Chapter 48 Principles and Practices of Neurological Rehabilitation
Neurological rehabilitation fosters assessments and practices that extend into every aspect of the care of patients with acute and chronic neurological disabilities. Managing the rehabilitation needs of patients should be a priority for the clinical neurologist who seeks opportunities to lessen impairments and disabilities and to meet the requests of patients to increase their ability to participate in daily home and community activities (Dimyan et al., 2008). To best assist patients, the clinician must determine how a patient’s physical and cognitive deficits cause disabilities; consider what tasks patients can and cannot perform independent of assistance and at the speed and accuracy necessary for daily activities; design practice and training paradigms with health-related professionals to lessen disabilities that are important to patients and their caregivers; consider interventions that manipulate the fundamental mechanisms that induce neural adaptations for cerebral reorganization and learning; and anticipate and manage the neuromedical and psychosocial complications of immobility, loss of motor control, cognitive impairment, and functional dependence.
Goals and Structure of Rehabilitation
Personnel and Strategies
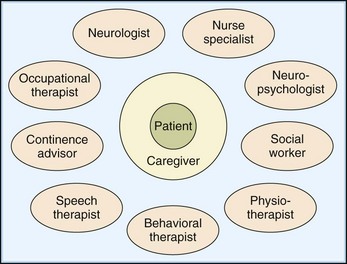
A team approach to inpatient and outpatient care best manages the diverse problems faced by disabled patients and their families (Fig. 48.1). In a multidisciplinary model, each member with specialty training treats particular disabilities. In an interdisciplinary model, roles blend. An interdisciplinary approach is oriented toward problem-solving to improve functional outcomes, rather than being bound by individual disciplines. For example, training procedures for motor and cognitive learning or behavioral modification are reinforced by all members of an interdisciplinary group, using agreed-on strategies. These interaction styles are not mutually exclusive. Most teams move between the two models when they formally meet to discuss the patient’s progress and to adjust goals and treatments.
Physicians
The clinician superimposes the contributions of neurological, musculoskeletal, cardiopulmonary, and other impairments on a map of the patient’s functional abilities and disabilities. For example, does tender musculoligamentous tissue cause pain or limit movement, or does spasticity lead to loss of motor control? Does a medication or metabolic abnormality lessen concentration, the ability to learn, or endurance for exercise? Physicians tend to be the facilitators of the multidisciplinary team, especially during inpatient care. Here, the physician may conduct a weekly team conference that reviews the patient’s progress in reaching the functional goals that will permit a discharge to the home. To do this well, the physician must help build the team’s infrastructure and understand the practices of its disciplines. Rehabilitation physicians should serve as clinician-scientists as well. The physician can encourage therapists to weigh, formulate, and test strategies. Drawing on current literature and collaborating with basic and clinical researchers, the neurological rehabilitation specialist can optimally assess and develop interventions and creative solutions (Dobkin, 2003).
Physicians should explain to both patient and primary care doctor the indications for medications, measures for secondary prevention of complications, management of risk factors for recurrence or exacerbation of the disease, and the type and duration of rehabilitative interventions. Increasingly, doctors must address the risks and possible benefits of not only medications and usual rehabilitative approaches to care but also potential research interventions such as cellular transplantation (Dobkin et al., 2006b). During outpatient care, physicians must provide informed counseling about exercise and home practice for motor and cognitive retraining. The clinician reviews the details of what the patient is practicing to improve walking, the functional use of an affected upper extremity, language and memory skills, and socialization. Education should be offered about how task-specific practice may alter the brain’s representations of these activities and improve the patient’s abilities, even years after the initial neurological illness. For patients with chronic diseases that progress, such as multiple sclerosis (MS), practice is perhaps even more important because it may spur gradual neural reorganization to maintain function. Clinicians can encourage patients to increase their strength, speed, and precision of multijoint movements and to build cardiovascular fitness. The physician also should monitor outcomes with serial tests of the activities that are targeted to best determine the optimal dose of a treatment. For example, if the gait pattern is suboptimal, the clinician can test walking speed for 50 feet or the distance walked in 2 minutes to reassess progress in mobility at each visit. By documenting the effects of treatment, the physician can best advocate the continuing goals of rehabilitation to patients and insurers.
The Internet has many sites from which to develop educational materials that physicians and the therapy team can offer patients (e.g., http://www.uclahealth.org/body.cfm?id=1174), as well as lectures and articles about recovery and about experimental interventions.
Rehabilitation Nursing
Traditionally, the nursing role has been one of providing care and support during a phase of illness and doing for others the things they would normally do for themselves. Nurses have particular expertise in bowel and bladder management and have developed the post of continence advisor, particularly for teaching chronic intermittent self-catheterization (CISC) and scheduled voiding programs. They teach skin care and pressure sore management. In an inpatient unit, nurses are in constant contact with the patient undergoing rehabilitation. This extended contact with patients allows nurses to address the issue of carryover of skills from physical and occupational therapy sessions to other areas fundamental for function in the community. Each activity is integrated with others; for instance, continence management through CISC may require improvements in upper limb coordination, trunk control, mobility, lower limb tone, medications to optimize bladder and sphincter control, and strategies to facilitate problem solving. Nurses in community programs also have become involved in the management of individual chronic diseases including MS, amyotrophic lateral sclerosis (ALS), and Parkinson disease (PD), especially attending to gaps in services needed by patients. A nurse practitioner can be a valuable asset to the physician and team on a busy inpatient service, especially in a university hospital, where patients tend to have complex medical illnesses and needs. The Association of Rehabilitation Nurses has excellent resources for continuing education (www.rehabnurse.org).
Physical Therapists
Two broad categories of physical therapy, therapeutic exercise and the so-called neurophysiological and neurodevelopmental techniques, were the bulwark of the approaches used by therapists in the past (Box 48.1). Newer concepts related to practice-induced neuroplasticity, motor control, and skills learning have taken greater hold beginning in the past decade.
Box 48.1 Practices in Physical Therapy
Neurophysiological Schools
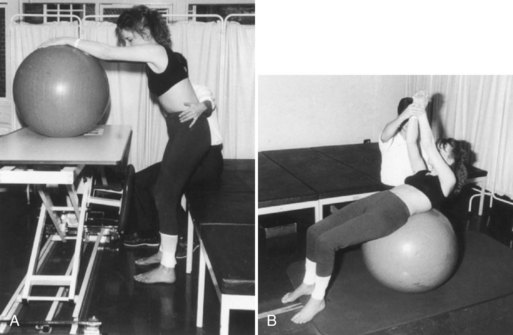
Neurodevelopmental practitioners believe that the optimal facilitation of movement requires normal postural responses, that abnormal motor behaviors are compensatory, and that the quality of motor experiences and the integration of whole-body somatosensory information will train patients for more normalized movements. The Bobath hands-on approach is a particularly popular neurodevelopmental technique. It aims to facilitate normal movement and desired automatic reactions and to restore postural control while inhibiting abnormal tone and reflex activity using specific motor patterns. Bobath therapists avoid provoking mass flexor synergies from the shoulder, elbow, and wrist or extensor synergies at the knee and ankle. The coordination of patterns of muscle group activity is viewed as more important than the actions of individual muscles. Most practitioners take a problem-solving and task-oriented approach with patients. A typical exercise routine may work on stance and trunk control with a large ball and careful hand placement by the therapist to evoke movements out of synergy (Fig. 48.2). These methods originally were developed for children with cerebral palsy but have been adapted for stroke and other neurological disorders. In comparison with other neurophysiological approaches and task-oriented motor learning for gait, upper extremity function, and overall level of functional independence, use of Bobath techniques has led to equivalent outcomes or, in several small trials, modestly inferior outcomes (van Vliet et al., 2005). Given the underlying theory of Bobath, however, outcome measures ought to examine the quality of task-related movements as much as functional gains.
