Chapter 102 Primary Vitrectomy in Rhegmatogenous Retinal Detachment
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Introduction
Currently, rhegmatogenous retinal detachment (RRD) continues to be an important cause of visual loss. The fundamental principles involved in reattachment of a retina include identification of all retinal breaks, and relief of the vitreous traction. Traditionally, scleral buckling (SB) was viewed as the gold standard treatment for uncomplicated RRD. Pars plana vitrectomy (PPV) was traditionally reserved for treatment of eyes with complications, such as those showing giant retinal tears or exhibiting significant proliferative vitreoretinopathy (PVR). In the 1980s, the indications for PPV in RRD patients were broadened to include less complicated instances, and the term “primary vitrectomy” was introduced by Klöti.1
As the necessary instrumentation for, and safety of, PPV continue to improve with developments in microscope technology, intensified endoillumination, and wide-angle viewing systems, the indications for vitrectomy in RRD have been further expanded to include most patients with RRD. Indeed, PPV is more useful than SB in eyes requiring simultaneous cataract extraction or those of pseudophakic status.2–8
Pathogenesis of Rhegmatogenous Retinal Detachment
Liquefaction of the vitreous occurs naturally upon aging, developing more rapidly in eyes with significant myopia, surgical or non-surgical trauma, and/or intraocular inflammation. Apart from liquefaction, alterations in the extracellular matrix of the vitreous facilitate detachment of the posterior vitreous from the underlying retina. PVD usually presents as an acute event, and is more prevalent in patients older than 50 years, with frequencies as high as 53%.9 PVD often precipitates RRD because the tractional forces necessary to generate retinal breaks are produced upon development of PVD, although not all retinal breaks progress to detachment. The reported incidence of retinal tears in patients with acute symptomatic PVD varies from 8–46%.10 In a study in a general population,11 18% of eyes with retinal breaks developed retinal detachment. Risk factors for progression included fresh, symptomatic, horseshoe-shaped tears; breaks suggestive of the presence of subclinical retinal detachment (RD); and pseudophakia/aphakia.
Categories of Rhegmatogenous Retinal Detachment
The number of aphakic/pseudophakic patients with RRD has increased significantly over the past decades. After cataract surgery, vitreous liquefaction accelerates, causing premature PVD and subsequent RD. Aphakic/pseudophakic eyes tend to have small (and sometimes multiple) anterior breaks. The status of the posterior capsule also influences the speed of vitreous liquefaction. One study found that Nd:YAG laser posterior capsulotomy increased the risk of RD after cataract extraction up to 4.9-fold.12 Because of the characteristics of the breaks and the status of the vitreous in aphakic/pseudophakic eyes, vitrectomy is preferred by many surgeons and has a good success rate.13
Patient selection for primary vitrectomy
SB and/or pneumatic retinopexy serve as the first treatment option(s) in patients with localized detachment in one quadrant together with single neighboring breaks. Young age and anteriorly located small holes in phakic patients encourage the use of SB. The success rates of final reattachment are 90–95%.14
Recent developments in vitrectomy instruments, including small-gauge systems, wide-angle viewing systems, and endoilluminators, as well as adjuvants, including triamcinolone acetonide suspension, perfluorocarbon liquids, and intraocular tamponades, led the choice of surgical technique for the treatment of RRD with medium-complexity shift more and more towards PPV.15–17 During the last 5 years, several surgeons have reported that primary vitrectomy is the method of choice in 40–80% of all patients with RRD.17,18
Several trials comparing PPV to SB in patients undergoing RRD surgery have been reported.19–23 In general, PPV was favored for treatment of pseudophakic eyes with unseen breaks, eyes featuring ocular hypotony, or eyes showing prolonged macular detachment. One large-scale prospective multicenter randomized trial, scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment study (the SPR Study), also supported the superiority of PPV in the pseudophakic/aphakic eyes, in terms of a primary success rate and a rate of retina-affecting secondary procedures. This study, however, showed no differences in phakic eyes.19
Surgical techniques
Primary vitrectomy is commonly performed using a wide-angle viewing system attached to an operating microscope. The surgical steps of primary vitrectomy using sutureless MIVS are described below (Figs 102.1–102.4).
