70 Pulmonary Embolism
• Appropriate testing for pulmonary embolism (PE) requires both an understanding of the pretest probability for each individual patient and knowledge of the characteristics of the diagnostic test used at your specific institution.
• A pretest probability of less than 10% with a negative D-dimer result that has a sensitivity of at least 95% is sufficient to rule out PE in most clinical circumstances.
• Right heart strain on an echocardiogram or electrocardiogram, decreased oxygen saturation, elevated brain natriuretic peptide, and elevated troponin may predict a worse short- and long-term outcome in patients in whom PE is diagnosed.
• Treatment of PE consists of heparin and initiation of warfarin to prevent additional clot formation.
• Treatment of shock associated with PE includes intravenous fluid, vasopressors, respiratory support, and thrombolytic therapy.
• PE represents a spectrum of severity determined by the size of the clot and baseline cardiopulmonary disease.
• The probability of mortality increases steeply once right ventricular dysfunction and hypotension occur.
• Diagnosis of PE as a cause of shock requires consideration of PE as a potential cause and a stepwise approach to simultaneous testing and resuscitation.
Epidemiology
The annual incidence of diagnosed pulmonary embolism (PE) is approximately 1.5 new cases per 1000 persons and is relatively similar among western populations.1 Dyspnea and chest discomfort are the most typical symptoms of PE, and these chief complaints are responsible for between 9 and 10 million annual patient visits to U.S. emergency departments (EDs).2 Physicians evaluate large numbers of patients for PE because the symptoms can be vague and severity may range from asymptomatic to shock and subsequent cardiac arrest.3
Pathophysiology
PE is part of the continuum of venous thromboembolism (VTE), which most often starts with deep vein thrombosis (DVT) in the leg. Patients with DVT often have concurrent PE when evaluated with imaging tests, and many patients with PE have concurrent DVT. Treatment is similar for both. Risk factors for VTE include the triad described by Virchow: injury, venous stasis, and hypercoagulability. These conditions are most commonly thought of as occurring at the level of the venous endothelium, but it is helpful to also think of them as occurring at the level of the patient. For instance, overall injury to the patient (trauma), stasis of the patient (immobility), and hypercoagulability of the patient (malignancy and known thrombophilic conditions) all result in elevated risk. Table 70.1 lists several risk factors for VTE. Understanding risk factors is critical to recognizing the potential for PE to exist.
| RISK FACTORS | SPECIFIC NOTES |
|---|---|
| Previous history of PE or DVT | Inquire about the setting and circumstance of the previous VTE |
| Recent trauma or surgery | In general, trauma requiring admission or surgery requiring general anesthesia within the previous month. Recent long-bone trauma or surgery may especially increase the risk |
| Cancer | In general, patients with currently treated cancer or palliative care. Remotely treated and inactive cancer probably does not increase the probability of PE |
| Age | Risk significantly increases above the age of 50 to 60 years |
| Oral contraceptives | Especially third-generation formulations |
| Hormone replacement therapy | Contemporary patients are less commonly receiving hormone replacement |
| Pregnancy | Risk increases along with the duration of pregnancy; it peaks at term and then decreases over a period of 4 to 6 weeks postpartum |
| Immobility | Includes casts and splints, as well as permanent limb or generalized body immobility |
| Factor V Leiden mutation | Most common in northern European populations. A heterozygous carrier state exists in 3% to 7% of many samples. Homozygous mutation is less common and confers three times greater risk for VTE relative to the normal genotype |
| Antiphospholipid antibody syndrome | Very potent risk factor that is associated with large and recurrent PE. It may be associated with anticardiolipin antibodies, CVA, MI, and first-trimester miscarriages |
| Prothrombin mutation | |
| Hyperhomocysteinemia | Can occur as a result of inadequate folate and vitamin B intake, as well as with a genetic mutation in methyltetrahydrofolate reductase |
| Deficient levels of clotting factors | Protein C, protein S, antithrombin III |
| Congestive heart failure | |
| Chronic obstructive pulmonary disease | |
| Air travel | Primary risk with travel of more than 5000 km (3100 miles) and concurrent other risk factors |
| Obesity | Elevated at a body mass index higher than 25 and even greater risk if higher than 29 |
CVA, Cerebrovascular accident; DVT, deep vein thrombosis; MI, myocardial infarction; PE, pulmonary embolism; VTE, venous thromboembolism.
