Prion diseases
 Creutzfeldt–Jakob disease (CJD) and variant Creutzfeldt–Jakob disease (vCJD).
Creutzfeldt–Jakob disease (CJD) and variant Creutzfeldt–Jakob disease (vCJD).
 Gerstmann–Sträussler–Scheinker disease (GSS).
Gerstmann–Sträussler–Scheinker disease (GSS).
Common to all of these diseases are:
 The accumulation of an abnormal form of a cellular protein (prion protein).
The accumulation of an abnormal form of a cellular protein (prion protein).
 Neuronal death, synaptic loss, and microvacuolation (spongiform change) in the brain.
Neuronal death, synaptic loss, and microvacuolation (spongiform change) in the brain.
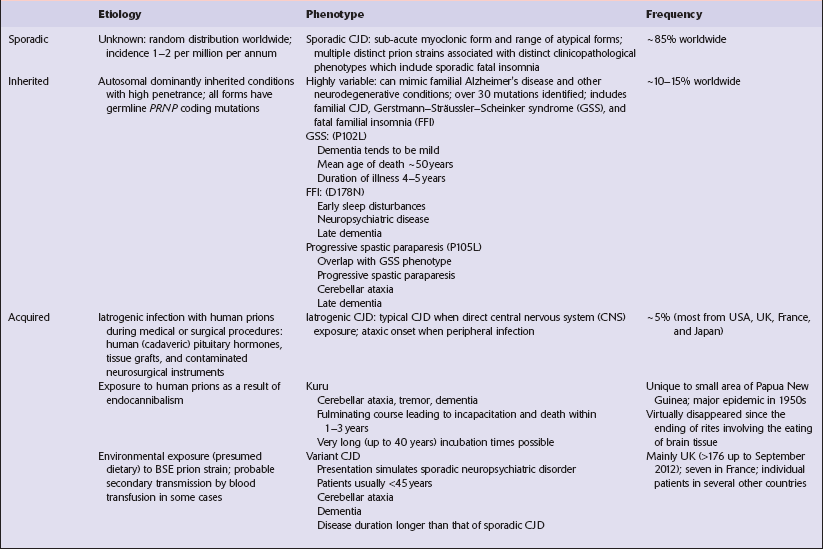
The commonest phenotype of prion disease is CJD. Patients typically present with subtle motor signs, which herald severe cerebellar ataxia, and progress to global dementia in under 1 year. Criteria for the clinical diagnosis of CJD have been proposed and widely adopted (Table 32.1).
Table 32.1
Clinical criteria for the diagnosis of CJD

aDepression, anxiety, apathy, withdrawal, delusions.
bThis includes both frank pain and/or unpleasant dysesthesia.
cGeneralized triphasic periodic complexes at approximately one per second.
dSpongiform change and extensive PrP deposition with florid plaques, throughout the cerebrum and cerebellum.
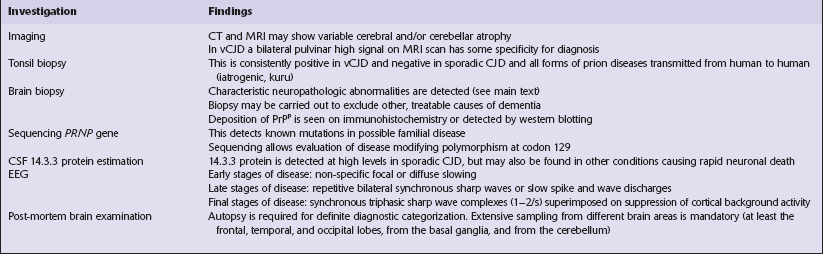
Several phenotypes of prion disease other than CJD have been identified (Table 32.2). In all of these, the mainstay of diagnosis is clinical examination supplemented by additional radiologic, electrophysiologic and neuropathologic investigations (Table 32.3).
PATHOLOGY
MACROSCOPIC APPEARANCES
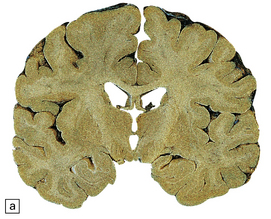
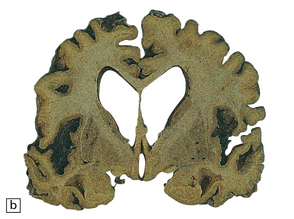
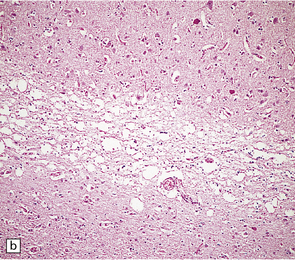
The brain may appear macroscopically normal, even in cases with long clinical histories. Most cases, however, show some atrophy (Fig. 32.8) and this may be severe, with a reduction in brain weight to as low as 850 g. In such cases, ventricular enlargement is marked and the atrophy often includes the caudate nucleus and thalamus. The hippocampus may be relatively spared, even in cases with severe atrophy elsewhere.
MICROSCOPIC APPEARANCES
The histologic features of prion diseases are:
 Spongiform change (intraneuronal vacuolation).
Spongiform change (intraneuronal vacuolation).
 Loss of synapses and subsequently of neurons.
Loss of synapses and subsequently of neurons.
 Paucity or absence of lymphocytic inflammation.
Paucity or absence of lymphocytic inflammation.
 Hyperphosphorylation of tau (immunohistochemical detection only).
Hyperphosphorylation of tau (immunohistochemical detection only).
