Chapter 87 Prevention of Sudden Cardiac Death with Implantable Cardiac Defibrillators and Cardiac Resynchronization Therapy
Introduction
Sudden cardiac death (SCD) can be defined as cessation of myocardial function within 1 hour of the onset of symptoms. Cardiac arrest leading to SCD is usually assumed to be caused by ventricular tachycardia (VT) or ventricular fibrillation (VF). However, SCD occurs from other causes, including bradycardia, pulmonary embolus, aortic dissection, and other noncardiac conditions. In the United States, SCD is the leading single cause of death and the second leading cause of death after all cancers combined.1 It claims nearly 325,000 lives annually, accounting for one death every 97 seconds. Globally, it accounts for more than 3 million deaths each year. Only 5% of the victims of SCD survive; for every minute in SCD without defibrillation, the mortality rate rises by as much as 10%.2,3 The average arrival time for the typical first responder after collapse is 7 to 8 minutes or even longer.4 Of those who survive to hospital admission, only 3% to 28% are ultimately discharged.5 In 1947, thoracic surgeon Claude Beck was credited as being the first individual to use a cardiac defibrillator successfully when he saved a 14-year-old boy who developed intraoperative VF during thoracic surgery.6 Before the advent of implantable devices, the mainstay of therapy to reduce the incidence of ventricular arrhythmias was primarily with Vaughan Williams class I and III antiarrhythmic drugs (AADs). However, these drugs have consistently failed to show a benefit in reducing SCD. More than 30 years later, in 1980, Mirowski and Mower were instrumental in developing the first implantable cardioverter-defibrillator (ICD) for humans.7 Today, rapid defibrillation is the sine qua non of therapy aimed at restoration of a stable rhythm in those in VT or VF. Additional reductions in the incidence of SCD among high-risk patients are observed with the appropriate use of β-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), aldosterone antagonists, aspirin, and statins. Initially, multiple randomized clinical trials proved that ICDs were superior to standard medical therapy, including AADs, in the secondary prevention of SCD.8–12 For primary prevention of SCD in several high-risk patient groups, ICDs have also been shown to be superior to optimal medical therapy (OMT) (Table 87-1).13–18 In high-risk patients with prolonged intraventricular conduction and progressive heart failure, biventricular pacing with or without ICD backup has been proven to offer additional benefit. Several well-designed clinical trials have shown reduction in SCD and all-cause mortality with cardiac resynchronization therapy (CRT) with or without the addition of cardioverter defibrillator capability.18–21 As with all clinical trials, interpretation of results must be viewed in the context of the patient population studied and the study design in each particular trial. Therefore, this review provides an in-depth summary of the role of ICDs and CRT with or without ICD in the primary and secondary prevention of SCD. Box 87-1 outlines current guidelines for ICD therapy.
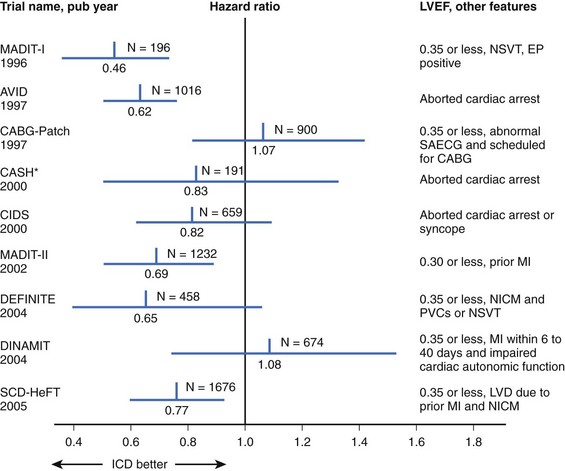
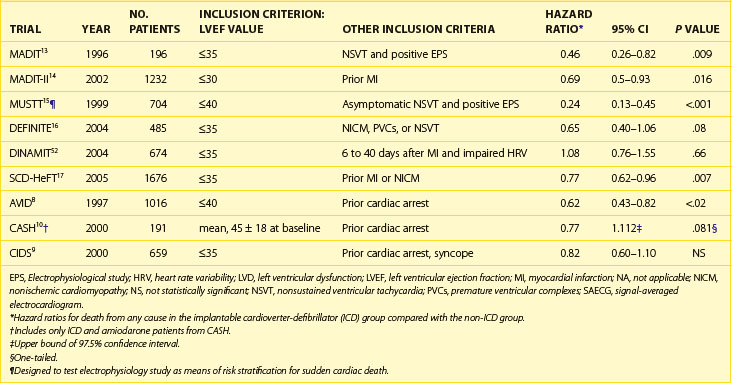
Table 87-1 Major Implantable Cardioverter-Defibrillator Trials for Prevention of Sudden Cardiac Death
Box 87-1 ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy for Prevention of Ventricular Arrhythmias
Class I
Class IIa
Class IIb
Class III
Modified from Epstein AE, DiMarco JP, Ellenbogen KA, et al: ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, J Am Coll Cardiol 51(21):e1–e62, 2008.
