Primary CNS lymphomas
CNS lymphomas may be primary or secondary:
 Primary CNS lymphomas (PCNSLs) are extra-nodal lymphomas that are confined to the brain or spinal cord at presentation. Most involve the cerebrum, but approximately 10% present in the cerebellum, and a few originate in the brain stem or spinal cord. Over 80% of PCNSLs are diffuse large B cell lymphomas, but differ from systemic diffuse large B cell lymphomas in their behavior, optimum management, and prognosis.
Primary CNS lymphomas (PCNSLs) are extra-nodal lymphomas that are confined to the brain or spinal cord at presentation. Most involve the cerebrum, but approximately 10% present in the cerebellum, and a few originate in the brain stem or spinal cord. Over 80% of PCNSLs are diffuse large B cell lymphomas, but differ from systemic diffuse large B cell lymphomas in their behavior, optimum management, and prognosis.
 Secondary CNS lymphomas spread to the brain or spinal cord from a primary site outside the nervous system. They are commoner than PCNSLs as 5–10% of systemic lymphomas involve the CNS, often occupying the subarachnoid space. Leukemias share this predilection for the meninges.
Secondary CNS lymphomas spread to the brain or spinal cord from a primary site outside the nervous system. They are commoner than PCNSLs as 5–10% of systemic lymphomas involve the CNS, often occupying the subarachnoid space. Leukemias share this predilection for the meninges.
MACROSCOPIC APPEARANCES
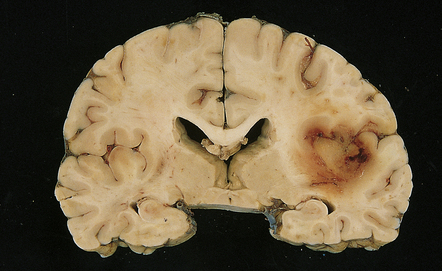
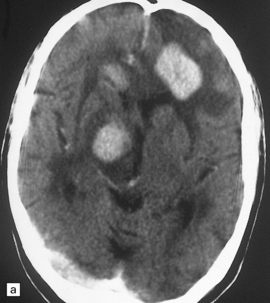
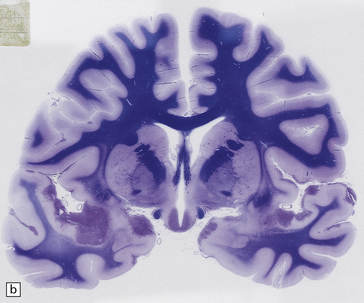
PCNSL in the post-mortem brain is often multifocal. The lesions appear quite well demarcated despite microscopic infiltration of surrounding tissue (Figs 41.1, 41.2). The affected brain tissue is softened, cream or brown in color, and may be focally hemorrhagic. Infiltrated meninges appear thick, and subependymal intraventricular spread may manifest as areas of irregularity and softening.
MICROSCOPIC APPEARANCES
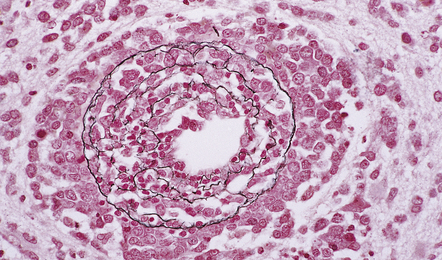
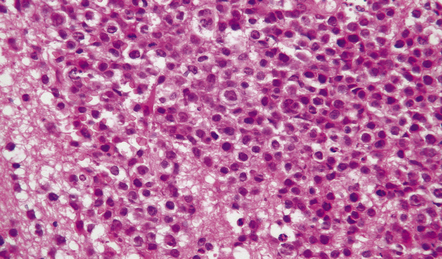
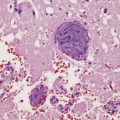
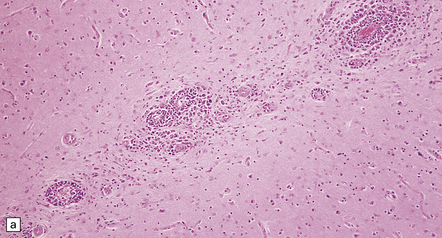
Many PCNSLs are characterized by sheets of lymphoma cells separated by areas of necrosis. The cells tend to invade the walls of small cerebral blood vessels and accumulate in perivascular spaces (Fig. 41.3). This produces a characteristic lacy pattern of reduplicated perivascular reticulin (Fig. 41.4), which is evident even in necrotic tissue. Small-cell PCNSLs tend to be more infiltrative and less necrotic than large-cell PCNSLs. At its infiltrative edge, PCNSL diffusely invades CNS tissue, mimicking a glioma (Fig. 41.5). Like some neuroepithelial neoplasms, PCNSL also spreads through the subarachnoid space and invades the pial surface of the brain. Meningeal and intraventricular spread can be extensive (Fig. 41.6, see also Fig. 41.2b).
