Chapter 140 Prognosis of Posterior Uveal Melanoma
Ocular prognosis of globe-conserving therapies
Treatment of primary choroidal melanoma without evidence of metastasis involves either globe-conserving therapy or enucleation. In a randomized clinical trial of patients with primary choroidal melanoma treated with globe-conserving iodine-125 brachytherapy versus enucleation, the Collaborative Ocular Melanoma Study (COMS) demonstrated no significant difference in mortality, 5, 10, and 12 years following treatment between brachytherapy and enucleation.1–3 Thus, increasing emphasis has been placed on globe-conserving therapy for choroidal melanoma. Reported rates of local treatment failure vary between treatment modalities and between centers using similar modalities. Radiation therapy overall results in lower rates of local treatment failure compared to nonradiation-based treatment approaches.
Radiation
Maintaining the integrity of the eye after globe-conserving treatment for choroidal melanoma is an important outcome in evaluating the success of a specific treatment. Local treatment failure or local tumor recurrence is associated with morbidity consisting of either need for re-irradiation or organ loss by enucleation. Iodine-125 plaque radiotherapy is the most common primary treatment modality for choroidal melanoma. Among the largest published series reporting local treatment failure with iodine-125, the COMS medium tumor trial reported the risk of treatment failure at 5 years, as measured by local recurrence rate in 650 patients to be 10.3%.4 A report by Sagoo et al. of 650 juxtapapillary tumors indicated a local treatment failure rate of 21% at 10 years.5 For iodine-125 brachytherapy the local treatment failure rate in the literature ranges from 0 to 27% among centers with varying follow-up intervals and sample sizes.6–22 Series by McCannel et al.6 and Tabandeh et al.8 reported very low local failure rates with iodine-125, 0% and 1.7%, respectively; both centers use intraoperative ultrasonography which minimizes the risk of edge-miss. Among the treatment modalities that involve radiation, Rouberol et al. reported a 21.7% and 24.3% rate of local recurrence after 5 and 10 years, respectively, after ruthenium brachytherapy in 213 patients treated from 1983 to 1995.23 Wilson also reported in a retrospective review a higher local treatment failure rate using ruthenium plaques compared to both proton beam irradiation and iodine-125 brachytherapy.24 In a series of 368 patients treated with proton beam, Mosci reported a local treatment failure rate of 8.4%.25 In another group of 1922 patients treated with proton beam irradiation, a local recurrence rate of 3.2% and 4.3% at 5 and 10 years, respectively, was reported.26 Published data indicate that the use of ruthenium results in higher rates of local treatment failure than iodine-125. Proton beam radiotherapy has a variable rate of local failure depending on the experience of the treatment center. Iodine-125 is the most commonly used form of local treatment for uveal melanoma in North America and rates of local treatment failure vary between centers.
Nonradiation therapy
Nonradiation local treatment of uveal melanoma includes laser photocoagulation, Visudyne photodynamic therapy, and transpupillary thermotherapy. With a reduction in primary enucleation for choroidal melanoma, and a general trend toward eye-salvaging therapy, developments in nonradiation therapies continue to emerge. The main theoretical advantages of laser over radiation include minimizing collateral damage to healthy ocular tissues compared to radiation, minimizing radiation exposure to the patient and operator, and a more simplified treatment approach not requiring radiation oncology or use of an operating theater. However, laser overall has been found to be less successful in achieving local tumor control compared with most forms of radiation-based treatment. The literature is sparse on reports utilizing argon laser photocoagulation to treat choroidal melanomas, but there are many reports of failures, and the prevailing opinion is that argon laser is not an adequate primary treatment for choroidal melanoma of any size.27–29 There are few reports of photodynamic therapy for uveal melanoma in the literature; only two case reports exist using this technique for small atypical amelanotic choroidal melanoma.30,31 Barbazetto et al. reported a case series of four patients treated by photodynamic therapy with recurrent tumors treated with other modalities, resulting in eventual enucleation of two eyes.32
Following the discontinuation of transpupillary thermotherapy (TTT) as a therapy for age-related macular degeneration, a noticeable interest in this form of thermal laser for use in posterior segment tumors occurred. However, the largest series using TTT reported high rates of local treatment failure. Shields et al. reported a 22% local treatment failure rate at 3 years in a series of 256 posterior uveal melanoma treated by transpupillary thermotherapy.33 Aaberg et al. reported a rate of 23% at 5 years in a series of 135 patients.34 Zaldivar described eight cases with histologic findings in eyes enucleated after failed TTT. Although the height of the tumor decreased, extrascleral extension and lateral spread of the tumor ensued.35 Harbour found that not only was there no significant difference in visual outcome between TTT and plaque radiotherapy, but also that the recurrences were substantially higher when TTT was used compared with iodine-125 plaque.36 Currently, the use of TTT has been limited to an adjunct to primary brachytherapy to compensate for edge-miss in plaque placement by some operators.37,38
Surgery
Peyman et al.39 studied a group of 34 patients treated by eye wall resection with an average follow-up of 5.3 years and reported that 11 (32.3%) required enucleation (mainly because of evidence of tumor cells in resected margins) and 13 (38.2%) retained 20/200 or better vision after treatment. No patient had local treatment failure, and two patients had died of metastatic melanoma at the time of that report. In a matched case–control study, 81 of 49 pairs of patients undergoing either transscleral resection at one center or iodine brachytherapy at another, Kivela and colleagues found fewer recurrences with plaques than resection, but no difference in 8-year mortality.40 Furthermore, only eyes undergoing radiation experienced neovascular glaucoma, radiation retinopathy, cataract or ischemic changes. Shields et al.41 described a series of 95 patients with choroidal or ciliary body tumors, or both, who were managed by partial lamellar sclerouvectomy. A total of 81 cases (85%) were uveal melanoma; vitreous hemorrhage occurred in 79 (83%); intraretinal or subretinal hemorrhage in 33 (35%); retinal detachment in 26 (28%), and cataract in 32 (34%) patients. Retinal detachment surgery was necessary in 16 patients (17%), and enucleation was necessary in 15 (16%) patients. Distant metastases developed in five patients (5%) with a median follow-up of 2.5 years. In the largest series of transscleral endoresection by Damato et al., the local treatment failure rate at 3 years was 20%.42 Puusaari et al. in a series of 33 patients reported a local treatment failure rate of 40% by 5 years.43 Garcia-Arumi44 reported in a series of 34 cases of vitrectomy with tumor endoresection with average follow-up of 70 months a local treatment failure rate of 5.8%, which is comparable with reports of others who have used vitrectomy with endoresection.45,46
Systemic prognosis for metastasis and death
The most common site for metastasis in uveal melanoma is the liver. At least 50% of all patients diagnosed with uveal melanoma develop metastatic disease.47 The COMS study reported liver metastasis with median survival once metastasis was diagnosed between 6 and 12 months.48 In an analysis of metastatic uveal melanoma patients by Rietschel et al., the authors found that the most common nonhepatic first-metastatic site to be diagnosed was the lung.49 Patients who were younger than age 60, had metastasis to the lung, had a longer interval between treatment of the melanoma and discovery of metastasis, had better survival after a diagnosis of metastatic disease.
