Pulmonary Hypertension
SPECIFIC DISORDERS ASSOCIATED WITH PULMONARY HYPERTENSION
Idiopathic Pulmonary Arterial Hypertension and Related Disorders (Group 1 PAH)
Pulmonary Hypertension Owing to Left Heart Disease (Group 2 PH)
Pulmonary Hypertension Owing to Lung Disease and/or Hypoxia (Group 3 PH)
Chronic Thromboembolic Pulmonary Hypertension (Group 4 PH)
Pulmonary Hypertension with Unclear Multifactorial Mechanisms (Group 5 PH)
The current classification for clinical categories of pulmonary hypertension is summarized in Table 14-1. Clarification of a few points is pertinent. First, the term pulmonary hypertension (PH) simply refers to elevated pulmonary arterial pressure, which may be due to a number of different mechanisms. The term pulmonary arterial hypertension (PAH) is reserved for specific types of PH—those categorized under Group 1 in the classification system in Table 14-1. Elevation of pulmonary arterial pressure may be acute or chronic and either reversible or irreversible, depending on the causative factors. In some cases, chronic PH is punctuated by further acute elevations in pressure, often as a result of exacerbations of the underlying disease. Second, the development of right ventricular hypertrophy is the consequence of chronic PH, whatever the primary cause of the latter. When PH is due to disorders of any part of the respiratory apparatus (airways, parenchyma and blood vessels, chest wall, respiratory musculature, or central nervous system controller), the term cor pulmonale is used to refer to the resulting right ventricular hypertrophy. This term should not be used to describe the right ventricular changes occurring as a consequence of primary cardiac disease or increased flow to the pulmonary vascular bed.
Table 14-1
UPDATED CLINICAL CLASSIFICATION OF PULMONARY HYPERTENSION (DANA POINT, 2008)
1. PULMONARY ARTERIAL HYPERTENSION (PAH)
1.5. Persistent pulmonary hypertension of the newborn
1′ Pulmonary Veno-Occlusive Disease (Pvod) and/or Pulmonary Capillary Hemangiomatosis (Pch)
2. PULMONARY HYPERTENSION OWING TO LEFT HEART DISEASE
3. PULMONARY HYPERTENSION OWING TO LUNG DISEASE AND/OR HYPOXIA
3.1. Chronic obstructive pulmonary disease
3.2. Interstitial lung disease
3.3. Other pulmonary diseases with mixed restrictive and obstructive pattern
3.4. Sleep-disordered breathing
3.5. Alveolar hypoventilation disorders
4. CHRONIC THROMBOEMBOLIC PULMONARY HYPERTENSION (CTEPH)
5. PULMONARY HYPERTENSION WITH UNCLEAR MULTIFACTORIAL MECHANISMS
5.1. Hematologic disorders: myeloproliferative disorders, splenectomy
5.2. Systemic disorders: sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis, neurofibromatosis, vasculitis
5.3. Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders
5.4. Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure on dialysis
Modified from Simonneau G, Robbins IM, Beghetti M, et al: Updated clinical classification of pulmonary hypertension, J Am Coll Cardiol 54(Suppl 1):S43–S54, 2009.
Pathogenesis
A number of factors contribute to the pathogenesis of PH, both acutely and chronically. First, occlusion of a sufficient cross-sectional area of the pulmonary arteries by material (e.g., pulmonary emboli) within the vessels is an important factor (discussed in Chapter 13). In acute embolism, in which massive pulmonary emboli occlude more than half to two thirds of the vasculature, pulmonary arterial pressure is elevated. The right ventricle may dilate in response to its acutely increased workload because of insufficient time for hypertrophy to occur. In contrast, in chronic embolic disease, multiple and recurrent pulmonary emboli may elevate pulmonary arterial pressures during a period sufficient for right ventricular hypertrophy to occur.