Spongiform change
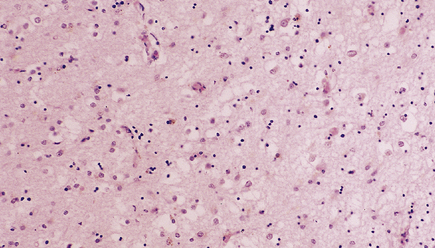
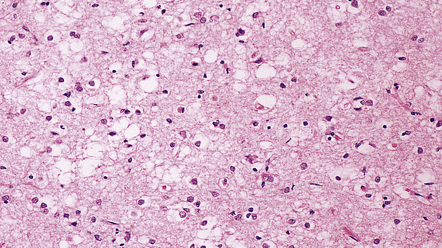
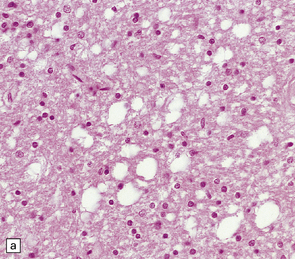
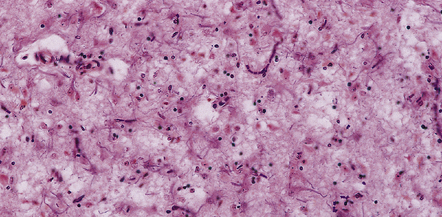
Microscopic examination of affected regions of the brain reveals vacuolation of the neuropil, an appearance termed spongiform change (Fig. 32.9). The vacuoles are typically round, relatively small (20–50 μm in diameter), and quite evenly distributed, but some can be large and irregular. The vacuoles are intracellular. Electron microscopy of early lesions shows that most of the vacuoles are within neuronal processes (Fig. 32.10). The vacuolation occurs mainly in gray matter. The distribution of pathology varies greatly between cases. The most consistently affected regions are the cerebral and cerebellar cortices, but the basal ganglia and thalamus are often involved. The distribution of lesions in the cerebral and cerebellar cortices is often patchy, affected regions alternating with seemingly unaffected areas, but may be confluent.
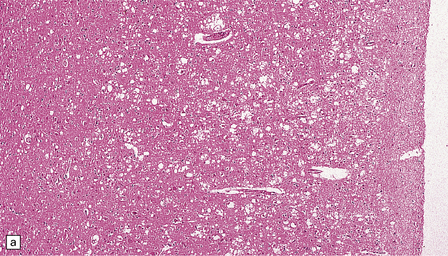
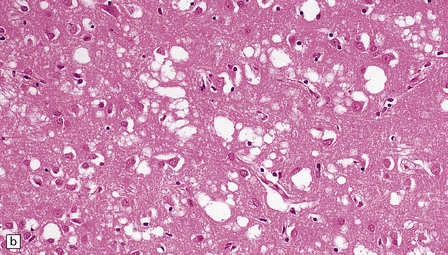
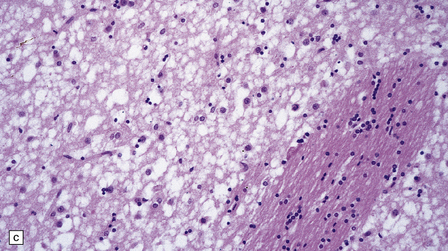
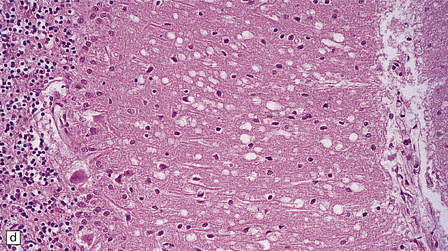
32.9 Spongiform change. There is fine vacuolation of the neuropil, even well away from blood vessels and neuronal somata (where tissue shrinkage during processing may give an artifactual appearance of vacuolation). The fine round vacuoles in the affected cortex and deep gray matter are interspersed with coarser vacuoles, some of which appear to be formed by the coalescence of smaller ones. (a) Spongiform change affecting all layers of the cerebral cortex. (b) Some of the larger vacuoles appear to result from the coalescence of smaller ones. (c) Spongiform change involving the putamen. (d) Spongiform change involving the cerebellum in which there are numerous small vacuoles in the molecular layer. The cerebellar cortex is commonly, but not invariably, affected in prion disease.
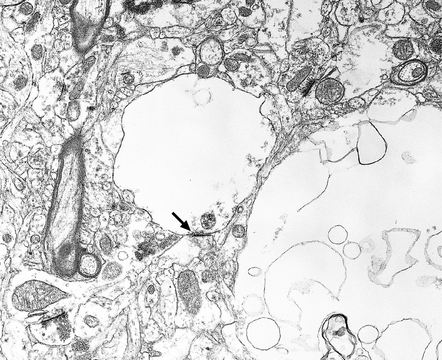
32.10 Electron microscopic appearance of spongiform change. Dendrites and axons contain vacuoles, which appear empty apart from fragments of membrane and occasional wisps of amorphous material. Note the synaptic density (arrow). Small vacuoles may be enclosed by a membrane. Larger vacuoles are usually incompletely enclosed or lack any discernible surrounding membrane.
Neuronal loss
In most cases there is a marked loss of neurons. This loss is greatest in cortical layers III–V and in focal regions of the caudate nucleus and thalamus. The pattern of loss is variable. There may be destruction of almost all neurons over large areas, causing a loss of lamination and coarse vacuolation (status spongiosus) (Fig. 32.15). Examination may reveal scattered shrunken remnants of neurons in the midst of apparently unaffected cells. In some cases residual neurons swell to form ballooned cells.
Astrocytosis
Loss of neurons is accompanied by massive activation and proliferation of astrocytes (Fig. 32.16). In severe cases, the neuronal component of cortical areas may have been replaced by reactive gliosis.
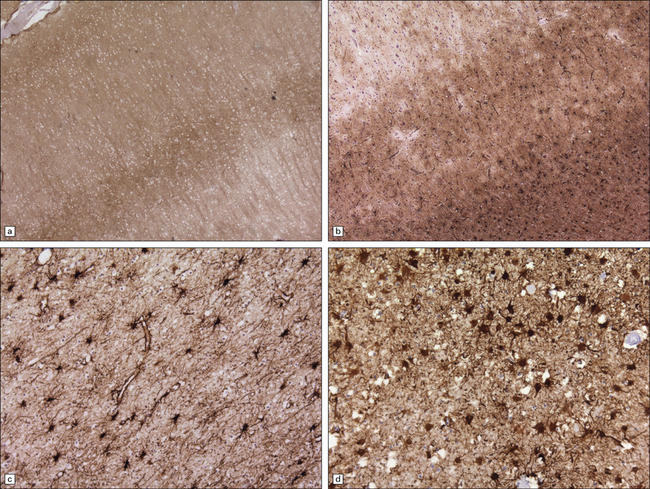
32.16 Gliosis in prion diseases. (a) Immunolabeling for abnormal PrP shows diffuse (synaptic) staining, with accentuation in cortical layers 3 and 4. (b) Immunolabeling of an adjacent section for GFAP reveals marked gliosis, particularly towards the junction with the white matter (bottom right of panel). Labeling for GFAP also highlights gliosis of the white matter (c) in sporadic CJD, and of the gray matter (d) in vCJD.
Synaptic loss
Electron microscopy has shown that axons and dendrites are damaged early in the disease. Quantitative studies of synaptic density in cases with obvious spongiform change have estimated synaptic loss at about 30% in the cerebral cortex. A synaptic loss of approximately 20% has been shown in atypical cases lacking obvious pathology at the light microscopic level (Fig. 32.17).