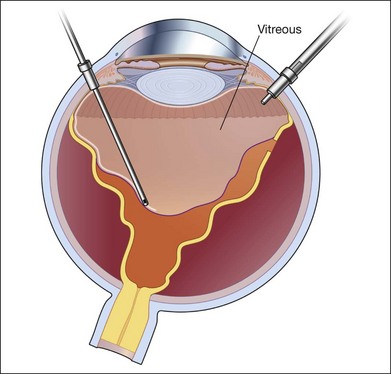
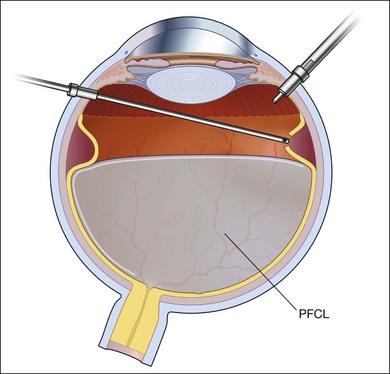
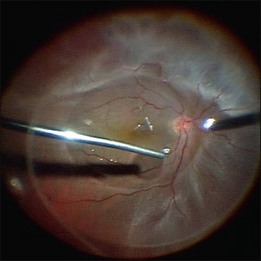
Fig. 102.1 Detached retina with posterior vitreous detachment is shown. Core vitrectomy is performed.
Core vitrectomy
First, the central vitreous is removed (Fig. 102.1). When PVD is absent, or even when PVD has occurred, triamcinolone acetonide can be injected to afford better visualization of the vitreous gel, to facilitate PVD, and to completely remove the residual vitreous. The posterior vitreous membrane can be removed using a diamond-dusted scraper; this prevents secondary macular pucker (Fig. 102.5).
Peripheral vitrectomy
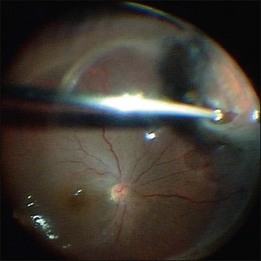
After core vitrectomy, PVD is further extended using a wide-angle viewing system. Usually, PVD is already present, up to the positions of the retinal breaks. However, abnormal attachment may be noted in other locations. If retinal breaks are located posterior to the equatorial zone, the vitreous bridge between the tears and the ora serrata must be separated and removed. If the breaks are peripheral to the equatorial zone, the vitreous on the flap should be shaved away, with scleral indentation. In case of bullous RD, perfluorocarbon liquid (PFCL) may be injected to flatten the detached retina, so that the peripheral vitreous can be safely shaved without creating an iatrogenic tear (Fig. 102.2).
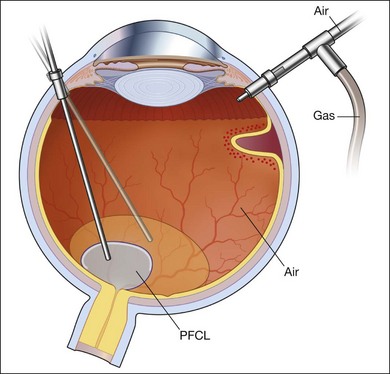
Fluid–air exchange
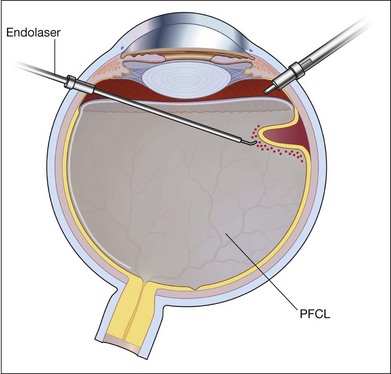
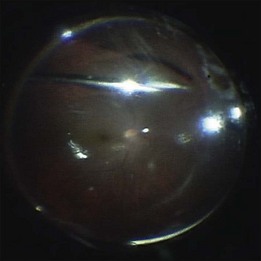
Subretinal fluid is removed through the original breaks using a backflush needle in the presence of air irrigation. PFCL may be injected, up to the level of the posterior edge of the break; the PFCL bubble displaces posterior subretinal fluid through the break. Next, air–fluid exchange at the top of the PFCL bubble may displace peripheral subretinal fluid through the break. PFCL is not indicated for all eyes. The preferable indications are bullous RD or RD that is associated with retinal breaks anterior to the equatorial zone; the subretinal fluid can thus be removed without creating a drainage retinotomy (Fig. 102.4).