There is a lack of substantial difference in survival for local globe-preserving therapies compared with enucleation in the early post-treatment years, which may be related to the possibility that metastases occur before the tumor is treated, perhaps before it is diagnosed.50 Another consideration in evaluating the apparent similarity in survival outcome of conservative therapies in comparison with enucleation is that selection of patients with small- to medium-sized tumors and other more favorable prognostic indicators for eye-sparing treatment methods may bias survival results in favor of these therapies. It is therefore important to control for these prognostic factors to the extent possible in nonrandomized studies. A data collection and management system for studies of prognosis and treatment was developed in the 1980s in order to standardize these measures in observational cross-sectional and longitudinal studies, and was used to identify several factors relevant to metastasis.51–54 In the initial studies evaluating prognosis after treatment with proton beam or enucleation,53,54 the Cox proportional hazards model was first used in ophthalmology to study prognosis and survival after treatment of uveal melanoma. Epidemiologic methods were applied to standardize variables and control for differences in demographic factors as well as tumor-specific variables: tumor height, tumor diameter, location of the tumor posterior to the equator, anterior to the equator, and involvement of the ciliary body. Larger tumor size and anterior location decreased rate of survival, and treatment type did not have a significant effect. Length of observation period or evidence of growth prior to treatment has not been shown to affect prognosis. Visual outcomes after proton beam irradiation were also evaluated using these methods.55,56
Radiation therapy
Metastasis and survival
Actuarial survival rates following radiation therapy of uveal melanoma have been reported to be 75%57 to 80%,58 and 89%59 5 years after treatment. For 96 patients treated by cobalt plaque, the 5-year survival rate (without death from metastatic melanoma) was approximately 75%.57 In a study of 128 patients treated by proton beam irradiation,58 with a median of 5.4 years of follow-up, the probability of metastasis-free survival at 5 years was 80%. Lommatzsch60 reported a 5-year survival of 89% in a group of 309 patients treated by ruthenium. In that study, tumors measured up to 5 mm in height and 15 mm in diameter, and the mean follow-up was 6.7 years. Similar survival estimates were observed in a series of ruthenium-treated patients in Germany,61 Sweden,62 and Finland.63 In an update of an earlier report, Char reported on long-term outcomes in 218 patients treated with helium ion irradiation and followed for an average of 12 years.64,65 The mean tumor thickness in this cohort was 6.7 mm, and the mean largest tumor dimension was 11.9 mm; 5-, 10-, and 15-year survival were estimated to be 73%, 61%, and 54%, respectively. Series of patients treated by iodine-125 irradiation66,67 have also been described.
Prognostic factors for patients treated by irradiation have been evaluated in several studies. Using the tumor registry and analytic methods developed by Seddon and colleagues,51–56 the first 780 patients treated by proton beam irradiation were evaluated,58 of whom 64 had developed metastasis at the time of the study. Similar to the earlier studies, leading predictors of metastasis were tumor diameter larger than 15 mm, ciliary body involvement, extrascleral extension, and age at treatment of 60 years or older. Surgical localization of the tumor and elevated pretreatment liver enzymes were not statistically significant prognostic factors. They found no survival advantage of proton beam irradiation compared with enucleation.53,68 In an investigation of the role of hormonal factors in the development of metastasis,69 among women of childbearing age who had been treated by proton beam irradiation for uveal melanoma neither postirradiation pregnancy history nor oral contraceptive use were associated with the rate of metastasis.
Augsburger and Goel studied tumor regression as a prognostic factor for metastasis following cobalt plaque irradiation. Findings of this preliminary study suggest that rapid tumor regression is a poor prognostic sign.70 Tumors regressing more rapidly were significantly more likely to metastasize concurrently with their regression in a series of patients treated by proton beam irradiation.71
Local tumor recurrence or local treatment failure may increase the risk of metastases from uveal melanoma.72,73 This appeared to be the case in a cohort of patients with juxtapapillary melanoma treated by brachytherapy.74 In that study, rates of melanoma metastasis were 13%, 16%, and 37% at 5, 10, and 15 years, respectively; tumor recurrence (defined as any amount of regrowth), larger tumor diameter, and superior location of the tumor were found to predict occurrence of distant metastasis. This may be due in part to technical difficulties in placement of the plaque, resulting in undertreatment of the tumor (or overtreatment of the normal retina). Harbour et al.75 evaluated the prognostic significance of patterns of tumor regrowth after irradiation. The relative risk for death resulting from metastasis was significantly higher among patients with increased tumor thickness than among those whose tumor spread at the margin (5.1 versus 2.2, respectively, relative to those without tumor recurrence). The mean rate of metastasis was also significantly higher in patients with increased tumor thickness compared with those with marginal spread. Reducing local treatment failure is critical in the management of primary uveal melanoma not only for the sake of preserving the eye, but more importantly for lowering the risk of systemic metastasis.
