Pulmonary Infections
After reading this chapter you will be able to:
 State the incidence of pneumonia in the United States and its economic impact.
State the incidence of pneumonia in the United States and its economic impact.
 Discuss the current classification scheme for pneumonia and be able to define hospital-acquired pneumonia, health care–associated pneumonia, and ventilator-associated pneumonia.
Discuss the current classification scheme for pneumonia and be able to define hospital-acquired pneumonia, health care–associated pneumonia, and ventilator-associated pneumonia.
 Recognize the pathophysiology and common causes of lower respiratory tract infections in specific clinical settings.
Recognize the pathophysiology and common causes of lower respiratory tract infections in specific clinical settings.
 List the common microbiologic organisms responsible for community-acquired and nosocomial pneumonias.
List the common microbiologic organisms responsible for community-acquired and nosocomial pneumonias.
 Describe the clinical findings seen in patients with pneumonia.
Describe the clinical findings seen in patients with pneumonia.
 State the radiographic findings seen in patients with pneumonia; state why some patients with pneumonia may have a normal chest radiograph.
State the radiographic findings seen in patients with pneumonia; state why some patients with pneumonia may have a normal chest radiograph.
 Describe the risk factors associated with increased morbidity and mortality in patients with pneumonia.
Describe the risk factors associated with increased morbidity and mortality in patients with pneumonia.
 State the criteria used to identify an adequate sputum sample for Gram stain and culture.
State the criteria used to identify an adequate sputum sample for Gram stain and culture.
 Describe the techniques used to identify the organism responsible for nosocomial pneumonia.
Describe the techniques used to identify the organism responsible for nosocomial pneumonia.
 List the latest recommendations regarding empiric and pathogen-specific antibiotic regimens used to treat various types of pneumonia.
List the latest recommendations regarding empiric and pathogen-specific antibiotic regimens used to treat various types of pneumonia.
 Discuss strategies that can be used to prevent pneumonia.
Discuss strategies that can be used to prevent pneumonia.
 Describe how the respiratory therapist aids in diagnosis and management of patients with suspected pneumonia.
Describe how the respiratory therapist aids in diagnosis and management of patients with suspected pneumonia.
Infection involving the lungs is termed pneumonia or lower respiratory tract infection and is a common clinical problem in the practice of respiratory care. In the late 1800s, Osler remarked that pneumonia is “captain of the men of death” because of its poor prognosis in the preantibiotic era. More than a century later, pneumonia remains a major cause of morbidity and mortality in the United States and around the world. Each year, 5 million people die from pneumonia worldwide. In the United States, it is estimated that 5 million cases of pneumonia occur annually, of which approximately 1 million require hospitalization, at a projected yearly cost of more than $20 billion.1 Pneumonia is the seventh leading cause of death in the United States and the most common cause of infection-related mortality.2
Classification
Pneumonia can be classified based on the clinical setting in which it occurs (Table 22-1). This classification is useful because it predicts the likely microbial causes and determines empiric antimicrobial chemotherapy while a definitive microbiologic diagnosis is awaited. (The term empiric therapy refers to treatment that is initiated based on the most likely cause of infection when the specific causative organism is still unknown.)
TABLE 22-1
Classifications and Possible Causes of Pneumonia
| Classification | Likely Organisms |
| Community-acquired: acute | |
| Typical | S. pneumoniae |
| H. influenzae | |
| Moraxella catarrhalis | |
| S. aureus | |
| Atypical | L. pneumophila |
| C. pneumoniae | |
| M. pneumoniae | |
| Viruses | |
| Coxiella burnetii | |
| Community-acquired: chronic | M. tuberculosis |
| H. capsulatum | |
| B. dermatitidis | |
| C. immitis | |
| Health care–associated | Mixed aerobic and anaerobic mouth flora |
| S. aureus | |
| Enteric gram-negative bacilli | |
| Influenza | |
| M. tuberculosis | |
| Immunocompromised host | P. jiroveci |
| Cytomegalovirus | |
| Aspergillus species | |
| Cryptococcus neoformans | |
| Reactivation tuberculosis or histoplasmosis | |
| Nosocomial | |
| Aspiration | Mixed aerobes and anaerobes Gram-negative bacilli |
| Health care–associated | S. aureus |
| Ventilator-associated | P. aeruginosa |
| Acinetobacter species | |
| Enterobacter species | |
| Klebsiella species | |
| S. maltophilia | |
| S. aureus |
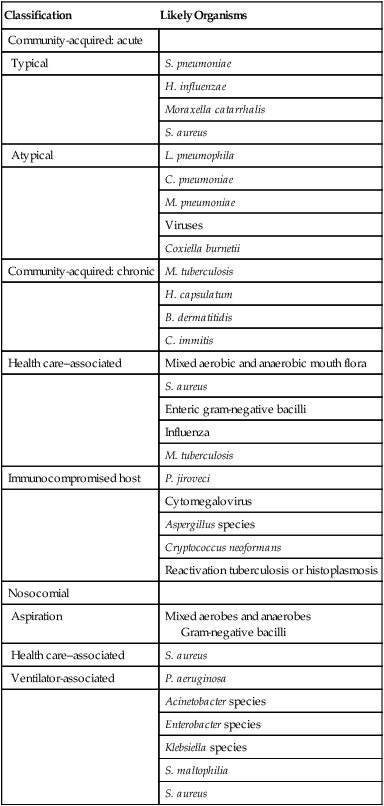
Pneumonia acquired in health care settings is often caused by different microorganisms than community-acquired pneumonia. Previously termed nosocomial pneumonia, this clinical entity has been further classified as health care–associated pneumonia (HCAP), hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP).3 HCAP is defined as pneumonia occurring in any patient hospitalized for 2 or more days in the past 90 days in an acute care setting or who in the past 30 days has resided in a long-term care or nursing facility; attended a hospital or hemodialysis clinic; or received intravenous antibiotics, chemotherapy, or wound care. HAP is defined as lower respiratory tract infection that develops in hospitalized patients more than 48 hours after admission and excludes community-acquired infections that are incubating at the time of admission. VAP is defined as lower respiratory tract infection that develops more than 48 to 72 hours after endotracheal intubation.