Photocoagulation/cryopexy of the retinal tear
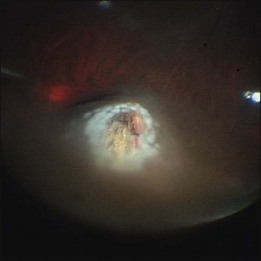
Whenever retinal breaks are found, endodiathermy may be used to mark the breaks. Once retinal reattachment is complete, endophotocoagulation is performed under air or a PFCL bubble. Two-to-three rows of laser burns can be applied, around each break and site of lattice degeneration (Fig. 102.3). Strong photocoagulation should be avoided on the thin retina detached by the residual subretinal fluid. Alternatively, cryopexy may be utilized if a break is located at the far periphery of phakic RD eyes.
Sutureless microincision vitrectomy surgery
Transconjunctival sutureless MIVS using 25-gauge (25G) instrumentation provides several advantages over conventional 20G surgery, including a shorter surgical time, less surgically induced inflammation, and a reduced risk of postoperative corneal astigmatism. These factors ultimately lead to improved patient comfort and faster visual recovery. The 2009 Preferences and Trends (PAT) surveys conducted by the American Society of Retinal Specialists reported that nearly 80% of respondents commonly employ small-gauge MIVS systems.24
The original MIVS procedure, introduced in 2002,25 had some limitations. The excessive flexibility of the first 25G instruments rendered it difficult for surgeons to adequately dissect the peripheral vitreous, to shave the vitreous base, and to rotate the eye. These limitations were particularly troublesome when operating on eyes with RRD. In addition, postoperative leakage of gas or silicone oil from the sclerotomies in the subconjunctival space was a further concern.
The introduction of 23G instruments solved several of these problems. The greater rigidity of such instruments, better illumination, and improved fluidics have facilitated manipulation of the peripheral retina, which is important when RRD is to be treated. MIVS technology continues to develop. Newly introduced 25G instruments, the 25G+ system, are substantially more rigid than their earlier counterparts. Further, an increase in instrument inner diameter has improved both infusion flow rates and illumination. In addition, a new trocar entry system and continued development of instrumentation have enhanced the performance of both 23- and 25G systems. Figures 102.6 to 102.9 show a surgical view of transscleral sutureless vitrectomy using the 25G+ system and a wide-angle viewing system.
The most recent vitrectomy system features a pneumatic dual-drive cutter with an ultrahigh cut rate of 5000 cpm that effectively reduces vitreoretinal traction.26,27 In this instrument, the port is also 50% closer to the tip than is the case with conventional cutters, and the inner lumen diameter is enlarged. Therefore, surgeons may work remarkably close to a detached retina, with minimal movement of mobile tissue. Other features include IOP compensation via direct control of infusion pressure, and direct control of the duty cycle (this controls the proportion of time during which the port is open, in terms of the total time of the cutting cycle). These features afford excellent control of flow rate with a constant cut rate, thus increasing precision without sacrificing speed.
A new scleral entry system has been introduced to create a smaller and tighter wound. This system uses an MVR-style blade that features a trailing edge, so that the cannula can be inserted via a linear incision. Such incisions are associated with a need for less force during trocar insertion, and retain shape well, resulting in less postoperative leakage than is the case when chevron incisions are made.26,27
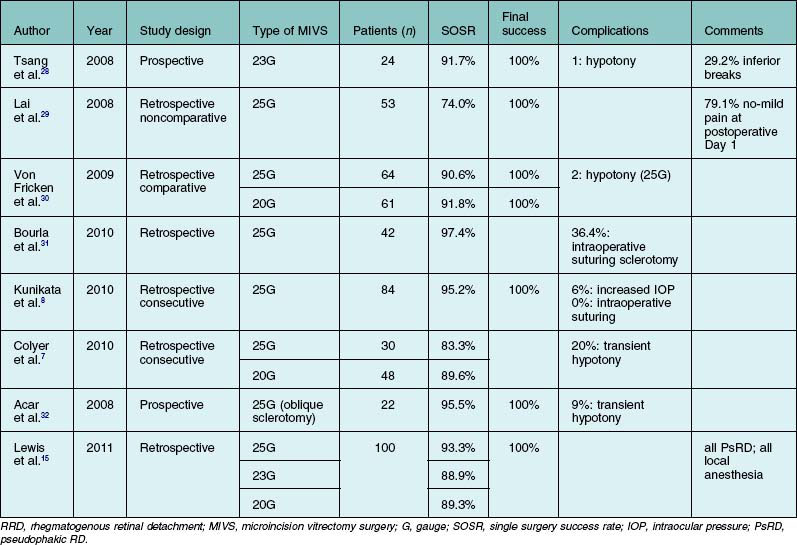
