypopharynx has been suctioned to clear debris.
Make sure the hypopharynx has been suctioned to clear debris.
 Using the laryngoscope blade to visualize the vocal cords, insert the ET tube to the appropriate depth. (Limit intubation attempts to approximately 20 seconds to avoid reflex bradycardia.)
Using the laryngoscope blade to visualize the vocal cords, insert the ET tube to the appropriate depth. (Limit intubation attempts to approximately 20 seconds to avoid reflex bradycardia.)
 Institute manual ventilation while holding the tube in a secure position.
Institute manual ventilation while holding the tube in a secure position.
 Listen for equal breath sounds on both sides of the chest.
Listen for equal breath sounds on both sides of the chest.
 Auscultate over the stomach to make sure there is not an esophageal intubation.
Auscultate over the stomach to make sure there is not an esophageal intubation.
 Watch for symmetric chest rise. Give just enough positive pressure to initiate chest rise.
Watch for symmetric chest rise. Give just enough positive pressure to initiate chest rise.
 Use of a CO2 detector may assist in reassuring that you are in the airway.
Use of a CO2 detector may assist in reassuring that you are in the airway.
In a depressed infant with gasping or absent respirations, 100% oxygen should be given by positive pressure ventilation. Depending on the extent of asphyxia (and depression of heart rate), cardiac compressions are usually initiated within 30 seconds. If there is no response (i.e., increased heart rate) after at least 30 seconds of positive pressure ventilation with 100% oxygen and chest compressions, epinephrine is indicated. As Kattwinkel et al. explain, “The recommended IV dose is 0.01 to 0.03 mg/kg per dose. Higher IV doses are not recommended because animal and pediatric studies show exaggerated hypertension, decreased myocardial function, and worse neurologic function after administration of IV doses in the range of 0.1 mg/kg. If the endotracheal route is used, doses of 0.01 or 0.03 mg/kg will likely be ineffective. Therefore, IV administration of 0.01 to 0.03 mg/kg per dose (0.1-0.3 mL of the 1:10,000 solution), is the preferred route.” 16
NRP 2010 states that “chest compressions are indicated for a heart rate that is less than 60 per minute despite adequate ventilation with supplementary oxygen for 30 seconds. Because ventilation is the most effective action in neonatal resuscitation and because chest compressions are likely to compete with effective ventilation, rescuers should ensure that assisted ventilation is being delivered optimally before starting chest compressions. Compressions should be delivered on the lower third of the sternum to a depth of approximately one third of the anterior-posterior diameter of the chest. Two techniques have been described: compression with 2 thumbs with fingers encircling the chest and supporting the back (the 2 thumb–encircling hands technique) or compression with 2 fingers with a second hand supporting the back. Because the 2 thumb–encircling hands technique may generate higher peak systolic and coronary perfusion pressure than the 2-finger technique, 76–80, the 2 thumb–encircling hands technique is recommended for performing chest compressions in newly born infants.” 17
53. What are the common medications used for newborn resuscitation? How are they given, and in what doses?
If the heart rate remains below 60 beats per minute despite adequate ventilation with 100% oxygen and chest compressions, administration of epinephrine or volume expansion (or both) may be indicated. Rarely, buffers, narcotic antagonists, or vasopressors may be useful after resuscitation; they are not recommended in the delivery room. The recommended IV dose of epinephrine is 0.01 to 0.03 mg/kg per dose. Higher doses are not recommended.
“Volume expansion should be considered when blood loss is known or suspected (pale skin, poor perfusion, weak pulse) and the baby’s heart rate has not responded adequately to other resuscitative measures. An isotonic crystalloid solution or blood is recommended for volume expansion in the delivery room. The recommended dose is 10 mL/kg, which may need to be repeated. When resuscitating premature infants, care should be taken to avoid giving volume expanders rapidly, because rapid infusions of large volumes have been associated with intraventricular hemorrhage.” 72
Hypoglycemia can be very damaging to the developing nervous system. It can result when hepatic glycogen stores are depleted as a result of stress. A blood glucose level below 40 mg/dL warrants treatment. The clinician should infuse 10% dextrose in water at a dose of 2 mL/kg over 10 to 15 minutes in an attempt to correct hypoglycemia. The target glucose concentration is greater than 45 to 50 mg/dL before each feeding. It is not necessary to use higher concentrations of glucose (e.g., D25W) in such circumstances. After the hypoglycemia has been corrected, normoglycemia can usually be maintained by an infusion rate of 5 to 8 mg/kg/min. In some circumstances hypoglycemia may not be corrected until the infusion rate is 8 to 12 mg/kg/min. Infants receiving levels this high who continue to demonstrate hypoglycemia may have an islet cell adenoma of the pancreas that is producing hyperinsulinemia. 19
These clinical signs are pulse rate and quality, capillary refill time, and urine output.
 Cranial injuries: caput succedaneum, subconjunctival hemorrhage, cephalohematoma, subgaleal hemorrhage, skull fractures, intracranial hemorrhage, cerebral edema
Cranial injuries: caput succedaneum, subconjunctival hemorrhage, cephalohematoma, subgaleal hemorrhage, skull fractures, intracranial hemorrhage, cerebral edema
 Spinal injuries: spinal cord transection
Spinal injuries: spinal cord transection
 Peripheral nerve injuries: brachial palsy (Erb–Duchenne palsy, Klumpke paralysis), phrenic nerve and facial nerve paralysis
Peripheral nerve injuries: brachial palsy (Erb–Duchenne palsy, Klumpke paralysis), phrenic nerve and facial nerve paralysis
 Visceral injuries: liver rupture or hematoma, splenic rupture, adrenal hemorrhage
Visceral injuries: liver rupture or hematoma, splenic rupture, adrenal hemorrhage
 Skeletal injuries: fractures of the clavicle, femur, and humerus
Skeletal injuries: fractures of the clavicle, femur, and humerus
No precise answer is possible because clinical circumstances and responses are variable. However, in one study of 58 newborns with an Apgar score of 0 at 10 minutes despite appropriate resuscitative efforts, only 1 of 58 survived, and that infant had profound CP. Studies of therapeutic hypothermia, however, show that initiaton of cooling within 6 hours improves outcomes. According to NRP guidelines (see first reference below), “In a newly born baby with no detectable heart rate, it is appropriate to consider stopping resuscitation if the heart rate remains undetectable for 10 minutes. The decision to continue resuscitation efforts beyond 10 minutes with no heart rate should take into consideration factors such as the presumed etiology of the arrest, the gestation of the baby, the presence or absence of complications, the potential role of therapeutic hypothermia, and the parents’ previously expressed feelings about acceptable risk of morbidity.”
Prolonged resuscitation has a very high risk of ischemic injury to the brain, resulting in cystic encephalomalacia, CP, severe microcephaly, and developmental delay. Failure of response after more than 10 to 15 minutes should prompt the clinician to consider cessation of therapy, as difficult as that always is to do. 202122
Radiology of Pulmonary Disorders of the Neonate
61. Where should the tip of an umbilical arterial catheter in satisfactory position project on an anteroposterior (AP) radiograph of the chest and abdomen?
 Careful placement under sterile conditions
Careful placement under sterile conditions
 Daily evaluation of ease of injection and withdrawal of blood
Daily evaluation of ease of injection and withdrawal of blood
 Assessment of the pressure waveform on the monitor screen
Assessment of the pressure waveform on the monitor screen
 Inspection of the site for erythema and induration
Inspection of the site for erythema and induration
 Daily evaluation of urine output and blood pressure
Daily evaluation of urine output and blood pressure
 Prompt removal of the line as soon as it is no longer needed
Prompt removal of the line as soon as it is no longer needed
Umbilical catheters may be left in place for many days as long as the aforementioned conditions are satisfactorily met. The typical goal is to remove umbilical lines within 7 days of birth. In extreme cases a catheter can be kept in place for 3 weeks without complication. One of the most common errors that is made in neonatal medicine, however, is to leave a catheter in place that is no longer necessary. Because it is not needed, the catheter is often not checked religiously and the risk of complications rises dramatically.
62. Where should the tip of an umbilical venous catheter (UVC) be placed for satisfactory projection on an AP radiograph of the chest and abdomen?
63. What is the best position, as seen on an AP radiograph of the chest, for the tip of an ET tube in an intubated neonate?
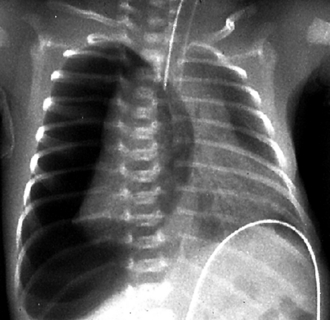
The classic RDS picture in a premature neonate has a diffuse increase in lung density (opacity) with a fine, reticulogranular (grainy) or ground-glass appearance, air bronchograms (a darker appearance to the branching central airway in contrast to the opacity of the lungs), and low lung volumes ( Fig. 18-4).
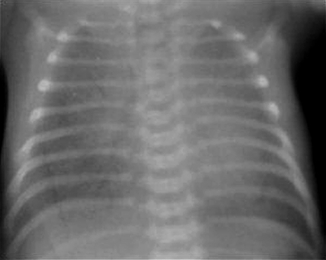
Figure 18-4 Radiograph of a baby with severe respiratory distress syndrome. Note the generalized haziness caused by atelectasis and the air bronchograms throughout the lung.
66. What would be a typical appearance of the lungs in a newborn with significant meconium aspiration?
Affected babies often have a coarse, irregular increase in lung markings accompanied by hyperinflation of the lungs. The pneumonic process here is one of patchy atelectasis and overdistention ( Fig. 18-5). Pneumomediastinum and pneumothorax are frequent accompanying abnormalities as well.
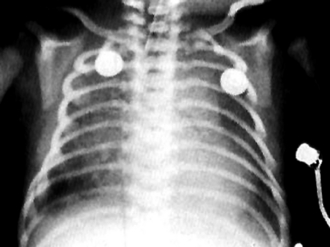
Figure 18-5 Radiograph of an infant with severe meconium aspiration syndrome, marked by hazy densities throughout the lung.