Task-Oriented Practice
Studies of the efficacy of particular schools of therapy have not revealed differences between approaches. These studies, primarily in patients with stroke, used outcome measures that emphasize independence in ADLs and not an outcome directly related to the primary focus of the specific technique of physiotherapy, which is motor performance and patterns of movement. Studies of efficacy should concentrate on the best well-defined practice for an important goal such as reaching, grasping items, and walking that may in theory be modulated by the intervention. Another emphasis must be on the optimal intensity and duration of sessions of training. Most therapists take an eclectic experimentalist’s approach, not unlike what physicians do in daily practice, but this may not be conducive to an optimal outcome for the patient. Indeed, the best evidence to date is that a mixed approach leads to more functional independence than a placebo or no active therapy, but no one approach to a particular set of upper extremity or mobility functions has been shown to be superior to another (Pollack et al., 2008).
Adaptive Equipment
Wheelchairs are of two main types, companion-operated and patient-operated. The latter can be manual or power-assisted. Lightweight and very lightweight patient- or companion-operated wheelchairs must be fitted with at least a dozen characteristics in mind (Box 48.2). Severe spasticity, poor head or trunk control, the amount of upper extremity function, and the type of work and sports engaged in may necessitate additional modifications. Very lightweight wheelchairs tend to be most manageable and durable for the patient with paraplegia. Models with power-assisted wheels have become more affordable and are practical for use by patients with weakness or pain in the upper extremities. Motorized wheelchairs can be maneuvered by joystick switches and chin or sip-and-puff mouth controls. The high cost of custom-designed wheelchairs means that therapists, vendors, patients, and families need to work together to obtain what is most appropriate and cost-effective. Wheelchairs also require maintenance. A wobbly front wheel or poorly aligned main rear wheel adds to the energy cost of mobility and may cause shoulder and wrist injuries. Most rehabilitation centers that manage patients with myelopathies offer a wheelchair clinic and have close links to durable medical equipment suppliers.
Occupational Therapists
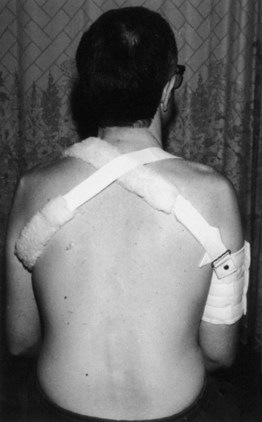
A program may include the use of appliances to improve independence, ranging from simple devices (e.g., a thickened grip to better grasp cutlery or a pen) to complex ones (e.g., use of an environmental control unit). Such adaptive aids for daily living are listed in (Box 48.3). Hemicuff and Bobath slings are used to reduce shoulder subluxation and prevent pain in patients with upper limb paralytic disorders (Fig. 48.3). A balanced forearm orthosis can support the upper arm and allow a modest biceps and triceps contraction to swing the arm over a table, which may be especially effective for the patient with a level C5 SCI. For patients with stroke and brain injury, OTs work closely with the neuropsychologist to address visuospatial inattention, memory loss, apraxia, difficulties in problem solving, and the skills needed for return to school or employment. Some OTs manage dysphagia and interpret modified barium swallow (MBS) studies.
Task-oriented and motor learning strategies have gained attention in formal occupational therapy research. Using this approach, the OT presents activities in a way that elicits the retention and transfer of particular skills for use in a functional setting. For example, in one study, limb kinematics improved in normal and hemiparetic subjects when training included purposeful goals with relevant items used daily. Thus, practice in object-related tasks, rather than simple repetition of reaching and grasping of items that have no significance for the client, may provide more concrete sensory information and offer rewards that motivate performance. In many instances, OT strategies evolve from problems that arise in daily living that require a solution. For example, adaptive equipment and an OT educational intervention in stroke patients to remediate the lack of confidence and increase the amount of information patients had available to them reduced the barriers to outdoor mobility and participation in the community (Logan et al., 2004).
Speech and Cognitive Therapists
Treatment for aphasia generally is based on clinical evaluation of the patient’s cognitive and linguistic assets and deficits. The therapy plan is fine-tuned according to standardized language and neuropsychological test results, knowledge of the cortical and subcortical structures damaged, and the ongoing response to specific therapies. Speech therapists attempt to circumvent, deblock, or help the patient compensate for defective language behaviors. Stimulation-facilitation approaches, listed in (Box 48.4), are commonly employed. Views on the value of speech therapy for aphasia vary. Most randomized controlled trials demonstrate a significant benefit for aspects of expression and comprehension in moderately impaired subjects. The amount of practice is a key variable for enhancing outcomes for any particular approach.
Box 48.4
Stimulation-Facilitation Approaches for Aphasia Therapy
 Gestural expression and pointing
Gestural expression and pointing
 Verbal cueing for words and sentence completion
Verbal cueing for words and sentence completion
 Phonemic and semantic word retrieval strategies
Phonemic and semantic word retrieval strategies
 Auditory processing at phrase level and then sentence level
Auditory processing at phrase level and then sentence level
 Word-, phrase-, then sentence-level reading
Word-, phrase-, then sentence-level reading
 Graphic tasks: tracing, copying, word completions
Graphic tasks: tracing, copying, word completions
 Pragmatic linguistic and nonlinguistic conversational skills
Pragmatic linguistic and nonlinguistic conversational skills
Adapted with permission from Dobkin, B., 2003. The Clinical Science of Neurologic Rehabilitation. Oxford University Press, New York.
Orthotists and Bracing
With shortened inpatient rehabilitation stays, especially after stroke, ankle-foot orthoses (AFOs) that fit inside a shoe tend to be used early to more quickly assist foot clearance during ambulation in patients with a central or peripheral lesion. Observation of gait usually is enough to determine the need for a trial with an AFO in a patient with hemiparesis or footdrop. Indications include inadequate dorsiflexion for initial heel contact or for toe clearance during early and mid-swing, excessive hip-hiking during swing, medial-lateral subtalar instability during stance, tibial instability during stance, and uncontrolled foot placement caused by sensory loss. An orthosis also may be needed after operative heel cord lengthening. If the knee of the hemiplegic buckles during stance, angling the AFO in slight plantar flexion will extend the knee earlier. Dorsiflexing the AFO by 5 degrees can decrease knee hyperextension and help prevent the snapping back that causes instability and pain in midstance. Ankle inversion may necessitate greater rigidity and longer anterior foot trim. The AFO worn in a shoe ought to improve weight-bearing on the affected leg, increase single-limb stance time, and perhaps lessen postural sway. This may improve safety, especially on uneven surfaces, and walking velocity. Fig. 48.4 shows a thermoplastic AFO that fits in a shoe to limit plantar flexion and rotation and help control the knee. Orthoses may be static, such as a rest splint worn at night, or dynamic, with joints that may be lockable or free moving. Fig. 48.5 shows a thermoplastic AFO with a hinged joint that allows flexibility on rising to stand and at heel strike to start the stance phase of the gait cycle. Toe clawing can be managed with a metatarsal pad that spreads the toes. Some patients who have footdrop from a neuropathy such as Charcot-Marie-Tooth disease or diabetes may find that a fashionable boot with a flat heel that fits snugly above the ankle can improve gait by lessening its steppage quality, yet allow toe clearance. An orthotist can assess the potential for shoe modifications and inserts to improve balance and pressure points. Multiple small trials in patients with hemiplegic stroke show that walking activity increases and impairments in walking and balance decrease with the use of an AFO (Tyson and Kent, 2009). Functional electrical stimulation of the peroneal nerve timed to the gait cycle has equivalent or somewhat better effects on walking speed.