Second, remodeling of the pulmonary arterial walls causing diminution of cross-sectional area is a potential factor. Disorders acting by this mechanism are characterized by intimal and medial changes (see Pathology) that lead to thickening of the arterial and arteriolar walls and narrowing or obliteration of the lumen. This group of disorders with pulmonary arterial pathology includes idiopathic pulmonary arterial hypertension (IPAH, formerly called primary pulmonary hypertension). The familial form of this condition, called heritable pulmonary arterial hypertension, in most cases is related to mutations of the gene on chromosome 2 that encodes the bone morphogenetic protein receptor type 2 (BMPR2). Abnormalities in this receptor are believed to lead to dysregulation of proliferative responses in the endothelium and pulmonary arterial smooth muscle cells, producing the well-described pathologic changes in small pulmonary arteries and arterioles (again, see Pathology). Lesions pathologically similar to those seen in IPAH are also observed in other conditions associated with PAH (e.g., scleroderma, portal hypertension, human immunodeficiency virus [HIV] infection) or with exposure to drugs and toxins (e.g., cocaine, methamphetamine, certain diet drugs). When compromise of the pulmonary vasculature and increased resistance to flow are sufficiently pronounced in these primary disorders of the vessel wall, the level of PH can be quite severe, both at rest and with exercise.
A fourth mechanism of PH is vasoconstriction in response to hypoxia and, to a lesser extent, to acidosis. The importance of this mechanism is related to its potential reversibility when normal PO2 and pH values are restored. In several causes of cor pulmonale, particularly chronic obstructive pulmonary disease (COPD), hypoxia is the single most important factor leading to PH and is potentially the most treatable. Acidosis, either respiratory or metabolic, causes pulmonary vasoconstriction and, although it is less important than hypoxia, may augment the vasoconstrictive response to hypoxia (discussed in Chapter 12).
Pathology
Although PH is classified into different clinical categories (see Table 14-1), as the disease progresses and remodeling occurs, the pathologic findings in the pulmonary arteries of patients with PH are similar regardless of the underlying cause. This section focuses on these general changes, which are particularly well illustrated in the lungs of patients with IPAH.
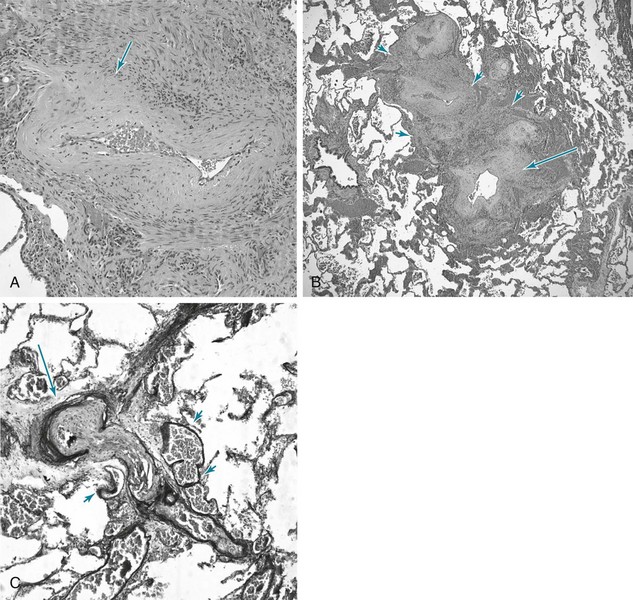
The most prominent abnormalities are seen in pulmonary arterial tree vessels with a diameter of less than 1 mm: the small muscular arteries (0.1–1 mm) and the arterioles (<0.1 mm). The muscular arteries show hypertrophy of the media, composed of smooth muscle, and hyperplasia of the endothelial cells that make up the intimal layer lining the vessel lumen. In the arterioles, a significant muscular component to the vessel wall is not normally present, but with PH, these vessels undergo “neomuscularization” of their walls (Fig. 14-1, A). In addition, the arteriolar intima proliferates. As a result of medial hypertrophy and encroachment of proliferating endothelial cells into the vessel, the luminal diameter is significantly decreased, and the pulmonary vascular resistance is elevated. Ultimately, the lumen may be completely obliterated and the overall number of small vessels greatly diminished. In some cases of severe PH, particularly when due to IPAH or secondary to congenital intracardiac shunts, cells originating in the vessel wall (smooth muscle cells, endothelial cells, and fibroblasts) will form so-called plexiform lesions, appearing as a plexus of small, slitlike vascular channels (Fig. 14-1, B). Although the pathogenesis of these lesions is not precisely understood, disordered endothelial cell growth has been documented in patients with IPAH. It appears likely that the endothelial cells in many patients with severe PH have acquired a dysfunctional pro-proliferative phenotype that is resistant to apoptosis (cell death).
