CHAPTER 33 Production and Flow of Cerebrospinal Fluid
Continually generated and flowing cerebrospinal fluid (CSF) is vital for brain homeostasis.1 Day and night, the choroid plexus churns out fluid into the ventricles. CSF formation is about 0.4 mL/min per gram of choroid plexus. Nascent CSF is more than a passive ultrafiltrate of plasma.2 Active secretion by choroidal epithelium is exquisitely modulated so that intracranial pressure (ICP) is stable if CSF absorption is normal. A tenth of the choroidal blood flow of about 4 mL/min per gram3,4 becomes new CSF in the ventricular spaces.
Multisource evidence indicates that 70% to 80% of CSF is formed by the plexuses in the lateral, third, and fourth ventricles. Human CSF elaboration is mechanistically similar to that in many mammalian species. Extrachoroidal formation of a CSF-like fluid occurs at the cerebral capillary wall,5 but it is less efficient compared with choroid plexus generation. A lavish turnover of fluid at the blood-CSF interface (choroidal epithelium) is engendered by a high blood flow to the plexus, substantial activities of Na+,K+-ATPase6,7 and carbonic anhydrase,8,9 and a plethora of ion transporters. Stable composition is the hallmark of CSF. Extracellular ion stability is essential to neurotransmission.
Intricate Fluid Balance Within the Central Nervous System
To preserve sound ICP and volume, the central nervous system (CNS) relies on a battery of choroidal and extrachoroidal fluid-regulating mechanisms.2 Orderly CSF percolation depends on simultaneous, precisely controlled solute and water fluxes at several transport interfaces among blood, CSF, and brain.10 The choroid plexus at the blood-CSF border is at the heart of fluid dynamics in the CNS. This industrious secretory epithelium provides a steady fluid output that has an impact on the biochemical and biophysical integrity of the brain. Disturbance of CSF flow disrupts extensive exchange between large-cavity CSF and brain interstitial fluid.11 Accordingly, the diminution of CSF formation and turnover rate in disease12 and senescence sets into motion many pathophysiologic cascades.
Variation in Cerebrospinal Fluid Production
Because CSF dynamics have an impact on brain metabolism, it is important to assess optimal CSF formation rate in health. Adult humans normally form CSF at about 0.35 mL/min.1,2 Nocturnally elevated CSF production13 may relate to altered cerebral metabolism during sleep. CSF formation also fluctuates in disease.14 Neurosurgeons face pathophysiologic situations in which the choroid plexus forms too much or not enough fluid. With hypersecreting choroid plexus papillomas,15,16 surgical excision often reduces ICP. With hyposecreting choroid plexus, as in normal-pressure hydrocephalus (NPH) and Alzheimer’s disease (AD), the stagnated CSF turnover rate17,18 may contribute to cerebral dysfunction. Disease-modified fluid formation by the choroid plexus can thus harm CSF dynamics and the extracellular environment of neurons.11 Accordingly, imbalanced CSF formation necessitates surgical or pharmacologic remediation.
Mechanisms of Cerebrospinal Fluid Formation by Choroid Plexus
Penetration of ions and water into the CNS occurs predominantly across the choroid plexus.2 Control of brain fluid balance therefore starts with a thorough knowledge of choroidal transport mechanisms. Fluid secretion into the ventricles is mediated by an array of ion transporters asymmetrically positioned at the blood- and CSF-facing membranes.2 Structurally and functionally, the choroid plexus epithelium resembles the kidney proximal tubule.19 Renal-like organs are designed to transfer a copious volume of fluid.
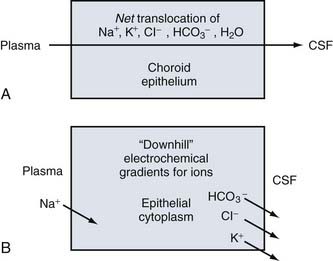
CSF production is directly proportional to the net transfer of Na+ and Cl− from blood to ventricles.20–22 Conversely, when CNS-inward Na+ and Cl− transport across the choroid plexus is reduced, CSF formation is attenuated.23,24 The driving force for ion movements across choroid plexus membranes is a downhill (energetically speaking) concentration or electrochemical gradient. For the external limiting membranes of the choroid plexus, the direction of the gradients for Na+, Cl−, K+, and HCO3− is given in Figure 33-1. Na+ entry into choroid plexus epithelium is downhill (gradient-wise) from plasma across the basolateral membrane. At the other side of the choroid cell, K+, Cl−, and HCO3− move downhill across the apical membrane into CSF. However, both basolaterally and apically, these downhill ionic movements are facilitated by uphill active transport (requiring chemical energy as ATP) by the primary Na+ pump (Fig. 33-2). Active Na+ pumping into CSF keeps choroid cell Na+ concentration relatively low,7 thereby establishing a basolateral inward driving force for Na+ from plasma into epithelium.25
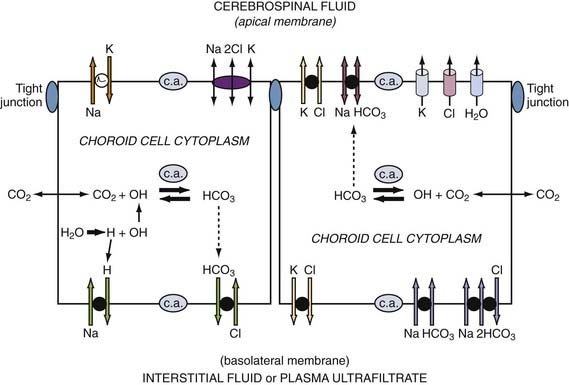
Epithelial transport polarity is fundamental to fluid formation. Polar distribution (sidedness) of specific active transporters and passive channels enables net fluid movement from blood to CSF (see Fig. 33-2). Basolateral (vascular) and apical (CSF) transporters and channels thus mediate the streaming of ions and water. Directionally, the fluxes are mainly from interstitium to parenchyma to ventricles. Figure 33-2 schematizes the polar distribution of primary and secondary active ion transporters. Channels allow passive diffusion of K+ and Cl− (apical efflux) into nascent CSF.26 Many ionic species are involved in CSF production (e.g., K+, Mg2+, and Ca2+). However, fluid formation is primarily (quantitatively) generated by net secretion of Na+, Cl−, and HCO3−. Water osmotically follows ion transport across the apical membrane (see Fig. 33-2). Such transfers occur by stepwise and parallel processes described in the following sections.
