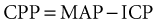Chapter 45 Principles of Neurointensive Care
Clinical Assessment of Critically Ill Neurological Patients
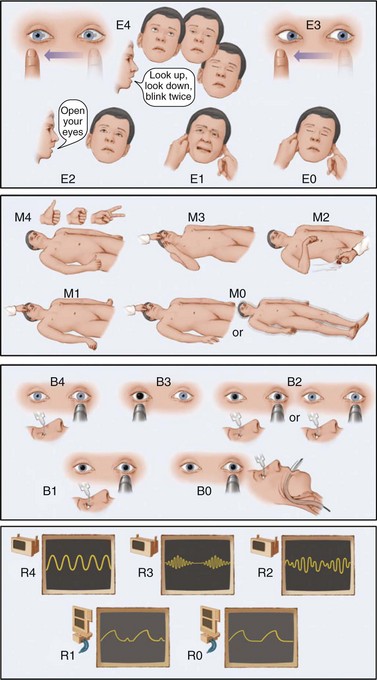
The neurological examination for a NICU patient should always begin by defining the level and content of consciousness. Level of consciousness measures the degree of arousal or wakefulness of the patient. Scales are useful to facilitate communication and monitor changes over serial examinations; the Glasgow Coma Scale (GCS) is the most widely used (see Chapter 5, Table 5.4). However, it loses accuracy in patients who are intubated or develop cerebral ptosis (inability or only partial ability to open the eyes [by contracting the frontalis muscle] because a brain lesion impairs control of eye-opening mechanisms) and fails to provide information on brainstem function and respiratory status. A new scale (the FOUR score) that addresses these shortcomings has been validated in various patient populations and merits consideration as an alternative (Wijdicks et al., 2005) (Fig. 45.1). For patients with localized structural brain diseases, the National Institutes of Health (NIH) Stroke Scale may be used to grade and track focal neurological deficits.
In patients with altered consciousness, the results of one of these scales should be complemented with documentation of additional neurological features. Detailed description of the location and movements of the eyes, brainstem reflexes (pupillary light reactions, corneal, oculocephalic, oculovestibular, gag, cough), spontaneous movements and motor responses to pain, lateralizing signs, and breathing pattern must be recorded. In patients with delirium, the clinician must note the predominant behavioral abnormalities, degree of motor activity, and ability to interact with the environment. It is always important to dedicate special attention to any abnormal or adventitious movements, since seizures in critically ill patients may present with very subtle motor manifestations (e.g., nystagmoid eye movements). Fundoscopy may also offer valuable information and should be attempted; however, to avoid confounding future pupillary evaluations, mydriatic agents should not be administered. The reader is referred to Chapters 4 and 5 for further information relative to clinical evaluation of comatose and delirious patients.
Another essential aspect of the examination in critically ill patients is evaluating neuromuscular respiratory weakness. Timely recognition of signs of impending neuromuscular respiratory failure may avoid potentially devastating complications. Among them, use of accessory muscles and paradoxical breathing pattern are most indicative of problems. Paradoxical breathing is defined as the loss of synchronicity in chest and abdominal movements during respiration (i.e., abnormal sinking of the abdomen during inspiration) and represents an unequivocal sign of diaphragmatic failure (Rabinstein and Wijdicks, 2003b).
Monitoring in the Neurointensive Care Unit
Systemic Monitoring
Systemic monitoring in the NICU typically includes cardiac telemetry, frequent scheduled noninvasive BP measurements (by automatic cuff inflation) or continuous invasive arterial BP recording, pulse oximetry, and core body temperature. Continuous arterial BP monitoring is accomplished by inserting an indwelling cannula into a medium-caliber artery (e.g., radial arterial line). The invasiveness of the procedure is justified by the precise real-time information it provides. Continuous arterial BP monitoring is especially recommended in patients treated with induced hypertension (e.g., symptomatic vasospasm after SAH), cases requiring very strict BP control to avoid hemorrhagic complications (e.g., ruptured arteriovenous malformations), and patients with hypotension (e.g., sepsis), compromised cerebral perfusion pressure (CPP) (e.g., TBI with raised intracranial pressure [ICP]), or autonomic instability (e.g., Guillain-Barré syndrome). Arterial lines provide the additional advantage of eliminating the need for repeated arterial punctures to measure arterial blood gases. However, although generally safe, placement of an arterial line may be complicated by local infection, leading to bacteremia and thrombosis with risk of digital ischemia. Careful attention to proper technique and adherence to strict sterile conditions during placement and manipulation of the catheter are mandatory (Tegtmeyer et al., 2006).
The most accurate method of measuring core body temperature is a pulmonary artery catheter thermistor, but since most patients in the NICU do not require pulmonary artery catheter insertion, bladder or rectal probes are most frequently used. Bladder and rectal probes correlate well with pulmonary artery catheter thermistor readings, but there is a lag in the detection of temperature changes by the probes. The site of temperature recording becomes particularly important in patients treated with cooling measures. Thus, monitoring esophageal temperatures is recommended when using certain intravascular cooling devices (De Georgia et al., 2004).
Central venous catheters allow monitoring of central venous pressure while providing access for fluid and drug administration. They are, however, a frequent source of infection. Rigorous sterile techniques at the time of catheter insertion, cutaneous antisepsis with chlorhexidine (rather than povidone-iodine), topical application of antiinfective ointment or a chlorhexidine-impregnated dressing to the insertion site, and catheters with an antiinfective surface may reduce the risk of catheter-related bloodstream infection (Safdar et al., 2002). The role of pulmonary artery catheters in ICUs is shrinking as studies consistently demonstrate that their use is associated with higher rates of complications (Sandham et al., 2003; Wheeler et al., 2006).
Brain Monitoring
Multiple brain monitoring methods are now available. They are most useful when applied in combination, a practice known as multimodality monitoring (Diedler and Czosnyka, 2010). It is important to be aware, however, that the endpoints of most studies validating the use of brain monitoring methods modalities have been surrogate physiological measures rather than actual assessments of patients’ functional outcome. In fact, there is no class I evidence proving that the use of multimodality brain monitoring results in improved clinical outcomes. Currently, the clinical application of brain monitoring techniques is restricted to large centers, especially those treating numerous TBI patients.
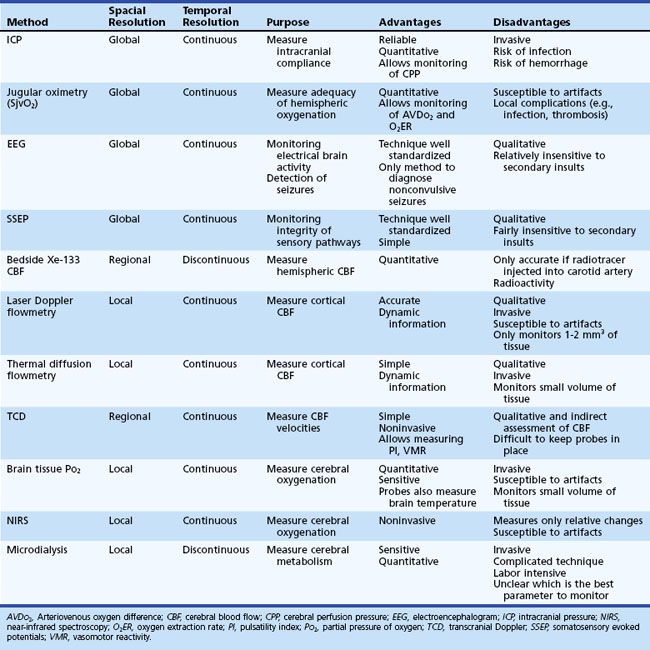
Methods for cerebral monitoring are divided into three main categories according to their spatial resolution: global, regional, and local brain monitoring (Table 45.1). Global brain monitoring techniques measure ICP, CPP, electrical potentials, and venous oxygen saturation. Regional and local brain monitoring methods include cerebral blood flow (CBF), cerebral blood flow velocities (BFV), brain tissue metabolism, temperature, and oxygenation.
Global Brain Monitoring Techniques
Intracranial Pressure Monitoring
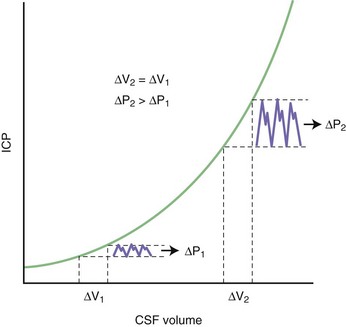
The intracranial space is occupied by three constituent compartments: the brain (accounting for 80% to 90% of the intracranial volume), the blood, and the cerebrospinal fluid (CSF). Because the skull is rigid, any expansion of one of these compartments must be compensated by a reduction in size of the others (a physiological principle known as the Monro-Kellie doctrine). When this compensation is insufficient, the ICP rises. Small increases in intracranial volume can be initially accommodated with little or no effect on the ICP, but as more volume is added, intracranial compliance falls until it reaches a critical point beyond which a minimal increase in volume causes an exponential rise in ICP. This pressure-volume relationship is depicted in Fig. 45.2. In other words, the initial physiological response to an increase in brain volume is a reduction in the CSF and venous blood volumes by shifting these fluids out of the intracranial space (except in the cases of hydrocephalus and venous thrombosis). Once these compensatory mechanisms are exhausted, the system becomes noncompliant, and further increments in brain volume compromise arterial blood flow and eventually lead to herniation of brain tissue.