Prognosis after enucleation
Metastasis from uveal melanoma may occur at any time after the onset of tumor diagnosis. Jensen,76 in his 25-year follow-up of Danish patients, found that the peak incidence of metastasis occurred during the first year after enucleation, and over half of those who developed metastasis did so within 3 years of treatment. However, it is a well-known but poorly understood fact that patients may develop metastasis decades after enucleation. Due to hypotheses that enucleation itself might cause increased mortality,77 the COMS study compared enucleation to pre-enucleation radiation for large tumors, and did not find a difference in mortality.47
Comprehensive and long-term studies of survival after enucleation derive from Denmark and Finland. These reports have the advantage of being population based with few losses to follow-up. Raivio compiled a roster of all patients diagnosed with uveal melanoma between 1923 and 1966 in Finland.78 Of 359 identified cases, survival status at 10 years was known for all but five patients; 314 cases (89%) had 15 or more years of follow-up available, and 214 cases (60%) had at least 20 years. The 5-, 10-, and 15-year survival rates based on melanoma-related deaths were 65%, 52%, and 46%, respectively. Of the 42 patients who survived at least 20 years, nine later developed metastasis. Jensen76 evaluated survival at least 25 years after enucleation in Danish patients with uveal melanoma. The majority of patients included in the original series had died (82%); 51% of those deaths were due to metastasis. Actuarial survival rates at 5, 10, and 15 years were similar to those reported by Raivio.78 A novel deficit survival analysis of 230 patients in 1984 by Lavin and colleagues indicated no overall survival advantage after enucleation.79
Metastasis from uveal melanoma usually occurs within the first few years after enucleation. The liver is usually the first site of metastasis after treatment.80–83 There is some evidence to suggest that metastasis may occur several years before the diagnosis of hepatic metastasis is made.50,84,85 Other organs that may be affected include the lung, bone, skin, and central nervous system.82 The majority of patients with hepatic involvement succumb within a few months of detection of the metastatic lesion.83
Visual prognosis and ocular morbidity
Rates of complications and visual loss after radiation depend on the type of treatment and the size and location of the tumor within the eye. Severe complications requiring enucleation have been reported to occur in 10%86 to 22%65 of patients. Rubeosis iridis and neovascular glaucoma87 and radiation retinopathy or optic neuropathy60,81,88,89 have been reported as the major complications that may occur in irradiated eyes. Lens opacification is a common complication of helium ion90 and proton beam91 radiotherapy, occurring in over 40% of cases in published series. Development of cataracts is also relatively common after episcleral plaque therapy67,92,93 with reported 5-year incidence ranging from about 20%67 to 37%.93
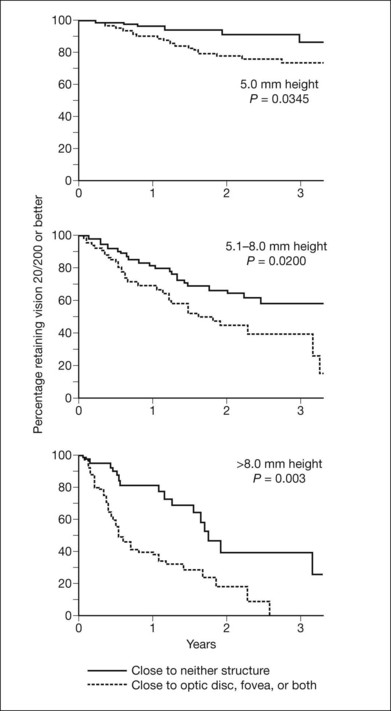
Vision after proton beam irradiation has been studied,55,56 and greater tumor thickness and proximity to the disc or macula (or high dose to these structures) were found to increase the risk of vision loss to worse than 20/200. Curves demonstrating cumulative probability of visual acuity loss according to height and distance of the tumor from the optic disc and fovea are presented in Figure 140.1. When patients were evaluated according to combined tumor characteristics, the probability of retaining vision of 20/200 or better at 3 years was 91% in the low-risk group (tumors 5 mm in height and more than two disc diameters from the macula and disc), 61% in the intermediate-risk group (tumors that were tall or close to the disc or macula), and 24% in the high-risk group (tumors that were tall and close to the disc or macula).
Visual field deficits have also been demonstrated after proton beam irradiation.94 Visual acuity outcomes similar to those in proton beam-irradiated patients were seen after iodine-125 plaque.95 In a review of 93 patients undergoing plaque therapy for juxtapapillary lesions,74 72% of patients lost at least three lines of visual acuity as measured on a Snellen chart by 4–5 years after treatment. The risk of losing this amount of vision was related to initial visual acuity; patients with better initial visual acuity (i.e. 20/20–20/40) were more likely to experience a loss of three or more lines than those with initial visual acuity of 20/50–20/100 or worse than 20/100 (67%, 64%, and 8%, respectively, at 5 years after treatment). In another study of 186 patients treated by helium ion irradiation, 49% retained 20/200 or better vision in the treated eye, with a median follow-up time of 26 months.96
In addition to measured visual acuity, visual functioning is a major concern after treatment for uveal melanoma. Augsburger et al.97 evaluated vision-related changes in employment and ability to drive, read, and watch television by standardized interview in patients treated by plaque radiotherapy or enucleation. Among 51 patients in the plaque radiotherapy group followed for a mean interval of 87 months after treatment, over 90% had no loss of vision-related performance. Similar results were reported in 51 enucleated patients followed for an average of 89 months. Thus, despite potential acuity loss in the affected eye, the vast majority of patients treated with plaque radiotherapy or enucleation retained full functioning in vision-related activities.
Clinical prognostic indicators for metastasis
Tumor size
Tumor size is an important prognostic factor for eventual metastasis. Size has been variously defined in different studies as the largest tumor dimension: height and diameter;51–56 largest diameter in contact with the sclera;98 combination of largest diameter and height;99 area of the tumor base,100 and tumor volume.101,102 These differences should be considered when comparing studies of tumor size and prognosis. Kaplan–Meier survival curves that show steadily worsening prognosis with every 2 mm increase in largest tumor dimension are presented in Figure 140.2.54
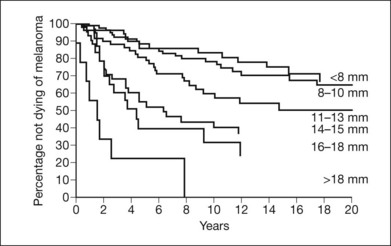
Fig. 140.2 Cumulative probability of not dying of melanoma and largest tumor dimension.
(Reproduced with permission from Seddon JM, Albert DM, Lavin PJ et al. A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol 1983;101:1894–1899. ©1983 American Medical Association. All rights reserved.)
Flocks et al.101 were among the first to recognize the importance of tumor size. Using Armed Forces Institute of Pathology data, they estimated tumor volume as the product of the tumor’s largest diameter, diameter perpendicular to the largest diameter, and height. When tumors were divided into two groups, based on whether they were larger or smaller than the median value of 1344 mm3, the 5-year mortality rates were 15% and 54% for the smaller and larger tumors, respectively. McLean et al.102 reported a somewhat higher 6-year mortality for tumors less than 1400 mm3 (25%), one difference being that Flocks et al. had excluded tumors with extrascleral extension.