Sodium
Energetically, the pivotal initiating step in CSF formation is the primary active transport of Na+ from choroidal epithelium to ventricle.27 Na+,K+-ATPase activity empowers this Na+ pumping by generating ATP (see Fig. 33-2). To stabilize choroid pH and epithelial volume25,28 while CSF is elaborated, the Na+ efflux (apically) is balanced by continual Na+ influx (basolaterally) through Na+-H+ exchange and Na+ inward transport coupled with HCO3−.29,30
Chloride
As the main anion in CSF secretion, Cl− is actively transported across the basolateral membrane in exchange for cellular HCO3−.31 This pulls plasma Cl− into the epithelium for accumulation above electrochemical equilibrium.32 Under some conditions, intraepithelial Cl− diffuses into CSF through the efflux arm of the Na+-K+-Cl− cotransporter.33 However, the downhill diffusion of Cl− into CSF by way of apical Cl− channels is likely to be the main pathway by which Cl− accesses the ventricles to sustain fluid formation.26
Bicarbonate
HCO3− in choroid plexus has a dual source. First, carbonic anhydrase catalyzes the hydration of CO2 to form H+ and HCO3− ions in choroid plexus epithelial cells.8 In addition, HCO3− is pulled from plasma into the epithelium by Na+-coupled HCO3− transport.29 On accumulation, the HCO3− is available for release across the CSF-facing membrane by two mechanisms. Through one, HCO3− in the epithelium diffuses downhill through an anion channel into CSF.34 By another putative route, HCO3− is transferred by an electrogenic Na+-coupled HCO3− cotransporter at the apical membrane.28,35 HCO3−-rich nascent CSF reflects facilitated movement of this anion into ventricles as CSF is produced.36
Water
CSF is 99% water. The watery medium of CSF enables multiple buffering, distributive, and excretory functions.1 Therefore, it is important to thoroughly characterize water movement across the blood-CSF interface. After Na+, Cl−, and HCO3− transport into CSF, water chases these osmotically active ions into the ventricles by diffusing down its chemical potential gradient through aquaporin 1 (AQP1) channels in the apical membrane.37 AQP1 channel involvement in CSF formation is deduced from AQP1 knockout mice displaying substantially reduced fluid movement into the ventricles.38 As a result, ICP is lowered.39 Transcellular water diffusion across the choroid plexus is potentially a drug target in modulating CSF dynamics.
Regulation of Cerebrospinal Fluid Formation
CSF formation rate adjusted downward or upward is relevant to management of CSF disorders, such as elevated ICP and ventriculomegaly.2,40 Manipulation of choroid plexus ion transporters and channels is the key to achievement of finer control of epithelial fluid output. From a cellular physiology standpoint, there are multiple loci where CSF formation can be regulated. Two main targeting sites or strategies attempt to modulate choroid plexus secretory function: (1) manipulation of the concentrations of neurotransmitter and neuropeptide ligands that have receptors on choroid epithelial membranes that interface with the extracellular fluid and (2) use of diuretic-type agents to interfere with membrane-bound transporter proteins that effect ion-water fluxes. Many studies focus on reduction of CSF formation because both nature and clinicians try to prevent rises in ICP.
Neurohumoral Ligands and Receptors
Apical and basolateral membranes of choroid plexus contain receptors for biogenic amines and fluid-regulating peptides. Neurogenic tone on CSF formation is commonly inhibitory in nature.41 Adrenergic regulation of choroid plexus epithelium is substantial, including modulation of the activities of Na+,K+-ATPase and carbonic anhydrase. The superior cervical ganglion innervates the lateral ventricle choroid plexuses. Sympathetic nerve stimulation or resection, respectively, decreases or increases CSF production by 30%.42 Pharmacologic and biochemical evidence indicates that sympathomimetic lowering of CSF formation results from a combined β-receptor inhibition of epithelial secretion and α-receptor stimulation (vasoconstriction and reduced plexus blood flow).41 Cholinergic agents exogenously administered also decrease CSF production, indicating muscarinic receptor inhibition of the choroid plexus. Both sympathetic and parasympathetic tone autonomically regulate CSF formation.
Serotonin 5-HT2C receptors in choroid plexus are highly expressed and therefore widely used in pharmacologic investigations,43 including those of CSF formation. Serotonin and serotoninergic agonists perfused through the ventricles reduce CSF production.44 Localization of 5-HT2C receptors to choroid plexus apical membrane points to control of CSF formation by centrally released serotonin. CSF serotonin derived from 5-HT fibers coursing through the ependymal wall45 is a potential source of biogenic amine released into the ventricles for convection to the choroid plexus. Such binding of 5-HT to choroid plexus apical receptors would inhibit CSF formation.
Fluid-modulating peptides such as arginine vasopressin (AVP), angiotensin II, and atrial natriuretic peptide (ANP) reduce CSF formation when they are exogenously placed on the ventricular side. This fits with central neuroendocrine-like control of CSF mediated by receptors for these neuropeptides at the apical membrane.2 Moreover, the CSF concentration of many neuropeptides, including AVP and ANP, is regulated independently of plasma. This implies neuroendocrine regulation of CSF dynamics by stimulation of receptors at the central or apical side of the choroid plexus.
Peptides figure prominently in transport, permeability, and synthetic and modulatory phenomena at the blood-CSF interface.46,47 Neuropeptide regulation of choroid plexus fluid output helps adjust ICP elevation. Both AVP and ANP induce dark, neuroendocrine-type choroid epithelial cells that inhibit CSF production, especially in hydrocephalus.48,49 AVP modulation of CSF formation includes complex functional interactions in the choroid plexus with basic fibroblast growth factor50 and angiotensin II.51 AVP directly inhibits epithelial ion transport and constricts choroid plexus vessels, thereby reducing choroidal blood flow, which can be rate limiting for CSF formation.
ANP is the focus of substantial interest in the peptidergic control of CSF dynamics. Autoradiographic mapping of choroid plexus binding sites provides solid evidence for plasticity of ANP receptors49 in response to hydrocephalus and CSF fluid shifting (as in space flight). In humans, the ANP concentration in CSF is independent of plasma levels52 and rises in proportion to increments in ICP.53 Interestingly, intracerebroventricular ANP reduces the CSF formation rate in animal models54 in the face of increasing plexus blood flow. Systemically, ANP unloads expanded plasma volume by inducing natriuresis. ANP may also unload CSF excess. ANP thus deserves more attention in the CNS as a regulator of CSF pressure and volume by feedback servomechanistic effects on ion transport (through cGMP) and fluid production by the choroid plexus.
Diuretic Agents and Ion Transporters
Both weak and high-ceiling diuretics reduce CSF production. The strategy is to suppress fluid formation without altering CSF composition by interfering with choroid plexus ion transporters at apical or basolateral membranes. One desirable clinical outcome is to lower ICP pressure by decreasing fluid input to the ventricles. To interpret hydrophilic drug effects, a significant factor is whether the inhibiting agent is administered on the blood side (intravenously or intraperitoneally) or the CSF side (intracerebroventricularly). Tight junctions between choroid epithelial cells limit penetration of water-soluble agents by diffusion across the blood-CSF barrier. Hydrophilic agents such as ouabain, a potent inhibitor of apical Na+,K+-ATPase, therefore do not inhibit CSF formation when they are presented on the blood side of the barrier.7 Drug access to the transporter target is critical.