It has also been argued that the main purpose of ICP monitoring is maintenance of normal CPP (normal, >70 mm Hg) because the latter may be more related to secondary ischemic injury (Rosner et al., 1995). The relative importance of ICP and CPP as main targets of therapy remains a matter of debate.
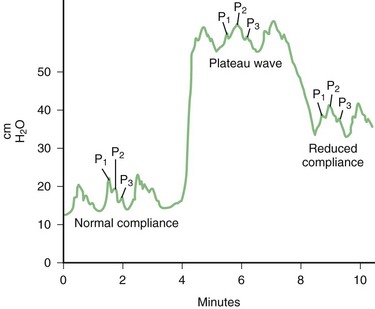
ICP is pulsatile and has systolic and diastolic components. In addition to the value of mean ICP, these components must also be evaluated carefully. The normal ICP waveform consists of a 3-peaked wave (Fig. 45.3). P1, the first and generally the tallest peak, is also known as the percussion wave and corresponds to the transmitted systolic BP; P2 (the tidal wave) and P3 (the dicrotic wave) are normally smaller peaks, and the notch between them corresponds to the dicrotic notch of the arterial waveform. As ICP increases, P2 and P3 rise and eventually surpass P1. Ultimately, with continued elevation of ICP, the waveform loses distinct peaks and assumes a triangular morphology. Intracranial pathology leading to sustained elevations of ICP may produce plateau waves, also known as A-waves of Lundberg (see Fig. 45.3). These waves reflect a sudden dramatic rise in ICP to levels of 40 to 100 mm Hg, often lasting 5 to 20 minutes. Plateau waves indicate critically low intracranial compliance leading to marked changes in ICP, even with very small variations in intracranial volume. Although their pathophysiology is not fully elucidated, experimental observations suggest that plateau waves may be generated by brief episodes of systemic hypotension leading to exaggerated cerebral vasodilation in patients with abnormal vasomotor reactivity (Rosner and Becker, 1984).
ICP may be monitored using intraparenchymal, intraventricular, epidural, or subdural devices (Brain Trauma Foundation, 2007). Intraventricular monitoring remains the gold standard because of its precision. It consists of a ventricular catheter connected to an external transducer which allows continuous ICP readings. Advantages of this technique are providing reliable ICP measurements and allowing external drainage of CSF. Hence, ventricular monitoring is indicated in patients with hydrocephalus and often preferred in those with refractory intracranial hypertension. Major drawbacks are higher risk of infection (rate of ventriculitis is 3% to 8% and increases with duration of the ventriculostomy) (Flibotte et al., 2004; Holloway et al., 1996; Martinez-Manas et al., 2000), risk of bleeding at the time of catheter placement (especially in patients with underlying coagulopathy or recent use of antithrombotics), and system malfunction (dampening of the waveform may be caused by apposition of the catheter tip against the ventricular wall or obstruction of the catheter by a blood clot or an air bubble). Risks may be minimized by careful placement of the catheter and maintenance of the system under strict sterile conditions, use of antibiotic prophylaxis (e.g., cefotaxime 2 g every 6 hours from the time of catheter insertion until 24 to 48 hours after its removal) (Flibotte et al., 2004), and withdrawal of the catheter as soon as possible (Holloway et al., 1996). Exchange of the catheter every 5 days, although a common practice, does not appear to decrease the risk of infection (Holloway et al., 1996; Lozier et al., 2002); in fact, repeated catheter insertions have been found to be associated with higher risk of ventriculitis (Arabi et al., 2005).
Intraparenchymal fiberoptic monitors are also quite accurate. When compared with intraventricular catheters, the measurements provided by intraparenchymal monitors differ on average by ± 2 to 5 mm Hg. Advantages of this monitoring system include simple and safe insertion technique, easy maintenance, relative lack of substantial drift (even after several days), and low risk of infection. Disadvantages include high cost, technical complications (e.g., breakage of the optical fiber), and most importantly, inability to drain CSF. Epidural and subdural monitors are less reliable and therefore rarely used but are a valuable option for patients with severe coagulopathy (e.g., liver failure with cerebral edema), given their lower risk of hemorrhagic complications (Vaquero et al., 2005).
ICP should be monitored in patients with severe TBI and a GCS sum score below 9 and an abnormal computed tomography (CT) scan, or a normal CT scan with two or more of the following criteria: age older than 40, unilateral or bilateral motor posturing, and systolic BP less than 90 mm Hg (Brain Trauma Foundation, 2007). It is difficult to extrapolate the value of these guidelines to patients with diagnoses other than trauma, owing to lack of specific data on ICP monitoring in those other conditions. Some experts advocate monitoring ICP in comatose patients with a large intracranial mass lesion (hematoma, abscess, large infarctions, etc.) causing radiologically documented tissue shift. Patients with SAH, ICH, or cerebellar ischemic or hemorrhagic strokes producing acute hydrocephalus typically have their ICP monitored once a ventriculostomy catheter has been placed primarily for drainage purposes.
Jugular Bulb Oximetry
Jugular bulb oximetry measures the oxygen saturation of venous blood returning from the brain (normal 50% to 65%) by means of a fiberoptic catheter (Feldman and Robertson, 1997). The main goal of jugular venous oxygen saturation (Sjvo2) monitoring is to provide a continuous measure of the changing balance between cerebral oxygen delivery and cerebral oxygen consumption. Simultaneous determination of Sjvo2 using the jugular bulb catheter and arterial oxygen saturation (Sao2) allows for the calculation of the intracranial arteriovenous oxygen difference (AVDo2) (normal 24%-42%). Cerebral oxygen consumption can be calculated as the product of AVDo2 and CBF. The cerebral oxygen extraction rate (O2ER) is derived from the ratio of cerebral oxygen consumption to cerebral oxygen delivery.
Jugular venous desaturations denote relative reductions of global cerebral oxygenation. Sjvo2 below 50% for 15 minutes or more are deemed indicative of ischemia. Sjvo2 monitoring has been mostly tested in patients with severe TBI. In these patients, jugular venous desaturations have been shown to correlate with the occurrence of secondary brain insults and poor outcome (Gopinath et al., 1994; Robertson et al., 1995). High Sjvo2 should not simply be equated with hyperemia; it may also be associated with poor outcome in comatose patients, possibly indicating lack of oxygen utilization after extensive neuronal death (Cormio et al., 1999). Favorable experience with jugular bulb oximetry has been reported in patients with SAH and ICH (Heran et al., 2004; von Helden et al., 1993), but interpreting Sjvo2 may be difficult in patients with severe hemispheric ischemic strokes (Keller et al., 2002). This technique is also used to monitor cerebral oxygenation during neurosurgical procedures.
Therapeutic interventions in response to information provided by jugular bulb oximetry have been proposed, including adjustment of the degree of hyperventilation, timing and intensity of osmotherapy, adjustment of MAP, and treatment of anemia (Macmillan and Andrews, 2000). There is no proof, however, that these interventions improve functional outcome. As shown by the negative results observed in studies testing therapies guided by pulmonary artery catheters, the clinical value of aggressive interventions aimed at optimizing physiological parameters must be proven before we incorporate these into clinical practice.
Advantages of the jugular bulb catheter as a monitoring modality include the practicality of continuous bedside monitoring, the capability of confirming the oximeter reading by drawing blood through the catheter, and the numerous physiological parameters that can be derived from the Sjvo2 to ascertain cerebral oxygen balance. Disadvantages of the catheter include its susceptibility to positioning artifacts and the complications associated with catheter insertion, including carotid puncture, infection, accidental misplacement, and jugular thrombosis (Coplin et al., 1997; Coplin et al., 1998; Latronico et al., 2000).
Electroencephalography
Continuous bedside electroencephalography (EEG) monitoring is based on four of its major neurobiological features (Jordan, 1995): (1) its close relationship to cerebral metabolic rate; (2) its sensitivity in detecting hypoxic-ischemic neuronal dysfunction at an early stage; (3) its obvious primacy as a monitor of seizure activity; and (4) its value in cerebral localization. Continuous EEG recording has been advocated as a valuable routine tool to monitor critically ill neurosurgical and neurological patients.
Status epilepticus is the most common indication for EEG monitoring, because the clinical ascertainment of ongoing seizure activity is often obscured by the effect of sedatives and analgesic agents. The EEG is essential for monitoring the effects of treatment, especially when barbiturates or general anesthetics are administered to achieve a burst-suppression pattern. Detection of nonconvulsive seizures and nonconvulsive status epilepticus (NCSE) can only be accomplished by EEG monitoring. Timely diagnosis of NCSE is important because delayed recognition may be associated with increased mortality (Young et al., 1996).
