Prolongation of succinylcholine effect
Pharmacology
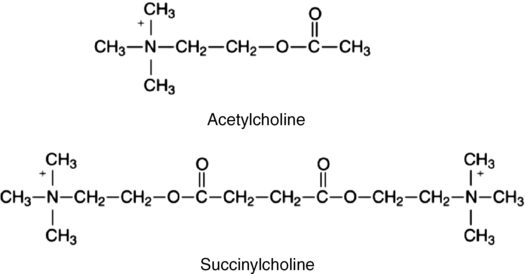
Succinylcholine consists of two molecules of ACh linked by methyl groups (Figure 81-1). Succinylcholine attaches to the nicotinic cholinergic receptor and mimics the action of ACh, thus producing depolarization of the postjunctional membrane. Compared with ACh, the hydrolysis of succinylcholine is slow, resulting in a sustained depolarization. Yet, compared with other neuromuscular blocking agents, the duration of action of succinylcholine is brief (3 to 5 min), owing to hydrolysis by butyrylcholinesterase (BChE), also known as plasma cholinesterase or pseudocholinesterase. This rapid breakdown of succinylcholine, to succinylmonocholine and choline, allows only a fraction (approximately 5%-10%) of the administered dose of the drug to reach the neuromuscular junction. The initial metabolite, succinylmonocholine, is 1⁄20 to 1⁄90 as potent as the parent compound. Succinylmonocholine is subsequently hydrolyzed to succinate and choline. There is little or no BChE at the neuromuscular junction, so the action of succinylcholine is terminated by diffusion from the end plate to extracellular fluid. Thus, by controlling the rate at which succinylcholine is hydrolyzed before it reaches, and after it leaves, the neuromuscular junction, BChE influences the onset and duration of action of the drug.
Measurement of butyrylcholinesterase activity
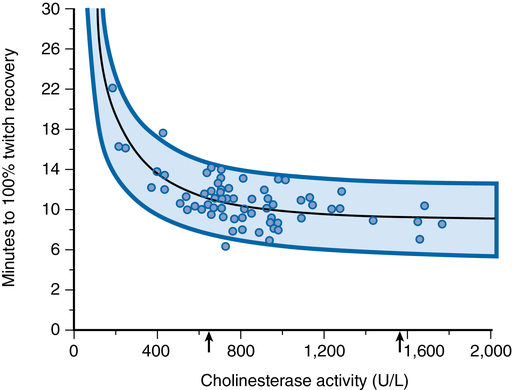
The activity of BChE refers to the number of succinylcholine molecules hydrolyzed per unit of time, expressed in international units. The BChE proteins produced by genetic variations may differ in enzyme amount (quantitative difference) or in enzyme performance (qualitative difference) when compared with normal BChE. Changes in either quantity or quality of the enzyme will cause alterations in BChE activity. The presence of succinylcholine interferes with both quantitative and qualitative assays; therefore, it is preferable to postpone testing until the day after an episode of prolonged neuromuscular blockade associated with the use of succinylcholine to ensure accurate results. Figure 81-2 illustrates the correlation between the duration of succinylcholine action and BChE activity. There is a wide normal range of BChE activity; prolonged muscle relaxation after administration of succinylcholine is clinically significant only with extreme depression of BChE activity.
Qualitative analysis
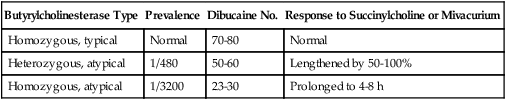
Atypical variants were originally described by Kalow, who identified individuals whose BChE could not metabolize succinylcholine but were only partially inhibited by dibucaine, a local anesthetic. Whereas a normal individual’s hydrolysis of benzoylcholine was inhibited 70% to 80% by dibucaine, affected individuals showed only 20% to 30% inhibition of hydrolysis (Table 81-1). The dibucaine number is defined as the percentage inhibition of BChE in the presence of 40 μmol/L of dibucaine:
Table 81-1
Dibucaine Number and Duration of Succinylcholine or Mivacurium Neuromuscular Blockade
| Butyrylcholinesterase Type | Prevalence | Dibucaine No. | Response to Succinylcholine or Mivacurium |
| Homozygous, typical | Normal | 70-80 | Normal |
| Heterozygous, atypical | 1/480 | 50-60 | Lengthened by 50-100% |
| Homozygous, atypical | 1/3200 | 23-30 | Prolonged to 4-8 h |
Adapted, with permission, from Naguib M, Lien CA. Pharmacology of muscle relaxants and their antagonists. In: Miller RD, Eriksson LI, Fleisher LA, et al, eds. Miller’s Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2009: Table 29.1.
