Prolactin
The Evolutionary Biology of Prolactin
The Ontogeny and Physiology of Prolactin Secretion
Pathophysiology of Prolactin Secretion
Prolactin Receptors and Signal Transduction
The Physiology and Pathophysiology of Prolactin Actions in Mammals
Prolactin (PRL) was the first of the pituitary hormones to be biochemically identified and purified,1,2 and hyperprolactinemia caused by hormone-secreting tumors is the most common human pituitary disease. Only recently, however, have the physiology and biochemistry of prolactin actions yielded to contemporary analytic methods to reveal a biology that is at once elegantly simple and sublimely complex. PRL has been identified in the pituitary glands of members of all vertebrate classes, and it has diverse effects on osmoregulation, metabolism, reproduction, metamorphosis, migratory behavior, parental behavior, and lactation.3–6 In most species, especially mammals, PRL has a specialized role in the postmating phase of reproduction. The predominant mammalian actions of PRL are stimulation of lactation and maternal behavior, and inhibition of reproductive function. Associated with the specialization of PRL in mammals, novel genes that encode placentally derived lactogens have evolved. PRL does not perform any indispensable function for the survival of the individual, but gestation and lactation lie at the core of the mammalian life cycle, and they place extreme demands on physiology. Adaptations in the control of PRL secretion and its physiologic actions have therefore been integral to the biology of all mammals, and abnormalities of PRL secretion are a relatively common cause of endocrine disease. The deepening understanding of PRL actions on both physiologic and molecular levels has facilitated improved therapeutic approaches to diseases of PRL secretion and has opened opportunities to use the physiology of PRL and lactation in new ways.
The Evolutionary Biology of Prolactin
PRL and growth hormone (GH) are related at the primary amino acid sequence level.7 PRL has been identified in all of the vertebrate classes, and it has been inferred that the PRL and GH genes arose from a duplication of an ancestral gene at least 400 million years ago, at about the time of the origin of vertebrates.8 Deeper relationships with other hormones are less certain, but erythropoietin shares substantial primary sequence similarity, as well as three-dimensional structural features, with PRL and GH, suggesting that all three of these hormones share an ancient common ancestry. In addition to PRL and GH, which have been conserved in all vertebrate lineages, a wide variety of derivative genes have appeared in specific vertebrate groups by duplication of the PRL or GH gene. The most familiar of these are the various mammalian placental lactogens.9,10
Placental Lactogens
Placental lactogens (PLs) are synthesized during pregnancy in most, but not all, eutherian mammals. Species that apparently do not produce any placental lactogens are distributed among many mammalian families and include familiar species such as pigs, horses, and dogs.11 Primates (including humans) synthesize a PL that is encoded by a gene duplicated within the GH locus.8 The GH locus in humans encompasses five genes spanning a region of about 50 kb on the long arm (q22-24) of chromosome 17. This locus includes two PL or, preferably, chorionic somatomammotropin (CS) genes (ICSH-1 and ICSH-2). The ICSH-1 and ICSH-2 genes, although slightly divergent at the nucleotide sequence level, encode identical proteins, and the genes are coexpressed in the placenta during gestation. In nonprimates (e.g., rodents, ruminants), the PLs have descended evolutionarily from duplications of the PRL gene.8 Multiple PL genes and nonlactogenic PRL-like genes have evolved from PRL. In mice, placental lactogen-I (PL-I) is synthesized early during gestation, appearing immediately after implantation. PL-I expression is extinguished at about midgestation and is replaced by PL-II. Both of the mouse PL genes are synthesized in trophoblast giant cells. In species that synthesize PLs, including humans, the major stimulus to mammary gland development during pregnancy is presumably PLs, rather than pituitary PRL. PL levels generally rise in correlation with placental growth, and their secretion is controlled by both positive and negative regulators.12 Loss of PLs and placental steroids at parturition is accompanied by elevation of pituitary PRL secretion, and a corresponding shift to pituitary-dominated regulation of mammary gland function during lactation. The importance of this shift is that pituitary PRL is strongly regulated by a suckling-induced neuroendocrine reflex, which allows nursing activity to determine directly the lactational stimulus to the mammary glands.
Nonlactogenic Prolactin Relatives
Nonlactogenic members of the PRL gene family are synthesized by the placenta of nonprimates. Although the physiologic activities of these PRL-related proteins have not yet been established, the expression patterns for some of these proteins are tightly regulated during gestation,10 and this has been interpreted to indicate that the proteins are functionally important. In mice and rats, at least six nonlactogenic PRL-like proteins (PLP-A through PLP-F) are present. In addition, mice synthesize two proteins, namely, proliferin and proliferin-related protein, which have been proposed to act as regulators of angiogenesis.13 Nonlactogenic PRL-like protein genes have also been extensively characterized in cattle.14 One curious feature of the PRL-like gene family is the apparently rapid evolutionary divergence of members of this family. These proteins generally share less than 25% sequence identity with PRL, but all share two pairs of cysteine residues that are conserved throughout the PRL and GH superfamily.10 Information regarding receptors for the nonlactogenic PRL-related proteins is scant. Proliferin binds to the mannose-6-phosphate insulin-like growth factor-2 (IGF-2) receptor.15 In mice, some of the non-lactogenic PRL relatives are essential for preventing fetal death during physiologic stresses, such as hypoxia.16,17 Therefore, the species specificity of PRL relatives may reflect the reproductive stresses that were relevant during the evolutionary divergence of different mammalian groups.
Extrapituitary Prolactin
Many mammalian tissues, including the human mammary gland and uterine decidua, express the PRL gene. In addition, various tissues metabolize PRL to alternative forms that may be biologically active. PRL is synthesized by both the decidua and the uterine myometrium in humans.18 High concentrations of PRL are present in the amniotic fluid; these can be traced to both decidually synthesized hormone and plasma PRL that is transported across the placenta into the amniotic fluid. The synthesis of human PRL in extrapituitary sites is controlled by a promoter that is distinct from the pituitary PRL promoter. Human extrapituitary PRL messenger RNA (mRNA) has a distinct 5′ untranslated sequence corresponding to an additional exon (exon 1A)19 (Fig. 2-1). Exon 1A and the promoter elements associated with it are located about 8000 base pairs (bp) distal to the initiation site for pituitary PRL transcription. In rodents, the evidence for a distinct extrapituitary PRL promoter is less certain than in the human. It is conceivable that rodents use other mechanisms, such as growth factors that control the conventional pituitary PRL promoter, to provide for regulation of PRL synthesis in extrapituitary tissues. The mammary gland is an important site of PRL synthesis and secretion. PRL is present in significant concentrations in milk, and milk PRL is absorbed by the neonatal gut and causes changes in the maturation of the hypothalamic neuroendocrine system.20 Pituitary PRL is transported out of the circulation, across the mammary epithelium, and into the alveolar lumen, and locally synthesized PRL is secreted into milk.18 To date, no disease states have been connected to dysregulation of extrapituitary PRL secretion. The lack of any clear proof that symptoms are present that are caused by either hypersecretion of extrapituitary PRL—say, from a PRL-secreting ectopic tumor—or loss of extrapituitary PRL gene expression makes it difficult to surmise the normal functional roles of extrapituitary PRL in humans. It has been suggested that locally synthesized PRL in the mammary gland might act as a growth factor for both normal breast epithelium and breast cancer cells.21 In mice, decidual PRL prevents fetal loss late in pregnancy by inhibiting the expression of multiple genes that are detrimental to pregnancy, including inflammatory cytokines.22 A recent pilot study reports a lack of endometrial PRL expression in some women with unexplained infertility and patients with repeated miscarriages.23
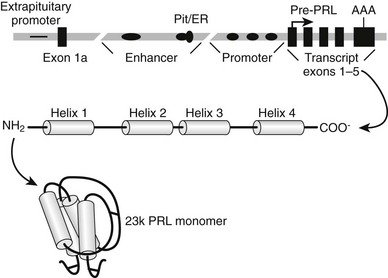
FIGURE 2-1 Biosynthesis of prolactin (PRL). The PRL gene is depicted at the top of the figure as consisting of five exons (black rectangles) encoding the structural gene for preprolactin (pre-PRL). The translation start site in exon 1 is marked by an arrow, and the polyadenylation site in exon 5 is marked AAA. The region labeled promoter includes multiple binding sites for the pituitary-specific transcription factor-1 (Pit-1; black ellipses), but only three are depicted in the diagram. The line that depicts the DNA sequence is broken by two interruptions to indicate that the upstream regulatory regions are separated from the promoter by several thousand base pairs. A distal regulatory region (enhancer) includes binding sites for Pit-1 and other factors, including a complex site that binds both Pit-1 and the estrogen receptor (Pit/ER). Exon 1a is transcribed in extrapituitary tissues and is controlled by a distinct “extrapituitary promoter.” After transcription and translation, the PRL protein consists of four α-helical regions, which are labeled helix 1 through 4, and intervening β-strand regions. The protein spontaneously folds into a globular structure in which three disulfide bridges connect β-strand regions, and this mature structure is depicted as the 23k PRL monomer.
The Biochemistry of Prolactin
Human PRL is synthesized as a prehormone that is encoded by an mRNA with an open reading frame of 684 bases. The native gene for PRL is divided into five exons, and the initiation site for translation is in exon 124 (see Fig. 2-1). Preprolactin (pre-PRL) is 227 amino acids in length, with a deduced molecular weight of nearly 26,000. Cleaving the signal peptide from the N-terminus of pre-PRL results in a mature polypeptide that is 199 residues in length and has a molecular weight of nearly 23,000 (23k PRL). On the basis of the fact that the bacterially synthesized recombinant 23k PRL monomer binds to the PRL receptor (PRL-R) and transduces functional signals, it is clear that no additional modifications are essential for the core functions of PRL. PRL folds itself into a tertiary structure that includes three intrachain disulfide bridges, two of which are conserved in all members of the PRL-GH family, and one that links residues 4 and 11 in the N-terminus, which is unique to PRL and its closest relatives.7 Four α-helical domains in PRL are arranged so that helices 1 and 2 run antiparallel to helices 3 and 4. This general molecular architecture of PRL has been conserved with GH and other homologous proteins and also has evolved independently in several families of cytokines.25 The convergent evolution of hormone and cytokine ligand architecture apparently has been driven by the properties of receptors that bind these hormones and transduce signals to the intracellular space.