HAP is a common clinical problem and represents the second most common nosocomial infection in the United States, accounting for 15% to 18% of all such infections.4,5 Current estimates suggest that more than 250,000 individuals develop this complication each year. HAP increases hospital length of stay 7 to 9 days at an average incremental per-patient cost of $40,000. In selected patient populations, such as patients in the intensive care unit (ICU) and bone marrow transplant recipients, the crude mortality rate from HAP may approach 30% to 70%, with attributable mortality of 33% to 50%. Certain microorganisms, such as Pseudomonas aeruginosa and Acinetobacter species, are associated with higher rates of mortality.6
Pathogenesis
Six pathogenetic mechanisms may contribute to the development of pneumonia (Table 22-2). To minimize nosocomial spread, knowledge of these mechanisms is important to the understanding of the various disease processes and to the formulation of effective infection control strategies within the hospital. The fact that tuberculosis is acquired by inhalation of infectious particles is the basis for a policy whereby patients with suspected or proven tuberculosis who are coughing are placed in respiratory isolation, minimizing the risk of disease transmission within the hospital setting.
TABLE 22-2
Pathogenetic Mechanisms Responsible for the Development of Pneumonia
| Mechanism of Disease | Examples of Specific Infections |
| Inhalation of aerosolized infectious particles | Tuberculosis |
| Histoplasmosis | |
| Cryptococcosis | |
| Blastomycosis | |
| Coccidioidomycosis | |
| Q fever | |
| Legionellosis | |
| Aspiration of organisms colonizing the oropharynx | Community-acquired bacterial pneumonia |
| Aspiration pneumonia | |
| Hospital-acquired pneumonia | |
| Ventilator-associated pneumonia | |
| Direct inoculation of organisms into the lower airway | Hospital-acquired pneumonia |
| Ventilator-associated pneumonia | |
| Spread of infection to the lungs from adjacent structures | Mixed anaerobic and aerobic pneumonia from subdiaphragmatic abscess |
| Amebic pneumonia from rupture of amebic liver abscess into the lung | |
| Spread of infection to the lung through the blood | S. aureus pneumonia arising from right-sided bacterial endocarditis |
| Parasitic pneumonia: strongyloidiasis, ascariasis, hookworm | |
| Reactivation of latent infection, usually resulting from immunosuppression | P. jiroveci pneumonia |
| Reactivation tuberculosis | |
| Cytomegalovirus |
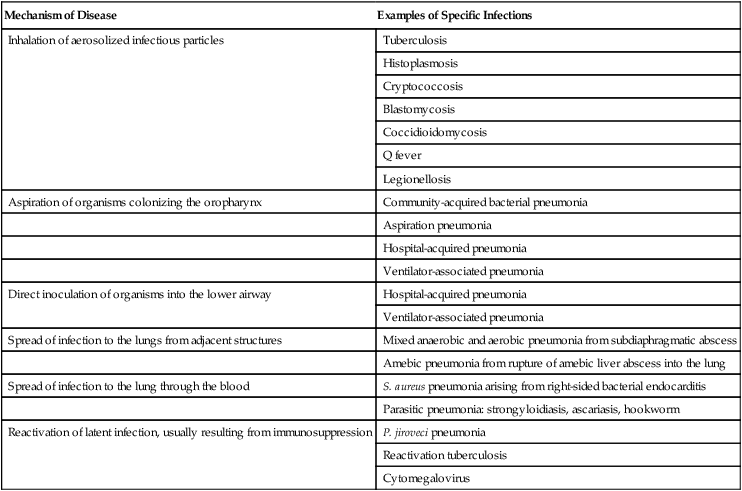
Aspiration seems to be the major mechanism responsible for the development of some types of mixed aerobic and anaerobic, gram-negative, and staphylococcal HAP. In intubated patients, chronic aspiration of colonized secretions through a tracheal cuff has been linked to the subsequent occurrence of pneumonia,4 which has led to the development of novel strategies to prevent HAP, such as continuous suctioning of subglottic secretions in mechanically ventilated patients and elevation of the head of the bed.7,8
Pneumonia also may develop when a latent infection, acquired earlier in life, is reactivated. This reactivation may occur for no apparent reason, as in the case of reactivation pulmonary tuberculosis, but most often it is attributable to the development of cellular immunodeficiency. Pneumocystis jiroveci (previously called Pneumocystis carinii) pneumonia is a prime example of lower respiratory tract infection arising as a result of this mechanism. In developed countries, most healthy individuals have acquired P. jiroveci by age 3 years and show serologic evidence of prior infection. The organism remains dormant in the lung but may reactivate later in life and produce pneumonia in individuals with compromised cell-mediated immunity, such as patients with human immunodeficiency virus (HIV) infection or recipients of long-term immunosuppressive therapy. Cytomegalovirus pneumonia is another example of a latent infection that can reactivate during chronic immunosuppression, especially in solid organ and bone marrow transplant recipients. Immunosuppressive drugs used to modify inflammatory diseases, such as tumor necrosis factor inhibitors, have been associated with pulmonary and extrapulmonary tuberculosis.9
Microbiology
In most studies, S. pneumoniae, also called pneumococcus, has been the most commonly identified cause of community-acquired pneumonia, accounting for 20% to 75% of cases (Table 22-3). Various other organisms have been implicated with varying frequencies. H. influenzae, Staphylococcus aureus, and gram-negative bacilli each account for 3% to 10% of isolates in many reports.10 Legionella species, Chlamydophila pneumoniae, and Mycoplasma pneumoniae together account for 10% to 20% of cases. These latter organisms, called atypical pathogens, vary in frequency in more recent reports, depending on the age of the patient population, the season of the year, and geographic locale. Legionellosis and C. pneumoniae, in particular, seem to exhibit significant geographic variation in incidence.