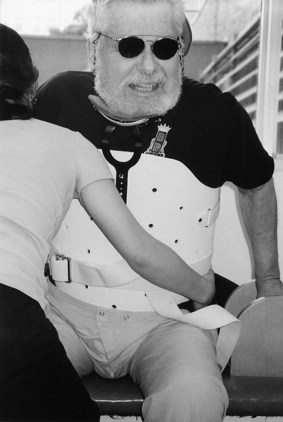
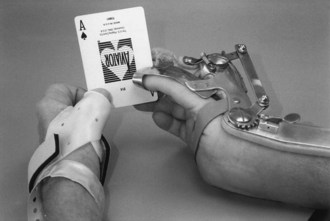
The thoracolumbar support shown in Fig. 48.6 was molded to the patient to lessen neck motion after a high thoracic vertebral injury and surgical stabilization. Static orthoses allow no motion of the primary joint. Solid wrist-hand orthoses usually are set between neutral and 30 degrees of extension. Upper limb orthoses, however, have not been shown to improve arm or hand function, increase range of motion or lessen the incidence of pain, based on small trials after stroke. Dynamic orthoses use elastic, wire, or powered levers that compensate for weakness or an imbalance in strength and allow some controlled movement. Fig. 48.7 shows the paretic left hand of a patient with a level C6 SCI holding a playing card with the aid of a thumb opposition splint. The weaker right hand needs wrist extension to mechanically oppose the thumb to the second and third digits. Such custom-made devices can be produced with lightweight metals and plastics. Functional neuromuscular electrical stimulation electrodes can be embedded in a wrist splint to enable grasp and release or over the peroneal nerve to trigger ankle dorsiflexion at appropriate times during the stepping cycle.
Measurement Tools
Outcome measurement is essential to demonstrate the effectiveness of an intervention for an individual and for clinical trials that aim to develop better evidence-based practices. Many of these measurement tools can also be employed within usual clinical care to monitor for declines. The complete discussion of this topic is available in the online version of this book at www.expertconsult.com.
Measures
Neurorehabilitation employs measurement tools to characterize the impairments, disabilities, activities, and psychosocial needs of patients, as well as to monitor interventions, assess outcomes, and document services. To provide a comprehensive assessment of the impact of disease, outcomes may be considered at four levels: pathophysiology and related impairments, disability and level of functional activity, handicap and ability to participate, and health-related quality of life. The design and applications of these scales across diseases are described in Table 48.1.
Table 48.1 World Health Organization System of Classification of Impairments, Disabilities, and Handicaps
Each outcome level addresses distinct aspects of the disease process. The relationships among them are complex. The first two levels entail physician-oriented outcomes, whereas those at the third and fourth levels are more accurately called patient-based outcomes, although only the fourth incorporates the patient’s perspective. Rehabilitation is especially interested in measures of the impact of disease, which is contained within the World Health Organization (WHO) International Classification of Impairments, Disabilities, and Handicaps. As shown in Table 48.1, the focus is on the consequences of the disease or health condition rather than the disease alone (i.e., a classification of disablements and functioning). A classification available on the WHO website (www.who.int/icidh/) emphasizes whether people actually perform the tasks, especially those that require a lot of time and energy. The dimensions of the WHO classification also include personal and environmental factors that affect functioning. The emphasis on activities, participation, and contextual factors interacting with impairments and disabilities may influence the design of new measurement tools.
Two types of measures exist for each of these domains: measures that are specific to a given disease and generic ones that may, with caution, be applied across a range of diseases (see Table 48.1). The former may be more sensitive in detecting changes particular to the effects of an individual disease, whereas the latter may allow comparisons across different diseases and serve as an off-the-shelf tool. Two generic measures of disability are the 10-item Barthel Index (BI), which can be totaled to a maximum score of 20 or 100 (Table 48.2), and the 18-item Functional Independence Measure (FIM) (Box 48.5), which includes a modestly responsive cognitive component for a total of 126 points (Box 48.6). The FIM is used more often in the United States, especially for studies with large numbers of subjects, than in Europe or Asia, where its applicability for clinical trials has been questioned. The FIM, the BI, and two other scales—the Glasgow Outcome Scale for Recovery of Consciousness and Function and the Rankin Disability Scale (Tables 48.3 and 48.4)—commonly are used in clinical trials. The latter correlate closely with one other, in part because their definitions at the levels of 3 and 4 not well defined in terms of residual impairments and specific disabilities. In many respects, the BI and FIM reflect the level of care needed by patients. These and most scales have floor or ceiling effects and variable sensitivity to change, especially if used during outpatient care.
| Score | ||
|---|---|---|
| Activity | Help | Independent |
| 1.Feeding (if food has to be cut up = help) | 5 | 10 |
| 2.Moving from wheelchair to bed and return (includes sitting up in bed) | 5-10 | 15 |
| 3.Personal toilet (wash face, comb hair, shave, clean teeth) | 0 | 5 |
| 4.Getting on and off toilet (handling clothes, wipe, flush) | 5 | 10 |
| 5.Bathing self | 0 | 5 |
| 6.Walking on level surface (or if unable to walk, propel wheelchair*) | 10 (0*) | 15 (5*) |
| 7.Ascend and descend stairs | 5 | 10 |
| 8.Dressing (include tying shoes, fastening fasteners) | 5 | 10 |
| 9.Controlling bowels | 5 | 10 |
| 10.Controlling bladder | 5 | 10 |
* Score only if patient is unable to walk.
Box 48.5 Functional Independence Measure (FIM)
Box 48.6 Description of Functional Independence Measure Levels of Function
Independent
Dependent
Another person is needed for supervision or physical assistance for the activity to be performed, or it is not performed (requires helper).
Modified dependence. Patient expends half (50%) or more of the effort. Levels of assistance needed are the following:
Complete dependence: patient expends less than 50% of the effort. Maximal or total assistance is needed; otherwise, the activity is not performed. Levels of assistance needed are the following:
Table 48.3 Glasgow Outcome Scale for Recovery of Consciousness and Function
| Score | Outcome |
|---|---|
| 1 | Death |
| 2 | Vegetative: unresponsive and speechless |
| 3 | Severe disability: dependent on others for all or part of care or supervision because of mental or physical disability |
| 4 | Moderate disability: disabled but independent in activities of daily living and community |
| 5 | Good recovery: resumption of normal life; may have minor neurological or psychological deficits |
Table 48.4 Rankin Disability Scale
| Score | Disability |
|---|---|
| 1 | No disability |
| 2 | Slight disability: unable to carry out some previous activities but looks after own affairs without assistance |
| 3 | Moderate disability: needs some help but walks without assistance |
| 4 | Moderately severe disability: unable to walk and do bodily care without help |
| 5 | Severe disability: bedridden, incontinent; constant nursing care needed |
Measures for Clinical Trials
Rehabilitation interventions have progressed considerably in conforming to scientifically designed clinical trials. Although the efficacy of general rehabilitation has been the subject of trials in patients with stroke, MS, and other diseases, trials are especially needed to assess a particular intervention for subjects with a particular set of disabilities that are the target of the well-defined intervention. Physical interventions are far more difficult to provide in a reproducible fashion than drug therapies. Outcome measures also are less easy to develop and apply when the primary outcome is levels of disability or severity of a specific disability, rather than outcomes such as mortality, morbidity, survival time, or subjects who survive without disability, which are typical measures of drug and surgical studies. Recent multicenter randomized controlled trials (MRCTs) of locomotor training after stroke (Duncan et al., 2007; Hidler et al., 2009) and SCI (Dobkin et al., 2006a), of constraint-induced movement therapy (Wolf et al., 2006), and of cortical electrical stimulation (Harvey et al., 2009) have demonstrated the feasibility of developing complex treatment strategies provided by many therapists at many cooperating sites. Ordinal scales like the BI dominate the outcome measures for most trials, but continuous or ratio measurement scales such as walking speed or time to complete tasks were the primary outcomes for these MRCTs. The relationship between walking faster over ground after an intervention and how well you can participate in home and community as a result of a mobility intervention is moot. Dichotomous outcome measures (Duncan et al., 2007) that divide subjects into those who achieve walking speeds compatible above or below a level that correlates with levels of home and community ability to ambulate is closer to an ideal measure of participation. Future trials related to mobility and arm use, however, may draw upon tiny triaxial accelerometers, gyroscopes, and global positioning satellite or cell phone devices. Using mathematical algorithms, these and other inexpensive wireless sensors may allow clinicians and trialists to directly monitor the quality and amount of activity and participation.