A variety of biochemical variants of 23k PRL, which appear to have altered functions, have been identified. PRL has a tendency to aggregate and form intermolecular disulfide bridges spontaneously when in solution at high concentrations. High-molecular-weight variants (sometimes referred to as “big” PRLs) may arise by virtue of multimerization, glycosylation, or cross-linking with other proteins. Only a small fraction of human PRL is glycosylated, whereas in some other species, such as swine, glycosylated PRL represents a large portion of both pituitary and plasma hormone.26 Glycosylation may alter the relative potency of PRL by changing its receptor-binding characteristics or by modifying its pharmacokinetic properties in the animal (plasma half-life, partitioning between plasma and interstitial compartments, etc.). PRL is metabolized by tissue uptake and by proteolysis in the circulation or in cells. Proteolysis also produces a 16kDa PRL fragment that has been proposed to have antiangiogenic bioactivity.27
Recent studies implicate the cathepsin-cleaved 16kDa PRL as causative of postpartum (or peripartum) cardiomyopathy (PPCM). PPCM is the acute onset of heart failure in women during late-stage pregnancy or the first several months post partum. Hilfiker-Kleiner and colleagues discovered that PPCM developed in mice with selective deletion of the STAT3 gene in cardiac myocytes.28 STAT3 has been shown to be a critical factor modulating cardiac angiogenesis, an important part of the normal cardiac hypertrophy that occurs during pregnancy.29 In a series of elegant studies, they demonstrated that the absence of STAT3 led to increased production of reactive oxygen species, upregulation of cathepsin-D, and elevation of the 16kDa fragment of PRL. Treatment of these mice with bromocriptine, which reduces secretion of 23kDa PRL from the pituitary, resulted in enhanced cardiac function and prevention of postpartum mortality. Preliminary studies have shown that circulating levels of 16kDa are barely detectable in healthy nursing women but are elevated in some women with PPCM.28 A few clinical case reports have found that women with PPCM may respond favorably to treatment with bromocriptine.28,30,31
Phosphorylated PRL has reduced potency in standard bioassays, and it antagonizes the actions of the predominant unphosphorylated form.32 Actions of kinases or phosphatases in either the pituitary or individual target tissues may have an important effect on the bioactivity of PRL in vivo.
The Ontogeny and Physiology of Prolactin Secretion
PRL is synthesized by lactotrophs, which are acidophilic cells that represent 20% to 50% of the anterior pituitary cell population. The lactotrophs are the last of the pituitary cell types to fully differentiate and, coincidentally, the most likely to give rise to pituitary adenomas. Pituitary PRL mRNA synthesis begins at 12 weeks in human gestation and is preceded by GH synthesis by at least 4 weeks.8,33 In rodents, the pattern is similar, with the GH gene being expressed several days before PRL, and with dual-functioning somatolactotrophs being observed before fully differentiated lactotrophs.34 Control of pituitary development and lactotroph differentiation depends on the orchestrated expression of a series of intrinsic, tissue-specific regulatory molecules that act as “molecular switches” to induce the sequence of developmental changes that lead up to full pituitary differentiation. Many of the intrinsic factors that have been implicated in pituitary development are evolutionarily related to “homeotic mutation” genes, which were first identified by their dramatic effects on development in fruit flies.33 Some genetic diseases of the pituitary, pituitary tumors, and physiologic states of hormone deficiency or excess can be attributed to dysfunction of these regulatory molecules.
The homeobox transcription factors are a diverse class of developmental regulatory proteins that share sequence similarities in their DNA-binding regions and are sequentially activated during organogenesis. Two pituitary homeobox proteins (Ptx1 and Ptx2) are expressed in multiple anterior (head and face) tissues before the development of Rathke’s pouch, and they continue to be expressed in some differentiated pituitary cells. Rathke’s pouch homeobox protein (Rpx) is expressed first in neural structures associated with the head region and then in Rathke’s pouch. During the formation of Rathke’s pouch, a subgroup of LIM-related homeobox proteins are synthesized (P-LIM, Lhx3, and Lhx4), and these genes continue to be expressed in specific regions of the pituitary throughout life. Properly timed extinction of expression is, for certain genes, as important during development as is their appropriate induction. Rpx must be turned off after Rathke’s pouch has been formed, so that genes that are specific to later stages of pituitary differentiation can be turned on. The transcription factor that downregulates Rpx expression is PROP-1 (Prophet of Pit-1). PROP-1 turns off Rpx and turns on Pit-1, leading to differentiation of some of the hormone-producing cells of the pituitary gland, including lactotrophs.35
In the adult pituitary gland, a population of stem-like cells provides new progenitors for all of the hormone-secreting cells, including lactotrophs.36
Pit-1 is essential for differentiation of both PRL- and GH-secreting cells, hence its alternative name, GH factor-1 (GHF-1).37 An early developing subpopulation of thyrotrophs is also dependent on Pit-1. The Pit-1 protein shares close sequence similarity with two other transcription factors within regions referred to as the POU (Pit, Oct, Unc)-specific domain, and the POU-homeodomain.38 Pit-1 expression in the developing pituitary gland precedes the synthesis of hormones and is necessary for the expression of GH, PRL, and thyroid-stimulating hormone (TSH) in fetal pituitaries. Variant forms of Pit-1 are encoded by alternatively spliced mRNAs and may differentially control expression of individual hormones. Pit-1 binds not only to DNA sequences in the GH, PRL, and TSH genes, but also to autoregulatory sites in the Pit-1 promoter. Autoactivation of Pit-1 transcription is one means of preserving phenotypic stability in differentiated pituitary cells. The factors that act after Pit-1 to drive the differentiation of lactotrophs from somatotroph progenitors are not known. Estrogen receptors synergize with Pit-1 to induce PRL, but not GH, gene expression. Estrogen therefore may be one of the factors that drive the ultimate differentiation of lactotrophs.39–42
Several extrinsic factors are involved in lactotroph differentiation. Estrogen is an important positive regulator of lactotroph development. Lactotrophs are greater in number and contain more PRL per cell in females during their reproductive years. Estrogen acts directly on lactotrophs to stimulate PRL synthesis and cell proliferation. Estrogen-induced galanin secretion from lactotrophs is an important mediator of these estrogen actions43,44 and involves signaling through the classic estrogen receptor isoform alpha (ERα).45 Paracrine factors produced by other anterior pituitary cell types include basic fibroblast growth factor (B-FGF or FGF-2), which has a specific positive stimulatory effect on lactotrophs.46 Likewise, epidermal growth factor (EGF) stimulates lactotrophs and may act as a developmental regulatory factor as well as a physiologic stimulator of PRL secretion.47 As will be presented in subsequent sections, the same factors that drive vectorial differentiation of pituitary cells can participate in regulating the tides of hormone secretion on a physiologic time scale and in disorders of hormone secretion.
Regulation of Pituitary Prolactin Synthesis and Secretion
In mammals, PRL secretion is normally restrained by the action of dopamine (DA), which is secreted from the hypothalamus.48 Although the levels of other pituitary hormones are modulated by inhibitory secretagogues such as somatostatin, PRL is the only such hormone that is secreted at unrestrained high levels when completely isolated from the positive trophic influences of the hypothalamus. This unconventional situation is unique to mammals. Control of PRL secretion in birds and other nonmammals is more conventional in the sense that positively acting secretagogues are the predominant regulators of PRL secretion.49,50 Lactotrophs are excitable cells in that they display spontaneous membrane depolarizations associated with calcium ion influx, and their resting membrane potential is influenced by neurotransmitters and peptide neuromodulators.
The normal secretory pattern of PRL is a series of daily pulses, occurring every 2 to 3 hours, which vary in amplitude so that the bulk of the hormone is secreted during rapid eye movement (REM) sleep. REM sleep is the dominant organizer in men and nonparous women and occurs mostly during the latter half of the sleep phase. Thus, the highest levels of PRL generally occur during the night in humans.51 In nocturnal rodents, the relationship to the light cycle is reversed, so higher PRL secretion occurs during the daytime, which is the inactive phase. It is unclear how REM and PRL secretion are linked. Infusion of PRL increases REM activity in the electroencephalogram (EEG),52,53 suggesting that it is PRL that induces REM sleep, and not vice versa.
Dopamine
As was mentioned earlier, the major regulatory input to lactotrophs is inhibitory, provided in the form of DA produced within the hypothalamus. The primary PRL-regulating DA neurons are the tuberoinfundibular dopaminergic (TIDA) cells, which have their cell bodies in the arcuate nucleus of the hypothalamus; they release DA in the median eminence and the pituitary stalk (Fig. 2-2). A secondary tuberohypophysial dopaminergic system has cell bodies in the rostral caudate and paraventricular nuclei, and these neurons release DA in the posterior pituitary.48 The type 2 isoforms (D2) of the DA receptor mediate the direct inhibitory actions of DA on PRL secretion, synthesis, and cell proliferation. Targeted disruption of the D2 receptor in mice leads to a phenotype of PRL hypersecretion and lactotroph proliferation.54
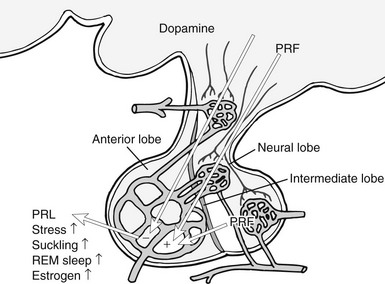
FIGURE 2-2 Control of pituitary secretion of prolactin (PRL). Dopamine from the hypothalamus is the predominant inhibitory regulator of pituitary PRL secretion. Multiple factors act as PRL-releasing factors (PRF; see text), and these come from both the hypothalamus and the posterior pituitary. Physiologic states that stimulate PRL release are listed on the figure. REM, Rapid eye movement.