The great majority of subsequent studies have confirmed the influence of tumor size on prognosis. Most of these studies have considered small tumors to be those no larger than 10 mm in largest diameter and 2–3 mm in height. Warren99 studied 108 patients from eastern Iowa with five or more potential years of follow-up. No deaths from metastases occurred in the group of ten patients with tumors up to 10 mm in diameter and 2 mm in thickness. Tumors were considered medium-sized if they were no larger than 15 mm in diameter and 5 mm in height, and nine of 24 patients (37%) in this group died of metastasis. Mortality was highest in patients with large tumors; over half of patients (57%) died of the disease in this group. Shammas and Blodi98 updated this series with additional cases, bringing the total to 293, and classified tumors according to largest diameter in contact with the sclera. Actuarial 6-year survival rates dropped with increasing tumor diameter from 87% for tumors 10 mm or less, to 30% for tumors larger than 12 mm. Long-term survival was found by Jensen100 to be quite poor even in patients with small tumors. He reported that of the 30 patients whose tumors were less than 10 mm in diameter and 3 mm in height, 12 (40%) died of metastatic disease. The corresponding figure for tumors with largest cross-sectional area >100 mm2 was 63%. Among clinical parameters available before treatment, largest tumor dimension (classified as <10 mm, 10–15 mm, or >15 mm) was found to be the most useful predictor of metastasis and mortality in a series of 111 patients.79 In that study, the sensitivity, specificity, and positive and negative predictive value of largest tumor dimension were similar to those of cell type. Other reports including meta-analyses and reviews of large series of patients with uveal melanoma confirm the importance of tumor size.103,104
Histopathologic prognostic indicators for metastasis
Histopathology and immunogenetics
In 1931, Callender105 published the first classification system of uveal melanoma according to histopathologic type. Callender described five histologic types: spindle cell subtypes A and B, epithelioid, fascicular, and mixed cell-type tumors composed of both spindle cells and epithelioid cells. Although this original system has undergone some refinement, it still serves as the basis for histologic typing of uveal melanomas.
The Callender classification system has been found in many studies to be a useful method for distinguishing tumors with varying degrees of malignancy. Jensen100 evaluated mortality according to cell type in his series of 226 Danish patients and compared his results with those of the previously published series of Zimmerman et al.106 In these reports, 10-year mortality ranged from 11% to 19% in spindle A tumors; 21–36% in spindle B tumors; 63–79% in mixed-cell tumors, and 72–100% in epithelioid tumors.
Limitations of the Callender system have been recognized. First, there are no histologic criteria to distinguish spindle A cell tumors from nevi. Second, there are no criteria for classification of a tumor composed of a mixture of spindle A and spindle B cells and no differentiation among the large group of tumors classified as mixed. To quantify these histologic observations, a standard measurement was created and new parameters were assessed including the number of epithelioid cells per high-power field and inverse standard deviation of nucleolar area,107 which were found to predict metastatic death. These methods require enucleation of the globe in order to obtain histopathology. Increasingly, globe-conserving therapies are the preferred treatment approach for local tumor control when possible. Furthermore, the cytology provided by the use of fine-needle aspiration biopsy in non-enucleated tumors is considered inadequate for evaluation of cellular morphology. Thus, molecular risk factors, discussed below, are of increasing value for determining prognosis.
Tumor microvasculature
The presence of epithelioid cells,107 extravascular matrix patterns that reflect the arrangement of tumor microcirculation,108–111 high microvascular density112–114 and large numbers of tumor-infiltrating macrophages115,116 in primary uveal melanoma, are independently associated with shorter time to metastatic death. These associations may be either markers of aggressive tumors without direct contribution to the metastatic cascade or they may indicate direct participation in tumor progression to metastasis. Toivonen et al. found that the presence of epithelioid cells and microvascular density were closely associated with progression of uveal melanoma from primary tumor to metastasis, in a cross-sectional histopathologic analysis of hepatic metastasis and the corresponding primary choroidal tumor. High microvascular density may help to predict survival after detection of hepatic metastases.117
Microvascular patterns within tumor tissue have been studied. Folberg et al. described nine distinct vascular patterns observed in tissue from 234 enucleated uveal melanomas.108 Two observers independently reviewed slides from each tumor and assessed the presence or absence of each vascular pattern. The presence of networks of three or more contiguous closed vascular loops was highly predictive of melanoma-related and all-cause mortality. Kaplan–Meier estimates of 10-year survival were 50.7% when networks were present and 88.3% in the absence of networks. McLean,110 in a series of 496 eligible uveal melanoma patients from the registry of ophthalmic pathology, confirmed Folberg’s observation of the prognostic significance of vascular loops, despite the use of different measurement techniques. Foss et al. investigated vascular patterns and microvessel density in a cohort of 120 patients with available tissue blocks and survival follow-up (mean, 77 months).113 Vessel count was highly predictive of melanoma-related death; vascular patterns as described by Folberg,108 were less clearly correlated with melanoma mortality in this study. Principal component analysis of vascular patterns from this cohort of patients118 identified three combinations of patterns, two of which – disordered growth and emergence of rapidly growing subclones – were predictive of melanoma mortality. However, as with histopathology, tumor microvasculature cannot be evaluated in this manner unless the tumor is enucleated.
Extrascleral extension
Another factor shown in many studies to increase the risk of tumor-related death after enucleation is extrascleral extension of the tumor. Histopathologic evidence of orbital extension is usually found in 8–14% of cases.119–121 Shammas and Blodi121 reported that only eight of 30 patients with extrascleral extension and 5 potential years of follow-up survived to 5 years. The majority died within 16 months after enucleation. The 5-year survival rate was 26% in patients with orbital extension and 78% in those without extension. In Jensen’s 25-year follow-up of patients,100 the long-term survival estimate for patients with orbital extension was 29%. The relationship between the probability of not dying of melanoma and the presence of extrascleral extension was evaluated in another group of patients using new statistical methods in 1983, including Kaplan–Meier survival curves and Cox proportional hazards models.54 Much poorer survival was seen in the group with extrascleral extension. There are no data to suggest that exenteration improves mortality in patients with extrascleral extension.119,121,122
Molecular prognostic indicators for metastasis
Tumor cytogenetic studies have revealed that abnormalities in chromosomes 3, 6 and 8 in the tumor tissue itself have been linked to metastatic death.123–129 A loss of chromosome 3 is associated with a poor prognosis; gains in chromosome 8 are associated with a worse prognosis, and abnormalities in chromosome 6 are associated with a good prognosis. Rather than simply markers of tumor progression, gross chromosomal changes may be associated with specific mutations that become manifest with chromosomal aberration.