Presenting Signs and Symptoms
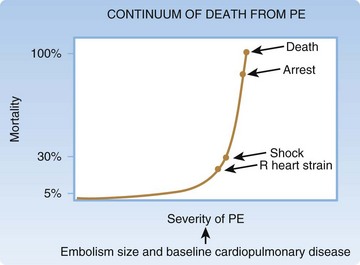
Dyspnea and chest pain are the most common findings in patients with PE. Brief, resolved chest pain in the absence of any shortness of breath or any respiratory signs or symptoms is not a typical manifestation. Other symptoms that can be associated with PE include syncope, cough, flank pain, abdominal pain, and even fever (Box 70.1). The severity of symptoms in a given patient is a function of two factors: the baseline cardiopulmonary status of the patient and the size of the clot4 (Fig. 70.1). This is why large clots are occasionally tolerated fairly well in young patients with no cardiopulmonary disease whereas a much smaller clot burden may result in hypotension, hypoxemia, and deterioration in patients with preexisting cardiopulmonary disease. Older patients often have a worse clinical course and outcome with PE, largely as a consequence of having worse cardiopulmonary status at baseline rather than simply being elderly.
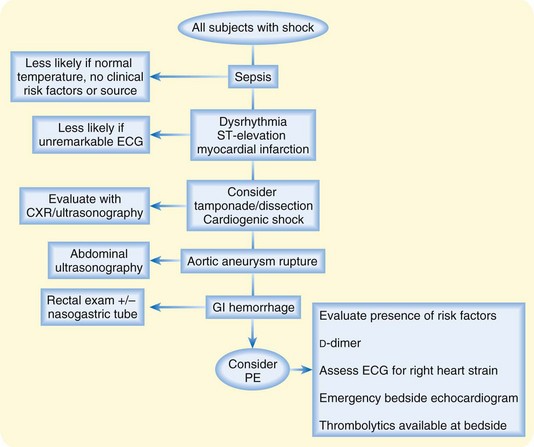
Shock may be a primary sign of PE. Patients may not be able to provide a history of symptoms or risk factors for PE and may not be sufficiently stable to allow imaging outside the ED, yet consideration of empiric treatment of PE with anticoagulation may be warranted. A rapid bedside evaluation to search for clues to non-PE diagnoses can be done promptly and is described in Figure 70.2. A potential pitfall is to attribute shock to a primary cardiac etiology despite an electrocardiogram (ECG) with no significant ischemic changes and no significant dysrhythmia and a chest radiograph with no evidence of pulmonary vascular congestion or cardiomegaly. If patients can be stabilized, imaging with CT should be done. If not, emergency bedside echocardiography to look for signs of massive PE (right ventricular dilation and hypokinesis, septal shift to the left, tricuspid regurgitation) should be done as an alternative means of heightening certainty of the diagnosis of PE.
Differential Diagnosis and Medical Decision Making
Because of the vague yet common nature of dyspnea and chest pain, the differential diagnosis is broad. However, a targeted diagnostic approach should be used rather than a “chest pain work-up” in an attempt to test for every diagnosis possible without regard to the pretest probability or negative consequences of overtesting. This is particularly important for PE because tests are not 100% sensitive or specific and the consequences of a false-positive diagnosis may include 6 months of oral anticoagulation with high direct and indirect costs to the patient and society. This is especially true in young patients with limitations in activity, as well as in older patients at risk for falls, medication interaction, and bleeding. Table 70.2 lists potential alternative diagnoses in ED patients evaluated for PE and clues to assist in rapid decision making. It is not surprising that many of these conditions are common entities such as pneumonia, bronchitis, asthma, musculoskeletal pain, gastroesophageal reflux or spasm, and anxiety or panic attack. Many of the most threatening alternative diagnoses can initially be evaluated with a chest radiograph, an ECG, bedside cardiac ultrasound, and cardiac enzyme testing during the first hour in the ED.
