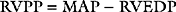Chapter 24 Pulmonary Hypertension
Pulmonary hypertension and right ventricular dysfunction are common problems in the cardiothoracic intensive care unit (ICU). In this chapter the causes, implications, and treatment of pulmonary hypertension are discussed. The focus is on pulmonary hypertension that occurs in the context of underlying cardiac or pulmonary disease, rather than pulmonary hypertension that is idiopathic, familial, or associated with collagen vascular disease.
CLASSIFICATION AND PATHOPHYSIOLOGY
Definition and classification
The normal ranges for the systolic, diastolic, and mean pulmonary artery pressures are 20 to 30, 5 to 15, and 10 to 20 mmHg, respectively. Pulmonary vascular resistance index is normally 150 to 250 dyne.s.cm−5 (1.8 to 3.2 Wood units). The transpulmonary gradient is the difference between the mean pulmonary artery pressure and the left atrial pressure, and is normally 5 to 10 mmHg. The transpulmonary gradient is commonly used as a surrogate for pulmonary vascular resistance. If pulmonary vascular resistance is normal and left atrial pressure is greater than 5 mmHg, pulmonary artery diastolic pressure is about equal to left atrial pressure. The normal regulation of pulmonary vascular resistance is outlined in Chapter 1. Pulmonary arterial hypertension is defined as a sustained increase in mean pulmonary artery pressure above 25 mmHg at rest and 30 mmHg with exercise, with a pulmonary artery wedge pressure (PAWP) and left-ventricular end-diastolic pressure of less than 15 mmHg.1 However, in many patients with pulmonary hypertension secondary to cardiac disease, PAWP is (or has been) greater than 15 mmHg. The classification of pulmonary hypertension is shown in shown in Table 24-1.
Table 24-1 Revised World Health Organization Classification of Pulmonary Hypertension
Modified from Simonneau G, Galie N, Rubin LJ, et al: Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43:5S-12S, 2004. ASD, atrial septal defect; HIV, human immunodeficiency virus; PDA, patent ductus arteriosus; VSD, ventricular septal defect; COPD, chronic obstructive pulmonary disease.
Pathophysiology
In clinical practice, pulmonary arterial pressure is commonly assumed to be reflective of pulmonary vascular resistance, and in most circumstances this is the case. However, based on Equation 1-6, it is clear that pulmonary arterial pressure is also dependent on cardiac output and left atrial pressure. An increase in cardiac output can lead to an increase in pulmonary arterial pressure despite a fall in pulmonary vascular resistance. Conversely, an acute rise in pulmonary vascular resistance resulting in acute right ventricular failure and thus a fall in cardiac output may cause a fall in pulmonary arterial pressure.
The extent to which the right ventricle can cope with increased pulmonary vascular resistance depends on the time period over which the increased resistance has occurred. An untrained, thin-walled right ventricle is sensitive to acute rises in afterload. Thus, an abrupt rise in pulmonary vascular resistance (e.g., due to a massive pulmonary embolism) that requires a mean pulmonary arterial pressure of more than 40 mmHg to perfuse the pulmonary bed will rapidly lead to right ventricular failure. In contrast, if pulmonary vascular resistance increases gradually, right ventricular hypertrophy develops, which allows resting cardiac output to be maintained in the face of very high pulmonary vascular resistance (e.g., >20 Wood units). In this situation, pulmonary arterial pressure may approach or even exceed systemic arterial pressure.
With raised left atrial pressure, increased pulmonary arterial pressure can occur without raised pulmonary vascular resistance (see Equation 1-6). This is termed passive pulmonary hypertension. In passive pulmonary hypertension, the increase in pulmonary arterial pressure is usually mild and the transpulmonary gradient (see Chapter 1) is normal. The common causes of passive pulmonary hypertension are mitral valve disease and left ventricular dysfunction. The term active pulmonary hypertension denotes pulmonary hypertension due to vasoconstriction or anatomic restriction within the pulmonary bed in association with normal left atrial pressure. Pulmonary vascular resistance and transpulmonary gradient are elevated. Causes of active pulmonary hypertension include chronic lung disease and chronically elevated flow through the pulmonary circulation, such as that which occurs with uncorrected systemic-to-pulmonary shunts. The term reactive pulmonary hypertension refers to active pulmonary hypertension due to long-standing passive pulmonary hypertension. Chronically elevated left atrial pressure eventually causes pathologic changes within the pulmonary arterioles. The most common cause of reactive pulmonary hypertension in cardiac surgery patients is chronic mitral valve disease, particularly mitral stenosis. Rarely, left ventricular failure leads to reactive pulmonary hypertension. Pulmonary hypertension may be severe.