Hyperphosphorylation of tau
This can occur in response to the cerebral accumulation of amyloid (Aβ in Alzheimer’s disease, ABri and ADan in familial British dementia and familial Danish dementia), and in prion disease. This is particularly prominent in areas of plaque formation but can also be seen in the context of synaptic PrPSc. The deposits are distinct from those seen in Alzheimer’s disease or British dementia, in that neurofibrillary tangles or threads are not formed. PrP-associated tau forms short stubs or rods (Fig. 32.18).
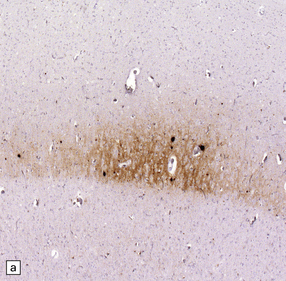
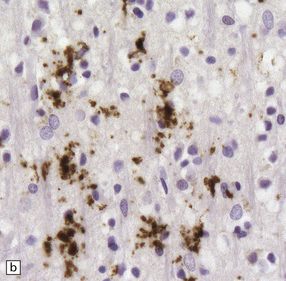
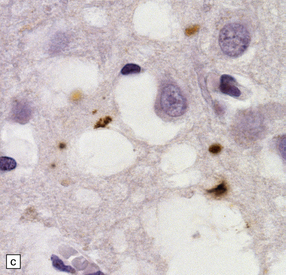
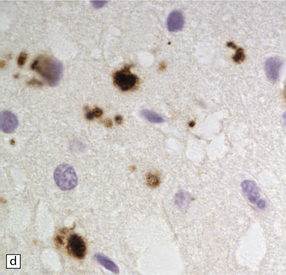
32.18 Deposition of hyperphosphorylated tau in prion disease. (a) sCJD with deposition of abnormal PrP in the deep cortical layer with formation of occasional small plaques. (b) Detection of dense granular or rod-shaped hyperphosphorylated tau in the vicinity of plaques (same area shown in the box in ‘a’). (c) Tau deposits in the cortex of sCJD near large vacuoles or (d) in the cerebellum in vCJD. (Courtesy of Reiniger L, Lukic A, Linehan J, Rudge P, Collinge J, Mead S, Brandner S. Tau, prions and Ab: the triad of neurodegeneration. Acta Neuropathol. 2010 Jan;121(1):5-20)
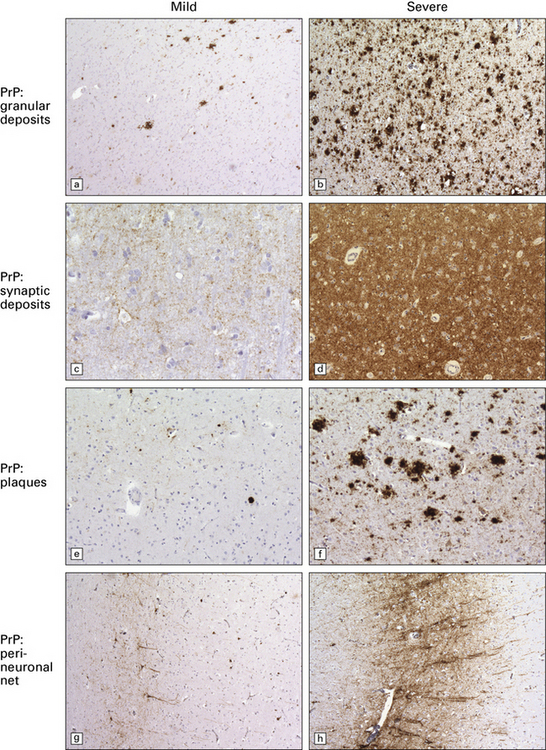
Accumulation of PrP: PrP accumulates in the brain in prion diseases and can be detected by immunohistochemistry. There are several patterns of accumulation:
 a diffuse synaptic pattern (Fig. 32.17)
a diffuse synaptic pattern (Fig. 32.17)
 perivacuolar deposits (Fig. 32.17)
perivacuolar deposits (Fig. 32.17)
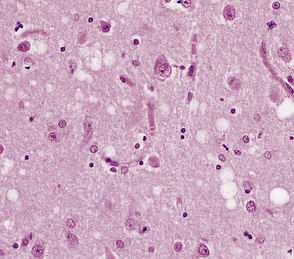
 larger deposits or plaques (Fig. 32.19) in which some of the PrP may be in the form of amyloid. There are five main types of plaque in prion disease:
larger deposits or plaques (Fig. 32.19) in which some of the PrP may be in the form of amyloid. There are five main types of plaque in prion disease:
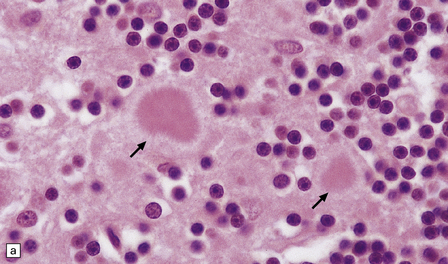
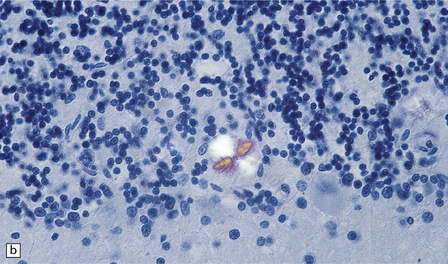
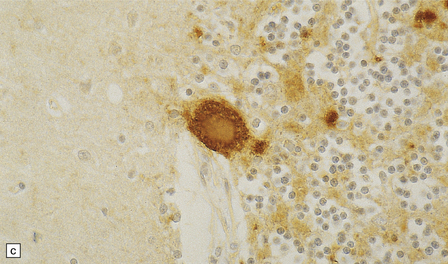
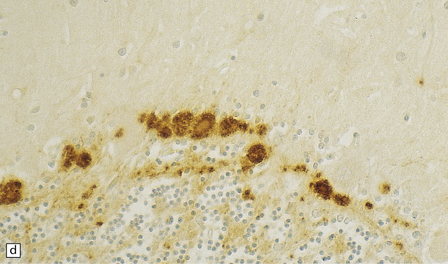
32.19 Plaques in prion disease. About 15% of brains from patients with prion disease contain amyloid plaques, usually restricted to the cerebellum. The plaques occur within the molecular layer, the granule cell layer, and the Purkinje cell layer. (a) They appear as homogeneous round or oval eosinophilic structures, and show birefringence when stained with Congo red and viewed under polarized light (b). (c) The plaques show immunoreactivity for PrP. (d) Plaques within the Purkinje cell layer.
