Chapter 53 Principles of Antineoplastic Drug Use
One of the fundamental advances made in oncology in the last few decades is the recognition that cancer is a genetic disease. This does not mean that all cancers are inherited (although numerous genetic diseases are associated with a predisposition to cancer), but rather that neoplastic cells have an altered genetic content. This was first recognized in leukemias, which were all found to be associated with an abnormal karyotype. Eventually it was noted that most malignant cells have chromosomal rearrangements, and even cells with apparently normal karyotypes can almost always be found to have definable abnormalities (e.g., translocations, deletions).
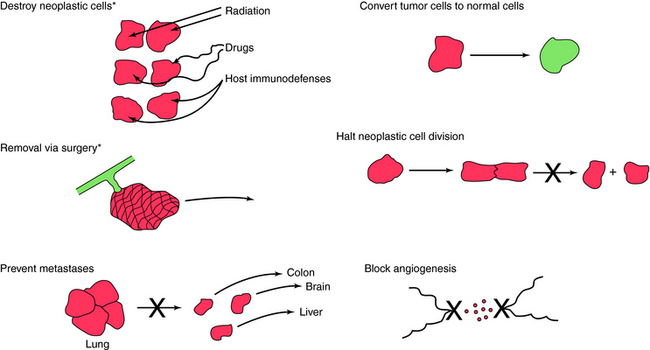
The generally accepted approach in the therapy of neoplastic diseases (Fig. 53-1) remains the removal or destruction of the neoplastic cells while minimizing toxic effects on non-neoplastic cells. It has been a long-standing question whether drugs effective against one type of neoplasm should be effective against all types. Clinical experience, however, has shown a wide range of drug activities among different types of tumors (sarcoma, carcinoma, leukemia, and lymphoma) and among tumors in different anatomical locations (breast, colon, and lung). Therefore interest has focused on treating each of the more than 100 clinically important forms of cancer as distinct diseases. Some of the therapeutic approaches listed in Figure 53-1 are not available for clinical use but represent experimental approaches that are under study. For example, drugs that function specifically to return neoplastic cells to normal differentiating cells and drugs that prevent metastases are not available or are highly experimental.
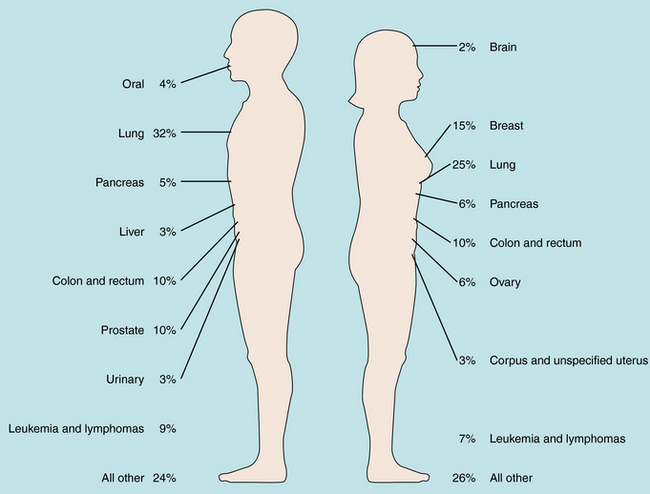
It is of interest to compare the tumor types in which therapy has been aided greatly by antineoplastic drugs with the leading causes of cancer mortality (Fig. 53-2). Unfortunately, chemotherapy is only minimally effective in management of the most common forms of neoplastic diseases. Overall, carcinoma of the lung accounts for the greatest number of cancer deaths in men and women, and although chemotherapy can produce objective responses, it is not curative in this setting. Thus, despite progress, there is still a great need for more effective chemotherapy for the major neoplastic diseases.