When PH becomes marked, other changes are commonly seen in the larger (elastic) pulmonary arteries (Fig. 14-1, C). These vessels, which normally have much thinner walls than comparably sized vessels in the systemic circulation, develop thickening of the wall, particularly in the media. They also develop the types of atherosclerotic plaques generally seen only in the higher-pressure systemic circulation.
Pathophysiology
The pathophysiologic hallmark of PH is, by definition, an increase in pressure within the pulmonary circulation. If the primary component of the vascular change occurs at the precapillary level in the pulmonary arteries or arterioles, as in the case of IPAH or cor pulmonale, pulmonary arterial pressures (both systolic and diastolic) rise, but the pressure within pulmonary capillaries remains normal. On the other hand, if PH is secondary to pulmonary venous and pulmonary capillary hypertension, as in the case of mitral stenosis or left ventricular failure, pulmonary capillary pressure is elevated above its normal level. Of note, fluid leaks from the pulmonary capillaries and accumulates in the interstitium or alveolar spaces when either intracapillary pressures are elevated (cardiogenic pulmonary edema) or pulmonary capillary permeability is increased (non-cardiogenic pulmonary edema; see Chapter 28). In contrast, patients with precapillary PH and with normal pulmonary capillary pressures typically do not develop pulmonary edema.
Diagnostic Features
Definitive diagnosis of PH and precise quantification of its hemodynamics require cardiac catheterization. Measurements of right ventricular, pulmonary arterial, and pulmonary capillary wedge pressures are important in confirming the diagnosis, determining disease severity, and assessing the response to acute vasodilator testing to guide the patient’s subsequent management (see Chapter 12 for discussion of pulmonary artery catheterization).
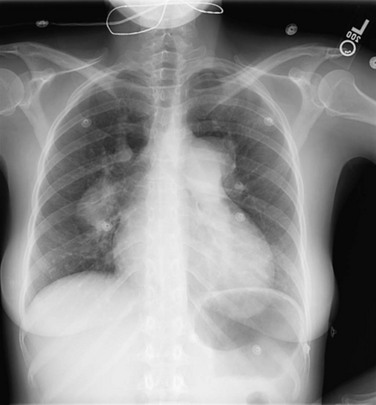
Clues to the status of the pulmonary vessels can be provided by chest radiography in some patients. With mild PH originating at the arterial or arteriolar level, frequently no abnormalities are seen. As PAH becomes more significant, the central (hilar) pulmonary arteries increase in size, and the vessels often rapidly taper such that the distal vasculature appears attenuated (Fig. 14-2). With hypertrophy of the right ventricle, the cardiac silhouette may enlarge. This feature is most apparent on the lateral radiograph, which shows bulging of the anterior cardiac border.
Computed tomographic angiography (CTA) or perfusion lung scanning can be valuable adjuncts in the assessment of patients with PH, primarily to look for chronic thromboembolic disease. CT scanning can also identify occult parenchymal disease not evident on chest radiograph (see Chapters 3 and 13). When CTA or perfusion scanning is positive, pulmonary angiography may be used to confirm the diagnosis and assess the surgical accessibility of the obstructing lesions.
Specific Disorders Associated with Pulmonary Hypertension
PH is currently classified according to the scheme given in Table 14-1, which is very useful in categorizing patients based on clinical aspects of their disease. It is important to recognize, however, that there is much pathophysiologic overlap among the categories, and as we better understand the pathobiology of PH, the classification system will likely evolve.