• Unicentric plaques, consisting of an amyloid core and radiating spicules of amyloid, which together form a spherical deposit. The amyloid is periodic acid–Schiff (PAS)-positive. This type is often called a kuru plaque.
• Multicentric plaques, consisting of several dense core regions of amyloid with radiating spicules, which form multilobed structures as if made by the fusion of many unicentric plaques. The amyloid is PAS-positive. This form is characteristic of GSS.
• Unicentric plaques, consisting of a dense amyloid core that lacks surrounding radiating spicules of amyloid material. The amyloid is PAS-positive.
• Florid plaques, appearing as unicentric amyloid deposits with radiating spicules of amyloid and a surrounding rim of spongiform change. The amyloid is PAS-positive. This type is characteristic of vCJD.
• Diffuse plaques consisting of large (100–200 μm diameter) ill-defined deposits of PrP that are visible on immunostaining. The deposits are not PAS-positive.
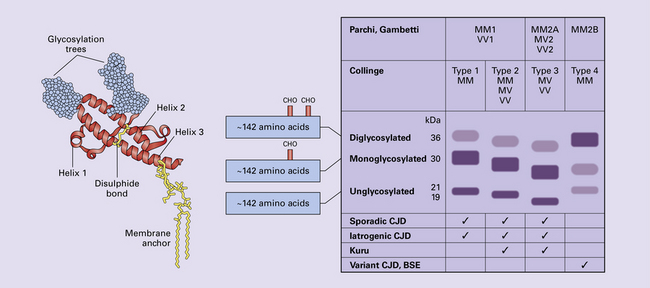
SPORADIC CJD
 129MM homozygote and 129MV heterozygote with PrPP type 1 (CJDMM1 and CJDMV1) are common, between them accounting for about 70% of sporadic cases, and characterized by a rapid clinical course, with early dementia, myoclonus, and periodic sharp waves on the EEG. Histologically, there is mild to moderate spongiosis and gliosis in the cerebral cortex, striatum, thalamus, and cerebellar cortex, with general sparing of the brain stem, hippocampus, and hypothalamus. PrP deposition is mainly of the synaptic pattern, in areas of spongiosis.
129MM homozygote and 129MV heterozygote with PrPP type 1 (CJDMM1 and CJDMV1) are common, between them accounting for about 70% of sporadic cases, and characterized by a rapid clinical course, with early dementia, myoclonus, and periodic sharp waves on the EEG. Histologically, there is mild to moderate spongiosis and gliosis in the cerebral cortex, striatum, thalamus, and cerebellar cortex, with general sparing of the brain stem, hippocampus, and hypothalamus. PrP deposition is mainly of the synaptic pattern, in areas of spongiosis.
 129VV homozygote with PrPP type 2 (CJDVV2) accounts for just under 20% of cases and is characterized by cerebellar signs early in the disease, and late dementia. The deep cerebral nuclei, brain stem, and cerebellum are predominantly affected, with relative sparing of the neocortex. White matter is involved in disease of long duration.
129VV homozygote with PrPP type 2 (CJDVV2) accounts for just under 20% of cases and is characterized by cerebellar signs early in the disease, and late dementia. The deep cerebral nuclei, brain stem, and cerebellum are predominantly affected, with relative sparing of the neocortex. White matter is involved in disease of long duration.
 129MV heterozygote with PrPP type 2 (CJDMV2) accounts for about 10% of cases and is characterized by both cognitive and cerebellar signs. The deep cerebral nuclei, brain stem, and cerebellum are predominantly affected, with relative sparing of the neocortex. White matter is involved in disease of long duration.
129MV heterozygote with PrPP type 2 (CJDMV2) accounts for about 10% of cases and is characterized by both cognitive and cerebellar signs. The deep cerebral nuclei, brain stem, and cerebellum are predominantly affected, with relative sparing of the neocortex. White matter is involved in disease of long duration.
 129MM homozygote with PrPP type 2 (CJDMM2) is rare and characterized by unusual clinical features. One form corresponds to sporadic fatal insomnia with a thalamic-based pathology, as seen in fatal familial insomnia (see below). Other cases are characterized by a gradual clinical course with dementia but without myoclonus or periodic sharp waves. There is severe neuronal loss with status spongiosus, and PrP deposition is coarse rather than punctate.
129MM homozygote with PrPP type 2 (CJDMM2) is rare and characterized by unusual clinical features. One form corresponds to sporadic fatal insomnia with a thalamic-based pathology, as seen in fatal familial insomnia (see below). Other cases are characterized by a gradual clinical course with dementia but without myoclonus or periodic sharp waves. There is severe neuronal loss with status spongiosus, and PrP deposition is coarse rather than punctate.
 129VV homozygous with PrPP type 1 (CJDVV1) is very rare and has been reported in only a small number of cases in which there was clinical dementia, with a young age of onset and absence of a typical EEG.
129VV homozygous with PrPP type 1 (CJDVV1) is very rare and has been reported in only a small number of cases in which there was clinical dementia, with a young age of onset and absence of a typical EEG.