Table 70.2 Other Diagnoses That Should Be Considered Along with Pulmonary Embolism
| DIAGNOSIS | MEANS OF RAPIDLY OBTAINING CLUES |
|---|---|
| Potential Threats to Life | |
| Myocardial ischemia, cardiogenic shock, dysrhythmia, congestive heart failure | ECG/CXR |
| Pneumothorax | CXR |
| Cardiac tamponade | Bedside cardiac ultrasound |
| Pneumonia | CXR |
| Esophageal rupture | CXR |
| Pulmonary malignancy (metastatic or primary) | CXR, history |
| Asthma | Examination, history |
| Aortic dissection | History |
| Pericarditis | ECG |
| Non–Life-Threatening | |
| Bronchitis | History |
| Chest wall pain | History |
| Pleuritis, pleurisy | History |
| GERD, esophageal spasm, peptic ulcer disease | History |
| Panic attack | History |
CXR, Chest x-ray; ECG, electrocardiogram; GERD, gastroesophageal reflux disease.
It is most helpful to think of PE as a continuum of cardiopulmonary stress as shown in Figure 70.1. Even in normotensive patients, in-hospital or 30-day mortality in those with diagnosed PE is approximately 8% to 13%.5–9 This mortality in PE patients without shock is greater than that for acute myocardial infarction.10 When early signs of right heart dysfunction occur, mortality begins to curve upward. This is followed by compensated shock, which may initially respond to intravenous fluid. Later, as left-sided filling is decreased because of septal shift into the left ventricle, as well as decreased filling of the left atrium, overt shock is present and mortality is in excess of 30%. If untreated, cardiac arrest ensues, with pulseless electrical activity being the most likely first rhythm.11,12
Diagnostic Testing
Pretest probability is the probability that the physician believes to be present before any test results are obtained. It is typically calculated by either a scoring system or the physician’s own “gestalt” estimation. Despite the fact that debate exists over the relative accuracy of using gestalt versus a structured means of assessing pretest probability, the American College of Emergency Physicians practice guideline of 2003 recommended pretest probability assessment in patients being evaluated for PE.13
The main structured pretest probability system with the longest track record of use and investigation is the Canadian score derived by Wells et al., which results in a score for an individual patient that can be used to estimate ranges of pretest probability14 (Table 70.3 and Fig. 70.3). Widespread clinical application of this scoring system can be challenging because of difficulty in physician recall15 and the fact that it provides a range of probability rather than an exact estimate. An alternative to these structured pretest probability systems is unstructured or implicit estimation systems whereby physicians arrive at their own pretest probability based on their experience and overall integration of clinical information for a unique patient. This is commonplace but imprecise and subject to wide variability.16 Recent data from a large multicenter study of ED patients in the United States found that several variables not in the Wells score may be significantly associated with the outcome of PE in ED patients who are tested. These variables are noted in Figure 70.3 and may be important in addition to the Wells score in formulating overall pretest probability.17
| WELLS SCORE | POINTS |
|---|---|
| Alternative diagnosis less likely | 3 |
| Signs and symptoms of DVT | 3 |
| History of PE or DVT | 1.5 |
| Surgery or immobilization within 1 mo | 1.5 |
| Pulse > 100 beats/min | 1.5 |
| Hemoptysis | 1 |
| Cancer | 1 |
| With a score of 4 or less, the pretest probability is 5% or less; with a score higher than 4, the pretest probability is greater than 5%. | |
Other factors to consider include estrogen use (oral contraceptive pill or hormone replacement therapy), thrombophilic condition, pleuritic nature of the chest pain if present, family history of PE or DVT, and oxygen saturation lower than 95%.