Organization of Services
Service Provision
Inpatient Rehabilitation Unit
Several studies of TBI suggest the benefit of coordinated care starting during the inpatient stay. Investigators randomly assigned 120 active-duty military personnel who suffered moderate TBI to a standardized inpatient milieu–based cognitive rehabilitation program or to a home program with weekly phone support from a nurse and mental and physical exercises carried out on their own for about 30 minutes a day (Salazar et al., 2000). The inpatient program used both didactic cognitive and functional experiential approaches. Treatment lasted 8 weeks. Outcomes did not differ 1 year later in standard cognitive tests, social adaptation, mood, behavior, or fitness for military duty. More than 90% returned to work. Aggression increased in both groups, suggesting the need for ongoing support.
Community-Based Services
Although community-based rehabilitation appears to have a number of advantages from the perspective of the disabled patient, few studies have addressed its efficacy (Legg, 2004). This lack may relate at least in part to the methodological difficulties in defining the training team’s level of expertise, the patient’s level of disability, the amount of caregiver support, and the frequency, duration, and type of therapy. One large randomized stroke trial compared rehabilitation at home after an average 12-day inpatient stay with another week of inpatient care followed by hospital-based outpatient treatment. Patients who lived alone either were independent in transfers when they left the hospital or needed to be assisted by a caregiver. Similar outcomes 12 months after a stroke were achieved at lower cost because of less use of hospital beds by the early discharge group. An intention-to-treat randomized trial with 250 subjects showed that rehabilitation in an inpatient unit after a brief stay in an acute stroke unit or general medical ward produced better outcomes in moderate to severely disabled patients (BI score less than 50) compared with rehabilitation treatment in the community. No differences in quality of life were found, and levels of activity outside the home were not measured. Smaller trials confirm similar positive outcomes at 3 to 6 months for home to those for various forms of outpatient care, with the home group having fewer in-hospital days and greater gains in instrumental ADLs.
A brief stint of outpatient therapy for a well-defined goal, such as to improve transfers in a patient who declines from MS or to make walking safe again after an illness that causes deconditioning in a patient with chronic hemiparesis, is an invaluable means for patients to maintain their highest level of independence and avoid placement in a nursing home. A few therapy sessions a week for a few weeks plus a home program may accomplish this when provided for a task-specific goal that relieves caregivers of new burdens. For example, a bout of therapy for aerobic conditioning and muscle strengthening lessened disability in patients with the chronic effects of a prior stroke. A randomized trial assigned 110 patients with mostly chronic TBI to an outreach program in the community or to provision of written material about resources for patients and families (Powell et al., 2002). The outreach group met about twice a week for a mean of 27 weeks. The outreach group made modest but significant gains in scores on the BI and a brain injury outcome measure 2 years after the start of this late intervention.
Biological Bases for Rehabilitative Interventions
The potential to enhance neurological recovery by manipulating the biological adaptability of the brain, spinal cord, and peripheral nerves has become remarkably relevant to clinical practice (Dobkin, 2003). Basic neuroscience studies suggest that physical and cognitive training, extrinsic stimulation, and pharmacological interventions, along with natural biological reactions to injury, could inhibit or enhance the restoration of motor and cognitive functions. Box 48.7 lists some of the potential mechanisms that contribute to changes in impairments and disabilities. These are not discrete mechanisms. They overlap, and many depend on each other over periods of variable duration. In addition, biological therapeutic approaches such as use of tissue implants may enhance these mechanisms when applied to human studies of recovery in the near future. These potential mechanisms, drawn mostly from in vitro and in vivo experiments with invertebrates and vertebrates, are the subject of much ongoing investigation (Carmichael, 2008; Blesch and Tuszynski, 2008). Although care must be taken in extrapolating from animal studies of recovery to their implications for human interventions, at least a few of these potential mechanisms suggest strategies that the rehabilitation team can use to improve outcomes.
Box 48.7
Mechanisms That May Support Recovery of Function
Network Plasticity
Neuronal Plasticity
Altered efficacy of synaptic activity:
Regeneration and sprouting from injured and uninjured axons and dendrites:
Adapted with permission from Dobkin, B., 2003. The Clinical Science of Neurologic Rehabilitation. Oxford University Press, New York.
Recovery of Neuronal Excitability
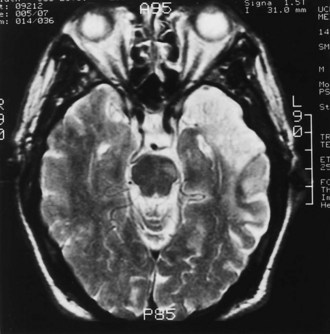
Reversal of toxic-metabolic insults to neurons, axons, and glia plays an early role in recovery of function. Recovery of impairments may be delayed until intracellular and extracellular edema, acidosis, and ion fluxes resolve and until protein synthesis restarts, the mass and toxic effects of blood from a hemorrhage lessen, and other intracellular and membrane functions return to normal. Some of the gains in the first days to few weeks after TBI, SCI, and stroke seem related to these mechanisms. Acute medical interventions to spare the penumbra of hypometabolic tissue on the edge of an infarct have been a mainstay of approaches to neuroprotection in stroke. The extent of the recovery of cortical penumbral and peri-infarct tissue has been modestly correlated with recovery of function in patients after stroke. The surviving penumbra may be a locus for long-term potentiation (LTP), representational plasticity and synaptogenesis, and activity-driven structural reorganization, as well as for the promotion of axonal regeneration and the attraction of new neural cells from the subventricular zone (Cramer, 2008). Infarcts, diffuse axonal injury after TBI, and much of the damage after SCI occur within white matter tracts, so concepts of penumbral sparing may not apply.
Activity in Partially Spared Pathways
Partial wallerian degeneration of the corticospinal tract sometimes can be seen on MRI scans after stroke (Fig. 48.8). On computed tomography (CT) images, sparing of more than 60% of the cerebral peduncle, including the medial portion, predicts the recovery of a precision grip and, to a lesser degree, the force of the grip. Greater sparing of the primary motor cortex (M1) and less extensive wallerian degeneration are associated with better hand function in studies that involved functional neuroimaging. The typical hemiplegic posture of elbow, wrist, and finger flexion followed 60% shrinkage, which corresponds to a loss of roughly 88% of the descending fibers. Better motor and functional outcomes also have been associated with sparing of metabolic activity in the ipsilesional primary motor cortex, the thalamic-and-basal ganglia–frontal network, and with sparing of the basal ganglia ipsilateral to the stroke. Approximately 20% sparing of the ventral or ventrolateral funiculi after SCI permits fair motor function, and 35% sparing of the dorsal funiculus allows appreciation of dorsal column sensory inputs.