Pulmonary Hypertension Owing to Lung Disease and/or Hypoxia (Group 3 PH)
The most important aim of treatment for cor pulmonale in the setting of obstructive and interstitial disease is correction of alveolar hypoxia and hypoxemia by administration of supplemental O2. The goal is to maintain arterial PO2 at a level greater than approximately 60 mm Hg, above which hypoxic vasoconstriction is largely eliminated. Other forms of therapy aimed more specifically at the underlying disease are discussed in Chapters 6, 10, and 11.
In addition to these two categories of lung disease, other disorders of the respiratory apparatus associated with hypoxemia and hypercapnia may be complicated by development of cor pulmonale. Specifically, disorders of control of breathing, of the chest bellows, and of the neural apparatus controlling the chest bellows may be complicated by cor pulmonale. These disorders are discussed in more detail in Chapters 18 and 19.
Chronic Thromboembolic Pulmonary Hypertension (Group 4 PH)
The typical presentation of chronic thromboembolic pulmonary hypertension (CTEPH) is with insidious onset of dyspnea and findings related to PH, rather than with a history suggesting one or more known acute episodes of pulmonary embolism (see Chapter 13). Presumably, by the time a patient presents with CTEPH, the emboli have been occurring over months to years. Because chronic thrombi are organized and extensively infiltrated with fibroblasts and connective tissue, anticoagulation alone is not effective therapy. In some cases, the organized thromboemboli are primarily located within the large proximal pulmonary arteries, causing significant obstruction. In these patients, surgical removal of the proximal organized thrombi (pulmonary thromboendarterectomy) may be a feasible and highly effective therapeutic option. In other cases, there is extensive thromboembolic occlusion of smaller, surgically inaccessible vessels. Although this type of small vessel occlusion has generally been assumed to result from multiple small pulmonary emboli, primary thrombosis of the microvasculature, perhaps secondary to endothelial damage, has also been suggested to play a role. For the small vessel or microvascular form of chronic pulmonary thromboembolism, therapy involves anticoagulation and agents similar to those used for IPAH.
Pulmonary Hypertension with Unclear Multifactorial Mechanisms (Group 5 PH)
A miscellaneous group of diseases listed in Table 14-1 under Group 5 may be associated with PH. The most common disorder in this category is sarcoidosis. The underlying mechanisms responsible for PH in these disorders are not entirely clear and are often believed to be multifactorial.
Archer, SL, Weir, EK, Wilkins, MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066.
Barst, RJ, Ertel, SI, Beghetti, M, Ivy, DD. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J. 2011;37:665–677.
El Chami, H, Hassoun, PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2012;55:218–228.
Mandel, J, Taichman, D. Pulmonary vascular disease. Philadelphia: Saunders Elsevier; 2007.
McLaughlin, VV, Archer, SL, Badesch, DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. ACCF/AHA. Circulation. 2009;119:2250–2294.
Saggar, R, Sitbon, O. Hemodynamics in pulmonary arterial hypertension: current and future perspectives. Am J Cardiol. 2012;110(6 Suppl):9S–15S.
Sakao, S, Tatsumi, K, Voelkel, NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2010;43:629–634.
Shah, SJ. Pulmonary hypertension. JAMA. 2012;308:1366–1374.
Simonneau, G, Robbins, IM, Beghetti, M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(Suppl 1):S43–S54.
Tuder, RM, Marecki, JC, Richter, A, Fijalkowska, I, Flores, S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23–42.
Pulmonary Arterial Hypertension and Related Disorders
Abenhaim, L, Moride, Y, Brenot, F, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335:609–616.
Badesch, DB, Raskob, GE, Elliott, CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387.
Barst, RJ, Rubin, LJ, Long, WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301.
Chan, SY, Loscalzo, J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44:14–30.
Davies, RJ, Morrell, NW. Molecular mechanisms of pulmonary arterial hypertension: role of mutations in the bone morphogenetic protein type II receptor. Chest. 2008;134:1271–1277.
Dupuis, J, Hoeper, MM. Endothelin receptor antagonists in pulmonary arterial hypertension. Eur Respir J. 2008;31:407–415.