DVT, Deep vein thrombosis; PE, pulmonary embolism.
The most typical approach to testing for PE is to determine whether the pretest probability is sufficiently low (Wells score ≤ 4 or physician’s gestalt of “low risk”) and then to use a sensitive D-dimer blood test that detects the presence of fibrin breakdown products (see Fig. 70.3). If D-dimer is normal in these low-risk patients, the posttest probability of PE is below a sufficient test threshold (<1% to 2%), and PE can be considered to be sufficiently excluded in these patients.18,19 Typically, two types of D-dimer tests are available for ED testing: (1) quantitative tests, including enzyme-linked immunosorbent assay or immunoturbidimetric tests, which are considered to be abnormal at a particular cutoff, typically ≥500 ng/mL, and (2) qualitative whole blood agglutination or immunofiltration tests, which yield a binary result of positive or negative. Benefits of the quantitative tests include higher sensitivity. Benefits of the qualitative tests include rapid results because of bedside testing. Table 70.4 describes how the two different types of D-dimer tests may be used to rule out PE. These are estimates of diagnostic test performance, and individual tests will vary in their actual sensitivity, specificity, and likelihood ratio, thus emphasizing the importance of clinician knowledge of the specific test at their institution.
Table 70.4 Comparison of Two Different Types of D-Dimer Tests
| Pretest probability | 10% or less | 5% or less |
|---|---|---|
| Type of D-dimer | Quantitative: ELISA or turbidimetric test | Qualitative: whole blood agglutination or immunofiltration tests |
| Approximate sensitivity | 94% | 85%* |
| Approximate specificity | 55% | 60% |
| Likelihood ratio with a negative result | 0.1 | 0.25 |
| Probability of pe after a negative test (posttest probability) | ≤1.2% | ≤1.3% |
ELISA, Enzyme-linked immunosorbent assay; PE, pulmonary embolism.
* low sensitivity makes this test unfavorable.
For these reasons, as well as the significant negative impact that a false-positive diagnosis may have for the patient because of months of anticoagulation, attempts to reduce testing in very low-risk settings have been proposed. One such method is the PE rule-out criteria shown in Box 70.2.18,20 It was derived from a multicenter U.S. sample and provides simple, easy-to-apply clinical criteria that when satisfied, indicate that the baseline probability of PE is below a test threshold (<1.8%) and testing in this setting may be more likely to lead to patient harm than benefit. This is only an attempt to support clinician judgment when the probability of PE is already very low and it is not intended to replace or dictate individual patient-based decision making. Patients who meet the PE rule-out criteria but for other reasons are thought to need testing for PE should undergo the standard evaluation.
CT has largely supplanted the use of  scans because of the perception that they produce a binary positive/negative result, as well as the fact that other chest pathology may be seen with CT.21 The sensitivity and specificity of chest CT angiography have improved with the advent of multidetector CT with higher spatial resolution and lower image acquisition time. Overall, well-accepted sensitivity and specificity data are lacking because of diverse differences in the equipment used, image acquisition technique, and image interpretation, but spiral CT is generally regarded to have greater than 90% sensitivity and specificity. One systematic review based on 3500 patients in 15 studies reported a pooled negative predictive value of 99.1% and a likelihood ratio for a negative result of 0.07. Newer CT scan machines with 128 detector systems and sophisticated image postprocessing will become more commonplace and improve the diagnostic accuracy of current imaging. Even with these newer machines, motion artifact, inadequate contrast bolus timing, and varied experience of readers may result in a surprising level of indeterminate or nondiagnostic results.22 Pretest probability assessment and D-dimer testing will still be important to avoid unnecessary radiation exposure and possible false-positive CT results in a large number of patients who have a very low likelihood of PE.