Dopamine is synthesized by a two-step reaction in which tyrosine conversion to levodopa is catalyzed by tyrosine hydroxylase, and levodopa is converted to DA by the action of aromatic amine decarboxylase. As is the case for catecholamine synthesis in other cells, the momentary rate of DA synthesis in the TIDA neurons is determined by the activity of tyrosine hydroxylase. The negative feedback mechanism for controlling PRL release is to increase tyrosine hydroxylase activity in the TIDA neurons, thereby increasing the amount of DA available for release from the median eminence. PRL receptors are located in both the arcuate nucleus (site of the TIDA perikarya) and the median eminence.55 Therefore, circulating PRL may feed back on TIDA neurons at their terminals, which lay outside the blood-brain barrier, or systemic PRL may enter the cerebrospinal fluid via the choroid plexus. The choroid plexus expresses high levels of a short isoform of the PRL-R, which may serve to transport PRL across the blood-brain barrier. Levels of PRL in the cerebrospinal fluid reflect changes in PRL in the systemic circulation.56 Isolated PRL deficiency resulting from targeted gene disruption in the mouse causes decreased DA in the median eminence but does not affect DA levels in other regions of the hypothalamus.57
Activation of D2 receptors in lactotrophs has at least two main actions that result in inhibition of PRL. D2 receptors are members of the heptahelical G protein–coupled receptor superfamily, and they activate the αi subunits, which leads to inhibition of cyclic adenosine monophosphate (cAMP) synthesis.48 In addition, D2 receptors activate a G protein–coupled, inwardly rectifying potassium channel, which instantaneously causes hyperpolarization of the lactotroph membrane and closes voltage-gated calcium channels.58 Cytoplasmic calcium levels fall because of decreased influx of extracellular calcium, and the reduction in cytosolic free calcium decreases the exocytosis of secretory vesicles.
Dopamine-induced membrane hyperpolarization opposes the actions of some stimulatory factors such as thyrotropin-releasing hormone (TRH), which acts predominantly to increase influx of extracellular calcium by depolarizing the lactotroph membrane. Inhibition of cAMP by DA also opposes the actions of stimulatory factors such as vasoactive intestinal peptide (VIP), which acts via a positive effect on cAMP. This action decreases PRL release in the short to intermediate term. Second, because cAMP is mitogenic in lactotrophs, as well as in other pituitary cells, activation of Gi signaling by DA is antimitogenic. Lactotroph proliferation is important for physiologic elevation of PRL release during lactation. The proliferative action of cAMP on lactotrophs is understood to be an important promoter of pituitary tumor growth, thereby contributing to pathologic hyperprolactinemia.35
Other hypothalamic factors, as well as DA, and local pituitary peptides can inhibit PRL secretion. Somatostatin inhibits PRL secretion and acts through both cAMP-dependent and cAMP-independent mechanisms.59 Calcitonin has been shown to inhibit PRL secretion and may be secreted from the hypothalamus.60 Endothelin-1 is produced by lactotrophs and inhibits PRL secretion; transforming growth factor-β1 can act as a paracrine inhibitor of PRL.61,62 The biologic significance of these factors in pituitary development and physiology has not yet been established.
Prolactin-Releasing Factors
A wide variety of stimulatory PRL secretagogues have been identified over the years, and it is likely that additional PRFs will be identified in the future. Known stimulators of PRL secretion include, but are not limited to, steroids (estrogen63), hypothalamic peptides (TRH, oxytocin, VIP,64,65 pituitary adenylate cyclase activating peptide [PACAP],66 and galanin67), and local pituitary factors (growth factors such as EGF68 and FGF-2,69 angiotensin II,70 and, again, PACAP71 and galanin43).
TRH is a potent and rapid stimulator of PRL release in vitro via a set of calcium-mediated pathways activated by a Gq-coupled receptor. However, the relative contribution of TRH to physiologic control of lactotrophs is not clear. VIP acts through cAMP to stimulate PRL synthesis and release on an intermediate to long-term basis. The importance of VIP as a positive lactotrophic factor is supported by two types of evidence. With the use of antibodies against VIP, the secretion of PRL can be inhibited to a very low level.47 In addition, VIP appears to be the primary PRF in birds and other nonmammals,72,73 suggesting that this positive mechanism may have been in place before the evolution of the dopaminergic inhibitory system in mammals. Oxytocin secretion is tightly coupled with PRL secretion during lactation, and both are secreted in response to nipple stimulation. The potential role of oxytocin as a PRF, given that it can reach the anterior pituitary through the short portal system, has remained controversial. Oxytocin antagonism partially suppresses PRL secretion,48 so this peptide is likely to provide some portion of the physiologic stimulus for PRL release. PACAP stimulates PRL synthesis and release. Galanin is synthesized in both the pituitary and the hypothalamus. In the pituitary, it colocalizes with PRL in lactotroph secretory granules and acts by autocrine and paracrine mechanisms to stimulate lactotrophs.43
A putative PRL-releasing peptide (PrRP) from the hypothalamus was identified by searching for ligands that activate an orphan pituitary G protein–coupled receptor. The mature peptide that was identified from bovine hypothalamus is a 20 amino acid molecule that originally was reported to cause rapid secretion of PRL from isolated pituitary cells.74 However, subsequent studies have failed to confirm that PrRP acts on lactotrophs to stimulate PRL release.75 Rather, PrRP may act within the hypothalamus to indirectly elevate PRL by inhibiting DA release. Antagonists of serotonin or opioid receptors inhibit PRL secretion under physiologically meaningful stimuli. Conversely, antidepressants that inhibit serotonin reuptake (fluoxetine [Prozac], etc.) may increase PRL secretion in humans and in laboratory animals. Serotonin and opioids are important indirect regulators of PRL by virtue of their actions on DA and releasing factor secretion in the hypothalamus.
Lactotrophs display a large degree of functional heterogeneity within the anterior pituitary. This heterogeneity is manifested as differences in morphology (i.e., secretory granule size and density), basal hormone release, electrical activity, and response to releasing and inhibiting factors. Assay of hormone release from single cells has revealed not only substantial cell-to-cell variations in function, but also marked temporal variations in a single cell.76
Transcription regulators that control the development of the anterior pituitary lactotrophs also participate in controlling PRL synthesis during adult life. Prominent among these factors is the Pit-1 protein. Pit-1 binds to two regions of the human PRL gene, the proximal promoter (within 250 bp of the transcription start) and a distal enhancer (beyond −1300 bp) (see Fig. 2-1). Multiple Pit-1–binding sites are present in each of these regions. Transcription regulators such as cAMP and estrogen receptors can control PRL gene expression by influencing Pit-1 activity.39,63
Pathophysiology of Prolactin Secretion
Normal plasma PRL concentrations in women who are neither pregnant nor lactating range from 4 to <20 ng/mL. In men, the values, on average, are several units lower. Late pregnancy and lactational levels normally range from 100 to 200 ng/mL, and the highest levels occur following active bouts of nursing. PRL is normally measured by radioimmunoassay (RIA). Although glycosylation and other chemical modifications of PRL can affect its immunoreactivity and therefore can lead to aberrant RIA results,26 pathologic levels generally are readily detected by RIA. The original method for bioassay of PRL involved measuring the growth of the pigeon crop sac mucosal epithelium.77 This method still is used occasionally and serves as the basis for the international standardization of PRL bioactivity. However, the method has been largely supplanted by a simpler bioassay that takes advantage of the ability of PRL to stimulate the proliferation of rat Nb2 lymphoma cells in culture.78
Prolactin Deficiency
When PRL deficiency occurs, it is normally one component of a combined pituitary hormone deficiency. However, a few cases of PRL deficiency without evidence of other pituitary defects have been reported in women. Isolated PRL deficiency results in lactational failure and reproductive difficulty with no other obvious problems.79–82 No cases of isolated PRL deficiency have been reported in men. These results in a few humans are consistent with the phenotype of mice in which the PRL gene has been disrupted by a targeted mutation. In mice with disruptions of either PRL or its receptor genes, mammary gland development is defective; the females fail to reproduce, but the males do not have any overt symptoms.57,84,85 The concordance of these results from humans and mice is remarkable, given the possible differences between PRL physiology in humans and rodents. One important difference is that progesterone secretion in the rodent corpus luteum requires PRL, but in the human, it does not. This difference in luteal control probably explains why women who have isolated PRL deficiency are merely subfertile,79–82 whereas PRL-deficient mouse females are completely infertile.84,85
Mice with a targeted mutation of the PRL gene develop pituitary hyperplasia57 and adenomas86 that are more severe in females. Mice with targeted disruption of the PRL receptor gene also exhibit this lactotroph hyperplasia and prolactinoma development.87 Loss of PRL feedback in both of these genetic models leads to decreased hypothalamic DA, and the deficiency of DA leads to poorly restrained pituitary growth. Some forms of combined pituitary hormone deficiency have been identified in which PRL, GH, and TSH are hyposecreted as a consequence of mutations in important developmental factors. Familial inheritance of defects in the Pit-1 gene or PROP-1 results in individuals who fail to develop lactotrophs, somatotrophs, and thyrotrophs, and consequently are dwarfed and hypothyroid, as well as PRL deficient. Two spontaneous mutations that cause dwarfism in mice have been shown to correspond to these human conditions. In Snell dwarf mice, a mutation of the Pit-1 gene occurs, and in Ames dwarfs, the PROP-1 gene is mutated.35,88,89
Hyperprolactinemia
Hypersecretion of PRL is among the most common of pituitary disorders. Medications that elevate PRL secretion and may cause hyperprolactinemia include commonly used antiemetics, antipsychotics, antidepressants, and narcotics. These medications alter PRL secretion by antagonizing DA action, or by elevating serotonin or endorphin bioactivity. Reserpine and methyldopa increase PRL secretion as a result of DA depletion. DA receptor antagonists, such as haloperidol and phenylthiazines, increase PRL secretion. Serotonin reuptake inhibitors, such as fluoxetine, elevate serum PRL.48 It is uncommon for any of these medications to cause clinical signs of hyperprolactinemia, because the levels of PRL seldom reach more than 30 to 50 ng/mL with these drugs. One might imagine that subtle hormonal effects may be noted after long-term treatment.
Hyperprolactinemia that manifests clinical symptoms is most commonly a consequence of a lactotroph adenoma. These tumors may secrete high levels of PRL alone or of both PRL and GH. Any intracranial mass or trauma that causes compression or disruption of the pituitary stalk can cause hyperprolactinemia because of the loss of dopaminergic tone from the hypothalamus. Pituitary adenomas have been discovered to be much more common than was once believed, with more than 20% of individuals harboring tumors measuring at least 3 mm at autopsy.35 Tumors that do not hypersecrete hormones are usually of gonadotroph or lactotroph origin. Some symptoms of prolactinomas may be caused by tumor mass effects. These include visual field defects, associated with pressure on the medial aspect of the optic chiasm, and alterations in temperature regulation, feeding patterns, or other effects caused by hypothalamic compression. However, effects associated with the physiologic actions of the hormone are the more common presenting symptoms.