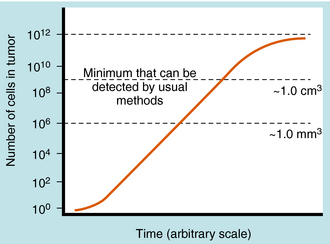
One of the difficulties in treating neoplastic diseases is that the tumor burden often is excessive by the time the diagnosis is made. This is shown in Figure 53-3, where the number of cells in a typical solid tumor is shown versus time, with 109 cells roughly equivalent to a volume of 1 cubic centimeter, and representing the minimum size tumor that can usually be detected. It takes approximately 30 doublings for a single cell to reach 109 cells. On the other hand, it takes only 10 additional doublings for 109 cells to reach a population of 1012 cells, which is no longer compatible with life. The significance of a large number of cells already established at the time of detection becomes readily evident, with 1012 to 1013 tumor cells leading to death. Thus by the time a tumor is detected, only a small number of doublings are required before it is fatal. Of course, not all tumor cells are cycling, so no meaningful predictions about longevity can be made purely on the basis of doubling times. Also, doubling times of human tumors vary greatly. For acute lymphocytic leukemia, the doubling time during log-phase (first-order) growth is 3 to 4 days, whereas the doubling time for lung squamous cell carcinoma is approximately 90 days. Thus in roughly 100 days, two lymphocytic leukemia cells in theory could keep doubling and reach 109 cells. Such a situation is extremely difficult to treat only with drugs and is cited here to emphasize the difficulty of the therapeutic task using currently available diagnostic timetables. In addition, by the time a tumor is clinically detectable, it already has a well-developed vascular supply and most probably has already metastasized. Mathematical models suggest that it also has a high likelihood of being resistant to cytotoxic agents that function through the same pathways.
Primary versus Adjuvant Therapy
The objective of chemotherapy in any given individual patient may be:
| Therapeutic Overview |
|---|
| Cancers in which complete remissions to chemotherapy are common and cures are seen even in advanced disease* |
| Acute lymphocytic leukemia (adults and children) |
| Acute myelogenous leukemia |
| Hodgkin’s disease (lymphoma) |
| Non-Hodgkin’s lymphoma |
| Choriocarcinoma |
| Testicular cancer |
| Burkitt’s lymphoma |
| Ewing’s sarcoma |
| Wilms’ tumor |
| Small-cell lung cancer |
| Ovarian cancer |
| Hairy cell leukemia |
| Cancers in which objective responses are seen but chemotherapy does not have curative potential in advanced disease |
| Multiple myeloma |
| Breast cancer |
| Head and neck cancer |
| Colorectal carcinomas |
| Chronic lymphocytic leukemia |
| Chronic myelogenous leukemia |
| Transitional cell carcinoma of bladder |
| Gastric adenocarcinomas |
| Cervical carcinomas |
| Medulloblastoma soft-tissue sarcoma |
| Neuroblastoma |
| Endometrial carcinomas |
| Insulinoma |
| Osteogenic sarcoma |
| Non-small cell lung cancer |
| Cancers in which only occasional objective responses to chemotherapy are seen |
| Melanoma |
| Renal tumor |
| Pancreatic carcinomas |
| Hepatocellular carcinoma |
| Prostate carcinomas (hormone nonresponsive) |
* Depending on tumor type, complete remission may result in cure.
Many sophisticated approaches have been used for selecting drugs and dosing schedules for combination chemotherapy, but the results have been disappointing. Some approaches have included drugs that have different mechanisms of action, in the hope that one mechanism would succeed where the others fail. Another goal of the multiple-mechanism approach has been the discovery of synergistic combinations. Several combinations, including sequential methotrexate-5-fluorouracil, doxorubicin-cyclophosphamide, and cisplatin-etoposide, have been found to be synergistic when tested against tumor cells cultured in vitro. A major problem of this approach, however, is that the observed in vivo clinical results often do not correlate with the in vitro data.
The relative sequence of drugs and the timing of drug administration may also play a significant role. As noted in Chapter 54, most drugs are more effective against tumor cells that are cycling rather than cells resting in the Go phase, but cells may be present in any part of the cycle in vivo. The effectiveness of some drug combinations may be in part attributable to their activation of cells in the Go phase to start cycling or to cycle more rapidly (see Fig. 54-1). A greater number of cells are then positioned in portions of the cell cycle where antineoplastic drugs can exert their cytotoxic actions.