TABLE 22-3
Frequency of Pathogens in Community-Acquired Pneumonia
| Cause | Cases (%) |
| S. pneumoniae | 20-75 |
| Aspiration | 6-10 |
| C. pneumoniae | 4-11 |
| H. influenzae | 3-10 |
| Gram-negative bacilli | 3-10 |
| S. aureus | 3-5 |
| Legionella species | 2-8 |
| Viruses | 2-16 |
| Moraxella catarrhalis | 1-3 |
| M. pneumoniae | 1-24 |
| P. jiroveci | 0-13 |
| M. tuberculosis | 0-5 |
| No diagnosis | 25-50 |
Many studies examining the epidemiology and microbiology of community-acquired pneumonia are potentially biased because they focus on patients requiring hospitalization. In patients with less severe illnesses not requiring hospitalization, more recent studies suggest that organisms such as M. pneumoniae and C. pneumoniae account for 38% of cases and may be more common than typical bacterial pathogens such as pneumococcus and H. influenzae.11 In patients who are ill enough to require admission to the ICU, Legionella species, gram-negative bacilli, and pneumococcus are disproportionately more common.1 A virulent strain of methicillin-resistant S. aureus (MRSA) has emerged as a cause of severe necrotizing community-acquired pneumonia.12
In urban settings that have a high incidence of endemic HIV infection, P. jiroveci may be a more common cause of community-acquired pneumonia and, according to one report, may account for 13% of cases.13 Viruses such as influenza, respiratory syncytial virus, and adenovirus are occasional causes of community-acquired pneumonia, especially in patients with milder illnesses not requiring hospitalization and encountered in the late fall and winter months. A worldwide pandemic of H1N1 influenza during 2009-201014 and ongoing sporadic cases of transmission of H5N1 influenza from birds to humans have led to heightened international awareness of influenza epidemiology, pathogenesis, and prevention.
The outbreak in 2000-2001 of inhalation anthrax in the United States adds another microbial differential diagnostic consideration in patients with fulminant community-acquired lower respiratory tract infection.15 To date, inhalation anthrax remains a rare disease. However, it must be considered in selected clinical and epidemiologic settings (see later). A new human pathogen, severe acute respiratory syndrome–associated coronavirus, emerged and spread worldwide in 2002-2003. No cases have been identified since 2004, but this virus should also be considered in the appropriate clinical and epidemiologic setting.16
• Inability of many patients to produce sputum
• Failure to perform numerous serologic studies routinely in all patients
• The fact that many organisms (e.g., viruses and anaerobic bacteria) were not routinely sought
• Failure, until more recently, to recognize “new” pneumonia pathogens, such as C. pneumoniae
The common microbial agents producing HCAP, HAP, and VAP are summarized in Table 22-1 and include gram-negative bacilli, S. aureus, Legionella species, and, rarely, viruses such as influenza or respiratory syncytial virus. The last-mentioned viruses are considerations only during the winter months, when they are endemic in the community and may be brought into the hospital by health care workers, visitors, or patients with incubating or active infections.
Clinical Manifestations
In the past, clinicians often distinguished between typical and atypical clinical syndromes as a means of predicting the most likely microbial causes. A typical presentation consisted of the sudden onset of high fever, shaking, chills, and cough with purulent sputum. Such a presentation was considered more common with bacterial pathogens such as pneumococcus and H. influenzae. An atypical presentation was an illness characterized by the gradual onset of fever, headache, constitutional symptoms, diarrhea, and cough, often with minimal sputum production. Coughing was often a relatively minor symptom at the outset, and the illness was initially dominated by nonrespiratory symptoms. Such a presentation was thought to be more common with pathogens such as M. pneumoniae, C. pneumoniae, Legionella species, and viruses. More recent studies have shown that these distinctions are not ironclad and that considerable overlap exists in the clinical presentations of pneumonia with typical and atypical pathogens.17 The occurrence of concomitant diarrhea, previously considered indicative of legionellosis, is now known to be common in pneumococcal and mycoplasmal pneumonia.
As noted previously, inhalation anthrax is a rare disease, but it warrants mention because of the small epidemic believed to have been an act of bioterrorism.15 This outbreak affected mainly postal workers who were exposed to mail containing anthrax spores. Most patients presented with a febrile flulike illness of several days’ duration accompanied by dry cough and shortness of breath. Some patients in whom the diagnosis was not quickly considered went on to develop septic shock, meningitis, and disseminated intravascular coagulation over several days, culminating in death.
Because of a lack of prior host immunity or unique viral virulence factors, patients infected with pandemic influenza strains may have unusually severe presentations. During the 2009-2010 pandemic of H1N1 influenza, clinical presentations varied from mild upper respiratory syndromes to fulminant pneumonias with acute respiratory distress syndrome (ARDS) and shock.14 Severe acute respiratory syndrome manifests with high fever and myalgia for 3 to 7 days followed by nonproductive cough and progressive hypoxemia with progression to mechanical ventilation in 20%.16
Chest Radiograph
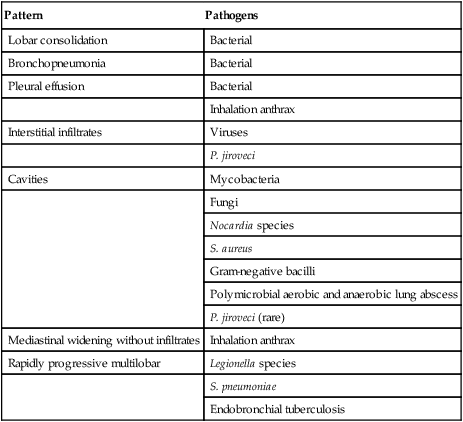
A normal chest radiograph does not exclude the diagnosis of pneumonia. The chest radiograph may be normal in patients with early infection, dehydration, or P. jiroveci infection. The pattern of radiographic abnormality is not diagnostic of the causative agent, although specific radiographic findings should suggest specific microbial differential diagnoses (Table 22-4).
TABLE 22-4
Radiographic Patterns Produced by Pathogens in Community-Acquired Pneumonia
| Pattern | Pathogens |
| Lobar consolidation | Bacterial |
| Bronchopneumonia | Bacterial |
| Pleural effusion | Bacterial |
| Inhalation anthrax | |
| Interstitial infiltrates | Viruses |
| P. jiroveci | |
| Cavities | Mycobacteria |
| Fungi | |
| Nocardia species | |
| S. aureus | |
| Gram-negative bacilli | |
| Polymicrobial aerobic and anaerobic lung abscess | |
| P. jiroveci (rare) | |
| Mediastinal widening without infiltrates | Inhalation anthrax |
| Rapidly progressive multilobar | Legionella species |
| S. pneumoniae | |
| Endobronchial tuberculosis |
Risk Factors for Mortality and Assessing the Need for Hospitalization
Many cases of community-acquired pneumonia can be managed successfully on an outpatient basis. The challenge for the clinician is to identify individuals at higher risk of morbidity and mortality for whom hospitalization is indicated. Over the past 20 years, numerous studies have analyzed risk factors for mortality in patients with community-acquired pneumonia.17–19 Risk factors predictive of a high risk of death are summarized in Box 22-1.