Prolactin Receptors and Signal Transduction
The PRL-R is a member of the type 1 cytokine receptor family,90 and its nearest relative is the GH receptor. Several hematopoietic cytokine receptors, such as those for erythropoietin, most interleukins, and granulocyte-macrophage colony-stimulating factor, are also very similar to PRL and GH receptors. Other receptors, such as those for the interferons, are members of a broader superfamily of proteins that includes cell adhesion proteins. Features that define the type 1 cytokine receptor family include two signature motifs in the extracellular domain and one in the intracellular domain. Four cysteine residues in the extracellular domain are absolutely conserved among all of the type 1 cytokine receptors, and they form two disulfide bridges that are essential for the proper tertiary folding of the ligand-binding domain. A short sequence, which includes a tandem repeat of tryptophan-serine interrupted by a single amino acid (the WSXWS motif), is the second signature motif in the extracellular domain. This sequence is highly conserved near the base of the extracellular domain, but the function of these residues has not yet been proven with any degree of certainty. The structure of the PRL-R extracellular domain, like that of the GH and other cytokine receptors, has been analyzed extensively by x-ray crystallography, as well as by biochemical methods.91 This domain comprises two 100 amino acid subdomains, which are structurally related to the type III repeats of fibronectin. Each of the type III subdomains includes a conserved series of seven β-strands folded into two β-sheets that run in an antiparallel orientation. These type III subdomains are connected by a short, flexible hinge peptide, and residues that contact the ligand span this connector to include amino acids in each of the type III subdomains. Across the vertebrate lineages, substantial conservation of the major features of the PRL-Rs is evident, with some notable exceptions. In birds (pigeons, chickens), the extracellular domain has duplicated and diverged, and in cattle, the distal C-terminus has been truncated, thus eliminating a tyrosine residue that is conserved in other lineages (Fig. 2-3). Neither of these evolutionary changes appears to have functional significance.92,93
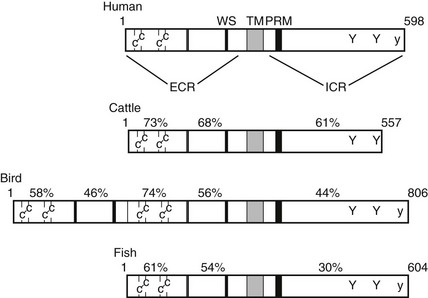
FIGURE 2-3 Prolactin receptor structure and function. Schematic diagram of the linear sequences of representative prolactin receptors. Pertinent structural features are two pairs of cysteines, the flexible hinge (double line), and the WS × WS repeat in the extracellular region (ECR). The transmembrane-spanning sequence (TM) marks the separation between the ECR and the intracellular region (ICR). In the ICR, the conserved motifs are the proline rich box 1 motif (PRM) and conserved tyrosine residues. The uppercase Y indicates ubiquitously conserved tyrosines, and the lowercase y is a tyrosine that is conserved in all known species except cattle. The percentage of identical amino acid residues in each region, compared with the human receptor, is labeled above each receptor.
Within the intracellular region of the PRL-R an 8 amino acid proline-rich motif, referred to as box 1, is the third conserved signature motif that characterizes type 1 cytokine receptors. These amino acids interact directly with the tyrosine kinases that are activated on ligand binding to the extracellular domain, and mutations in box 1 completely disable PRL-R signaling.5 Multiple PRL-R isoforms vary in terms of length and amino acid sequence of the intracellular domain. The long isoform, which has been identified in all species to date, has an intracellular domain that is about 350 amino acids in length. Short isoforms (<100 intracellular residues) have been identified in rodents, humans, and several other mammalian species. The short forms of the PRL-R include box 1 but lack other regions of the intracellular domain that are required for signal transduction. In particular, conserved tyrosine residues in the distal portion of the long form of the receptor are phosphorylated after ligand binding, and these tyrosines are required for normal signal transduction.
Ligand binding appears to facilitate conformational changes or dimerization of the PRL-Rs as the first step toward signal transduction. The first evidence favoring dimeric receptor interactions as a physiologically important step in PRL signaling was derived from experiments in which antibodies were used to artificially induce receptor dimerization and consequent signaling in PRL-responsive cells. Results from this creative experimental approach were ultimately proved to be correct when hormone-receptor complexes for human GH and PRL receptors were biochemically and crystallographically mapped.5,91,94,95 Formation of 1:2 complexes of the hormone with its receptor (Fig. 2-4) appears to be the essential first step in the transmission of the biologic signal within target cells. Transcriptional activation requires homodimerization of the long-form PRL-R. Heterodimers of short and long receptors, or short homodimers, do not mediate normal signal transduction.96
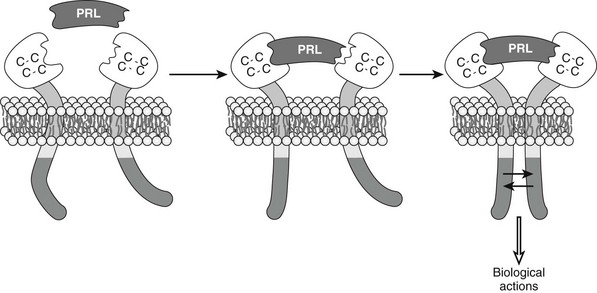
FIGURE 2-4 Dimerization and conformational changes in the prolactin (PRL) receptor cause activation. Based on studies described in the text, PRL is understood to cause receptor interaction between the receptors and associated proteins, leading to activation of appropriate biological actions.
The PRL-R gene, which is located on the long arm of human chromosome 5 (p13-14), is composed of at least 10 coding exons. Multiple transcripts, reflecting alternative splicing variants and transcription start sites, account for some of the variability in PRL-R structure and tissue distribution.5
A human mutation in the PRL-R gene was identified in patients with benign multiple fibroadenomas.97 The mutation encodes a constitutively active form of the receptor.
Tyrosine Kinase Activation
JAK2 (Janus kinase-2) is a protein kinase that is associated with the PRL-R through binding to the box 1 motif. Its activation is the first intracellular event in a complex and incompletely understood web of interactions that mediate PRL effects within its target cells (Fig. 2-5). JAK2 has been shown to be the essential PRL-regulated protein kinase by both biochemical and genetic experiments,98,99 but this kinase is also essential for signaling by other cytokines.100 Although it is presumed today that JAK2 binds directly to the PRL-R, it remains possible that another protein could mediate this association. This possibility is raised by the observation that JAK2 is associated with unliganded PRL-Rs, whereas in the case of GH signaling, in which JAK2 is also the important receptor-activated kinase, ligand binding is necessary before the kinase can bind to the GH receptor. On ligand-induced dimerization of PRL-Rs, JAK2 phosphorylates specific tyrosine residues on the receptor intracellular domain and autophosphorylates residues within the kinase. These phosphotyrosines serve as docking sites for additional signal transduction proteins. The actions of the kinase are counteracted by multiple tyrosine phosphatases, which rapidly dephosphorylate specific proteins and maintain the steady-state level of tyrosine phosphorylation at a very low level in the absence of hormonal stimulation.
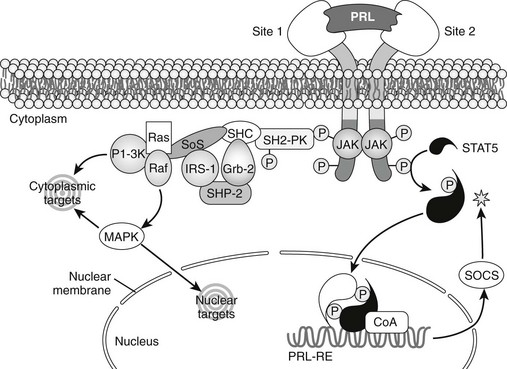
FIGURE 2-5 Intracellular signal transduction by prolactin (PRL). Janus kinase 2 (JAK2) is associated with the PRL receptor and becomes active after receptor dimerization. A signal transducer and activator of transcription protein (STAT5) is phosphorylated (P), dimerizes, associates with Co-activators (Co-A), and binds to appropriate genes through prolactin response elements (PRL-RE). Activation of the JAK2-STAT5 pathway is inhibited (asterisk) by suppressors of cytokine signaling proteins (SOCS). JAK2 also activates other Src-related protein kinases (SH2-PK), and these couple with an array of signaling molecules that can activate cytoplasmic or nuclear target molecules, including mitogen-activated protein kinases (MAPK).
In addition to the signal transduction and activators of transcription (STAT)-dependent events triggered by JAK2 activation, other STAT-independent signaling pathways can be activated when PRL binds to its receptor, as shown in Figure 2-6. Src-family kinases may be involved in PRL signaling by virtue of their ability to couple to multiple signaling intermediates. Phosphotidylinositol-3′-kinase, mitogen-activated protein kinases (MAPKs), and protein kinase C have each been observed to be activated by PRL in some systems.5 The tyrosine phosphatase short heterodimer partner (SHP)-2 is essential for PRL signaling.100
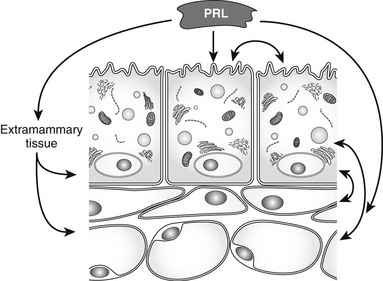
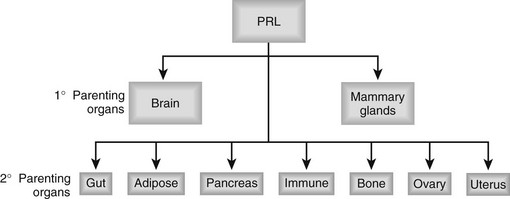
FIGURE 2-6 Mammary gland regulation by prolactin. Acting directly on mammary epithelial cells, and indirectly through extramammary tissues such as the ovaries and other cells within the mammary glands, prolactin causes growth and functional differentiation of the epithelium. Interactions among the cell types may be supportive for prolactin-induced functions or homeostatic negative feedbacks.