67. In a newborn with suspected transient tachypnea of the newborn (e.g., wet lung syndrome, transient respiratory distress of the newborn, delayed reabsorption of fetal lung fluid), approximately how long should it take for the chest radiograph to return to a normal appearance to be consistent with this diagnosis?
68. What should the clinician look for on the chest radiograph of a newborn in whom congenital diaphragmatic hernia is suspected (usually by antenatal sonography of the fetus)?
69. If a unilateral pneumothorax is suspected in a newborn, what is the best projection of the chest to confirm or exclude this diagnosis?
Early air leaks are often difficult to diagnose. The most obvious finding is a separation of the edge or margin of the lung from the inner margin of the chest wall, with no lung markings definable in that space. An AP decubitus view of the chest with the side of suspected pneumothorax to the top (nondependent) is also helpful. For example, if you suspect a left-sided pneumothorax, you should order a “right decubitus AP chest radiograph,” which means the right side of the patient will be dependent and the left side nondependent. If a pneumothorax is present, look for a zone of lucency representing pleural air collecting between the lateral chest margin and the adjacent lung ( Fig. 18-6).
Respiratory Distress Syndrome
The small one collapses more quickly because of surface tension. The LaPlace relationship states P = 2T/R, where P is the pressure across the wall of the sphere, T is surface tension of the substance forming the bubble (i.e., its tendency to collapse), and R is the radius of the sphere. The smaller the radius, the greater the collapsing pressure ( Fig. 18-7).
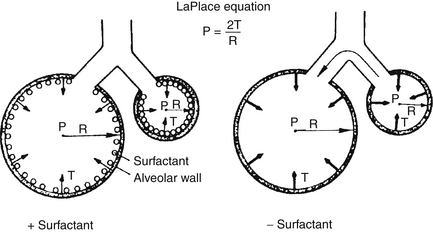
Figure 18-7 The LaPlace relationship. In the absence of surfactant, the smaller alveolus has a greater surface tension and tends to empty into the larger alveolus. In the presence of surfactant, the compacting of surface tension–reducing surfactant acts to “splint” the lung against further collapse. (Courtesy F. Netter, CIBA-Geigy Corp., Ardsley, New York.)
71. What are the physiologic, physical, and biochemical factors that result in pulmonary vasodilation at the time of birth?
Within minutes after delivery, pulmonary artery pressure falls, and blood flow increases in response to birth-related stimuli, such as ventilation, increased PO2, and shear stress. Physical stimuli, including increased shear stress, lung inflation, ventilation, and increased oxygen, cause pulmonary vasodilation in part by increasing production of vasodilators, nitric oxide, and prostacyclin. Pretreatment with the nitric oxide synthase inhibitor, nitro-L-arginine, attenuates pulmonary vasodilation after delivery by 50% in near-term fetal lambs. These findings suggest that a significant part of the rise in pulmonary blood flow at birth may be related directly to the acute release of nitric oxide. Each of the birth-related stimuli can stimulate nitric oxide release independently, followed by vasodilation through cyclic guanosine monophosphate kinase–mediated stimulation of K channels. Although the endothelial isoform of nitric oxide synthase III has been presumed to be the major contributor of nitric oxide at birth, recent studies suggest that other isoforms (neuronal type I and inducible type II) may be important sources of nitric oxide release in utero and at birth. Other vasodilators, especially prostacyclin, also modulate changes in pulmonary vascular tone. Rhythmic lung distention and shear stress stimulate both prostacyclin and nitric oxide production in late gestation. Increasing oxygen tension also triggers nitric oxide activity and overcomes the effects of prostacyclin inhibition at birth. Thus, although nitric oxide does not account for the entire fall in pulmonary vascular resistance at birth, nitric oxide synthase activity appears important in achieving postnatal adaptation of lung circulation.
More recently, vasculoendothelial growth factor (VEGF), which is really a family of growth factors, and angiopoietins 1 through 4 have been also shown to have important angiogenic roles during fetal development of the pulmonary vascular bed. These agents also appear to be closely related to the release and influence of nitric oxide in pulmonary development and vasodilation. Additional proteins have been elaborated that may play critical roles in this entire process, demonstrating the increasing complexity of our understanding of neonatal lung development. 23
Surfactant is inactivated in the alveolar space without large changes in amounts of its components. The monolayer does break down as protein and lipid dissociate. Surfactant changes to a small aggregate form that minimally reduces surface tension. These aggregates are then absorbed by macrophages and type II cells, which recycle lipid and protein components. 24
Analyses of more recent studies challenge this view. When studies that allowed for routine stabilization on CPAP are evaluated by meta-analysis, they demonstrated a decrease in the risk of chronic lung disease or death in infants stabilized on CPAP. Recent large trials that reflect current practice (including greater utilization of maternal steroids and routine postdelivery stabilization on CPAP) do not support the differences seen in earlier studies and demonstrate less risk of chronic lung disease or death when using early stabilization on CPAP with selective surfactant administration to infants requiring intubation. 2526
Should infants with RDS receive high-frequency ventilation? The primary pathology of RDS comes from an inability to maintain lung inflation and fluid leak into the alveolar space. Secondary pathology originates from positive pressure reexpansion of collapsed alveoli. A ventilation strategy that maintains lung volume and avoids large distending pressure seems ideal. That is the idea behind high-frequency ventilation for RDS. The lung is inflated, and lung volumes are maintained while gas exchange occurs, using tidal volumes less than dead space. High-frequency ventilator technology is improving, and its applicability as the first-line treatment for RDS continues to be evaluated. Consensus has not yet been reached, however. 272829
CPAP is continous positive airway pressure. CPAP is applied to an infant’s airway using a variety of devices to maintain positive pressure in the airway during spontaneous breathing. These devices include a head hood, face chamber, face mask, several types of nasal cannulae, nasopharyngeal tube, and ET tube. Nasal cannulae inserted into the nares are used most often. Not all CPAP devices are equal, and they have varying degrees of success. They have been associated with a number of problems, such as difficulty with gaining access to the baby and maintaining connection to the airway, increase in dead space, and increase in airway resistance.
 Increase transpulmonary pressure and functional residual capacity
Increase transpulmonary pressure and functional residual capacity
 Prevent alveolar collapse and decrease intrapulmonary shunting
Prevent alveolar collapse and decrease intrapulmonary shunting
These include but are not limited to the following:
 Diseases with a low functional residual capacity (e.g., RDS)
Diseases with a low functional residual capacity (e.g., RDS)
 Apnea and bradycardia of prematurity
Apnea and bradycardia of prematurity
 Airway closure disease (e.g., bronchiolitis, BPD)
Airway closure disease (e.g., bronchiolitis, BPD)
 Pneumothorax (<2%): usually occurs in the acute phase and is usually more benign than when it occurs during mechanical ventilation. Pneumothorax is not a contraindication for CPAP therapy.
Pneumothorax (<2%): usually occurs in the acute phase and is usually more benign than when it occurs during mechanical ventilation. Pneumothorax is not a contraindication for CPAP therapy.
 Nasal obstruction: obstruction caused by secretions or improper positioning of CPAP prongs. Secretions in nasal cavities should be suctioned every 4 hours or as needed.
Nasal obstruction: obstruction caused by secretions or improper positioning of CPAP prongs. Secretions in nasal cavities should be suctioned every 4 hours or as needed.
 Abdominal distention from swallowed air: This is usually benign and occurs more commonly in the chronic than acute phase. Abdominal distention can be treated by intermittent aspiration of the stomach. For severe distention an indwelling orogastric tube may be required.
Abdominal distention from swallowed air: This is usually benign and occurs more commonly in the chronic than acute phase. Abdominal distention can be treated by intermittent aspiration of the stomach. For severe distention an indwelling orogastric tube may be required.
 Nasal or septal erosion or necrosis: This finding is a concern in a VLBW premature infant with sensitive skin who may need CPAP therapy for weeks. However, this can be prevented by choosing a properly sized CPAP cannula and avoiding compression of the septum. A snug cap is used to hold the tubings securely in place, and self-adhesive Velcro is used to keep the cannulae away from the septum.
Nasal or septal erosion or necrosis: This finding is a concern in a VLBW premature infant with sensitive skin who may need CPAP therapy for weeks. However, this can be prevented by choosing a properly sized CPAP cannula and avoiding compression of the septum. A snug cap is used to hold the tubings securely in place, and self-adhesive Velcro is used to keep the cannulae away from the septum.
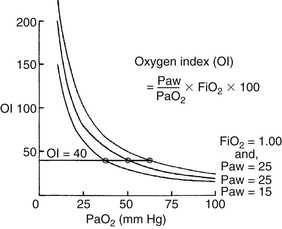
The oxygenation index (OI) is used to express the severity of the respiratory disease.
where MAP = mean airway pressure, FiO2 = fractional concentration of oxygen in inspired gas, PaO2 = partial pressure of oxygen in arterial blood, PIP = peak inspiratory pressure, PEEP = positive end-expiratory pressure, TI = inspiration time, and TE = expiration time.
Note that the MAP is influenced by all respirator controls except the FiO2. However, without a uniform ventilation strategy, the oxygenation index cannot be universally applied as an expression of severity of respiratory disease. This statement is especially true in the NICU, where patients may be hyperventilated; in these patients the MAP, and thus the oxygenation index, is elevated regardless of the severity of disease.
Meconium-Stained Amniotic Fluid and Meconium Aspiration Syndrome
No. Those who have an initial heart rate greater than 100 bpm, good respiratory effort, and reasonable tone will not benefit from intubation and suctioning. In fact, some vigorous infants may be injured in the process of suctioning because they are so difficult to restrain. 3031
83. How long has meconium been present in the amniotic fluid if an infant has evidence of meconium staining?
85. What pulmonary disorder is most frequently associated with persistent pulmonary hypertension of the newborn (PPHN)?
Causes of aspirated meconium are as follows:
 Alveolar and parenchymal inflammation
Alveolar and parenchymal inflammation
 Alveolar and parenchymal edema
Alveolar and parenchymal edema
 Altered pulmonary vasoreactivity leading to pulmonary vasoconstriction, increased pulmonary resistance, and right-to-left shunting
Altered pulmonary vasoreactivity leading to pulmonary vasoconstriction, increased pulmonary resistance, and right-to-left shunting
 Direct toxicity of meconium constituents on pulmonary parenchyma leading to ischemia and necrosis
Direct toxicity of meconium constituents on pulmonary parenchyma leading to ischemia and necrosis
 Surfactant dysfunction (inactivation and decreased production of SP-A and SP-B)
Surfactant dysfunction (inactivation and decreased production of SP-A and SP-B)
 Altered lung elastic forces (increased resistance, decreased compliance) ( Fig. 18-8)
Altered lung elastic forces (increased resistance, decreased compliance) ( Fig. 18-8)
87. What disorder makes up the largest proportion of neonates who are treated with extracorporeal membrane oxygenation (ECMO)?