Implantable Cardioverter-Defibrillators and Secondary Prevention of Sudden Cardiac Death
Secondary prevention of SCD can be defined as that achieved in those patients who had a previous episode of resuscitated ventricular tachyarrhythmia. The Antiarrhythmics Versus Implantable Defibrillators (AVID) trial, which was published in 1997, was the first of its kind to show that in survivors of VF or sustained ventricular tachycardia causing severe symptoms, an ICD was superior to AADs in increasing overall survival.8 AVID was a multi-center, randomized comparison of single-lead ICD versus amiodarone or, in a few instances, sotolol. The devices used in the trial were single-lead transvenous devices with tiered therapy, defibrillation, cardioversion, bradycardia pacing, and anti-tachycardia pacing (ATP) capabilities. A device without ATP was permitted only if a thoracotomy was otherwise required for device implantation.22 Patients were required to be clinically acceptable for amiodarone therapy to be included in the study. Key inclusion criteria were a history of ventricular arrhythmia as described above and, if VT was the index arrhythmia, an ejection fraction (EF) of 40% or greater. Exclusion criteria were designed to limit the study to patients at high risk for SCD but with life expectancy of greater than 1 year. Key exclusions were New York Heart Association (NYHA) class IV heart failure, inotropic or mechanical support, less than 5 days from revascularization or acute myocardial infarction (AMI), atrial fibrillation (AF), and SCD with a transient or correctable cause. Patients who had transient or reversible causes of SCD and otherwise would have met inclusion criteria were enrolled in a separate AVID registry.
Stratified regression analysis of the results from the AVID registry suggested that the differences in outcomes in the two arms attributable to differences in baseline patient characteristics only accounted for 8% of the relative reduction in the primary endpoint. The imbalance in β-blocker use in the ICD arm was examined by Cox regression analysis, and it was found that this imbalance reduced the beneficial effect of ICD only minimally (unadjusted hazard ratio [HR] for the ICD group of 0.62 compared with adjusted HR of 0.67). The benefit of β-blocker use in congestive heart failure (CHF) has been shown to reduce mortality rate in similar patients in other studies.5 However, an additional benefit may have been observed in patients with paroxysmal AF or sustained VT by reducing inappropriate shocks. Patients in AVID with an index event of VT were more likely to receive shocks or ATP during follow-up. Further retrospective subgroup analysis showed several interesting findings. When the outcomes of those with a left ventricular EF (LVEF) of 35% or less were compared, ICD conferred a 40% relative mortality rate reduction. Of the patients, 39% had an EF less than 35% to 40%. In this subgroup, no statistical benefit in reducing overall mortality rate was observed in the ICD arm.23 The lack of significant mortality benefit may have been caused by a protective effect of amiodarone in this specific population. Previous observational studies have suggested that amiodarone may have a weak protective effect in preventing arrhythmic death and that this benefit may be more significant in patients with higher LVEF.24–26 Spielman et al found that in patients with recurrent sustained VT, higher LVEF and the absence of left ventricular aneurysm or hypokinesis were associated with VT suppressibility.27 In the amiodarone group, survival was strongly correlated with left ventricular systolic function.28 A retrospective analysis of the data from AVID attempted to identify subgroups that had secondary prevention the lowest rates of recurrent ventricular arrhythmias.29 The criteria were VF as the index event, no history of cerebrovascular disease, greater EF, no tachyarrhythmia history, and need for revascularization. Taken together, these findings suggested that important electrophysiological differences exist between these two patient populations.
