CHAPTER 9 Principles of Elbow Rehabilitation
ESTABLISH A COMPLETE AND ACCURATE DIAGNOSIS
Successful rehabilitation is predicated on a complete understanding of the anatomic and physiologic factors pertaining to a particular elbow disorder. The elbow joints (humeroulnar, humeroradial, proximal radioulnar), nerves, vessels, capsule and ligaments, and muscles, as well as adjacent articulations (distal radioulnar and shoulder) should be considered. Anatomic alterations in these tissues will define initial motion restrictions as well as the potential for restoration of motion and stability. However, it is ultimately the patient’s physiologic age and biologic healing potential that will determine how much of this potential is realized. Some patients heal poorly and may be prone to ongoing instability, whereas others exhibit a propensity for scar formation and will develop stiffness despite the best efforts of the treatment team.32 From our perspective, this sometimes dramatic individual variation in the healing response assumes a dominant role in the recovery of some patients.
Throughout the rehabilitation process, the physiologic stage of healing directly affects the rehabilitation program.50 During the inflammatory stage, the primary goals are pain and edema control and adherence to stable arcs of motion to protect tissues at risk. During the fibroblastic phase, controlled stresses may be increased to promote more normal collagen formation, and low-level strengthening is implemented to re-establish neuromuscular control. Finally, during the remodeling phase, stretching and strengthening exercises are advanced, and functional restoration is pursued. The clinician must be constantly aware of the physiologic status of the elbow. The elbow is an unforgiving articulation with significant bony congruity and a tendency to develop inflammation and stiffness.32 Overzealous rehabilitation efforts can quickly regress the elbow from the fibroblastic phase back into the inflammatory phase. Consequently, clinicians should constantly monitor the status of the elbow and modify the rehabilitation program accordingly. This process requires appropriate follow-up, patient education, and constant communication between members of the treatment team.
REDUCE PAIN AND INFLAMMATION
During the acute post-traumatic or postsurgical period, the primary goal is to control pain and inflammation. The elbow tends to get stiff as a result of adhesion formation and muscular cocontraction.32,50 PRRICEMM principles are applied to reduce pain, edema, and inflammation—Protection, relative Rest, Ice, Compression, Elevation, Medications, and Modalities.
Protection and Relative Rest
Appropriate protection and relative rest require balancing the need to protect healing tissues with the adverse effects of immobility. Total immobility can precipitate rapid deconditioning, whereas tenuous tissues can be easily damaged by aggressive motion.2,4,21,50 Diagnosis-specific safe elbow motion arcs guide early motion and are discussed in the next section. Bracing or splinting is often prescribed to protect healing tissues and are discussed in Chapter 11. Patients can immediately initiate general aerobic fitness programs (e.g., Exercycle) and exercises with their three unaffected limbs. With respect to the affected limb, patients may perform wrist-hand and shoulder motions while avoiding injurious elbow positions or loads. For example, shoulder abduction will produce a varus elbow stress and therefore is contra-indicated in the early post-traumatic/post-operative period after lateral collateral ligament complex injury/reconstruction.41
Ice
Physiologically, ice can reduce inflammation, modulate pain and control muscle spasm.10 Ice is applied regularly in the acute post-traumatic/postoperative period, and intermittently postexercise/postactivity in the later phases of healing.50 Caution should be exercised when applying ice over traversing nerves, particularly those that have been surgically transposed.7
Compression and Elevation
Compression wrapping and elevation above heart level promote edema control. Both static and intermittent air-compression devices have been successfully used in the early stages of rehabilitation. Although published scientific investigation is lacking, a case-control pilot study from our institution documented a statistically significant advantage with respect to edema control for a compression cryotherapy device (Aircast) applied following total elbow arthroplasty (Fig. 9-1).1
Medications
Medication use is determined by the specific diagnosis, healing stage, and physician preference. Narcotics, nonsteroidal anti-inflammatory drugs (NSAIDs), and acetaminophen are used based upon an individualized risk-benefit ratio analysis. Although NSAIDs may provide short-term analgesic benefits in lateral elbow tendinopathy (“tennis elbow”),20 they are typically used with more caution in the post-traumatic/postsurgical elbow. Some NSAIDs inhibit platelets and may result in hemorrhage, whereas others may actually inhibit the healing response.30,48 Nonetheless, controlling inflammation with medication is an important element of the postinjury/postoperative recovery period.