88. Meconium-stained amniotic fluid (MSAF) is found across all races and socioeconomic strata in humans. Additionally, MSAF and MAS are noted frequently in domestic animals. How do farmers and veterinarians manage MSAF in an effort to prevent MAS?
89. Is thin-consistency MSAF more likely to enter the airways and cause MAS or other forms of respiratory distress compared with thick-consistency MSAF?
No. The thicker the consistency of MSAF, the greater the likelihood of MAS or other respiratory distress. There is at least a sevenfold increase in the incidence of respiratory disorders among infants born through “pea-soup” MSAF compared with those born through watery-consistency MSAF.
90. What mechanisms of meconium aspiration into the lungs contribute to ventilatory failure, and what is the role of surfactant therapy in the treatment of this condition?
Meconium-induced lung injury is associated with many pulmonary changes that contribute to respiratory failure. These include airway obstruction, inflammation with release of vasoactive substances, and surfactant dysfunction. Meconium has the ability to inactivate surfactant both in vivo and in vitro and has direct effects on type II pneumocyte function. In both animal models and human infants who have aspirated meconium and who are undergoing pulmonary fluid analysis, inflammatory cell numbers and total protein are significantly elevated compared with infants in the control group. Various inflammatory mediators, including myeloperoxidase and interleukin-8, are increased. Maximal influx of inflammatory cells occurs by 16 hours of age with some recovery by 72 hours. These findings support the role of surfactant replacement in infants with MAS that requires ventilatory support. 3233
Persistent Pulmonary Hypertension of the Newborn
92. When was PPHN first described? Why is persistent fetal circulation not an accurate term to describe PPHN?
The shunting across the foramen ovale and ductus arteriosus as a result of suprasystemic pulmonary arterial pressure seen in PPHN is very similar to fetal circulation. However, the exclusion of placental circulation and the fact that ductus venosus may or may not be patent preclude the use of the term persistent fetal circulation to describe this condition. The term persistent pulmonary hypertension of the newborn describes the pathophysiology of the disease more accurately, indicating that the critical problem in this situation is the failure of the pulmonary circulation to decrease to normal pressures. 34353637
 Labile hypoxemia or cyanosis disproportionate to the level of respiratory distress may be present. These infants are extremely sensitive to environmental stimuli.
Labile hypoxemia or cyanosis disproportionate to the level of respiratory distress may be present. These infants are extremely sensitive to environmental stimuli.
 Infants with significant ductal shunting have higher oxygen saturation in the right hand (preductal) than in the legs (postductal). Similarly, arterial PO2 in the right radial artery is significantly greater than that obtained from the umbilical artery. Infants with predominant shunting at the level of foramen ovale have similar preductal and postductal oxygen levels.
Infants with significant ductal shunting have higher oxygen saturation in the right hand (preductal) than in the legs (postductal). Similarly, arterial PO2 in the right radial artery is significantly greater than that obtained from the umbilical artery. Infants with predominant shunting at the level of foramen ovale have similar preductal and postductal oxygen levels.
 Cardiac murmur compatible with tricuspid insufficiency is present.
Cardiac murmur compatible with tricuspid insufficiency is present.
 Chest radiograph may reveal cardiomegaly. The underlying disease (e.g., congenital diaphragmatic hernia, RDS) alters the radiologic picture. Infants with idiopathic PPHN have clear and undervascularized lung fields (“black-lung” PPHN).
Chest radiograph may reveal cardiomegaly. The underlying disease (e.g., congenital diaphragmatic hernia, RDS) alters the radiologic picture. Infants with idiopathic PPHN have clear and undervascularized lung fields (“black-lung” PPHN).
 Echocardiography is important to rule out cyanotic congenital heart disease and establish the diagnosis. In infants with PPHN, shunting at the atrial and ductal level can be demonstrated. Tricuspid insufficiency, right ventricular hypertrophy, septal deviation to the left, and prolonged right ventricular systolic intervals support the diagnosis of PPHN.
Echocardiography is important to rule out cyanotic congenital heart disease and establish the diagnosis. In infants with PPHN, shunting at the atrial and ductal level can be demonstrated. Tricuspid insufficiency, right ventricular hypertrophy, septal deviation to the left, and prolonged right ventricular systolic intervals support the diagnosis of PPHN.
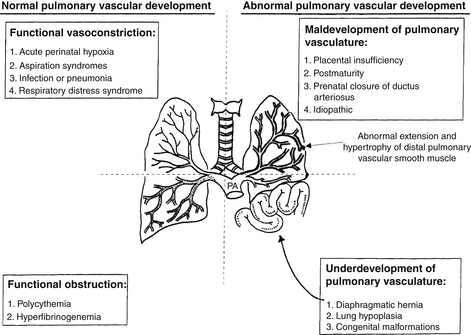
The common causes of PPHN are summarized in the mnemonic DIAPHRAGMATIC:
 Diaphragmatic hernia (hypoplastic lungs)
Diaphragmatic hernia (hypoplastic lungs)
 Infection (including pneumonia), especially GBS
Infection (including pneumonia), especially GBS
 Aspiration syndromes (e.g., meconium, amniotic fluid)
Aspiration syndromes (e.g., meconium, amniotic fluid)
 Hyperviscosity (polycythemia, hyperfibrinogenemia)
Hyperviscosity (polycythemia, hyperfibrinogenemia)
 RDS (i.e., hyaline membrane disease)
RDS (i.e., hyaline membrane disease)
 Growth retardation (placental insufficiency)
Growth retardation (placental insufficiency)
 Maternal nonsteroidal antiinflammatory drug ingestion
Maternal nonsteroidal antiinflammatory drug ingestion
 Transient tachypnea of newborn
Transient tachypnea of newborn
 Idiopathic (“black lung” PPHN)
Idiopathic (“black lung” PPHN)
 Congenital anomalies of the lung, alveolar-capillary dysplasia
Congenital anomalies of the lung, alveolar-capillary dysplasia
The causes of PPHN can also be classified according to the predominant abnormality involved (see Fig. 18-2).
Persistently elevated pulmonary vascular resistance increases right ventricular afterload and oxygen demand and impairs oxygen delivery to cardiac muscle. Ischemic damage to the myocardium, papillary muscle necrosis, and tricuspid regurgitation can occur. Increased right ventricular pressure displaces the septum into the left ventricle, impairs left ventricular filling, and decreases cardiac output. Myocardial dysfunction is an important cause for mortality in PPHN ( Fig. 18-9).
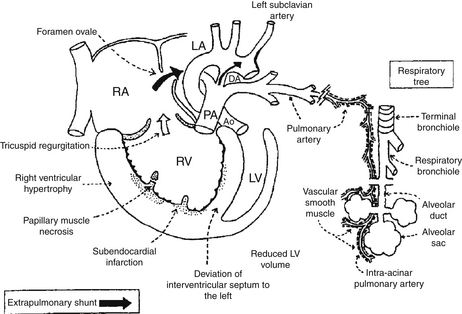
Figure 18-9 Pathophysiology of persistent pulmonary hypertension (PPHN) of the newborn. Suprasystemic pulmonary arterial pressure results in right ventricular (RV) hypertrophy and deviation of the intraventricular septum to the left. This reduces the left ventricular (LV) volume and decreases systemic output. Extrapulmonary right-to-left shunting occurs at the foramen ovale from the right atrium (RA) to the left atrium (LA) and at the level of the ductus arteriosus (DA) from the pulmonary artery (PA) to the aorta (Ao). Normally, PAs distal to the level of terminal bronchioles are not muscular. Abnormal extension and hypertrophy of distal pulmonary vascular smooth muscle (shown as interrupted lines), sometimes to the level of intra-acinar arteries, is seen in severe PPHN.
In the past the mortality for infants with PPHN ranged from 20% to 40%, and the incidence of neurologic handicap ranged from 12% to 25%. With recent advances in conservative management, survival and neurodevelopmental outcome have improved considerably. In most centers inhaled nitric oxide and ECMO have further reduced mortality associated with severe PPHN. Survival rates between 76% and 93% have been reported for infants with pneumonia, meconium aspiration, and idiopathic PPHN who require ECMO. The outlook for infants with diaphragmatic hernia requiring ECMO has not been as dramatic, and survival is still only about 60% to 80%.
 Infants presenting with more severe parenchymal disease may have persistent tachypnea and bronchospasm.
Infants presenting with more severe parenchymal disease may have persistent tachypnea and bronchospasm.
 Neurologic development may be impaired in children with PPHN, especially if they are severely asphyxiated.
Neurologic development may be impaired in children with PPHN, especially if they are severely asphyxiated.
 An increased incidence of sensorineural hearing loss among infants with PPHN treated with hyperventilation and alkalization has been reported. 3839
An increased incidence of sensorineural hearing loss among infants with PPHN treated with hyperventilation and alkalization has been reported. 3839
Inhaled Nitric Oxide
Several randomized control trials indicate that inhaled nitric oxide reduces the incidence of the combined end point of death or need for ECMO compared with patients not offered treatment with inhaled nitric oxide. This reduction seems to be entirely due to a reduction in the use of ECMO insofar as mortality is not reduced. 40
101. Inhaled nitric oxide acts like an endothelium-relaxing factor and is a major regulator of vascular smooth muscle tone. Why doesn’t it also dilate the systemic vascular system?