GERSTMANN–STRÄUSSLER–SCHEINKER DISEASE (GSS)
Several clinical conditions are encompassed by the designation GSS, as defined by the presence of multicentric PrP amyloid plaques in the brain (Fig. 32.20):
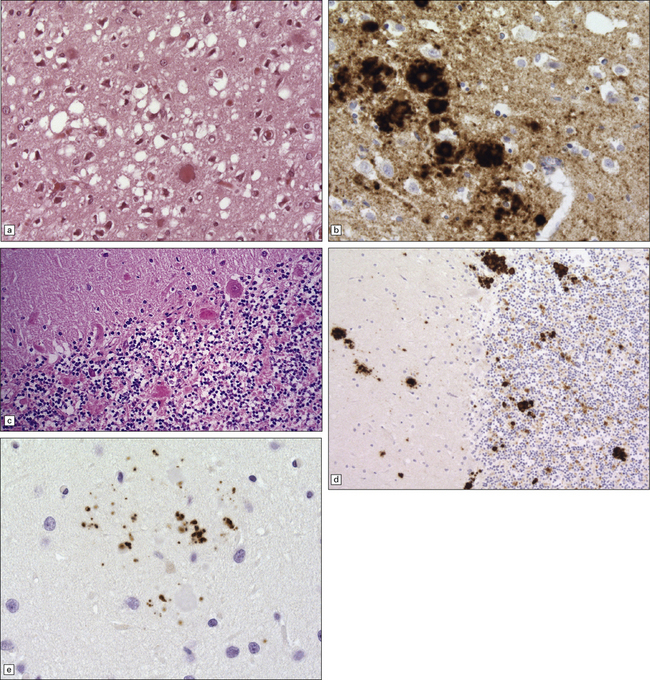
32.20 Cortical and cerebellar plaques in GSS (P102L). (a) The cerebral cortex contains dense core amyloid plaques and very significant spongiform change with formation of large vacuoles. Often vacuolation is less prominent but the extent can vary considerably within a single brain. (b) Immunohistochemistry for abnormal PrP highlights numerous, multicentric amyloid plaques but also synaptic PrP, which can be a feature in GSS. (c) Amyloid plaques in the cerebellar cortex. (d) Labeling of cerebellar plaques with PrP antibody. (e) Hyperphosphorylated tau forms small rod-shaped deposits in the area of a cerebellar plaque.
 Ataxic GSS (PRNP P102L) in which ataxia is combined with varying degrees of dementia. Unicentric and multicentric amyloid plaques are present in the molecular layer of the cerebellar cortex and in smaller numbers in the cerebral cortex. Usually spongiform change is not prominent. The severity of neuronal loss from cerebral and cerebellar cortices is variable.
Ataxic GSS (PRNP P102L) in which ataxia is combined with varying degrees of dementia. Unicentric and multicentric amyloid plaques are present in the molecular layer of the cerebellar cortex and in smaller numbers in the cerebral cortex. Usually spongiform change is not prominent. The severity of neuronal loss from cerebral and cerebellar cortices is variable.
 Telencephalic GSS (PRNPA117V) in which a dementia syndrome predominates, but ataxia has been the clinical presentation in one family. Multicentric and diffuse plaques are present in neocortical regions and occasionally in the cerebellum. Unicentric amyloid plaques are present in white matter. Spongiform change is absent. Severe neuronal loss and astrocytosis are evident in the basal ganglia and thalamus.
Telencephalic GSS (PRNPA117V) in which a dementia syndrome predominates, but ataxia has been the clinical presentation in one family. Multicentric and diffuse plaques are present in neocortical regions and occasionally in the cerebellum. Unicentric amyloid plaques are present in white matter. Spongiform change is absent. Severe neuronal loss and astrocytosis are evident in the basal ganglia and thalamus.
 NFT GSS (PRNPF198S) in which there is a cognitive decline accompanying ataxia, and parkinsonism, is a late feature. Multicentric and unicentric PrP amyloid plaques are seen in the neocortex and cerebellar cortex. There are Aβ plaques and tau-immunoreactive neurofibrillary tangles in a distribution compatible with early Alzheimer’s disease. Plaques with combined Aβ and PrP immunoreactivities are a rare finding. Neuronal loss affects the neocortex and cerebellar cortex. (Tangles may be seen in other familial cases PRNP Q217R, PRNP Y145*, and some cases with PRNP P105L and PRNP A117V.)
NFT GSS (PRNPF198S) in which there is a cognitive decline accompanying ataxia, and parkinsonism, is a late feature. Multicentric and unicentric PrP amyloid plaques are seen in the neocortex and cerebellar cortex. There are Aβ plaques and tau-immunoreactive neurofibrillary tangles in a distribution compatible with early Alzheimer’s disease. Plaques with combined Aβ and PrP immunoreactivities are a rare finding. Neuronal loss affects the neocortex and cerebellar cortex. (Tangles may be seen in other familial cases PRNP Q217R, PRNP Y145*, and some cases with PRNP P105L and PRNP A117V.)
 Progressive spastic paraparesis GSS (PRNPP105L) in which a clumsy hand is an early feature. This is followed by gait disturbance, spastic paraparesis, and ataxia. Cognitive decline and dysarthria are late features. No spongiform change is seen. Plaques occur mainly in the cerebral cortex with less involvement of the cerebellum and basal ganglia.
Progressive spastic paraparesis GSS (PRNPP105L) in which a clumsy hand is an early feature. This is followed by gait disturbance, spastic paraparesis, and ataxia. Cognitive decline and dysarthria are late features. No spongiform change is seen. Plaques occur mainly in the cerebral cortex with less involvement of the cerebellum and basal ganglia.
 GSS (PRNPY145*) produces a slowly progressive dementia. The histology is characterized by blood vessel-related amyloid plaques. Tangles and neuropil threads may be seen in the cerebral cortex.
GSS (PRNPY145*) produces a slowly progressive dementia. The histology is characterized by blood vessel-related amyloid plaques. Tangles and neuropil threads may be seen in the cerebral cortex.
 GSS (PRNP octapeptide repeat), in which ataxia precedes dementia. Cerebellar plaques are found, with less involvement of the cerebral cortex. Spongiform change is present.
GSS (PRNP octapeptide repeat), in which ataxia precedes dementia. Cerebellar plaques are found, with less involvement of the cerebral cortex. Spongiform change is present.