Fine and associates19 performed a meta-analysis of 127 cohorts of patients with community-acquired pneumonia. The study examined risk factors for fatal outcome. The overall mortality for the 33,148 patients in these cohorts was 13.7%. Eleven prognostic variables were significantly associated with mortality, including male sex, absence of pleuritic chest pain, hypothermia, systolic hypotension, tachypnea, diabetes mellitus, cancer, neurologic disease, bacteremia, leukopenia, and multilobar infiltrates on chest radiograph. Mortality varied according to the infecting agent and was highest for P. aeruginosa (61.1%), Klebsiella species (35.7%), Escherichia coli (35.3%), and S. aureus (31.8%). Mortality rates for more common pathogens were lower but still substantial and included Legionella species (14.7%), S. pneumoniae (12.3%), C. pneumoniae (9.8%), and M. pneumoniae (1.4%).
Because some variables are unknown at the time a patient seeks treatment for pneumonia, such as the causative agent and whether bacteremia is present, more recent studies have sought to assess the risk of fatal outcome by using clinical and laboratory data that are readily available at the time of the initial evaluation. Based on an analysis of more than 40,000 patients regarding 30-day mortality, Fine and associates20 proposed a prediction rule to identify low-risk and high-risk patients with community-acquired pneumonia. Their algorithm uses the demographic, clinical, and laboratory data available at presentation to stratify the risk of fatal outcome and the criteria for hospitalization in outpatient groups. Points are assigned for the presence of numerous variables, and cumulative point scores are used to stratify patients into one of five different risk groups with predictable mortality rates (Tables 22-5 and 22-6). In this model, which has been validated in large prospective cohorts of patients, the patients at the lowest risk of death fall into groups I and II. In most instances, these patients may be treated successfully as outpatients, unless they are hypoxic, vomiting and unable to take oral antibiotics, noncompliant, or immunocompromised. Patients in group I are patients younger than 50 years without comorbid illnesses and abnormal physical findings at presentation (see Box 22-1 and Table 22-5). This group of patients has a risk of fatal outcome of 0.1%.
TABLE 22-5
Scoring System for Stratifying Risk of 30-Day Mortality in Adults With Community-Acquired Pneumonia
| Variable | Points Assigned |
| Age | |
| Men | Age (yr) |
| Women | Age (yr) − 10 |
| Nursing home resident | +10 |
| Comorbid illnesses | |
| Cancer | +30 |
| Liver disease | +20 |
| Kidney disease | +10 |
| Cerebrovascular disease | +10 |
| CHF | +10 |
| Physical findings | |
| Altered mentation | +20 |
| Tachypnea >30 breaths/min | +20 |
| Systolic hypotension <90 mm Hg | +20 |
| Temperature <35° C or >40° C | +15 |
| Heart rate >125 beats/min | +10 |
| Laboratory and radiographic findings | |
| Acidemia (arterial pH <7.35) | +30 |
| Azotemia (BUN >30 mg/dl) | +20 |
| Hyponatremia (sodium <130 mmol/L) | +20 |
| Hypoxia (PaO2 < 60 mm Hg) | +10 |
| Hyperglycemia (glucose >250 mg/dl) | +10 |
| Anemia (hematocrit <30%) | +10 |
| Pleural effusion | +10 |
Modified from Fine MJ, Auble TE, Yealy DM, et al: A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336:243–250, 1997.
TABLE 22-6
| Risk Class (Cumulative Point Score) | Mortality Rate (%) |
| I | 0.1 |
| II (≤70) | 0.6 |
| III (71-90) | 2.8 |
| IV (91-130) | 8.2 |
| V (>130) | 29.2 |
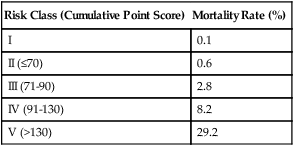
Note: Patients in risk class I are <50 years old and lack existing illness or physical findings listed in Table 19-5. Points are assigned to patients in risk classes II and higher.
Modified from Fine MJ, Auble TE, Yealy DM, et al: A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336:243–250, 1997.
Because of the complexity of the pneumonia severity index (PSI), many practitioners prefer a simpler stratification system, the Confusion, Urea, Respiratory rate, Blood pressure, age >65 (CURB-65). Risk criteria in this system include confusion, blood urea nitrogen greater than 20 mg/dl, respiratory rate greater than 30 breaths/min, systolic blood pressure less than 90 mm Hg or diastolic blood pressure less than 60 mm Hg, and age older than 65 years. Based on mortality in the derivation and validation cohorts, the authors recommend that patients with one or two risk criteria be treated outside the acute care setting, patients with two criteria be treated on general hospital wards, and patients with three or more criteria be admitted to the ICU.21
Patients with HAP are, by definition, already hospitalized at the time pneumonia develops, and a decision regarding the need for hospitalization is unnecessary. Many studies have examined risk factors for the development of HAP and VAP, which in broad terms can be divided into (1) factors that interfere with host defense and (2) factors that facilitate exposure to large numbers of bacteria.6 Examples of the factors that interfere with host defense include the following:
• Underlying illnesses such as diabetes mellitus, malignancy, chronic heart and lung disease, and renal failure
• Critical illnesses such as sepsis syndrome and ARDS
• Therapeutic interventions such as endotracheal intubation, tracheostomy, and administration of medications such as sedatives and corticosteroids
Although numerous studies have highlighted the substantial mortality rate (20% to 50%) for patients who develop HAP or VAP, few studies have examined the specific risk factors associated with mortality in hospital-acquired lower respiratory tract infection. For nonventilated patients, risk factors for mortality include bilateral infiltrates, respiratory failure, and infection with high-risk organisms.22,23 In mechanically ventilated patients, factors associated with fatal outcome include the following:23,24
Diagnostic Studies
Community-Acquired Pneumonia
Many patients with community-acquired pneumonia who are treated as outpatients never have an established microbiologic diagnosis. Many are treated based on the history and on compatible findings on physical examination, with or without a chest radiograph to confirm the presence of an infiltrate. Patients who are sick enough to warrant hospitalization or consideration of hospitalization should undergo numerous studies to stratify risk of mortality and to establish a microbiologic diagnosis (Box 22-2). Complete blood count, blood glucose, serum sodium, and blood urea nitrogen all are necessary to derive a point score for estimating the risk of mortality. An arterial blood gas analysis is used to detect the presence of hypoxemia and acidemia, which indicate a more serious illness.