White et al.130 performed cytogenetic analyses on 54 tumor patients and correlated the results with the patient clinical outcome. It was found that abnormalities in both chromosomes 3 and 8 were important in predicting metastasis; abnormalities in chromosome 6, despite abnormalities in 3 and 8 portended a good prognosis. Prescher et al.131 examined 180 uveal melanoma patients in whom primary enucleation was the selected treatment. Tumor histology, cytogenetics and clinical data were obtained from the tumor specimens. The tumors of 30 patients had monosomy 3. This resulted in 57% of these patients succumbing to metastasis and the 3-year relapse-free survival rate was 50%. Of the 24 patients with a normal chromosome 3, none developed metastasis. Monosomy 3, tumor location and tumor diameter were the most significant predictors of poor prognosis. Furthermore, histological subtype, age, sex, extrascleral growth and tumor thickness had no predictive value. Scholes et al.132 also found that monosomy 3 and the development of metastasis did not correlate with tumor size or histologic subtype, emphasizing the likely inadequacy of size and histology alone as good predictors for metastasis. Thus, cytogenetically speaking, uveal melanoma appears to fall into two largely mutually exclusive cytogenetic groups: those patients who will go on to develop fatal metastasis (monosomy 3 of the tumor) and those patients who will not (absence of monosomy 3). Work by Onken et al.133 has demonstrated the results of gene expression profiling in 25 primary choroidal melanoma specimens. The tumors clustered into two groups which correlated strongly with metastatic risk.
Prognosticating the risk of developing metastatic uveal melanoma has traditionally been based on clinical and histopathologic characteristics of the primary tumor, as discussed previously. However, as the majority of uveal melanomas are now being treated with globe-sparing surgery, analysis of molecular and cytogenetic information from uveal melanoma fine-needle aspiration biopsy has evolved and is becoming increasingly common.134–137 Both DNA and RNA can be analyzed from the tumor. Cytogenetic testing can be performed for chromosomes of interest using fluorescence in situ hybridization (FISH). FISH testing may be performed in a hospital clinical cytogenetics laboratory. However, other assessment techniques that are investigational and less widely available include whole-genome single-nucleotide polymorphism (SNP), multiplex ligation-dependent probe amplification (MLPA) and microsatellite analysis (MSA).
1 Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. III. Initial mortality findings. COMS report no. 18. Arch Ophthalmol. 2001;119:969–982.
2 Collaborative Ocular Melanoma Study Group. Ten-year follow-up of fellow eyes of patients enrolled in Collaborative Ocular Melanoma Study randomized trials: COMS report no. 22. Ophthalmology. 2004;111:966–976.
3 The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. V. Twelve-year mortality rates and prognostic factors. COMS report no. 28. Arch Ophthalmol. 2006;123:1684–1693.
4 Jampol LM, Moy CS, Murray TG, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma. IV: Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology. 2002;109:2197–2206.
5 Sagoo MS, Shields CL, Mashayekhi A, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: tumor control in 650 consecutive cases. Ophthalmology. 2011;118:402–407.
6 McCannel TA, Chang MY, Burgess BL. Multi-year follow-up of fine needle aspiration biopsy in choroidal melanoma. Ophthalmology. 2012. [Epub ahead of print]
7 Correa R, Pera J, Gomez J, et al. (125)I episcleral plaque brachytherapy in the treatment of choroidal melanoma: a single-institution experience in Spain. Brachytherapy. 2009;8:290–296.
8 Tabandeh H, Chaudhry NA, Murray TG, et al. Intraoperative echographic localization of iodine-125 episcleral plaque for brachytherapy of choroidal melanoma. Am J Ophthalmol. 2000;129:199–204.
9 Packer S, Stoller S, Lesser ML, et al. Long-term results of iodine 125 irradiation of uveal melanoma. Ophthalmology. 1992;99:767–774.
10 Garretson BR, Robertson DM, Earle JD. Choroidal melanoma treatment with iodine 125 brachytherapy. Arch Ophthalmol. 1987;105:1394–1397.
11 Bosworth JL, Packer S, Rotman M, et al. Choroidal melanoma: I-125 plaque therapy. Radiology. 1988;169:249–251.
12 Sia S, Harper C, McAllister I, et al. Iodine-I25 episcleral plaque therapy in uveal melanoma. Clin Experiment Ophthalmol. 2000;28:409–413.
13 Quivey JM, Char DH, Phillips TL, et al. High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol Biol Phys. 1993;26:613–618.
14 Fontanesi J, Meyer D, Xu S, et al. Treatment of choroidal melanoma with I-125 plaque. Int J Radiat Oncol Biol Phys. 1993;26:619–623.
15 Jensen AW, Petersen IA, Kline RW, et al. Radiation complications and tumor control after 125I plaque brachytherapy for ocular melanoma. Int J Radiat Oncol Biol Phys. 2005;63:101–108.
16 Karlovits B, Trombetta MG, Verstraeten T, et al. Local control and visual acuity following treatment of medium-sized ocular melanoma using a contact eye plaque: A single surgeon experience. Brachytherapy. 2011;10:228–231.
17 Nag S, Wang D, Wu H, et al. Custom-made “Nag” eye plaques for 125I brachytherapy. Int J Radiat Oncol Biol Phys. 2003;56:1373–1380.
18 Lumbroso-Le Rouic L, Charif Chefchaouni M, Levy C, et al. 125I plaque brachytherapy for anterior uveal melanomas. Eye (Lond). 2004;18:911–916.
19 Sobrin L, Schiffman JC, Markoe AM, et al. Outcomes of iodine 125 plaque radiotherapy after initial observation of suspected small choroidal melanomas: a pilot study. Ophthalmology. 2005;112:1777–1783.
20 Leonard KL, Gagne NL, Mignano JE, et al. A 17-year retrospective study of institutional results for eye plaque brachytherapy of uveal melanoma using (125)I, (103)Pd, and (131)Cs and historical perspective. Brachytherapy. 2011;10:331–339.
21 Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579–1587.
22 Puusaari I, Damato B, Kivela T. Transscleral local resection versus iodine brachytherapy for uveal melanomas that are large because of tumour height. Graefes Arch Clin Exp Ophthalmol. 2007;245:522–533.
23 Rouberol F, Roy P, Kodjikian L, et al. Survival, anatomic, and functional long-term results in choroidal and ciliary body melanoma after ruthenium brachytherapy (15 years’ experience with beta-rays). Am J Ophthalmol. 2004;137:893–900.
24 Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579–1587.
25 Mosci C, Mosci S, Barla A, et al. Proton beam radiotherapy of uveal melanoma: Italian patients treated in Nice, France. Eur J Ophthalmol. 2009;19:654–660.