Nitric oxide has a high affinity for the iron of all heme proteins, including reduced hemoglobin, with which it forms nitrosyl hemoglobin (NOHb). The NOHb is then oxidized to methemoglobin with the production of nitrate. As a result, when given by inhalation, nitric oxide is inactivated before acting on any systemic vascular bed, while relaxing the pulmonary vascular smooth muscle through the cyclic GMP production. In normal development endogenous nitric oxide produced in endothelial cells from oxygen and L-arginine diffuses into smooth cells in the vascular wall and causes vasodilation. Nitric oxide that diffuses into the blood vessel lumen is avidly bound by hemoglobin and does not cause systemic vasodilatation.
102. Does inhaled nitric oxide reduce the use of ECMO in neonates with congenital diaphragmatic hernia?
Meta-analysis showed that infants with diaphragmatic hernia do not appear to share the benefits of inhaled nitric oxide that infants with other causes of hypoxemic respiratory failure experience. Indeed, there are suggestions that outcomes may be worse in infants with congenital diaphragmatic hernia who received inhaled nitric oxide compared with control subjects. This analysis showed that the incidence of death or requiring ECMO was 40 of 46 among control patients and 36 of 38 among patients treated with nitric oxide (relative risk, 1.09; 95% confidence interval [CI], 0.95 to 1.26). Mortality rates were similar in control and treatment patients (18 of 46 in the control group compared with 18 of 38 in the treatment group; relative risk of death, 1.20; 95% CI, 0.74 to 1.96), but there was a significant increase in the requirement for ECMO in infants treated with inhaled nitric oxide (31 of 46 in the control group compared with 32 of 38 in the treatment group; relative risk, 1.27; 95% CI, 1.00 to 1.62). 41
103. What are the major concerns regarding the weaning of nitric oxide and oxygen in infants with PPHN?
When nitric oxide and O2 come into contact, peroxynitrite (ONOO−), a potent oxidant, is formed. The relative amount of nitric oxide, O2−,ONOO−, and antioxidants in the airway will determine whether nitric oxide will be beneficial or potentially toxic. These oxidants can contribute to lung injury by enhancing lung inflammation, producing pulmonary edema, and reducing surfactant function. Furthermore, recent findings have shown that abrupt withdrawal of inhaled nitric oxide, even in infants with minimal or no response, can induce worsening pulmonary hypertension. The potential for pulmonary inflammatory injury can be decreased as the concentrations of inhaled nitric oxide and O2 are lowered. Most late preterm and term infants can be weaned off inhaled nitric oxide within 4 days. 42
104. What are the indications and the risks associated with the use of inhaled nitric oxide for the treatment of ventilatory failure in preterm infants?
One population that may be an exception is preterm infants born after prolonged rupture of membranes. Multiple small studies suggest that preterm infants with severe respiratory failure born after premature and prolonged rupture of membranes may benefit from inhaled nitric oxide 4344
Major Anomalies that Alter Pulmonary Function
105. Infants with fetal akinesia syndrome (Pena–Shokeir phenotype) frequently have pulmonary anomalies. Describe the pulmonary anomalies in this disorder.
Infants with Pena–Shokeir phenotype (also termed arthrogryposis multiplex congenita with pulmonary hypoplasia) have gracile ribs and reduced thoracic volume. Also present are a lack of fetal breathing activity; polyhydramnios resulting from a lack of fetal swallowing; and intrauterine constraint, resulting in muscular hypoplasia involving both intercostal and diaphragmatic musculature. Thoracic wall weakness, hypotonia of the muscles of respiration, and anterior horn cell atrophy or deficiency lead to reduced ventilatory drive, which may improve over time for some infants.
106. Fetal airway obstruction can be the direct result of intrinsic defects in the larynx or trachea, resulting in congenital high airway obstruction syndrome. What is the differential diagnosis of extrinsic fetal obstruction?
Mechanical Ventilation of the Neonate
 Time-cycled, pressure-limited ventilators have become the standard in neonatal mechanical ventilation because of the problems associated with volume-cycled ventilators. Time-cycled, pressure-limited ventilators have the advantage of providing continuous flow through the circuit, which allows the infant to take spontaneous breaths of fresh gas between mechanical breaths (the mechanical breaths are referred to as intermittent mandatory ventilation [IMV]). The system gives the operator direct control over the delivered peak inspiratory pressure (PIP) and allows for easier compensation for leakage around ET tubes, and the decelerating flow pattern allows better gas distribution within the lungs.
Time-cycled, pressure-limited ventilators have become the standard in neonatal mechanical ventilation because of the problems associated with volume-cycled ventilators. Time-cycled, pressure-limited ventilators have the advantage of providing continuous flow through the circuit, which allows the infant to take spontaneous breaths of fresh gas between mechanical breaths (the mechanical breaths are referred to as intermittent mandatory ventilation [IMV]). The system gives the operator direct control over the delivered peak inspiratory pressure (PIP) and allows for easier compensation for leakage around ET tubes, and the decelerating flow pattern allows better gas distribution within the lungs.
 Volume-cycled ventilators deliver a preset tidal volume, usually in a constant-flow fashion, generating whatever pressure is necessary to deliver the gas into the lungs. This results in a triangular pressure and volume waveforms with maximum volume and pressure being reached just before the onset of exhalation.
Volume-cycled ventilators deliver a preset tidal volume, usually in a constant-flow fashion, generating whatever pressure is necessary to deliver the gas into the lungs. This results in a triangular pressure and volume waveforms with maximum volume and pressure being reached just before the onset of exhalation.
The chief disadvantage is the fact that tidal volume is not directly controlled. The delivered tidal volume is determined by the interaction between PIP and lung compliance. Consequently, as compliance changes, so will the delivered tidal volume. Improving lung compliance can lead to excessive tidal volume and can cause lung injury. Conversely, worsening compliance can lead to hypoventilation and loss of lung volume. In addition, if an infant is breathing asynchronously with the ventilator, peak pressures are reached quickly, and volume is reduced. This situation may result in a serious deterioration of blood gases.
111. What are the ways to increase ventilation (improve CO2 removal) in an infant on time-cycled, pressure-limited ventilation?
Paw has been shown to be a major determinant of oxygenation. Adequate distending pressure is needed to maintain lung volume and prevent the diffuse microatelectasis that leads to ventilation–perfusion imbalance with consequent hypoxemia. 45
113. List the key ventilator variables that affect Paw in conventional time-cycled, pressure-limited ventilation.
Paw is the area under the pressure curve ( Fig. 18-10). Increasing the PEEP is usually the most effective means of increasing the Paw. The least recognized factor affecting the area under the curve is the slope of the upstroke of pressure, which determines the shape of the pressure waveform. Higher flow leads to more rapid upstroke and a more square-shaped curve, which has a larger area than one with a gradual upstroke and a more triangular shape.
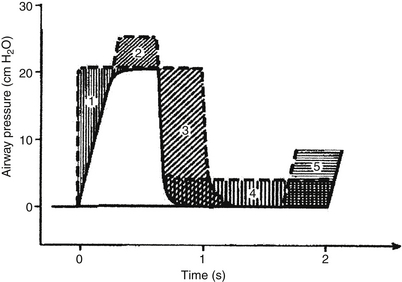
Figure 18-10 Effects of changes in airway pressures and timing on the respiratory waveform and mean airway pressure (Paw). Different waveforms will have different associated blood gases in many instances. (From Reynolds EOM. Pressure waveform and ventilator settings for mechanical ventilation in severe hyaline membrane disease. Int Anesthesiol Clin 1974;12:259.)
114. When placing an infant on conventional time-cycled, pressure-limited ventilation, how do you choose the initial PIP?
For any given PIP the delivered tidal volume will be determined by the compliance of the baby’s lungs. Select a pressure based on the best estimate of what the infant will need, and observe the result. If adequate chest rise, good breath sounds, and oxygenation are apparent, the pressure is appropriate. If the chest rise is excessive, reduce the PIP, and if the chest movement is inadequate, higher PIP is needed (assuming the ET tube is correctly positioned).
Note: Some devices measure VT at the point where the circuit attaches to the ventilator. This position is undesirable because it will give an artificially large VT measurement, ignoring the loss of VT to compression of gas in the circuit and circuit stretching. Furthermore, gadgets do malfunction, so continue to use your eyes and ears to verify that the “numbers” are believable. 4647
115. List as many possible causes of acute CO2 retention in an infant on mechanical ventilation as you can. (There are many more than you may think.)
 The ET tube is occluded with secretions.
The ET tube is occluded with secretions.
 The ET tube is up against the carina.
The ET tube is up against the carina.
 There is an accumulation of secretions in the airways (patient needs suctioning).
There is an accumulation of secretions in the airways (patient needs suctioning).
 The patient has a pneumothorax and some other condition that acutely decreased lung compliance.
The patient has a pneumothorax and some other condition that acutely decreased lung compliance.
 Acute bronchospasm is present.
Acute bronchospasm is present.
 Oversedation with suppression of spontaneous respiratory effort is occurring.
Oversedation with suppression of spontaneous respiratory effort is occurring.
 The ventilator is malfunctioning (e.g., leak in circuit, partial disconnection).
The ventilator is malfunctioning (e.g., leak in circuit, partial disconnection).
 There has been an acute onset of sepsis with loss of spontaneous respiratory effort.
There has been an acute onset of sepsis with loss of spontaneous respiratory effort.
 Acute abdominal distention or the presence of a large abdominal mass is leading to decreased diaphragmatic excursion.
Acute abdominal distention or the presence of a large abdominal mass is leading to decreased diaphragmatic excursion.
 Acute lung injury (barotrauma or volutrauma, such as pneumothorax, pneumomediastinum, pneumopericardium, pulmonary interstitial emphysema)
Acute lung injury (barotrauma or volutrauma, such as pneumothorax, pneumomediastinum, pneumopericardium, pulmonary interstitial emphysema)
 Chronic lung injury (chronic lung disease, BPD)
Chronic lung injury (chronic lung disease, BPD)
 Hemodynamic impairment caused by increased intrathoracic pressure
Hemodynamic impairment caused by increased intrathoracic pressure
 Intraventricular hemorrhage and periventricular leukomalacia
Intraventricular hemorrhage and periventricular leukomalacia
 Tracheal damage with subglottic stenosis
Tracheal damage with subglottic stenosis
 Palatal groove and damage to tooth buds of the upper incisors
Palatal groove and damage to tooth buds of the upper incisors
117. Which of the following infants, each ventilated and with PIP of 25 cm H2O, PEEP of 5 cm H2O, IMV of 90 breaths per minute, and an inspiratory time of 0.3 seconds, is least likely to experience hypercarbia, hemodynamic impairment, and air leakage resulting from incomplete exhalation (air trapping): (A) A 12-hour-old, 760-g premature infant of 26 weeks’ gestation who has RDS; (B) a 2-hour-old, 3.8-kg infant of 41 weeks’ gestation who has MAS; or (C) a 6-week-old, 1420-g, former 25 weeks’ gestation premature infant with severe chronic lung disease?