The value of Gram stain and culture of expectorated sputum has been debated for years.25 Many patients lack a productive cough, making collection of an adequate specimen difficult. Prior antibiotic therapy reduces the yield from both of these tests. Only approximately 50% of patients with bacteremic pneumococcal pneumonia have a positive sputum culture.26 Nevertheless, the finding of a predominant organism on Gram stain in an appropriately collected specimen has a high predictive value for the selection of appropriate antibiotic therapy.27 In addition, isolation of penicillin-resistant pneumococci from sputum has important implications for therapy. A routine sputum culture can be interpreted only within the context of the sputum Gram stain. Specimens contaminated with oropharyngeal epithelial cells are unsatisfactory for analysis.
The RT has an important role in the collection of an appropriate specimen of expectorated sputum. Patients should be advised to rid the mouth of contaminating saliva, either by rinsing with water or by spitting, and then to expectorate a specimen from deep within the tracheobronchial tree into a collection container. Prompt transportation to the laboratory is essential and improves the diagnostic yield from culture.11 Most microbiology laboratories screen the adequacy of the specimen by cytologic examination. A satisfactory specimen contains more than 25 leukocytes and less than 10 squamous epithelial cells per high-power field.28 In routine sputum culture, the isolation of bacteria, such as S. pneumoniae and H. influenzae, must be interpreted within the context of the Gram stain because these organisms can colonize the oropharynx, and their presence in culture may not signify true lower respiratory tract infection. The culture isolation of other organisms, such as Mycobacterium tuberculosis, Histoplasma capsulatum, Blastomyces dermatitidis, Coccidioides immitis, and Legionella species is diagnostic of disease because these organisms almost never colonize the respiratory tract.
Other stains and cultures of expectorated sputum should be obtained as dictated by the clinical circumstance, when management would be changed, or for purposes of tracking unusual or resistant organisms in an institution or population. In patients with suspected tuberculosis, the finding of acid-fact bacilli in stained specimens of sputum often prompts initiation of antituberculous therapy because culture isolation of M. tuberculosis may take 6 weeks. A direct fluorescent antibody stain of sputum for Legionella species may reveal the organism in 25% to 80% of individuals with Legionnaire’s disease, and cultures are positive in 50% to 70% of these individuals.29 Toluidine blue O stains of sputum may disclose the organism in 80% of patients with P. jiroveci pneumonia. Potassium hydroxide preparations of sputum disclose fungi in a few patients with histoplasmosis, blastomycosis, or coccidioidomycosis but are very helpful if positive. In inhalation anthrax, patients typically do not produce sputum, and the organism is usually absent if they do.
Blood cultures should be obtained in hospitalized patients with community-acquired pneumonia and may be helpful in establishing the diagnosis in patients with typical bacterial pathogens. Blood cultures are positive in approximately 30% of patients with pneumococcal pneumonia and 70% of patients with H. influenzae pneumonia.30 Blood cultures are often positive in patients with inhalation anthrax and should be collected if the diagnosis is suspected. They are not helpful in patients with legionellosis or M. pneumoniae, C. pneumoniae, P. jiroveci, or most viral infections. Collection of blood cultures within 24 hours of hospitalization in elderly patients with pneumonia has been associated with improved survival.31
Parapneumonic pleural effusions are common; they occur in 30% to 50% of cases of community-acquired pneumonia.8 Hemorrhagic pleural effusions are typically present in patients with inhalation anthrax. Thoracentesis is indicated for patients with large pleural effusions and for patients with smaller effusions who fail to respond to therapy or for whom the microbiologic diagnosis is not established. Pleural fluid should also be sampled in patients with suspected inhalation anthrax. Pleural fluid should be tested for cell count, glucose, protein, pH, lactate dehydrogenase, Gram and acid-fast bacilli stains, and routine (aerobic and anaerobic) and mycobacterial cultures. Patients with effusions with a pH less than 7.30 or an elevated white blood cell count require tube thoracostomy for drainage.32
Other studies may be helpful in establishing a microbiologic diagnosis in the appropriate clinical setting. L. pneumophila serogroup 1 accounts for 80% of cases of legionnaires disease.33 The urinary antigen test for L. pneumophila serogroup 1 is a sensitive and rapid test and usually becomes positive within 3 days of onset of illness. The test has limitations. First is its inability to detect the non–serogroup 1 L. pneumophila and non–L. pneumophila species that account for 20% of cases of Legionnaire’s disease. Second, the test may remain positive for 1 year, obviating the ability to distinguish new from remote infection in patients with a recent history of pneumonia.
Health Care–Associated Pneumonia, Hospital-Acquired Pneumonia, and Ventilator-Associated Pneumonia
The accurate diagnosis of nosocomial pneumonia is fraught with difficulty and has been the subject of intense investigation over the past 2 decades. Numerous techniques have been extensively reviewed (Box 22-3)34,35; none is absolutely sensitive and specific. Clinical diagnosis has been defined as the development of a new infiltrate on chest radiograph in the setting of fever, purulent tracheal secretions, and leukocytosis in a hospitalized patient. Clinical diagnosis lacks specificity because many other causes of pulmonary infiltrates exist in hospitalized patients, especially patients on mechanical ventilation.36 In addition, the upper airway commonly is colonized with nosocomial gram-negative bacilli and staphylococci, even in the absence of pneumonia. The qualitative culture isolation of these organisms from tracheal secretions correlates poorly with the presence or absence of pneumonia.