STAT-independent pathways have been proposed for PRL signaling, but the physiologic relevance of such mechanisms is not yet clear. It has been suggested that STAT-independent signaling mediates the mitogenic actions of PRL.101 This action would be consistent with findings in other cytokine-signaling systems, where STAT activation determines certain differentiation-related effector functions, whereas other pathways, such as MAPK activation, are involved in mitogenic signal transduction. However, it is unclear whether this analogy can be extended to PRL. In tissues where PRL has a growth-stimulating action, such as mammary gland or pigeon crop sac, it is not established whether the growth stimulus is direct or is mediated by local synthesis of growth factors other than PRL. The rat Nb2 lymphoma cell line, for which PRL acts as a direct mitogen, expresses a mutated form of the PRL-R, which may transduce an unbalanced set of intracellular signals. The role of specific signal transducers in the proliferative response of Nb2 cells has not yet been established.
Transcriptional Regulation
Analysis of PRL-induced genes led, in 1994, to the identification of cis-acting elements that bind members of the STAT family of transcription factors.25,103 PRL-regulated genes were shown to include conserved DNA motifs in their promoter regions, and these sequences were bound to STAT proteins.104 A novel STAT protein (STAT5) was cloned from lactating sheep mammary glands.105 Mammals synthesize two STAT5 proteins encoded by closely related genes. Genetic studies, making use of targeted gene disruption in mice, have made it clear that both STAT5a and STAT5b are partially responsible for mediating the primary PRL effects in the ovaries and mammary glands. STAT5a is more important in the mammary glands, whereas STAT5b is more important in the ovaries.106–108 The mouse genetic studies also have revealed a remarkable degree of concordance of the characteristics of animals that lack genes for the ligand (PRL), its receptor, or the PRL-regulated STAT5 transcription factors.84,85 The concordance among these studies convincingly demonstrates that known PRL-R and STAT5 proteins are the primary mediators of the physiologic actions of PRL. However, subtle differences are seen among the various animal models, which suggest that the STAT5-dependent mechanism might not be the only component of PRL signaling in mammalian cells. STAT5 is phosphorylated by JAK2 on an essential tyrosine residue in its C terminus.109 After its tyrosine phosphorylation is complete, STAT5 dimerizes through interactions between phosphotyrosine and src homology 2 (SH2) domains. Dimeric STAT complexes translocate into the nucleus, where they interact with specific sites in the promoters of PRL-regulated genes, leading to an increase in the rate of transcription of those genes. The exact mechanism by which STAT5 is transported into the nucleus is not known, nor is it known how this inducible transcription factor interacts with basal transcription machinery during activation of gene expression. Good evidence suggests that the glucocorticoid receptor (GR) collaborates with STAT5 during milk protein gene induction.110 This positive interaction depends on occupancy of the GR by its ligand.
STAT activation by PRL is regulated inside the cell by a negative feedback mechanism. CIS (cytokine-inducible SH2 protein) and SOCS (suppressor of cytokine signaling) are members of a class of proteins that are transcriptionally regulated by activated STAT proteins. These proteins feed back on the receptor complex to inhibit the coupling of JAK to either receptor or to STAT.111
Whereas STAT5 appears to be the exclusive mediator of the primary physiologic PRL actions in mammals, other STAT proteins, such as STAT1, may be activated in response to PRL in certain pathophysiologic or pharmacologic conditions. In a rat T lymphoma cell line (Nb2), PRL induces the expression of the interferon response factor-1 (IRF-1) gene. This effect of PRL is mediated by STAT1 and, paradoxically, is inhibited by STAT5.112 Although IRF-1 gene regulation probably is not involved in the normal functions of PRL, the regulation of this gene through STAT1 in Nb2 cells provides important lessons regarding potential derangements in PRL signaling that can mediate pathologic changes. Other experimental models in which the normal pathways of PRL signaling are subverted will provide additional important insights.
The Physiology and Pathophysiology of Prolactin Actions in Mammals
PRL is essential for lactation in all mammals, although the precise temporal dimensions of its actions vary among species. The first step in mammary gland organogenesis is the prenatal establishment of the mammary ductal rudiment. Parathyroid hormone-related peptide (PTHrP) is essential at this first stage of mammary gland development, but PRL is not.83,85,113 The epithelial rudiment and the fat pad grow isometrically until puberty, at which time the epithelial ductal system expands rapidly under the influence of estrogen, GH, and IGF-1.114,115 During the latter stages of puberty, lobular buds branch off from the ductal system under the influence of PRL and progesterone. As a consequence of the regular cycles of estrous or menstrual hormone surges, the complexity of mammary ductal branching increases progressively, and the epithelial cells undergo cyclic changes. If the female becomes pregnant before lobule budding and maturation are complete, these processes occur during the first pregnancy. As a general rule, progesterone induces ductal arborization, whereas PRL induces the formation of alveolar progenitors. However, the relative roles of progesterone and PRL in the adolescent development of the mammary glands have not been completely resolved at the organ level, and the genes that are induced by each of these hormones during development are not completely known.
During pregnancy, the lobuloalveolar epithelium undergoes extensive proliferation under the influence of PRL, PLs, progesterone, and local growth factors such as RANK-ligand and IGF-2116–118 (see Fig. 2-6). During and after parturition, progesterone, estrogen, and PLs fall precipitously, and PRL rises. This combination of hormone changes leads to functional lactogenesis and lactation. The lobuloalveolar epithelium is converted to a secretory phenotype, and the full complement of milk proteins and lactogenic enzymes is synthesized. At the end of lactation, involution of the lobuloalveolar system occurs in response to milk stasis and falling systemic lactogens.119 According to this scheme of development, PRL and PLs, each of which binds to the PRL-R, act during three stages of mammary gland development: lobule budding during organogenesis, lobuloalveolar expansion during pregnancy, and lactational differentiation after parturition.
Pioneering studies using surgical ablation of endocrine glands and hormone replacement established specific roles for estrogen and GH in ductal development, and for PRL, progesterone, and corticosteroids in lobuloalveolar development and lactogenesis.120 Transgenic and gene disruption techniques have added to our knowledge of hormone actions in mammary gland development in vivo. In laboratory mice, complete PRL deficiency results in the arrest of mammary organogenesis at an immature pubertal state. In this arrested developmental state, the epithelial component of the gland consists of a basic ductal system and terminal end buds, but none of the lobuloalveolar system.
PRL induces the differentiation and growth of alveolar progenitor cells from the ductal epithelium.121,122 This development of alveoli from precursor cells in the ductal epithelium may involve both clonal growth from committed precursors and induction of phenotypic changes in cells that are near specialized “organizer” cells. PRL is also an essential survival factor for lobuloalveolar cells during both pregnancy and lactation.123–126 During lactation, PRL regulates several secreted milk proteins, including the caseins, lactoglobulin (except in rodents), lactalbumin, and whey acidic protein. Enzymes such as lactose synthetase, lipoprotein lipase, and fatty acid synthase, which are essential for milk synthesis, are induced by PRL in the mammary gland.
Female Reproductive Tissues
PRL inhibits reproductive function by decreasing the hypothalamic drive for pulsatile luteinizing hormone (LH) secretion,127,128 inhibiting ovarian folliculogenesis,129 and inhibiting granulosa cell aromatase activity, which leads to lower estradiol synthesis.130,131 Elevated DA levels in the hypothalamus, secondary to high PRL levels, are one mechanism for the antigonadal effects of PRL. PRL contributes to the breakdown of the corpus luteum in many mammalian species, including humans. In rodents, however, PRL is essential to corpus luteum maintenance in early pregnancy. One of the well-characterized mechanisms of the luteotropic action of PRL is inhibition of 20α-hydroxysteroid dehydrogenase activity.132 This action prevents the conversion of progesterone to 20α-hydroxyprogesterone and therefore increases progesterone secretion from the corpus luteum.
The maintenance of early pregnancy in rodents depends on the establishment of a stereotypic pattern of twice-daily surges of PRL, which are established after coital stimulation of the cervix. In laboratory rodents, the luteal phase of the estrous cycle is transient, and implantation cannot occur unless the corpus luteum is maintained by high levels of PRL. The cervical stimulus drives a hypothalamic reflex, which alters the secretion of a variety of regulatory factors, including DA, opioids, and various putative PRFs. Although it is clear that diurnal and nocturnal PRL surges in early pregnancy are controlled by different sets of factors,133 neither the exact circuitry nor the essential hypophysiotropic factors that are responsible for each of the surges are yet known.
Male Reproductive Tissues
High levels of PRL are inhibitory to male reproductive function, much the same as they are to female function. Common presenting symptoms of human hyperprolactinemia in males are loss of libido and impotence. These symptoms may or may not be associated with galactorrhea. PRL inhibits GnRH and LH secretion in males as well as in females.134 PRL increases LH and follicle-stimulating hormone receptors in the testis, as well as androgen receptors in the prostate.135 The antigonadal actions of PRL are the most widely conserved PRL actions among mammals and nonmammalian vertebrates.
Male mice with a targeted disruption of either the PRL gene itself or the PRL-R gene are completely fertile.84,85 Consistent with this, no reports in the literature describe human males with isolated PRL deficiency. The prostate gland of PRL-deficient mice is smaller (by about 30%) than that of normal mice, and high levels of PRL cause prostate hyperplasia in mice.136 PRL secretion therefore may be a contributing factor in human prostate disease, although no data specifically addressing this possibility are yet available.
Ion Balance and Calcium Metabolism
PRL is an essential freshwater survival hormone in many species of fish and amphibians, and it has effects on all of the osmoregulatory epithelia in these species. Its actions include decreasing water permeability in the gills and skin and increasing salt reabsorption in the kidney and urinary bladder (which is evolutionarily homologous with the collecting ducts of the mammalian kidney).3 Similar actions have not been proved in mammals, which is not surprising, since the osmoregulatory challenges facing terrestrial mammals are not at all similar to those confronted by freshwater fishes. PRL does increase the absorption of a variety of minerals in the intestine of mammals,137 and this effect may be physiologically important during pregnancy and breastfeeding, which place large demands on water and solute homeostasis.