118. What is a time constant, and why is it important to consider when ventilating a newborn infant?
A time constant is the product of lung compliance and airway resistance (Tc = R × C). Conceptually, time constants reflect the time it takes for gas flow to cease and pressure to be fully equilibrated between the large airways and the alveoli when a sudden pressure change is applied to the airway opening (three time constants are needed for 95% equilibration) ( Fig. 18-11).
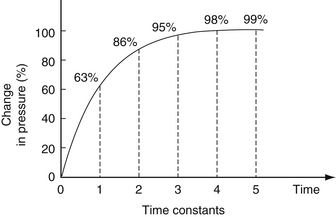
Figure 18-11 Time constants in the lung. A percentage change in pressure in relation to the time (in time constants) allowed for equilibration. As a longer time is allowed for equilibration, a higher percentage change in pressure occurs. (From Carlo WA, Martin RJ. Principles of assisted ventilation. Pediatr Clin North Am 1986;33:321.)
In acute RDS, compliance is low and airway resistance is also low (normal). Therefore short inspiratory times can be used. In addition, time constants are also a function of size (total compliance, not compliance per kilogram, is used). Consequently, large subjects such as adults or horses have long time constants, and small premature infants and hummingbirds have short time constants. Time constants are a major determinant of resting respiratory rate, which turns out to fall exactly where work of breathing is lowest. This is why adults at rest breathe at a rate of 14 breaths per minute, term infants breathe at 40 breaths per minute, and small premature infants breathe at about 60 breaths per minute. Mice and hummingbirds breathe significantly more quickly. In infants with acute respiratory distress, tachypnea is a reflection of shorter time constants as lung compliance decreases because of various causes. Asthmatics, on the other hand, prefer to breathe rather slowly because of their prolonged expiratory phase. The bottom line is this: Consider the underlying disease process and its pathophysiology before making decisions about ventilator settings.
 Avoidance of asynchrony (baby “bucking” or “fighting” the ventilator)
Avoidance of asynchrony (baby “bucking” or “fighting” the ventilator)
 Less need for sedation, neuromuscular blockade, or both
Less need for sedation, neuromuscular blockade, or both
 Lower airway pressures because the baby and the ventilator work in tandem
Lower airway pressures because the baby and the ventilator work in tandem
 Decreased risk of barotrauma, volutrauma, and intraventricular hemorrhage
Decreased risk of barotrauma, volutrauma, and intraventricular hemorrhage
 Preservation of respiratory muscle training (compared with muscle paralysis)
Preservation of respiratory muscle training (compared with muscle paralysis)
120. What is assist-controlled (A/C) ventilation? How does it differ from synchronized intermittent mandatory ventilation (SIMV)? When should it be used?
A/C ventilation is a form of mechanical ventilation in which the infant triggers the ventilator to cycle with each breath ( Fig. 18-12). With a small triggering effort, therefore, the baby can achieve a much higher level of ventilatory support than with spontaneous breathing. In general, A/C ventilation can be used very successfully to treat VLBW babies with RDS or pulmonary insufficiency of prematurity. It has become the most common way to initiate mechanical ventilation therapy in these clinical situations. It often enables patients to be ventilated at lower PIP levels than with conventional mechanical ventilation or SIMV. It differs from SIMV in that, with A/C, the baby will trigger a ventilator breath with each respiratory effort. In SIMV the ventilator is synchronized to the baby’s respiratory cycle so as to avoid stacking of the ventilator and infant breaths, but the baby is given only a preset amount of synchronized breaths. With modern ventilators, if the baby becomes apneic during either A/C ventilation or SIMV, the machine will deliver a preset number of breaths per minute. 48
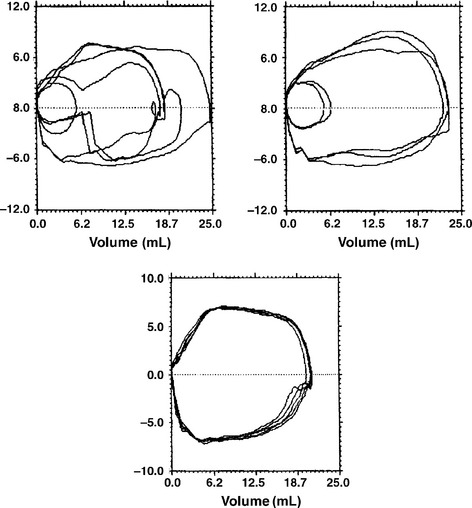
Figure 18-12 Flow-volume loops indicating the differences among conventional ventilation (top left), SIMV (top right), and assist-controlled ventilation (bottom). The loops are erratic with conventional ventilation. With synchronized intermittent mandatory ventilation, the loops are either triggered by the patient or the ventilator. In assist-controlled mode, all loops are ventilator generated, either triggered by the patient (if breathing above the set rate) or by the ventilator (if the infant’s respiratory rate falls below the set level). (From Goldmsith JP, Karotkin EH, editors. Assisted ventilation of the neonate. 4th ed. Philadelphia: Saunders; 2003. p. 208.)
121. An infant is now on PIP of 18 cm H2O, IMV rate of 30 breaths per minute, and FIO2 of 0.3. Which is the correct way of weaning the infant from mechanical ventilation in A/C mode: (A) progressively decrease the PIP and IMV; (B) progressively lower the IMV, leaving the PIP unchanged; or (C) progressively lower the PIP, leaving the IMV unchanged?
C is correct. In A/C mode, every breath that the infant takes triggers a ventilator breath—that is, every breath is supported. As a result, the baby is in control of the ventilatory rate. The IMV rate is only a back-up rate in case the infant is apneic or the triggering mechanism is not functioning. Decreasing the IMV rate does not actually decrease the level of support the infant is receiving. Weaning occurs by lowering the degree of support for each breath (i.e., the PIP). Ultimately, when the PIP is down to where the ventilator is generating only enough pressure to overcome the resistance of the ET tube and circuit, the baby is ready for extubation (usually about 10 cm H2O).
 A chest radiograph should be obtained as a baseline so that postextubation changes can be compared.
A chest radiograph should be obtained as a baseline so that postextubation changes can be compared.
 The child should be given nothing by mouth for at least 4 hours before extubation and should be given intravenous fluids during that time.
The child should be given nothing by mouth for at least 4 hours before extubation and should be given intravenous fluids during that time.
 A CPAP setup or oxygen should be available to help the child’s transition to spontaneous breathing.
A CPAP setup or oxygen should be available to help the child’s transition to spontaneous breathing.
 A laryngoscope and a new ET tube should be at hand in case the child does poorly.
A laryngoscope and a new ET tube should be at hand in case the child does poorly.
 It is not necessary to initiate steroids or methylxanthines before extubation in all children. However, these adjuncts may be useful if one or two prior attempts at extubation have failed. Bear in mind that the evidence for the value of steroids is mostly anecdotal.
It is not necessary to initiate steroids or methylxanthines before extubation in all children. However, these adjuncts may be useful if one or two prior attempts at extubation have failed. Bear in mind that the evidence for the value of steroids is mostly anecdotal.
When the child is ready to be extubated, the tube should be carefully untaped from the face to prevent any abrasions. An anesthesia bag should be attached to the ET tube, and a long, slow, low-level (15 cm H2O), positive pressure breath should be administered as the ET tube is withdrawn from the airway. This breath overcomes the natural negative pressure created as the tube is withdrawn from the airway. The child should be given CPAP or oxygen and observed closely. Stridor or hoarseness is common and typically indicates upper airway edema. Marked retractions also may be seen and are worrisome, indicating either volume loss in the lung or upper airway obstruction. Adequate humidification of inspired gas is essential after extubation. Because of the initial inability to oppose the vocal cords, feeding should not be resumed for at least 6 to 12 hours after extubation. Clinical deterioration that occurs 24 to 48 hours after extubation may be caused by a number of factors, including increased atelectasis, upper airway edema and obstruction, and muscular fatigue. If reintubation is deemed necessary, it should be carried out promptly.
Neonatal High-Frequency Ventilation
127. What are the three types of high-frequency ventilation, and how are they distinct from one another?
No. Because there have been no comparison trials, each type has its advocates and critics.
129. What happens to tidal volume delivery to the alveolus when frequency is increased during high-frequency oscillation?
This question emphasizes the importance of understanding the differences between high-frequency oscillation and conventional ventilation. In conventional ventilation increasing the rate will increase carbon dioxide elimination in most cases. With high-frequency ventilation turning up the rate generally causes a decrease in minute ventilation owing to the loss of tidal volume delivery. When ventilation is inadequate during high-frequency ventilation, turning the rate down can increase carbon dioxide elimination.
 Spike theory: This theory postulates that the resistance along the periphery of the airway is higher than in the center so that a spike is produced that extends far down the center of the airway, bypassing much of the lung’s dead space.
Spike theory: This theory postulates that the resistance along the periphery of the airway is higher than in the center so that a spike is produced that extends far down the center of the airway, bypassing much of the lung’s dead space.
 Pendelluft: The rapid to-and-fro movement of air between lungs or between lung segments may be enhanced at higher frequencies.
Pendelluft: The rapid to-and-fro movement of air between lungs or between lung segments may be enhanced at higher frequencies.
 Brownian diffusion: This may increase at higher frequencies.
Brownian diffusion: This may increase at higher frequencies.
 Coaxial flow: This theory speculates that gas flow in the airway is not simply a to-and-fro movement. Rather, inhaled gas spikes down the center of the airway, whereas the exhaled carbon dioxide moves along the periphery in a circuitous fashion. As frequencies increase, a whirlpool may actually arise within the airway that literally pulls the small-volume puffs of gas to a very deep region of the lung ( Fig. 18-13).