Pulmonary hypertension may be fixed or responsive. Changes in the pulmonary vascular bed that accompany active or reactive pulmonary hypertension include vasoconstriction, endothelial and smooth muscle proliferation, thrombosis, and fibrosis. Pulmonary hypertension that is associated with extensive thrombosis, fibrosis, or tissue destruction tends to be fixed, whereas pulmonary hypertension that is associated with abnormal vasoconstriction may be responsive to pulmonary vasodilating agents. Impaired production of vasodilator substances, such as nitric oxide, prostacyclin, and vasoactive intestinal peptide, and increased production of vasoconstrictor substances, such as endothelin-1 and thromboxane-A2, have been identified for a range of conditions associated with pulmonary hypertension.1 Alveolar hypoxemia is a potent stimulus for pulmonary vasoconstriction, and it contributes to pulmonary hypertension in patients with chronic lung disease.
DIAGNOSIS
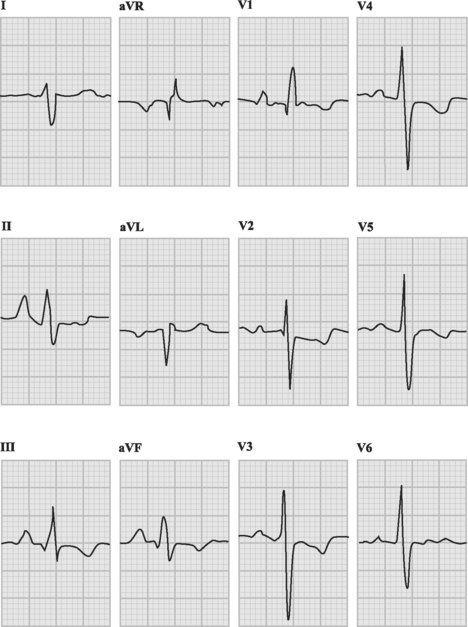
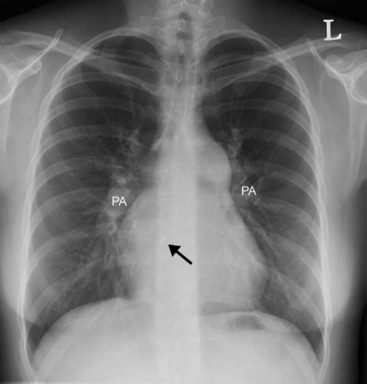
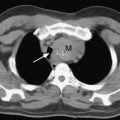
With severe pulmonary hypertension, the electrocardiogram (ECG) typically demonstrates right ventricular hypertrophy and right axis deviation (Fig. 24-1). On the chest radiograph, characteristic features of pulmonary hypertension include enlargement of the main and central hilar pulmonary arteries (Fig. 24-2) and reduced vascular markings in the peripheries (pruning of the vessels). Signs of left ventricular dysfunction or lung disease may be evident.
Pulmonary Artery Catheterization
Pulmonary artery catheterization may be used as part of a diagnostic workup (see Chapter 5) or in the ICU as a guide to management (see Chapter 8). Diagnostic right heart catheterization involves measurement of pulmonary arterial pressure, measurement of cardiac output, calculation of pulmonary vascular resistance, assessment of reversibility with pulmonary vasodilators (100% oxygen, nitroprusside, nitric oxide) and, in patients with intracardiac shunting, measurements of various oxygen saturations so as to allow estimation of the ratio of pulmonary to systemic flow (described in Chapter 5).
Echocardiography
With chronic pulmonary hypertension, the right ventricle is typically hypertrophied (wall thickness >0.5 cm and occasionally >1 cm) and dilated but, in the absence of ventricular failure, systolic function is preserved. Chronic right ventricular pressure overload leads to characteristic abnormalities in the shape and motion of the interventricular septum, which are described in Chapter 7. In the absence of right heart failure, tricuspid regurgitation is typically mild. If pulmonary valve function is normal, the velocity of the tricuspid regurgitant jet can be used to estimate systolic pulmonary arterial pressure (from Equation 7-5). A velocity greater than 2 m/sec is consistent with pulmonary hypertension; greater than 4 m/sec, it is consistent with severe pulmonary hypertension. If right atrial pressure exceeds left atrial pressure, the interatrial septum bulges abnormally toward the left. If a patent foramen ovale (PFO) is present there may be right-to-left shunting across the atrial septum.