Dual apical transporter targets are the Na+ pump and the Na+-K+-Cl− cotransporter. Directly inhibited Na+ pumping is accomplished with cardiac glycosides. Intraventricular ouabain reduces CSF formation,55 but it elevates the CSF K+ concentration7 and is not therapeutically feasible. Digoxin, more lipid soluble, permeates the blood-CSF barrier to reach target sites at the CSF. Patients treated with digoxin have a decline in CSF formation of about 25%.56 Geriatric patients receiving digoxin may have a neurotoxicity risk due to reduced CSF turnover added to an already low baseline CSF production in senescence.18 Another apical target is Na+-K+-Cl− cotransport, which is bumetanide sensitive.57 Bumetanide acts on the kidney to reduce swelling and fluid retention. It has also been tested on the choroid plexus, the “kidney” of the CNS.19 When it is presented intraventricularly (0.1 mM) in dogs, bumetanide curtails CSF production up to 50%.58 Bumetanide administered intravenously, however, affects CSF formation negligibly,59 presumably because of poor systemic access to choroid plexus apical membrane. Furosemide, another high-ceiling diuretic, also reduces CSF formation and ICP.60 At high doses, furosemide alters choroid plexus blood flow and carbonic anhydrase activity as well as Na+-K+-Cl− cotransport. A third pharmacologic target on the CSF-facing membrane is the Na+-HCO3− cotransporter.35 Awaiting elucidation is the role of this HCO3− cotransporter in CSF formation as well as the use of novel agents to access it after systemic administration.
Basolateral membrane targets for choroid plexus fluid production are the Na+-H+ (NHE) and Cl−-HCO3− (AE) exchangers as well as newly identified HCO3−-loading transporters. Amiloride, a diuretic agent, inhibits Na+-H+ exchange, but relatively high doses are needed to lower CSF formation rate.61 Acetazolamide, used clinically to suppress CSF formation,62 indirectly slows Na+-H+ exchange by reducing availability of cellular protons for basolateral exchange with interstitial Na+.8 With regard to Cl−-HCO3− exchange, the disulfonic stilbene agent DIDS interferes with Cl− uptake by the choroid plexus and reduces CSF formation,63 but its experimental use is complicated by ion-CO2 imbalances in the periphery. Recently delineated expressions of HCO3−-loading transporters on the plasma side of the choroid plexus epithelium64,65 need to be assessed physiologically in relation to CSF dynamics. Finer pharmacologic control of fluid formation40 promotes better management of disease-induced changes in CSF pressure, volume, and flow.2
Cerebrospinal Fluid Formation Rate in Hydrocephalus
Structurally and functionally, the choroid plexus is markedly altered in hydrocephalus.48 There is a decrease in choroidal solute fluxes66–68 and CSF formation rate12,49 in congenital and adult chronic hydrocephalus, which is in response to augmented CSF volume and pressure. Reduced choroid plexus blood flow, epithelial cell shrinkage or damage, and diminution of blood-CSF surface area for transport contribute to reduced fluid turnover in hydrocephalus.48,66,69
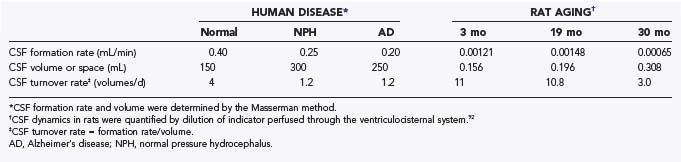
In humans with NPH, the CSF formation rate decreases 40% as estimated by the Masserman technique.12 In adult rats injected with kaolin, an NPH-like model, the CSF formation at 3 weeks is also reduced by 40%.72 Overall, the elevated CSF pressure in human NPH and kaolin models devastates the CNS interior (i.e., choroid plexuses, ependyma, and periventricular zones). Therapeutic agent stabilization of gradually increasing CSF pressure in adult chronic hydrocephalus, or in later progression to AD,73 may minimize deterioration in the aged, dementia-prone CNS.
Cerebrospinal Fluid Flow
Freely flowing CSF is biophysically necessary for efficient CNS absorption of the arterial pressure pulse.74 Biochemically, the uninterrupted flow of CSF sustains an optimal level of substrate to stabilize metabolism and neuronal functions.2 Overall, CSF flow rate complexly depends on upstream choroidal, mainstream ventriculocisternal, and downstream arachnoidal fluid throughput. Slowing of CSF flow disturbs both trophic and excretory functions in the CNS.11 CSF flow characteristics and effects have been studied less than pressure and volume phenomena. Substantial brain injury incurred from severely attenuated CSF flow is prompting research on regional ventricular CSF interactions with nearby interstitial fluid and cerebral blood flow.75
CSF flow is neither laminar nor constantly unidirectional. Pulsatile CSF movement during each cardiac pumping cycle occurs as anterograde caudal flow is followed by retrograde cranial flow. This cyclic forward and backward fluid motion, as commonly monitored in the aqueduct, is a rhythmic passive response to arterial pulsations and brain compliance. Thus, the cyclic flow of CSF is driven by a dynamically changing hydrostatic pressure gradient, which is superimposed on the static pressure gradient driving continuous bulk flow of CSF (volume transmission1) from the proximal choroid plexus origin to the distal arachnoidal drainage sites.
Various methods have been employed for measurement of hyperdynamic pulsatile flow in the aqueduct, the most common being average flow rate (in milliliters per minute) and stroke volume (i.e., net caudal flow during one cardiac cycle, in microliters). Average flow rates greater than 18 mL/min can be considered a good criterion for diagnosis of NPH; an average flow rate of 27 mL/min has been reported in chronic hydrocephalus. Although early studies using aqueductal stroke volume as the flow measure reported favorable outcome from shunt surgery (i.e., improvement of the Hakim triad of NPH symptoms) for patients with stroke volume greater than 42 µL, more recent studies have questioned this assumption.76,77 In general, although hyperdynamic flow greater than 18 mL/min may be considered diagnostic of NPH, patients with mild or normal pulsatile flow in the cerebral aqueduct cannot be excluded from this diagnosis.76,77 Other regions that can be assessed with this method include the third ventricle floor (third ventriculostomy context78), the cervical (C2) subarachnoid space,79 and the prepontine cistern.80 Although questions persist, hyperdynamic CSF assessment may become increasingly valuable for diagnosis and management of CSF diversion in hydrocephalus. Phase-contrast balanced steady-state free precession, with its higher signal-to-noise ratio and insensitivity to turbulent (nonlaminar) fluid flow, improves CSF flow quantitation.80
CSF flow is tightly and intricately coupled to cerebral blood flow. Analyses of hydrodynamic and hemodynamic relationships provide differentials in disease diagnosis. Throughout the cardiac cycle, a typical MRI-generated CSF flow curve has separate and opposite phases covering flush and fill periods. Mechanical coupling of the CSF and vascular circulations is revealed by analysis of peak and latency phenomena during each cardiac cycle. Peak latencies on MRI-generated flow curves, for CSF and cerebral blood flow, are expressed as points over the cardiac cycle (0% to 100%). In healthy individuals, CSF flow follows a regular pattern (Table 33-1). At the start of systole, there is increased cerebral blood volume and thus elevated ICP. The consequent flushing of cervical CSF relieves subarachnoid space pressure, thereby enhancing jugular venous peak flow. There is then peak CSF flow (downward flushing) through the third ventricle–aqueduct that helps equilibrate cerebral pressure. Following this sequence of flow distributions in systole, there are successive reverse phases during diastole to refill the aqueduct. CSF flushing phenomena in systole have been precisely timed (see Table 33-1). Such control data enable diagnostic evaluation of altered CSF-blood dynamic relationships in disease and aging.81
TABLE 33-1 Dynamic Fluid Flow Throughout the Systolic Phase of Each Cardiac Cycle
| PHASE NUMBER | PERCENTAGE OF CARDIAC CYCLE | DESCRIPTION OF FLOW PHENOMENA IN VARIOUS CNS COMPARTMENTS |
|---|---|---|
| 1 | 0 | Systolic arterial fill flow peak occurs in internal carotid arteries, which causes instantaneous increase in the cerebral blood volume. |
| 2 | 5 | The cervical CSF initially responds to brain expansion by flushing through the subarachnoid spaces. |
| 3 | 11-20 | In response to the cervical CSF displacement, there is a fall in subarachnoid space pressure, leading to peak flow (flush) through jugular veins. |
| 4 | 20-25 | In response to subarachnoid space pressure decrease, the third ventricle–aqueductal CSF surges distally (downward). |
| 5 | 25-35 | Cerebral pressures equilibrate as arterial and venous flows equalize, and the cervical CSF flow becomes negligible. |
Data are recapitulated from Stoquart-ElSankari et al.81
CSF flow data from phase-contrast cine MRI are also useful for noninvasive analysis of intracranial compliance.82 With aging, neurodegenerative disease, and CSF overloading, the CNS becomes less compliant, that is, it is not as able to adjust to stressful increases in pressure and volume. A compliance index (Ci), defined as Δ volume/Δ pressure, is calculated with data for intracranial volume changes (derived from CSF and blood flows during a cardiac cycle) and the CSF pressure gradient (derived from CSF flow data). Ci values in NPH from subarachnoid hemorrhage are lower than those in patients with brain atrophy or asymptomatic ventricular dilation.83 MRI flow analysis at the C2 subarachnoid space83 can thus be used along with net blood flow determination (carotid and vertebral arteries and jugular veins) to assess brain compliance in NPH diagnostics.