FATAL FAMILIAL INSOMNIA (FFI)
 marked neuronal loss and astrocytic gliosis in the thalamus
marked neuronal loss and astrocytic gliosis in the thalamus
 inconsistent and mild neuronal loss and gliosis in the cerebral cortex
inconsistent and mild neuronal loss and gliosis in the cerebral cortex
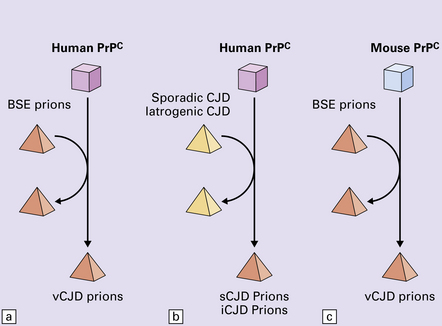
VARIANT CJD (VCJD)
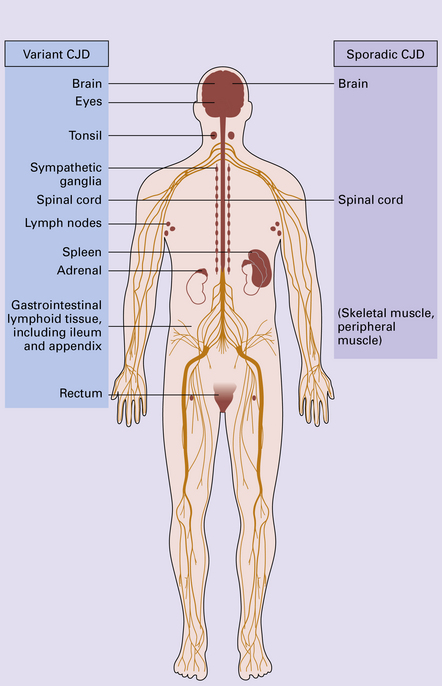
vCJD has a distinct neuropathological profile. The cerebral cortex and cerebellum typically contain numerous amyloid plaques which are distinct from those in Kuru or inherited forms of prion disease (Fig. 32.21). Many plaques are surrounded by vacuoles and have been termed florid plaques (Fig. 32.22). Spongiform change, neuronal loss, and astrocytic gliosis are generally very severe and are most evident in the thalamus and basal ganglia. Within the CNS, immunohistochemistry typically shows an abundant accumulation of PrP (Fig. 32.23). Additionally, and in remarkable contrast to all other forms of prion diseases, there is accumulation of PrPSc in lymphoreticular tissues such as the tonsil, lymph nodes, spleen, and appendix (Fig. 32.23). This finding is unique to vCJD and has not been observed in sporadic, inherited or, importantly, in acquired prion diseases in which human prions were transmitted (i.e. iatrogenic forms and Kuru). This unique property of the vCJD strain allows pre-mortem diagnosis of vCJD by tonsil biopsy. PrPSc can be demonstrated in follicular dendritic cells of lymphoid tissue by immunohistochemistry.
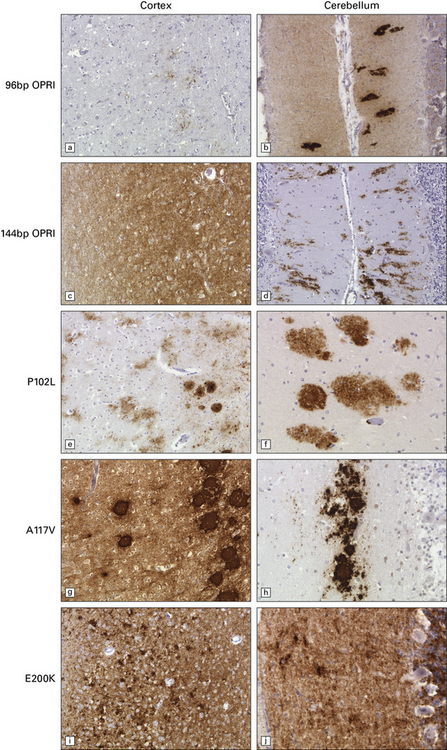
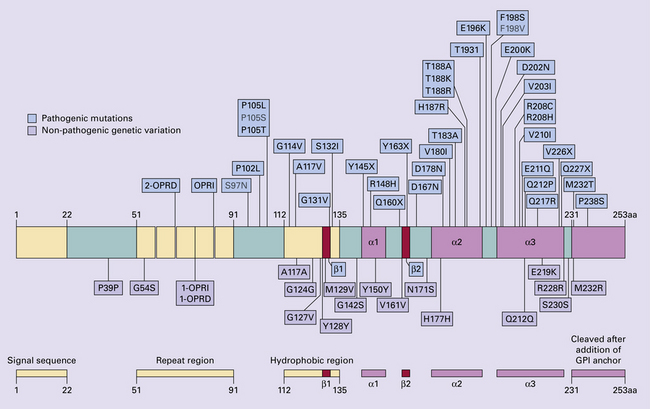
32.21 Phenotypic variability of genetic (inherited) prion disease. (a-j) The very variable appearance of the PrPSc deposits associated with PRNP mutations is shown in these panels. OPRI = octapeptide repeat insert. The other PRNP mutations are indicated using the conventional abbreviation ‘A number B’, where the number is the position of the mutant codon, A is the single-letter code for the amino acid at that position in wild-type PrP, and B is the code for the amino acid at that position in the mutant PrP.
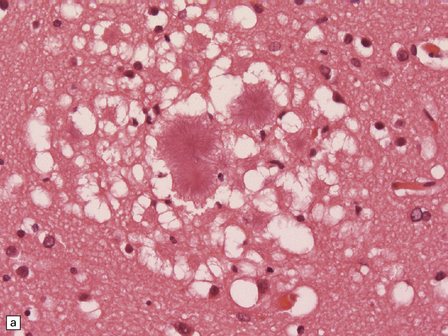
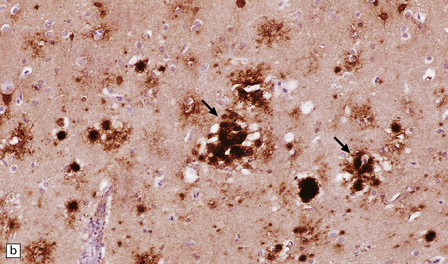
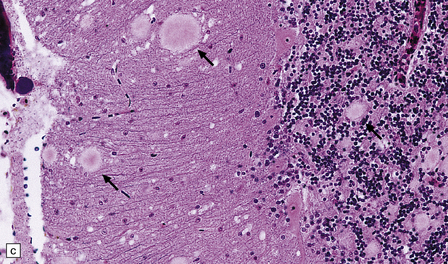
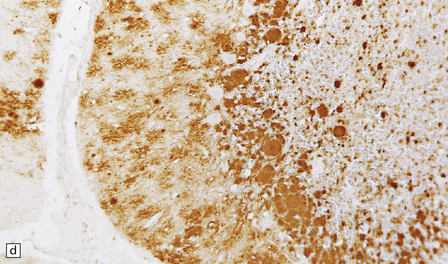
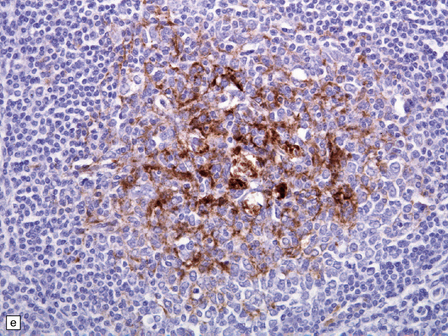
32.22 vCJD. (a) The plaques of vCJD are typically of ‘florid’ type (arrows), consisting of an amyloid core with relatively coarse surrounding vacuoles in a pattern that has been likened to the petals of a daisy. (b) The coarsely granular amyloid cores are strongly immunoreactive for PrP (arrows). (c) Large numbers of plaques (arrows) are usually present in the cerebellum. (d) The cerebellum shows abundant deposition of PrP on immunostaining. (e) Germinal center from tonsil showing positive immunostaining for PrP. Staining is concentrated in follicular dendritic cells.