PRL may have important physiologic actions on calcium metabolism in mammals, and these actions directly relate to changes in calcium balance during pregnancy and lactation. In the mammary gland, PRL induces the secretion of PTHrP, which can act as a local or systemic effector of calcium homeostasis.138
Hyperprolactinemia in humans has been associated with decreased bone density, which is normalized when elevated PRL levels are corrected medically. Decreased estrogen, due to the antigonadal effects of PRL, may explain part of the bone loss in hyperprolactinemia, but there appears to be a component of bone loss that is due to direct actions of PRL independent of estrogen loss.139,140 Recent genetic evidence has shown that the PRL-R is essential to normal bone formation and calcium homeostasis.140 PRL-R–deficient mice displayed reductions in bone mineral density and bone mineral content, as well as a deceleration in the apposition rate for new bone. Plasma total calcium and parathyroid hormone (PTH) were each higher in the receptor-deficient mice. The phenotypic characteristics of bone growth and calcium homeostasis in PRL-R–deficient mice argue that there must be multiple sites of PRL action that influence calcium metabolism, including both direct effects on bone cells and systemic actions on other hormones or carriers.141 PRL-R mRNA levels are very high in bone during development,142 and PLs, as well as PRL per se, could contribute to prenatal control of bone growth.
Brain and Behavior
The vertebrate brain is a target tissue for numerous PRL actions, many of which are related directly to the parental care of offspring. The first evidence that PRL is a brain-regulating hormone was seen in birds, where systemic or intracranial PRL infusion stimulates behaviors associated with brooding and migration.3,73 In rats, PRL infusions increase the intensity of parental attendance to offspring or shorten the time required for inexperienced adults to begin showing parental behaviors.143 Mice that lack the PRL-R are profoundly deficient in maternal behaviors.144 The neuroanatomic and neurochemical substrates that mediate the PRL-regulated parental behaviors in mammals are not yet known. However, sensory stimuli are clearly important cues for these behaviors and elevated PRL, such as that seen during pregnancy, stimulates neurogenesis in the olfactory lobe of mice.145
Whereas stereotypic maternal behavior patterns in animals such as birds, mice, and rats have been quantified and studied objectively, it has not been possible to characterize such behaviors in humans in a way that would allow one to determine whether PRL has a similar role in human parenting. Human PRL increases DA turnover in the nucleus accumbens, corpus striatum, and median eminence, but it decreases DA turnover in the substantia nigra, ventral tegmentum, and cingulate nucleus. It has been proposed that human hyperprolactinemia can be one component of an organic response to psychologic trauma (particularly deprivation from parental attention), and that the behavior patterns associated with high PRL levels (a “maternal subroutine”) may be an adaptive psychologic response.146
Behavioral actions of PRL that are not directly related to parenting, but may be indirectly supportive, include stimulation of appetite (orexia) and analgesia and increases in REM sleep activity.52,53,147–149 The analgesia caused by PRL is blocked by naloxone, indicating that the effect occurs through an opioid pathway.
Hematopoiesis and Immunoregulation
In humans, PRL secretion is correlated with disease severity in systemic lupus erythematosus, an autoimmune disease that affects primarily women of child-bearing age.150 In a rat model of immunosuppression following acute hemorrhagic shock, PRL stimulates immune effector cell functions as well as normal cytokine secretion.151 Whereas PRL can act as a positive stimulus for immune cells when given to animals by injection or to cells in culture, PRL deficiency does not significantly impair immune function or hematopoiesis.84 Elevated PRL can block lymphocyte apoptosis, and PRL secretion during stress, pregnancy, and lactation may be sufficient to affect immune cells.152 It is also conceivable that the higher level of PRL secretion in females compared with males is one factor that contributes to a sexual difference in immune responses.
Metabolism
PRL-Rs are present in the liver, gut, pancreas, and adipose tissue.5 PRL causes splanchnomegaly (gut growth) and accelerates liver regrowth after partial hepatectomy.153,154 Bile acid secretion and taurocholate transport in liver are elevated by PRL during lactation.155 PRL and placental lactogens stimulate growth of pancreatic β cells during pregnancy and lactation. Beta cell proliferation is both a direct response to PLs and PRL156 and an adaptive response to gestational insulin resistance. In general, the actions of PRL on organs that control whole-body metabolism are consistent with the metabolic alterations that support successful gestation and lactation. Many hormones in addition to PRL contribute to these metabolic adjustments.
Summary
PRL, along with PLs in many species, plays a central role in ensuring successful reproduction by acting after fertilization to promote a variety of developmental, metabolic, and behavioral adaptations (Fig. 2-7). Hypothalamic DA inhibits PRL secretion and is the dominant PRL regulator in mammals but not in other species. The specialization of the mammalian life cycle to include not only maternal gestation but also postpartum nurturing of offspring has been accompanied by a wide range of physiologic adaptations. Breast milk secretion in mammals, as well as milk-like secretions that occur in certain nonmammalian vertebrates, are direct responses to PRL. PRL suppression of gonadal development and sexual drive in males and females is mediated both centrally and peripherally. The physiologic actions of PRL are pathologically exaggerated in human hyperprolactinemia.
References
1. Riddle, O, Bates, RW, Dykshorn, SW. The preparation, identification and assay of prolactin: A hormone of the anterior pituitary. Am J Physiol. 1933;105:191.
2. Stricker, P, Grueter, F. Action du lobe antérior de l’hypophyse sur la montée laiteuse. C R Seances Soc Biol Fil. 1928;99:1978.
3. Horseman, ND. Models of prolactin action in nonmammalian vertebrates. In: Rillema JA, ed. Actions of Prolactin on Molecular Processes. Boca Raton, FL: CRC Press; 1987:41.
4. Bern, HA, Nicoll, CS. The comparative endocrinology of prolactin. Recent Prog Horm Res. 1968;24:681.
5. Bole-Feysot, C, Goffin, V, Edery, M, et al. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225.
6. Nicoll, CS. Physiological actions of prolactin. In: Knobil E, Sawyer WH, eds. Handbook of Physiology. Washington, DC: American Physiology Society; 1974:253.
7. Li, CH, The chemistry of prolactin. Hormonal Proteins and Peptides. Li, CH, eds. Hormonal Proteins and Peptides, vol 8. New York: Academic Press, 1980:2.
8. Cooke, NE, Liebhaber, SA. Molecular biology of the growth hormone-prolactin gene system. Vitam Horm. 1995;50:385.
9. Soares, MJ, Faria, TN, Roby, KF, et al. Pregnancy and the prolactin family of hormones: Coordination of anterior pituitary, uterine, and placental expression. Endocr Rev. 1991;12:402.
10. Soares, MJ, Muller, H, Orwig, KE, et al. Uteroplacental prolactin family and pregnancy. Biol Reprod. 1998;58:273.
11. Talamantes, F, Ogren, L, Markoff, E, et al. Phylogenetic distribution, regulation of secretion, and prolactin-like effects of placental lactogens. Fed Proc. 1980;39:2582.
12. Handwerger, S. Clinical counterpoint: The physiology of placental lactogen in human pregnancy. Endocr Rev. 1991;12:329.
13. Jackson, D, Volpert, OV, Bouck, N, et al. Stimulation and inhibition of angiogenesis by placental proliferin and proliferin-related protein. Science. 1994;266:1581.
14. Kessler, MA, Schuler, LA. Purification and properties of placental prolactin-related protein-I. Placenta. 1996;18:29.
15. Lee, SJ, Nathans, D. Proliferin secreted by cultured cells binds to mannose 6-phosphate receptors. J Biol Chem. 1988;263:3521.
16. Ain, R, Dia, G, Dunmore, JH, et al. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci USA. 2004;101:16543.
17. Ho-Chen, JK, Bustamante, JJ, Soares, MJ. Prolactin-like protein-F subfamilty of placental hormones/cytokines: Responsiveness to maternal hypoxia. Endocrinology. 2007;148:559.
18. Ben-Jonathan, N, Mershon, JL, Allen, DL, et al. Extrapituitary prolactin: Distribution, regulation, functions, and clinical aspects. Endocr Rev. 1997;17:639.
19. Gellerson, B, Dimattia, GE, Friesen, HG, et al. Prolactin (PRL) mRNA from human decidua differs from pituitary PRL mRNA but resembles the IM-9-P3 lymphoblast PRL transcript. Mol Cell Endocrinol. 1989;64:127.
20. Kacsóh, B, Veress, Z, Tóth, BE, et al. Bioactive and immunoreactive variants of prolactin in milk and serum of lactating rats and their pups. J Endocrinol. 1993;138:243.
21. Clevenger, CV, Furth, PA, Hankinson, SE, et al. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1.
22. Bao, L, Tessier, C, Prigent-Tessier, A, et al. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology. 2007;148:2326.
23. Garzia, E, Borgato, S, Cozzi, V, et al. Lack of expression of endometrial prolactin in early implantation failure: A pilot study. Hum Reprod. 2004;19:1911.
24. Miller, WL, Baxter, JD, Eberhardt, NL, Peptide hormone genes: Structure and evolution. Brain Peptides. Krieger, DT, Brownstein, MJ, Martin, JB, eds. Brain Peptides, vol 21. New York: John Wiley, 1983:16.
25. Horseman, ND, Yu-Lee, L-Y. Transcriptional regulation by the helix bundle peptide hormones: GH, PRL, and hematopoietic cytokines. Endocr Rev. 1994;15:627.
26. Sinha, YN. Structural variants of prolactin: Occurrence and physiological significance. Endocr Rev. 1995;16:354.
27. Lee, H, Struman, I, Clapp, C, et al. Inhibition of urokinase activity by the antiangiogenic factor 16K prolactin: Activation of plasminogen activator inhibitor 1 expression. Endocrinology. 1998;139:3696.
28. Hilfiker-Kleiner, D, Kaminski, K, Podewski, E, et al. A Cathepsin D-cleaved 16 kDa form of prolactis mediates postpartum cardiomyopathy. Cell. 2007;128:589.
29. Hilfiker-Kleiner, D, Limbourg, A, Drexler, H. STAT3-mediated activation of myocardial capillary growth. Trends Cardiovasc Med. 2005;15:152.
30. Jahns, B, Stein, W, Hilfiker-Kleiner, D, et al. Peripartum cardiomyopathy—a new treatment option by inhibition of prolactin secretion. Am J Obstet Gynecol. 2008;199:e5.
31. Hilfiker-Kleiner, D, Meyer, GP, Schieffer, E, et al. Recovery from postpartum cardiomyopathy in two patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol. 2007;50:2354.
32. Wang, Y-F, Walker, AM. Dephosphorylation of standard prolactin produces a more biologically active molecule: Evidence for antagonism between nonphosphorylated and phosphorylated prolactin in the stimulation of Nb2 cell proliferation. Endocrinology. 1993;133:2156.
