Chapter 108 Prognostic Factors, Surgical Outcomes, and Guidelines for Managing Metastatic Spine Cancer
The spine is the most common site for skeletal metastasis.1 Approximately 5% to 10% of all cancer patients develop spine metastasis.2,3 Although Jaffee1 reported in 1958 a 70% incidence of vertebral metastasis, others asserted that spinal involvement ranges from 33.4% to 56%.4–6 Spinal cord compression occurs in 10% to 25% of treated patients with preexisting spinal metastasis.2,6–12 It is well established that if metastatic vertebral disease is left untreated, it may lead to paraplegia,13–16 which is the most dreadful complication of metastatic vertebral disease. In this situation, surgery plays an important role. Several surgical options exist; however, their indications and outcomes must be scrutinized for better medical care. The discovery of vertebral metastases intensifies an already complex situation requiring highly technical management, sustained coherence of the entire care-provider team, and their awareness of the patient’s increased need to be heard, informed, and supported.
Surgical Outcomes
The following are essential when evaluating surgical outcomes for vertebral metastases:
According to published reports, the anticipated improvement after surgery is expected to range from 55% to 87% of cases as a result of overall upgrade of neurologic deficit (Table 108-1); 36% to 89% because of restoration of sphincter control (Table 108-2); 47% to 100% due to pain relief (Table 108-3); and 40% to 100% as a result of restoration of ambulation (Table 108-4). Apparently restoration of ambulation after dorsal decompression alone fares worse. The anticipated survival rates are depicted in Table 108-5. The complication rates range from 12% to 32% (Table 108-6). In our series of 70 patients, the complication rate was 25%, including two deaths. In one case, surgery was aborted, while in progress, because of massive intraoperative bleeding, despite preoperative transarterial embolization of the region.17 Other complications were profuse bleeding in nine patients, infections in three, wound dehiscence in three, and incomplete surgery in three patients. The 4- to 6-week mortality rates after surgery, reoperation rates for complications, and local recurrences after surgery are listed in Tables 108-7, 108-8, and 108-9, respectively. Local tumor recurrence is more likely to occur in patients with certain tumors and particularly in those for whom chemotherapy and radiation therapy are ineffective. This is particularly the case in such cancers as renal cell carcinoma, in which the reported recurrence is expected to be as high as 40% to 50%.18–21 The overall patient satisfaction was rated in the range of 55% to 80%.18,22
TABLE 108-1 Reported Overall Neurologic Improvement after Surgery
| Study | Improvement |
|---|---|
| Kostuik J et al.68 | 55% |
| Ernstberger T et al.80 | 57% |
| Weigel B et al.18 | 58% |
| King GJ et al.19 | 60% |
| Villavicencio AT et al.81 | 60% |
| Rompe JD et al.82 | 63% |
| Atanasiu JP et al.30 | 64% |
| O’Neil J et al.32 | 68% |
| Harrington KD37 | 68% |
| Hatrick N et al.83 | 69% |
| Tomita T et al.72 | 73% |
| Hammerberg KW66 | 74% |
| Bauer HCF84 | 76% |
| Gokalsan ZL et al.46 | 76% |
| Onimus M et al.85 | 79% |
| Hosono N et al.86 | 81% |
| King et al.19 | 88% |
| Hertlein H et al.87 | 88% |
| Shimizu K et al.34 | 87% |
TABLE 108-2 Restoration of Sphincter Control after Surgery
| Study | Successful Restoration |
|---|---|
| King GJ et al.19 | 36% |
| Kocialkowski A, Webb JK88 | 43% |
| Kostuik J et al.68 | 47% |
| Sinardet D et al.89 | 51% |
| Tomita T et al.72 | 77% |
| Klekamp J, Samii H31 | 89% |
| Study | Success Rate |
|---|---|
| Sinardet D et al.89 | 47% |
| Touboul E et al.90 | 71% |
| Hirabayashi H et al.25 | 77% |
| Kocialkowski A, Webb JK88 | 79% |
| Kostuik J et al.68 | 81% |
| Sundaresan N et al.91 | 85% |
| Ernstberger T et al.80 | 85% |
| Villavicencio AT et al.81 | 85% |
| King GJ et al.19 | 88% |
| Weigel B et al.18 | 89% |
| Hatrick N et al.83 | 90% |
| Hammerberg KW66 | 91% |
| Gokalsan ZL et al.46 | 92% |
| Hertlein H et al.87 | 92% |
| Harrington KD37 | 94% |
| O’Neil J et al.32 | 94% |
| Hosono N et al.86 | 94% |
| Atanasiu JP et al.30 | 95% |
| Tomita T et al.72 | 95% |
| Shimizu K et al.34 | 100% |
| Bilsky MH et al.92 | 100% |
TABLE 108-4 Restoration of Ambulation after Ventral or Dorsal Approach
| Study | Successful Restoration |
|---|---|
| Livingston KE, Perrin RG93 | 40% |
| Kostuik J et al.68 | 44% |
| Gokalsan ZL et al.46 | 51% |
| Sinardet D et al.89 | 52% |
| Tomita T et al.72 | 60% |
| Patchel RA et al.94 | 62% |
| Hosono N et al.86 | 64% |
| King GJ et al.19 | 64% |
| Weigel B et al.18 | 70% |
| Onimus M et al.85 | 70% |
| Hammerberg KW66 | 76% |
| Hatrick NC et al.83 | 78% |
| Siegal T, Siegal T95 | 80% |
| O’Neil J et al.32 | 82% |
| Villavicencio AT et al.81 | 100% |
| Sundaresan N et al.91,96 | 100% |
| Restoration of Ambulation after Laminectomy Alone | |
| Klekamp J, Samii H31 | 23% |
| Kennady JC, Stern WE97 | 23% |
| Schoeggl A et al.98 | 33% |
| Wright RL99 | 35% |
| White WA et al.100 | 36% |
| Siegal T, Siegal T95 | 47% |
TABLE 108-5 Anticipated Survival Rates after Surgery
| Study | Survival Rate |
|---|---|
| 3 month | |
| Kocialkowski A, Webb JK88 | 50.0% |
| Wai EK et al.52 | 76.0% |
| 6 month | |
| Klekamp J, Samii H31 | 58.8% |
| Rompe JD et al.82 | 72.0% |
| Hosono N et al.86 | 78.0% |
| 9 month | |
| Falicov A et al.33 | 50.0% |
| 12 month | |
| Kocialkowski A, Webb JK88 | 25.0% |
| Klekamp J, Samii H31 | 48.8% |
| Rompe JD et al.82 | 50.0% |
| Villavicencio AT et al.81 | 65.0% |
| Gokalsan ZL et al.46 | 62.0% |
| 19 month | |
| Rompe JD et al.82 | 4.7% |
| 60 month | |
| Klekamp J, Samii H31 | 5% |
| Median month | |
| Schoeggl A et al.98 | 6.5% |
| Sioutos PJ et al.35 | 10% |
| Hirabayashi H et al.25 | 10.6% |
| Lewandrowski KU et al.101 | 14% |
| Sundaresan N et al.102 | 30% |
| Mean month | |
| Chataigner H, Onimus M20 | 8% |
| Hertlein H et al.87 | 8.5% |
| O’Neil J et al.32 | 9% |
| Livingston KE, Perrin RG93 | 9% |
| Atanasiu JP et al.30 | 11% |
| Ernstberger T et al.80 | 15.6% |
| Weigel B et al.18 | 13.1% |
| Wise JJ et al.67 | 15.9% |
| Cahill DW, Kumar R103 | 15.9% |
| Kocialkowski A, Webb JK88 | 12.0% |
TABLE 108-6 Complication Rates
| Study | Percentage Experiencing Complications |
|---|---|
| Bilsky MH et al.7 | 12% |
| Wai EK et al.52 | 12% |
| Hosono N et al.86 | 16% |