As previously mentioned, patients screened for AVID but excluded for various reasons were enrolled in a registry. In the AVID registry, after adjusting for multiple variables, patients with transient reversible causes of SCD such as electrolyte abnormalities, new ischemia or infarction, and proarrhythmic drug adverse events actually had poorer outcomes compared with those with primary VT or VF. Patients who were excluded because they had VT that was hemodynamically stable had an overall prognosis similar to that of the patients included in the trial with unstable or pulseless VT.30 These findings argue against the commonly held belief that those with reversible causes of SCD or stable VT have a better prognosis and may not benefit from ICD implantation. Perhaps other clinical characteristics such as the presence of heart failure, stroke, ischemia, or left ventricular dysfunction may be more important discriminators of risk stratification of SCD.
The Canadian Implantable Defibrillator Study (CIDS), which published results in 2000, included a similar study population as AVID. A total of 659 patients were eligible for the study if, in the absence of recent AMI (≤72 hours) or electrolyte abnormalities, they had the following: (1) a history of cardiac arrest, (2) documented sustained VT or VF, (3) sustained VT of at least 150 beats/min, or (4) unmonitored syncope, with depressed LVEF and inducible sustained monomorphic ventricular arrhythmia by programmed ventricular stimulation. Key exclusion criteria were (1) likely survival for less than 1 year, (2) physician discretion against amiodarone or ICD, (3) excessive perioperative ICD implantation risk, (4) previous amiodarone therapy for 6 weeks or more, and (5) long QT syndrome. Patients were randomized to ICD or amiodarone with prespecified stratification by LVEF of 35% or less or greater than 35%. Patients randomized to ICD received a device capable of bradycardia pacing, ATP, cardioversion, and defibrillation. Approxmately 90% of leads were implanted via the transvenous approach, with only 33 undergoing thoracotomy. The primary endpoint was all-cause mortality, and the secondary endpoint was arrhythmic death. Nearly half the patients had VF or cardiac arrest. Similar to AVID, 89% of patients had NYHA class II or less heart failure but, unlike AVID, the population included a small cohort with NYHA class IV heart failure. Ischemic cardiomyopathy was present in 83%. At hospital discharge, β-blockers other than sotolol were prescribed for only 21.4% of patients in the amiodarone arm and 33.5% in the ICD arm, with these differences remaining through follow-up. Less than 10% of the patients were taking class I AADs. However, class I AADs were four times as likely to be used in the ICD arm and have since been shown to increase mortality rate in this patient population.31 Mean duration of follow-up was 2.9 years and 3 years in the amiodarone and ICD cohorts, respectively.
The observed risk reduction in CIDS was less than that of AVID, although both trials were similarly designed. However, substantial overlap exists in the confidence intervals (CIs) of the two studies, suggesting that the results are likely similar. At 1 year, 23.7% of those in CIDS in the AAD arm were taking a β-blocker (including sotolol), which was greater than the 16.8% in AVID. The actual benefit of ICD may have been underestimated in CIDS because of a 20% crossover to ICD in the amiodarone arm as well as the greater use of β-blockers in the ICD arm. As previously noted, data suggest that the combined use of amiodarone and β-blockers may potentiate their antiarrhythmic effects.24,25 The former findings would lessen the impact of ICD benefit in the ICD arm. After the results of CIDS were published, patients were followed up for an average of 5.6 years. The CIDS long-term substudy found progressive increase in benefit of ICD over time.32
The results of CIDS were closely followed by the Cardiac Arrest Study of Hamburg (CASH).10 The study design was more complex than those of previous secondary prevention trials. Enrollment of 407 patients began 4 years before the increased mortality rate associated with class I AADs in patients with structural heart disease was well known.31 Patients were randomized in a 1 : 3 fashion to ICD or medical therapy with one of three drugs intended to reduce ventricular arrhythmias. ICD programming was limited to a shock-only device, with rate as the only criterion for the detection of a sustained ventricular arrhythmia. Within the medical therapy arm, patients were randomized to amiodarone, metoprolol, or propafenone. Inclusion criteria were documented sustained ventricular arrhythmias and aborted cardiac arrest. Exclusion criteria were similar to earlier secondary prevention trials. Patients were excluded if the arrest occurred within 72 hours of AMI, cardiac surgery, electrolyte abnormalities, or