Patients with inflammatory arthropathies may benefit from rheumatologic consultation to optimize systemic medications. In tennis elbow, corticosteroid injections do provide reliable relief for 4 to 6 weeks in most cases but may not affect long-term outcome and often cause temporary symptom exacerbation.27,45 Further investigation is needed to clarify the initial positive results reported for topical nitric oxide,42 platelet-rich plasma injections,31 and botulinum toxin injections24 in tennis elbow. Interested readers are referred to Chapter 44 for a more in depth discussion.
Modalities
Other than ice, the role of modalities in the acute post-traumatic/postsurgical period remains poorly defined. During periods of muscle inhibition, high-voltage galvanic stimulation (HVGS)–induced muscle contractions have been used to reduce pain and edema.50 As elbow motion improves, electromyographic (EMG)-biofeedback can be used to reduce muscle co-contractions, initially inhibiting the antagonists and subsequently cuing on the agonists.13,50 These modalities should be applied carefully in conjunction with constant reassessment.
With respect to tennis elbow, iontophoresis appears to offer some short-term benefit,38 the roles of acupuncture and shock wave therapy remain inconclusive,8,11 and pulsed electrical magnetic stimulation47 and laser therapy8 appear to have no role.
IMPLEMENT EARLY ATRAUMATIC MOTION
The elbow exhibits a marked tendency to rapidly develop intra-articular and periarticular adhesions, resulting in motion loss that may eventually compromise outcome.32 Early motion is desirable to minimize or prevent adhesion formation,32,51 mitigate against the deleterious effects of immobility,2,4,21,50 facilitate lymphatic and venous drainage,13 and modulate pain through proprioceptive mechanisms.4,13,52 These benefits must be weighed against the risk of irritating healing tissues, thereby deleteriously affecting the rehabilitation program.
The long-term range-of-motion goals for the patient must be established, and the patient must have a clear understanding of this goal. The potential range of motion is defined by the specific diagnosis and any post-traumatic/postsurgical anatomic alterations. Whether this potential is achieved depends on the ability of the rehabilitation program to optimize the patient’s physiology, as well as the patient’s compliance with the program. Normal elbow range of motion is 0 to 5 degrees of hyperextension, 134 to 145 degrees of flexion, 75 degrees pronation, and 85 degrees supination.28,35 Most activities of daily living are performed within 30 to 130 degrees flexion, 50 degrees pronation, and 50 degrees supination.28,35 However, some daily activities (e.g., reaching to the opposite side of the head), as well as many recreational and vocational activities, may require greater motion.17,36 The patient and the treatment team must understand the expected limitations of the elbow in the context of the potential functional needs, thereby defining realistic functional expectations for “success.”
To implement early, atraumatic motion, the clinician must completely understand the patient’s anatomy and physiologic healing stage with respect to all tissues involved—joints, capsule, ligaments, muscle-tendon units, nerves, and vessels. In addition, the elbow must be constantly reassessed for increased pain, swelling, or motion loss, and the rehabilitation program modified accordingly. The initial arc of protected elbow motion is diagnosis specific. For example, a dislocated elbow with lateral ulnar collateral ligament (LUCL) insufficiency is most stable in flexion and pronation,15,33,40 whereas a dislocation with bilateral ligament injuries (LUCL and medial ulnar collateral ligament [MUCL]) and a radial head fracture is kept in neutral pronation-supination to modulate humeroradial contact stress while balancing ligamentous tension.13,32 A normally located ulnar nerve may be irritated by excessive or prolonged flexion, whereas a transposed ulnar nerve, particularly if adhered, may be irritated by excessive or prolonged extension.50 Interested readers are referred to Chapters 28, 29, and 48 for more diagnoses-specific in-depth discussion.
As previously discussed, elbow bracing or splinting may be protective in the postoperative/post-traumatic period by controlling position and forces, as well as providing external stability.9 As healing progresses to the fibroblastic and remodeling phases, braces may be used to restore motion.9 Appropriate prescription, application, and compliance are the keys to success.9,18,26 Specific applications are discussed in Chapter 11.