Several groups have reported good surgical outcomes when RRD was treated with transconjunctival MIVS, either the 25- or 23G systems (Table 102.1).7,8,15,28–32 Although a few reports found that such MIVS afforded a lower single-operation success rate (SOSR) than did 20G vitrectomy7,29 most authors attained success rates (91.7–97.4%) similar to that of 20G surgery.8,28,30,31 The major reported advantages of MIVS include no or mild pain on the first postoperative day in the majority of patients,29 a shorter operation time,8 and faster postoperative visual recovery.33 Although some instances of transient hypotony have been reported following MIVS, no patient experienced significant postoperative leakage or endophthalmitis.7,8,15,28–33
Surgical outcomes
After the occurrence of any type of RD, approximately 40% of patients will not achieve reading ability, 10–40% will need more than one surgical procedure, and approximately 5% will suffer permanent anatomical and functional failure. The reported primary success rates for RRD repair by PPV range from 64–94%.3–5,17,19,22
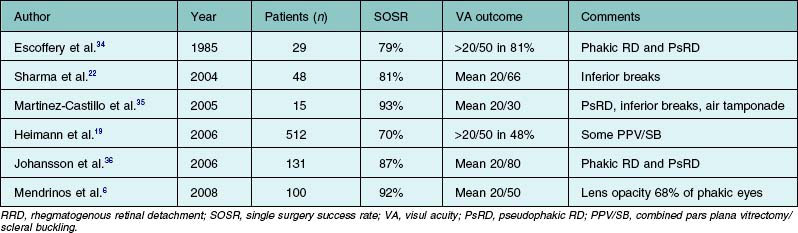
In the time since the first report of the use of PPV without concomitant SB to treat RD appeared in 1985,34 numerous case series have been reported (Table 102.2).6,19,22,34–36 Although primary vitrectomy had historically been indicated principally for treatment of pseudophakic RD with superior retinal breaks, several studies found that the technique was also useful in phakic RD patients and in those with inferior breaks.22,35 In general, the SOSR and the improvement in visual acuity appear to be comparable to those achieved using SB, in a wide variety of patients. The poorest outcomes were reported in series containing patients with chronic detachment and evidence of PVR. Exploiting the recent advances in surgical technology including MIVS, several recent case series reported that the SOSR improved to over 90% and that the final average visual acuity was 20/40 or better.
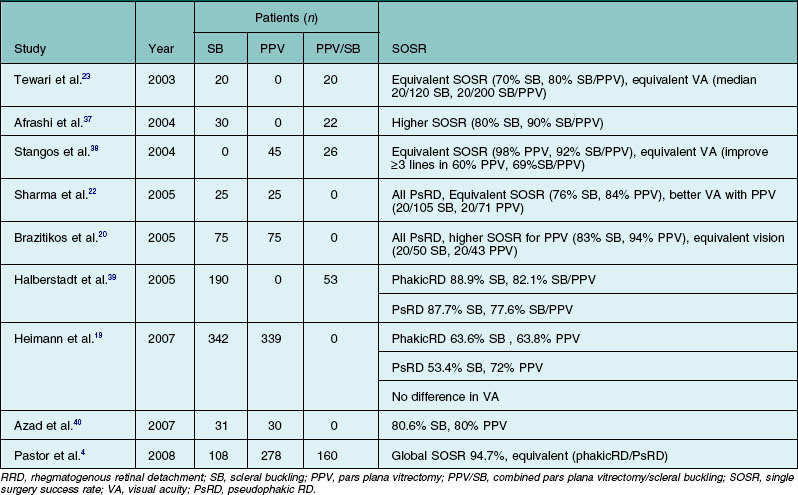
Several retrospective series and prospective clinical trials comparing SB, PPV, and/or combined SB/PPV, found no statistically significant difference in SOSR among the various procedures (Table 102.3).4,19,20,22,23,37–40 However, a few studies reported that PPV was superior in terms of either anatomic or visual outcome in comparison with SB alone.19,20,22,40 In addition, several reports comparing PPV and combined PPV/SB also found that the use of a combined procedure did not significantly influence anatomical or visual outcomes.17,38,39
A meta-analysis of 29 studies on patients with pseudophakic RD found that both PPV and combined PPV/SB were associated with a higher SOSR and better visual acuity outcomes than was SB alone41; another review of nine reports comparing PPV to SB found no statistically significant difference with respect to SOSR or visual outcomes.42
Between 1998 and 2003, a prospective, multicenter randomized trial of SB versus PPV to treat RRD was conducted in Europe, and the data were reported in 2007.19 This is the first prospective large-scale randomized multicenter clinical trial with good numbers of centers, surgeons, and patients. Patients were classified on the basis of lens status (pseudophakia/aphakia or phakia), and the group results were separately analyzed. In the pseudophakic/aphakic group, patients treated with PPV showed a significantly greater SOSR and a lower rate of reoperation than the SB group. In the phakic trial, no difference was seen in terms of anatomical success or postoperative PVR between the two groups, although a greater rate of cataract progression was evident in the PPV group.