IATROGENIC CJD
There are typically widespread neuronal loss and spongiform change in the cerebellar cortex and to a lesser degree in the cerebral cortex (Fig. 32.24). Plaques are common. PrP immunohistochemistry reveals heavy deposits in the cerebellum and a linear pattern of labeling involving the deeper laminae of the cerebral cortex.
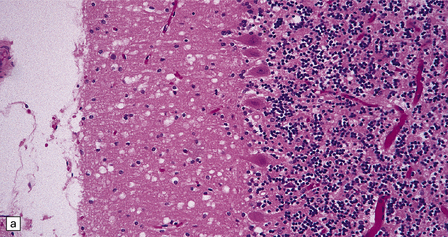
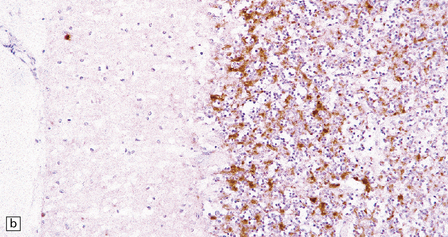
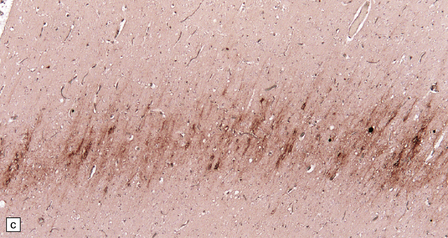
32.24 Iatrogenic CJD. A recipient of human pituitary derived growth hormone, this patient presented with ataxia and later developed dementia. (a) Spongiform change is present in the molecular layer of the cerebellum. (b) Immunohistochemistry for PrP reveals scattered deposits in the cerebellum and (c) a linear pattern of immunoreactivity involving the deeper laminae in the cerebral cortex.
PANENCEPHALOPATHIC CJD
The panencephalopathic form of CJD is characterized by extensive involvement of white matter as well as cerebral cortex (Fig. 32.25). It has been suggested that this is an end-stage pattern that reflects relatively prolonged disease.
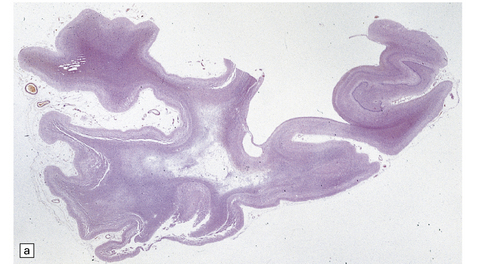
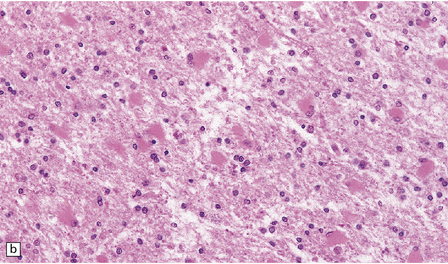
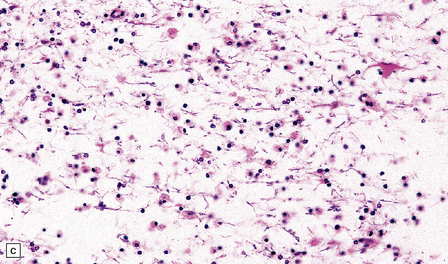
32.25 Panencephalopathic CJD. (a) Severe white matter rarefaction and focal cavitation in panencephalopathic CJD. This patient had typical spongiform change in the basal ganglia and thalamus. (b) Marked reactive astrocytosis in the cerebral white matter. (c) Elsewhere, the white matter is severely rarefied and contains scattered lipid-laden macrophages as well as reactive astrocytes.
The brain generally shows severe atrophy. Marked neuronal loss and spongiform change in the cerebral cortex are associated with an intense astrocytosis. There is diffuse myelin pallor in the hemispheric white matter, which appears spongy and may become cavitated. These changes are accompanied by moderate astrocytosis and an accumulation of lipid in macrophages (Fig. 32.25).
KURU
Clinical presentation and the neuropathology of Kuru are distinct from the majority of patients with sporadic CJD, in that Kuru presents with progressive cerebellar ataxia. Dementia is a late and less prominent feature. In contrast to vCJD there is no accumulation of abnormal PrP in lymphoreticular tissue (Fig. 32.26).
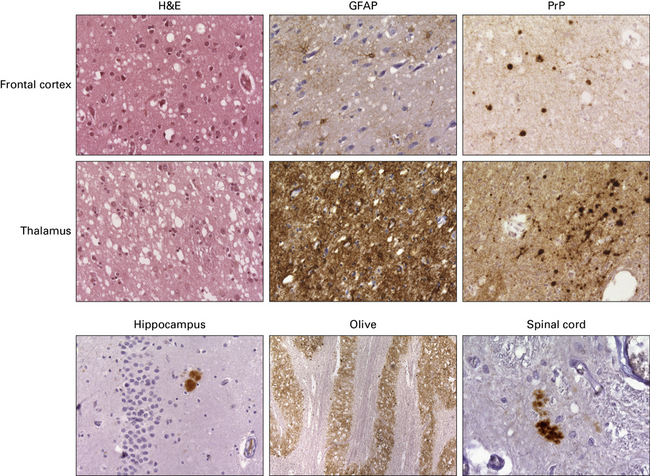
32.26 Neuropathology of Kuru. These images are from a patient in whom there was a 40-year latent interval between infection and the development of the first symptoms. The time from onset of symptoms to death was 2 years. Spongiform changes are highly variable in Kuru, as are the degree of gliosis and intensity and type of PrP deposition. Note that PrP accumulates both in small, spiculated, so-called Kuru plaques, but that many regions also show synaptic deposition of abnormal PrP. The codon 129 genotype in this case was 129MV and the pattern on western blotting type 3/type 2MV.