scans because of the perception that they produce a binary positive/negative result, as well as the fact that other chest pathology may be seen with CT.21 The sensitivity and specificity of chest CT angiography have improved with the advent of multidetector CT with higher spatial resolution and lower image acquisition time. Overall, well-accepted sensitivity and specificity data are lacking because of diverse differences in the equipment used, image acquisition technique, and image interpretation, but spiral CT is generally regarded to have greater than 90% sensitivity and specificity. One systematic review based on 3500 patients in 15 studies reported a pooled negative predictive value of 99.1% and a likelihood ratio for a negative result of 0.07. Newer CT scan machines with 128 detector systems and sophisticated image postprocessing will become more commonplace and improve the diagnostic accuracy of current imaging. Even with these newer machines, motion artifact, inadequate contrast bolus timing, and varied experience of readers may result in a surprising level of indeterminate or nondiagnostic results.22 Pretest probability assessment and D-dimer testing will still be important to avoid unnecessary radiation exposure and possible false-positive CT results in a large number of patients who have a very low likelihood of PE.
Treatment
The defining characteristic of the emergency medicine physician is simultaneous treatment and evaluation. Oxygen should be given to patients suspected of having PE. Correction of local hypoxemia assists in decreasing reflexive vasoconstriction in the pulmonary vasculature. Patients who are thought to be at high risk for PE and have a high pretest probability should be considered candidates for empiric treatment with anticoagulation while awaiting the results of tests, provided that they have no contraindications. Low-molecular-weight heparin (LMWH) has become standard therapy despite being more costly than unfractionated heparin. It has benefit in achieving appropriate anticoagulation rapidly without the need to monitor coagulation levels, and it is also associated with a less likelihood of heparin-induced thrombocytopenia with thrombosis. Some studies have suggested that LMWH may have somewhat higher efficacy in terms of time to thrombus resolution and recurrence in patients with DVT.23 Unfractionated heparin is still a safe and effective treatment and may be especially useful if a shorter term of anticoagulation with the ability to rapidly cease anticoagulation is desired. Unfractionated heparin and LMHW do not act to break down existing thrombus but rather act to decrease thrombus propagation and extension. Endogenous mechanisms of antithrombin and plasmin activation are responsible for gradually effecting thrombus breakdown. The dose of enoxaparin, the most commonly used LMHW, is 1 mg/kg subcutaneously every 12 hours. The dose of unfractionated heparin is typically a 80-U/kg bolus intravenously and then an 18-U/kg/hr intravenous infusion.
Risk Stratification
It is critical to assess the likelihood for further deterioration. Figure 70.1 demonstrates the increase in mortality that occurs gradually with right heart strain and then more steeply with shock. It is possible to gather data in the ED to estimate the likelihood of right heart strain and early shock. The ECG is the starting point, and the presence of T-wave inversion in leads V1 to V4, incomplete or complete right bundle branch block, an S wave in lead I, a Q wave in lead III, and an inverted T wave in lead III may all be evidence of right heart strain. Pulse oximetry is an important predictor of 30-day mortality in normotensive ED patients with PE when less than 95% on room air.7 Other means of risk stratification include brain natriuretic peptide (BNP) and troponin blood testing. Though not specific for PE and not helpful in establishing the diagnosis, these factors are being seen in emerging studies to predict a more complicated course both in the hospital and several months later. The most accepted means of risk stratification is the echocardiogram. Though often not available on an emergency basis in many EDs, it is a rapid means of identifying right heart strain (Box 70.3).