Although the data are not entirely consistent, most surgeons agree that PPV without buckling effectively treats pseudophakic RD and results in better long-term visual and anatomical outcomes than does conventional SB.6 The rationale for use of primary PPV alone to treat pseudophakic RD patients is that visualization of the peripheral retina is good, the causative retinal breaks can be properly identified and treated, buckling-related complications are absent, and the risk of PPV-induced cataracts is absent. When it is considered that the need for re-operation is a (negative) indicator of the quality and efficacy of surgical techniques, the SPR study showed that the risk of such surgery was significantly reduced when PPV was used, compared to SB, in pseudophakic eyes, but increased after use of PPV compared with SB in phakic eyes. The results also showed that the prognostic factors differed between the pseudophakic and phakic subgroups.43
Prognostic factors
Identification of patients at high risk of failure at the time of initial presentation would allow surgeons to individually tailor patient management. Reported risk factors for surgical failure after PPV in the repair of RRD include duration of symptoms, older age, the extent of RD, macular detachment, involvement of inferior quadrants, absence of detectable retinal breaks, high myopia, hypotony, and PVR-related risk factors such as pseudophakia/aphakia, uveitis, vitreous hemorrhage, and preoperative PVR.6,17,22,41–45
The extent of RD can be considered to serve as a surrogate marker of RD duration, although the location of retinal breaks may affect the speed of detachment after onset. Nevertheless, such patients may have a higher level of retinal glial upregulation and RPE cell dispersion, and may also develop PVR, which is an important risk factor in terms of surgical failure.46
A number of studies have found that previous lens extraction was a risk factor for development of PVR and was associated with worse outcomes.47,48 The association between pseudophakia/aphakia and surgical failure may be related to the observations that (1) pseudophakic/aphakic patients often have small, sometimes multiple anterior breaks which may be missed at the time or surgery; or (2) such patients tend to develop new retinal breaks after vitrectomy, caused by residual vitreous base traction. Many features of pseudophakic eyes, including anterior or posterior capsular fibrosis, the presence of cortical remnants, poor pupillary dilatation, vitreous opacity, and optic aberrations secondary to IOL placement per se, may seriously compromise the surgical view, resulting in poor outcomes.
Macular detachment and subsequent structural change detected by OCT appear to cumulatively reduce final visual acuity.45 An experimental study showed that rapid retinal reattachment reversed the retinal degeneration associated with detachment.49 As complete intraoperative attachment of the macula is achieved in most patients during vitrectomy, visual recovery after surgery is faster. Delayed or incomplete visual recovery after SB for macula-off RD may be associated with macular subretinal fluid persistence, which can also be assessed using OCT.50,51
Inferior breaks have also been reported to be associated with surgical failure following PPV for RRD.3,22,35 The functional study of the SPR group showed that inferior detachment with breaks below the 4 and 8 o’clock positions was a significant risk factor in pseudophakic/aphakic subtrial.44 However, other studies have failed to show any advantage of combined PPV with SB, compared with PPV alone, in treatment of inferior retinal breaks.45
Complications
The most frequent intraoperative complications are relatively high rates of iatrogenic retinal breakage (0.78–24%),6,20,22,23,37,38,40 and crystalline lens damage (0.03–9%).21,37,52 Several less serious complications include retinal incarceration at retinotomy sites (0.6–2.9%), corneal abrasion (0.6%), scleral rupture (0.2%), and choroidal effusion (0.5%), as reported in one national audit.53