Nonconvulsive seizures were reported in up to one-third of unselected NICU patients, frequently involving the presence of NCSE (Jordan, 1995). NCSE has also been noted in 8% of comatose patients in medical ICUs (Towne et al., 2000), and nearly a third of septic patients with encephalopathy may have electrographic seizures without obvious clinical manifestations (Oddo et al., 2009). Continuous EEG monitoring has documented nonconvulsive seizures after severe TBI, ischemic stroke, poor-grade SAH, ICH, and after termination of generalized convulsive status epilepticus (DeLorenzo et al., 1998; Dennis et al., 2002; Vespa et al., 1999). These events might exacerbate excitotoxic injury in vulnerable brains and have been associated with high mortality (Young et al., 1996). But while their prognostic value is fairly well established, the impact of aggressive treatment of nonconvulsive seizures on clinical outcome remains to be determined (Hirsch, 2004).
Continuous EEG monitoring has also been used as an aid for early detection of ischemia in patients with SAH at high risk for vasospasm. Although early experience is promising (Claassen et al., 2006; Vespa et al., 1997), it is too early to recommend continuous EEG for this indication. Intracortical EEG (based on the use of deep electrodes) may be substantially superior to scalp EEG for detecting changes related to secondary neurological insults in patients with various forms of acute brain injury (Waziri et al., 2009). Furthermore, recurrent cortical spreading depolarizations may exacerbate local brain hypoxia in patients with TBI or SAH (Bosche et al., 2010), but the value of monitoring for these changes with intracortical EEG remains to be conclusively determined.
Other applications of EEG in the ICU, especially in comatose patients, include evaluating metabolic encephalopathy (EEG serves to substantiate the diagnosis by showing diffusely slow, low-amplitude activity, and often triphasic waves, but does not distinguish between different causes of the condition), recognizing psychogenic unresponsiveness, and confirming brain death (Wijdicks, 2001). After cardiac arrest, near-complete suppression, burst-suppression, nonreactive alpha or theta rhythms (alpha or theta coma), status epilepticus and generalized periodic complexes are considered malignant patterns (Rossetti et al., 2007; Synek, 1990). Although valuable for the prognostication of anoxic-ischemic encephalopathy, EEG data should not be interpreted in isolation in these patients (Wijdicks et al., 2006).
Evoked Potentials
Evoked potentials have a more restricted role in the NICU (Moulton et al., 1998). The median nerve somatosensory evoked potential (SSEP) has been mostly used; technical details are discussed in Chapter 32A. Bilateral absence of the N20 response 1 to 3 days after cardiopulmonary resuscitation accurately predicts poor chances of recovery of awareness (Zandbergen et al., 2006). Unfortunately, presence of these responses after anoxic brain injury lacks meaningful prognostic value.
Regional/Focal Brain Monitoring Techniques
Transcranial Doppler Ultrasonography
TCD ultrasonography is a noninvasive technique used to evaluate mean CBF velocity in the large intracranial arteries at the level of the circle of Willis. TCD is easy to learn and use, noninvasive, and safe. It measures CBF velocity rather than CBF, and the linear relationship between CBF and BFV depends on the angle of insonation. Still, TCD provides a wealth of useful clinical information including the presence or absence of blood flow, its velocity (systolic, diastolic, and mean), and direction. It also allows calculation of the pulsatility index (PI = peak systolic velocity minus end diastolic velocity divided by mean BFV), which represents the downstream resistance to blood flow. Increases in BFV are observed in patients with cerebral vasospasm, hyperventilation (which produces vasoconstriction), and anemia. Cerebral vasospasm may be distinguished from hyperdynamic status by measuring the hemispheric index or Lindegaard ratio (ratio of middle cerebral artery to extracranial internal carotid artery mean BFV) (Lindegaard et al., 1989). A ratio greater than 3 is considered indicative of vasospasm; a low ratio is more suggestive of hyperemia. TCD also allows assessment of vasomotor reactivity (Ng et al., 2000). Impairment of vasomotor reactivity is a well-established poor prognostic factor in patients with TBI and may portend the occurrence of symptomatic vasospasm in patients with SAH (Czosnyka et al., 1997; Frontera et al., 2006b). TCD may also be used as a confirmatory test for the diagnosis of brain death (severely diminished mean cerebral BFV associated with absent diastolic flow, reversed flow, and severely elevated PI).
The diagnosis of cerebral vasospasm in patients with SAH remains the main indication of TCD monitoring in the NICU. The criteria for vasospasm in the middle cerebral artery territory is a mean BFV greater than 120 cm/sec with a hemispheric index greater than 3, or an increment greater than 50 cm/sec within a 24-hour period (Suarez et al., 2002). A specialized headset allows continuous monitoring of BFV and may be a useful adjunct in monitoring patients at high risk for vasospasm. TCD monitoring in patients with cerebral vasospasm has good correlation with angiographic vasospasm and is comparable to conventional angiography in the prognostication of delayed ischemia in these patient, although neither technique is uniformly diagnostic (Rabinstein et al., 2004).
Local Cerebral Oxygenation Monitoring Techniques
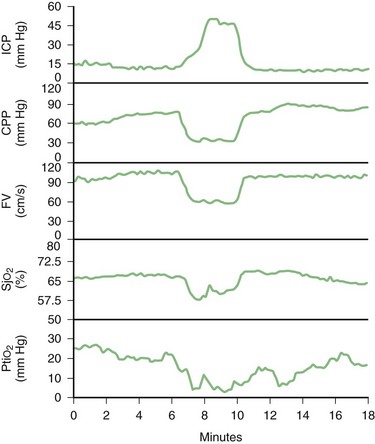
Brain tissue oxygen probes and near-infrared spectroscopy allow assessment of local oxygenation. Brain tissue oxygen may be measured by invasive probes such as the Licox catheter. Apart from tissue Po2, this catheter allows measurement of brain temperature. Brain tissue Po2 measures the diffusion of dissolved plasma oxygen across the blood brain barrier (rather than CBF, arterial delivery of oxygen, or brain metabolism) in a relatively small area of brain tissue (approximately 15 mm3) (Rosenthal et al., 2009). Factors that determine brain tissue Po2 include Pao2, arterial Paco2, systemic BP, and CBF. Normal brain tissue Po2 values range from 25 to 30 mm Hg. The major disadvantages of brain oxygen probes include their invasiveness, limited spatial resolution, and susceptibility to artifacts (due to inappropriate calibration and head movement, among other factors). Its use has been recommended by experts in various major centers for patients with severe head injuries and poor-grade SAH (Maloney-Wilensky et al., 2009). It is best used when applied in the setting of multimodality monitoring, along with jugular oximetry and perhaps microdialysis (Andrews et al., 2008) (Fig. 45.4).
Near-infrared spectroscopy (NIRS) is based on the property of a near-infrared light (700–1000 nm) to pass through tissues while being both scattered and absorbed. The absorption of a near-infrared light is proportional to the local concentration of certain chromophores, most notably hemoglobin. Thus, the absorption of near-infrared light changes according to the oxygenation state of hemoglobin. The probes illuminate up to a volume of 10 mL of brain tissue. All measurements are expressed as absolute concentration changes from a baseline zero at the start of the measurement. Normal values of oxygenated hemoglobin are reported to be 60% to 80%, and ischemic threshold is estimated to be below 47% saturation (Casati et al., 2006). However, the reliability of this technique has been questioned. It is susceptible to extraneous light, motion artifact, and signal drift. The measurement may also become unreliable when obtained through intracranial hematomas or through blood in the CSF.
Microdialysis
The basic concept of microdialysis involves inserting a fine catheter into the brain parenchyma, then perfusing the catheter with a physiological solution such as Ringer’s lactate, thereby facilitating the exchange of molecules between the perfusate and the extracellular fluid across a dialysis membrane located within the catheter tip. The dialysate is sampled under sterile conditions at hourly or other regular intervals and put through a microdialysis analyzer at the bedside. Insertion artifacts make measurements unreliable for the first hour after placement (Bellander et al., 2004).
Microdialysis allows monitoring of brain pH, lactate and pyruvate, glucose, glycerol, glutamate, urea, and potentially other soluble molecules of interest (Bellander et al., 2004; Nilsson et al., 1999; Vespa et al., 1998). Changes in lactate concentration, lactate/pyruvate ratio, and glutamate concentration have been used as indices of cerebral ischemia. A lactate/pyruvate ratio greater than 25 is probably the best indicator of ischemia (Andrews et al., 2008; Bellander et al., 2004). Rises in glycerol are believed to reflect phospholipid breakdown as a result of cell membrane damage. Because of this, cerebral microdialysis has been employed in the NICU to monitor for cerebral vasospasm and delayed cerebral ischemia in SAH, to identify secondary insults after severe brain trauma, and to follow extracellular glutamate concentration peri-ictally in patients with epilepsy.
Several aspects of microdialytic analysis remain controversial, such as where to place the catheter (Andrews et al., 2008), whether the lactate/pyruvate ratio alone or in combination with other parameters is a better indicator of early cerebral ischemia, and why there has been no correlation between microdialysis measures and clinical outcome in some studies. Other problems are the invasiveness and labor intensiveness of the technique. It currently represents a valuable research tool, but its widespread clinical use cannot yet be recommended.