Interruption of Cerebrospinal Fluid Flow at Various Loci
In chronic adult hydrocephalus,84 CSF flow can be compromised at several anatomic locations along the neuraxis. To specify NPH conditions, it is important to elucidate the differential impact on brain and ventricular functions caused by flow disruption at sequential points in the pathway: sylvian aqueduct, basal cisterns, and subarachnoid space.
Aqueduct
Cerebral aqueduct narrowing enhances the resistance to CSF flow through the brain interior, often inducing an NPH-like syndrome. Late-onset idiopathic aqueductal stenosis usually presents with chronic onset; younger patients present with headache, and older ones have NPH symptoms.85 Late-onset idiopathic aqueductal stenosis is relieved by endoscopic third ventriculostomy,85 especially when CSF outflow resistance is assessed by test infusion of an indwelling ventricular catheter. In both animal and human models, late-onset idiopathic aqueductal stenosis has been linked to venous malfunctions: elevated venous pressure and greater collateral flow.86
Basal Cisterns
MRI-viewed enlargement of the basal cisterns supports the diagnosis of shunt-responsive idiopathic NPH.87 Kaolin injection into basal cisterns88 creates communicating hydrocephalus that allows CSF outflow across the more proximal fourth ventricle–cisterna magna interface (foramen of Luschka). Thus, the more distal CSF blockage experimentally in the basal cisterns (compared with standard kaolin injection into cisterna magna) allows the modeling of extraventricular, nonobstructive hydrocephalus clinically observed in children. Tracking of the progression of the basal cistern destabilization is important in understanding how early-life “decompensating” hydrocephalus might evolve to NPH.
Cortical Subarachnoid Space
There is a paucity of systematic analyses of abnormal flow in the cortical subarachnoid space during NPH and AD.89 In animal models, intrathecally injected kaolin disrupts orderly CSF flow in the subarachnoid space. Kaolin administration into the cortical subarachnoid space induces backstream ventriculomegaly, but the degree and rate of expansion of the lateral ventricles are not as great as with injection into basal cisterns.88 Slower dilation of the ventricles after cortical subarachnoid space injection therefore provides a model that mimics the protracted development of NPH in adults. In aging, NPH, and AD, the structural changes in the subarachnoid space and arachnoid membrane increase the impedance to CSF flow.90,91
Cerebrospinal Fluid Turnover Rate
CSF turnover rate is distinguished from CSF formation rate and flow. Defined as CSF formation rate divided by CSF volume, the turnover rate measures the frequency of total CSF renewal. With advanced aging comes markedly compromised CSF dynamics.14 Senescence is characterized by a decreasing formation rate even as the ventricular system becomes more voluminous. Ventriculomegaly is the cardinal feature of chronic hydrocephalus (NPH). In older animals and humans, the reduced choroid plexus formation of fluid, accompanied by expanding ventricles, is manifested by curtailed CSF turnover rate.18,92 Moreover, as AD progresses, the deepening sulci on the brain surface represent an enhanced volume of subarachnoid space. This increment in extracellular fluid volume places even more stress on the choroid plexus management of CSF composition.17
CSF circulation viewed from the standpoint of turnover rate is compromised in senescence, NPH, and AD.18 Table 33-2 summarizes CSF turnover rates, human versus rodent. CSF turnover rate is lower in humans than in rats but declines in both species substantially in late life. Relative stasis of CSF in aging is problematic for the brain, which lacks a lymphatic drainage system. Devoid of true lymphatic capillaries, the CNS is highly dependent on the quasi-lymphatic CSF sink action. The CSF sink effect is akin to drainage that removes proteins and harmful catabolites from interstitial fluid. Sink clearance action is feasible because of the low concentration of proteins and neural catabolites in nascent CSF. This sets up a concentration gradient for macromolecules and parenchymal catabolites, directed from brain interstitial fluid into ventricles and subarachnoid space. This downhill concentration gradient facilitates diffusion of harmful substances from neural tissue to CSF, which are subsequently convected to lymph and venous blood.
Stagnated CSF bulk flow in aging and hydrocephalus93 is problematic for brain clearance of peptides such as Aβ1-42. With advancing age, there is reduced outward clearance of interstitial fluid Aβ peptide across cerebral capillaries. This places a burden on CSF to clear amyloid from CNS, but unfortunately, the bulk flow clearance capacity also diminishes in senescence because of less fluid production by the choroid plexus.17,94 Reduced interstitial clearance of Aβ promotes amyloid plaque deposition, creating additional metabolic effects that eventually destabilize the neuronal cytoskeleton. Given that brain interstitial fluid convection is interdependent with CSF dynamics, the latter need preservation to support healthy neuronal networks.
Pharmacologic strategies can be directed at stabilization of choroid plexus function throughout life to maintain or even increase the rate of CSF formation.40 Consequently, improved CSF turnover rate curtails neurodegeneration by providing a cleaner, less oxidatively stressful environment for neurons. Brain well-being depends on a balance of CSF formation, flow, and turnover. Maintenance of fluid percolation through the ventricular-subarachnoid spaces also contributes to homeostasis of CSF pressure, volume, and composition.