Residual neurons and axons of an injured tract may need rehabilitation to help drive training-induced, activity-dependent plasticity. When the number of corticospinal fibers that synapse with spinal motor pools is too small to generate adequate excitatory postsynaptic potentials, a descending volley will not excite the spinal neurons. Recovery of an adequate excitatory postsynaptic potential, however, may follow practice that strengthens synaptic efficacy. Other contributors to spared pathway functioning include changes in ion channels along partially demyelinated axons, increased strength of output from undamaged cortical and brainstem neurons that also descend onto spinal neurons such as propriospinal pathways, dendritic sprouting, and up-regulation of the number of motor neuron and interneuron excitatory receptors. In addition, the ipsilateral motor cortex, via the uncrossed ventral corticospinal tract, often has been invoked as a pathway that may provide some of this input, thereby compensating for a contralateral cerebral injury. The fibers of the ventral tract synapse especially with motor neurons for axial and limb girdle muscles. Some projections of the lateral corticospinal tract cross and then recross under the central canal of the cord. Thus, deafferentation of one side of the ventral horn from loss of descending fibers may induce further sprouting of recrossing fibers to enhance motor control of the affected limb (Rosenzweig et al., 2009). Functional neuroimaging studies of the upper extremity, leg, and language suggest that better gains with training are associated with greater cortical activation near the primary representation than when homologous regional activity of the intact hemisphere is most apparent (Baron et al., 2004).
Alternative Behavioral Strategies
Many functional activities can be accomplished by compensatory behavioral adaptations that allow greater independence in ADLs despite persistent impairments. Motor functions can appear to have fully recovered when in fact residual neural activity is supporting behavioral plasticity (Levin et al., 2009). For example, after a unilateral pyramidal lesion in the rodent or monkey, reaching for a pellet of food gradually improves and at first glance may appear to have recovered fully. A closer analysis of the movement reveals better control of the proximal than of the distal portion of the affected limb. The animal reaches with a grasp, brings the pellet to its mouth without the normal supination of the hand and forearm, turns its head to chase after the food, and cannot easily release its grip. The hand-to-mouth movement pattern of the hemiparetic patient is similar to the lesioned animal’s strategy. Functional ambulation often improves in part by biomechanical adaptations that are revealed by kinematic deviations in the gait pattern and by use of braces and assistive devices. Behavioral adaptations, of course, also have a neural substrate that includes training-induced cortical representational reorganization and increased efficiency in the incorporation of residual pathways. Cognitive impairments that affect learning and problem solving, however, may limit the use of compensatory strategies. Rehabilitation efforts often train patients to use compensatory behavioral strategies to accomplish a task. Rather than performing tasks using the original neural network, alternative strategies may be emphasized. Substitution by a different neural network, however, may interfere with the activity-dependent reorganization that is needed to achieve further recovery from a specific impairment or disability.
Distributed Networks of Neuronal Circuitry
A distributed system is a collection of processing units (i.e., dynamic neuronal assemblies with similar functional properties and anatomical connections) that are spatially separate and exchange messages. Hierarchical, parallel, and quasi-serial linking operations are made with the afferent and efferent systems of the brain (Box 48.8). The nodes in such systems cooperate to manage the diverse information necessary for the rapid and highly flexible control of, for example, cognition and multijoint movements. These circuits lend themselves to an understanding of the neuroplasticity that may contribute to spontaneous and training-induced recovery of function.
Box 48.8 Characteristics of Distributed Systems in Neural Circuitry
 Signal flow follows a number of pathways.
Signal flow follows a number of pathways.
 Action may be initiated at any of the nodal loci in a system.
Action may be initiated at any of the nodal loci in a system.
 Local lesions in a system may degrade but not completely eliminate a function.
Local lesions in a system may degrade but not completely eliminate a function.
 Dynamic reorganization may be more important than modification of structural connections.
Dynamic reorganization may be more important than modification of structural connections.
 In a reentrant system, nodes are open to externally and internally generated signals.
In a reentrant system, nodes are open to externally and internally generated signals.
The executive motor system has been the subject of recovery-related studies. The primate primary motor cortex (M1) has separate clusters of output neurons that can facilitate the activity of a single spinal motoneuron. Also, a single cortical motor neuron can project to the spinal motoneurons for several muscles, even those that may act across a joint. In addition, neurons in overlapping M1 territories are activated to produce movements of different body parts. This overlapping organization contributes to the control of the complex muscle synergies for voluntary movement of the arm and leg within the ordinary workspace of the body (Graziano et al., 2002). Thus, complex movement representations within cortical and subcortical maps may be reexpressed after injury as usual patterns of movements. It seems likely, then, that retraining ought to engage patients in the practice of complex movements rather than single joint actions or exercise of single muscles.
The capacity of motor-related cortices to redistribute their function is apparent from PET and functional magnetic resonance imaging (fMRI) studies that have looked at subjects with recovered hand function after a stroke. For example, after a striatocapsular infarction in patients who recovered finger tapping and hand squeezing, bilateral rather than just contralateral activation of cortical motor neurons was found, along with greater involvement of motor areas that ordinarily would not be visibly activated for that task. Other regions related to selective attention and intention also show increases in blood flow and metabolism, which suggests that they must play a larger role when the substrates for a movement reorganize. Regions that are activated in normal subjects when greater force is exerted, such as insular, premotor, and anterior opercular cortex, may also be activated during movement of a hemiparetic limb (Baron et al., 2004), in parallel with the increased effort needed to move that some patients describe.
Cortical and Subcortical Representational Adaptations
The combination of mutable neuronal assemblies that represent movements and sensation and multiple representational maps in a parallel distributed system offers a sound basis for developing rehabilitation interventions. Behaviorally relevant tasks that are shaped by an optimal schedule of practice and feedback to neural networks may potentially increase functional gains. The optimal duration and intensity of training are uncertain for human rehabilitation strategies, but greater intensity of practice seems to enhance subsequent performance (Kwakkel et al., 2004). Unfortunately, most patients get only a few months of formal inpatient and outpatient retraining of modest intensity that is spread across many tasks.
Biological Interventions
Published cell transplant experiments in humans include numerous interventions for PD; most have been unsuccessful overall, but recent trials are more promising and aim to eliminate induced movement disorders. Studies in animal models already have led to several safety studies of human interventions for stroke. These include intravenous injection of autologous mesenchymal stem cells about 40 days after a hemispheric stroke and implantation of human neuronal cells (LBS neurons) into the edge of deep infarcts near the basal ganglia. Further studies of bone marrow–derived stem cells are anticipated. Safety studies in SCI have proceeded with injection of human fetal spinal cord tissue into a syrinx, autologous activated macrophage injections into the acute injury, and injections of porcine oligodendrocyte progenitors into chronic injuries, as well as recent dural or intrathecal infusions of Rho and Nogo inhibitors. No serious complications have been described to date, but reports are often vague. Recent uncontrolled but prospective safety trials from Australia and Portugal point to the safety of autologous olfactory ensheathing glia implanted in the spinal cord. At conferences and on websites, but not in peer-reviewed published reports, clinicians at hospitals in China (www.stemcellschina.com) and at least 100 sites around the world have built stem-cell spas to treat a broad range of neurological and other diseases. They claim to use fetal, olfactory ensheathing glia, bone marrow stromal, and other cell types for injection or implantation in uncontrolled and poorly documented experimental therapies in patients (Dobkin et al., 2006b). The sites offer hope at $20,000 to $50,000 for their services. These unpublished interventions tend to be based on a misinterpretation of preclinical experiments in rodents. Of interest, the patients who go for these interventions and then commit to a lengthy postoperative course of rehabilitation are usually the ones who report very modest changes.