Several complications may be encountered after primary vitrectomy in RRD patients. Transient or persistent intraocular pressure increases have been reported in 15–24% of patients.6,36–38,40,53 Phakic patients are at significant risk of developing nuclear cataracts; this is especially true of patients older than 50 years (21–86%).19,37,40,52–54 Other anterior segment problems, occurring less frequently, include hemorrhage, subluxation or capture of intraocular lenses, posterior capsular opacification, and synechia.3,6,19,31,36–38,40,52,53
Major postoperative complications include retinal redetachment, with or without PVR, usually by new or missed breaks, or reopening of former breaks. As discussed above in terms of surgical outcomes, recent advances in surgical technology, including the development of MIVS and wide-angle viewing systems, have reduced the frequency of anatomic failures. Apart from recurrent detachment, several posterior segment complications may occur; these include cystic macular edema, macular pucker, and macular hole.3,6,37,53–56 Although very rare, endophthalmitis has been reported,53 as has sympathetic ophthalmia. The incubation period for the latter condition after vitrectomy varies from 4 weeks to 2 years.57–59
Recently, as MIVS has become more popular, the risks of postoperative hypotony and endophthalmitis have become of concern. However, recent case series have found that endophthalmitis did not occur more frequently than after conventional vitrectomy, and postoperative hypotony following MIVS was transient and resolved spontaneously in most patients.7,8,22,23,28,30–32
1 Klöti R. Amotio-Chirurgie ohne Skleraeindellung. Primäre Vitrektomie. Klin Monatsbl Augenheilkd. 1983;182:474–478.
2 Sodhi A, Leung L-S, Do DV, et al. Recent Trends in the management of rhegmatogenous retinal detachment. Surv Ophthalmol. 2008;53:50–67.
3 Heimann H, Zou X, Jandeck C, et al. Primary vitrectomy for rhegmatogenous retinal detachment: an analysis of 512 cases. Graefes Arch Clin Exp Ophthalmol. 2006;244:69–78.
4 Pastor JC, Fernandez I, Rodriguez de la RE, et al. Surgical outcomes for primary rhegmatogenous retinal detachments in phakic and pseudophakic patients: the Retina 1 Project – report 2. Br J Ophthalmol. 2008;92:378–382.
5 Schwartz SG, Flynn HW. Pars plana vitrectomy for primary rhegmatogenous retinal detachment. Clin Ophthalmol. 2008;2:57–63.
6 Mendrinos E, Dang-Burgener NP, Stangos AN, et al. Primary vitrectomy without scleral buckling for pseudophakic rhegmatogenous retinal detachment. Am J Ophthalmol. 2008;145:1063–1070.
7 Colyer MH, Barazi MK, von Fricken MA. Retrospective comparison of 25-gauge transconjunctival sutureless vitrectomy to 20-gauge vitrectomy for the repair of pseudophakic primary inferior rhegmatogenous retinal detachment. Retina. 2010;30:1678–1684.
8 Kunikata H, Nishida K. Visual outcome and complications of 25-gauge vitrectomy for rhegmatogenous retinal detachment; 84 consecutive cases. Eye (Lond). 2010;24:1071–1077.
9 Pischel DK. Detachment of the vitreous as seen by slitlamp examination; with notes on the technique of slitlamp microscopy of the vitreous cavity. Am J Ophthalmol. 1953;36:1497–1507.
10 Boldrey EE. Risk of retinal tears in patients with vitreous floaters. Am J Ophthalmol. 1983;96:783–787.
11 Davis MD. Natural history of retinal breaks without detachment. Arch Ophthalmol. 1974;92:183–194.
12 Sheu SJ, Ger LP, Ho WL. Late increased risk of retinal detachment after cataract extraction. Am J Ophthalmol. 2010;149:113–119.
13 Minihan M, Tanner V, Williamson TH. Primary rhegmatogenous retinal detachment: 20 years of change. Br J Ophthalmol. 2001;85:546–548.
14 Ah-Fat FG, Sharma MC, Majid MA, et al. Trends in vitreoretinal surgery at a tertiary referral centre: 1987 to 1996. Br J Ophthalmol. 1999;83:396–398.
15 Lewis SA, Miller DM, Riemann CD, et al. Comparison of 20-, 23-, and 25-gauge pars plana vitrectomy in pseudophakic rhegmatogenous retinal detachment repair. Ophthalmic Surg Lasers Imaging. 2011;42:107–113.
16 Albrieux M, Rouberol F, Bernheim D, et al. Comparative study of 23-gauge vitrectomy versus 20- gauge vitrectomy for the treatment of rhegmatogenous retinal detachment. Grafes Arch Clin Exp Ophthalmol. 2011;249:1459–1468.