Principles of Managing Critically Ill Neurological Patients
Analgesia and Sedation
Awakening is typically seen within minutes of discontinuation of the infusion, but time to awakening may be significantly prolonged when the drug has been used in large doses for several days, because propofol is redistributed to the fat tissue from where it is only slowly released. Hypotension is the most common side effect of propofol infusion, especially when administered as a bolus. Falls in BP are more frequent and pronounced in patients who are hypovolemic. Other adverse effects include caloric overload (1 mL of propofol contains 1 kilocalorie), hypertriglyceridemia, and withdrawal myoclonus (often confused with seizures). Propofol can also be used to treat elevated ICP and status epilepticus (Parviainen et al., 2006). However, administration of high doses of propofol for prolonged periods of time (i.e., >4-5 mg/kg/h for more than 48 hours) can cause the propofol infusion syndrome (Kam and Cardone, 2007). This is a serious complication characterized by metabolic acidosis, rhabdomyolysis, refractory bradycardia, myocardial depression, and when most severe, cardiac arrest (Iyer et al., 2009). Even strict surveillance for these manifestations may fail to prevent this life-threatening complication. Consequently, propofol should be used with great caution for the treatment of recalcitrant intracranial hypertension and status epilepticus, indications in which high doses of the medication are often necessary for up to several days to achieve the therapeutic goal.
Midazolam and lorazepam are the two most commonly used benzodiazepines in the NICU. The advantages of midazolam are its rapid effect and short duration of action (half-life 1.9 hours); it has only one active metabolite. Clearance is fast, but accumulation may occur after 3 days of continuous infusion. Patients who receive midazolam for several days can be expected to exhibit delayed awakening. Clearance of midazolam is diminished by hepatic and renal failure. Lorazepam has a much longer half-life (14 hours), which leads to a much slower emergence from sedation. However, continuous infusion of lorazepam can produce severe metabolic acidosis from propylene glycol toxicity (Arroliga et al., 2004). The main side effect of benzodiazepines is respiratory depression in patients who are not mechanically ventilated. They can also induce hypotension in patients with reduced intravascular volume. Risk of withdrawal symptoms is small. Benzodiazepines are effective in the treatment of status epilepticus, but pharmacoresistance emerges over time and requires progressive increase in the rate of infusion of the drug. They do not have a significant effect on ICP.
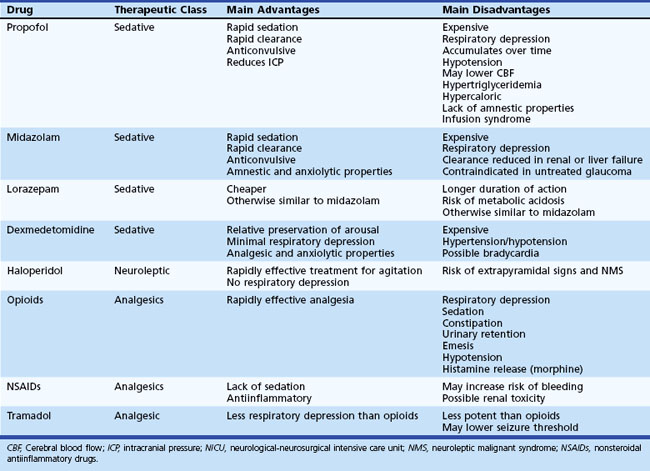
Opiates represent the mainstay of analgesic treatment in acutely ill neurological patients. Options include morphine, fentanyl, sufentanil, alfentanil, and codeine. They all produce analgesia, reduced level of consciousness, and respiratory depression. Hypotension may occur in hypovolemic patients or when using high doses of these medications. Fentanyl is preferred to morphine because it provokes fewer cardiovascular side effects and does not produce histamine release. Codeine is a much less potent agent, and its role is limited in the NICU. The action of opioids may be reversed by using naloxone, a competitive antagonist. Hypertension and cardiac arrhythmias are potential side effects of naloxone use. For milder forms of pain, nonsteroidal antiinflammatory agents (e.g., ketorolac), tramadol, and acetaminophen may be used. Table 45.2 summarizes key pharmacokinetic and pharmacodynamic information for the most commonly used sedative and analgesic agents in the NICU.
Airway and Ventilatory Assistance
At the time of endotracheal intubation, the two main complications in neurological patients are a rise in ICP and exacerbation of hypoxia. Rapid-sequence intubation is the safest approach for patients with increased ICP (Wijdicks and Borel, 1998). Rapid-sequence intubation proceeds in three phases: (1) preoxygenation to prevent worsening hypoxia during intubation—this can be achieved by providing effective bag-valve-mask (AMBU) ventilation; (2) pretreatment with drugs to mitigate the hemodynamic changes that may increase ICP upon intubation (e.g., lidocaine, thiopental); and (3) sequential administration of a potent sedative (e.g., propofol) and, when necessary, a rapidly acting nondepolarizing neuromuscular blocking agent (e.g., rocuronium, vecuronium). Succinylcholine should be avoided because it may increase ICP due to widespread muscle fasciculations, increased central venous pressure, and hypercarbia, and because it can produce dangerous hyperkalemia in patients with underlying muscle disease. In cases of TBI, it is also essential to maintain in-line stabilization of the cervical spine. When cervical spine injury is suspected, fiberoptic-assisted intubation is preferred.
In patients with acute brain disorders, level of consciousness may a limiting factor when considering extubation. Despite successful weaning, the stuporous patient may be considered unsafe for extubation because of concerns about airway safety. Keeping patients intubated once they have fulfilled the ventilatory criteria for extubation is a common but questionable practice. In patients with TBI, this practice may be associated with a higher risk of ventilator-associated complications (Coplin et al., 2000). Thus, safety of extubation in patients with adequate respiratory function but persistently depressed level of consciousness is a problem that demands further research (Manno et al., 2008).
Cardiovascular Care and Blood Pressure Management
Cardiac disorders are common in critically ill neurological patients, and they may precede or accompany the neurological illness. They are often related to the massive catecholamine release associated with the acute brain insult (Banki et al., 2005). The most common forms of cardiac complications in the NICU are acute coronary syndrome, cardiac arrhythmias, and congestive heart failure.
Acute Coronary Syndrome
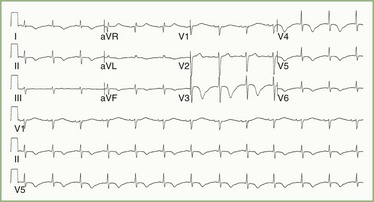
Electrocardiographic (ECG) and clinical abnormalities suggestive of myocardial ischemia are fairly common in patients with acute brain injury (e.g., large ischemic stroke, SAH, large intraparenchymal hematoma, TBI with contusions, status epilepticus). Typical ECG abnormalities in patients with acute brain damage include symmetrically inverted T waves (Fig. 45.5) and sometimes ST-segment elevation across all the precordial leads. Elevation of serum troponin levels should be considered indicative of myocardial injury, whereas elevation of serum creatinine kinase is much less specific in patients with acute brain damage (Woodruff et al., 2003). Yet, troponin elevation is seen in patients with SAH as an expression of ventricular dysfunction secondary to the neurogenic (adrenergic-induced) injury (Deibert et al., 2003).
Cardiac Arrhythmias
Cardiac arrhythmias in acute neurological patients may be due to preexisting cardiac disease. They may also be responsible for the acute neurological disorder, as occurs in patients with atrial fibrillation presenting with embolic stroke. On the other hand, arrhythmias and conduction abnormalities may be due to acute brain disease. Decreased high rate viability, increased risk for arrhythmias, and even increased risk for sudden death have been documented in patients with insular strokes (Abboud et al., 2006). Cardiac arrhythmias may also develop as a complication of seizures. Dysregulation of autonomic function may provoke life-threatening arrhythmias in patients with Guillain-Barré syndrome.
Congestive Heart Failure
Apical ballooning syndrome is a characteristic form of cardiomyopathy seen after acute neurological insults (Lee et al., 2006). Sudden sympathetic hyperstimulation of the myocardium causes a specific pattern of myocardial stunning (Prasad et al., 2008), and its diagnosis depends on echocardiographic demonstration of apical hypokinesis or akinesis with sparing of basal segments. Consequently, the heart takes on the form of an octopus catcher pot (takotsubo in Japanese, hence the name takotsubo cardiomyopathy sometimes given to this condition). Patients with apical ballooning syndrome have reductions in left ventricular ejection fraction and may develop acute congestive heart failure with pulmonary edema. The presentation may also mimic myocardial ischemia. Cardiac function typically returns to baseline after 2 or 3 weeks (Lee et al., 2006; Prasad et al., 2008).
Blood Pressure Management
BP management represents one of the most crucial aspects of neurocritical care. The three main goals of BP management in critically ill neurological and neurosurgical patients are to ensure adequate cerebral perfusion, prevent intracranial bleeding, and avoid exacerbation of cerebral edema. These goals must often be balanced in individual cases in which the risk of hypoperfusion and worsening ischemia coexist with the danger of new or enlarging hemorrhage and progression of brain swelling. Although guidelines and practice parameters have been published to guide BP treatment in various acute neurological conditions (Table 45.3), there are still areas of debate in regard to what should be considered optimal BP targets in patients with some of the most common disorders treated in the NICU.