Fadini, GP, Avogaro, A, Ferraccioli, G, Agostini, C. Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J. 2010;35:418–425.
Farber, HW, Loscalzo, J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665.
Frost, AE, Badesch, DB, Barst, RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest. 2011;139:128–137.
Geraci, MW, Bull, TM, Tuder, RM. Genomics of pulmonary arterial hypertension: implications for therapy. Heart Fail Clin. 2010;6:101–114.
Gomberg-Maitland, M, Olschewski, H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31:891–901.
Krowka, MJ. Portopulmonary hypertension. Semin Respir Crit Care Med. 2012;33:17–25.
Landzberg, MJ. Congenital heart disease associated pulmonary arterial hypertension. Clin Chest Med. 2007;28:243–253.
Le Pavec, J, Humbert, M, Mouthon, L, Hassoun, PM. Systemic sclerosis-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181:1285–1293.
Morrell, NW, Adnot, S, Archer, SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S20–S31.
Rai, PR, Cool, CD, King, JAC, et al. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:558–564.
Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118:2372–2379.
Stamm, JA, Mathier, MA. Overview of current therapeutic approaches for pulmonary hypertension. Pulm Circ. 2011;1:286–298.
Wilkins, MR. Pulmonary hypertension: the science behind the disease spectrum. Eur Respir Rev. 2012;21:19–26.
Pulmonary Hypertension Related to Lung Disease
Behr, J, Ryu, JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J. 2008;31:1357–1367.
Chaouat, A, Naeije, R, Weitzenblum, E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–1385.
Nathan, SD, Noble, PW, Tuder, RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med. 2007;175:875–880.
Poor, HD, Girgis, R, Studer, SM. World Health Organization Group III pulmonary hypertension. Prog Cardiovasc Dis. 2012;55:119–127.
Ruggiero, RM, Bartolome, S, Torres, F. Pulmonary hypertension in parenchymal lung disease. Heart Fail Clin. 2012;8:461–474.
Ryu, JH, Krowka, MJ, Pellikka, PA, Swanson, KL, McGoon, MD. Pulmonary hypertension in patients with interstitial lung diseases. Mayo Clin Proc. 2007;82:342–350.
Wrobel, JP, Thompson, BR, Williams, TJ. Mechanisms of pulmonary hypertension in chronic obstructive pulmonary disease: a pathophysiologic review. J Heart Lung Transplant. 2012;31:557–564.
Chronic Pulmonary Thromboembolism
Auger, WR, Kim, NH, Trow, TK. Chronic thromboembolic pulmonary hypertension. Clin Chest Med. 2010;31:741–758.
Fedullo, P, Kerr, KM, Kim, NH, Auger, WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:1605–1613.
Klok, FA, Mos, IC, van Kralingen, KW, Vahl, JE, Huisman, MV. Chronic pulmonary embolism and pulmonary hypertension. Semin Respir Crit Care Med. 2012;33:199–204.
Piazza, G, Goldhaber, SZ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2011;364:351–360.
Pulmonary Hypertension Associated with Left Heart Disease
Dalen, JE, Matloff, JM, Evans, GL, et al. Early reduction of pulmonary vascular resistance after mitral-valve replacement. N Engl J Med. 1967;277:387–394.
Guazzi, M, Borlaug, BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990.
Shah, RV, Semigran, MJ. Pulmonary hypertension secondary to left ventricular systolic dysfunction: contemporary diagnosis and management. Curr Heart Fail Rep. 2008;5:226–232.
Pulmonary Hypertension Associated with Multifactorial Mechanisms
Baughman, RP. Pulmonary hypertension associated with sarcoidosis. Arthritis Res Ther. 2007;9(Suppl 2):S8.
Bunn, HF, Nathan, DG, Dover, GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116:687–692.
Golbin, JM, Somers, VK, Caples, SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc. 2008;5:200–206.
Le Pavec, J, Humbert, M, Mouthon, L, Hassoun, PM. Systemic sclerosis-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;181:1285–1293.