| Weigel B et al.18 | 19% |
| Jansson KA, Bauer HC104 | 20% |
| Wise JJ et al.67 | 25% |
| Klimo P Jr et al. (review of 24 surgical reports)105 | 23% |
| Falicov A et al.33 | 29% |
| Gokalsan ZL et al.46 | 32% |
TABLE 108-7 Postsurgery Mortality Rates (4 to 6 weeks)
| Study | Mortality Rate |
|---|---|
| Gokalsan ZL et al.46 | 3.0% |
| Klimo PJr et al.105 | 6.3% |
| Wise JJ et al.67 | 6.0% |
| Siegal TJ et al.106 | 6.0% |
| Weigel B et al.18 | 7.0% |
| Sundaresan N et al.96 | 8.0% |
| Harrington KD37 | 8.0% |
| Sinardet D et al.89 | 9.0% |
| Sioutos PJ et al.35 | 11.0% |
| Turner PL et al.78 | 10.0% |
| Hertlein H et al.87 | 10.0% |
| Chataigner H, Onimus M20 | 10.2% |
| Bilsky MH et al.92 | 12.0% |
| Jansson KA, Bauer HC104 | 13.0% |
| Kocialkowski A, Webb JK88 | 16.0% |
| Fidler MW107 | 18.0% |
TABLE 108-8 Reoperation Rates for Complications or Local Recurrences
| Study | Percentage Undergoing Reoperation |
|---|---|
| Weigel B et al.18 | 7.0% |
| Jansson KA, Bauer HC104 | 10.2% |
| Hertlein H et al.87 | 12.1% |
| Bauer HCF84 | 24.0% |
| Chataigner H, Onimus M20 | 15.8% |
| King GJ et al.19 | 56.2% |
TABLE 108-9 Local Recurrences after Surgery
| Study | Percentage Experiencing Recurrence |
|---|---|
| Falicov A et al.33 | 3.5% |
| Review of 9 surgical reports | 8.0% |
| Klimo P Jr et al.105 | |
| Bilsky MH et al.92 | |
| Foumey DR et al.108 | |
| Hammerberg KW66 | |
| Hosono N et al.86 | |
| King GJ et al.19 | |
| Muhlbauer M et al.109 | |
| Siegal T et al.106 | |
| Weigel B et al.18 | |
| Chataigner H, Onimus M20 | 8.4% |
| Nazarian S110 | 11.0% |
| King GJ et al.19 | 12.1% |
| Hirabayashi H et al.25 | 21.0% |
| Weigel B et al.18 | 22.0% |
| Bridwell KH et al.71 (after laminectomy) | 31.6% |
| Sundaresan N et al.102 | 32.0% |
| Hertlein H et al.87 | 49.0% |
Prognostic Factors
It has been observed that certain factors, irrespective of treatment selection, may influence the prognosis when malignant tumors develop spinal metastases. The original claim that the histopathology of the tumor had no bearing on the ultimate prognosis23 has not been duplicated by others.
Origin of Tumor
The origin of the primary tumors was postulated by several authors to influence the prognosis. Thus, metastatic vertebral tumors from unsuspected adenocarcinoma, stomach, esophagus, pancreas, and lungs, portend the worst possible prognosis, whereas carcinoid, thyroid, breast, prostate, and myeloma carry the best prognosis. Distinction should also be made between the different types of lung cancer.24 In general, non–small cell lung cancer (NSCLC) patients with spinal metastases had survival rates of 37.1%, 14.6%, and 2.1% at follow-up of 6 months, 1 year, and 2 years, respectively. The median survival time was 4.5 months, and the mean, 6.2 months. However, for small-cell lung cancer (SCLC), the corresponding survival rates were 36.8%, 5.3%, and 0% at 6 months, 1 year, and 2 years follow-up, respectively. In both NSCLC and SCLC with spinal metastases, the presence of hypercalcemia or hypoalbuminemia was indicative of a gloomy prognosis, with a survival period of less than 3 months.24
The aggressiveness of cancer can also be classified as slow growth (breast, thyroid, prostate), moderate growth (kidney, uterus), and rapid growth (lung, stomach, liver, colon, unknown).25–29
Concurrent Visceral Metastases
The influence on survival with visceral metastasis was also observed by Tokuhashi et al.28 and Tomita et al.29 The average survival with no visceral metastases was 36.8 months (range, 5–84 months), treatable visceral metastases, 16.5 months (4–31 months), and untreatable visceral metastases, 8.9 months (range, 1–24 months). The grave prognostic factor of extraskeletal metastatic lesions on the survival rate is also in agreement with Weigel et al.,18 who demonstrated that a patient survival period without extraskeletal metastases at surgery was significantly longer than with extraskeletal metastases (23.5 months vs. 5.8 months; P < .0001).
Location of Spinal Lesion
The location of the metastatic lesion was also implicated as a survival factor. Atanasiu et al.30 reported that lesions in the upper cervical spine had an adverse effect on life expectancy, with average survival of 1.8 months; however, this was challenged by Klekamp and Samii,31 who found no differences in prognosis between the upper and lower spine.
Number of Affected Vertebrae
The number of affected vertebrae also had an influence on survival. Patients with single-level involvement had a better survival rate (average survival, 12.9 months) than did patients with multiple-level involvement (average, 7.9 months),28,32,33 even after effective surgical treatment.34
Neurologic Deficit
Neurologic dysfunction was observed to be associated with survival rates. Some believe that ambulatory patients survive longer than nonambulatory patients with sphincter incontinence.19,35 The state of sphincter incontinence as a bad prognostic sign is also emphasized by some.36 The rate of neurologic deterioration was related to prognosis; slow progression had a good prognosis as opposed to rapid progression, which was correlated with dismal results. The latter was compounded when the onset of treatment was delayed.36,37 However, the notion of preoperative intact neurologic status (ambulatory vs. nonambulatory) as a predictor of longer survival was challenged by others.18,38
Likewise, timing for adjunctive radiation treatment (preoperative radiation vs. postoperative radiation) was also questioned as a significant prognostic predictor for outcomes.39 Weigel18 and Hirabayashi et al.25 emphasized that postoperative ambulation definitely has a positive prognostic influence on survival, rather than preoperative ambulation.
Timing of Surgery
The timing of treatment can also influence the results. Some authors36,37 observed that delayed onset of treatment may compromise the effectiveness of neurologic recovery. More specifically, Fürstenberg et al.40 addressed the timing of surgery and concluded that the effectiveness of decompression, when undertaken less than 48 hours after the development of symptoms, was significantly better for neurologic recovery (71.4%, unchanged 28.6%), compared with those with delayed surgical treatment (improvement 28.6%, unchanged 42.8%, deterioration 28.6%). Furthermore, they noted that normal bladder function may be considered a good prognostic factor for neurologic recovery after appropriate decompression.