proarrhythmic drug effect, or if they had NYHA class IV CHF. More than half the participants had mild heart failure (NYHA class II). In the ICD cohort, 55% of the patients underwent epicardial lead placement via thoracotomy. The index arrhythmia was VF in 84% of patients. The mean LVEF was 46%, with 10% of patients having structurally normal hearts. Another 19% in the ICD arm and 21% in the drug arm underwent revascularization during the index hospitalization for SCD. The propafenone arm was discontinued after 119 patients were enrolled and interim analysis revealed a 61% increase in all-cause mortality in the drug group. This difference was noted at a mean of 11.3 months of follow-up and was clustered in the propafenone arm. The remaining 288 patients enrolled after 1992 were randomized in a 1 : 2 fashion to ICD, amiodarone, or metoprolol. Unlike CIDS and AVID, crossover rates in both groups in this study were relatively low and equal in each arm. In the ICD arm, 5.1% of patients died in the perioperative period versus 1.1% in the drug arm during the same period (P = .029). In addition, the overall complication rate in the ICD arm was 23% through the completed follow-up period. Over a mean follow-up of 57 ± 34 months, the crude death rates were 36.4% in the ICD arm and 44.4% in the drug arm, excluding patients who had been on propafenone. This resulted in a nonsignificant 23% reduction in all-cause mortality (P = .081), with the majority of the benefit in the ICD arm seen in the first 5 years. The ICD group had a significant improvement in survival free of SCD (HR, 0.423, P = .005). The results in the β-blocker arm from CASH helped shed light on previous suggested interaction caused by β-blocker use in the two arms of AVID and CIDS. No significant difference was observed in the crude death rates between the amiodarone and metoprolol groups (29.5% and 35.1%, respectively). As in CIDS, baseline characteristics of patients, lower risk of SCD than predicted, and study design may have limited a demonstrable difference between the ICD and drug arms. Previous studies had suggested those with LVEF greater than 35% may not receive as much benefit from ICD as those with lower LVEF.23 The mean LVEF in CASH was 46% and one tenth of these patients had no organic heart disease. In addition, the observed 2-year overall mortality rate was 19.6%, which was approximately half as high as projected in the original study design. Thus the study was underpowered to detect any significant differences between the two groups. The significant increase in perioperative death in the ICD arm was much higher than current estimates and likely negated any benefit of ICDs on mortality rate.33,34 A nonsignificant trend toward greater benefit with ICD was seen in those with lower LVEF and higher NYHA functional class.
The Midlands Trial of Empiric Amiodarone Versus Electrophysiology-Guided Interventions and Implantable Cardioverter-Defibrillators (MAVERIC) study sought to evaluate the efficacy of electrophysiology study (EPS) in predicting outcomes in patients implanted with ICDs or managed with AADs alone.12 It is important to note that the primary aim of this trial was not to compare ICD versus drug therapy but to determine the role of EPS in this patient population. Patients were randomized to one of two treatment strategies. One group was started on empiric amiodarone therapy. The other underwent a complex EPS-guided algorithm in which implantation of ICD was determined by inducibility of VT or VF as well as the origin of the VT. Those with right ventricular outflow tract VT, bundle branch re-entrant VT, and fascicular tachycardia were referred for ablation and did not undergo ICD implantation. If VT or VF was not inducible by programmed electrical stimulation (PES), a Holter monitor was used to further guide treatment. If the Holter monitor did not reveal more than 30 premature ventricular complexes per hour, an ICD was then implanted. As part of the protocol, all patients were evaluated and treated for ischemia, if present. Survivors of SCD in the absence of AMI in the past 48 hours met the inclusion criteria. Premenopausal women with life expectancy of less than 1 year were excluded. The primary endpoint was all-cause mortality with secondary endpoints of VT or VF recurrence and crossover in treatment. In total, 214 patients were enrolled in the trial. Two important differences existed between this study and AVID, CASH, and CIDS. First, more than half the patients included in this study had hemodynamically stable VT. Second, amiodarone was compared with EPS-guided therapy and not directly with ICD implantation. This study was limited by the small number of patients receiving ICDs (24% in the EPS arm and 5% in the amiodarone arm). More than half the patients had LVEF greater than 35%. The patients in the Massachusetts Veterans Epidemiology Research and