Coaxial flow: This theory speculates that gas flow in the airway is not simply a to-and-fro movement. Rather, inhaled gas spikes down the center of the airway, whereas the exhaled carbon dioxide moves along the periphery in a circuitous fashion. As frequencies increase, a whirlpool may actually arise within the airway that literally pulls the small-volume puffs of gas to a very deep region of the lung ( Fig. 18-13).
133. In neonates with poor lung inflation, should high-frequency oscillation be used at lower, the same, or higher Paw than that being used on conventional ventilation?
The strategy with which high-frequency oscillation is used is important. Patients with diffuse loss of lung volume (i.e., atelectasis) should be treated with a lung recruitment strategy. High-frequency oscillation allows the use of higher Paws than conventional ventilation because the small tidal volumes promote ventilation without causing lung overinflation. This approach has been studied in animal models of hyaline membrane disease and has been shown to improve lung inflation, decrease acute lung injury, decrease pulmonary air leaks, and promote survival. Often referred to as a “high mean airway pressure strategy,” the real goal is not a high Paw but rather optimal lung inflation. Unfortunately, measures of optimal lung inflation are not available. Clinically, the goal is to promote lung recruitment while avoiding lung overinflation, cardiac compromise, and lung atelectasis. 4950
134. When high-frequency ventilation is used, what measurements help guide choice of ventilation settings?
In oscillatory ventilation Paw can be altered directly by changing that setting on the ventilator. With jet ventilation Paw is a measured value that is a combination of several factors: PIP, PEEP, duration of inspiratory phase (jet valve on time), and background sigh rate.
137. Has high-frequency ventilation been conclusively shown to reduce the use of ECMO in neonates with MAS?
No. Only anecdotal evidence exists to support the efficacy of high-frequency ventilation in neonates with MAS. In fact, in neonates with MAS and signs of air trapping, high-frequency ventilation may be dangerous. Reported success in MAS with high-frequency ventilation is between 30% and 40%. 51
138. Theoretically, how does high-frequency ventilation prevent acute lung injury in hyaline membrane disease?
139. What other tools are used in neonatology to promote better lung inflation and reduce the injury associated with ventilating a collapsed lung?
Neonatal Extracorporeal Membrane Oxygenation
ECMO is a modification of standard cardiopulmonary bypass techniques used in the operating room during open heart surgery. It was adapted in a simplified circuit to provide artificial life support to pulmonary patients in an intensive care unit setting. Neonatal ECMO was the first clinically successful application of this technology to treat severe and progressive cardiorespiratory failure caused by MAS and complicated by persistent pulmonary artery hypertension occurring in the first week of life. At the core of ECMO technology are the heart–lung pump (a semiocclusive roller device) and the innovative Kolobow polycarbonate-spooled, silicone membrane oxygenator ( Fig. 18-14). Both devices are sufficiently powerful to completely support cardiac output and lung function in neonates.
ECLS includes ECMO, hemofiltration, hemodialysis, and indwelling oxygenator filaments (i.e., intravenous oxygenator). Many of these other techniques can be incorporated with an ECMO circuit or can be applied separately. 525354
The definitive randomized trial establishing the effectiveness of neonatal ECMO was conducted by the National Health Service in the United Kingdom. Of 93 infants referred to ECMO centers, 30 (or 32%) died compared with 54 of 92 (59%) receiving conventional care. The relative risk for reduced mortality with ECMO was 0.55 (95% CI, 0.39 to 0.77; P <0.0005). Of the survivors, one child in each group was severely disabled at 1 year, and ten ECMO patients (compared with six conventionally treated infants) were disabled to a lesser degree. The UK Collaborative ECMO Trial Group concluded that ECMO support should be actively considered for mature neonates with severe but potentially reversible respiratory failure. 5556
The success of ECMO relies on the physician’s ability to recognize, within the first week of illness, those near-term or term newborn infants with reversible pulmonary disease and to exclude infants with irreversible pulmonary disease. According to the ECLS Organization’s Registry data estimates, only one in approximately 1700 infants can benefit from ECMO. Criteria for ECMO patient selection have been widely debated during the past decade, and two controversial questions have arisen: (1) Is less invasive therapy likely to succeed? (2) With constantly improving neonatal ventilatory and pharmacologic techniques, must physicians continually reassess ECMO criteria? In general, the earlier the ECMO physician can identify the infant with a high probability of dying as a result of disease (before iatrogenic consequences of conventional therapy), the better the patient selection and outcome will be. The following inclusion and exclusion criteria provide general neonatal ECMO guidelines that are currently widely accepted:
 <2 weeks’ postnatal age (or ≤10 days’ high-pressure mechanical ventilation, relative age)
<2 weeks’ postnatal age (or ≤10 days’ high-pressure mechanical ventilation, relative age)
 Reversible cardiopulmonary condition
Reversible cardiopulmonary condition
 No syndromes with an unsurvivable prognosis
No syndromes with an unsurvivable prognosis
 No uncontrollable bleeding diathesis (e.g., disseminated intravascular coagulation with bleeding uncontrolled despite multiple component transfusions or progressive parenchymal brain hemorrhage)
No uncontrollable bleeding diathesis (e.g., disseminated intravascular coagulation with bleeding uncontrolled despite multiple component transfusions or progressive parenchymal brain hemorrhage)
Once the aforementioned inclusion and exclusion criteria have been considered, one of several pulmonary indices is used to assess the severity of respiratory illness and the likelihood of death if the infant is treated conventionally. The simplest and most popular index is the oxygenation index ( Fig. 18-15). Briefly, the oxygenation index is equivalent to the Paw generated during mechanical ventilation multiplied by the FiO2 (both of these values indicate the level of conventional ventilatory support) divided by the postductal arterial oxygen tension in the blood (a sensitive indicator of both ventilation and perfusion of the baby’s lung). The resulting value is multiplied by 100. The relative importance of the ratio between Paw and arterial oxygen tension in the calculation of oxygenation index performed at 1.00 (FiO2) is further demonstrated graphically in Figure 18-15. Once the arterial PO2 is below 40 mm Hg in the denominator of the oxygenation index equation, a geometric rise in the oxygenation index occurs. This rise parallels increased pulmonary vascular resistance with increased right-to-left shunting in the patient with severe pulmonary arterial hypertension.
147. How may vascular access for ECMO be achieved, and what are the benefits and liabilities of venoarterial (VA) versus venovenous (VV) bypass?
 Less effective cardiac support
Less effective cardiac support
 Lower arterial PO2 with mixing in right atrium
Lower arterial PO2 with mixing in right atrium
 Recirculation into double-lumen cannula
Recirculation into double-lumen cannula
 Mixed venous saturation (SvO2) and SaO2 monitors unreliable; must follow arterial PO2, pH to judge oxygen sufficiency
Mixed venous saturation (SvO2) and SaO2 monitors unreliable; must follow arterial PO2, pH to judge oxygen sufficiency
SvO2 from the jugular venous cannula drain is monitored continuously during bypass using a fiberoptic device inserted directly into the blood path coming out of the patient. SvO2 does not so much reflect pulmonary function (as does the systemic arterial saturation) as it represents the adequacy of tissue oxygen delivery from the native heart and the ECMO circuit combined. If the oxygen delivered by ECMO is sufficient to meet tissue oxygen demand, then the SvO2 is generally greater than 70%. Failure to meet tissue oxygen demand results in the progressive desaturation of venous blood returning from the capillary beds into the right atrium. An SvO2 below 65% to 70% indicates marginal oxygen delivery, and an SvO2 below 60% may be associated with lactic acid production through anaerobic metabolism. Therefore the single most important parameter monitored during ECMO and used to assess the adequacy of bypass is the SvO2. Notably, during VV ECMO the SvO2 may be artificially elevated because of recirculation of arterialized blood back into the drainage side of the double-lumen cannula; however, trends in SvO2 may still be useful, and the patient may be taken off bypass briefly to assess a true SvO2.
The typical ECMO course transpires over 3 to 7 days, while awaiting spontaneous lung recovery.
Cardiac recovery and mobilization of capillary leak edema usually precede lung recovery and weaning the ECMO pump flow rate. As the tissue edema is mobilized, fluid is transferred back into the intravascular space, increasing the baby’s native cardiac output. Therefore the infant’s systemic arterial saturation and arterial PO2 may actually decrease during recovery (as ECMO support is weaned and the infant’s native cardiac output drives right-to-left shunting of deoxygenated blood through fetal channels in an accelerated fashion). During this early improvement phase on ECMO, diuretic therapy (e.g., furosemide, mannitol) or hemofiltration may assist in reducing this native circulation of desaturated blood. Thereafter, as the mixed venous saturation improves in the jugular venous cannula (above 80%), the ECMO pump flow is reduced in 10 mL/min decrements until a pump idle rate is reached of approximately 100-mL/min minimum flow (to prevent stasis and clotting within the circuit). Frequent arterial and venous blood gas assessments are important during the weaning process. Recent reports have suggested that pulmonary function testing demonstrating increased functional residual capacity (>15 mL/kg) and improved dynamic lung compliance may be useful in determining more exactly when lung recovery is sufficient to warrant coming off bypass.
Air Leak Syndromes
Pneumothorax is the most common form of air leak, and, fortunately, pneumopericardium is the least common. Pneumopericardium must be recognized promptly because of its high morbidity and mortality risk. In the era before surfactant, pulmonary interstitial emphysema was more common and in many cases preceded other forms of air leak. Pneumomediastinum is uncommon but the most difficult to treat because there is no easy way to evacuate mediastinal air.
153. You are called to the bedside of a baby who has suddenly become cyanotic while on a ventilator. You listen to the chest, and you hear better breath sounds on the right side. You call for a chest radiograph, but the x-ray technician is on a break. Neither the senior resident nor the neonatologist is available, and you are on your own. What do you suspect, and how can you tell whether you are correct?
 You could transilluminate the chest with a high-intensity fiberoptic light. If a pneumothorax is present, the left side should light up, whereas the right will transilluminate less.