Antineoplastic drugs are used as the primary mode of therapy when the tumor is known to be sensitive, or when surgical removal or radiation destruction of the main tumor mass is not particularly feasible. A more difficult situation is posed by a patient who presents with metastatic disease of a tumor type that is not responsive to chemotherapy. In this situation the best option is treatment with a newer experimental agent. An easier situation is the patient in whom drugs can be used as an adjuvant therapy after surgical or radiation removal of the primary tumor. Adjuvant therapy is commonly used in management of completely resected breast cancer and colorectal cancer. Significant improvement in survival is seen in patients with either of these tumors who receive adjuvant chemotherapy. Some examples of common combination drug regimens are given in Table 53-1.
| Terminology | Cancer | Drugs |
|---|---|---|
| MOPP | Hodgkin’s | Mechlorethamine, vincristine, procarbazine, prednisone |
| ABVD | Hodgkin’s | Doxorubicin, bleomycin, vinblastine, dacarbazine |
| CMF | Breast | Cyclophosphamide, methotrexate, 5-fluorouracil |
| CAF | Breast | Cyclophosphamide, doxorubicin, 5-fluorouracil |
| Acute lymphocytic leukemia | Vincristine, prednisone, asparaginase, daunorubicin | |
| Acute myelogenous leukemia | Cytarabine, plus mitoxantrone or idarubicin or daunorubicin | |
| Chronic myelogenous leukemia | Hydroxyurea, interferon | |
| Wilms’ | Actinomycin D, vincristine, doxorubicin | |
| Small cell (lung) | Etoposide-cisplatin | |
| Non-small cell (lung) | Cisplatin, etoposide | |
| PVC | Anaplastic oligodendrogliomas | Procarbazine, vincristine, CCNU |
| BEP | Germ cell cancers | Bleomycin, etoposide, cisplatin |
| Ovary | Paclitaxel, carboplatin | |
| CHOP | Lymphoma | Cyclophosphamide, doxorubicin, vincristine, prednisone |
| Head and neck | 5-fluorouracil, cisplatin | |
| Colon/rectum | 5-fluorouracil, leucovorin |
There is a growing trend toward the use of high-dose protocols in an effort to push higher concentrations of drug into the tumor cells. This is also true for drugs that are rapidly degraded in plasma such as cytarabine. The emergence of granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor as agents that can reduce chemotherapy-induced neutropenia and infections have accelerated this trend toward high-dose therapy.
Nausea and vomiting can be expected in a high fraction of patients receiving antineoplastic drugs. Some of the drugs most and least likely to trigger emesis are listed in Box 53-1. Although the clinical management of nausea and vomiting can become a serious problem, many drugs now exist to successfully control these symptoms.
Targeted Therapies and Biological Response Modifiers
Targeted therapies are becoming increasingly important in treatment of cancer. Examples include bevacizumab, which targets vascular endothelial growth factor; I131 tositumomab and Y-90-ibritumomab tiuxetan, which target CD20 and are used for treatment of chemotherapy-refractory non-Hodgkin’s lymphoma; and gefitinib and the antibody cetuximab, which target the epidermal growth factor receptor pathway. Biological response modifiers comprise a class of agents that stimulate the human immune system to destroy tumor cells. The α and β human interferons are efficacious in hairy cell leukemia and in certain skin cancers and may become aids for treating chronic myelogenous leukemia and non-Hodgkin’s lymphoma. Interleukin-2 is another endogenous compound that may prove beneficial in treating lung, renal, colorectal, and several other tumor types. Still other compounds include tumor necrosis factor, human growth factors, and monoclonal antibodies (see Chapter 6).
New Horizons
Although significant advances have been made in the treatment of the hematological neoplastic diseases over the past several decades, less progress has been made in the treatment of most solid tumors. During this same time there has been an explosion in our understanding of the basic science of cancer. For example, the past few decades have witnessed the description of RNA and deoxyribonucleic acid tumor viruses, oncogenes, and anti-oncogenes (tumor suppressor genes) as well as dramatic advances in our understanding of cell-cycle regulation, apoptosis, and the signaling pathways in deoxyribonucleic acid damage responses. Many gene products involved in these pathways are new targets in the treatment of malignancies (see Chapter 54). Over the next several years many cancer treatments are certain to be devised based on our increased understanding of these basic molecular mechanisms, thereby narrowing the chasm between the molecular biology of cancer and clinical oncology.
Mina etal 2007 Mina L, Soule SE, Badve S, et al. Predicting response to primary chemotherapy: Gene expression profiling of paraffin-embedded core biopsy tissue. Breast Cancer Res Treat. 2007;103:197-208.
Tiseo M, Loprevite M, Ardizzoni A. Epidermal growth factor receptor inhibitors: A new prospective in the treatment of lung cancer. Curr Med Chem Anti-Canc Agents. 2004;4:139-148.
Workman P. Strategies for treating cancers caused by multiple genome abnormalities: From concepts to cures? Curr Opin Investig Drugs. 2003;4:1410-1415.