Direct visualization by bronchoscopy of the lower airway in ventilated patients is sometimes helpful in supporting the diagnosis of VAP. In one study, the presence of distal, purulent secretions; persistence of secretions surging from distal bronchi during exhalation; and a decrease in the PaO2/FiO2 ratio of less than 50 were independently associated with the presence of pneumonia. The presence of two of three of these factors had a sensitivity of 78% in the diagnosis of nosocomial pneumonia, and these factors were absent 89% of the time when there was no pneumonia (89% specific).37
Because the specificity of qualitative sputum cultures has been unreliable, several studies have examined the role of quantitative cultures of endotracheal aspirates using various breakpoints ranging from 103 to 107 colony-forming units (CFU) per milliliter of respiratory secretions. Results with this technique have been best using a breakpoint of 106 CFU/ml, but sensitivities have been only 68% to 82% with specificities of 84% to 96% with this modality.38,39
Protected specimen brush (PSB) was developed in the 1970s and uses a special double-catheter brush system to minimize contamination by upper airway flora. Specimens obtained with this technique are cultured quantitatively. Numerous studies have validated the sensitivity of PSB in the diagnosis of nosocomial pneumonia.40,41 However, the usefulness of PSB may be suboptimal for cases in which antibiotic therapy has already been initiated, in cases of early infection, and in cases in which the wrong lobe is sampled.34
Nonbronchoscopic techniques using telescoping protected catheters have been developed to obtain specimens for quantitative culture from the lower airway. In most studies, sensitivity has been comparable with bronchoscopic techniques, but discordant results, compared with bronchoscopy, have been noted in 20% of cases.34
Bronchoalveolar lavage (BAL), in which a lung segment is lavaged with sterile saline through the bronchoscope and recovered fluid is quantitatively cultured, has been studied extensively as a tool for diagnosing nosocomial pneumonia. Some studies have supported the usefulness of this technique, whereas others have questioned its specificity because of upper airway contamination.40,41 BAL has proved useful for obtaining alveolar cells for microscopic analysis, and several studies have suggested that the presence of intracellular bacteria in 3% to 5% of BAL cells distinguishes patients with nosocomial pneumonia from patients without pneumonia.40,41 In one study, the combination of PSB cultures and microscopic examination of BAL cells for intracellular bacteria was 100% sensitive and 96% specific in identifying patients with nosocomial pneumonia.40
Mini-BAL performed by RTs has been advocated for diagnosing VAP. In one study, results obtained using this technique were comparable with results obtained by bronchoscopy using PSB.42 Some centers use this technique as the primary method of sampling respiratory secretions in suspected nosocomial pneumonia. Transthoracic ultrathin needle aspiration of the lung in nonventilated patients with nosocomial pneumonia also has been studied and in one report was found to have a sensitivity of 60%, a specificity of 100%, and a positive predictive value of 100%.43
Antibiotic Therapy
Community-Acquired Pneumonia
The selection of antibiotic therapy for patients with community-acquired pneumonia should be guided by several considerations, including the age of the patient, severity of the illness, presence of risk factors for specific organisms, and results of initial diagnostic studies. Pathogen-specific therapy should be used when clinical circumstances and initial evaluation strongly suggest the microbiologic diagnosis or when cultures or other studies confirm the cause. In many instances, initial studies fail to establish a diagnosis, and empiric therapy must be initiated. Major classes of antibiotics used to treat pneumonia are listed in Table 22-7. Consensus guidelines for therapy have been published by the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA) (Table 22-8).44–46 Therapy initiated within 4 hours of hospital admission has been associated with improved survival.31
TABLE 22-7
Major Classes of Antibiotics Used in the Treatment of Pneumonia
| Antibiotic Class | Representative Drugs |
| Penicillins | Penicillin G, ampicillin |
| Ureidopenicillins | Ticarcillin, piperacillin, mezlocillin |
| Semisynthetic penicillins | Oxacillin, nafcillin |
| First-generation cephalosporins | Cefazolin |
| Second-generation cephalosporins | Cefuroxime |
| Third-generation cephalosporins | Cefotaxime, ceftriaxone, ceftizoxime |
| Antipseudomonal cephalosporins | Ceftazidime, cefepime |
| Carbapenems | Imipenem, meropenem, ertapenem |
| Monobactams | Aztreonam |
| Beta-lactam/beta-lactamase inhibitor combinations | Ticarcillin/clavulanate, piperacillin/tazobactam, ampicillin/sulbactam |
| Quinolones | Ciprofloxacin, levofloxacin, moxifloxacin, gemifloxacin |
| Macrolides | Erythromycin, clarithromycin, azithromycin |
| Tetracyclines | Doxycycline |
| Glycopeptides | Vancomycin |
| Oxazolidinones | Linezolid |
TABLE 22-8
Empiric Regimens for Treatment of Hospitalized Adults With Community-Acquired Pneumonia
| Patient Group | Likely Pathogens | Empiric Regimens |
| Hospitalized on ward | S. pneumoniae, H. influenzae, C. pneumoniae, S. aureus, M. pneumoniae, anaerobes, viruses | Respiratory fluoroquinolone (levofloxacin, moxifloxacin, gemifloxacin) alone or beta-lactam (cefotaxime, ceftriaxone, ampicillin, ertapenem) and macrolide |
| Critically ill, ICU | S. pneumoniae, Legionella species, S. aureus, gram-negative bacilli, M. pneumoniae, C. pneumoniae | If P. aeruginosa unlikely: beta-lactam (cefotaxime, ceftriaxone, ampicillin-sulbactam) plus either azithromycin or a respiratory fluoroquinolone |
| If P. aeruginosa possible: IV antipseudomonal beta-lactam (piperacillin-tazobactam, cefepime, imipenem, meropenem) plus fluoroquinolone (ciprofloxacin or levofloxacin) or IV antipseudomonal beta-lactam plus aminoglycoside plus either IV macrolide or fluoroquinolone |
IV, Intravenous; PO, by mouth.
Adapted from Mandell MA, Wunderink RG, Anzueto A, et al: Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44:S27–S72, 2007.
For hospitalized patients who are not critically ill and who are admitted to the ward, an empiric regimen of a respiratory fluoroquinolone alone or an advanced macrolide plus a beta-lactam (cefotaxime, ceftriaxone, or ampicillin) is recommended (see Table 22-8). For critically ill patients requiring admission to the ICU, the IDSA and ATS recommend as empiric therapy a beta-lactam (cefotaxime, ceftriaxone, or ampicillin-sulbactam) plus either an advanced macrolide or a respiratory fluoroquinolone. Certain pathogens require specific consideration in the ICU setting. If Pseudomonas is a concern, recommended regimens include an antipseudomonal beta-lactam (piperacillin-tazobactam, cefepime, imipenem, or meropenem) and ciprofloxacin or levofloxacin; an antipseudomonal beta-lactam, an aminoglycoside, and azithromycin; or an antipseudomonal beta-lactam, an aminoglycoside, and a respiratory fluoroquinolone (see Table 22-8). When MRSA is a concern, addition of vancomycin or linezolid is recommended.