The causes of pulmonary hypertension encountered in the cardiothoracic ICU are listed in Table 24-2. Clinically important pulmonary hypertension that occurs during the perioperative period is usually caused by the combination of chronically elevated pulmonary vascular resistance and an acute increase due to the effects of cardiopulmonary bypass (CPB), cardiac or thoracic surgery, mechanical ventilation, or impaired gas exchange.
Table 24-2 Causes of Pulmonary Hypertension in the Cardiothoracic Intensive Care Unit
| Cardiac Surgery |
| Mitral surgery |
| Heart transplantation |
| Thoracic Surgery |
| Lung transplantation |
| Pneumonectomy |
| Chronic left ventricular dysfunction |
| Chronic Lung Disease |
| COPD |
| Interstitial lung disease |
| Obesity hypoventilation syndrome |
| Uncorrected chronic pulmonary-to-systemic shunts (including Eisenmenger syndrome) |
| Acute lung injury |
| Acute respiratory distress syndrome |
| Pulmonary edema |
| Pulmonary Embolus |
| Massive pulmonary embolus |
| Chronic pulmonary thromboembolism |
COPD, chronic obstructive pulmonary disease.
ETIOLOGY
Increased Pulmonary Vascular Resistance During the Perioperative Period
CPB is associated with the systemic inflammatory response syndrome which, among other effects, causes an acute increase in pulmonary vascular resistance (see Chapter 2). Pulmonary vascular resistance may also be increased by raised intrathoracic pressure due to mechanical ventilation (see Chapter 29) and impaired gas exchange (acidemia and low alveolar oxygen tension; see Chapter 1). Elevated pulmonary vascular resistance is also a feature of acute lung injury and acute respiratory distress syndrome (ARDS). Acute, severe increases in pulmonary vascular resistance may occur due to ventilator dysynchrony, in which intrathoracic pressure increases dramatically concurrent with a sudden deterioration in gas exchange. Pulmonary hypertension is exacerbated by elevated left atrial pressure due to heart dysfunction. These perioperative effects add to any underlying chronic pulmonary hypertension and can precipitate acute right ventricular decompensation.
Congenital Heart Disease
A range of congenital cardiac abnormalities (see Table 24-1) that are associated with systemic-to-pulmonary (i.e., left-to-right) shunting are associated with pulmonary hypertension due to chronically high pulmonary blood flow. As with mitral valve disease, increased pulmonary vascular resistance is initially responsive to pulmonary vasodilators, but over time it becomes fixed. If pulmonary vascular resistance exceeds systemic vascular resistance, shunt reversal occurs, leading to systemic hypoxemia and the risk of paradoxic embolism. This condition is termed Eisenmenger syndrome (see Chapter 15). Once Eisenmenger syndrome has developed, shunt closure is contraindicated because closure will precipitate right ventricular failure. Even without Eisenmenger syndrome, shunt closure in the presence of raised pulmonary vascular resistance may still precipitate right ventricular failure because of the higher pulmonary resistance and impaired right ventricular function that accompany surgery.
RIGHT VENTRICULAR FAILURE
Right ventricular failure can occur because of (1) systolic dysfunction; (2) volume overload; or (3) pressure overload (see Table 20-7). In this chapter, only right ventricular decompensation due to acute and chronic pressure overload is considered.
Echocardiography shows the right ventricle to be grossly dilated and hypokinetic. There is often severe tricuspid regurgitation. Ventricular septal motion is typically abnormal and has features of acute right ventricular volume overload (see Fig. 7-15). The interventricular septum is displaced to the left in late diastole, making the left ventricle appear hypovolemic. The interatrial septum bulges abnormally to the left.
Hypoxemia and a Patent Foramen Ovale
A particular feature of acute right ventricular failure is the potential for the development of hypoxemia due to right-to-left shunting across a PFO. A PFO is present in about 25% of adults; it is a flap-like atrial septal defect that allows flow only from right to left. Because right atrial pressure is normally less than left atrial pressure, under normal circumstances no flow occurs across a PFO. With right ventricular dysfunction, right atrial pressure typically exceeds left atrial pressure and right-to-left shunting occurs, leading to hypoxemia. This is important because in ventilated patients, a clinician’s first response to hypoxemia is likely to be to increase the level of positive end-expiratory pressure (PEEP), which may worsen right ventricular function and increase shunting. Thus, any patient with right ventricular dysfunction who develops unexplained hypoxemia despite normal lung compliance and an unremarkable chest radiograph should undergo an urgent, ideally transesophageal, echocardiogram to search for right-to-left shunting across a PFO.