26 Gragoudas ES, Lane AM, Munzenrider J, et al. Long-term risk of local failure after proton therapy for choroidal/ciliary body melanoma. Trans Am Ophthalmol Soc. 2002;100:43–48. discussion 48–49
27 Eide N. Primary laser photocoagulation of “small” choroidal melanomas. Acta Ophthalmol Scand. 1999;77:351–354.
28 Jaffe GJ, Mieler WF, Burke JM, et al. Photoablation of ocular melanoma with a high-powered argon endolaser. Arch Ophthalmol. 1989;107:113–118.
29 Tsai T, O’Brien JM, Engstrom R, et al. Extrascleral extension of a choroidal melanoma after argon photocoagulation and transpupillary thermotherapy. Br J Ophthalmol. 2002;86:358–359.
30 Donaldson MJ, Lim L, Harper CA, et al. Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin Experiment Ophthalmol. 2005;33:548–549.
31 Soucek P, Cihelkova I. Photodynamic therapy with verteporfin in subfoveal choroidal melanoma (A controlled case). Neuro Endocrinol Lett. 2006;27:145–148.
32 Barbazetto IA, Lee TC, Rollins IS, et al. Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol. 2003;135:898–899.
33 Shields CL, Shields JA, Perez N, et al. Primary transpupillary thermotherapy for small choroidal melanoma in 256 consecutive cases: outcomes and limitations. Ophthalmology. 2002;109:225–234.
34 Aaberg TM, Jr., Bergstrom CS, Hickner ZJ, et al. Long-term results of primary transpupillary thermal therapy for the treatment of choroidal malignant melanoma. Br J Ophthalmol. 2008;92:741–746.
35 Zaldivar RA, Aaberg TM, Sternberg P, et al. Clinicopathologic findings in choroidal melanomas after failed transpupillary thermotherapy. Am J Ophthalmol. 2003;135:657–663.
36 Harbour JW, Meredith TA, Thompson PA, et al. Transpupillary thermotherapy versus plaque radiotherapy for suspected choroidal melanomas. Ophthalmology. 2003;110:2207–2214. discussion 2215
37 Bartlema YM, Oosterhuis JA, Journee-De Korver JG, et al. Combined plaque radiotherapy and transpupillary thermotherapy in choroidal melanoma: 5 years’ experience. Br J Ophthalmol. 2003;87:1370–1373.
38 Damato B, Lecuona K. Conservation of eyes with choroidal melanoma by a multimodality approach to treatment: an audit of 1632 patients. Ophthalmology. 2004;111:977–983.
39 Peyman GA, Juarez CP, Diamond G, et al. Ten years’ experience with eye wall resection for uveal malignant melanomas. Ophthalmology. 1984;91:1720–1725.
40 Kivela T, Puusaari I, Damato B. Transscleral resection versus iodine brachytherapy for choroidal malignant melanomas 6 millimeters or more in thickness: a matched case–control study. Ophthalmology. 2003;110:2235–2244.
41 Shields JA, Shields CL, Shah P, et al. Partial lamellar sclerouvectomy for ciliary body and choroidal tumors. Ophthalmology. 1991;98:971–983.
42 Damato BE, Paul J, Foulds WS. Risk factors for residual and recurrent uveal melanoma after trans-scleral local resection. Br J Ophthalmol. 1996;80:102–108.
43 Puusaari I, Damato B, Kivela T. Transscleral local resection versus iodine brachytherapy for uveal melanomas that are large because of tumour height. Graefes Arch Clin Exp Ophthalmol. 2007;245:522–533.
44 Garcia-Arumi J, Zapata MA, Balaguer O, et al. Endoresection in high posterior choroidal melanomas: long-term outcome. Br J Ophthalmol. 2008;92:1040–1045.
45 Kertes PJ, Johnson JC, Peyman GA. Internal resection of posterior uveal melanomas. Br J Ophthalmol. 1998;82:1147–1153.
46 Karkhaneh R, Chams H, Amoli FA, et al. Long-term surgical outcome of posterior choroidal melanoma treated by endoresection. Retina. 2007;27:908–914.
47 Hawkins BS, Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol. 2004;138:936–951.
48 Diener-West M, Reynolds SM, Agugliaro DJ, et al. Collaborative Ocular Melanoma Study Group. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123:1639–1643.
49 Rietschel P, Panageas KS, Hanlon C, et al. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23:8076–8080.
50 Eskelin S, Pyrhönen S, Summanen P, et al. Tumor doubling times in metastatic malignant melanoma of the uvea: tumor progression before and after treatment. Ophthalmology. 2000;107:1433–1439.
51 Seddon JM, Gragoudas ES, Egan K, et al. Standardized data collection and coding in eye disease epidemiology: The uveal melanoma data system. Ophthalmic Surg. 1991;22:127–136.
52 Seddon JM, Polivogianis L, Gragoudas ES, et al. Enucleation vs cobalt 60 irradiation of melanomas. Arch Ophthalmol. 1986;104:175–176.
53 Seddon JM, Gragoudas ES, Albert D, et al. Comparison of survival rates for patients with uveal melanoma after treatment with proton beam irradiation or enucleation. Am J Ophthalmol. 1985;99:282–290.
54 Seddon JM, Albert DM, Lavin PT, et al. A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol. 1983;101:1894–1899.
55 Seddon JM, Gragoudas ES, Polivogianis L, et al. Visual outcome after proton beam irradiation of uveal melanoma. Ophthalmology. 1986;93:666–674.
56 Seddon JM, Gragoudas ES, Egan K, et al. Uveal melanomas near the optic disc or fovea: visual results after proton beam irradiation. Ophthalmology. 1987;94:354–361.
57 Augsburger JJ, Gamel JW, Sardi VF, et al. Enucleation vs. cobalt plaque radiotherapy for malignant melanomas of the choroid and ciliary body. Arch Ophthalmol. 1986;104:655–661.
58 Gragoudas ES, Seddon JM, Egan M, et al. Metastasis from uveal melanoma after proton beam irradiation. Ophthalmology. 1988;95:992–999.
59 Gragoudas ES, Seddon JM, Egan KM, et al. Long-term results of proton beam irradiated uveal melanomas. Ophthalmology. 1987;94:349–353.
60 Lommatzsch PK. Results after B-irradiation (106Ru/106Rh) of choroidal melanomas: 20 years’ experience. Br J Ophthalmol. 1986;70:844–851.
61 Kleineidam M, Guthoff R, Bentzen SM. Rates of local control metastasis and overall survival in patients with posterior uveal melanomas treated with ruthenium-106 plaques. Radiother Oncol. 1993;28:148–156.
62 Seregard S, Trampe E, Lax I, et al. Results following episcleral ruthenium plaque radiotherapy for posterior uveal melanoma. Acta Ophthalmol Scand. 1997;75:11–16.