You could transilluminate the chest with a high-intensity fiberoptic light. If a pneumothorax is present, the left side should light up, whereas the right will transilluminate less.
 Also, check the position of the ET tube. Make sure it is in a good position and has not changed (look at the numeric value of the ET tube and compare with where it is supposed to be). If there is evidence that the position of the ET tube has changed and it is out or has been pushed in too far, secure the airway and make sure it is in appropriate position. If the acute deterioration is caused by ET tube malposition, repositioning of the tube should lead to rapid improvement in gas exchange. Repositioning that does not lead to improvement supports the diagnosis of air leak as a possible cause for the deterioration.
Also, check the position of the ET tube. Make sure it is in a good position and has not changed (look at the numeric value of the ET tube and compare with where it is supposed to be). If there is evidence that the position of the ET tube has changed and it is out or has been pushed in too far, secure the airway and make sure it is in appropriate position. If the acute deterioration is caused by ET tube malposition, repositioning of the tube should lead to rapid improvement in gas exchange. Repositioning that does not lead to improvement supports the diagnosis of air leak as a possible cause for the deterioration.
154. A baby is breathing asynchronously on a conventional ventilator, and you are concerned that she is at risk for a pneumothorax. What can you can do to decrease the risk of air leak in this patient?
 Increasing the gas temperature may slightly decrease the incidence of air leak.
Increasing the gas temperature may slightly decrease the incidence of air leak.
 Decreasing the inspiratory time will decrease Paw and could decrease the risk of pneumothorax, but it may reduce oxygenation.
Decreasing the inspiratory time will decrease Paw and could decrease the risk of pneumothorax, but it may reduce oxygenation.
 Increasing the ventilatory rate may allow you to take over ventilation and decrease the baby’s effort, but you need to watch for air trapping.
Increasing the ventilatory rate may allow you to take over ventilation and decrease the baby’s effort, but you need to watch for air trapping.
 Use of a synchronized mode of ventilation (SIMV or A/C) will help the baby breathe with the ventilator breaths.
Use of a synchronized mode of ventilation (SIMV or A/C) will help the baby breathe with the ventilator breaths.
The primary treatment goal unilateral pulmonary interstitial emphysema is to allow the affected lung to deflate. Selective bronchial intubation will allow the contralateral lung to deflate (of course, selective left mainstem intubation may be technically difficult), but it may pose problems because the perfusion to the ventilated lung may not be sufficient for gas exchange in all cases. A randomized trial of high-frequency jet ventilation did show effectiveness in treating pulmonary interstitial emphysema by lowering the Paw, which may allow the emphysema to resolve. In infants the lung in the superior position will receive more of the ventilation. Placing the affected lung in the downward position may be helpful in deflating that lung. 5758
Ideally, a chest tube should be placed in that part of the thoracic cavity where it will do the most good with the least risk to the infant. Positioning of the infant is the key to the entire procedure. All too commonly, the baby is allowed to remain supine. When the clinician enters the chest in that position, the catheter hits the lung and moves posteriorly ( Fig. 18-16). However, if the child is placed nearly vertical to start the procedure, it is easy to angle the catheter anteriorly for optimal placement. The thoracostomy tube is inserted through an incision made in the fifth interspace in the midaxillary line. After the incision is made, the clinician tunnels up an interspace with a hemostat, which is used to pop through the strong muscular wall of the chest (a remarkably tough structure even in a tiny premature infant). If a pneumothorax is present, a gush of air should be seen when the chest is opened. The catheter should be advanced so that no end holes lie outside the chest wall. If the catheter is inserted too far, it must be pulled back. The chest tube is then connected to a suction apparatus. The suction rarely needs to be greater than −10 to −15 cm H2O.
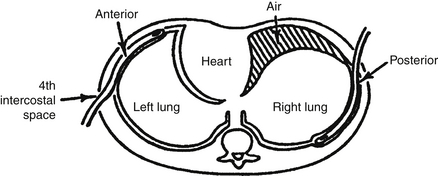
Figure 18-16 Appropriate chest tube placement is shown on the left side of the figure, with the chest tube in the proper position to reach the air that sits in the superior part of the thorax. On the right side of the figure, the chest tube has migrated to a posterior position, preventing it from evacuating the air lying superiorly.
Bronchopulmonary Dysplasia
BPD is the chronic lung disease that often follows RDS in VLBW babies. First described by Northway in 1967, it has become the greatest foe of all neonatologists and the focal point of perhaps more studies than any other clinical syndrome in neonatology. BPD was not a disease until the neonate became a patient. Once people attempted to save critically ill neonates with lung disease, a certain percentage developed BPD. In most nurseries the BPD rate is between 20% and 30% in infants who weigh less than 1500 g. BPD is defined as a need for oxygen at either 28 days of life or, more recently, at 36 weeks’ postconception, with radiographic changes consistent with chronic lung disease. The incidence of BPD increases with decreasing gestational age at birth, decreasing birth weight, and increasing severity of lung disease at birth. In addition, testing (physiologic test) to determine if an infant is able to tolerate being in room air at 36 weeks’ postconceptional age influences the use of the term BPD. 5960
In a series of open lung biopsies from VLBW infants ages 14 days to 7 weeks who were receiving ventilatory support with radiographic changes consistent with chronic lung disease, Coalson and colleagues described a consistent lack of alveolarization with variably widened alveolar septae and minimal changes in the airways. Mild to moderate septal fibrosis was also apparent. These widened alveolar septae were hypercellular with disordered capillary growth. Typically, the alveolar spaces were laden with numerous alveolar macrophages and neutrophils.
Transmission electron microscopy demonstrated poor differentiation of type I and type II lung epithelial cells. These epithelial cells had relatively abundant cytoplasm and extensive glycogen stores; however, lamellar bodies were extremely rare to totally absent. There was no progression of alveolarization with enlarged simplified terminal air spaces or minimal and focal saccular fibroplasia. The interstitium of the lung contained myofibroblasts, and there was focal deposition of elastin and collagen fibers. Most saccular walls showed blunted “outpouchings” or secondary crest formation. 61626364
 Pulmonary edema (resulting from a patent ductus arteriosus, overhydration, or delayed diuresis)
Pulmonary edema (resulting from a patent ductus arteriosus, overhydration, or delayed diuresis)
 Pulmonary air leak (e.g., interstitial emphysema, pneumothorax)
Pulmonary air leak (e.g., interstitial emphysema, pneumothorax)
Both animal and human studies indicate that chronic steroid use may result in reduced amounts of neural tissue mass. Neurologic handicap rates are higher in infants treated with dexamethasone. Somatic growth may also be adversely affected. 6566
166. Why is it that a child can recover from BPD, but an adult cannot repair the lung injury seen in emphysema?
 Provision of optimal PEEP for tracheobronchomalacia
Provision of optimal PEEP for tracheobronchomalacia
 Fluid restriction (it is difficult in neonates to give many calories and restrict fluids at the same time)
Fluid restriction (it is difficult in neonates to give many calories and restrict fluids at the same time)
 Diuretic therapy (short-term therapy may be helpful, but long-term use has not been shown to improve outcomes)
Diuretic therapy (short-term therapy may be helpful, but long-term use has not been shown to improve outcomes)
 Chloride supplementation to prevent metabolic alkalosis from diuretics if they are used
Chloride supplementation to prevent metabolic alkalosis from diuretics if they are used
 Methylxanthines (both caffeine and theophylline decrease work of breathing and apnea)
Methylxanthines (both caffeine and theophylline decrease work of breathing and apnea)
Apnea of Prematurity
Apnea is the cessation of breathing. Although this problem affects people of all ages in many different forms, it is most prevalent in premature infants younger than 36 weeks’ gestation. Pathologic apnea refers to cessation of breathing for more than 20 seconds; cessation of breathing for less than 20 seconds and accompanied by bradycardia 20% below the baseline heart rate; or cessation of breathing for less than 20 seconds with oxygen desaturation below 80%. Apnea in a newborn is classified as central, obstructive, or mixed. Most apnea of prematurity is classified as central apnea ( Fig. 18-17), in which there is complete absence of respiratory effort. Obstructive apnea occurs when an infant makes a respiratory effort but no airflow is present because of the presence of obstruction (see Figure 18-17, bottom). Obstructive apnea can be associated with gastroesophageal reflux. Mixed apnea is a combination of central and obstructive apnea.
Figure 18-17 A, An episode of central apnea, with bradycardia and oxygen desaturation. B, An episode of obstructive apnea with recurring desaturation.
Periodic breathing is a type of central apnea characterized by brief pauses in breathing of less than 10 seconds, followed by periods of regular respiration of less than 20 seconds’ duration. This pattern repeats itself for at least three cycles and often many more times ( Fig. 18-18). The significance of this form of breathing is unknown at present. Many prematurely born infants demonstrate periodic breathing for as much as 20% to 30% of total sleep time. Because of the frequency of this finding, some neonatologists consider periodic breathing to be a normal maturational process. On the other hand, it also may be a reflection of significant immaturity of respiratory control and a variant of apnea.
In the short term, particularly in the NICU, extremely premature infants can have prolonged apneic episodes that may be fatal. As they mature, most infants will have more self-limited episodes. Less clear are the long-term effects on infants who have had a history of severe apnea of prematurity. Clinical studies suggest apnea of prematurity, particularly with oxygen desaturation, may affect learning and other aspects of childhood development. 6768
Although in most cases apnea of prematurity is gone by 37 weeks’ postconceptional age, in many cases it can persist even beyond 45 weeks’ postconceptional age (and occasionally longer). Recent evidence indicates that apnea persists longest in the most immature infants. 69
Caffeine is the preferred treatment because of its once-a-day dosing and fewer side effects. Caffeine therapy for apnea of prematurity reduces the rates of CP and cognitive delay at 18 months of age. The improved outcomes seen at 18 months were not seen at 5 years after birth, but the trends toward improvement in outcome still favored use of caffeine over placebo for the treatment of apnea. Patients are typically loaded with 20 mg/kg of caffeine citrate and maintained on 7 to 8 mg/kg daily. 70
Sudden Infant Death Syndrome
The SIDS rate slowly declined from 1985 to 1994, then it began to drop precipitously from about 2 deaths per 1000 births in 1992 to the present 0.55 per 1000 births. This rapid decline paralleled the institution of the “Back to Sleep” campaign sponsored by the National Institutes of Health, the SIDS Alliance, and the American Academy of Pediatrics. This initiative followed the discovery that the simple act of changing infants’ sleeping positions from the stomach to the back was responsible for a dramatic reduction in the SIDS rate in England and Australia. In the United States the rate of infants sleeping on their backs has risen from 15% to over 70% in the past 5 years. It is likely that the SIDS rate will decrease even more as increasing numbers of infants sleep on their backs. 71
In medicine, as in all aspects of life, uncomplicated and elegant observations can make great differences. Although the exact physiology is unclear, it is likely that sleeping on the back reduces the rebreathing of carbon dioxide; adjusts the position of the airway, thus reducing obstruction; and reduces the possibility of poor oxygenation and ventilation through the mattress. The effects on the baby’s thermal environment and the ability to eliminate heat may also be important.