TREATMENT OF PULMONARY HYPERTENSION DURING THE PERIOPERATIVE PERIOD
This section outlines treatment strategies to be used in the ICU environment for right ventricular dysfunction due to acute (or acute-on-chronic) pressure overload. The management of right ventricular dysfunction caused by volume overload and that caused by systolic dysfunction is similar and is described in Chapter 20.
General Supportive Measures
Although mechanical ventilation may impair right ventricular function by increasing right ventricular afterload and reducing right ventricular preload (see Chapter 29), when gas exchange is impaired, the benefits of mechanical ventilation greatly outweigh the risks, and this intervention should not be withheld if indicated. However, high airway pressures and high levels of PEEP should be avoided.
Fluid Therapy
Judicious volume loading may have a role in treating patients with right ventricular failure due to systolic dysfunction (see Chapter 20). However, with right ventricular pressure overload it is likely to be harmful because it increases RVEDP and reduces perfusion pressure to the right ventricle. Fluid administration also increases the severity of tricuspid regurgitation and leads to further leftward displacement of the interventricular septum, worsening left ventricular filling.
Vasoactive Support
A critical component of treating right ventricular failure due to pressure overload is maintaining right ventricular perfusion pressure by supporting blood pressure.2 Norepinephrine is the vasopressor of choice.2
Systemically Administered Vasodilators
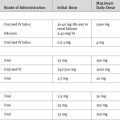
Commonly used vasodilators (e.g., nitroglycerin, sodium nitroprusside, prostacyclin, and diltiazem) and inodilators (e.g., milrinone, dobutamine, and isoproterenol) all cause pulmonary arteriolar vasodilatation. If introduced at low doses and titrated to effect, these drugs may significantly improve right ventricular function—though it is important to remember that the goal is improvement in right ventricular function, not reduction in pulmonary arterial pressure. Sildenafil (see subsequent material) is used predominantly to treat chronic pulmonary hypertension, but in one report, 50 mg administered enterally was used successfully to treat pulmonary hypertension following cardiac surgery.3
Inhaled Therapy
Prostacyclin
Prostacyclin (prostaglandin I2, epoprostenol) is a short-acting prostaglandin with vasodilatory and antiplatelet effects. It may be given by intravenous infusion (see subsequent material) or by inhalation. Inhaled prostacyclin is well described for treating pulmonary hypertension following cardiac surgery and is similar to inhaled nitric oxide in its ability to reduce pulmonary vascular resistance, improve oxygenation, and augment cardiac output. Impaired platelet function has been observed but it has not been associated with increased postoperative bleeding.8,9 Doses in the range of 5 to 50 ng/kg/min have been used.8,9 Because of its short half-time, prostacyclin must be given by continuous nebulization via a low-flow jet nebulizer placed in the inspiratory limb of the ventilator circuit. The syringe and nebulizer should be foil-wrapped to protect the drug from light. One method of drug delivery involves formulating a solution such that a dose of 50 ng/kg/min is delivered at a volume of 8 ml/hour via a syringe driver into the jet nebulizer.9,10 (Thus, for a 70 kg patient, 1.58 mg of prostacyclin is diluted into a 60 ml syringe.) The infusion is started at 8 ml/hr, and the rate is adjusted downward as tolerated.
Iloprost
Iloprost is an analog of prostacyclin that has been used successfully to treat pulmonary hypertension following cardiac surgery.11 Like prostacyclin, it has vasodilatory and antiplatelet effects, but it has a longer half-life (20 to 30 minutes) and improved solubility in saline than prostacyclin, making it suitable for administration by intermittent nebulization. When given by the inhaled route, iloprost is well tolerated and has minimal systemic hemodynamic effects. To treat acute pulmonary hypertension, iloprost may be given at a dose of 12 to 20 μg every 4 to 6 hours by intermittent nebulization. The contents of one iloprost ampoule (50 μg/0.5 ml) can be diluted with saline in a 10-ml syringe, and 3 ml (15 μg) can be given per dose. If refrigerated, the solution is stable for 24 hours.