63 Summanen P, Immonen I, Heikkonen J, et al. Survival of patients and metastatic and local recurrent tumor growth in malignant melanoma of the uvea after ruthenium plaque radiotherapy. Ophthalmic Surg. 1993;24:82–90.
64 Char DH, Castro J, Kroll S, et al. Five-year follow-up of helium ion therapy for uveal melanoma. Arch Ophthalmol. 1990;108:209–214.
65 Char DH, Kroll SM, Castro J. Ten-year follow-up of helium-ion therapy for uveal melanoma. Am J Ophthalmol. 1998;125:81–89.
66 Packer S, Stoller S, Lesser ML, et al. Long-term results of iodine-125 irradiation of uveal melanoma. Ophthalmology. 1992;99:767–773.
67 Quivey JM, Char DH, Phillips TL, et al. High intensity 125-iodine (125I) plaque treatment of uveal melanoma. Int J Radiat Oncol. 1993;26:613–618.
68 Seddon JM, Gragoudas ES, Egan KM, et al. Relative survival rates after alternative therapies for uveal melanoma. Ophthalmology. 1990;97:769–777.
69 Egan KM, Walsh SM, Seddon JM, et al. An evaluation of the influence of reproductive factors on the risk of metastases from uveal melanoma. Ophthalmology. 1993;100:1160–1165.
70 Augsburger JJ, Gamel JW, Shields JA, et al. Post-irradiation regression of choroidal melanomas as a risk factor for death from metastatic. Ophthalmology. 1987;94:1173–1177.
71 Glynn RJ, Seddon JM, Gragoudas ES, et al. Evaluation of tumor regression and other prognostic factors for early and late metastasis after proton irradiation of uveal melanoma. Ophthalmology. 1989;96:1566–1573.
72 Gragoudas ES, Egan KM, Seddon JM, et al. Intraocular recurrence of uveal melanoma after proton beam irradiation. Ophthalmology. 1992;99:760–766.
73 Vrabec TR, Augsburger JJ, Gamel JW, et al. Impact of local tumor relapse on patient survival after cobalt 60 plaque radiotherapy. Ophthalmology. 1991;98:984–988.
74 DePotter P, Shields CL, Shields JA, et al. Plaque radiotherapy for juxtapapillary choroidal melanoma: visual acuity and survival outcome. Arch Ophthalmol. 1996;114:1357–1365.
75 Harbour JW, Char DH, Kroll S, et al. Metastatic risk for distinct patterns of post-irradiation local recurrence of posterior uveal melanoma. Ophthalmology. 1997;104:1785–1793.
76 Jensen OA. Malignant melanomas of the human uvea: 25-year follow-up of cases in Denmark, 1943–1952. Acta Ophthalmol. 1982;60:161–182.
77 McLean IW, Foster WD, Zimmerman LE, et al. Interred natural history of uveal melanoma. Invest Ophthalmol Vis Sci. 1980;19:760–770.
78 Raivio I. Uveal melanoma in Finland: an epidemiological, clinical, histological, and prognostic study. Acta Ophthalmol Suppl. 1977;133:3–64.
79 Lavin P, Albert D, Seddon J. A deficit survival analysis to assess the natural history of uveal melanomas. J Chronic Dis. 1984;37:481–487.
80 Coleman K, Baak JP, Van Diest P, et al. Prognostic factors following enucleation of 111 uveal melanomas. Br J Ophthalmol. 1993;77:688–692.
81 Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98:383–390.
82 Kath R, Hayungs J, Bornfeld N, et al. Prognosis and treatment of disseminated uveal melanoma. Cancer. 1993;72:2219–2223.
83 Rajpal S, Moore R, Karakousis CP. Survival in metastatic ocular melanoma. Cancer. 1983;52:334–336.
84 Singh AD. Uveal melanoma: implications of tumor doubling time. Comment in Ophthalmology. 2001;108:831–832.
85 Manschot WA, van Strik R. Uveal melanoma: therapeutic consequences of doubling times and irradiation results; a review. Int Ophthalmol. 1992;16:91–99.
86 Egan KM, Gragoudas ES, Seddon JM, et al. The risk of enucleation after proton beam irradiation of uveal melanoma. Ophthalmology. 1989;96:1377–1382.
87 Paul EV, Parnell BL, Fraker M. Prognosis of malignant melanomas of the choroid and ciliary body. Ophthalmol Clin. 1962;2:387–402.
88 Garretson JP, Robertson DM, Earle JD. Choroidal melanoma treatment with iodine-125 brachytherapy. Arch Ophthalmol. 1987;105:1394–1397.
89 Char DH, Quivey J, Castro J, et al. Helium ions versus iodine-125 brachytherapy in the management of uveal melanoma: a prospective, randomized, dynamically balanced trial. Ophthalmology. 1993;100:1547–1554.
90 Meecham WJ, Char DH, Kroll S, et al. Anterior segment complications after helium ion radiation therapy for uveal melanoma: radiation cataract. Arch Ophthalmol. 1994;112:197–203.
91 Gragoudas ES, Egan KM, Walsh SM, et al. Lens changes after proton beam irradiation for uveal melanoma. Am J Ophthalmol. 1995;119:157–164.
92 Kleineidam M, Augsburger JJ, Hernandez C, et al. Cataractogenesis after Cobalt-60 eye plaque radiotherapy. Int J Radiat Oncol Biol Phys. 1993;26:625–630.
93 Summanen P, Immonen I, Kivela T, et al. Radiation related complications after ruthenium plaque radiotherapy of uveal melanoma. Br J Ophthalmol. 1996;80:732–739.
94 Park SS, Walsh SM, Gragoudas ES. Visual-field deficits associated with proton beam irradiation for parapapillary choroidal melanoma. Ophthalmology. 1996;103:110–116.
95 Fontanesi J, Meyer D, Xu S, et al. Treatment of choroidal melanoma with I-125 plaque. Int J Radiat Oncol Biol Phys. 1993;26:619–623.
96 Lindstadt D, Char DH, Castro J, et al. Vision following helium ion radiotherapy of uveal melanoma: a northern California Oncology group study. Int J Radiat Oncol Biol Phys. 1988;25:347–352.
97 Augsburger JJ, Goel SD. Visual function following enucleation or episcleral plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol. 1994;112:786–789.
98 Shammas HF, Blodi FC. Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol. 1977;95:63–69.
99 Warren RM. Prognosis of malignant melanomas of the choroid and ciliary body. In: Blodi FC, ed. Current concepts in ophthalmology. Mosby: St Louis, 1974.