Transport Phenomena in Hydrocephalus: Cerebrospinal Fluid–Bordering Cells Versus Blood-Brain Barrier
To prevent or repair dwindling fluid turnover rates in the degenerating CNS, attention should be directed to the transport interfaces at the internal brain surface.40 In the aging choroid plexus,94 progressive interstitial fibrosis2 and deposition of immune complexes interfere with efficient fluid movement across the blood-CSF barrier.95 Also contributing to disrupted ventricular hydrodynamics is the senescence- and NPH-associated breakdown of the ependymal wall.96 Denudation of ependymal cells in some regions and fibrillary (rosette) formation at other CSF-brain loci97 contribute to modified fluid movement between ventricles and CSF. In the brain proper, untoward ischemic effects on the arteries and arterioles98,99 cause downstream destruction of human capillaries in NPH and AD,96,99 presumably leading to inefficient generation of interstitial fluid by microvessels. Kaolin-induced hydrocephalus mimics accelerated aging, thereby informing on the evolving pathologic changes of the blood-brain barrier, blood-CSF barrier, and ependymal wall from aging or NPH88,93 to dementia. Stabilization of chronic hydrocephalus therefore entails therapeutic targets at CSF transport interfaces2 as well as the neural tissue and cerebrovasculature.96,100
Cerebrospinal Fluid Diagnostic and Management Issues in Adult Chronic Hydrocephalus
Refined diagnosis and management of NPH, both idiopathic and secondary, are being driven by the burgeoning geriatric population and improving evidence-based medicine. Diagnostic categories of NPH have been presented as possible, likely, and definite and therefore reflect intensification of stages. Along with ventriculomegaly, new MRI biomarking approaches emphasize dilation of the sylvian fissure and high convexity tightness.101 Hyperdynamic peak CSF flow velocity and the lumbar CSF tapping are useful analytic measures to corroborate the clinical diagnosis of NPH.102 Maximal systolic flow through the sylvian aqueduct has also been useful in differentiating NPH from aged control patients.103 Although CSF hydrodynamic data usefully distinguish non-NPH from NPH,104 these MRI flow parameters do not always predict favorable response to surgical shunting.86
Unstable CSF stroke volumes, which increase during early NPH progression but then decrease precipitously 3 to 4 years after onset, point to irreversible NPH. Regressing CSF-brain functions in patients refusing shunting provide insight into the natural course of aqueductal dynamics in surgically untreated NPH. After NPH onset, the aqueductal stroke volume typically increases for 18 months or longer.105 This progressive hyperdynamics is followed by a plateau in stroke volume and then a slight decline for another 18 months before a precipitous reduction in aqueductal flow. The continual fall in CSF flow in unshunted NPH, eventually decelerating rapidly, is attributed to worsening cerebral ischemia and irreversible hydrocephalus.105
Pathophysiologic overlapping of altered CSF dynamics between NPH and AD creates a challenge to pinpoint differences among NPH, AD, and NPH-AD hybrid patients and to manage them by shunting versus medical regimens. Although the brain amyloid burden follows a continuum from aging to NPH to AD, the CSF hydrodynamics do not necessarily proceed in a progressive pattern. In one study of intracranial hydrodynamics and compliance, AD patients had aqueductal stroke volumes midway between those of NPH and controls.86 In assessing NPH flow characteristics, the hydrodynamics of AD in comorbid patients thus complicates stroke volume interpretation.
Challenges in Rectifying Distorted Cerebrospinal Fluid Dynamics
What is the nature and potential reversibility of altered CSF flow coupling to brain metabolism? Multiple central mechanisms, including recruitment of alternative CSF outflow pathways, partially cope with failing CSF dynamics in chronic hydrocephalus. As CSF turnover rate decreases substantially, however, brain metabolism is severely affected. At some juncture, then, brain metabolic impairment is decoupled from CSF flow in that metabolic neuropathy is not improved after correction of fluid dynamics.106 The best animal model for NPH (i.e., intrathecal kaolin) can be used to evaluate the time course of shunting improvement as chronic hydrocephalus intensifies. Earlier identification of cerebral metabolic distortions, by way of novel biomarkers in CSF,106 will likely help stave off dementia development in advanced adult hydrocephalus. Another important dimension in treating NPH is the discovery of new agents40 to more finely adjust CSF formation as well as outflow. Overall, a combination of improved biomarking107 and shunt technology, as well as pharmacologic advances, will help remediate flow disorders in the adult CSF system.
Al-Zain FT, Rademacher G, Lemcke J, et al. [Idiopathic normal-pressure hydrocephalus. Flow measurement of cerebrospinal fluid using phase contrast MRI and its diagnostics importance.]. Nervenarzt. 2007;78:181-187.
Bargallo N, Olondo L, Garcia AI, et al. Functional analysis of third ventriculostomy patency by quantification of CSF stroke volume by using cine phase-contrast MR imaging. AJNR Am J Neuroradiol. 2005;26:2514-2521.
Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957-970.
Brusco A, Lopez-Costa JJ, Tagliaferro P, Pecci Saavedra J. Serotonergic ependymal fibres in rat and monkey: light and electron microscopic immunocytochemical study. Biocell. 1998;22:115-122.
Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2136-R2146.
Doczi T, Joo F, Vecsernyes M, Bodosi M. Increased concentration of atrial natriuretic factor in the cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage and raised intracranial pressure. Neurosurgery. 1988;23:16-19.
Faraci FM, Mayhan WG, Heistad DD. Effect of vasopressin on production of cerebrospinal fluid: possible role of vasopressin (V1)–receptors. Am J Physiol. 1990;258(pt 2):R94-R98.
Fukuhara T, Luciano MG. Clinical features of late-onset idiopathic aqueductal stenosis. Surg Neurol. 2001;55:132-136.
Husted RF, Reed DJ. Regulation of cerebrospinal fluid bicarbonate by the cat choroid plexus. J Physiol. 1977;267:411-428.
Johanson CE, Donahue JE, Spangenberger A, et al. Atrial natriuretic peptide: its putative role in modulating the choroid plexus–CSF system for intracranial pressure regulation. Acta Neurochir Suppl. 2006;96:451-456.
Johanson C, Silverberg G, Donahue J, et al. Choroid plexus and CSF in Alzheimer’s disease: altered expression and transport of proteins and peptides. In: Zheng W, Chodobski A, editors. The Blood–Cerebrospinal Fluid Barrier. Boca Raton: CRC Press; 2004:307-339.
Johanson CE, Szmydynger-Chodobska J, et al. Altered formation and bulk absorption of cerebrospinal fluid in FGF-2–induced hydrocephalus. Am J Physiol. 1999;277(pt 2):R263-R271.
Klinge PM, Samii A, Niescken S, et al. Brain amyloid accumulates in aged rats with kaolin-induced hydrocephalus. Neuroreport. 2006;17:657-660.
Lorenzo AV, Hornig G, Zavala LM, et al. Furosemide lowers intracranial pressure by inhibiting CSF production. Z Kinderchir. 1986;41(Suppl 1):10-12.