TREATMENT OF CHRONIC PULMONARY HYPERTENSION IS UNRELATED TO CARDIAC OR PULMONARY DISEASE
Patients with severe chronic pulmonary arterial hypertension that is unrelated to cardiac or pulmonary disease12 (predominantly groups 1 and 5 in Table 24-1) are admitted to the ICU only rarely, usually following a noncardiac surgical procedure. Occasionally, such patients are offered lung transplantation or, with chronic pulmonary embolic disease, pulmonary thromboendarterectomy (see Chapter 23). Occasionally, patients with end-stage pulmonary hypertension present for the first time in an acutely unwell state, in which case mechanical ventilation and vasoactive support may be instituted until the diagnosis is established. However, aggressive treatment of patients with end-stage disease is not appropriate in the ICU unless there is a clearly identified reversible problem, which is rare.
Pharmacotherapy that should be considered in all symptomatic patients consists of anticoagulation with warfarin, diuretics for right ventricular failure, and oxygen for hypoxemia. In a minority of patients with severe pulmonary hypertension, marked reversibility is documented during right heart catheterization. These patients may be treated by administering a calcium channel blocker. However, most patients with severe pulmonary hypertension have limited reversibility or significant right ventricular dysfunction. These patients should be considered for treatment by one or more of the following: bosentan,13,14 sildenafil,15 or prostacyclin16; all of these drugs have been shown to improve functional capacity in patients with severe symptomatic disease.
Intravenous prostacyclin (prostaglandin I2, epoprostenol) is also effective in improving functional capacity, but its use is limited by its very short duration of effect and side effects. It must be administered by continuous intravenous infusion, usually via a tunneled central venous catheter. Doses in the range of 15 to 40 ng/kg/min have been used. Side effects include jaw pain, headache, diarrhea, flushing, leg pain, and nausea. Serious complications are caused primarily by catheter-related sepsis. Alternatives to epoprostenol include the prostacyclin analogues treprostinil, beraprost, and iloprost. Treprostinil has a longer duration of action than epoprostenol and may be administered by continuous subcutaneous infusion. Use of the drug is limited by local pain at the infusion site. Beraprost is an orally active form of prostacyclin. It has been shown to provide improved functional capacity at 6 months, but this benefit was not sustained at 12 months.17 Inhaled iloprost avoids the needs for intravenous or subcutaneous injection and improves functional capacity in patients with severe pulmonary hypertension.18 However, it must be given 6 to 12 times per day, which limits its tolerability.
1 Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655-1665.
2 Mebazaa A, Karpati P, Renaud E, et al. Acute right ventricular failure—from pathophysiology to new treatments. Intens Care Med. 2004;30:185-196.
3 Madden BP, Sheth A, Ho TB, et al. Potential role for sildenafil in the management of perioperative pulmonary hypertension and right ventricular dysfunction after cardiac surgery. Br J Anaesth. 2004;93:155-156.
4 Maxey TS, Smith CD, Kern JA, et al. Beneficial effects of inhaled nitric oxide in adult cardiac surgical patients. Ann Thorac Surg. 2002;73:529-532.
5 Kieler-Jensen N, Lundin S, Ricksten SE. Vasodilator therapy after heart transplantation: effects of inhaled nitric oxide and hintravenous prostacyclin, prostaglandin E1, and sodium hnitroprusside. J Heart Lung Transplant. 1995;14:436-443.
6 Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603-1609.
7 Manktelow C, Bigatello LM, Hess D, et al. Physiologic determinants of the response to inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology. 1997;87:297-307.
8 Lowson SM. Inhaled alternatives to nitric oxide. Anesthesiology. 2002;96:1504-1513.
9 Lowson SM. Inhaled alternatives to nitric oxide. Crit Care Med. 2005;33:S188-S195.
10 Lowson SM, Doctor A, Walsh BK, et al. Inhaled prostacyclin for the treatment of pulmonary hypertension after cardiac surgery. Crit Care Med. 2002;30:2762-2764.
11 Theodoraki K, Rellia P, Thanopoulos A, et al. Inhaled iloprost controls pulmonary hypertension after cardiopulmonary bypass. Can J Anaesth. 2002;49:963-967.
12 Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351:1425-1436.
13 Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896-903.
14 Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119-1123.
15 Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148-2157.
16 Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296-302.
17 Barst RJ, McGoon M, McLaughlin V, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:2119-2125.
18 Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322-329.
19 Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S-12S.