Age
Weigel et al.18 also reported that the patient age can be considered as a prognostic factor for survival, because patients younger than 60 years survived significantly longer than older patients (20.1 months vs. 6.2 months; P = .028). Age as a prognostic factor has not been incorporated into the Tokuhashi scoring system and needs to be duplicated by others.
More Than Two Prognostic Factors
A report indicates that when more than two of the “prognostic factors” are present, they have a compounding adverse effect on survival.35 For example, lung cancer, neurologic deficit, and involvement of multiple vertebrae in the same patient would have a greater adverse effect on survival than the combined effect of the same type of lung cancer involving a single vertebra and without neurologic deficit. However, these findings have not been substantiated by others. When estimating survival, one should take into consideration that, invariably, most of the data from different reports were based on metastatic tumor of the spine that became symptomatic.
Instruments for Outcomes Assessment
Evaluation of patient functional status should be based on (1) pain assessment, (2) profile of mood states, and (3) overall performance status. Because performance depends not only on pain and disability but also on neurologic status, a detailed neurologic assessment is an integral part of the evaluation of these patients and provides reliable and straightforward clinical documentation. However, the assessment of pain disability and bodily functional performance of the patient can be appreciated only through psychometric instruments, which should be simple and reproducible.41
Because treatment is seldom curative in metastatic disease,42 the treating physician should keep in mind that patients with vertebral metastasis differ from noncancer patients. Therefore, the sensitivity of these instruments is limited to monitoring changes of the patient’s feelings of well-being.43,44
Pain is an alarming consequence of vertebral metastatic disease. Specific efforts should be made to avoid crude assessment and try to quantify and locate the pain. The McGill Pain Questionnaire is a sensitive tool. A visual analogue scale (VAS) for pain measurement combined with a pain diagram that indicates the site of pain and its spread is a simple and reliable method for quantifying and depicting pain. The VAS, although a subjective assessment of pain, has been shown to be more reliable than other types of pain assessment.45
A distinction should be made between radicular pain and axial pain. Even the surgical approach may be responsible for severe pain. A report46 detailing outcomes of pain demonstrated that in 72 patients who underwent a ventral approach through thoracotomy, 90% had postoperative pain (only 23% achieved complete resolution, 60% had significant improvement, and 8%, no change or worsening of thoracotomy pain). Quantification of pain is useful in monitoring the effectiveness of pain management, as well as in helping establish pain-control goals for the individual patient.47
Cancer patients often have emotional and psychological problems. Because anxiety and depression have a direct correlation with pain intensity, these two factors may need to be addressed. The Zung Self-Rating Anxiety Scale48 and the Hamilton Depression Scale49 are suitable instruments in this situation. The Memorial Pain Assessment Card (MPAC)50 (Fig. 108-1) is a modified visual analogue with multidimensional characteristics, practically equivalent to a full assessment combining the McGill Pain Questionnaire, the Hamilton Depression Rating Scale (HDRS), and the Zung Self-Rating Anxiety Scale.
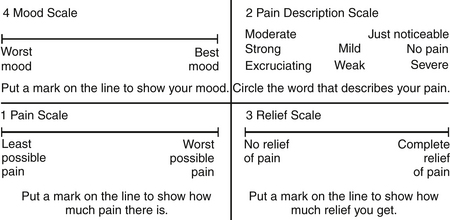
FIGURE 108-1 The Memorial Pain Assessment Card.
(From Fishman B. Pasternak S, Wallenstein SL, et al: The Memorial Pain Assessment Card: a valid instrument for the evaluation of cancer pain. Cancer 60:1151, 1987.)
The Edmonton Symptom Assessment Scale (ESAS) is a validated patient-centered questionnaire designed specifically to address the overall quality of life in patients with terminal cancer.43,44,51 ESAS measures nine domains (pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, shortness of breath, and other problems). After surgery, the largest improvement is reported in the pain domain.52
The 36-Item Short Form Health Survey (SF-36) by Townsend et al. is a physician-determined assessment of the patient’s physical disability. Patients are classified as having (1) normal, pain-free function, (2) normal function with pain, (3) significantly limited function requiring some type of assistance (e.g., a walker, cane), and (4) nonfunctional (e.g., wheelchair bound, bedridden).53
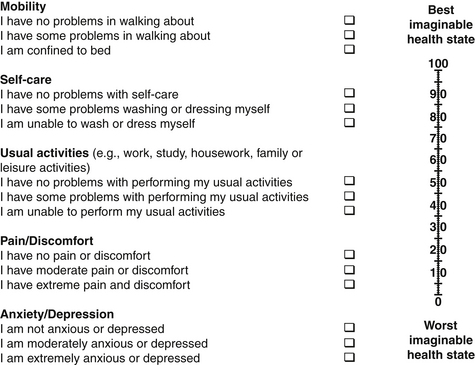
Other validated outcome measures in patients suffering from vertebral metastasis are the Health Utility Index Mark 3 (HUI-MARK 3; Table 108-10),54 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30),55 Eastern Cooperative Oncology Group (ECOG; Table 108-11),56 EuroQol 5d (EQ-5D; Fig. 108-2),57 and Karnofsky Performance Status (PS; Table 108-12), which have been successfully applied in patients with vertebral metastases.58
| Scale | Description |
|---|---|
| 1 | FREE OF PAIN AND DISCOMFORT |
| 2 | MILD TO MODERATE PAIN Pain does not prevent activity |
| 3 | MODERATE PAIN Pain prevents few activities |
| 4 | MODERATE TO SEVERE PAIN Pain prevents some activities |
| 5 | SEVERE PAIN Pain prevents most activities |
TABLE 108-11 ECOG Performance Status
| Grade | ECOG |
|---|---|
| 0 | Fully active, able to carry on all predisease performance without restriction |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light housework, office work |
| 2 | Ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours |
| 3 | Capable of only limited self-care; confined to bed or chair more than 50% of waking hours |
| 4 | Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair. |
| 5 | Dead |
ECOG, Eastern Cooperative Oncology Group.
TABLE 108-12 Rating of Karnofsky Performance Status
| Rating | Status | Description |
|---|---|---|
| 0 | Dead | Unable to care for self; requires equivalent of institutional or hospital care; disease may be progressing rapidly. |
| 10% | Moribund Fatal processes progressing rapidly |
|
| 20% | Very sick; hospitalization necessary Active support treatment necessary |
|
| 30% | Severely disabled; hospitalization is indicated, although death not imminent | |
| 40% | Disabled Requires special medical care and assistance |
Unable to work; able to live at home and care for most personal needs; varying amount of assistance needed. |
| 50% | Requires considerable assistance and frequent medical care | |
| 60% | Requires occasional assistance, but is able to care for most needs | |
| 70% | Cares for self Unable to carry on normal activity or to do active work |
|
| 80% | Normal activity with effort Some signs or symptoms of disease |
Able to carry on normal activity and to work; no special care needed. |
| 90% | Able to carry on normal activity Minor signs or symptoms of disease |
|
| 100% | Normal No complaints No evidence of disease |
From Karnofsky DA: Clinical evaluation of anticancer drugs: cancer chemotherapy. GANN Monogr 2:223, 1967.