Lung Abnormalities
Pulmonary sequestrations are either extralobar or intrapulmonary. Extralobar pulmonary sequestrations include lesions with lung tissue surrounded by its own pleura. Intrapulmonary sequestrations, also known as intralobar sequestrations, have no discernible pleural tissue.
186. What is a congenital cystic adenomatoid malformation of the lung? How does it generally present?
1American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 2003;111(4 Pt 1):914–7.
3Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics 2000;105(1 Pt 1):1–7.
4Kattwinkel J, Bloom RS, American Academy of Pediatrics. American Heart Association. Textbook of neonatal resuscitation. [Elk Grove Village, Ill.]; [Dallas, Tex.]: American Academy of Pediatrics; American Heart Association; 2011.
5Kattwinkel J, Bloom RS, American Academy of Pediatrics. American Heart Association. Textbook of neonatal resuscitation. [Elk Grove Village, Ill.]; [Dallas, Tex.]: American Academy of Pediatrics ; American Heart Association; 2011.
6Davis PG, Tan A, O’Donnell CP, et al. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet 2004;364:1329–33.
7Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: a systematic review and meta-analysis. Resuscitation. 2007;72:353–63.
73Kattwinkel J, Bloom RS, American Academy of Pediatrics. American Heart Association. Textbook of neonatal resuscitation. [Elk Grove Village, Ill.]; [Dallas, Tex.]: American Academy of Pediatrics; American Heart Association; 2011.
9Hankins GDV, Speer M. Defining the pathogenesis and pathophysiology of neonatal encephalopathy and cerebral palsy. Obstet Gynecol 2003;102(3):628-36.
10Lie KK, Groholt EK, Eskild A. Association of cerebral palsy with Apgar score in low and normal birthweight infants: population based cohort study. BMJ 2010;341:c4990.
11Kent A. Apgar scores and cerebral palsy. Rev Obstet Gynecol 2011;4(1):33–4.
12Martin-Ancel A, Garcia-Mix A, Gaya F, et al. Multiple organ involvement in perinatal asphyxia. J Pediatr 1995;127:786–93.
13Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363.
14Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 2011;159(5):851–8.
15Jain L, Vidyasagar D. Cardiopulmonary resuscitation of newborns: its application to transport medicine. Pediatr Clin North Am 1993;40:287–301.
16Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2010;126(5):e1400–e1413.
17Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2010;126(5):e1400–e1413.
72Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2010;126(5):e1400-e1413.
19Committee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011;127(3):575–9.
20Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2010;126(5):e1400–e1413.
21Kattwinkel J, Bloom RS, American Academy of Pediatrics. American Heart Association. Textbook of neonatal resuscitation. [Elk Grove Village, Ill.]; [Dallas, Tex.]: American Academy of Pediatrics; American Heart Association; 2011.
22Jain L. Cardiopulmonary resuscitation of apparently stillborn infants: survival and long-term outcome. J Pediatr 1991;118:778–82.
23Konduri GG. The role of nitric oxide in lung growth and function in the newborn lung. In: Bancalari E, editor. 2nd ed. Philadelphia: Saunders; 2012. p. 111–32.
24Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol 2010;135(2):223–35.
25Engle WA, Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics 2008;121(2):419–32.
26Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2012 Mar 14;3:CD000510:CD000510.
27Cools F, Askie LM, Offringa M, et al. Elective high-frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta-analysis of individual patients’ data. Lancet 2010;375(9731):2082–91.
28Courtney SE, Durand DJ, Asselin JM, et al. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N Engl J Med 2002;347:643–52.
29Johnson AH, Peacock JL, Greenough A, et al. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med 2002;347:633–42.
30Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet 2004;364:597–602.
31Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics 2000;105(1 Pt 1):1–7.
32Wiswell TE. Advances in the treatment of the meconium aspiration syndrome. Acta Paediatr Suppl 2001;90(436):28–30.
33Soll RF, Dargaville P. Surfactant for meconium aspiration syndrome in full term infants. Cochrane Database Syst Rev 2000;(2):CD002054.
34Gersony WM, Due GV, Sinclair JC. “PFC” syndrome (persistence of fetal circulation). Circulation 1969;40:3–9.
35Askie LM, Ballard RA, Cutter GR, et al. Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials. Pediatrics 2011 Oct;128(4):729–39.
36Tissot C, Beghetti M. Review of inhaled iloprost for the control of pulmonary artery hypertension in children. Vasc Health Risk Manag 2009;5(1):325–31.
37Abman SH, Chatfield BA, Hall SL, et al. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol 1990;259(6 Pt 2):H1921–H1927.
38Levison J, Halliday R, Holland AJ, et al. A population-based study of congenital diaphragmatic hernia outcome in New South Wales and the Australian Capital Territory, Australia, 1992-2001. J Pediatr Surg 2006;41(6):1049–53.
39Davis PJ, Firmin RK, Manktelow B, et al. Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: the UK experience. J Pediatr 2004;144(3):309–15.
40Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 2001;2:CD000399.
41Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 2001;2:CD000399.
42Clark RH, Kueser TJ, Walker MW, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 2000;342(7):469–74.
43Askie LM, Ballard RA, Cutter GR, et al. Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials. Pediatrics 2011;128(4):729–39.
44Ball MK, Steinhorn RH. Inhaled nitric oxide for preterm infants: a marksman’s approach. J Pediatr 2012;161(3):379–80.
45Clark RH, Gerstmann DR, Jobe AH, et al. Lung injury in neonates: causes, strategies for prevention, and long-term consequences. J Pediatr 2001;139(4):478–86.
46Klingenberg C, Wheeler KI, Davis PG, et al. A practical guide to neonatal volume guarantee ventilation. J Perinatol 2011;31(9):575–85.
47Keszler M. Volume-targeted ventilation. Early Hum Dev 2006;82(12):811–8.
48Goldsmith JP, Karotkin EH, editors. Assisted ventilation of the neonate. Philadelphia: Saunders; 2004.
49Cools F, Askie LM, Offringa M, et al. Elective high-frequency oscillatory versus conventional ventilation in preterm infants: a systematic review and meta-analysis of individual patients’ data. Lancet 2010;375(9731):2082–91.
50Krebs J, Pelosi P, Tsagogiorgas C, et al. Open lung approach associated with high-frequency oscillatory or low tidal volume mechanical ventilation improves respiratory function and minimizes lung injury in healthy and injured rats. Crit Care 2010;14(5):R183.
51Henderson-Smart DJ, De Paoli AG, Clark RH, et al. High frequency oscillatory ventilation versus conventional ventilation for infants with severe pulmonary dysfunction born at or near term. Cochrane Database Syst Rev 2009;3:CD002974.
52Kim K, Mazor RL, Rycus PT, et al. Use of venovenous extracorporeal life support in pediatric patients for cardiac indications: a review of the Extracorporeal Life Support Organization registry. Pediatr Crit Care Med 2012;13(3):285–9.
53Lazar DA, Cass DL, Olutoye OO, et al. The use of ECMO for persistent pulmonary hypertension of the newborn: a decade of experience. J Surg Res 2012;177(2):263–7.
54Haines NM, Rycus PT, Zwischenberger JB, et al. Extracorporeal Life Support Registry Report 2008: neonatal and pediatric cardiac cases. ASAIO J 2009;55(1):111–6.
55UK Collaborative ECMO Trial Group: UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet 1996;348:75–82.
56Petrou S, Bischof M, Bennett C, et al. Cost-effectiveness of neonatal extracorporeal membrane oxygenation based on 7-year results from the United Kingdom Collaborative ECMO trial. Pediatrics 2006;117(5):1640–9.
57Keszler M, Donn SM, Bucciarelli RL, et al. Multicenter controlled trial comparing high-frequency jet ventilation and conventional mechanical ventilation in newborn infants with pulmonary interstitial emphysema. J Pediatr 1991;119(1 Pt 1):85–93.
58Keszler M, Durand DJ. Neonatal high-frequency ventilation. Past, present, and future. Clin Perinatol 2001;28(3):579–607.
59Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease. N Engl J Med 1967;276:357–68.
60Walsh MC, Wilson-Costello D, Zadell N, et al. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol 2003;23:451–6.
61Coalson JJ, Winter VT, Siler KT, et al. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 1999;160:1333–46.
62Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003 Feb;8(1):73–81.
63Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol 2006 Aug;30(4):179–84.
64Ambalavanan N, Carlo WA. Ventilatory strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol 2006;30(4):192–9.
65Yeh TF, Lin YJ, Lin HC, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 2004;350:1304–13.
66Watterberg KL. Policy statement—postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010;126(4):800–8.
67Hunt CE, Corwin MJ, Baird T, et al. Cardiorespiratory events detected by home memory monitoring and one-year neurodevelopmental outcome. J Pediatr 2004;145:465–71.
68Mattia FR, deRegnier RA. Chronic physiologic instability is associated with neurodevelopmental morbidity at one and two years in extremely premature infants. Pediatrics 1998;102:E35.
69Lorch SA, Srinivasan L, Escobar GJ. Epidemiology of apnea and bradycardia resolution in premature infants. Pediatrics 2011;128(2):e366–e373.
70Schmidt B, Anderson PJ, Doyle LW, et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012;307(3):275–82.
71Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics 2011;128(5):e1341–e1367.