Table 45.3 Guidelines for Blood Pressure Management in the Most Common Conditions Treated in the NICU
| Diagnosis | Recommendation |
|---|---|
| Acute ischemic stroke | Keep <180/110 mm Hg if thrombolysis |
| Treat only BP >220/120 if no thrombolysis | |
| Intracerebral hemorrhage | Keep SBP <180 and MAP <130 mm Hg |
| (ideal SBP <160 and MAP <110 mm Hg) | |
| Subarachnoid hemorrhage | Keep SBP <160 mm Hg before aneurysm treated |
| Do not lower BP after aneurysm treated | |
| Traumatic brain injury | Keep adequate MAP to maintain CPP >60 mm Hg |
BP, Blood pressure; CPP, cerebral perfusion pressure; MAP, mean arterial pressure; NICU, neurological-neurosurgical intensive care unit; SBP, systolic blood pressure.
Acute Ischemic Stroke
Sudden and profound reductions of BP are associated with neurological decline in patients with acute ischemic stroke (Oliveira-Filho et al., 2003). This is likely related to insufficient perfusion in areas already affected by ischemic penumbra. In fact, elevation of BP appears to be a protective physiological response that occurs after occlusion of a cerebral vessel, as suggested by the spontaneous resolution of hypertension in patients who achieve successful recanalization (Mattle et al., 2005). Furthermore, low BP (diastolic BP <70, systolic BP <155, or MAP <100 mm Hg) on initial evaluation in the emergency department and greater BP fluctuations within the first 3 hours have been shown to correlate with increased 90-day mortality in patients with acute cerebral ischemic infarction (Stead et al., 2005; Stead et al., 2006).
Current practice guidelines advocate a very conservative approach to treating hypertension after acute ischemic stroke. In patients ineligible for thrombolysis, antihypertensive therapy is only recommended for patients with systolic BP higher than 220 mm Hg or diastolic BP higher than 120 mm Hg (Adams, Jr. et al., 2007). Intermittent doses of IV labetalol or continuous infusion of nicardipine are the preferred treatment options; when diastolic BP exceeds 140 mm Hg, sodium nitroprusside should be infused instead. The initial objective of treatment should be to reduce the BP by 10% to 15%. However, it is important to acknowledge that this permissive approach to hypertension is not based on direct evidence from randomized trials. In fact, preliminary data indicate that modest BP reduction early after cerebral ischemia could actually be beneficial (Potter et al., 2009), so this important clinical matter demands more investigation.
There is limited but promising evidence suggesting that pharmacological elevation of BP may be beneficial for certain patients with acute ischemic stroke (Mistri et al., 2006). Further research is needed to determine the safety of this intervention and which patients could be optimal candidates for this type of aggressive hemodynamic treatment.
Intracerebral Hemorrhage
The treatment of hypertension in patients with spontaneous (hypertensive) intraparenchymal hematomas is more controversial. There is abundant (Fogelholm et al., 1997; Leira et al., 2004; Terayama et al., 1997) although not uniform (Brott et al., 1997; Jauch et al., 2006; Qureshi et al., 1999) evidence that extreme hypertension is associated with greater risk of hematoma expansion, a major determinant of poor outcome and increased mortality in ICH (Davis et al., 2006). Meanwhile, solid demonstration that areas of hypoperfusion are frequently present around parenchymal hematomas (Kidwell et al., 2001; Mayer et al., 1998; Rosand et al., 2002) has supported the argument that aggressive BP reduction could precipitate ischemia in these regions. This theoretical risk is, however, not substantiated by PET studies showing decreased oxygen extraction fraction in the hypoperfused perihematoma tissue (as opposed to the increased oxygen extraction that would be expected in areas of ischemic penumbra) (Zazulia et al., 2001) and preserved CBF in those regions after acute BP reduction (Powers et al., 2001).
There are valid arguments both in favor of and against aggressive BP reduction in acute ICH. Current guidelines advise keeping the SBP ideally below 160 mm Hg and the MAP ideally below 110 mm Hg unless there is suspicion of intracranial hypertension, in which case ICP monitoring is recommended to target therapy to maintain CPP between 60 and 80 mm Hg (Broderick et al., 2007). The initial phases of ongoing randomized controlled trials have shown that more aggressive BP reduction is feasible and most likely safe (Anderson et al., 2008; Qureshi et al., 2010).
Subarachnoid Hemorrhage
In patients with acute aneurysmal SAH, it is often recommended to keep a systolic BP below 160 mm Hg until the ruptured aneurysm is secured in order to prevent re-bleeding. It should be noted that this widespread practice of aggressive BP lowering is not based on solid scientific data. After the aneurysm is secured, BP should not be lowered, since these patients are at risk for delayed ischemia from vasospasm. Hemodynamic augmentation therapy, often including the use of vasopressors, is indicated in patients with symptomatic vasospasm (Rabinstein et al., 2010).
Traumatic Head Injury
Maintenance of an adequate CPP is one of the principal therapeutic goals in the intensive care of severe TBI patients, since secondary ischemic insults are known to have a major detrimental impact on prognosis (Sarrafzadeh et al., 2001). It is advisable to keep the CPP above 60 mm Hg, although it is unclear whether raising the MAP or lowering the ICP should be the main therapeutic strategy to achieve this goal (Brain Trauma Foundation, 2007). Aggressive fluid resuscitation is the mainstay of hemodynamic treatment in TBI. Vasopressors should be reserved for patients with persistent hypotension after aggressive fluid replacement.
Fluid and Electrolytes
Acute renal failure in acutely ill neurological or neurosurgical patients is most commonly iatrogenic. Mannitol can rapidly cause prerenal azotemia when adequate hydration is not provided to compensate for the fluid lost from osmotic diuresis. This complication can be reliably avoided by adjusting the fluid intake to prevent negative fluid balance, while monitoring serum osmolality. If serum osmolality exceeds 320 mOsm/kg, mannitol infusion is typically withheld to protect renal function. If continuation of osmotherapy is indispensable, mannitol may be continued with relatively low risk of kidney failure, so long as concomitant aggressive hydration is provided. Hypertonic saline may be a valuable alternative in these cases; it is a safer choice than mannitol in patients with chronic renal insufficiency. Radiocontrast-induced nephropathy can be prevented by preemptive hydration, N-acetylcysteine, and bicarbonate infusion (Merten et al., 2004; Tepel et al., 2000). Acute interstitial nephritis from drug toxicity (e.g., antibiotics) and (less commonly) pyelonephritis (in patients with chronic indwelling catheters) are also causes of acute renal failure in the NICU.
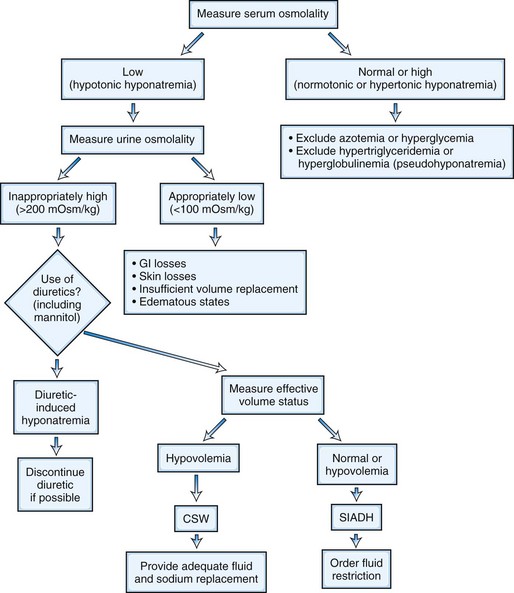
Hyponatremia is the most common electrolyte imbalance encountered in critically ill neurological patients. The two most common mechanisms of hyponatremia in these patients are cerebral salt-wasting syndrome (CSWS) and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) (Rabinstein and Wijdicks, 2003a). Both mechanisms produce hypotonic hyponatremia with high concentration of urinary sodium (secondary to increased sodium excretion in CSWS and increased water reabsorption in SIADH). In fact, determination of extracellular fluid volume remains the only reliable distinguishing feature between these two conditions: SIADH is a state of volume expansion, while CSWS is a state of volume depletion (Table 45.4). The practical importance of this concept needs to be highlighted because fluid restriction—adequate therapy for SIADH—may be enormously deleterious in patients with CSWS, as is the case in SAH. Symptomatic acute hyponatremia requires tightly controlled infusion of hypertonic saline. Excessively rapid correction of profound chronic hyponatremia may precipitate severe osmotic myelinolysis. The rate of correction should not exceed 10 mmol/L over any 24-hour period to avoid this potentially devastating complication (Laureno and Karp, 1997). Fig. 45.6 presents an algorithm for the diagnosis and management of hyponatremia in critically ill neurological patients.