When a microbiologic diagnosis is established, the antimicrobial regimen should be tailored to the isolated pathogen. Pathogen-specific treatment recommendations from the IDSA and ATS are summarized in Table 22-9. For isolates of S. pneumoniae susceptible to penicillin, penicillin remains the preferred agent. Many strains of H. influenzae produce beta-lactamase, rendering them resistant to penicillin. Second-generation or third-generation cephalosporins and amoxicillin/clavulanate are the agents of choice. Legionellosis should be treated with a macrolide or with a fluoroquinolone alone. Pneumonia caused by M. pneumoniae and C. pneumoniae should be treated with a macrolide or doxycycline. Trimethoprim-sulfamethoxazole is the drug of choice for P. jiroveci pneumonia. However, 50% of HIV-infected patients may develop fever or a rash while taking this medication. Pentamidine is an acceptable alternative. Treatment for staphylococcal or gram-negative pneumonias is dictated by the antibiotic susceptibility profiles of the offending organism. For patients with staphylococcal pneumonia, vancomycin is preferred, pending antibiotic susceptibility results. If the isolate is methicillin susceptible, a semisynthetic penicillin, such as oxacillin or nafcillin, should be used; in seriously ill patients, rifampin or an aminoglycoside may be added. In patients with suspected or proven inhalation anthrax, doxycycline, ciprofloxacin, or levofloxacin should be administered along with one or two of the following antibiotics: penicillin, ampicillin, vancomycin, rifampin, chloramphenicol, imipenem, clindamycin, or clarithromycin.
TABLE 22-9
| Pathogen | Recommended Regimen |
| S. pneumoniae | |
| Penicillin susceptible | Penicillin G or amoxicillin |
| Penicillin resistant | Ceftriaxone, cefotaxime, fluoroquinolone, or vancomycin |
| H. influenzae | Second- or third-generation cephalosporin, azithromycin, or TMP-SMX |
| Legionella species | Macrolide ± rifampin or fluoroquinolone alone |
| M. pneumoniae | Macrolide or doxycycline |
| C. pneumoniae | Macrolide or doxycycline |
| S. aureus | |
| Methicillin susceptible | Semisynthetic penicillin ± rifampin or gentamicin |
| Methicillin resistant | Vancomycin or linezolid |
| Enterobacteriaceae | Third-generation cephalosporin ± aminoglycoside or carbapenem |
| P. aeruginosa | Aminoglycoside + antipseudomonal beta-lactam or carbapenem |
| Influenza with suspected secondary pneumococcal or staphylococcal infection | Neuraminidase inhibitor (oseltamivir or zanamivir) and vancomycin or linezolid |
TMP-SMX, Trimethoprim-sulfamethoxazole.
From Mandell MA, Wunderink RG, Anzueto A, et al: Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44:S27–S72, 2007.
The duration of therapy of community-acquired pneumonia is guided by the specific pathogen and the patient’s clinical course. Recommendations have evolved from the traditional 14 days to a minimum of 5 days of therapy with clinical stability. Exceptions include Legionnaire’s disease or staphylococcal pneumonia, for which a minimum of 2 weeks of therapy is recommended. Older individuals and patients with comorbidities may also require longer courses of treatment. When fever has resolved and patients begin to improve clinically, oral therapy may be used to complete the treatment program. Failure of the patient’s temperature to normalize within 4 or 5 days suggests the following possibilities: a missed pathogen, a metastatic or closed-space infection (e.g., empyema), drug fever, or the presence of an obstructing endobronchial lesion. Empyema should be treated with tube thoracostomy. Abnormal findings on physical examination may persist beyond 1 week in 20% to 40% of patients, despite clinical improvement. By 1 month, radiographic resolution occurs in 90% of individuals younger than 50 years.47 After 1 month, radiographic abnormalities may persist in 70% of cases involving older individuals or in patients with significant underlying illnesses.47
Health Care–Associated Pneumonia, Hospital-Acquired Pneumonia, and Ventilator-Associated Pneumonia
Empiric and definitive therapy of nosocomial pneumonia is dictated by institution-specific data regarding the most common organisms and their antibiotic-susceptibility profiles and by patient-specific risk factors. Although general guidelines have been published,3 the importance of local data cannot be overemphasized because considerable geographic and institutional variability exists regarding the prevalence and susceptibility profiles of specific pathogens.
Generally, in-hospital aspiration should be treated with a regimen that provides coverage against anaerobes and gram-negative bacilli, such as a beta-lactam/beta-lactamase inhibitor combination or clindamycin with a third-generation cephalosporin. Although vancomycin has been the traditional treatment agent of choice for MRSA pneumonia, evolving data suggest that linezolid may be superior to vancomycin in this role.48 For VAP, empiric coverage may be targeted at organisms known to colonize the patient’s oropharynx or pathogens endemic to the ICU. Patients with P. aeruginosa pneumonia usually are treated with two agents, such as ureidopenicillin or antipseudomonal cephalosporin together with an aminoglycoside or fluoroquinolone. Other gram-negative pneumonias generally are treated with a single agent except in cases involving critically ill patients, for whom a second drug is sometimes added. If legionellosis is endemic as a nosocomial infection within an institution, a macrolide may be added to the empiric regimen.
Similar to community-acquired pneumonia, the duration of therapy for cases of nosocomial pneumonia is dictated by the clinical course. A study comparing 8 days versus 15 days of therapy in patients with VAP found that short-course therapy was associated with comparable outcomes to long-course therapy, although the rate of relapse was slightly higher in patients with Pseudomonas or Acinetobacter infections.48 More prolonged courses of therapy may be required in patients slow to respond but are associated with a greater risk of new colonization with other organisms. Failure of the patient to improve should prompt the following considerations: the presence of an occult empyema; an unrecognized pathogen; a new, unrelated nosocomial infection; or other noninfectious causes of fever common in the ICU, such as deep venous thrombosis, drug fever, occult pancreatitis, or acalculous cholecystitis (gallbladder inflammation without gallstones).
Some organisms, such as P. aeruginosa and Acinetobacter species, are associated with a poor prognosis in VAP despite optimal therapy.49 The mortality rate for these organisms may approach 90% despite appropriate treatment.
Prevention
Community-Acquired Pneumonia
Preventive strategies for community-acquired pneumonia have focused on immunization of high-risk individuals against influenza and S. pneumoniae. Influenza is a risk factor for subsequent development of community-acquired pneumonia during the fall and winter months. Immunization is indicated for individuals older than 60 years because it reduces the incidence of illness for this age group by half.50 Immunization also is indicated for individuals with chronic lung or heart disease or for whom the morbidity of influenza may be substantial. More recent studies have suggested that widespread immunization of healthy working adults may be cost-effective because the number of sick days taken and the number of visits to a physician are reduced.51 Health care workers, including RTs, should be immunized annually to prevent transmission of influenza to patients.