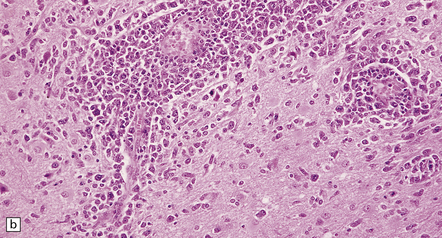
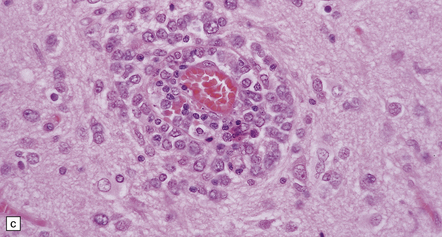
41.3 PCNSL.
(a) Perivascular neoplastic cells in the cerebal cortex at the edge of a diffuse large B cell lymphoma. (b) Large neoplastic cells spread from the perivascular spaces into the parenchyma. (c) Small reactive lymphocytes mixed with larger neoplastic cells around a cerebral blood vessel.
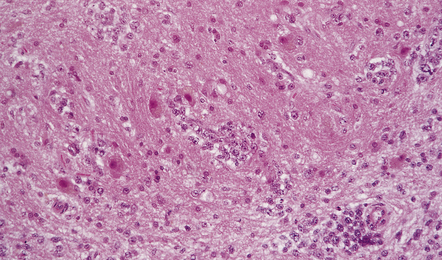
41.5 Diffuse large B cell lymphoma of the cerebellum.
Neoplastic cells have infiltrated the dentate nucleus.
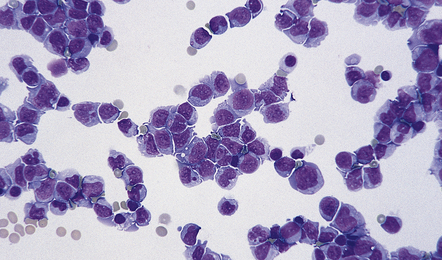
41.6 CSF from a patient with PCNSL.
Meningeal involvement with invasion of the subarachnoid space is common in PCNSL.
Diffuse large B cell lymphomas (DLBCLs) account for more than 95% of PCNSLs. All morphological variants of DLBCL, including centroblastic, immunoblastic, and anaplastic, occur in the CNS. They contain cells somewhat resembling centrocytes, centroblasts or immunoblasts, but their histology is not identical to that of nodal diffuse large B cell lymphomas. Neoplastic cells are admixed with smaller cells, including reactive T cells (Fig. 41.7), and there are many mitotic figures and apoptotic bodies.
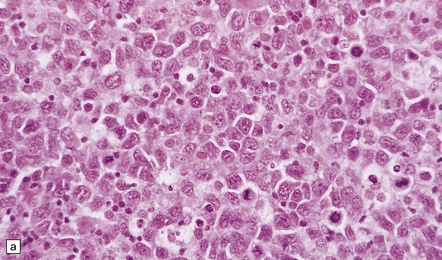
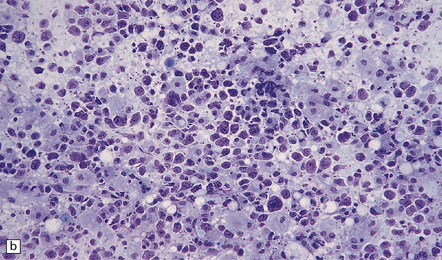
41.7 Diffuse large B cell PCNSL.
(a) Polymorphous cells are seen, some of which resemble centroblasts and centrocytes. (b) Intraoperative smear preparation of a biopsy of PCNSL from a patient with AIDS and multiple cerebral masses.
Fewer than 5% of PCNSLs are low grade B cell lymphomas with lymphocytic or lymphoplasmacytoid cytology. Cells in these neoplasms are more monomorphic than those in their high grade counterparts (Fig. 41.8). The low grade marginal zone B cell (or MALT) lymphoma characteristically presents as a meningeal mass (Fig. 41.9).
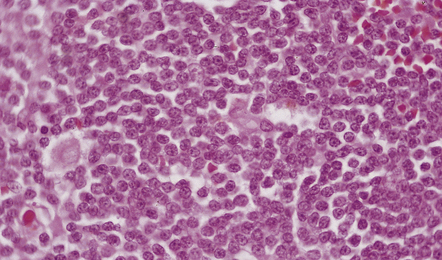
41.8 Lymphoplasmacytoid PCNSL.
Cells in this CNS lymphoma are smaller than those in other B cell PCNSLs, and some cells show plasmacytic differentiation.