Clinical trials in neural repair will be complex. No single intervention is likely to succeed, a similar situation to trials of neuroprotective agents. Too many biological pathways must be manipulated together and in series over time to get robust regrowth and effective connectivity or to incorporate new cells. To optimize the applicability of animal models to human translational research, clinicians will need to put the results of models into perspective and participate with basic neuroscientists in combining training and transplant technologies. In addition, strict adherence to best scientific practices for the conduct of randomized clinical trials will be needed, as suggested by guidelines from the International Campaign for Cures of Spinal Cord Injury Paralysis. Clinicians should encourage patients to read the educational materials about participating in neural repair experiments, such as the guidelines available at www.icord.org.
Pharmacological Interventions
Drugs that affect neurotransmission, intracellular second messenger signaling, excitation and inhibition, and the cascades associated with long-term potentiation and long-term depression are candidates to enhance learning and the reacquisition of skills after a brain injury or an SCI. At least five neurotransmitter projections have modulating effects on wide regions of the cortex and spinal cord. A lesion may transsynaptically interrupt this neurotransmitter output to cortex and cord, limiting the drive to uninjured regions and producing behavioral deficits. For example, dextroamphetamine augments specific cognitive processes by increasing the signal-to-noise ratio; cholinergic projections serve as a gate for behaviorally relevant sensory information. Cholinergic modulation is essential for motor cortex learning (Conner et al., 2005). Animal studies have provided preliminary evidence that a variety of pharmacological agents may facilitate or inhibit the rate or degree of recovery of sensorimotor function and walking after a cerebral injury. After being given dextroamphetamine, both rats and cats that underwent a unilateral or bilateral ablation of the sensorimotor or frontal cortex exhibited an accelerated rate of recovery, although not necessarily greater gains than in the control subjects, in the ability to walk across a beam. This effect endured well past the single or intermittent dosing schedule of the drug. Amphetamine and other drugs to be discussed have shown promise in small human trials. Thus, specific training paradigms combined with agents may enhance gains. Functional imaging studies with transcranial magnetic stimulation and fMRI may aid in the selection of drugs to combine with rehabilitation (Butefisch et al., 2002).
Drugs that may affect neural repair have also been tried, such as fibroblast growth factor, but side effects have exceeded expectations. Erythropoietin, granulocyte stimulating growth factor, an anti-Nogo antibody, and other factors are currently in clinical trials for patients with stroke and spinal cord injury. The designs of these trials suggest uncertainty about the mechanism of action, neuroprotection versus plasticity, in that they are initiated within 1 to 5 days of onset. Some drugs have more clear-cut mechanisms of action. For example, the conduction of action potentials along demyelinated axons may be increased by pharmacological agents such as 4-aminopyridine, which blocks potassium channels. This approach has met with some success in the responders with MS, who improved their ability to walk (Goodman et al., 2009). In some patients with motor neuron disease and Guillain-Barré syndrome, peripheral anticholinesterase-inhibiting medications, acting on neuromuscular junctions with altered structure and function, have reduced fatigability and improved strength in a modest way.
Neuromedical Problems During Rehabilitation
Frequency of Complications
Complications in Patients with Stroke
Medical complications often interfere with a patient’s ability to participate in therapy (Table 48.5). Medical and neurological complications occur at rates of approximately 4 and 0.6 per patient, respectively, during an average course of inpatient rehabilitation. A urinary tract infection, urinary retention, musculoskeletal pain, or depression will develop in approximately one-third of patients. Up to 20% will experience a fall, exhibit a rash, or need continuous management of blood pressure, hydration, nutrition, and glucose levels. In approximately 10%, a transient toxic-metabolic encephalopathy, pneumonia, cardiac arrhythmias, pressure sores, or thrombophlebitis will develop. Up to 5% will have a pulmonary embolus, seizures, gastrointestinal bleeding, heart failure, or other systemic complications. When feasible, prophylactic measures for these potential problems are essential. Because many patients in the United States are transferred from the acute hospital to a rehabilitation unit less than 7 days after a stroke, the rate of neuromedical complications may be higher at some centers. Side effects during the adjustment of new medications are especially prevalent, including orthostatic hypotension from antihypertensives or dialysis, drowsiness from anticonvulsants and analgesics, and a statin-induced myopathy with normal serum creatine kinase. Across rehabilitation centers, 5% to 15% of patients must be transferred back to an acute hospital setting.
Table 48.5 General Frequency of Inpatient Stroke Rehabilitation Neuromedical Complications
| Complication | % of Patients |
|---|---|
| Urinary tract infection | 40 |
| Musculoskeletal pain | 30 |
| Depression | 30 |
| Urine retention | 25 |
| Falls | 25 |
| Fungal rash | 20 |
| Hypertension | 20 |
| Hypotension | 15 |
| Incipient pressure sores | 15 |
| Hypoglycemia or hyperglycemia | 15 |
| Azotemia | 15 |
| Toxic-metabolic encephalopathy | 10 |
| Pneumonia | 10 |
| Arrhythmia | 10 |
| Congestive heart failure | 5 |
| Angina | 5 |
| Thrombophlebitis | 5 |
| Allergic reaction | 5 |
| Gastrointestinal bleeding | 5 |
| Pulmonary embolus | <5 |
| Myocardial infarction | <5 |
| Decubitus ulcer | <5 |
| Recurrent stroke | <5 |
| Seizure | <5 |
Adapted with permission from Dobkin, B., 2003. The Clinical Science of Neurologic Rehabilitation. Oxford University Press, New York.
Complications in Patients with Spinal Cord Injury
Medical complications of a somewhat different nature are common in the acute and chronic phases after SCI. In this younger group of patients, chronic comorbid systemic medical problems are less common than in patients with stroke. Prior substance abuse and emotional and behavioral disorders, however, are much more likely to complicate therapy. In the first 6 weeks after SCI, reparative operative procedures affect what can be done in rehabilitation. Approximately half of patients undergo spinal fusion and internal fixation. Acute spinal care also entails the use of external stabilization techniques such as a halo vest and Philadelphia collar for cervical injuries and a thermoplastic, custom-molded, thoracolumbar fixation shell (see Fig. 48.6). These devices limit head and trunk mobility, which makes self-care tasks that involve balance, management of the lower extremities, and CISC more difficult. Lower extremity fractures, especially of the femur, occur in about 5% of patients with acute SCI, which can further limit mobility and increase the risk of deep vein thrombosis and skin breakdown. Table 48.6 lists the complications found during a prospective clinical trial of methylprednisolone for acute SCI in 487 patients.