Milhorat TH, Hammock MK, Davis DA, Fenstermacher JD. Choroid plexus papilloma. I. Proof of cerebrospinal fluid overproduction. Childs Brain. 1976;2:273-289.
Millar ID, Brown PD. NBCe2 exhibits a 3 HCO3−:1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun. 2008;373:550-554.
Nakamura S, Hochwald GM. Effects of arterial pCO2 and cerebrospinal fluid volume flow rate changes on choroid plexus and cerebral blood flow in normal and experimental hydrocephalic cats. J Cereb Blood Flow Metab. 1983;3:369-375.
Pollay M, Hisey B, Reynolds E, et al. Choroid plexus Na+/K+-activated adenosine triphosphatase and cerebrospinal fluid formation. Neurosurgery. 1985;17:768-772.
Praetorius J. Water and solute secretion by the choroid plexus. Pflugers Arch. 2007;454:1-18.
Rubenstein E. Relationship of senescence of cerebrospinal fluid circulatory system to dementias of the aged. Lancet. 1998;351:283-285.
Scollato A, Tenenbaum R, Bahl G, et al. Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2008;29:192-197.
Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci. 2003;8:s515-s521.
Sharma AK, Gaikwad S, Gupta V, et al. Measurement of peak CSF flow velocity at cerebral aqueduct, before and after lumbar CSF drainage, by use of phase-contrast MRI: utility in the management of idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg. 2008;110:363-368.
Zou R, Park EH, Kelly EM, et al. Intracranial pressure waves: characterization of a pulsation absorber with notch filter properties using systems analysis: laboratory investigation. J Neurosurg Pediatr. 2008;2:83-94.
1 Johanson C. Choroid plexus–CSF circulatory dynamics: impact on brain growth, metabolism and repair. In: Conn P, editor. Neuroscience in Medicine. Totowa, N.J.: The Humana Press; 2008:176-200.
2 Johanson CE, Duncan JA3rd, Klinge PM, et al. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10.
3 Szmydynger-Chodobska J, Chodobski A, Johanson CE. Postnatal developmental changes in blood flow to choroid plexuses and cerebral cortex of the rat. Am J Physiol. 1994;266(pt 2):R1488-R1492.
4 Faraci FM, Mayhan WG, Heistad DD. Effect of vasopressin on production of cerebrospinal fluid: possible role of vasopressin (V1)–receptors. Am J Physiol. Jan 1990;258(pt 2):R94-R98.
5 Cserr HF. Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann N Y Acad Sci. 1988;529:9-20.
6 Parmelee JT, Johanson CE. Development of potassium transport capability by choroid plexus of infant rats. Am J Physiol. 1989;256(pt 2):R786-R791.
7 Smith QR, Johanson CE. Effect of ouabain and potassium on ion concentrations in the choroidal epithelium. Am J Physiol. 1980;238:F399-F406.
8 Johanson CE. Differential effects of acetazolamide, benzolamide and systemic acidosis on hydrogen and bicarbonate gradients across the apical and basolateral membranes of the choroid plexus. J Pharmacol Exp Ther. 1984;231:502-511.
9 Johanson CE, Parandoosh Z, Dyas ML. Maturational differences in acetazolamide-altered pH and HCO3 of choroid plexus, cerebrospinal fluid, and brain. Am J Physiol. 1992;262(pt 2):R909-R914.
10 Johanson C, McMillan P, Palm D, et al. Volume transmission-mediated protective impact of choroid plexus–CSF growth factors on forebrain ischemic injury. In: Sharma H, Westman J, editors. Blood–Spinal Cord and Brain Barriers in Health and Disease. San Diego: Academic Press; 2003:361-384.
11 Rubenstein E. Relationship of senescence of cerebrospinal fluid circulatory system to dementias of the aged. Lancet. 1998;351:283-285.
12 Silverberg GD, Huhn S, Jaffe RA, et al. Downregulation of cerebrospinal fluid production in patients with chronic hydrocephalus. J Neurosurg. 2002;97:1271-1275.
13 Nilsson C, Stahlberg F, Gideon P, et al. The nocturnal increase in human cerebrospinal fluid production is inhibited by a beta1-receptor antagonist. Am J Physiol. 1994;267(pt 2):R1445-R1448.
14 Silverberg GD, Heit G, Huhn S, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology. 2001;57:1763-1766.
15 Milhorat TH, Hammock MK, Davis DA, Fenstermacher JD. Choroid plexus papilloma. I. Proof of cerebrospinal fluid overproduction. Childs Brain. 1976;2:273-289.
16 Fujimura M, Onuma T, Kameyama M, et al. Hydrocephalus due to cerebrospinal fluid overproduction by bilateral choroid plexus papillomas. Childs Nerv Syst. 2004;20:485-488.
17 Johanson C, Silverberg G, Donahue J, et al. Choroid plexus and CSF in Alzheimer’s disease: altered expression and transport of proteins and peptides. In: Zheng W, Chodobski A, editors. The Blood–Cerebrospinal Fluid Barrier. Boca Raton: CRC Press; 2004:307-339.
18 Silverberg GD, Mayo M, Saul T, et al. Alzheimer’s disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2003;2:506-511.
19 Spector R, Johanson CE. The mammalian choroid plexus. Sci Am. 1989;261:68-74.
20 Knuckey NW, Fowler AG, Johanson CE, et al. Cisterna magna microdialysis of 22Na to evaluate ion transport and cerebrospinal fluid dynamics. J Neurosurg. 1991;74:965-971.
21 Johanson CE, Palm DE, Dyas ML, Knuckey NW. Microdialysis analysis of effects of loop diuretics and acetazolamide on chloride transport from blood to CSF. Brain Res. 1994;641:121-126.
22 Smith QR, Woodbury DM, Johanson CE. Kinetic analysis of [36Cl]-, [22Na]- and [3H]mannitol uptake into the in vivo choroid plexus–cerebrospinal fluid brain system: ontogeny of the blood brain and blood-CSF barriers. Brain Res. 1982;255:181-198.
23 Smith QR, Johanson CE. Effect of carbonic anhydrase inhibitors and acidosis in choroid plexus epithelial cell sodium and potassium. J Pharmacol Exp Ther. 1980;215:673-680.
24 Johanson CE, Murphy VA, Dyas M. Ethacrynic acid and furosemide alter Cl, K, and Na distribution between blood, choroid plexus, CSF, and brain. Neurochem Res. 1992;17:1079-1085.
25 Murphy VA, Johanson CE. Na+-H+ exchange in choroid plexus and CSF in acute metabolic acidosis or alkalosis. Am J Physiol. 1990;258(pt 2):F1528-F1537.
26 Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957-970.
27 Johanson CE, Reed DJ, Woodbury DM. Active transport of sodium and potassium by the choroid plexus of the rat. J Physiol. 1974;241:359-372.
28 Johanson CE, Murphy VA. Acetazolamide and insulin alter choroid plexus epithelial cell [Na+], pH, and volume. Am J Physiol. 1990;258(pt 2):F1538-F1546.