Currently available pneumococcal vaccines provide protection against the 23 serotypes of S. pneumoniae, which cause 85% to 90% of invasive pneumococcal infections in the United States. Vaccination is indicated for all individuals older than 65 years and for individuals older than 2 years who have functional or anatomic asplenia. Vaccination is also indicated in patients with chronic illnesses such as CHF, chronic lung disease, or chronic liver disease; alcoholism; cerebrospinal fluid leaks; or conditions characterized by impaired immunity.52 Routine pneumococcal vaccination of all health care workers is not currently recommended; pneumococcal vaccination is recommended to health care workers who possess one of the specific indications for vaccination outlined previously.
Immunity against Bordetella pertussis wanes over time, leading to transmission from older adults to other adults and infants. Because secondary bacterial pneumonia occurs in a significant number of cases of pertussis, the Advisory Committee on Immunization Practices has recommended that the tetanus-diphtheria-acellular pertussis (Tdap) vaccine replace the tetanus-diphtheria (Td) vaccine in the adult immunization schedule.53
Health Care–Associated Pneumonia, Hospital-Acquired Pneumonia, and Ventilator-Associated Pneumonia
The prevention of nosocomial pneumonia has been an area of intense investigation over the past 20 years. Table 22-10 summarizes currently available strategies and their relative efficacy. No preventive strategy is uniformly effective. Many institutions now employ a “ventilator bundle” including several of these measures.
TABLE 22-10
Strategies for Prevention of Nosocomial Pneumonia
| Strategy | Efficacy |
| Handwashing | Probably effective |
| Isolation of patients with resistant organisms | Probably effective |
| Infection control and surveillance | Probably effective |
| Enteral feeding, rather than TPN | Possibly effective |
| Semierect position | Possibly effective |
| Sucralfate for bleeding prophylaxis | Possibly effective |
| Careful handling of respiratory therapy equipment | Possibly effective |
| Subglottic secretion aspiration | Possibly effective |
| Selective digestive decontamination | Unproved efficacy |
| Topical tracheobronchial antibiotics | Unproved efficacy |
In patients requiring nutrition support, the use of enteral feeding via jejunostomy has been associated with a lower risk of nosocomial pneumonia than the use of total parenteral nutrition.54 In addition, patients who are fed enterally have a lower incidence of pneumonia if kept semierect rather than recumbent.7
Two studies have suggested that gastrointestinal bleeding prophylaxis with sucralfate is associated with a lower risk of pneumonia compared with antacid or H2-blockers.55,56 Careful handling of respiratory therapy equipment may reduce the risk of lower respiratory tract infection in ventilated patients. Condensate within the tubing may be colonized with bacteria and should be drained away from the patient because passage of this material into the airway may facilitate colonization with nosocomial pathogens. One study found that continuous subglottic aspiration of secretions was effective in reducing the incidence of nosocomial pneumonia in intubated patients.57 Many studies have failed to show the efficacy of selective digestive decontamination in the prevention of nosocomial pneumonia, a strategy that uses topical antibiotics in the oropharynx and gastrointestinal tract along with a brief course of systemic therapy. A meta-analysis suggested that topical oral decontamination may reduce the incidence of VAP but not mortality, duration of mechanical ventilation, or length of ICU stay.58
Tuberculosis
Epidemiology
Tuberculosis has increasingly become a disease affecting individuals of lower socioeconomic status in whom homelessness or crowded living conditions, poor access to health care, and unemployment have contributed to the resurgence of the disease.59 Other risk factors for tuberculosis include the presence of hematologic malignancies, head and neck cancer, or celiac disease (a bowel disease characterized by poor absorption) and the receipt of certain medications such as corticosteroids and tumor necrosis factor-alpha antagonists.59–62
Pathophysiology
Primary Tuberculosis
Symptomatic primary tuberculosis occurs in a few individuals shortly after exposure. Primary tuberculous pneumonia is a more common clinical presentation in children and in HIV-infected individuals compared with non–HIV-infected adults. Fever is the most common symptom and occurs in 70% of patients; it persists for 14 to 21 days on average.63 Chest pain occurs in about 25%; cough is even less common. The chest radiograph shows hilar lymphadenopathy (enlargement of the lymph nodes in the area where the pulmonary arteries emerge from the mediastinum) in 65%, pleural effusion in one-third, and an infiltrate in about 25%. The disease may be difficult to diagnose given the infrequency of cough and a pulmonary infiltrate.
Precautions
A 5 TU PPD may be performed in individuals with suspected tuberculosis. A positive skin test supports the diagnosis in the appropriate clinical setting, but a negative skin test does not exclude the diagnosis. Patients with HIV infection, other causes of immunodeficiency, advanced age, or other comorbidities may be anergic (i.e., have decreased immune responsiveness to skin tests) and unable to mount a positive skin test. Interferon gamma release assays may be used to support diagnosis of tuberculosis when clinical suspicion is high, but decreased sensitivity in immunocompromised persons limits their usefulness in diagnosis of active tuberculosis.64
Treatment
Treatment recommendations for tuberculosis have been published by the ATS, U.S. Centers for Disease Control (CDC), and IDSA.65 The goals of therapy are to cure the patient and to prevent transmission of M. tuberculosis to others. Treatment must address clinical and social issues and should be customized to the patient’s circumstance. At the outset, daily observed therapy should be part of the treatment program; this consists of observing the patient taking his or her antituberculous medications. Treatment programs that use comprehensive case management and daily observed therapy have a higher rate of successful completion of therapy than other treatment strategies. Social service support, housing assistance, and treatment for substance abuse may be required for selected individuals with tuberculosis and should be part of the treatment plan. Patients with tuberculosis must be promptly reported to the local department of public health so that contact tracing can be performed. Contact tracing includes identification, if possible, of the index case from whom the patient has contracted the infection and identification of close personal contacts to whom the patient may have transmitted M. tuberculosis. Identification of key contacts should also occur at the time of the patient’s hospitalization, and appropriate counseling and referral for medical evaluation should be provided to potential at-risk individuals.
Isoniazid, rifampin, pyrazinamide, and ethambutol are first-line antituberculous medications. Pending antimicrobial susceptibility results, treatment with four drugs at the outset is recommended. In patients with drug-susceptible pulmonary tuberculosis, many 6- to 9-month treatment regimens have been shown to be effective as outlined in guidelines by the ATS, CDC, and IDSA.65 Patients with multidrug-resistant tuberculosis may require more prolonged courses of therapy with multidrug regimens.