TIMING
Once safe motion arcs have been defined, motion can be prescribed based on the stage of tissue healing.13,50 A complete clinical examination is necessary to determine the healing stage, as well as the specific tissue or tissues responsible for the motion loss.32 Assessing both the quality and quantity of motion loss is necessary to accurately prescribe range of motion within the restrictions. Bony motion blocks will not respond to rehabilitation efforts, and well-established soft tissue contractures with a hard end-feel will not respond as reliably as those with a springier end-feel.50 During the inflammatory phase, range of motion must not be aggressive, and must strictly adhere to restrictions while monitoring for regression. As the elbow enters the fibroblastic and eventually remodeling phases, range of motion may become more aggressive because tissues have healed sufficiently to absorb additional forces that will be beneficial to promote collagen reformation. During these latter phases, the clinician must constantly monitor for signs of inflammation and modify the program accordingly. Four types of range of motion are typically used during elbow rehabilitation: active assisted, active, passive, and resisted.
Active Assisted Range of Motion
Active assisted range of motion (AAROM) is typically implemented earliest, including during the inflammatory phase. Goals are prevention of intra-articular and periarticular adhesions, promotion of cartilage healing, edema control, and pain modulation.13 Maintaining low levels of voluntary muscle activation minimizes elbow joint compression and shear forces. Gravity-assisted motion is often used during this phase, as is continuous passive motion (CPM) (see Chapter 10). Although CPM is by definition “passive,” in the early post-traumatic/postoperative period, its benefits parallel those of AAROM.
During gravity-assisted flexion, the patient is positioned supine on the table, upper limb flexed to 90 degrees, and the elbow allowed to flex under the pull of gravity, as guided and assisted by the well arm.13 Gravity-assisted extension may be performed sitting with the upper limb supported, and the elbow allowed to extend under the influence of gravity, assisted by the well arm.13 Both exercises may be initiated during the inflammatory phase with diagnosis specific restrictions. As pain and edema subside, the amount of gravity and well arm assistance may be decreased, eventually transitioning to active range of motion.
Active Range of Motion (AROM)
Active range of motion (AROM) may initially be performed in gravity-eliminated positions (e.g., table top flexion-extension) before transitioning to antigravity positions (e.g., sagittal plane flexion-extension). Benefits of AROM parallel those of AAROM, with added benefit that AROM voluntarily activates the elbow muscles, thus stimulating neuromuscular control. AROM is performed within safe flexion-extension and pronation-supination motion arcs while monitoring loads placed on the elbow due to upper limb positioning (e.g., effect of forearm pronation on humeroradial compression force,34 effect of shoulder abduction on elbow varus force).41
Passive Range of Motion (PROM)
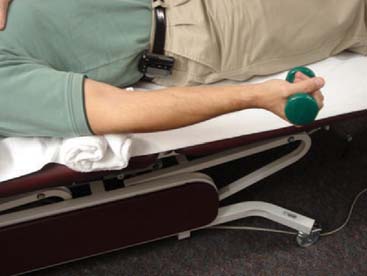
Passive range of motion (PROM) may be initiated as patients enter the fibroblastic phase, or during the remodeling phase. The goal of PROM, in the form of splinting or stretching, is to induce permanent tissue length changes to gain motion.50 Clinical research supports the efficacy of appropriately applied progressive static splinting for elbow contractures19 (see Chapter 11). Although no form of stretching has been proven superior in the elbow, low-load, long-duration (LLLD) stretches are commonly used based upon supportive basic science research22 and clinical experience.50 The LLLD stretch for the anterior elbow is performed with the patient’s elbow supported, forearm held in supination to optimize anterior capsule tension19 and a small weight or low-resistance exercise band held in the hand50 (Fig. 9-2). Patients perform several repetitions of a 20-second to 2- to 3-minute stretch, or one or two repetitions of a 10- to 12-minute stretch.22,46,50 In either case, stretches are followed by AROM to re-establish neuromuscular control within the newly obtained motion arcs. The process is completed by icing the affected area in a lengthened position to reduce inadvertent inflammation produced during the stretch and to allow cooling in a lengthened position.46