33. Kenyon, C. If birds can fly, why can’t we? Homeotic genes and evolution. Cell. 1994;78:175.
34. Frawley, SL. Mammosomatotropes: Current status and possible functions. Trends Endocrinol Metab. 1989;1:31.
35. Asa, SL, Ezzat, S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr Rev. 1998;19:798.
36. Vankelecom, H. Stem cells in the postnatal pituitary? Neuroendocrinology. 2007;85:110.
37. Theill, LE, Castrillo, J-L, Wu, D, et al. Dissection of functional domains of the pituitary-specific transcription factor GHF-1. Nature. 1989;342:945.
38. He, X, Treacy, MN, Simmons, DM, et al. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989;340:35.
39. Ingraham, HA, Chen, R, Mangalam, HJ, et al. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988;55:519.
40. Simmons, DM, Voss, JW, Ingraham, HA, et al. Pituitary cell phenotypes involve cell-specific Pit-1 mRNA translation and synergistic interactions with other classes of transcription factors. Genes Dev. 1990;4:695.
41. Morris, AE, Kloss, B, McChesney, RE, et al. An alternatively spliced Pit-1 isoform altered in its ability to trans-activate. Nucleic Acids Res. 1992;20:1355.
42. Seyfred, MA, Kladde, MP, Gorski, J. Transcriptional regulation by estrogen of episomal prolactin gene regulatory elements. Mol Endocrinol. 1989;3:305.
43. Cai, A, Bowers, RC, Moore, JPJ, et al. Function of galanin in the anterior pituitary of estrogen-treated Fischer 344 rats: Autocrine and paracrine regulation of prolactin secretion. Endocrinology. 1998;139:2452.
44. Wynick, D, Small, CJ, Bacon, A, et al. Galanin regulates prolactin release and lactotroph proliferation. Proc Natl Acad Sci U S A. 1998;95:12671.
45. Shen, ES, Hardenburg, JL, Meade, EH, et al. Estradiol induces galanin gene expression in the pituitary of the mouse in an estrogen receptor alpha-dependent manner. Endocrinology. 1999;140:2628.
46. Schweppe, RE, Frazer-Abel, AA, Gutierrez-Hartmann, A, et al. Functional components of fibroblast growth factor (FGF) signal transduction in pituitary cells: Identification of FGF response elements in the prolaction gene. J Biol Chem. 1997;272:30852.
47. Zhang, K, Kulig, E, Jin, L, et al. Effects of estrogen and epidermal growth factor on prolactin and Pit-1 mRNA in GH3 cells. Proc Soc Exp Biol Med. 1993;202:193.
48. Ben-Jonathan, N. Regulation of prolactin secretion. In: Imura H, ed. The Pituitary Gland. 2d ed. New York: Raven Press; 1994:261.
49. Lea, RW, Vowles, DM. Vasoactive intestinal polypeptide stimulates prolactin release in vivo in the ring dove (Streptopelia risoria). Experientia. 1986;42:420.
50. Lea, RW, Talbot, RT, Sharp, PJ. Passive immunization against chicken vasoactive intestinal polypeptide suppresses plasma prolactin and crop sac development in incubating ring doves. Horm Behav. 1991;25:283.
51. Sassin, JF, Frantz, AG, Kapen, S, et al. The nocturnal rise of human prolactin is dependent upon sleep. J Clin Endocrinol Metab. 1973;37:436.
52. Obál, F, Jr., Payne, L, Kacsoh, B, et al. Involvement of prolactin in the REM sleep-promoting activity of systemic vasoactive intestinal peptide (VIP). Brain Res. 1994;645:143.
53. Roky, R, Obal, F, Valatx, J-L, et al. Prolactin and rapid eye movement sleep regulation. Sleep. 1995;18:536.
54. Kelly, M, Rubinstein, M, Asa, S, et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103.
55. Pi, X, Grattan, DR. Expression of prolactin receptor mRNA is increased in the preoptic area of lactating rats. Endocrine. 1999;1:91.
56. Login, IS, MacLeod, RM. Prolactin in human and rat serum and cerebrospinal fluid. Brain Res. 1977;132(3):477.
57. Steger, RW, Chandrashekar, V, Zhao, W, et al. Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology. 1998;139:3691.
58. Gregerson, K, Flagg, T, Anderson, M, et al. Identification of the G-protein-coupled, inward rectifying potassium channel gene products in rat anterior pituitary gland. Endocrinology. 2001;142:2820.
59. Koch, BD, Blalock, JB, Schonbrunn, A. Characterization of the cyclic AMP-independent actions of somatostatin in GH cells: I. An increase in potassium conductance is responsible for both the hyperpolarization and the decrease in intracellular free calcium produced by somatostatin. J Biol Chem. 1988;263:216.
60. Shah, GV, Pedchenko, V, Stanley, S, et al. Calcitonin is a physiological inhibitor of prolactin secretion in ovariectomized female rats. Endocrinology. 1996;137:1814.
61. Kanyicska, B, Lerant, A, Freeman, ME. Endothelin is an autocrine regulator of prolactin secretion. Endocrinology. 1998;139:5164.
62. Sarkar, DK, Kim, KH, Minami, S. Transforming growth factor β-1 messenger RNA and protein expression in the pituitary gland: Its action on prolactin secretion and lactotropic growth. Mol Endocrinol. 1992;6:1825.
63. Seyfred, MA, Gorski, J. An interaction between the 5′ flanking distal and proximal regulatory domains of the rat prolactin gene is required for transcriptional activation by estrogens. Mol Endocrinol. 1990;4:1226.
64. Yan, G-Z, Pan, WT, Bancroft, C. Thyrotropin-releasing hormone action on the prolactin promoter is mediated by the POU protein Pit-1. Mol Endocrinol. 1991;5:535.
65. Johnston, CA, Negro-Vilar, A. Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology. 1988;122:341.
66. Bredow, S, Kacsóh, B, Obál, F, Jr., et al. Increase of prolactin mRNA in the rat hypothalamus after intracerebroventricular injection of VIP or PACAP. Brain Res. 1994;660:301.
67. López, FJ, Merchenthaler, I, Ching, M, et al. Galanin: A hypothalamic-hypophysiotropic hormone modulating reproductive functions. Proc Natl Acad Sci U S A. 1991;88:4508.
68. Pickett, CA, Gutierrez-Hartmann, A. Ras mediates Src but not epidermal growth factor-receptor tyrosine kinase signaling pathways in GH4 neuroendocrine cells. Proc Natl Acad Sci U S A. 1994;91:8612.
69. Porter, TE, Wiles, CD, Frawley, LS. Stimulation of lactotrope differentiation in vitro by fibroblast growth factor. Endocrinology. 1994;134:164.
70. Aguilera, G, Hyde, CL, Catt, KJ. Angiotensin II receptors and prolactin release in pituitary lactotrophs. Endocrinology. 1982;111:1045.
71. Koves, K, Molnar, J, Kantor, O, et al. New aspects of the neuroendocrine role of PACAP. Ann N Y Acad Sci. 1996;805:648.
72. El Halawani, ME, Burke, WH, Millam, JR, et al. Regulation of prolactin and its role in gallinaceous bird reproduction. J Exp Zool. 1984;232:521.
73. Horseman, ND, Buntin, JD. Regulation of pigeon crop milk secretions and parental behaviors by prolactin. Annu Rev Nutr. 1995;15:213.
74. Hinuma, S, Habata, Y, Fujii, R, et al. Prolactin-releasing peptide in the brain. Nature. 1998;393:272.
75. Samson, WK, Keown, C, Samson, CK, et al. Prolactin-releasing peptide and its homology RFRP-1 act in hypothalamus but not in anterior pituitary gland to stimulate stress hormone secretion. Endocrine. 2003;20:59.
76. Castano, JP, Kineman, RD, Frawley, LS. Dynamic fluctuations in the secretory activity of individual lactotropes as demonstrated by a modified sequential plaque assay. Endocrinology. 1994;135:1747.
77. Nicoll, CS. Bioassay of prolactin. Analysis of the pigeon crop-sac response to local protein injection by objective and quantitative methods. Endocrinology. 1967;80:641.
78. Gout, PW, Beer, CT, Noble, RL. Prolactin-stimulated growth of cell cultures established from malignant Nb rat lymphomas. Cancer Res. 1980;40:2433.
79. Kauppila, A, Chatelain, P, Kirkinen, P, et al. Isolated prolactin deficiency in a woman with puerperal alactogenesis. J Clin Endocrinol Metab. 1987;64:309.
80. Falk, RJ. Isolated prolactin deficiency: A case report. Fertil Steril. 1992;58:1060.
81. Douchi, T, Nakae, M, Yamamoto, S, et al. A woman with isolated prolactin deficiency. Acta Obstet Gynecol Scand. 2001;80:368.
82. Zargar, AH, Masoodi, SR, Laway, BA, et al. Familial puerperal alactogenesis: Possibility of a genetically transmitted isolated prolactin deficiency. Br J Obstet Gynaecol. 1997;104:629.
83. Vomachka, AJ, Pratt, SL, Lockefeer, JA, et al. Prolactin gene-disruption arrests mammary gland development and retards T-antigen-induced tumor growth. Oncogene. 2000;19:1077.
84. Horseman, ND, Zhao, W, Montecino-Rodriguez, E, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926.
85. Ormandy, C, Camus, A, Barra, J, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167.
86. Cruz-Soto, ME, Scheiber, MD, Gregerson, KA, et al. Pituitary tumorigenesis in prolactin gene-disrupted mice. Endocrinology. 2002;143:4429.
87. Schuff, KG, Hentges, ST, Kelly, MA, et al. Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and independent mechanisms. J Clin Invest. 2002;110:973.
88. Radovick, S, Nations, M, Du, Y, et al. A mutation in the POU-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science. 1992;257:1115.
89. Voss, JW, Rosenfeld, MG. Anterior pituitary development: Short tales from dwarf mice. Cell. 1992;70:527.
90. Cosman, D, Lyman, SD, Idzerda, RL, et al. A new cytokine receptor superfamily. Trends Biochem Sci. 1990;15:265.
91. Somers, W, Ultsh, M, De Vos, AM, et al. The x-ray structure of a growth hormone-prolactin receptor complex. Nature. 1994;372:478.
92. Chen, X, Horseman, ND. Cloning, expression, and mutational analysis of the pigeon prolactin receptor. Endocrinology. 1994;135:269.
93. Schuler, LA, Nagel, RJ, Gao, J, et al. Prolactin receptor heterogeneity in bovine fetal and maternal tissues. Endocrinology. 1997;138:3187.