Table 48.6 Medical Complications Within 6 Weeks of Acute Spinal Cord Injury
| Complication | % of Patients |
|---|---|
| Urinary tract infection | 46 |
| Pneumonia | 28 |
| Decubitus ulcer | 18 |
| Paralytic ileus | 9 |
| Arrhythmia | 6 |
| Sepsis | 6 |
| Thrombophlebitis | 5 |
| Wound infection | 4 |
| Gastrointestinal hemorrhage | 3 |
| Pulmonary embolus | 3 |
| Congestive failure | 1 |
Complications in Patients with Traumatic Brain Injury
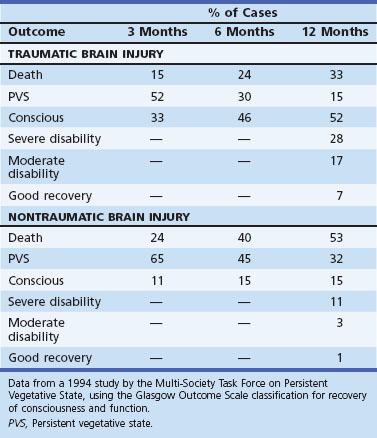
Patients who are in a persistent vegetative state often are evaluated by the rehabilitation team for prognostication and for a trial of stimulation. After TBI, the prognosis is not quite so grim as after hypoxic-ischemic coma unless hypotension accompanied the TBI. Table 48.7 shows the outcomes during the first year after brain injury determined for a 1994 study by the Multi-Society Task Force on Persistent Vegetative State.
Management of Neuromedical Problems
Dysphagia
Dysphagia management must be addressed through all phases of recovery from stroke and TBI and during the progression of disorders such as PD, ALS, and myasthenia gravis (see Chapter 12B). Lesions in the pathways for swallowing interfere with the oral, oral preparatory, and reflex or pharyngeal phases of deglutition. Common deviations include loss of the ability to form a bolus and inadvertent trapping of liquids in the vallecula, with subsequent trickling over the vocal cords. The initial emphasis for care is placed on protecting the airway and maintaining adequate alimentation and hydration, with use of a nasopharyngeal feeding tube if necessary. A large single-site randomized trial found that a standardized daily therapy for swallowing and dietary modification for up to 1 month after stroke, compared with usual care or with standard care provided only 3 days a week, reduced dysphagia-related medical complications such as pulmonary infection and significantly increased the number of patients who regained swallowing function. As in many other rehabilitation trials, it may be that the intensity of a treatment is as important as the strategy to achieve greatest efficacy.
Swallowing may improve by downsizing a tracheostomy tube or eliminating it as soon as possible. Oral pooling of secretions and drooling, which predispose the patient to aspiration, can be managed with suctioning and sometimes with use of a low titrated dose of an anticholinergic drug such as glycopyrrolate. Good oral hygiene and prevention of tooth caries by oral rinsing with chlorhexidine gluconate and stannous fluoride can lessen the likelihood of carriage of bacterial infection into the lungs by oral secretions. Therapeutic efforts focus on the use of stimulation and compensatory approaches designed to reduce the swallowing impairment or to minimize the functional disability resulting from that impairment (Table 48.8). For example, postural adjustments may be made on the basis of the MBS findings. A chin tuck narrows the airway opening, tilting the head to the stronger side of the pharynx directs a bolus away from the weak side, and head rotation toward the weak pharyngeal muscle channels a bolus toward the stronger side. Modification of diet texture includes choosing thickened or gelled liquids, which are less likely to be aspirated than thin liquids. Purees and formable solids such as applesauce or mashed potatoes usually are safer than foods that require chewing and oral manipulation. Pharyngeal stimulation by the therapist may help drive representational plasticity for these muscles on the unaffected side of the brain after stroke to improve swallowing.
| Compensation | Sensorimotor Exercises | Direct Interventions |
|---|---|---|
| Postural adjustments: | Oral sensory stimulation (thermal, vibration) | Palatal prosthesis |
| Head positioning | Resistance and placement exercises of tongue and jaw | Surgery |
| Chin tuck | Chewing, oral manipulation of bolus | Cricopharyngeal myotomy |
| Head rotation to weak pharyngeal side | Laryngeal adduction | Epiglottopexy |
| Dietary modifications: | Biofeedback | |
| Softer food | ||
| Thicker liquids | ||
| Lower intake volume | ||
| Slower intake pace | ||
| Compensatory maneuvers: | ||
| Double swallow | ||
| Supraglottic swallow | ||
| Laryngeal elevation |
Bowel and Bladder Dysfunction
After SCI, the bladder detrusor reflex may not return for 6 weeks to 12 months. In the absence of spontaneous bladder emptying, intermittent catheterization is done on a schedule that prevents the accumulation of more than 400 mL. Patients should measure their output from time to time and develop a voiding schedule that takes into account variations in fluid intake and the use of alcohol and caffeine. Catheters can be washed, stored in a plastic bag, and reused. If sensorimotor impairments persist, nearly all patients with an SCI lesion that spares the S2 to S4 micturition center will develop dyssynergia between the detrusor and the external sphincter. These uncoordinated contractions lead to incontinence and intermittent outlet obstruction. Urodynamic studies and an intravenous pyelogram are indicated as a baseline and to assist in therapy, especially in patients with SCI (see Chapter 50C). Obstruction can cause recurrent infections, urosepsis, vesicourethral reflux, urolithiasis, and hydronephrosis.
Pharmacological treatment can be understood in relation to problems in bladder filling, storage, and bladder emptying with any neurological disease (Table 48.9). Urodynamic studies often aid in the choice of drug trial. The goals are continence and regular emptying that is achieved without high intravesicular pressure and with less than 100 mL of residual volume after voiding. Many males after stroke who have an enlarged prostate benefit from an alpha-blocker. If the goal for micturition is not achieved, several alternatives to the use of an indwelling catheter on a long-term basis are available. To prevent inadequate emptying at low pressure at the price of external sphincterotomy and resultant incontinence, long-term CISC can be used in combination with anticholinergic medications or bladder infusion of botulinum toxin to partially paralyze detrusor function. This procedure has become increasingly accepted as an effective alternative in the management of low intravesicular pressure. An external collecting device also can be used to ensure dryness. The patient must be trained to adjust fluid intake and the timing of catheterization to meet the flexible needs of community living. The absence of an effective external collecting device for female patients makes it necessary to continue long-term intermittent catheterization in women with paralysis of detrusor function. A waterproof undergarment may be worn between catheterizations to avoid embarrassment. The difficulties of this regimen cause many women to choose constant indwelling catheter drainage despite its drawbacks. Augmentation enterocystoplasty, reservoirs, conduits, and electrical stimulation techniques are less often needed. The VOCARE Bladder System for patients with UMN SCI allows patients to stimulate sacral nerves that have been implanted with electrodes to empty the bladder and bowel.
Table 48.9 Pharmacological Manipulation of Bladder Dysfunction
| Medication | Indication(s) | Mechanism of Action |
|---|---|---|
| Bethanechol, 25 mg bid-50 mg qid | Facilitate emptying | Increase detrusor contraction |
| Prazosin, 1 mg bid-2 mg tid; or tamsulosin, 0.4 mg daily | Decrease outlet obstruction | Alpha blockade of external sphincter to decrease tone |
| Prostatic hypertrophy | ||
| Hyoscyamine, 0.125 mg hs-0.25 mg tid; oxybutynin 2.5 mg hs-5 mg qid | Urge incontinence | Relax detrusor, increase internal sphincter tone, decrease detrusor contractions |
| Tolterodine, 2 mg daily; or imipramine, 25-100 mg hs | Frequency | |
| Enuresis |
bid, Twice daily; hs, at bedtime; qid, four times daily; tid, three times daily.
Central Pain
A major source of disability can be pain from a thalamoparietal stroke or SCI. One of the most common patient complaints a year or more after SCI is burning pain at and below the level of the lesion. Approximately half of these patients gauge their symptoms as moderate or severe. Some patients need only assurance that the pain does not represent a serious complication or a warning signal of another stroke. Others will need the help of the physician in setting goals and identifying interventions to moderate the severity, frequency, and duration of the pain, also taking into consideration the usual time of day for pain onset. Tricyclic and selective serotonin reuptake inhibitor antidepressants, carbamazepine, clonidine, gabapentin, lamotrigine, benzodiazepines, and baclofen are among the drugs that diminish dysesthetic or lancinating pain in some patients. Intrathecal clonidine, baclofen, and morphine can be efficacious when oral approaches fail (see Chapter 44).