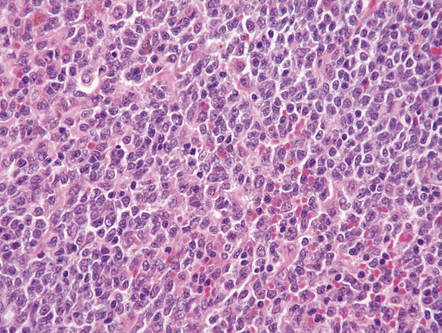
41.9 Meningeal marginal zone B cell lymphoma.
This CNS lymphoma is characterized by a relatively monomorphic population of cells resembling centrocytes.
T cell PCNSLs are very rare, and comprise monomorphic and pleomorphic neoplasms. They often have a subcortical location and vasculitic features (Figs 41.10, 41.11).
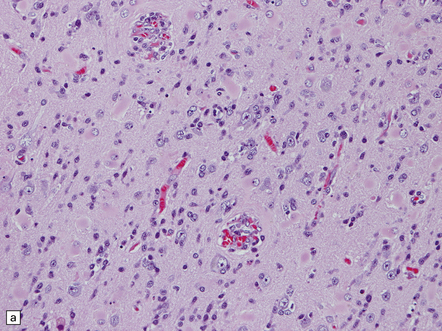
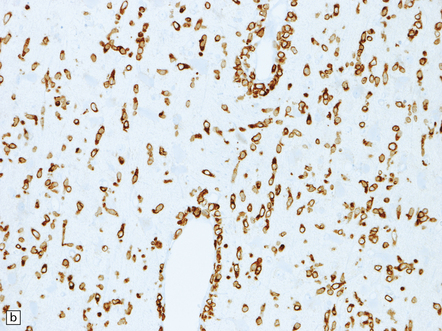
41.10 T cell PCNSL (peripheral T cell lymphoma).
(a) Neoplastic cells invade the cerebral cortex, causing reactive astrocytosis, and are immunolabeled with an anti-CD3 antibody (b).
The neoplastic cells of the rare CNS angiotropic lymphoma (previously termed malignant angioendotheliomatosis), which is usually a large B cell lymphoma, fill the lumina of small cerebral vessels and invade the surrounding parenchyma in only a few places (Fig. 41.12). These neoplasms tend to cause vascular occlusion and hemorrhagic infarcts. Most are systemic lymphomas that preferentially affect the CNS and the skin.
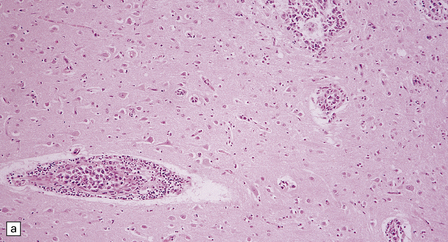
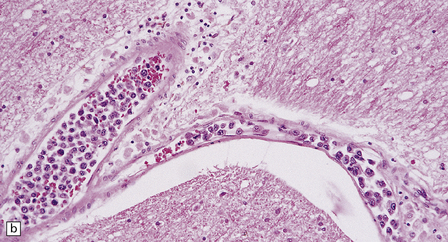
41.12 Angiotropic large B cell lymphoma.
(a,b) Intravascular and perivascular neoplastic lymphoid cells are evident, as are smaller reactive T cells.
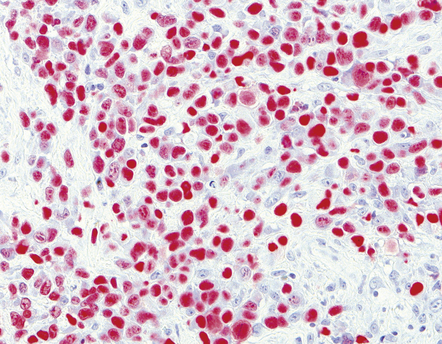
Immunohistochemistry with a panel of antibodies allows characterization of PCNSLs (Fig. 41.13). PCNSL may be distinguished from gliomas and metastatic small-cell carcinomas by immunolabeling of neoplastic cells with an antibody to CD45 (leukocyte common antigen). B cell lymphomas can be differentiated from T cell lymphomas by antibodies to the following antigens:
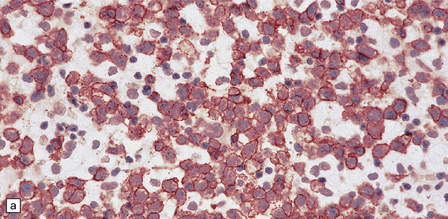
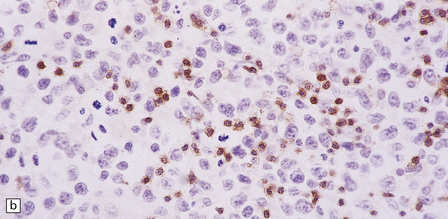
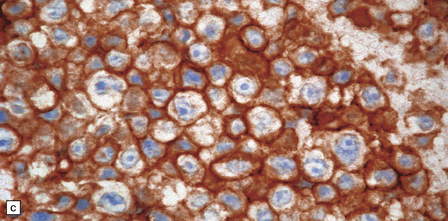
41.13 PCNSL.
(a) Antibody to CD20 labeling neoplastic cells in a B cell lymphoma. (b) Antibody to CD3 labeling reactive T cells in a B cell lymphoma. (c) Antibody to CD45RO labeling neoplastic cells in a T cell lymphoma.
Immunohistochemistry with antibodies to κ and λ light chains may show light chain restriction in some lymphomas. However, interpretation of these preparations can be difficult, and light chain restriction may be better demonstrated by in situ hybridization. Epstein–Barr virus may be demonstrated in some PCNSLs by immunohistochemistry or in situ hybridization (Fig. 41.14).
REFERENCES
Abrey, L.E., Ben-Porat, L., Panageas, K.S., et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol.. 2006;24:5711–5715.
Adams, J.H., Howatson, A.G. Cerebral lymphomas: review of 70 cases. J Clin Pathol.. 1990;43:544–547.
Ahsan, H., Neugut, A.I., Bruce, J.N. Trends in incidence of primary malignant brain tumors in USA, 1981–1990. Int J Epidemiol.. 1995;24:1078–1085.
Bataille, B., Delwail, V., Menet, E., et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg.. 2000;92:261–266.
Cady, F.M., O’Neill, B.P., Law, M.E., et al. Del(6)(q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. J Clin Oncol.. 2008;26:4814–4819.
Ellison, D.W., Wilkins, B.S. Lymphoma and the nervous system. Curr Top Pathol.. 2001;95:239–265.
Gerstner, E.R., Abrey, L.E., Schiff, D., et al. CNS Hodgkin lymphoma. Blood.. 2008;112:1658–1661.
Grois, N., Prayer, D., Prosch, H., et al. Neuropathology of CNS disease in Langerhans cell histiocytosis. Brain.. 2005;128:829–838.
Harris, N.L., Jaffe, E.S., Diebold, J., et al. The World Health Organization classification of neoplastic diseases of the haematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Histopathology.. 2000;36:69–86.
Harris, N.L., Jaffe, E.S., Stein, H., et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood.. 1994;84:1361–1392.
Hoffman, S., Propp, J.M., McCarthy, B.J. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol.. 2006;8:27–37.
Iwamoto, F.M., Abrey, L.E. Primary dural lymphomas: a review. Neurosurg Focus.. 2006;21:E5.
Montesinos-Rongen, M., Brunn, A., Bentink, S., et al. Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia.. 2008;22:400–405.
Paulus, W. Classification, pathogenesis and molecular pathology of primary CNS lymphomas. J Neuro Oncol.. 1999;43:203–208.
Porter, A.B., Giannini, C., Kaufmann, T., et al. Primary central nervous system lymphoma can be histologically diagnosed after previous corticosteroid use: a pilot study to determine whether corticosteroids prevent the diagnosis of primary central nervous system lymphoma. Ann Neurol.. 2008;63:662–667.
Schwechheimer, K., Braus, D.F., Schwarzkopf, G., et al. Polymorphous high-grade B cell lymphoma is the predominant type of spontaneous primary cerebral malignant lymphomas. Histological and immunomorphological evaluation of computed tomography-guided stereotactic brain biopsies. Am J Surg Pathol.. 1994;18:931–937.
Shenkier, T.N., Blay, J.Y., O’Neill, B.P., et al. Primary CNS lymphoma of T-cell origin: a descriptive analysis from the international primary CNS lymphoma collaborative group. J Clin Oncol.. 2005;23:2233–2239.
Tomlinson, F.H., Kurtin, P.J., Suman, V.J., et al. Primary intracerebral malignant lymphoma: a clinicopathological study of 89 patients. J Neurosurg.. 1995;82:558–566.
Tu, P.H., Giannini, C., Judkins, A.R., et al. Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol.. 2005;23:5718–5727.
Tun, H.W., Personett, D., Baskerville, K.A., et al. Pathway analysis of primary central nervous system lymphoma. Blood.. 2008;111:3200–3210.