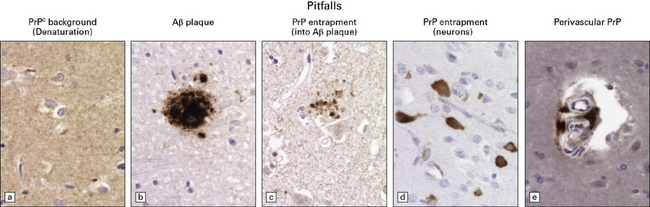
PITFALLS IN THE HISTOPATHOLOGICAL DIAGNOSIS OF PRION DISEASE
The diagnosis of prion diseases by examination of brain biopsies can be complicated by a number of pitfalls, some related to technical difficulties associated with PrP immunostaining, others to the biochemical properties of PrPC. See Figure 32.27 for details.
REFERENCES
Aguzzi, A., Weissmann, C. Prion research: the next frontiers. Nature. 1997;389:795–798.
Baldwin, M.A., Stahl, N., Reinders, L.G., et al. Permethylation and tandem mass spectrometry of oligosaccharides having free hexosamine: analysis of the glycoinositol phospholipid anchor glycan from the scrapie prion protein. Anal Biochem.. 1990;191:174–182.
Beck, J.A., Poulter, M., Campbell, T.A., et al. PRNP allelic series from 19 years of prion protein gene sequencing at the MRC Prion Unit. Hum Mutat.. 2010;31(7):E1551–E1563.
Bell, J.E., Ironside, J.W. How to tackle a possible Creutzfeldt–Jakob disease necropsy. J Clin Pathol.. 1993;46(3):193–197.
Brandner, S., Whitfield, J., Boone, K., et al. Central and peripheral pathology of kuru: pathological analysis of a recent case and comparison with other forms of human prion disease. Philos Trans R Soc Lond B Biol Sci.. 2008;363(1510):3755–3763.
Budka, H., Aguzzi, A., Brown, P., et al. Neuropathological diagnostic criteria for Creutzfeldt–Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol.. 1995;5(4):459–466.
Budka, H., Aguzzi, A., Brown, P., et al. Tissue handling in suspected Creutzfeldt–Jakob disease (CJD) and other human spongiform encephalopathies (prion diseases). Brain Pathol.. 1995;5(3):319–322.
Collinge, J. Molecular neurology of prion disease. J Neurol Neurosurg Psychiatry.. 2005;76:906–919.
Collinge, J., Rossor, M. A new variant of prion disease. Lancet.. 1996;347(9006):916–917.
Collinge, J., Whitfield, J., McKintosh, E., et al. A clinical study of kuru patients with long incubation periods at the end of the epidemic in Papua New Guinea. Philos Trans R Soc Lond B Biol Sci.. 2008;363(1510):3725–3739.
Collinge, J., Sidle, K.C., Meads, J., et al. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature.. 1996;383(6602):685–690.
Gambetti, P., Cali, I., Notari, S., et al. Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol.. 2011;121(1):79–90.
Hill, A.F., Zeidler, M., Ironside, J., et al. Diagnosis of new variant Creutzfeldt–Jakob disease by tonsil biopsy. Lancet.. 1997;349:99.
Hill, A.F., Desbruslais, M., Joiner, S., et al. The same prion strain causes vCJD and BSE [letter]. Nature.. 1997;389(6650):448–450.
Hilton, D.A., Ghani, A.C., Conyers, L., et al. Accumulation of prion protein in tonsil and appendix: review of tissue samples. BMJ.. 2002;325:633–634.
Ironside, J.W., Bell, J.E. Florid plaques and new variant Creutzfeldt–Jakob disease [letter]. Lancet.. 1997;350(9089):1475.
Ironside, J.W., Bell, J.E. The ‘high-risk’ neuropathological autopsy in AIDS and Creutzfeldt–Jakob disease: principles and practice. Neuropathol Appl Neurobiol.. 1996;22(5):388–393.
Kovacs, G.G., Seguin, J., Quadrio, I., et al. Genetic Creutzfeldt–Jakob disease associated with the E200K mutation: characterization of a complex proteinopathy. Acta Neuropathol (Berl).. 2011;121(1):39–57.
Medori, R., Montagna, P., Tritschler, H.J., et al. Fatal familial insomnia: a second kindred with mutation of prion protein gene at codon 178. Neurology.. 1992;42(3 Pt 1):669–670.
Palmer, M.S., Dryden, A.J., Hughes, J.T., et al. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt–Jakob disease. Nature.. 1991;352(6333):340–342.
Parchi, P., Castellani, R., Capellari, S., et al. Molecular basis of phenotypic variability in sporadic Creutzfeldt–Jakob disease. Ann Neurol.. 1996;39(6):767–778.
Parchi, P., Strammiello, R., Notari, S., et al. Incidence and spectrum of sporadic Creutzfeldt–Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neuropathol (Berl).. 2009;118:659–671.
Reiniger, L., Lukic, A., Linehan, J., et al. Tau, prions and Ab: the triad of neurodegeneration. Acta Neuropathol (Berl).. 2011;121(1):5–20.
Wadsworth, J.D., Hill, A.F., Beck, J.A., et al. Molecular and clinical classification of human prion disease. Br Med Bull.. 2003;66:241–254.
Wadsworth, J.D., Joiner, S., Linehan, J.M., et al. Phenotypic heterogeneity in inherited prion disease (P102L) is associated with differential propagation of protease-resistant wild-type and mutant prion protein. Brain.. 2006;129(Part 6):1557–1569.
Weber, T., Tumani, H., Holdorff, B., et al. Transmission of Creutzfeldt–Jakob disease by handling of dura mater [letter]. Lancet.. 1993;341(8837):123–124.
Whittington, M.A., Sidle, K.C., Gowland, I., et al. Rescue of neurophysiological phenotype seen in PrP null mice by transgene encoding human prion protein. Nat Genet.. 1995;9:197–201.
Will, R.G., Ironside, J.W., Zeidler, M., et al. A new variant of Creutzfeldt–Jakob disease in the UK. Lancet.. 1996;347(9006):921–925.
Young, K., Clark, H.B., Piccardo, P., et al. Gerstmann–Sträussler–Scheinker disease with the PRNP P102l mutation and valine at codon 129. Mol Brain Res.. 1997;44(1):147–150.
Zerr, I., Giese, A., Windl, O., et al. Phenotypic variability in fatal familial insomnia (D178N-129M) genotype. Neurology.. 1998;51(5):1398–1405.
Zou, W.Q., Puoti, G., Xiao, X., et al. Variably protease-sensitive prionopathy: a new sporadic disease of the prion protein. Ann Neurol.. 2010;68(2):162–172.