Spasticity and Upper Motor Neuron Syndrome
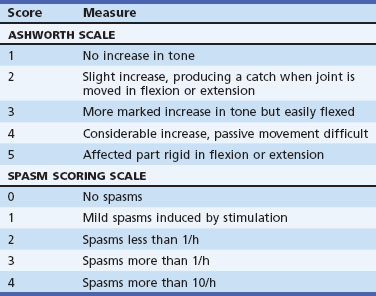
Assessments
For routine assessments and for clinical trials of antispasticity interventions, a number of measures of hypertonicity have been used. The Ashworth Scale (Table 48.10) has had good interrater reliability in studies of stroke, SCI, and MS. The relationship between this score and disability is not so evident, however. Hypertonicity, clonus, and spasms vary in relation to positioning of the limbs, posture, and activities. A clenched, plegic hand may lead to disability if pain or maceration of the palm develops, but a treatment that lowers the Ashworth score is unlikely to improve motor control. Other measurement techniques require instrumentation.
| Score | Measure |
|---|---|
| ASHWORTH SCALE | |
| 1 | No increase in tone |
| 2 | Slight increase, producing a catch when joint is moved in flexion or extension |
| 3 | More marked increase in tone but easily flexed |
| 4 | Considerable increase, passive movement difficult |
| 5 | Affected part rigid in flexion or extension |
| SPASM SCORING SCALE | |
| 0 | No spasms |
| 1 | Mild spasms induced by stimulation |
| 2 | Spasms less than 1/h |
| 3 | Spasms more than 1/h |
| 4 | Spasms more than 10/h |
Physical Modalities
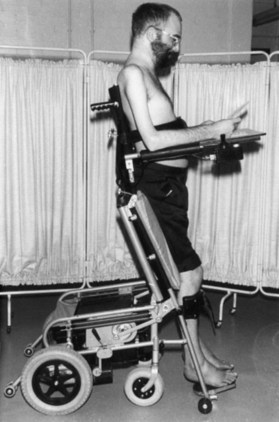
Slow stretching movements and daily passive range-of-motion exercises will reduce motion-sensitive symptoms of spasticity and the risk of contractures. Static stretching with splints probably will not prevent contractures when motor control is absent. Serial casting can reduce stretch reflex activity and contractures as the joint angle gradually increases. Muscle cooling, tendon vibration, pressure exerted over a tight muscle belly, postural adjustments, loading a limb by weight bearing on an extended arm or, for paraplegics, standing in a support frame (Fig. 48.9), and electromyographic BFB can complement a stretching program. Electrical stimulation of motor and sensory nerves, muscles, and dermatomes by a variety of paradigms may reduce tone at the ankle and knee and in the forearm and finger flexors. A single session of stimulation usually decreases resistance and clonus for a few hours.
Pharmacotherapy
Controlled trials of antispasticity agents have varied widely in the target symptoms managed and the outcome assessments used. Functional gains related to locomotion and voluntary use of the upper extremity usually are marginal in any UMN disease. A medication that prevents disabling spasms, however, may improve quality of life. Table 48.11 lists useful first- and second-line drugs.
Table 48.11 Dosages of Medications for Symptomatic Spasticity
| Drug | Dosage |
|---|---|
| FIRST-LINE AGENTS | |
| Diazepam | 2 mg bid-15 mg qid |
| Dantrolene | 25 mg bid-100 mg qid |
| Baclofen | 5 mg bid-40 mg qid |
| Clonidine | 0.05 mg daily-0.2 mg tid |
| Tizanidine | 2 mg bid-8 mg qid |
| Gabapentin | 400 mg tid-900 mg qid |
| OCCASIONALLY USEFUL ADDITIONS | |
| Intrathecal baclofen | 50-150 µg trial dosages; intrathecal infusion with pump |
| l-Dopa or carbidopa | 25 or 100 mg bid, respectively |
| Phenytoin | Serum concentration 10-20 µg/dL |
| Phenobarbital | Serum concentration 10-30 µg/dL |
| Cyproheptadine | 4 mg bid-8 mg qid |
| Chlorpromazine | 10 mg daily-50 mg tid |
| Dronabinol | 2.5 mg daily–tid |
bid, Twice daily; qid, four times daily; tid, three times daily.
After an SCI, about 25% of patients are discharged with an antispasticity agent, and half are still using medication by 1 year. Patients with American Spinal Injury Society (ASIA) grades A and D (see Chapter 50C) are less likely to have been treated than those with grades B and C. Baclofen, tizanidine, and clonidine are especially useful in reducing clonus and extensor spasms caused by a myelopathy. The latter two drugs are α2-agonists that inhibit the excitatory influences of peripheral sensory inputs on motoneurons. Medications with short-lasting effects, such as tizanidine, may be especially useful in limiting spasms during sleep or brief activities like transferring from wheelchair to bed. Baclofen, dantrolene, and the benzodiazepines can cause muscular weakness and difficulty with weight bearing. Children with cerebral palsy and patients with hemiplegic stroke often need their extensor tone to ambulate on a paretic leg. Dantrolene tends to be most useful in managing hypertonicity of the upper extremity after stroke and TBI. Less than 0.5% of users develop hepatotoxicity after several months of intake. l-Dopa may be of additive value to lessen spasms in selected adults after stroke or SCI. All these drugs can also cause sedation, confusion, or hypotension; may add to bowel and bladder dysfunction; and can produce other central and systemic side effects. Great care must be taken in using them in the patient who has neurogenic dysphagia or a pseudobulbar palsy and is at risk for aspiration. Whenever a drug appears to be useful, it is worth tapering the dosage down from time to time so that the patient can help reassess continued benefits.
Chemical Blocks
Botulinum injections have seemed most efficacious for the wrist and finger flexors and plantar flexors of the ankle. Interpretation of the results of clinical trials using botulinum toxin requires attention to how well the outcome measure reflects clinical effectiveness for an important problem. Is a change in ease of passive range of motion, as in the Ashworth Scale, as meaningful as an increase in functional use of the limb? A few trials in children with cerebral palsy and spastic diplegia reveal modest 10% increases in walking speed after injections. The great majority of studies report a 1- or 2-point decrease in the Ashworth Scale score and support this finding by offering a global physician score that in reality probably reflects a perception of a decrease in tone around one or more joints. A randomized trial compared injection of type A toxin into forearm muscles with vehicle injection in patients after stroke who scored 3 or more on the Ashworth Scale for the wrist and 2 or more for the fingers (Brashear et al., 2002). A statistically significant decrease in the Ashworth score occurred at 6 and 12 weeks (e.g., a change from 0.1 in controls to 0.8 in the finger flexors of the treated group). Significant changes also were found in the Disability Assessment Scale, which was said to reflect functional disability. This disability is only a subjective measure of change in hand hygiene, pain, positioning, and dressing that does not require use of the hand. It would be easy to misinterpret the data as revealing better functional use of the hand after botulinum toxin injection, but the real meaning is that with the wrist and finger flexors loosened, the hand was easier to keep open passively, and the arm may then be easier to manage when, for example, the patient tries to put it through a shirt sleeve. Trials of botulinum toxin that are sponsored by the pharmaceutical industry have not included a control intervention that uses rehabilitative passive or active range of motion or treatments for pain that may decrease tone. Also, these studies have not tried to maintain the effect of greater passive ranging by adding physical therapy after an injection to try to prolong any benefit.