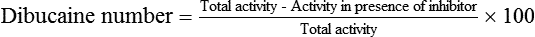
Total activity
Fluoride-resistant (F) variants have been found, the enzyme of which is resistant to inhibition by sodium fluoride. People with one of several extremely rare silent (S) variant genes produce little or no BChE. Individuals with K (Kalow) variants inherit genes producing low levels of normal BChE; similar J and H variants also have been discovered. High-activity (C5) variant families have been identified with cholinesterase activity three times normal. Human BChE variants are shown in Table 81-2.
Table 81-2
Human Butyrylcholinesterase Variants
| Common Name | Phenotypic Description | Amino Acid Alteration | DNA Alteration |
| Usual | Normal | None | None |
| Atypical | Dibucaine-resistant | 70 Asp → Gly | nt 209 (GAT → GGT) |
| Silent-1 | Silent, no activity | 117 Gly → frameshift | nt 351 (GGT → GGAG) |
| Silent-2 | Silent, no activity | 6 Ile → frameshift | nt 16 (ATT → TT) |
| Silent-3 | Silent, no activity | 500 Tyr → stop | nt 1500 (TAT → TAA) |
| Fluoride-1 | Fluoride-resistant | 243 Thr → Met | nt 728 (ACG → ATG) |
| Fluoride-2 | Fluoride-resistant | 390 Gly → Val | nt 1169 (GGT → GTT) |
| K variant | K polymorphism | 539 Ala → Thr | nt 1615 (GCA → ACA) |
| H variant | H polymorphism | 142 Val → Met | nt 424 (GTG → ATG) |
| J variant | J polymorphism | 497 Glu → Val 539 Ala → Thr |
nt 1490 (GAA → GTA) nt 1615 (GCA → ACA) |
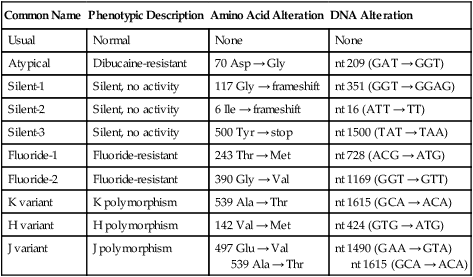
Reprinted, with permission, from Bartels CF, Jensen FS, Lockridge O, et al. DNA mutation associated with the human butyrylcholinesterase K-variant and its linkage to the atypical variant mutation and other polymorphic sites. Am J Hum Genet. 1992;50:1086-1103.
Phase I versus phase II block
A phase II neuromuscular blockade can cause prolongation of the action of succinylcholine; it is a risk of using repeated doses or an infusion of the drug. Phase II blockade may occur when the dose (usually >6 mg/kg) or duration (>30 min continuous infusion) of succinylcholine use is excessive. The exact mechanism for the transition from phase I to phase II blockade is not known but is thought to occur when the postjunctional membrane has become repolarized but still does not respond normally to ACh. The onset of phase II block coincides with tachyphylaxis, as more succinylcholine is required for the same effect. Phase II blockade can be avoided if succinylcholine administration is stopped when train-of-four fade becomes evident. Reversal of a phase II blockade is controversial; it should not be attempted until spontaneous recovery of the twitch has occurred. Administration of neostigmine or edrophonium when spontaneous recovery of the twitch response has been observed for 20 to 30 min and has reached a plateau has been suggested to promote return of the train of four to normal. Characteristics of phase I, transition, and phase II neuromuscular blockade during succinylcholine infusion are shown in Table 81-3.
Table 81-3
Clinical Characteristics of Phase I, Transition, and Phase II Neuromuscular Blockade during Succinylcholine Infusion
| Characteristic | Phase I | Transition | Phase II |
| Tetanic stimulation | No fade | Slight fade | Fade |
| Posttetanic facilitation | None | Slight | Moderate |
| Train-of-four | |||
| Fade | None | Moderate | Marked |
| Ratio | >0.7 | 0.4-0.7 | <0.4 |
| Edrophonium | Augments | Has little effect | Antagonizes |
| Recovery | Rapid | Rapid to slow | Increasingly prolonged |
| Dose requirements (mg/kg)* | 2-3 | 4-5 | >6 |
| Tachyphylaxis | No | Yes | Yes |
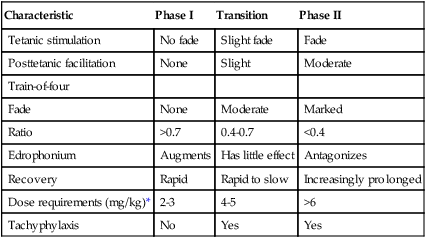
*Cumulative dose of succinylcholine given by intravenous infusion with patient under N2O anesthesia supplemented with intravenously administered agents. The dose required to cause a phase II block is lower in the presence of potent inhalation anesthetic agents.
Modified, with permission, from Lee C, Katz RL. Neuromuscular pharmacology. A clinical update and commentary. Br J Anaesth 1980;52:173-188.