During the fibroblastic and remodeling phases, additional modalities may be beneficial to facilitate motion. Use of superficial heat in the form of hot packs, whirlpool, fluidotherapy, or a heating pad will increase local blood flow and tissue extensibility while decreasing stiffness and muscle spasm.3,49 Therefore, superficial heat may serve an adjunctive role to stretching and splinting, provided that no signs of inflammation are present. There exists a paucity of data investigating the role of ultrasound (US) in specific clinical conditions about the elbow.8,44 Limited data suggest that low-intensity, pulsed US may promote wound healing and increase fibroblastic activity while controlling inflammation,39,48 whereas high-intensity US may increase the extensibility of scar tissue through heating, particularly when accompanied or followed by stretching.8,39,44,46 There are insufficient data to support or refute the role of US in chronic tendinopathies, which can be considered a dysfunctional fibroblastic phase, although the majority of data suggest that phonophoresis (US with corticosteroid) does not offer any additional benefit over US alone.8,45
Joint Mobilization and Neural Gliding
This approach has been advocated as an adjunctive technique to increase motion during elbow rehabilitation.12,50 In theory, joint mobilizations may reduce pain, spasm, and stiffness, but supportive scientific data are lacking.13,50 In practice, therapists will initiate low-amplitude oscillatory motions and progress to higher amplitude distraction techniques as tissue healing allows.13,50 Joint mobilizations may be preceded by heat, and are followed by PROM/stretching to gain motion, and thereafter by AROM to control the newly gained motion, as noted above. Neural gliding consists of gentle exercises to promote nerve mobility with respect to surrounding tissues and has been promoted to disperse intraneural edema, optimize axonal transport, reduce nerve adhesions, and relieve nerve-related pain.12 At this time, there is insufficient evidence to support or refute the practice of joint oscillations or neural glide techniques during the elbow rehabilitation process. Consequently, prescription should be based on the assessment of the risk-benefit ratio, availability of qualified practitioners, and financial and time constraints.
RESTORE REGIONAL NEUROMUSCULAR CONTROL
Consideration of the status of the joint is essential with this component of rehabilitation. If the joint cartilage is compromised or if the joint surface is compromised, this element of rehabilitation should be described primarily to accomplish activities of daily living.
If the joint allows, in order to optimize elbow function, neuromuscular control (NMC) must be re-established. NMC includes strength, endurance, and coordinated muscle contractions. NMC is a prerequisite to capitalize on the functional potential provided by the range-of-motion gains during the rehabilitation program. In addition, NMC about the elbow may provide some ability to compensate for persistent instabilities despite optimal treatment. For example, coordinated muscle contraction can dynamically improve elbow stiffness,5 and at least partially compensate for instability after LUCL15 and MUCL14,43 injury or reconstruction.
Early NMC training is achieved through the AAROM exercises, as previously discussed. Positions and motions are chosen based on diagnosis-specific restrictions and modified accordingly over time. As healing allows, RROM (i.e., strengthening) exercises are initiated. Strengthening exercises are prescribed in the context of a graduated program, initially emphasizing low-load, low-repetition exercise sessions performed multiple times per day (e.g., one to two sets of 10 to 20 repetitions three to five times/day). This scheme re-establishes motor control pathways while minimizing edema and overload risk.50 Finger flexion-extension, wrist ulnar-radial deviation, forearm pronation-supination, and elbow flexion-extension are included. Initial isometric contractions can minimize atrophy and may provide some strength gains while controlling joint stress. Typically, 3- to 6-second submaximal isometric contractions are followed by 3 to 6 seconds of rest, repeated 10 to 20 times. Isometric exercises are initially completed in the most stable position for the patient, and are repeated two to three times per day. As the clinical situation allows, frequency is increased, less stable positions are included, and external resistance in the form of free weights, cables, or resistance tubing/bands is added. The optimal repetition-load scheme for strengthening about the elbow remains elusive, but frequency should be stressed over load, at least initially.5,13 It is not uncommon to start resisted elbow flexion exercises with a 1/4 or 1/2 kg weight, performing one set of 10 repetitions three to four times per day. As the rehabilitation progresses, loads and repetitions may be adjusted to meet the specific strength-endurance needs of the patient. Although eccentric-biased strengthening may have a role in chronic tennis elbow,29 this strengthening mode has a limited role in general elbow rehabilitation.