29 Praetorius J. Water and solute secretion by the choroid plexus. Pflugers Arch. 2007;454:1-18.
30 Chen LM, Kelly ML, Rojas JD, et al. Use of a new polyclonal antibody to study the distribution and glycosylation of the sodium-coupled bicarbonate transporter NCBE in rodent brain. Neuroscience. 2008;151:374-385.
31 Lindsey AE, Schneider K, Simmons DM, et al. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci U S A. 1990;87:5278-5282.
32 Smith QR, Johanson CE. Active transport of chloride by lateral ventricle choroid plexus of the rat. Am J Physiol. 1985;249(pt 2):F470-F477.
33 Keep RF, Xiang J, Betz AL. Potassium cotransport at the rat choroid plexus. Am J Physiol. 1994;267(pt 1):C1616-C1622.
34 Millar ID, Bruce J, Brown PD. Ion channel diversity, channel expression and function in the choroid plexuses. Cerebrospinal Fluid Res. 2007;4:8.
35 Millar ID, Brown PD. NBCe2 exhibits a 3 HCO3−:1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun. 2008;373:550-554.
36 Husted RF, Reed DJ. Regulation of cerebrospinal fluid bicarbonate by the cat choroid plexus. J Physiol. 1977;267:411-428.
37 Johansson PA, Dziegielewska KM, Ek CJ, et al. Aquaporin-1 in the choroid plexuses of developing mammalian brain. Cell Tissue Res. 2005;322:353-364.
38 Oshio K, Song Y, Verkman AS, Manley GT. Aquaporin-1 deletion reduces osmotic water permeability and cerebrospinal fluid production. Acta Neurochir Suppl. 2003;86:525-528.
39 Oshio K, Watanabe H, Song Y, et al. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel aquaporin-1. FASEB J. 2005;19:76-78.
40 Johanson CE, Duncan JA, Stopa EG, Baird A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus–CSF route. Pharm Res. 2005;22:1011-1037.
41 Lindvall M, Owman C. Autonomic nerves in the mammalian choroid plexus and their influence on the formation of cerebrospinal fluid. J Cereb Blood Flow Metab. 1981;1:245-266.
42 Lindvall M, Edvinsson L, Owman C. Sympathetic nervous control of cerebrospinal fluid production from the choroid plexus. Science. 1978;201:176-178.
43 Backstrom JR, Westphal RS, Canton H, Sanders-Bush E. Identification of rat serotonin 5-HT2C receptors as glycoproteins containing N-linked oligosaccharides. Brain Res Mol Brain Res. 1995;33:311-318.
44 Boyson SJ, Alexander A. Net production of cerebrospinal fluid is decreased by SCH-23390. Ann Neurol. 1990;27:631-635.
45 Brusco A, Lopez-Costa JJ, Tagliaferro P, Pecci Saavedra J. Serotonergic ependymal fibres in rat and monkey: light and electron microscopic immunocytochemical study. Biocell. 1998;22:115-122.
46 Chodobski A, Szmydynger-Chodobska J. Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech. 2001;52:65-82.
47 Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56:1765-1791.
48 Weaver C, McMillan P, Duncan JA, et al. Hydrocephalus disorders: their biophysical and neuroendocrine impact on the choroid plexus epithelium. In: Hertz L, editor. Non-Neuronal Cells of the Nervous System: Function and Dysfunction, vol 31. Amsterdam: Elsevier; 2004:269-293.
49 Johanson CE, Donahue JE, Spangenberger A, et al. Atrial natriuretic peptide: its putative role in modulating the choroid plexus–CSF system for intracranial pressure regulation. Acta Neurochir Suppl. 2006;96:451-456.
50 Szmydynger-Chodobska J, Chun ZG, Johanson CE, Chodobski A. Distribution of fibroblast growth factor receptors and their co-localization with vasopressin in the choroid plexus epithelium. Neuroreport. 2002;13:257-259.
51 Chodobski A, Szmydynger-Chodobska J, Johanson CE. Vasopressin mediates the inhibitory effect of central angiotensin II on cerebrospinal fluid formation. Eur J Pharmacol. 1998;347:205-209.
52 Doczi T, Joo F, Vecsernyes M, Bodosi M. Increased concentration of atrial natriuretic factor in the cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage and raised intracranial pressure. Neurosurgery. 1988;23:16-19.
53 Yamasaki H, Sugino M, Ohsawa N. Possible regulation of intracranial pressure by human atrial natriuretic peptide in cerebrospinal fluid. Eur Neurol. 1997;38:88-93.
54 Steardo L, Nathanson JA. Brain barrier tissues: end organs for atriopeptins. Science. 1987;235:470-473.
55 Pollay M, Hisey B, Reynolds E, et al. Choroid plexus Na+/K+-activated adenosine triphosphatase and cerebrospinal fluid formation. Neurosurgery. 1985;17:768-772.
56 Allonen H, Anderson KE, Iisalo E, et al. Passage of digoxin into cerebrospinal fluid in man. Acta Pharmacol Toxicol (Copenh). 1977;41:193-202.
57 Bairamian D, Johanson CE, Parmelee JT, Epstein MH. Potassium cotransport with sodium and chloride in the choroid plexus. J Neurochem. 1991;56:1623-1629.
58 Javaheri S, Wagner KR. Bumetanide decreases canine cerebrospinal fluid production. In vivo evidence for NaCl cotransport in the central nervous system. J Clin Invest. 1993;92:2257-2261.
59 Vogh BP, Langham MRJr. The effect of furosemide and bumetanide on cerebrospinal fluid formation. Brain Res. 1981;221:171-183.
60 Lorenzo AV, Hornig G, Zavala LM, et al. Furosemide lowers intracranial pressure by inhibiting CSF production. Z Kinderchir. 1986;41(Suppl 1):10-12.
61 Murphy VA, Johanson CE. Acidosis, acetazolamide, and amiloride: effects on 22Na transfer across the blood-brain and blood-CSF barriers. J Neurochem. 1989;52:1058-1063.
62 Poca MA, Sahuquillo J. Short-term medical management of hydrocephalus. Expert Opin Pharmacother. 2005;6:1525-1538.
63 Deng QS, Johanson CE. Stilbenes inhibit exchange of chloride between blood, choroid plexus and cerebrospinal fluid. Brain Res. 1989;501:183-187.
64 Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2136-R2146.
65 Praetorius J, Nejsum LN, Nielsen S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol. 2004;286:C601-C610.
66 Knuckey NW, Preston J, Palm D, et al. Hydrocephalus decreases chloride efflux from the choroid plexus epithelium. Brain Res. 1993;618:313-317.
67 Nakamura S, Hochwald GM. Spinal fluid formation and glucose influx in normal and experimental hydrocephalic rats. Exp Neurol. 1983;82:108-117.
68 Marlin AE, Wald A, Hochwald GM, Malhan C. Kaolin-induced hydrocephalus impairs CSF secretion by the choroid plexus. Neurology. 1978;28(pt 1):945-949.
69 Nakamura S, Hochwald GM. Effects of arterial pCO2 and cerebrospinal fluid volume flow rate changes on choroid plexus and cerebral blood flow in normal and experimental hydrocephalic cats. J Cereb Blood Flow Metab. 1983;3:369-375.