Information Center (another MAVERIC) trial were in a high-risk group, and many of those who were not implanted would meet the current class I recommendations for ICD placement. The net outcome of the trial was neutral with no demonstrable difference between empiric amiodarone versus EPS-guided treatment. Despite the limitations of this study, several points were gleaned from this trial. ICD recipients did consistently better than non-ICD recipients with a greater benefit shown by those with hemodynamically unstable ventricular arrhythmias as their index arrhythmia. This contradicts the subgroup analysis from AVID, which showed no difference in ICD benefit based on hemodynamic stability. As in the previous trials, the authors concluded that advanced age, CHF, and LVEF less than 35% were independently associated with death. In addition, diabetes was an independent predictor of poorer outcomes. Nonrandomized comparison of ICD and non-ICD cohorts revealed an HR of 0.54 for percent alive at the end of follow-up. On the basis of study design, these can be only considered hypothesis-generating results. More important, in the setting of secondary prevention, EPS added no additional benefit to conventional treatment strategies. Therefore EPS has no role in determining whether a survivor of sudden cardiac arrest (SCA) will benefit from ICD implantation.
Another secondary prevention trial performed in a more select group of participants was the Defibrillator Versus Beta Blockers for Unexplained Death in Thailand (DEBUT) trial.11 In this two-phase study, survivors of sudden unexplained death syndrome (SUDS) were randomized to ICD or the β-blocker propranolol. As a cohort largely with Brugada syndrome, patients in SUDS tended to have ST elevation in the right precordial leads (V1 to V3) and incomplete right bundle branch block.35 The primary endpoint was all-cause mortality and the secondary endpoints were VT, VF, or cardiac arrest. No differences were observed in baseline characteristics between the two groups, and all patients had structurally normal hearts, with a mean EF of 67%. The results of this trial have somewhat limited applicability because of the significant variability of the underlying pathophysiology of arrhythmia. In this study, 86 patients were randomized to either treatment strategy. The trial was stopped early after 3 years because of an 18% mortality rate in the drug arm and no deaths in the ICD arm. The patients in DEBUT had no contributing factors to death other than VF, which therefore suggests that the ICD fully prevented death.
The results of the aforementioned key secondary prevention trials have been examined further. A meta-analysis published by Connolly et al analyzed the results from AVID, CASH, and CIDS compared with ICD with amiodarone.36 They found significant relative reductions in total mortality rate of 28% and a significant 50% reduction in arrhythmic death. Over a period of 6 years, implantation of an ICD yielded a 4.4-month survival benefit over AAD therapy. The mortality rate reduction with ICD was realized regardless of the presence of structural heart disease, type of presenting arrhythmia (VT or VF), β-blocker use, or prior surgical revascularization. Although benefit occurred in all groups regardless of LVEF, the benefit was greater in those with lower EF. A second similarly designed meta-analysis by Lee et al in 2003 confirmed the findings of Connolly, thus adding to the statistically nonsignificant benefit of ICD implantation in CIDS and CASH.37 A subgroup analysis of the three main secondary prevention trials performed by Oseroff, Retyk, and Bochoeyer found that ICDs confer a 28% RR reduction in all-cause mortality. This benefit was greatest in those with LVEF 20% to 34%. Similar to the findings of MAVERIC, those with inducible VT or VF had no worse mortality rates than those who were noninducible with PES at EPS.38
Primary Prevention of Sudden Cardiac Death
The results of previous secondary prevention trials showed a benefit of ICDs in reducing the incidence of SCD. However, most patients do not survive SCD, so it was logical to explore the use of ICDs for primary prevention in high-risk patients with no history of sustained ventricular arrhythmias (Figure 87-1). The first major published study of primary prevention of SCD was the Multicenter Automatic Defibrillator Implantation Trial (MADIT), published in 1996.13 In this trial, patients with ischemic cardiomyopathy with an EF of 35% or less and NYHA class I to III heart failure, a history of nonsustained VT (NSVT), and inducible nonsupressible sustained VT by PES were randomized to ICD or OMT. The primary endpoint was all-cause mortality. Notable exclusion criteria were surgical revascularization in the past 2 months, angioplasty in the preceding 3 months, recent MI (≤3 weeks), criteria for ICD by secondary prevention standards, and life expectancy of