Table 45.4 Clinical and Laboratory Features of CSWS and SIADH
| Variable | CSWS | SIADH |
|---|---|---|
| Extracellular fluid volume | ↓ | ↑ |
| Body weight | ↓ | ↑ |
| Fluid balance | Negative | Positive |
| Urine volume | ↔ or ↑ | ↔ or ↓ |
| Tachycardia | + | − |
| Hematocrit | ↑ | ↔ |
| Albumin | ↑ | ↔ |
| Serum bicarbonate | ↑ | ↔ or ↓ |
| Blood urea nitrogen | ↑ | ↔ or ↓ |
| Serum uric acid | ↔ or ↓ | ↓ |
| Urinary sodium | ↑ | ↑ |
| Sodium balance | Negative | Neutral or positive |
| CVP/PCWP | ↓ | ↔ or slightly ↑ |
↔, Absent or minor variable change; ↓, decreased; ↑, increased; CSWS, Cerebral salt-wasting syndrome; CVP, central venous pressure; PCWP, pulmonary capillary wedge pressure; SIADH, syndrome of inappropriate secretion of antidiuretic hormone.
Nutrition and Metabolic Derangements
Adequate nutrition is essential for the recovery of critically ill patients, including those with primary acute neurological conditions. Depressed level of consciousness and abnormal swallowing function are very prevalent in NICU patients, who consequently often require tube feedings. Meanwhile, gastroparesis is also common and may increase the risk of aspiration in patients receiving enteral nutrition. This potential risk demands close monitoring of gastric residuals and positioning of the feeding tube in the distal part of the stomach or first portion of the duodenum. Agents that promote gastric motility (e.g., metoclopramide) may be added in the most severe cases (Booth et al., 2002).
Enteral feeding is preferred whenever possible to help maintain the integrity of the intestinal mucosal lining. It is recommended to start feeding patients early (ideally within 48 hours of admission); early feeding has been associated with a trend toward better survival and less disability in patients with TBI (Yanagawa et al., 2002). The optimal timing of percutaneous gastrostomy in neurological patients has not been sufficiently studied. There is some evidence that gastrostomy should be performed in patients with dysphagia from stroke persisting after 14 days (Norton et al., 1996).
Hyperglycemia is the most frequent metabolic derangement in critically ill neurological patients. There is solid evidence that hyperglycemia activates neurotoxic oxidative and inflammatory responses after acute ischemia. In patients with acute ischemic stroke, hyperglycemia has been associated with increased risk of hemorrhagic transformation and hyperacute worsening and lower rates of recanalization after thrombolysis (Alvarez-Sabin et al., 2004; Leigh et al., 2004; Ribo et al., 2005). It has also been found to correlate with infarct expansion and worse functional outcome (Baird et al., 2003; Bruno et al., 2002). Similarly, functional recovery is poorer in hyperglycemic patients with ICH and SAH (Frontera et al., 2006a; Passero et al., 2003). Intensive insulin therapy to maintain strict normoglycemia is no longer recommended for critically ill patients, after this practice led to increased mortality in a large randomized trial (Finfer et al., 2009). However, strict blood sugar control could decrease the rate of critical illness polyneuropathy (Van den et al., 2005). In the NICU, intensive insulin therapy carries the risk of inducing neuroglycopenia (with the ensuing risk of energy failure), which may occur in patients with acute brain insults even with serum glucose concentrations within the usual normal range (Godoy et al., 2010; Oddo et al., 2008). Therefore, the treatment of hyperglycemia must be particularly cautious in patients with acute brain disease, and serum glucose concentrations below 100 to 110 mg/dL should be avoided.
Fever and Infections
Fever in a patient with acute brain disease demands prompt diagnostic investigation to determine its cause and symptomatic treatment to avoid the deleterious impact of hyperthermia on the injured brain. Experimental models have consistently shown that even mild hyperthermia worsens cerebral damage after ischemia or trauma (Baena et al., 1997; Dietrich et al., 1996; Kim et al., 1996). Fever has been associated with poor functional outcome in patients with ischemic infarction (Reith et al., 1996; Wang et al., 2000), ICH (Schwarz et al., 2000), SAH (Oliveira-Filho et al., 2001), and TBI (Jiang et al., 2002). Increased metabolic expenditure, exacerbation of excitotoxicity, and elevated ICP may be responsible for the detrimental effects of hyperthermia (Rossi et al., 2001; Thompson et al., 2003).
Fever is very prevalent among NICU patients. Although disturbances in central thermoregulation occur frequently in patients with acute brain disorders (Rabinstein et al., 2007), infections are a common cause of fever in the NICU and should always be excluded (Commichau et al., 2003). Pneumonia, urinary tract infection, and bloodstream infection are the most frequent infectious complications (Dettenkofer et al., 1999). Ventriculitis must always be ruled out in patients with ventriculostomy. The appearance of fever in an NICU patient should be evaluated with cultures of blood, urine, respiratory secretions (ideally collected by bronchoalveolar lavage), and CSF (always in patients with ventriculostomy and when deemed clinically indicated in others). Chest radiograph and urine microscopic analysis are also pertinent. CT scan of the sinuses may be added to the diagnostic evaluation in febrile patients who have been intubated for several days. CT scan of the abdomen and pelvis is sometimes necessary to detect abscesses, pancreatitis, or cholecystitis. The skin should be thoroughly searched for signs of cellulitis or phlebitis. Osteomyelitis and discitis must be included in the differential diagnosis of fever after spine surgery.
Drug reactions, DVT, ethanol withdrawal, pancreatitis, and gout are relatively common causes of noninfectious fever in the NICU. Drug fever is most frequently caused by phenytoin in neurological patients. Signs of anticonvulsant hypersensitivity syndrome (rash, lymphadenopathy, hepatomegaly, eosinophilia, elevation of liver transaminases) must be readily recognized, since failure to discontinue the culprit medication promptly may have the devastating consequence of Stevens-Johnson syndrome (Schlienger and Shear, 1998). Detailed physical examination, venous Doppler, and measurement of liver and pancreatic enzymes should be performed in NICU patients with fever of unclear cause. In chronically immobile patients (especially those with spinal cord injury), heterotopic ossification may be a cause of persistent fever; it may be suspected by marked elevation of the C-reactive protein and sedimentation rate and confirmed by bone scintigraphy.
Central fever remains a diagnosis of exclusion. It occurs most frequently in patients with SAH, and in those patients it is associated with increased risk of vasospasm (Oliveira-Filho et al., 2001; Rabinstein et al., 2007). Patients with central fever often have prolonged hyperthermia with failure to return to normal body temperature, as opposed to the spikes of fever followed by normothermia typically observed with infections. Among patients with TBI, high fevers may be accompanied by other manifestations of paroxysmal sympathetic hyperactivity (tachycardia, hypertension, diaphoresis, dystonia) (Rabinstein, 2007).
Multiple measures can be used to normalize body temperature in febrile patients. Antipyretic medications (acetaminophen, ibuprofen) are sufficient in milder cases. However, mechanical cooling methods must be added in patients with more severe or refractory hyperthermia. Ice packs, air- or water-circulating cooling blankets, and effective cooling vests are alternative methods of conductive cooling (Mayer et al., 2004; Seder and Van der Kloot, 2009). Endovascular cooling devices may offer greater control of temperature modulation but require placement of a central venous catheter (De Georgia et al., 2004; Seder and Van der Kloot, 2009). Patients should be monitored for the appearance of shivering, which can be treated with warming gloves, buspirone, meperidine (in patients without high risk for seizures), magnesium infusion, or dexmedetomidine in patients who are awake, but it may necessitate neuromuscular paralysis when severe.
Hematological Complications
The risk of DVT is increased in immobilized patients. The incidence of clinical DVT after acute ischemic stroke ranges between 1% and 5%, and clinical PE occurs in 0.5% to 3.5% of these patients (Kamphuisen et al., 2005). However, the incidence of subclinical DVT is much higher when assessed by ultrasound, venography, or nuclear scans (Kamphuisen et al., 2005). The risk of DVT is also increased after craniotomy (Hamilton et al., 1994), ICH (Lacut et al., 2005), and in patients with severe neuromuscular weakness. The main diagnostic test for DVT is noninvasive bedside vascular ultrasound (venous Doppler). Physical examination is relatively insensitive to detect DVT in acutely ill hospitalized patients. When PE is suspected, spiral CT angiogram of the chest should be performed.
Early mobilization should be promoted in all patients. Options for prevention of thromboembolic complications in immobilized patients include mechanical methods (compressive stockings, intermittent pneumatic compression) and antithrombotics. Current evidence supports the use of subcutaneous anticoagulants for most patients with acute ischemic stroke (Adams, Jr. et al., 2007; Kamphuisen et al., 2005) and after craniotomy (Iorio and Agnelli, 2000). Unfortunately, similar data are not available to guide the management of thromboprophylaxis in patients with ICH or large ischemic cerebral infarction. Enoxaparin (40 mg daily) was found to be superior to unfractionated heparin (5000 units twice daily) in one randomized controlled trial of patients with acute ischemic stroke (Sherman et al., 2007). In cases of documented DVT and high risk of hemorrhagic complications from anticoagulation, placement of a Greenfield filter in the inferior vena cava is a valuable alternative.
Cerebral Protection after Cardiac Arrest
Therapeutic hypothermia has been shown to improve clinical outcomes after witnessed out-of-hospital ventricular fibrillation arrest (Bernard et al., 2002; the Hypothermia after Cardiac Arrest Study Group, 2002), and induction of hypothermia has become standard of care for the management of this condition. In fact, many centers have adopted the practice of inducing hypothermia after all cardiac arrests, regardless of the initial rhythm. Therapeutic hypothermia serves primarily as a neuroprotective modality, allowing more patients to recover awareness and improving functional outcomes among survivors.