Treatment of Patients With Confirmed Pulmonary Embolism and Shock
In a practice guideline statement the American College of Emergency Physicians recommended fibrinolytic therapy for hemodynamically unstable patients with confirmed PE as a class B recommendation.13 (Class B represents a moderate level of certainty.) Despite controversy over the use of thrombolytics and mortality in patients with PE and shock, in selected samples it seems clear that fibrinolytic therapy has been associated with improved perfusion and better right heart function. Catheter-based techniques of thrombus fragmentation, as well as surgical embolectomy, are alternatives to pharmacologic fibrinolysis. These techniques are unlikely to be available outside dedicated interventional radiology and cardiovascular surgery centers and are highly operator dependent. No evidence has shown that they are superior to pharmacologic approaches.
When the use of fibrinolytics has been studied in patients without overt shock but with right ventricular dysfunction, they have been associated with decreased need to escalate therapy with vasopressors, as well as reduced rates of intubation or additional thrombolytic treatment.24 The Food and Drug Administration–approved on-label use of fibrinolytic therapy for PE is alteplase, 15 mg as an intravenous bolus and then 85 mg intravenously over a 2-hour period (heparin infusion should be stopped during alteplase administration). Other off-label dosing regimens have been described, including a 15-mg bolus with an 85-mg infusion over a 90-minute period, which has been used in the near-arrest setting.25 In conclusion, it is accepted that fibrinolytic therapy is appropriate in patients with confirmed PE and shock. Identification of PE as a cause of shock can be aided by a stepwise approach to diagnosis with consideration of other causes of shock (see Fig. 70.2). If shock is present and no right heart abnormalities are seen on echocardiography, PE is a very unlikely explanation for the shock.
Follow-Up, Next Steps In Care, and Patient Education
Patients with low oxygen saturation (less than 95%), right heart strain on an echocardiogram or ECG, elevated BNP, or elevated troponin should be observed closely for deterioration. The decision for intensive care unit management should be based on the level of specialized care that patients need (Box 70.4).
Box 70.4 Indications for Intensive Care Unit Management of Patients with Pulmonary Embolism
Patients with a history of previous PE present several challenges. Recurrent PE, defined as an acute clot in a new or different anatomic location, should be treated as acute PE, and if patients are no longer taking anticoagulation agents, they need treatment as stated earlier. In contrast, a persistent or chronic clot is not uncommonly seen in the same areas as previous PE and may look radiographically distinct from acute PE on CT. If patients are appropriately anticoagulated and do not have other indications for admission, they may be discharged from the ED, provided that no other inpatient evaluation for the symptoms is warranted. However, patients with no new clot visualized on CT may be suffering from chronic venous thromboembolic syndrome, a condition in which pulmonary hypertension and chronic vascular changes in the lung can lead to chronic disabling dyspnea and other symptoms that may prompt ED evaluation. Depending on oxygenation and functional status, these patients should be admitted or, if discharged, referred for outpatient echocardiography and pulmonary consultation, but no specific therapy as yet exists for this condition, which may affect 1% to 3% of all patients with PE (Box 70.5).
Box 70.5 Future Trends in the Diagnosis, Prognosis, Treatment, and Disposition of Patients with Pulmonary Embolism
Electronic decision support aids to provide more accurate point-of-care pretest probability
Effective risk stratification for short- and long-term adverse events
Improved understanding of thrombophilic states
Outpatient or emergency department observation-based treatment of selected low-risk new patients with pulmonary embolism
Oral rapid-acting anticoagulation medications as alternatives to warfarin and low-molecular-weight heparin
Fibrinolytic therapy for patients at high risk for immediate and delayed adverse events
![]() Patient Teaching Tips
Patient Teaching Tips
The decision to test for pulmonary embolism should always assume that if screening tests such as the D-dimer blood test are positive, more definitive tests will be undertaken. It is critical to explain this to patients so that they are not surprised and displeased when many of them have a false-positive D-dimer result and need to stay in the emergency department for imaging.
In pregnant patients, a detailed discussion with the patient, radiologist, obstetrician, and the patient’s family should occur at the outset even before tests are ordered and should include the probability of disease, the extremely low probability of radiation-associated negative effects, and the potential morbidity with undiagnosed pulmonary embolism.