17 Falkner-Radler CI, Myung JS, Moussa S, et al. Trends in primary retinal detachment surgery: Results of a Bicenter Study. Retina. 2011;31:928–936.
18 Uemura A. Indications of primary pars plana vitrectomy for rhegmatogenous retinal detachments. Jap J Ophthalmic Surg. 2010;23:573–576.
19 Heimann H, Bartz-Schmidt KU, Bornfeld N, et al. Scleral Buckling versus Primary Vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114:2142–2154.
20 Brazitikos PD, Androudi S, Christen WG, et al. Primary pars plana vitrectomy versus scleral buckle surgery for the treatment of pseudophakic retinal detachment: a randomized clinical trial. Retina. 2005;25:957–964.
21 Ahmadieh H, Moradian S, Faghihi H, et al. Anatomic and visual outcomes of scleral buckling versus primary vitrectomy in pseudophakic and aphakic retinal detachment. Six-month follow-up results of a single operation – report no. 1. Ophthalmology. 2005;112:1421–1429.
22 Sharma YR, Karunanithi S, Azad RV, et al. Functional and anatomic outcome of scleral buckling versus primary vitrectomy in pseudophakic retinal detachment. Acta Ophthalmol Scand. 2005;83:293–297.
23 Tewari HK, Kedar S, Kumar A, et al. Comparison of sclera buckling with combined scleral buckling and pars plana vitrectomy in the management of rhegmatogenous retinal detachment with unseen retinal breaks. Clin Experiment Ophthalmol. 2003;31:403–407.
24 American Society of Retina Specialists. Annual Preferences and Trends Survey. http://www.asrs.org, 2009. Online. Available at access for members only
25 Fujii GY, De Juan E, Jr., Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109:1807–1812.
26 Hariprasad SM. Microincision vitrectomy surgery for the repair of retinal detachment. Retinal Physician. 2009;Nov:103654.
27 Rizzo S, Genovesi-Ebert F. Comparative study of the standard 25-g vitrectomy system vs the new ultra-high-speed vitrectomy system. Insert to Retina Today. 2010;Sept:9.
28 Tsang CW, Cheung BT, Lam RF, et al. Primary 23-gauge transconjunctival sutureless vitrectomy for rhegmatogenous retinal detachment. Retina. 2008;28:1075–1081.
29 Lai MM, Ruby AJ, Sarrafizadeh R, et al. Repair of primary rhegmatogenous retinal detachment using 25-gauge transconjunctival sutureless vitrectomy. Retina. 2008;28:729–734.
30 Von Fricken MA, Kunjukunju N, Weber C, et al. 25-Gauge sutureless vitrectomy versus 20-gauge vitrectomy for the repair of primary rhegmatogenous retinal detachment. Retina. 2009;29:444–450.
31 Bourla DH, Bor E, Axer-Siegel R, et al. Outcomes and complications of rhegmatogenous retinal detachment repair with selective sutureless 25-gauge pars plana vitrectomy. Am J Ophthalmol. 2010;149:630–634.
32 Acar N, Kapran Z, Altan T, et al. Primary 25-gauge sutureless vitrectomy with oblique sclerotomies in pseudophakic retinal detachment. Retina. 2008;28:1068–1074.
33 Nam Y, Chung H, Lee JY, et al. Comparison of 25- and 23-gauge sutureless microincision vitrectomy surgery in the treatment of various vitreoretinal diseases. Eye. 2010;24:869–874.
34 Escoffery RF, Olk RJ, Grand MG, et al. Vitrectomy without scleral buckling for primary rhegmatogenous retinal detachment. Am J Ophthalmol. 1985;99:275–281.
35 Martinez-Castillo V, Verdugo A, Boixadera A, et al. Management of inferior breaks in pseudophakic rhegmatogenous retinal detachment with pars plana vitrectomy and air. Arch Ophthalmol. 2005;123:1078–1081.
36 Johansson K, Malmsjo M, Ghosh F. Tailored vitrectomy and laser photocoagulation without scleral buckling for all primary rhegmatogenous retinal detachments. Br J Ophthalmol. 2006;90:1286–1291.
37 Afrashi F, Erakgun T, Akkin C, et al. Conventional buckling surgery or primary vitrectomy with silicone oil tamponade in rhegmatogenous retinal detachment with multiple breaks. Graefes Arch Clin Exp Ophthalmol. 2004;242:295–300.