less than 1 year. Of the patients, 253 patients had inducible VT that could not be suppressed by intravenous procainamide, and ultimately 196 were then randomized. Mostly, monophasic pulse generators were used, and roughly half the devices were transthoracic implants (before the advent of transvenous systems). Baseline characteristics of the patients were similar, with a mean EF of 26%. Two thirds of patients in each group had a history of bypass surgery. One month after enrollment, amiodarone was the primary AAD in the OMT arm. The use of ACE inhibitors was similar between groups (60% and 55%). In addition, the overall use of β-blockers was low (25%) but more common in the ICD arm. Sixteen crossovers to ICD occurred. Eleven patients in the OMT arm underwent ICD implantation, and five patients in the ICD arm never had an ICD placed. Two patients had their devices inactivated during the trial. Perioperative mortality rate was 0%, and follow-up was completed in 92% of the ICD group and 86% in the OMT group. Over a mean follow-up of 27 months, an overall mortality rate of 39% occurred in the medical therapy arm and 16% in the ICD arm (HR, 0.46; 95% CI, 0.26 to 0.82; P = .009). This difference translated into a number needed to treat (NNT) of 3 over 36 months to save one life. Sixty percent of those in the defibrillator group received shocks at 2 years, although the appropriateness of these shocks was not well established given the absence of stored electrograms in many devices. This rate is significantly higher than contemporary estimates of appropriate shocks, and it is now known that ICD shocks predict an increase in mortality rate regardless of cause.39 The majority of the differences in death rates were seen in reduction in primary arrhythmia of unknown cause (occurred outside the monitored setting in the OMT arm). It is unclear if the differences in nonarrhythmic deaths were caused by a misclassification of cause of death or the possible adverse effects of AADs. A nonsignificantly greater overall mortality rate at 1 month was observed in the amiodarone group compared with the defibrillator group (36% and 26%, respectively). However, amiodarone was the most used AAD, and other data suggest that it can be used without excess mortality from heart failure.40 A Cox regression analysis failed to attribute the difference in the two groups to differences in medical therapy. In addition, the observed crossover would have only diluted observed differences in outcome between the two groups.
The Multicenter Unsustained Tachycardia Trial (MUSTT) investigators sought to determine if EPS with PES could identify high-risk patients who would benefit from empiric ICD implantation. Patients with an LVEF of 40% or less and asymptomatic NSVT were randomized to AAD therapy guided by EPS or no AAD therapy. Patients with inducible ventricular arrhythmias were then randomized to various AADs and after four to five repeated doses of PES. The exception was amiodarone, which could be used at the discretion of the investigator. If fewer than 15 ventricular complexes were induced, long-term therapy with that drug was initiated. If ventricular arrhythmia could not be suppressed, the patient could be discharged on an AAD, which resulted in induced hemodynamically stable ventricular arrhythmia. No empiric AAD therapy was used. Early in the trial, ICDs were implanted only if the patients failed three or more AADs. As practices changed during enrollment, patients could undergo ICD implantation after just one AAD failure as determined by PES. Patients were evaluated for obstructive coronary artery disease within 6 months of enrollment. NSVT had to occur within 6 months of enrollment and more than 96 hours after an AMI or a revascularization procedure. Key exclusion criteria included reversible causes of NSVT and VT or syncope more than 2 days after AMI. Of the study patients, 704 were randomized and 353 placed in the ICD arm. The mean EF was 30% in the EPS arm and 29% in the no-AAD arm. Fewer patients in the EPS arm were taking β-blockers (29% vs. 51%, P = .001). Distribution of patients across NYHA classes I to III was roughly even, with no class IV patients. Among the 351 patients randomized to EPS, 158 (45%) were discharged on AADs, more than half of which were class I AADs. Another 161 (46%) underwent ICD implantation. Twelve percent of patients initially discharged on AADs eventually underwent ICD implantation. At a mean follow-up of 5 years, a 7% absolute reduction and a 27% relative reduction in mortality were observed in the EPS arm versus the no-AAD arm (25% vs. 32%; 95% CI, 0.53 to 0.99). This difference was caused solely by the ICD in approximately half the patients in the EPS arm. The RR of cardiac arrest or death from arrhythmia in patients who received ICDs was significantly lower (9% vs. 37%) compared with that in patients discharged without ICDs