Tips and Tricks
The combination of normal oxygen saturation, no known risk factors, and no pulmonary symptoms is very uncommon.
A targeted diagnostic approach should be used rather than a “chest pain work-up” in an attempt to test for every diagnosis possible without regard to the pretest probability or negative consequences of overtesting.
Many of the most threatening alternative diagnoses can initially be assessed with a chest radiograph, electrocardiogram, bedside echocardiogram, and cardiac enzyme testing during the first hour.
Patients who are otherwise at low risk should not be automatically assumed to have a high-risk pretest probability just because they are pregnant.
Patients with a history of pulmonary embolism and no new clot on computed tomography may be suffering from chronic venous thromboembolic syndrome, a condition in which pulmonary hypertension and chronic vascular changes in the lung can lead to chronic disabling dyspnea.
![]() Red Flags
Red Flags
Mortality in patients who have PE without shock is greater than that in those with acute myocardial infarction.
Physician gestalt is useful only when the probability of PE is already very low; therefore, patients who meet the PE rule-out criteria but for other reasons are thought to need testing for PE should undergo the standard evaluation.
Patients at high risk by pretest probability assessment and have low or indeterminate  scans cannot be assumed to have PE ruled out.
scans cannot be assumed to have PE ruled out.
Patients with a high pretest probability are candidates for empiric treatment with anticoagulation agents while awaiting test results, provided that they have no contraindications.
Unfractionated heparin and LMWH do not act to break down existing thrombus but rather act to decrease thrombus propagation and extension.
The ACEP practice guideline recommends fibrinolytics for unstable patients with confirmed PE as a class B recommendation.
Patients without overt shock but with right ventricular dysfunction who are treated with fibrinolytics have a lower need for vasopressors and intubation.
If shock exists and no right heart abnormalities are seen on echocardiography, PE is a very unlikely explanation for the shock.
Patients with low oxygen saturation (less than 95%), right heart strain on echocardiography or electrocardiography, elevated BNP, or elevated troponin should be observed closely.
1 Goldhaber SZ. Pulmonary embolism. Lancet. 2004;363:1295–1305.
2 Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Rep. 2010;26:1–31.
3 Courtney DM, Kline JA. Identification of prearrest clinical factors associated with outpatient fatal pulmonary embolism. Acad Emerg Med. 2001;8:1136–1142.
4 Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905.
5 Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386–1389.
6 Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165–1171.
7 Kline JA, Hernandez-Nino J, Newgard CD, et al. Use of pulse oximetry to predict in-hospital complications in normotensive patients with pulmonary embolism. Am J Med. 2003;115:203–208.
8 Alpert JS, Smith R, Carlson J, et al. Mortality in patients treated for pulmonary embolism. JAMA. 1976;236:1477–1480.
9 The urokinase pulmonary embolism trial. A national cooperative study. Circulation. 1973;47(2 Suppl):II1–II108.
10 Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360.
11 Courtney DM, Sasser HC, Pincus CL, et al. Pulseless electrical activity with witnessed arrest as a predictor of sudden death from massive pulmonary embolism in outpatients. Resuscitation. 2001;49:265–272.
12 Courtney DM, Kline JA. Prospective use of a clinical decision rule to identify pulmonary embolism as likely cause of outpatient cardiac arrest. Resuscitation. 2005;65:57–64.
13 Clinical policy: critical issues in the evaluation and management of adult patients presenting with suspected pulmonary embolism. Ann Emerg Med. 2003;41:257–270.
14 Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–420.
15 Runyon MS, Richman PB, Kline JA. Emergency medicine practitioner knowledge and use of decision rules for the evaluation of patients with suspected pulmonary embolism: variations by practice setting and training level. Acad Emerg Med. 2007;14:53–57.
16 Runyon MS, Webb WB, Jones AE, et al. Comparison of the unstructured clinician estimate of pretest probability for pulmonary embolism to the Canadian score and the Charlotte rule: a prospective observational study. Acad Emerg Med. 2005;12:587–593.