38 Stangos AN, Petropoulos IK, Brozou CG, et al. Pars-plana vitrectomy alone vs vitrectomy with scleral buckling for primary rhegmatogenous pseudophakic retinal detachment. Am J Ophthalmol. 2004;138:952–958.
39 Halberstadt M, Chatterjee-Sanz N, Brandenberg L, et al. Primary retinal reattachment surgery: anatomical and functional outcome in phakic and pseudophakic eyes. Eye (Lond). 2005;19:891–898.
40 Azad RV, Chanana B, Sharma YR, et al. Primary vitrectomy versus conventional retinal detachment surgery in phakic rhegmatogenous retinal detachment. Acta Ophthalmol Scand. 2007;85:540–545.
41 Arya AV, Emerson JW, Engelbert M, et al. Surgical management of pseudophakic retinal detachments: a meta-analysis. Ophthalmology. 2006;113:1724–1733.
42 Saw SM, Gazzard G, Wagle AM, et al. An evidence-based analysis of surgical interventions for uncomplicated rhegmatogenous retinal detachment. Acta Ophthalmol Scand. 2006;84:606–612.
43 Heussen N, Hilgers RD, Heimann H, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment study (SPR Study): Multiple-event analysis of risk factors for reoperations. SPR Study report no. 4. Acta Ophthalmol. 2009;89:622–628.
44 Heussen N, Feltgen N, Walter P, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment study (SPR Study): predictive factors for functional outcome. Study report no. 6. Graefes Arch Clin Exp Ophthalmol. 2011;249:1129–1136.
45 Wickham L, Ho-Yen GO, Bunce C, et al. Surgical failure following primary retinal detachment surgery by vitrectomy: risk factors and functional outcomes. Br J Ophthalmol. 2010;95:1234–1238.
46 Sethi CS, Lewis GP, Fisher SK, et al. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–342.
47 Halberstadt M, Brandenburg L, Sans N, et al. Analysis of risk factors for the outcome of primary retinal reattachment surgery in phakic and pseudophakic eyes. Klin Monbl Augenheilkd. 2003;220:116–121.
48 Kon CH, Asaria RH, Occleston NL, et al. Risk factors for proliferative vitreoretinopathy after primary vitrectomy: a prospective study. Br J Ophthalmol. 2000;84:506–511.
49 Lewis GP, Charteris DG, Sethi CS, et al. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43:2412–2420.
50 Cavallini GM, Masini C, Volante V, et al. Visual recovery after scleral buckling for macula-off retinal detachments: an optical coherence tomography study. Eur J Ophthalmol. 2007;17:790–796.
51 Wolfensberger TJ. Foveal reattachment after macula-off retinal detachment occurs faster after vitrectomy than after buckle surgery. Ophthalmology. 2004;111:1340–1343.
52 Day S, Grossman DS, Mruthyunjaya P, et al. One-year outcomes after retinal detachment surgery among medicare beneficiaries. Am J Ophthalmol. 2010;150:338–345.
53 Thompson JA, Snead MP, Billington BM, et al. National audit of the outcome of primary surgery for rhegmatogenous retinal detachment. II. Clinical outcomes. Eye (Lond). 2002;16:771–777.
54 Melberg NS, Thomas MA. Nuclear sclerotic cataract after vitrectomy in patients younger than 50 years of age. Ophthalmology. 1995;102:1466–1471.
55 Kumagai K, Ogino N, Furukawa M, et al. Surgical outcomes for patients who develop macular holes after pars plana vitrectomy. Am J Ophthalmol. 2008;145:1077–1080.
56 Benzerroug M, Genevois O, Siahmed K, et al. Results of surgery on macular holes that develop after rhegmatogenous retinal detachment. Br J Ophthalmol. 2008;92:217–219.
57 Abu El-Asrar AM, Al Kuraya H, Al-Ghamdi A. Sympathetic ophthalmia after successful retinal reattachment surgery with vitrectomy. Eur J Ophthalmol. 2006;16:891–894.
58 Ozbek Z, Arikan G, Yaman A, et al. Sympathetic ophthalmia following vitreoretinal surgery. Int Ophthalmol. 2010;30:221–227.
59 Haruta M, Mukuno H, Nishijima K, et al. Sympathetic ophthalmia after 23-gauge transconjunctival sutureless vitrectomy. Clin Ophthalmol. 2010;4:1347–1349.