As NMC is re-established, it is important to continually monitor the status of the elbow to ensure that injury or irritation is not occurring. In addition, the clinician must remain cognizant of safe versus unsafe positions and motion arcs, and modify the rehabilitation programaccordingly. For example, initially after MUCL reconstruction or injury, the elbow is inherently more stable with the forearm supinated. In this position, resisted finger flexion, wrist flexion (Fig. 9-3A) and ulnar deviation (see Fig. 9-3B) exercises may be safely performed to strengthen the dynamic medial elbow stabilizers as long as valgus loads are avoided by appropriate positioning.5,14,43 After LUCL injury or reconstruction, wrist extensor strengthening may be performed with the forearm pronated and supinator strengthening with the forearm supinated, in both cases to avoid a varus load on the elbow.15 Following elbow dislocation, the elbow is initially exercised in flexion due to the consistent posterior force vectors of the elbow musculature.33
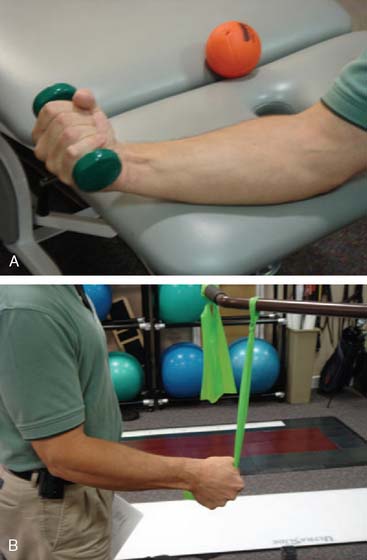
FIGURE 9-3 A, Resisted wrist flexion exercise with forearm supinated to minimize elbow valgus stress after medial ulnar collateral ligament injury/repair. B, Similar to Figure 9-3A, with resisted ulnar deviation (targeting the flexor carpi ulnaris [FCU]) performed in midprosupination to minimize elbow valgus stress.
INTEGRATION INTO THE KINETIC-KINEMATIC CHAIN
Regardless of pathoetiology, the elbow should not be rehabilitated in isolation of other joints. The elbow represents an important part of the kinetic-kinematic chain, whereby forces and motions are generated, transformed, and transferred through multiple body segments to the hand for the purpose of function.25 As a matter of fact, even if little is being done actively for the elbow, the other joints should be addressed during the recovery period. Throughout the rehabilitation process, patients are encouraged to exercise the entire body, including ipsilateral shoulder and wrist. Programs are tailored to each patient’s anticipated needs and generally include both resistance and aerobic exercise modes. As previously mentioned, the clinician should consider the position of the elbow, and consequent direct and indirect forces at all times during this process. As the elbow improves, integrated total body exercises are implemented and advanced with the goal of return to daily life, work, and sport.
Research continues to elucidate the affect of proximal kinetic-kinematic chain dysfunction on incurred elbow stress.25 Consequently, clinicians should evaluate the kinetic-kinematic chain for deficits in flexibility, strength, and coordination that may be pathoetiologic in the presenting elbow disorder. A technique coach or occupational medicine specialist may identify and rectify technique flaws that produce inefficient movement and consequently overstress the elbow.16,23 In some cases, it may be necessary to intentionally increase motion or strength, or both, at adjacent body segments to compensate for permanent elbow deficits (e.g., increased shoulder abduction and internal rotation compensating for forearm pronation loss).
Finally, a qualified professional should evaluate the patient’s work or sporting environment and relevant equipment to identify modifiable risk factors that may have contributed to the presenting elbow disorder.37 Placing the patient and the elbow back into the same situational stress may lead to symptom recurrence, and may compromise outcome and satisfaction.
SUMMARY
Elbow rehabilitation is a challenging and dynamic process. The commitment of the physician, therapists, and patient and understanding their subjective roles is essential for success. A complete understanding of elbow mechanics and pathomechanics, open communication between patients and all members of the rehabilitation team, and patience is essential. By following the general principles outlined in this chapter (Box 9-1), cliniciansmay successfully rehabilitate a wide variety of elbow disorders while minimizing complications.
1 Adams, R., and Morrey, B.: The effect of cryocompression on the elbow: a prospective randomized study. AAOS Annual Meeting. Anaheim, CA, Feb 1999.
2 Akeson W., Amiel D., Woo S. Immobility effects on synovial joints. The pathomechanics of joint contracture. Biorheology. 1980;17:95.
3 Allen R. Physical agents used in the management of chronic pain by physical therapists. Phys. Med. Rehabil. Clin. North Am.. 2006;17:315.