The overall distribution of HUI-MARK 3 utility calculates quality-adjusted life years (QuaLY) during the 1-year postoperative period.33
The ECOG grading system or the Karnofsky PS is used to assess performance status. The ECOG (see Table 108-11) performance status56 measures functional performance on a scale from 0 to 4 and is a good index of assessing the preoperative functional status.33
The Karnofsky PS details the condition of the patient’s health status58 (see Table 108-12). Scoring between 0 and 10% indicates poor health and total dependency on others, from 50% to 70% indicates an inability to work and requires assistance, and 80% to 100% represents the ability to carry on with normal activities. The Karnofsky PS was successfully applied by Tokuhashi et al.59 in clinical guidance for predicting cancer patient outcomes.
Frankel et al.60 and the American Spinal Injury Association (ASIA) impairment scores,61 although nonspecific enough to describe nuances of the physical state of locomotion, grade the functional outcomes for neurologic assessment. Four other scales also address the state of ambulation60,62–64 and can be used as complementary to the ASIA or Frankel scales, such as combining the Cooper and the Frankel grading systems.
Surgical Guidelines
The rationale of guidelines is to achieve better treatment outcomes. Their scientific strength depends on how well evidence-based knowledge is used.65 It is reasonable to assume that consensus-based guidelines supported by available evidence are more convincing than individual ones. Unfortunately, no such reported guidelines are available for the management of metastatic spinal tumors. Certain published guidelines are based on individual experiences without enough clinical evidence to support their effectiveness. These guidelines do not cover an integrated approach for the management of metastatic spinal tumors but are concerned with different aspects of the problem.
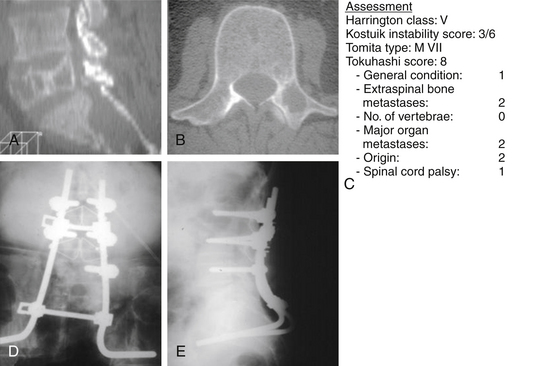
In 1990, Tokuhashi et al.59 devised a guideline scoring system for the preoperative evaluation of metastatic vertebral tumor, based on the general condition of the patient, as graded by the Karnofsky PS58 and five of the following prognostic factors: (1) number of extraspinal bone metastases, (2) number of metastases in the spine, (3) metastases to the major internal organs (lungs, liver, kidneys, and brain), (4) primary site of the cancer, and (5) severity of spinal cord impairment. Table 108-13 demonstrates the Tokuhashi prognostic scoring system for preoperative evaluation of the metastatic spinal lesions. Each parameter has a score ranging from 0 to 2 points (maximum score 12 points) that is related to the patient’s prognosis: the higher the score, the better the prognosis.
TABLE 108-13 Tokuhashi System for Preoperative Evaluation of Metastatic Spine Tumors
| Parameters | Score |
|---|---|
| General condition (performance status) | 0, 1, 2 |
| Extraspinal bone metastatic foci | 0, 1, 2 |
| Number of metastases in the spine (vertebrae) | 0, 1, 2 |
| Metastasis to major internal organs | 0, 1, 2 |
| Primary site of the cancer (histopathology) | 0, 1, 2 |
| Spinal cord palsy | 0, 1, 2 |
| Indications for Type of Surgery | Score |
| Excisional surgery | >9 |
| Palliative surgery | <5 |
In 1997, Tokuhashi et al.28 reported that the reliability for predicting the prognosis was 63.3%, tested in 128 patients with metastatic spinal tumors.
The original Tokuhashi scoring system was tested by Enkaoua et al.26 for three types of metastatic tumors: renal cell carcinoma, thyroid cancer, and unknown primary cancer. The median survival period was 5.3 months for a score of 7 or less and 23.6 months for a score of 8 or more. For cancer of an unknown primary site, the score should be 0 because the prognosis is invariably gloomy.
Subsequently, Tokuhashi et al.28 revised the original scoring system to improve its accuracy by providing more detailed prognostic values based on the origin of the primary tumor. The score was modified to range between 0 and 5 points. The score was designated as 0 for metastases originating in the lungs, osteosarcoma, stomach, bladder, esophagus, and pancreas; 1 for metastases from the liver, gallbladder, and unidentified sources; 2 for metastasis from other sites; 3 for renal or uterine malignancies; 4 for metastasis from the rectum; and 5 for tumors from the thyroid, breast, or prostate or a carcinoid tumor. Therefore, in the revised system, a total of 15 scores can be obtained. In the patients with a total score of less than 8 or multiple vertebral metastases, Tokuhashi et al. indicate conservative or palliative procedure (predicted survival period is less than 6 months), whereas they advocate excisional procedure when the score is 12 or more (predicted survival period, 1 year or more) and for scores between 9 and 11 (predicted survival period, 6 months or more). According to the authors, the consistency rate between the criteria for predicted prognosis and the actual survival period was high in patients within each score range (0–8, 9–11, 12–15), 86.4% in 118 patients evaluated prospectively after 1998, and 82.5% in all 246 patients evaluated retrospectively. No reports in the literature indicate that the actual value of each scoring point (0, 1, and 2) in each factor has been sufficiently scrutinized. Ogihara et al.24 reported that the Tokuhashi preoperative scoring system for patients with lung cancer did not correlate well with the survival period. The authors found that only the performance status ECOG (PS; see Table 108-11),56 which is similar to the Karnofsky test ,was correlated with the survival rate. However, this test was predictive only when applied postoperatively. The average survival rate for patients with a postoperative PS of 0 to 2 was 13.9 months, whereas that for patients with a postoperative PS of 3 or 4 was 2.0 months.
By using the Karnofsky PS, Weigel et al.18 found that the postoperative general state of health was 55%, a state of health that lies halfway between total dependency (50%) and partial dependency on others (60%). No one achieved 100%. The best scores were achieved with breast tumors (80% on the index); the second best results were seen in renal cell carcinoma patients (30% of the patients scoring 80–90%); and the poorest scores were seen in patients with melanoma (mean index, 48%), tumors of other sites (43%), and lung tumors (38%).
Chantaigner et al.20 asserted that when deciding on a surgical option in non-neurologic patients, the Karnofsky PS alone was more effective than the Tokuhashi score, which also includes the Karnofsky PS.