17 Courtney DM, Kline JA, Kabrhel C, et al. Clinical features from the history and physical examination that predict the presence or absence of pulmonary embolism in symptomatic emergency department patients: results of a prospective, multicenter study. Ann Emerg Med. 55, 2010. 307.e1–315.e1
18 Kline JA, Mitchell AM, Kabrhel C, et al. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247–1255.
19 Wells PS. Advances in the diagnosis of venous thromboembolism. J Thromb Thrombolysis. 2006;21:31–40.
20 Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6:772–780.
21 Richman PB, Courtney DM, Friese J, et al. Prevalence and significance of nonthromboembolic findings on chest computed tomography angiography performed to rule out pulmonary embolism: a multicenter study of 1,025 emergency department patients. Acad Emerg Med. 2004;11:642–647.
22 Courtney DM, Miller C, Smithline H, et al. Prospective multicenter assessment of interobserver agreement for radiologist interpretation of multidetector computerized tomographic angiography for pulmonary embolism. J Thromb Haemost. 2010;8:533–539.
23 Breddin HK, Hach-Wunderle V, Nakov R, et al. Clivarine: assessment of regression of thrombosis, efficacy, and safety. Effects of a low-molecular-weight heparin on thrombus regression and recurrent thromboembolism in patients with deep-vein thrombosis. for the CORTES Investigators. N Engl J Med. 2001;344:626–631.
24 Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. for the Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. N Engl J Med. 2002;347:1143–1150.
25 Goldhaber SZ, Nadel ES, King ME, et al. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 17-2004. A 42-year-old woman with cardiac arrest several weeks after an ankle fracture. N Engl J Med. 2004;350:2281–2290.


 , ventilation-perfusion.
, ventilation-perfusion. scan. If imaging tests are negative in these low-risk patients, PE can be considered to be sufficiently excluded.
scan. If imaging tests are negative in these low-risk patients, PE can be considered to be sufficiently excluded. scanning is an alternative to CT for patients with contrast allergy or renal insufficiency and for pregnant patients. It is important to remember that patients who are found to be at high risk by pretest probability assessment and have low or indeterminate
scanning is an alternative to CT for patients with contrast allergy or renal insufficiency and for pregnant patients. It is important to remember that patients who are found to be at high risk by pretest probability assessment and have low or indeterminate  scans cannot be assumed to have PE ruled out. These patients need anticoagulation and further testing—typically, duplex Doppler ultrasound of the lower extremities.
scans cannot be assumed to have PE ruled out. These patients need anticoagulation and further testing—typically, duplex Doppler ultrasound of the lower extremities. scans have been used over a long period in pregnant patients and are thought to provide minimal to no risk to the fetus. However, the need for experienced technicians and experienced nuclear medicine physicians may limit the availability of
scans have been used over a long period in pregnant patients and are thought to provide minimal to no risk to the fetus. However, the need for experienced technicians and experienced nuclear medicine physicians may limit the availability of  scans. Regardless of what imaging method is available or chosen, it is important to minimize the amount of potential radiation received by the fetus. Discussion with the patient, obstetrician, radiologist, and family needs to occur at the outset even before tests are ordered and should include comment on the probability of disease, extremely low probability of radiation-associated negative effects, and potential morbidity with undiagnosed PE. Tests should be performed if appropriate concern exists for PE. The use of
scans. Regardless of what imaging method is available or chosen, it is important to minimize the amount of potential radiation received by the fetus. Discussion with the patient, obstetrician, radiologist, and family needs to occur at the outset even before tests are ordered and should include comment on the probability of disease, extremely low probability of radiation-associated negative effects, and potential morbidity with undiagnosed PE. Tests should be performed if appropriate concern exists for PE. The use of  , ventilation-perfusion.
, ventilation-perfusion.