4 Amiel D., Akeson W., Harwood F., Frank C.B. Stress deprivation effect on metabolic turnover of the medial collateral ligament collage–a comparison between 9 and 12 week immobilization. Clin. Orthop. Rel. Res.. 1983;172:265.
5 An K., Hui F., Morrey B., Linscheid R.L., Chao E.Y. Muscles across the elbow joint: A biomechanical analysis. J. Biomech.. 1981;14:659.
6 An K.-N., Morrey B. Biomechanics of the elbow. In: Morrey B., editor. The Elbow and Its Disorders. 3rd ed. Philadelphia: W. B. Saunders Co.; 2000:43.
7 Bassett F., Kirkpatrick J., Engelhardt D., et al. Cryotherapy induced nerve injury. Am. J. Sports Med.. 1992;20:516.
8 Bisset L., Paungmali A., Vicenzino B., Beller E. A systematic review and meta-analysis of clinical trials on physical interventions for lateral epicondylalgia. Br. J. Sports Med.. 2005;39:411.
9 Blackmore S. Splinting for elbow injuries and contractures. Atlas Hand Clin.. 2001:21-50.
10 Bleakley D., McDonough S. The use of ice in the treatment of acute soft tissue injury: a systematic review of randomized controlled trials. Am. J. Sports Med.. 2004;32:251.
11 Buchbinder R., Green S., Youd J., Assendelft W.J.J., Barnsley L., Smidt N. Shock waves for lateral elbow pain. The Cochrane Library. 2006;4:1.
12 Butler D. Mobilization of the nervouse system. Melbourne, Australia: Churchill Livingstone, 1991.
13 Chinchalker S., Szekeres M. Rehabilitation of elbow trauma. Hand Clin.. 2004;20:363.
14 Davidson P., Pink M., Perry J., Jobe F.W. Functional anatomy of the flexor pronator muscle group in relation to the medial collateral ligament of the elbow. Am. J. Sports Med.. 1995;23:245.
15 Dunning C., Zarzour Z.D., Patterson S.D., Johnson J.A., King G.J. Muscle forces and pronation stabilize the lateral ligament deficient elbow. Clin. Orthop. Rel. Res.. 2001;338:118.
16 Elliot B., Fleisig G., Escamilla R. Technique effects on upper limb loading in the tennis serve. J. Sci. Med. Sport. 2003;6:76.
17 Fleisig G., Barrentine S., Escamilla R., Andrews J.R. Biomechanics of overhand throwing with implications for injuries. Sports Med. 1996;21:421-437.
18 Flowers K., LaStayo P. Effect of total end range time on improving passive range of motion. J. Hand Ther.. 1994;7:150.
19 Green D., McCoy H. Turnbuckle orthotic correction of elbow-flexion contractures after acute injuries. J. Bone Joint Surg.. 1979;61A:1092.
20 Green S., Buchbinder R., Barnsley L., Nall S., White M., Smidt N., Assendelft W.J. Non-steroidal anti-inflammatory drugs (NSAIDS) for treating lateral elbow pain in adults. Cochrane Database Systematic Review. 2002:2.
21 Halar E., Bell K. Immobility. In: DeLisa J., Gans B., editors. Rehabilitation Medicine: Principles and Practice. Philadelphia: Lippincott-Raven; 1998:1015.
22 Hardy M., Woodal W. Therapeutic effects of heat, cold, and stretch on connective tissue. J. Hand Ther.. 1998;11:148.
23 Hatch G., Pink M., Mohr K., Sethi P.M., Jobe F.W. The effect of tennis racket grip size on forearm muscle firing patterns. Am. J. Sports Med.. 2006;34:1977.
24 Keizer S., Rutten H., Pilot P., Moore N.N., Vos J.J., Verburg A.D.. Botulinum toxin injection versus surgical treatment for tennis elbow. Clin. Orthop. Rel. Res., 2002;401:125.
25 Kibler W., Sciasica A. Kinetic chain contributions to elbow function and dysfunction in sports. Clin. Sports Med.. 2004;23:545.
26 Lee M., LaStayo P., vonKersburg A. A supination splint worn distal to the elbow. J. Hand Ther.. 2003;16:190.
27 Lewis M., Hay E., Paterson S., Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Clin. J. Sports Med.. 2005;21:330.
28 Magermans D., Chadwick E., Veeger H., van der Helm F.C. Requirements for upper extremity motions during activities of daily living. Clin. Biomech.. 2005;20:591.