Some investigators are more selective than others in applying more vigorous selection in their inclusion criteria for surgery. Therefore, the overall survival period after surgery is not accurate. In some series, surgery is not indicated when the estimated survival rate is 3 months, whereas in others, the criterion for surgery is longer than 6 months. This explains the longer survival rates with different cancer patients and particularly in lung cancer (6–12 months) in some reports.39,66
Harrington37 described a classification guideline system based on both bone destruction and neurologic impairment, and distinguishes five classes of vertebral metastatic lesions (Table 108-14). He suggested surgical intervention for class IV and V lesions and conservative treatment for class I and II, while for class III, which lies in the gray zone, treatment depends on various circumstances. Harrington’s37 grading system is a practical guideline system for managing metastatic vertebral tumors, but it has some significant limitations: (1) it makes no recommendations for surgical options; (2) it does not take into account the biology of the tumor nor (3) the radiosensitivity of the metastatic lesion; and (4) it makes no provisions for the performance status of the patient. The applicability of the Harrington37 classification system for selecting the appropriate treatment in metastatic cancer of the spine was also applied by others.67
TABLE 108-14 Harrington Classification Based on Structural Defect and Neurologic Deficit
| Class | Neurologic Status | Structural Changes |
|---|---|---|
| I | Not significant | No vertebral collapse |
| II | Not significant | Vertebral involvement without collapse or instability (lytic or blastic lesion) |
| III | Major (sensory or motor) | No significant bone destruction or instability (lytic tumor) |
| IV | Not significant | Mechanical pain from vertebral collapse ± instability |
| V | Major | Retropulsion of hard discovertebral elements ± kyphotic deformity |
Harrington37 alluded to instability as one of the deciding factors for surgical stabilization because vertebral instability, caused by bone destruction, would provoke pain. However, he has not determined what constitutes instability in destructive metastatic lesions.
Kostuik et al.68,69 devised a vertebral body stability model by dividing the vertebral body into six segments. More specifically, the vertebral body is divided into an anterior column consisting of the vertebral body and a posterior column consisting of the pedicles, laminae, and spinous processes. The anterior column is further subdivided into four segments: anterior right, anterior left, middle right, and middle left. The posterior column is also subdivided into right and left segments. The spine is considered stable if no more than one or two of the six segments is destroyed. The spine is considered probably unstable if three or four of the segments are destroyed, and if five or six segments are affected, the spine is considered markedly unstable. Although this has not been biomechanically tested or clinically substantiated, it may have potential applications when describing instability in the Harrington classification.
According to Taneichi et al.,70 impending collapse is indicated (1) when more than 50% to 60% of the vertebral body is destroyed, or (2) when 25% to 30% of the vertebral structure is destroyed when there is an associated destruction of the costovertebral junction in the thoracic spine, or (3) when the destruction in the thoracolumbar spine ventrally is 35% to 40% or 20% to 25% involves the dorsal elements. Bridwell et al.71 believe that if less than 50% of the vertebral body has been resected, vertebral body reconstruction may not be necessary.
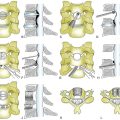
Tomita et al.,72 based on Enneking’s grading of skeletal resection,73 Denis’s three-column classification of fracture of the spine,74 and Weinstein’s zone classification75 (intraosseous, extraosseous, and distant spread), proposed a classification consisting of seven types of lesions (Fig. 108-3). This seems a reasonable classification for allowing the surgeon to choose the extent of surgical intervention—that is, radical excision versus stabilization alone.
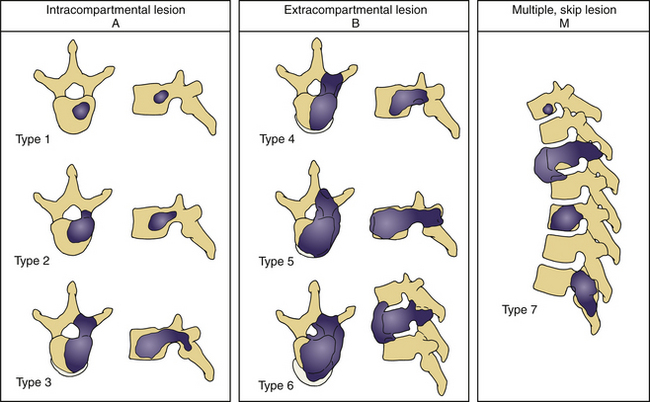
FIGURE 108-3 Tomita classification based on vertebral spread of metastasis.
(From Tomita K, Kawahara N, Baba H, et al: Total en bloc spondylectomy for solitary spinal metastases. Int Orthop 18:291, 1994.)
The most recent topographic classification system reported by Boriani et al.76 provides an excellent guideline for surgical excision of primary tumors of the spine and therefore is not specific for metastatic tumors.
A system that would encompass all these criteria would form a more comprehensive approach for the management of metastatic spinal malignancies (Fig. 108-4). For Harrington class I and II without significant neurologic involvement and without vertebral collapse, the management is nonsurgical and consists of chemotherapy, hormonal manipulation, and/or radiation therapy37 (Fig. 108-5). However, the treatment is contingent on the origin of the metastatic tumor. For example, in renal cell carcinoma, which is resistant to radiation therapy and chemotherapy, radical excision is indicated, even in class I. For Harrington class II tumors, as in the case of multiple myeloma or small lytic metastatic lesion, considerable and immediate relief of pain can be obtained by combining chemotherapy and a cement augmentation procedure (vertebroplasty or balloon kyphoplasty). See Figure 108-6.
For Harrington category III lesions, with a neurologic deficit in the absence of major destruction of bone or spinal instability, surgery should not be entertained unless the tumor is radioresistant or unresponsive to chemotherapy.37
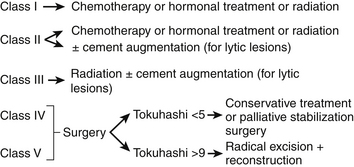
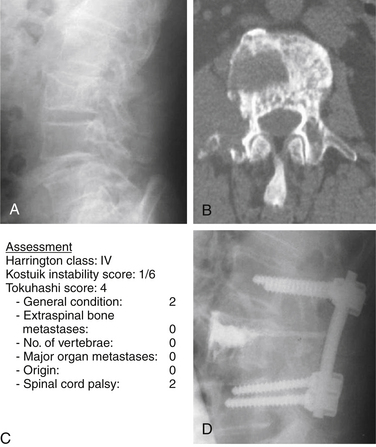
For class IV and V, surgery is the treatment of choice. For Harrington class IV, consisting of mechanical pain caused by vertebral collapse and/or instability, with a Tokuhashi score of less than 5 with the old system, or 8 with the revised system, the indications for treatment would be either conservative treatment or palliative surgery, consisting of stabilization and/or cement augmentation (Fig. 108-7).
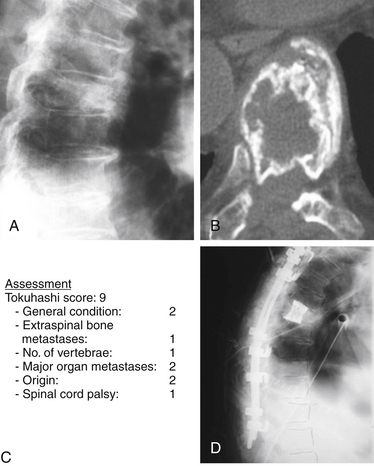
For a Harrington class IV or V with a Tokuhashi score greater than 9 (old system) or 12 (revised system) and a Tomita B score of 6 (widespread metastases), local radical excision, if feasible combined with stabilization, would be indicated (Fig. 108-8).