Abboud H., Berroir S., Labreuche J., et al. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol. 2006;59:691-699.
Adams H.P.Jr., Del Zoppo G., Alberts M.J., et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711.
Alvarez-Sabin J., Molina C.A., Ribo M., et al. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke. 2004;35:2493-2498.
Anderson C.S., Huang Y., Wang J.G., et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391-399.
Andrews P.J., Citerio G., Longhi L., et al. NICEM consensus on neurological monitoring in acute neurological disease. Intensive Care Med. 2008;34:1362-1370.
Arabi Y., Memish Z.A., Balkhy H.H., et al. Ventriculostomy-associated infections: incidence and risk factors. Am J Infect Control. 2005;33:137-143.
Arroliga A.C., Shehab N., McCarthy K., et al. Relationship of continuous infusion lorazepam to serum propylene glycol concentration in critically ill adults. Crit Care Med. 2004;32:1709-1714.
Baena R.C., Busto R., Dietrich W.D., et al. Hyperthermia delayed by 24 hours aggravates neuronal damage in rat hippocampus following global ischemia. Neurology. 1997;48:768-773.
Baird T.A., Parsons M.W., Phanh T., et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208-2214.
Banki N.M., Kopelnik A., Dae M.W., et al. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005;112:3314-3319.
Bellander B.M., Cantais E., Enblad P., et al. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30:2166-2169.
Bernard S.A., Gray T.W., Buist M.D., et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557-563.
Booth C.M., Heyland D.K., Paterson W.G. Gastrointestinal promotility drugs in the critical care setting: a systematic review of the evidence. Crit Care Med. 2002;30:1429-1435.
Bosche B., Graf R., Ernestus R.I., et al. Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann Neurol. 2010;67:607-617.
Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1-106.
Broderick J., Connolly S., Feldmann E., et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001-2023.
Brott T., Broderick J., Kothari R., et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1-5.
Bruno A., Levine S.R., Frankel M.R., et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59:669-674.
Casati A., Spreafico E., Putzu M., et al. New technology for noninvasive brain monitoring: continuous cerebral oximetry. Minerva Anestesiol. 2006;72:605-625.
Claassen J., Hirsch L.J., Frontera J.A., et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103-112.
Commichau C., Scarmeas N., Mayer S.A. Risk factors for fever in the neurologic intensive care unit. Neurology. 2003;60:837-841.
Coplin W.M., O’Keefe G.E., Grady M.S., et al. Thrombotic, infectious, and procedural complications of the jugular bulb catheter in the intensive care unit. Neurosurgery. 1997;41:101-107.
Coplin W.M., O’Keefe G.E., Grady M.S., et al. Accuracy of continuous jugular bulb oximetry in the intensive care unit. Neurosurgery. 1998;42:533-539.
Coplin W.M., Pierson D.J., Cooley K.D., et al. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161:1530-1536.
Cormio M., Valadka A.B., Robertson C.S. Elevated jugular venous oxygen saturation after severe head injury. J Neurosurg. 1999;90:9-15.
Czosnyka M., Smielewski P., Kirkpatrick P., et al. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11-17.
Davis S.M., Broderick J., Hennerici M., et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175-1181.
De Georgia M.A., Krieger D.W., Abou-Chebl A., et al. Cooling for acute ischemic brain damage (cool aid): a feasibility trial of endovascular cooling. Neurology. 2004;63:312-317.
Deibert E., Barzilai B., Braverman A.C., et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg. 2003;98:741-746.
DeLorenzo R.J., Waterhouse E.J., Towne A.R., et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833-840.
Dennis L.J., Claassen J., Hirsch L.J., et al. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery. 2002;51:1136-1143.
Dettenkofer M., Ebner W., Hans F.J., et al. Nosocomial infections in a neurosurgery intensive care unit. Acta Neurochir (Wien). 1999;141:1303-1308.
Diedler J., Czosnyka M. Merits and pitfalls of multimodality brain monitoring. Neurocrit Care. 2010;12:313-316.
Dietrich W.D., Alonso O., Halley M., et al. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38:533-541.
Feldman Z., Robertson C.S. Monitoring of cerebral hemodynamics with jugular bulb catheters. Crit Care Clin. 1997;13:51-77.
Finfer S., Chittock D.R., Su S.Y., et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283-1297.
Flibotte J.J., Lee K.E., Koroshetz W.J., et al. Continuous antibiotic prophylaxis and cerebral spinal fluid infection in patients with intracranial pressure monitors. Neurocrit Care. 2004;1:61-68.
Fogelholm R., Avikainen S., Murros K. Prognostic value and determinants of first-day mean arterial pressure in spontaneous supratentorial intracerebral hemorrhage. Stroke. 1997;28:1396-1400.
Frontera J.A., Fernandez A., Claassen J., et al. Hyperglycemia after SAH: predictors, associated complications, and impact on outcome. Stroke. 2006;37:199-203.
Frontera J.A., Rundek T., Schmidt J.M, et al. Cerebrovascular reactivity and vasospasm after subarachnoid hemorrhage: a pilot study. Neurology. 2006;66:727-729.
Godoy D.A., Di Napoli M., Rabinstein A.A. Treating hyperglycemia in neurocritical patients: benefits and perils. Neurocrit Care. 2010;13:425-438.
Gopinath S.P., Robertson C.S., Contant C.F., et al. Jugular venous desaturation and outcome after head injury. J Neurol Neurosurg Psychiatry. 1994;57:717-723.
Hamilton M.G., Hull R.D., Pineo G.F. Venous thromboembolism in neurosurgery and neurology patients: a review. Neurosurgery. 1994;34:280-296.
Heran N.S., Hentschel S.J., Toyota B.D. Jugular bulb oximetry for prediction of vasospasm following subarachnoid hemorrhage. Can J Neurol Sci. 2004;31:80-86.
Hirsch L.J. Continuous EEG monitoring in the intensive care unit: an overview. J Clin Neurophysiol. 2004;21:332-340.
Holloway K.L., Barnes T., Choi S., et al. Ventriculostomy infections: the effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85:419-424.
Iorio A., Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med. 2000;160:2327-2332.
Iyer V.N., Hoel R., Rabinstein A.A. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med. 2009;37:3024-3030.
Jauch E.C., Lindsell C.J., Adeoye O., et al. Lack of evidence for an association between hemodynamic variables and hematoma growth in spontaneous intracerebral hemorrhage. Stroke. 2006;37:2061-2065.
Jiang J.Y., Gao G.Y., Li W.P., et al. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J Neurotrauma. 2002;19:869-874.
Jordan K.G. Neurophysiologic monitoring in the neuroscience intensive care unit. Neurol Clin. 1995;13:579-626.
Kam P.C., Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690-701.
Kamphuisen P.W., Agnelli G., Sebastianelli M. Prevention of venous thromboembolism after acute ischemic stroke. J Thromb Haemost. 2005;3:1187-1194.
Keller E., Steiner T., Fandino J., et al. Jugular venous oxygen saturation thresholds in trauma patients may not extrapolate to ischemic stroke patients: lessons from a preliminary study. J Neurosurg Anesthesiol. 2002;14:130-136.
Kidwell C.S., Saver J.L., Mattiello J., et al. Diffusion-perfusion MR evaluation of perihematomal injury in hyperacute intracerebral hemorrhage. Neurology. 2001;57:1611-1617.
Kim Y., Busto R., Dietrich W.D., et al. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke. 1996;27:2274-2280.
Lacut K., Bressollette L., Le Gal G., et al. Prevention of venous thrombosis in patients with acute intracerebral hemorrhage. Neurology. 2005;65:865-869.
Latronico N., Beindorf A.E., Rasulo F.A., et al. Limits of intermittent jugular bulb oxygen saturation monitoring in the management of severe head trauma patients. Neurosurgery. 2000;46:1131-1138.
Laureno R., Karp B.I. Myelinolysis after correction of hyponatremia. Ann Intern Med. 1997;126:57-62.
Lee V.H., Connolly H.M., Fulgham J.R., et al. Takotsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: an underappreciated ventricular dysfunction. J Neurosurg. 2006;105:264-270.
Leigh R., Zaidat O.O., Suri M.F., et al. Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy. Stroke. 2004;35:1903-1907.
Leira R., Davalos A., Silva Y., et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461-467.
Lindegaard K.F., Nornes H., Bakke S.J., et al. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir (Wien). 1989;100:12-24.
Lozier A.P., Sciacca R.R., Romagnoli M.F., et al. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51:170-181.
Macmillan C.S., Andrews P.J. Cerebrovenous oxygen saturation monitoring: practical considerations and clinical relevance. Intensive Care Med. 2000;26:1028-1036.
Maloney-Wilensky E., Gracias V., Itkin A., et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med. 2009;37:2057-2063.
Manno E.M., Rabinstein A.A., Wijdicks E.F., et al. A prospective trial of elective extubation in brain injured patients meeting extubation criteria for ventilatory support: a feasibility study. Crit Care. 2008;12:R138.