29 Manias P., Stasinopoulos D. A controlled clinical pilot trial to study the effectiveness of ice as a supplement to the exercise programme fo rhte management of lateral elbow tendinopathy. Br. J. Sports Med.. 2006;40:81.
30 Mehallo C., Drezner J., Bytomski J. Practical management: nonsteroidal antiinflammatory drug (NSAID) use in athletic injuries. Clin. J. Sports Med.. 2006;16:170.
31 Mishra A., Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet rich plasma. Am. J. Sports Med.. 2006;34:1174.
32 Morrey B. The posttraumatic stiff elbow. Clin. Orthop. Rel. Res.. 2005;431:26.
33 Morrey B., An K.-N. Stability of the elbow: osseous constraints. J. Shoulder Elbow Surg.. 2005;14:174S.
34 Morrey B., An K., Stormont T. Force transmission through the radial head. J. Bone Joint Surg.. 1988;70A:250.
35 Morrey B., Askew L., Chao E.Y. A biomechanical study of normal functional elbow motion. J. Bone Joint Surg.. 1981;63A:872.
36 Murray I., Johnson G. A study of the external forces and moments at the shoulder and elbow while performing every day tasks. Clin. Biomech.. 2004;19:586.
37 Nirschl R. Prevention and treatment of elbow and shoulder injuries in the tennis player. Clin. Sports Med.. 1988;7:289.
38 Nirschl R., Rodin D., Ochiai D., Maartmann-Moe C., DEX-AHE-01-99 Study Group. Iontophoretic administration of dexamethasone sodium phosphate for acute lateral epicondylitis. Am. J. Sports Med.. 2003;31:189.
39 Nussbaum E. The influence of ultrasound on healing. J. Hand Ther.. 1998;11:140.
40 O’Driscoll S. Classification and spectrum of elbow instability: chronic instability. In: Morrey B., editor. The Elbow and Its Disorders. Philadelphia: W.B. Saunders Company; 1993:453.
41 O’Driscoll S., Bell D., Morrey B. Posterolateral rotary instability of the elbow. J. Bone Joint Surg.. 1991;73A:440.
42 Paoloni J., Appleyard R., Nelson J., Murrell G.A. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow. Am. J. Sports Med.. 2003;31:915.
43 Park M., Ahmad C. Dynamic contributions of the flexor-pronator mass to elbow valgus stability. J. Bone Joint Surg.. 2004;86A:2268.
44 Smidt N., Assendelft W., Arola H., Malmiuaara A., Green S., Buchbinder R., van der Windt D.A., Bouter L.M. Effectiveness of physiotherapy for lateral epicondylitis: a systematic review. Ann. Intern. Med.. 2003;35:51.
45 Smidt N., Assendelft W., van der Windt D. Corticosteroid injections for lateral epicondylitis: a systematic review. Pain. 2002;96:23.
46 Taylor D., Dalton J., Seaber A., Garrett W.E.Jr. Viscoelastic properties of muscle-tendon units: the biomechanical effects of stretching. Am. J. Sports Med. 1990;18:300.
47 Trudel D., Duley J., Zastrow I., Kerr E.W., Davidson R., MacDermid J.C. Rehabilitation for patients with lateral epicondylitis: a systematic review. J. Hand Ther. 2004;17:243.
48 Warden S., Avin K., Beck E., DeWolf M.E., Hagemeier M.A., Martin K.M. Low-intensity pulsed ultrasound accelerates and a non-steroidal anti-inflammatory drug delays knee ligament healing. Am. J. Sports Med. 2006;34:1094.
49 Warren C., Lehman J., Koblanski J. Heat and stretch procedures: an evaluation using rat tail tendon. Arch. Phys. Med. Rehabil. 1976;57:122.
50 Wilk K., Reinold M., Andrews J. Rehabilitation of the thrower’s elbow. Clin. Sports Med. 2004;23:765.
51 Dhert W.J., O’Driscoll S., van Royen B., Salter R.B. Effects of immoblization and continuous passive motion on post-operative muscle atrophy in mature rabbits. Can. J. Surg. 1988;31:185.
52 Wyke B. The neurology of joints. Ann. R. Coll. Surg. Engl. 1966;41:25.