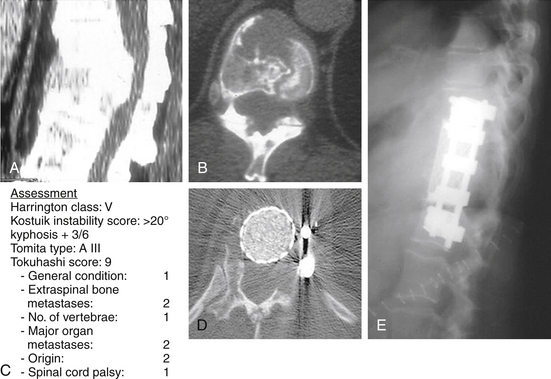
For class V, characterized by intrusion of hard discovertebral elements into the spinal canal that compromise the spinal cord, the only choice is surgery. Spinal kyphotic deformity with cord compromise should be categorized as class V. In this situation, radiation has no logical place, and surgery is the treatment of choice (Fig. 108-9).
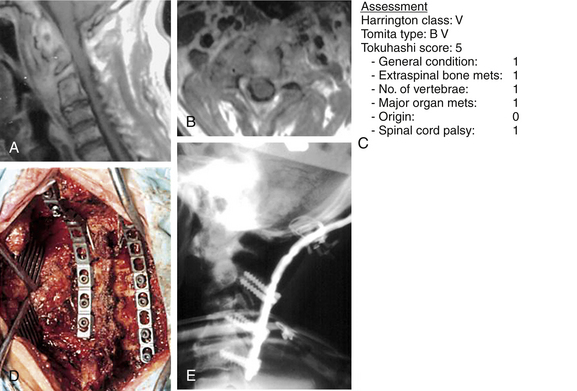
The ultimate intent of surgical management of metastatic spinal disease is to improve the patient’s overall quality of life, which is definitely apparent from the cited reports. The greatest effect occurs in largely the reduction of the level of the pain. In general, a minimum life expectancy of 3 to 6 months has been accepted as a prerequisite for surgery.67 However, even with a life expectancy shorter than 3 months, surgical intervention may be justified.77 In this situation, palliative surgery is indicated, and the anticipated surgical intervention should be performed, with the expectation of improving the remaining global quality of life, notwithstanding the morbidity of the procedure78 (Figs. 108-10 and 108-11). Some of the procedures for alleviating pain, such as cement augmentation (vertebroplasty, balloon kyphoplasty) and neuroaugmentation (morphine pump), are associated with minimal morbidity. Surgery is also indicated for patients who have reached spinal cord tolerance after external radiation beam treatment.
Summary
In this updated and upgraded version of a previously published chapter on this subject,79 the conclusion remains that survival outcomes for managing metastatic spinal tumors are still gloomy. When assessing surgical outcomes, the following published parameters should form the baseline for acceptable outcomes: neurologic improvement (55–87%) restoration of ambulation (40–100%), restoration of sphincter control (36–89%), pain relief (47–100%); local recurrences (8–31%), complication (16–32%), reoperation rate for complications (7–24%), and patient satisfaction (55–80%). Furthermore, the reported mortality rate 4 to 6 weeks postoperatively ranges from 3% to 10.2%, whereas the mean survival is 8 to 15.9 months. Pain shows the greatest response. In addition, the prognostic factors that can influence the final outcomes are origin of tumor, visceral metastasis, number of affected vertebrae, state of neurologic dysfunction, and onset of surgical treatment. It is important to quantify the patient’s pain, psychological distress, and performance status before establishing treatment goals. Certain outcome instruments have been validated for vertebral metastatic disease (EORTC, EQ-5D, QLOJL30, EQSD, HUI-MARK 3, ESAS, McGill Pain Questionnaire, VAS, MPAC). Appropriate surgical intervention is based on reasonable guidelines. The Harrington classification system combined with the Kostuik instability model, the Tomita grading system for vertebral tumor spread, and the Tokuhashi preoperative scoring system questionnaire are excellent guides for predicting outcomes of metastatic tumors and establishing methods for selecting the most appropriate treatment. The degree of the clinical validity of these guidelines has not been vigorously tested and, therefore, is lacking strong scientific support. Although they are not consensus- or evidence-based guidelines, they are useful tools for selecting a more appropriate surgical intervention. Furthermore, they can form the basis for establishing the standards for more widely acceptable guidelines.
Bauer H.C.F. Posterior decompression and stabilization for spinal metastases: analysis of sixty-seven consecutive patients. J Bone Joint Surg [Am]. 1997;79:514.
Enkaoua E.A., Doursounian L., Chatellier G., et al. Vertebral metastases: a critical appreciation of the preoperative prognostic Tokuhashi score in a series of 71 cases. Spine (Phila Pa 1976). 1997;22:2293.
Harrington K.D. Metastatic disease of the spine. J Bone Joint Surg [Am]. 1986;68:1110.
Karnofsky D.A. Clinical evaluation of anticancer drugs: cancer chemotherapy. GANN Monogr. 1967;2:223.
Patchel R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643.
Tokuhashi Y., Matsuzaki H., Oda H., et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005;30:2186.
Tokuhashi Y., Matsuzaki H., Toriyama S., et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 1990;15:1110.
1. Jaffe H.L. Tumors and tumorous conditions of the bones and joints. Philadelphia: Lea & Febinger; 1958.
2. Barron K.D., Hirano A., Araki S., et al. Experiences with metastatic neoplasms involving the spinal cord. Neurology. 1959;9:91.
3. Walsh G.L., Gokaslan Z.L., McCutcheon I.E., et al. Anterior approaches to the thoracic spine in patients with cancer: indications and results. Ann Thorac Surg. 1997;64:1611.
4. Drury R.A.B., Palmer P.H., Highman W.J. Carcinomatous metastasis to the vertebral bodies. J Clin Pathol. 1964;17:448.
5. Ortiz Gomez J.A. The incidence of vertebral body metastases. Int Orthop. 1995;19:309.
6. Schaberg J., Gainor B.J. A profile of metastatic carcinoma of the spine. Spine (Phila Pa 1976). 1985;10:19.
7. Bilsky M.H., Lis E., Raizer J., et al. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459.
8. Byrne T.N. Spinal cord compression from epidural metastases. N Engl J Med. 1992;27:614.
9. Gerszten P.C., Welch W.C. Current surgical management of metastatic spinal disease. Oncology (Williston Park). 2000;14:1013.
10. Healey J.H., Brown H.K. Complications of bone metastases: surgical management. Cancer. 2000;88(12 suppl):2940.
11. Saengnipanthkul S., Jirarattanaphochai K., Rojviroj S., et al. Metastatic adenocarcinoma of the spine. Spine (Phila Pa 1976). 1992;17:427.
12. Wang J.C., Boland P., Mitra N., et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients: invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:287.
13. Gilbert R.W., Kim J.H., Posner J.B. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3:40.
14. Greenberg H.S., Kim J.H., Posner J.B. Epidural spinal cord compression from metastatic tumor: results with a new treatment protocol. Ann Neurol. 1980;8:361.
15. Mones R.J., Dozier D., Berrett A. Analysis of medical treatment of malignant extradural spinal cord tumors. Cancer. 1842;19:1966.
16. Rodriguez M., Dinapoli R.P. Spinal cord compression: with special reference to metastatic epidural tumors. Mayo Clin Proc. 1980;55:442.