Martinez-Manas R.M., Santamarta D., de Campos J.M., et al. Camino intracranial pressure monitor: prospective study of accuracy and complications. J Neurol Neurosurg Psychiatry. 2000;69:82-86.
Mattle H.P., Kappeler L., Arnold M., et al. Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke. 2005;36:264-268.
Mayer S.A., Kowalski R.G., Presciutti M., et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508-2515.
Mayer S.A., Lignelli A., Fink M.E., et al. Perilesional blood flow and edema formation in acute intracerebral hemorrhage: a SPECT study. Stroke. 1998;29:1791-1798.
Merten G.J., Burgess W.P., Gray L.V., et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;19:2328-2334.
Mistri A.K., Robinson T.G., Potter J.F. Pressor therapy in acute ischemic stroke: systematic review. Stroke. 2006;37:1565-1571.
Moulton R.J., Brown J.I., Konasiewicz S.J. Monitoring severe head injury: a comparison of EEG and somatosensory evoked potentials. Can J Neurol Sci. 1998;25:S7-11.
Ng S.C., Poon W.S., Chan M.T., et al. Transcranial Doppler ultrasonography (TCD) in ventilated head injured patients: correlation with stable xenon-enhanced CT. Acta Neurochir (Suppl. 2000;76:479-482.
Nilsson O.G., Brandt L., Ungerstedt U., et al. Bedside detection of brain ischemia using intracerebral microdialysis: subarachnoid hemorrhage and delayed ischemic deterioration. Neurosurgery. 1999;45:1176-1184.
Norton B., Homer-Ward M., Donnelly M.T., et al. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ. 1996;312:13-16.
Oddo M., Carrera E., Claassen J., et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051-2056.
Oddo M., Schmidt J.M., Carrera E., et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;36:3233-3238.
Oliveira-Filho J., Ezzeddine M.A., Segal A.Z., et al. Fever in subarachnoid hemorrhage: relationship to vasospasm and outcome. Neurology. 2001;56:1299-1304.
Oliveira-Filho J., Silva S.C., Trabuco C.C., et al. Detrimental effect of blood pressure reduction in the first 24 hours of acute stroke onset. Neurology. 2003;61:1047-1051.
Parviainen I., Uusaro A., Kalviainen R., et al. Propofol in the treatment of refractory status epilepticus. Intensive Care Med. 2006;32:1075-1079.
Passero S., Ciacci G., Ulivelli M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology. 2003;61:1351-1356.
Potter J.F., Robinson T.G., Ford G.A., et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol. 2009;8:48-56.
Powers W.J., Zazulia A.R., Videen T.O., et al. Autoregulation of cerebral blood flow surrounding acute (6 to 22 hours) intracerebral hemorrhage. Neurology. 2001;57:18-24.
Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417.
Qureshi A.I., Bliwise D.L., Bliwise N.G., et al. Rate of 24-hour blood pressure decline and mortality after spontaneous intracerebral hemorrhage: a retrospective analysis with a random effects regression model. Crit Care Med. 1999;27:480-485.
Qureshi A.I., Palesch Y.Y., Martin R., et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol. 2010;67:570-576.
Rabinstein A.A. Paroxysmal sympathetic hyperactivity in the neurological intensive care unit. Neurol Res. 2007;29:680-682.
Rabinstein A.A., Friedman J.A., Weigand S.D., et al. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862-1866.
Rabinstein A.A., Lanzino G., Wijdicks E.F. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2010;9:504-519.
Rabinstein A.A., Sandhu K. Non-infectious fever in the neurological intensive care unit: incidence, causes and predictors. J Neurol Neurosurg Psychiatry. 2007;78:1278-1280.
Rabinstein A.A., Wijdicks E.F. Hyponatremia in critically ill neurological patients. Neurologist. 2003;9:290-300.
Rabinstein A.A., Wijdicks E.F. Warning signs of imminent respiratory failure in neurological patients. Semin Neurol. 2003;23:97-104.
Reith J., Jorgensen H.S., Pedersen P.M., et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347:422-425.
Ribo M., Molina C., Montaner J., et al. Acute hyperglycemia state is associated with lower tPA-induced recanalization rates in stroke patients. Stroke. 2005;36:1705-1709.
Robertson C.S., Gopinath S.P., Goodman J.C., et al. Sjvo2 monitoring in head-injured patients. J Neurotrauma. 1995;12:891-896.
Rosand J., Eskey C., Chang Y., et al. Dynamic single-section CT demonstrates reduced cerebral blood flow in acute intracerebral hemorrhage. Cerebrovasc Dis. 2002;14:214-220.
Rosenthal G., Hemphill J.C.III, Manley G. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury. Crit Care Med. 2009;37:379-380.
Rosner M.J., Becker D.P. Origin and evolution of plateau waves. Experimental observations and a theoretical model. J Neurosurg. 1984;60:312-324.
Rosner M.J., Rosner S.D., Johnson A.H. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949-962.
Rossetti A.O., Logroscino G., Liaudet L., et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology. 2007;69:255-260.
Rossi S., Zanier E.R., Mauri I., et al. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001;71:448-454.
Safdar N., Kluger D.M., Maki D.G. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters: implications for preventive strategies. Medicine (Baltimore). 2002;81:466-479.
Sandham J.D., Hull R.D., Brant R.F., et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5-14.
Sarrafzadeh A.S., Peltonen E.E., Kaisers U., et al. Secondary insults in severe head injury–do multiply injured patients do worse? Crit Care Med. 2001;29:1116-1123.
Schlienger R.G., Shear N.H. Antiepileptic drug hypersensitivity syndrome. Epilepsia. 1998;39(Suppl 7):S3-S7.
Schwarz S., Hafner K., Aschoff A., et al. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology. 2000;54:354-361.
Seder D.B., Van der Kloot T.E. Methods of cooling: practical aspects of therapeutic temperature management. Crit Care Med. 2009;37:S211-S222.
Sherman D.G., Albers G.W., Bladin C., et al. The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet. 2007;369:1347-1355.
Stead L.G., Gilmore R.M., Decker W.W., et al. Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology. 2005;65:1179-1183.
Stead L.G., Gilmore R.M., Vedula K.C., et al. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology. 2006;66:1878-1881.
Suarez J.I., Qureshi A.I., Yahia A.B., et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med. 2002;30:1348-1355.
Synek V.M. Value of a revised EEG coma scale for prognosis after cerebral anoxia and diffuse head injury. Clin Electroencephalogr. 1990;21:25-30.
Tegtmeyer K., Brady G., Lai S., et al. Videos in clinical medicine. Placement of an arterial line. N Engl J Med. 2006;354:e13.
Tepel M., Van der Giet, M., Schwarzfeld C., et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N. Engl J Med. 2000;343:180-184.
Terayama Y., Tanahashi N., Fukuuchi Y., et al. Prognostic value of admission blood pressure in patients with intracerebral hemorrhage. Keio Cooperative Stroke Study. Stroke. 1997;28:1185-1188.
The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-556.
Thompson H.J., Tkacs N.C., Saatman K.E., et al. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12:163-173.
Towne A.R., Waterhouse E.J., Boggs J.G., et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340-345.
Van den B.G., Schoonheydt K., Becx P., et al. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64:1348-1353.
Vaquero J., Fontana R.J., Larson A.M., et al. Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581-1589.
Vespa P., Prins M., Ronne-Engstrom E., et al. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg. 1998;89:971-982.
Vespa P.M., Nenov V., Nuwer M.R. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol. 1999;16:1-13.
Vespa P.M., Nuwer M.R., Juhasz C., et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607-615.
von Helden A., Schneider G.H., Unterberg A., et al. Monitoring of jugular venous oxygen saturation in comatose patients with subarachnoid haemorrhage and intracerebral haematomas. Acta Neurochir Suppl (Wien). 1993;59:102-106.
Wang Y., Lim L.L., Levi C., et al. Influence of admission body temperature on stroke mortality. Stroke. 2000;31:404-409.
Waziri A., Claassen J., Stuart R.M., et al. Intracortical electroencephalography in acute brain injury. Ann Neurol. 2009;66:366-377.
Wheeler A.P., Bernard G.R., Thompson B.T., et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213-2224.
Wijdicks E.F. The diagnosis of brain death. N Engl J Med. 2001;344:1215-1221. 19
Wijdicks E.F., Bamlet W.R., Maramattom B.V., et al. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58:585-593.
Wijdicks E.F., Borel C.O. Respiratory management in acute neurologic illness. Neurology. 1998;50:11-20.
Wijdicks E.F., Hijdra A., Young G.B., et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203-210.
Woodruff B.K., Britton J.W., Tigaran S., et al. Cardiac troponin levels following monitored epileptic seizures. Neurology. 2003;60:1690-1692.
Yanagawa, T., Bunn, F., Roberts, I., et al., 2002. Nutritional support for head-injured patients. Cochrane Database Syst Rev CD001530.
Young G.B., Jordan K.G., Doig G.S. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83-89.
Zandbergen E.G., Hijdra A., Koelman J.H., et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66:62-68.
Zazulia A.R., Diringer M.N., Videen T.O., et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804-810.