despite more nonsuppressible ventricular arrhythmias in the ICD cohort (RR, 0.24; 95% CI, 0.13 to 0.45; P < .001). Neither the rate of cardiac arrest nor death from arrhythmia was lower in the EPS arm assigned to AADs or in those assigned to no AADs (33% vs. 28%; P = not significant). Although this trial was meant to assess the efficacy of EPS in risk stratifying those at high risk for SCD, the impact of ICD was substantial. The requirement that patients undergoing implantation of an ICD failed at least one AAD suggests that those receiving ICDs had a worse overall prognosis. Despite this, patients receiving ICDs had better survival than those who did not receive defibrillators. Furthermore, half the patients on AAD therapy were taking class I drugs, which have since been shown to increase mortality in patients with structural heart disease.31 β-Blocker use was nearly twice as likely in the cohort not receiving AADs and ICDs (51%) compared with those who did (P = .001). All these differences in treatment would tend to offset the suggested benefit of ICDs in reducing mortality. The authors of this study concluded that AAD therapy guided by EPS does not improve survival. They suggested ICD should be implanted in those meeting the high-risk inclusion criteria of this trial in whom PES induces sustained ventricular arrhythmias. A follow-up study compared the outcomes of 1397 patients in the MUSTT database registry with the 353 with inducible tachyarrhythmias.41 They found that the negative predictive value of SCD of a negative EPS was 88% to 90% over 2 years. Overall mortality rate at 5 years was significantly higher in those with positive EPS (48% vs. 44%, P = .005). Although the risk is greater in inducible tachyarrhythmias, the difference between the groups was smaller than expected and indicated a surprisingly high risk despite a “negative” EPS. These findings are often incorporated into current practice. In patients at high risk for SCD with an EF of 36% to 40%, a positive EPS provides grounds for prophylactic ICD implantation.
After the publication of MUSTT, additional substudies were performed to determine if certain populations would derive greater benefit from ICDs. Of the patients in MUSTT who were not prescribed AADs or implanted with an ICD, mortality rate was higher in those with an LVEF of 30% or less compared with those with an LVEF of 30% to 40%.42 In addition to EF, a further analysis of the MUSTT data suggested that other clinical factors, including functional class, history of heart failure, NSVT unrelated to bypass surgery, age, QRS width, and the presence of AADs, were all linked to increased mortality and arrhythmic death.43 The authors also noted that the risk for arrhythmic events may be greater in patients with an EF greater than 30% but with the aforementioned risk factors compared with those with an EF less than 30% without additional risk factors.
The MADIT-II investigators studied the prophylactic implantation of defibrillators in patients with prior MI and reduced EF.14 They hypothesized that myocardial scar left by the previous infarction would serve as a substrate for ventricular arrhythmia and that these patients may benefit from empiric ICD implantation without additional risk stratification based on EPS, NSVT, or SAECG. Patients having ischemic cardiomyopathy with an MI more than 1 month in the past and an EF of 30% or less were randomized in a 3 : 2 fashion to ICD versus conventional medical therapy. Patients were excluded if they were NYHA class IV at enrollment, had been revascularized in the past 3 months, had advanced cerebrovascular disease, or had a coexisting condition that had a high likelihood of causing death during the trial. ICD recipients underwent implantation of a transvenous ICD with DFT testing to ensure 10-J safety margins. Approximately 45% of the patients had dual-chamber units; ATP was allowed; and the rate cutoff for pacing or shock was not mandated, but most investigators programmed the ICD units to a rate cutoff of 170 beats/min for ATP, shock, or both.44 This was the earliest of the recent ICD trials to closely mimic current guidelines with regard to medical therapy. The majority of patients in both arms were treated with β-blockers, ACE inhibitors, and statins. AAD use was neither mandated nor encouraged in the medical therapy arm, with only 10% of patients taking amiodarone and less than 3% prescribed class I AADs. Of the randomized patients, 35% were in NYHA class I, and the average EF was 23%. No baseline differences existed between groups. In this trial, 1232 patients were randomized, with 742 in the ICD group and 490 in the conventional medical therapy group. At the conclusion of the study, 4.5% of patients in the medical therapy arm crossed over into the ICD arm, and 4.3% of the patients randomized to ICD either did not have a device implanted or had the device removed.