17. Nader R., Afford B.T., Nauta H.J., et al. Preoperative embolization and intraoperative cryocoagulation as adjuncts in resection of hypervascular lesions of the thoracolumbar spine. J Neurosurg. 2002;97:294.
18. Weigel B., Maghsudi M., Neumann C., et al. Surgical management of symptomatic spinal metastases: postoperative outcome and quality of life. Spine (Phila Pa 1976). 1999;24:2240.
19. King G.J., Kostuik J.P., McBroom R.J., et al. Surgical management of metastatic renal carcinoma of the spine. Spine (Phila Pa 1976). 1991;16:265.
20. Chataigner H., Onimus M. Surgery in spinal metastasis without spinal cord compression: indications and strategy related to the risk of recurrence. Eur Spine J. 2000;9:523.
21. Hosono N., Ueda T., Tamura D., et al. Prognostic relevance of clinical symptoms in patients with spinal metastases. Clin Orthop Relat Res. 2005;436:196.
22. Neyt J., Samson M., Hoogmartens M. Surgical treatment of spinal metastases: long term follow-up. Acta Orthop Belg. 1993;59:83.
23. Kleinman W.B., Kiernan H.A., Michelsen W.J. Metastatic cancer of the spinal column. Clin Orthop Relat Res. 1978;136:166-172.
24. Ogihara S., Seichi A., Hozumi T., et al. Prognostic factors for patients with spinal metastases from lung cancer. Spine (Phila Pa 1976). 2006;31:1585.
25. Hirabayashi H., Ebara S., Kinoshita T., et al. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003;97:476.
26. Enkaoua E.A., Doursounian L., Chatellier G., et al. Vertebral metastases: a critical appreciation of the preoperative prognostic Tokuhashi score in a series of 71 cases. Spine (Phila Pa 1976). 1997;22:2293.
27. Tatsui H., Onomura T., Morishita S., et al. Survival rates of patients with metastatic spinal cancer after scintigraphic detection of abnormal radioactive accumulation. Spine (Phila Pa 1976). 1996;21:2143.
28. Tokuhashi Y., Matsuzaki H., Oda H., et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005;30:2186.
29. Tomita K., Kawahara N., Kobayashi T., et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001;26:298.
30. Atanasiu J.P., Badatcheff F., Pidhorz L. Metastatic lesions of the cervical spine: a retrospective analysis of 20 cases. Spine (Phila Pa 1976). 1993;18:1279.
31. Klekamp J., Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien). 1998;140:957.
32. O’Neil J., Gardner V., Armstrong G. Treatment of tumors of the thoracic and lumbar spinal column. Clin Orthop Relat Res. 1988;227:103.
33. Falicov A., Fisher C.G., Sparkes J., et al. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine. 2006;31:2849.
34. Shimizu K., Shikata J., Iida H., et al. Posterior decompression and stabilization for multiple metastatic tumors of the spine. Spine (Phila Pa 1976). 1992;17:1400.
35. Sioutos P.J., Arbit E., Meshulam C.F., et al. Spinal metastases from solid tumors: analysis of factors affecting survival. Cancer. 1995;76:1453.
36. Nather A., Bose K. The results of decompression of cord or cauda equina compression from metastatic extradural tumors. Clin Orthop Relat Res. 1982;169:103.
37. Harrington K.D. Metastatic disease of the spine. J Bone Joint Surg [Am]. 1986;68:1110.
38. Spiegel D.A., Sampson J.H., Richardson W.J., et al. Metastatic melanoma to the spine: demographics, risk factors, and prognosis in 114 patients. Spine (Phila Pa 1976). 1995;20:2141.
39. Sundaresan N., Bains M., McCormack P. Surgical treatment of spinal cord compression in patients with lung cancer. Neurosurgery. 1985;16:350.
40. Fürstenberg C.H., Wiedenhöfer B., Gerner H.J., et al. The effect of early surgical treatment on recovery in patients with metastatic compression of the spinal cord. J Bone Joint Surg [Br]. 2009;91:240.
41. Hadjipavlou A., Necessary J. Principles of psychometric studies for clinical outcomes and disability evaluation in chronic low back. J Disabil. 1988;7:1.
42. Cella D.F. Quality of life outcomes: measurement and validation. Oncology. 1996;10:233.
43. Bruera E., Kuehn N., Miller M.J., et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6.
44. Chang V.T., Hwang S.S., Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164.
45. Jensen M.P., Chen C., Brugger A.M. Postsurgical pain outcome assessment. Pain. 2002;99:101.
46. Gokaslan Z.L., York J.E., Walsh G.L., et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599.
47. Cleeland C.S. The impact of pain on the patient with cancer. Cancer. 1984;54:2635.
48. Zung W.W. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371.
49. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56.
50. Fishman B., Pasternak S., Wallenstein S.L., et al. The Memorial Pain Assessment Card: a valid instrument for the evaluation of cancer pain. Cancer. 1987;60:1151.
51. Kaasa T., Loomis J., Gillis K., et al. The Edmonton Functional Assessment Tool: preliminary development and evaluation for use in palliative care. J Pain Symptom Manage. 1997;13:10.
52. Wai E.K., Finkelstein J.A., Tangente R.P., et al. Quality of life in surgical treatment of metastatic spine disease. Spine (Phila Pa 1976). 2003;28:508.
53. Townsend P.W., Rosenthal H.G., Smalley S.R., et al. Impact of postoperative radiation therapy and other perioperative factors on outcome after orthopedic stabilization of impending or pathologic fractures due to metastatic disease. J Clin Oncol. 1994;12:2345.
54. Feeny D., Furlong W., Torrance G.W., et al. Multi-attribute and single-attribute utility functions for the Health Utilities Index Mark 3 system. Med Care. 2002;40:113.
55. Fayers P.M. Interpreting quality of life data: population-based reference data for the EORTC QLQ; C30. Eur J Cancer. 2001;37:1331.
56. Oken M.M., Creech R.H., Tormcy D.C., et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649.
57. Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095.
58. Karnofsky D.A. Clinical evaluation of anticancer drugs: cancer chemotherapy. GANN Monogr. 1967;2:223.
59. Tokuhashi Y., Matsuzaki H., Toriyama S., et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 1990;15:1110.
60. Frankel H.L., Hancock D.O., Hyslop G., et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179.
61. American Spinal Injury Association. International standards for neurological classifications of spinal cord injury (revised). Chicago: American Spinal Injury Association; 2000.
62. Brice J., Mckissock W. Surgical treatment of malignant extradural spinal tumours. Br Med J. 1965;22:1341.
63. Cooper P.R., Errico T.J., Martin R., et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1.
64. Tomita T., Galicich J.H., Sundaresan N. Radiation therapy for spinal epidural metastases with complete block. Acta Radiol Oncol. 1983;22:135.
65. Woolf S.H., Grol R., Hutchinson A., et al. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. Br Med J. 1999;318:527.
66. Hammerberg K.W. Surgical treatment of metastatic spine disease. Spine (Phila Pa 1976). 1992;17:1148.
67. Wise J.J., Fischgrund J.S., Herkowitz H.N., et al. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine (Phila Pa 1976). 1999;24:1943.