100 Jensen OA. Malignant melanomas of the uvea in Denmark. 1943–1952. Acta Ophthalmol Suppl. 1963;75:1–220.
101 Flocks M, Gerende JH, Zimmerman LE. The size and shape of malignant melanomas of the choroid and ciliary body in relation to prognosis and histologic characteristics: a statistical study of 210 tumors. Trans Am Acad Ophthalmol Otolaryngol. 1955;59:740–758.
102 McLean IW, Foster WD, Zimmerman LE. Prognostic factors in small malignant melanomas of choroid and ciliary body. Arch Ophthalmol. 1977;95:48–58.
103 Diener-West M, Hawkins BS, Markowitz JA, et al. A review of mortality from choroidal melanoma. Arch Ophthalmol. 1992;110:245–250.
104 Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–998.
105 Callender GR. Malignant melanotic tumors of the eye: a study of histologic types in 111 cases. Trans Am Acad Ophthalmol Otolaryngol. 1931;3:131–142.
106 Zimmerman LE, McLean IW, Foster WD. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells? Br J Ophthalmol. 1978;62:420–425.
107 Seddon J, Polivogianis L, Chung-c Hsieh, et al. Death from uveal melanoma: number of epithelioid cells and inverse SD of nucleolar area as prognostic factors. Ophthalmology. 1987;105:801–806.
108 Folberg R, Rummelt V, Parys-Van Ginderdeuren R, et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993;100:1389–1398.
109 Makitie T, Summanen P, Tarkkanen A, et al. Microvascular loops and networks as prognostic indicators in choroidal and ciliary body melanomas. J Natl Cancer Inst. 1999;91:359–367.
110 McLean IW, Keefe KS, Burnier MN. Uveal melanoma. Comparison of the prognostic value of fibrovascular loops, mean of the ten largest nucleoli, cell type, and tumor size. Ophthalmology. 1997;104:777–780.
111 Seregard S, Spangberg B, Juul C, et al. Prognostic accuracy of the mean of the largest nucleoli, vascular patterns, and PC-10 in posterior uveal melanoma. Ophthalmology. 1998;105:485–491.
112 Chen X, Maniotis AJ, Majumdar D, et al. Uveal melanoma cell staining for CD34 and assessment of tumor vascularity. Invest Ophthalmol Vis Sci. 2002;43:2533–2539.
113 Foss AJ, Alexander RA, Jefferies LW, et al. Microvessel count predicts survival in uveal melanoma. Cancer Res. 1996;56:2900–2903.
114 Makitie T, Summanen P, Tarkkanen A, et al. Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci. 1999;40:2471–2480.
115 Clarijs R, Schalkwijk L, Ruiter DJ, et al. Lack of lymphangiogenesis despite coexpression of VEGF-C and its receptor Flt-4 in uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1422–1428.
116 Makitie T, Summanen P, Tarkkanen A, et al. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421.
117 Toivonen P, Makitie T, Kujala E, et al. Microcirculation and tumor-infiltrating macrophages in choroidal and ciliary body melanoma and corresponding metastases. Invest Ophthalmol Vis Sci. 2004;45:1–6.
118 Foss AJ, Alexander RA, Hungerford JL, et al. Reassessment of the PAS patterns in uveal melanoma. Br J Ophthalmol. 1997;81:240–246.
119 Affeldt JC, Minckler DS, Azen SP, et al. Prognosis in uveal melanoma with extrascleral extension. Arch Ophthalmol. 1980;98:1975–1979.
120 Pach JM, Robertson DM, Taney BS, et al. Prognostic factors in choroidal and ciliary body melanomas with extrascleral extension. Am J Ophthalmol. 1986;101:325–331.
121 Shammas HF, Blodi FC. Orbital extension of choroidal and ciliary body melanomas. Arch Ophthalmol. 1977;95:2002–2005.
122 Kersten RC, Tse DT, Anderson RL, et al. The role of orbital exenteration in choroidal melanoma with extrascleral extension. Ophthalmology. 1985;92:436–443.
123 Tschentscher F, Prescher G, Zeschnigk M, et al. Identification of chromosomes 3, 6, and 8 aberrations in uveal melanoma by microsatellite analysis in comparison to comparative genomic hybridization. Cancer Genet Cytogenet. 2000;122:13–17.
124 White VA, McNeil BK, Horsman DE. Acquired homozygosity (isodisomy) of chromosome 3 in uveal melanoma. Cancer Genet Cytogenet. 1998;102:40–45.
125 Sisley K, Rennie IG, Parsons MA, et al. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer. 1997;19:22–28.
126 White VA, McNeil BK, Thiberville L, et al. Acquired homozygosity (isodisomy) of chromosome 3 during clonal evolution of a uveal melanoma: association with morphologic heterogeneity. Genes Chromosomes Cancer. 1996;15:138–143.
127 Speicher MR, Prescher G, du Manoir S, et al. Chromosomal gains and losses in uveal melanomas detected by comparative genomic hybridization. Cancer Res. 1994;54:3817–3823.
128 Horsman DR, White VA. Cytogenetic analysis of uveal melanoma. Consistent occurrence of monosomy 3 and trisomy 8q. Cancer. 1993;71:811–819.
129 Sisley K, Rennie IG, Cottam DW, et al. Cytogenetic findings in six posterior uveal melanomas: involvement of chromosomes 3, 6, and 8. Genes Chromosomes Cancer. 1990;2:205–209.
130 White VA, Chambers JD, Courtright PD, et al. Correlation of cytogenetic abnormalities with the outcome of patients with uveal melanoma. Cancer. 1998;83:354–359.
131 Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225.
132 Scholes AG, Damato BE, Nunn J, et al. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003;44:1008–1011.
133 Onken MD, Worley LA, Ehlers JP, et al. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209.
134 Young TA, Rao NP, Glasgow BJ, et al. Fluorescent in situ hybridization of for monosomy 3 via 30-gauge fine needle aspiration biopsy of choroidal melanoma in vivo. Ophthalmology. 2007;114:142–146.
135 Shields CL, Materin MA, Teixiera L, et al. Small choroidal melanoma with chromosome 3 monosomy on fine needle aspiration biopsy. Ophthalmology. 2007;114:1919–1924.
136 Shields JA, Shields CL, Materin MA, et al. Role of cytogenetics in the management of uveal melanoma. Arch Ophthalmol. 2008;126:416–419.
137 Shields CL, Ganguly A, Bianciotto CG, et al. Prognosis of uveal melanoma in 500 cases using genetic testing of needle aspiration biopsy specimens. Ophthalmology. 2011;118:396–401.