70 Pollay M, Stevens FA, Roberts PA. Alteration in choroid-plexus blood flow and cerebrospinal-fluid formation by increased ventricular pressure. In: Wood JH, editor. Neurobiology of Cerebrospinal Fluid, vol 2. New York: Raven Press; 1983:687-695.
71 Lindvall M, Owman C. Sympathetic nervous control of cerebrospinal fluid production in experimental obstructive hydrocephalus. Exp Neurol. 1984;84:606-615.
72 Hochwald GM, Nakamura S, Camins MB. The rat in experimental obstructive hydrocephalus. Z Kinderchir. 1981;34:403-410.
73 Silverberg G, Mayo M, Saul T, et al. Elevated cerebrospinal fluid pressure in patients with Alzheimer’s disease. Cerebrospinal Fluid Res. 2006;3:7.
74 Zou R, Park EH, Kelly EM, et al. Intracranial pressure waves: characterization of a pulsation absorber with notch filter properties using systems analysis: laboratory investigation. J Neurosurg Pediatr. 2008;2:83-94.
75 Wang YF, Gwathmey JK, Zhang G, et al. Cerebrospinal fluid may mediate CNS ischemic injury. Cerebrospinal Fluid Res. 2005;2:7.
76 Kahlon B, Annertz M, Stahlberg F, Rehncrona S. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery. 2007;60:124-129.
77 Dixon GR, Friedman JA, Luetmer PH, et al. Use of cerebrospinal fluid flow rates measured by phase-contrast MR to predict outcome of ventriculoperitoneal shunting for idiopathic normal-pressure hydrocephalus. Mayo Clin Proc. 2002;77:509-514.
78 Bargallo N, Olondo L, Garcia AI, et al. Functional analysis of third ventriculostomy patency by quantification of CSF stroke volume by using cine phase-contrast MR imaging. AJNR Am J Neuroradiol. 2005;26:2514-2521.
79 Wagshul ME, Chen JJ, Egnor MR, et al. Amplitude and phase of cerebrospinal fluid pulsations: experimental studies and review of the literature. J Neurosurg. 2006;104:810-819.
80 McCormack EJ, Egnor MR, Wagshul ME. Improved cerebrospinal fluid flow measurements using phase contrast balanced steady-state free precession. Magn Reson Imaging. 2007;25:172-182.
81 Stoquart-ElSankari S, Baledent O, Gondry-Jouet C, et al. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007;27:1563-1572.
82 Miyati T, Mase M, Kasai H, et al. Noninvasive MRI assessment of intracranial compliance in idiopathic normal pressure hydrocephalus. J Magn Reson Imaging. 2007;26:274-278.
83 Mase M, Miyati T, Yamada K, et al. Non-invasive measurement of intracranial compliance using cine MRI in normal pressure hydrocephalus. Acta Neurochir Suppl. 2005;95:303-306.
84 Edwards RJ, Dombrowski SM, Luciano MG, Pople IK. Chronic hydrocephalus in adults. Brain Pathol. 2004;14:325-336.
85 Fukuhara T, Luciano MG. Clinical features of late-onset idiopathic aqueductal stenosis. Surg Neurol. 2001;55:132-136.
86 Bateman GA, Loiselle AM. Can MR measurement of intracranial hydrodynamics and compliance differentiate which patient with idiopathic normal pressure hydrocephalus will improve following shunt insertion? Acta Neurochir (Wien). 2007;149:455-462.
87 Kitagaki H, Mori E, Ishii K, et al. CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. AJNR Am J Neuroradiol. 1998;19:1277-1284.
88 Li J, McAllister JP2nd, Shen Y, et al. Communicating hydrocephalus in adult rats with kaolin obstruction of the basal cisterns or the cortical subarachnoid space. Exp Neurol. 2008;211:351-361.
89 Rekate HL, Nadkarni TD, Wallace D. The importance of the cortical subarachnoid space in understanding hydrocephalus. J Neurosurg Pediatr. 2008;2:1-11.
90 Stopa EG, Berzin TM, Kim S, et al. Human choroid plexus growth factors: what are the implications for CSF dynamics in Alzheimer’s disease? Exp Neurol. 2001;167:40-47.
91 Eklund A, Smielewski P, Chambers I, et al. Assessment of cerebrospinal fluid outflow resistance. Med Biol Eng Comput. 2007;45:719-735.
92 Preston JE. Ageing choroid plexus–cerebrospinal fluid system. Microsc Res Tech. 2001;52:31-37.
93 Klinge PM, Samii A, Niescken S, et al. Brain amyloid accumulates in aged rats with kaolin-induced hydrocephalus. Neuroreport. 2006;17:657-660.
94 Johanson C, McMillan P, Tavares R, et al. Homeostatic capabilities of the choroid plexus epithelium in Alzheimer’s disease. Cerebrospinal Fluid Res. 2004;1:3.
95 Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci. 2003;8:s515-s521.
96 Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85:573-585.
97 Johanson CE, Szmydynger-Chodobska J, Chodobski A, et al. Altered formation and bulk absorption of cerebrospinal fluid in FGF-2–induced hydrocephalus. Am J Physiol. 1999;277(pt 2):R263-R271.
98 Stopa EG, Butala P, Salloway S, et al. Cerebral cortical arteriolar angiopathy, vascular beta-amyloid, smooth muscle actin, Braak stage, and APOE genotype. Stroke. 2008;39:814-821.
99 Zipser BD, Johanson CE, Gonzalez L, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977-986.
100 Klinge PM, Samii A, Muhlendyck A, et al. Cerebral hypoperfusion and delayed hippocampal response after induction of adult kaolin hydrocephalus. Stroke. 2003;34:193-199.
101 Ishikawa M, Hashimoto M, Kuwana N, et al. Guidelines for management of idiopathic normal pressure hydrocephalus. Neurol Med Chir (Tokyo). 2008;48(Suppl):S1-23.
102 Sharma AK, Gaikwad S, Gupta V, et al. Measurement of peak CSF flow velocity at cerebral aqueduct, before and after lumbar CSF drainage, by use of phase-contrast MRI: utility in the management of idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg. 2008;110:363-368.
103 Forner J, Florez N, Valero Merino C, et al. [Assessment of reliable quantification of the dynamics of cerebrospinal fluid by magnetic resonance imaging in idiopathic normal pressure hydrocephalus.]. Neurologia. 2007;22:213-220.
104 Al-Zain FT, Rademacher G, Lemcke J, et al. [Idiopathic normal-pressure hydrocephalus. Flow measurement of cerebrospinal fluid using phase contrast MRI and its diagnostics importance.]. Nervenarzt. 2007;78:181-187.
105 Scollato A, Tenenbaum R, Bahl G, et al. Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2008;29:192-197.
106 Kondziella D, Sonnewald U, Tullberg M, Wikkelso C. Brain metabolism in adult chronic hydrocephalus. J Neurochem. 2008;106:1515-1524.
107 Tullberg M, Blennow K, Mansson JE, et al. Cerebrospinal fluid markers before and after shunting in patients with secondary and idiopathic normal pressure hydrocephalus. Cerebrospinal Fluid Res. 2008;5:9.