68. Errico T.F., Kostuik J.P., Gleason T.F., et al. Spinal stabilization of vertebral column tumors. Spine (Phila Pa 1976). 1988;13:250.
69. Kostuik J.P., Weinstein J.N. Differential diagnosis and surgical treatment of metastatic spine tumors. In: Frymoyer J.W., editor. The adult spine. New York: Raven Press, pp 826–860, 1991.
70. Taneichi H., Kaneda K., Takeda N., et al. Risk factors and probability of vertebral body collapse in metastases of thoracic and lumbar spine. Spine (Phila Pa 1976). 1997;22:239.
71. Bridwell K.H., Jenny A.B., Saul T., et al. Posterior segmental spinal instrumentation (PSSI) with posterolateral decompression and debulking for metastatic thoracic and lumbar spine disease: limitations of the technique. Spine (Phila Pa 1976). 1988;13:1383.
72. Tomita K., Kawahara N., Baba H., et al. Total en bloc spondylectomy for solitary spinal metastases. Int Orthop. 1994;18:291.
73. Enneking W.E. Staging musculoskeletal tumors. In: Enneking W.F., editor. Musculoskeletal tumor surgery. New York: Churchill-Livingstone, 1983.
74. Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res. 1984;189:65.
75. Weinstein J. The adult spine: principles and practice. New York: Raven Press; 1991.
76. Boriani S., Weinstein J.N., Biagini R. Primary bone tumors of the spine: terminology and surgical staging. Spine. 1997;22:1036.
77. Lord C.F., Herndon J.H. Spinal cord compression secondary to kyphosis associated with radiation therapy for metastatic disease. Clin Orthop Relat Res. 1986;210:120.
78. Turner P.L., Prince H.G., Webb J.K., et al. Surgery for malignant extradural tumours of the spine. J Bone Joint Surg [Br]. 1988;70:451.
79. Hadjipavlou A., Szpalski M., Gunzburg R., et al. Surgical outcomes and guidelines for management of metastatic spine cancer (a point-of-view review). In: Gunzburg R., Szpalski M., Aebi M., editors. Vertebral tumors. Philadelphia,: Lippincott Williams & Wilkins, 2008. pp 196–216
80. Ernstberger T., Brüning T., König F. Vertebrectomy and anterior reconstruction for the treatment of spinal metastases. Acta Orthop Belg. 2005;71:459.
81. Villavicencio A.T., Oskouian R.J., Roberson C., et al. Thoracolumbar vertebral reconstruction after surgery for metastatic spinal tumors: long-term outcomes. Neurosurg Focus. 2005;19:E8.
82. Rompe J.D., Hopf C.G., Eysel P. Outcome after palliative posterior surgery for metastatic disease of the spine: evaluation of 106 consecutive patients after decompression and stabilization with the Cotrel-Dubousset instrumentation. Arch Orthop Trauma Surg. 1999;119:394.
83. Hatrick N.C., Lucas J.D., Timothy A.R., et al. The surgical treatment of metastatic disease of the spine. Radiother Oncol. 2000;56:335.
84. Bauer H.C.F. Posterior decompression and stabilization for spinal metastases: analysis of sixty-seven consecutive patients. J Bone Joint Surg [Am]. 1997;79:514.
85. Onimus M., Papin P., Gangloff S. Results of surgical treatment of spinal thoracic and lumbar metastases. Eur Spine J. 1996;5:407.
86. Hosono N., Yonenobu K., Fuji T., et al. Vertebral body replacement with a ceramic prosthesis for metastatic spinal tumors. Spine (Phila Pa 1976). 1995;15:2454.
87. Hertlein H., Mittlmeier T., Piltz S., et al. Spinal stabilization for patients with metastatic lesions of the spine using a titanium spacer. Eur Spine J. 1992;1:131.
88. Kocialkowski A., Webb J.K. Metastatic spinal tumours: survival after surgery. Eur Spine J. 1992;1:43.
89. Sinardet D., Chabane A., Khalil T., et al. Evolution neurologique de 152 patients operes de metastases. Neurochirurgie. 2000;46:4.
90. Touboul E., Camille R.R., Guerin R.A., et al. Tumeurs extra-durales rachidiennes metastatiques: a propos de cent trente cas. Semin Hop Paris. 1986;62:1785.
91. Sundaresan N., Galicich J.H., Lane J.M., et al. Treatment of neoplastic epidural cord compression by vertebral body resection and stabilization. J Neurosurg. 1985;63:676.
92. Bilsky M.H., Boland P., Lis E., et al. Single-stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases. Spine (Phila Pa 1976). 2000;25:2240.
93. Livingston K.E., Perrin R.G. The neurosurgical management of spinal metastases causing cord and cauda equina compression. J Neurosurg. 1978;49:839.
94. Patchel R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643.
95. Siegal T., Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: a prospective study. Neurosurgery. 1985;17:424.
96. Sundaresan N., Digiacinto G.V., Hughes J.E.O., et al. Treatment of neoplastic cord compression: results of a prospective study. Neurosurgery. 1991;29:645.
97. Kennady J.C., Stern W.E. Metastatic neoplasms of the vertebral column producing compression of the spinal cord. Am J Surg. 1962;104:155.
98. Schoeggl A., Reddy M., Matula C. Neurological outcome following laminectomy in spinal metastases. Spinal Cord. 2002;40:363.
99. Wright R.L. Malignant tumors in the spinal extradural space: results of surgical treatment. Ann Surg. 1963;157:227.
100. White W.A., Patterson R.H.Jr., Bergland R.M. Role of surgery in the treatment of spinal cord compression by metastatic neoplasm. Cancer. 1971;27:558.
101. Lewandrowski K.U., Hecht A.C., DeLancy T.F., et al. Anterior spinal arthrodesis with structural cortical allografts and instrumentation for spine tumor surgery. Spine (Phila Pa 1976). 2004;29:1150.
102. Sundaresan N., Rothman A., Manhart K., et al. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976). 2002;27:1802.
103. Cahill D.W., Kumar R. Palliative subtotal vertebrectomy with anterior and posterior reconstruction via a single posterior approach. J Neurosurg. 1999;90:42.
104. Jansson K.A., Bauer H.C. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J. 2006;15:196.
105. Klimo P.Jr., Thompson C.J., Kestle J.R., et al. A metaanalysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neurooncology. 2005;7:64.
106. Siegal T., Tiqva P., Siegal T. Vertebral body resection for epidural compression by malignant tumors: results of forty-seven consecutive operative procedures. J Bone Joint Surg [Am]. 1985;67:375.
107. Fidler M.W. Anterior decompression and stabilisation of metastatic spinal fractures. J Bone Joint Surg [Br]. 1986;68:83.
108. Fourney D.R., Schomer D.F., Nader R., et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21.
109. Muhlbauer M., Pfisterer W., Eyb R., et al. Noncontiguous spinal metastases and plasmacytomas should be operated on through a single posterior midline approach, and circumferential decompression should be performed with individualized reconstruction. Acta Neurochir (Wien). 2000;142:1219.
110. Nazarian S. Place de la chirurgie dans le traitement des metastases du rachis: synthese et conclusion. Rev Chir Orthop Reparatrice Appar Mot. 1997;83:167.