94. de Vos, AM, Ultsch, M, Kossiakoff, AA. Human growth hormone and extracellular domain of its receptor: Crystal structure of the complex. Science. 1992;255:306.
95. Gertler, A, Grosclaude, J, Djiane, J. Interaction of lactogenic hormones with prolactin receptors. Ann N Y Acad Sci. 1998;839:177.
96. Chang, W-P, Clevenger, CV. Modulation of growth factor receptor function by isoform heterodimerization. Proc Natl Acad Sci U S A. 1996;93:5947.
97. Bogorad, RL, Courtillot, C, Mestayer, C, et al. Identification of a gain-of-function mutation of the prolactin receptor in women with benign breast tumors. Proc Natl Acad Sci USA. 2008;105:14533.
98. Campbell, GS, Argentsinger, LS, Ihle, JN, et al. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci U S A. 1994;91:5232.
99. Gao, J, Hughes, JP, Auperin, B, et al. Interaction among JANUS kinases and the prolactin (PRL) receptor in the regulation of a PRL response element. Mol Endocrinol. 1995;10:847.
100. Parganas, E, Wang, D, Stravopodis, D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385.
101. Berchtold, S, Volarevic, S, Moriggl, R, et al. Dominant negative variants of the SHP-2 tyrosine phosphatase inhibit prolactin activation of Jak2 (janus kinase 2) and induction of Stat5 (signal transducer and activator of transcription 5)-dependent transcription. Mol Endocrinol. 1998;12:556.
102. Das, R, Vonderhaar, BK. Prolactin as a mitogen in mammary cells. J Mammary Gland Biol Neoplasia. 1997;2:29.
103. Darnell, JE, Jr., Kerr, IM, Stark, GR. Jak-Stat pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415.
104. Sidis, Y, Horseman, ND. Prolactin induces rapid p95/p70 tyrosine phosphorylation, and protein binding to GAS-like sites in the anx Icp35 and c-fos genes. Endocrinology. 1994;134:1979.
105. Wakao, H, Gouilleux, F, Groner, B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994;13:2182.
106. Liu, X, Robinson, GW, Wagner, K-U, et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179.
107. Udy, GB, Towers, RP, Snell, RG, et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A. 1997;94:7239.
108. Teglund, S, McKay, C, Schuetz, E, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841.
109. Gouilleux, F, Wakao, H, Mundt, M, et al. Prolactin induces phosphorylation of tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994;13:4361.
110. Stocklin, E, Wissler, M, Gouilleux, et al. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726.
111. Helman, D, Sandowski, Y, Cohen, Y, et al. Cytokine-inducible SH2 protein (CIS3) and Jak2 binding protein (JAB) abolish prolactin receptor-mediated Stat5 signaling. FEBS Lett. 1998;441:287.
112. Luo, G, Yu-Lee, L-Y. Transcriptional inhibition by Stat5. J Biol Chem. 1997;272:26841.
113. Wysolmerski, JJ, Stewart, AF. The physiology of parathyroid hormone-related protein: An emerging role as a developmental factor. Annu Rev Physiol. 1998;60:431.
114. Topper, YJ, Freeman, CS. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980;60:1049.
115. Kleinberg, DL. Early mammary development: Growth hormone and IGF-1. J Mammary Gland Biol Neoplasia. 1997;2:49.
116. Srivastava, S, Matsuda, M, Hou, Z, et al. Receptor activator of NF-kappaB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J Biol Chem. 2003;278:46171.
117. Hovey, RC, Harris, J, Hadsell, DL, et al. Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol Endocrinol. 2003;17:460.
118. Brisken, C, Ayyannan, A, Nguyen, C, et al. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell. 2002;3:877.
119. Schmitt-Ney, M, Happ, B, Hofer, P, et al. Mammary gland-specific nuclear factor activity is positively regulated by lactogenic hormones and negatively by milk stasis. Mol Endocrinol. 1992;6:1988.
120. Lyons, W, Li, CH, Johnson, RE. Hormonal control of mammary growth and lactation. Recent Prog Horm Res. 1958;14:219.
121. Chepko, G, Smith, GH. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 1997;29:239.
122. Smith, GH. Experimental mammary epithelial morphogenesis in an in vivo model: Evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21.
123. Travers, MT, Barber, MC, Tonner, E, et al. The role of prolactin and growth hormone in the regulation of casein gene expression and mammary cell survival: Relationships to milk synthesis and secretion. Endocrinology. 1996;137:1530.
124. Humphreys, RC, Hennighausen, L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ. 1999;10:685.
125. Capuco, AV, Li, M, Long, E, et al. Concurrent pregnancy retads mammary involution: Effects on apoptosis and proliferation of the mammary epithelium after forced weaning of mice. Biol Reprod. 2002;66:1471.
126. Bailey, JP, Nieport, K, Herbst, MP, et al. Prolactin and transforming growth factor-beta signaling exert opposing effects on mammary gland morphogenesis, involution, and the Akt-forkhead pathway. Mol Endocrinol. 2004;12:1171.
127. Sarkar, D, Yen, S. Hyperprolactinemia decreases the luteinizing hormone releasing hormone concentration in pituitary portal plasma: A possible role for β-endorphin as a mediator. Endocrinology. 1985;116:2080.
128. Cohen-Becker, I, Selmanoff, M, Wise, P. Hyperprolactinemia alters the frequency and ampitude of pulsatile lutenizing hormone secretion in the ovariectomized rat. Neuroendocrinology. 1986;42:328.
129. Larsen, J, Bhanu, A, Odell, W. Prolactin inhibition of pregnant mare’s serum stimulated follicle development in the rat ovary. Endocr Res. 1990;16:449.
130. Tsai-Morris, C, Ghosh, M, Hirshfield, A, et al. Inhibition of ovarian aromatase by prolactin in vivo. Biol Reprod. 1983;29:342.
131. Krasnow, J, Hickey, G, Richards, J. Regulation of aromatase mRNA and estradiol biosynthesis in rat ovarian granulosa and luteal cells by prolactin. Mol Endocrinol. 1990;4:13.
132. Albarracin, CT, Parmer, TG, Duan, WR, et al. Identification of a major prolactin-regulated protein as 20α-hydroxysteroid dehydrogenase: Coordinate regulation of its activity, protein content, and messenger ribonucleic acid expression. Endocrinology. 1994;134:2453.
133. Freeman, ME, Smith, MS, Nazian, SJ, et al. Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy. Endocrinology. 1974;94:875.
134. Voogt, JL, de Greef, WJ, Visser, TJ, et al. In vivo release of dopamine, luteinizing hormone-releasing hormone and thyrotropin-releasing hormone in male rats bearing a prolactin-secreting tumor. Neuroendocrinol. 1987;46:110.
135. Bex, FJ, Bartke, A. Testicular LH binding in the hamster: Modification by photoperiod and prolactin. Endocrinology. 1977;100:1223.
136. Wennbo, H, Kindblom, J, Isaksson, OG, et al. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology. 1997;138:4410.
137. Mainoya, JR, Bern, HA, Regan, JW. Influence of ovine prolactin on transport of fluid and sodium chloride by the mammalian intestine and gall bladder. J Endocrinol. 1974;63:311.
138. Ferrari, SL, Rizzoli, R, Bonjour, JP. Parathyroid hormone-related protein production by primary cultures of mammary epithelial cells. J Cell Physiol. 1992;150:304.
139. Klibanski, A, Neer, RM, Beitins, IZ, et al. Decreased bone density in hyperprolactinemic women. N Engl J Med. 1980;303:1511.
140. Klibanski, A, Greenspan, SL. Increase in bone mass after treatment of hyperprolactinemic amenorrhea. N Engl J Med. 1986;315:542.
141. Clément-Lacroix, P, Ormandy, C, Lepescheux, L, et al. Osteoblasts are a new target for prolactin: Analysis of bone formation in prolactin receptor knockout mice. Endocrinology. 1999;140:96.
142. Freemark, M, Nagano, M, Edery, M, et al. Prolactin receptor gene expression in the fetal rat. J Endocrinol. 1995;144:285.
143. Bridges, RS. The role of lactogenic hormones in maternal behavior in female rats. Acta Paediatr Suppl. 1994;397:33.
144. Lucas, BK, Ormandy, CJ, Binart, N, et al. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102.
145. Shingo, T, Gregg, C, Enwere, E, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117.
146. Sobrinho, LG. The psychogenic effects of prolactin. Acta Endocrinol. 1993;129:38.
147. Buntin, JD. Time course and response specificity of prolactin-induced hyperphagia in ring doves. Physiol Behav. 1989;45:903.
148. Sauve, D, Woodside, B. The effect of central administration of prolactin on food intake in virgin female rats is dose-dependent, occurs in the absence of ovarian hormones and the latency to onset varies with feeding regimen. Brain Res. 1996;729:75.
149. Ramaswamy, S, Pillai, NP, Bapna, JS. Analgesic effect of prolactin: Possible mechanism of action. Eur J Pharmacol. 1983;96:171.
150. Walker, SE, Allen, SH, McMurray, RW. Prolactin and autoimmune disease. Trends Endocrinol Metab. 1993;4:147.
151. Zellweger, R, Zhu, X-H, Wichmann, MW, et al. Prolactin administration following hemorrhagic shock improves macrophage cytokine release capacity and decreases mortality from subsequent sepsis. J Immunol. 1996;157:5748.
152. Krishnan, N, Thellin, O, Buckley, DJ, et al. Prolactin suppresses glucocorticoid-induced apoptosis in vivo. Endocrinology. 2003;144:2102.
153. Bates, RW, Riddle, O, Lahr, EL, et al. Aspects of splanchnomegaly associated with the action of prolactin. Am J Physiol. 1937;119:603.
154. Buckley, AR, Crowe, PD, Russell, DR. Rapid activation of protein kinase C in isolated rat liver nuclei by prolactin, a known hepatic mitogen. Proc Natl Acad Sci U S A. 1988;85:8649.
155. Liu, Y, Hyde, JF, Vore, M. Prolactin regulates maternal bile secretory function post partum. J Pharmacol Exp Ther. 1992;261:560.
156. Brelje, TC, Sorenson, RL. Role of prolactin versus growth hormone on islet B-cell proliferation in vitro: Implications for pregnancy. Endocrinology. 1991;128:45.