During an average follow-up period of 20 months, the all-cause mortality rate in the medical therapy arm was 19.8% versus 14.2% in the ICD arm (HR, 0.69; 95% CI, 0.51 to 0.93; P = .016). The mortality benefit began to appear 9 months after randomization. Subgroup analyses showed no significant difference in the benefit of defibrillator therapy with respect to LVEF, age, gender, NYHA class, or QRS width. Similarly, no differences existed in the benefit of ICD on survival with respect to diabetes, hypertension, left bundle branch block (LBBB), AF, time elapsed since MI, or use of a dual-chamber device. A post hoc analysis suggested that no benefit was derived from ICD in those with end-stage renal disease but that a significant benefit was experienced by those with mild to moderate renal dysfunction.45 Complication rates in the ICD arm were low, with 2.5% of patients over the duration of follow-up requiring surgical lead extraction for lead malfunction or infection. Interestingly, more patients in the ICD arm were hospitalized with heart failure (14.9% vs. 19.9%). This resulted in an overall trend of increased hospitalization in the ICD arm (11.3 vs. 9.4 patients hospitalized per 1000 months of active follow-up; P = .09). The reasons for the increases in hospitalization for heart failure in the ICD arm, though nonsignificant, are unclear. This may be a surrogate marker for increased survival as an effect of the ICD; that is, those who otherwise would have died from SCD lived longer to go on and develop more decompensated heart failure. However, in light of follow-up studies of these patients, this seems unlikely. Post hoc analysis of the MADIT-II participants revealed one or more inappropriate shocks occurred in 83 (11.5%) of the 719 patients in the ICD group.47 Inappropriate shock episodes constituted 184 of the 590 total shock episodes (31.2%). Inappropriate shocks were more likely to occur in patients with a history of smoking, prior AF, and diastolic hypertension, and antecedent appropriate shock predicted inappropriate shock occurrence. AF (44%) and sustained VT (36%) were the most common triggers for inappropriate shock, followed by abnormal sensing (20%). The stability detection algorithm was programmed less frequently in patients receiving inappropriate shocks (17% vs. 36%, P = .030), whereas other programming parameters did not differ significantly from those without inappropriate shocks. Importantly, patients with inappropriate shocks had a greater likelihood of all-cause mortality in follow-up (HR, 2.29; P = .025). By proportionately increasing mortality, inappropriate shocks may have weakened the overall mortality benefit afforded by the ICD, but it is unclear if this would have resulted in more hospitalizations for heart failure. The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), a more recent study of 829 patients with heart failure who were randomly assigned to ICD therapy, assessed the potential effects of both appropriate and inappropriate shocks.48 Over a median follow-up period of 45.5 months, 269 patients (33.2%) received at least one ICD shock, with 15% of patients receiving only appropriate shocks, 10% receiving only inappropriate shocks, and 7% receiving both types of shock. An appropriate ICD shock, compared with no appropriate shock, was associated with a significant increase in the subsequent risk of death from all causes (adjusted HR, 5.68; 95% CI, 3.97 to 8.12; P < .001). An inappropriate ICD shock, compared with no inappropriate shock, was also associated with a significant increase in the risk of death (HR, 1.98; 95% CI, 1.29 to 3.05; P = .002). For patients who survived longer than 24 hours after an appropriate ICD shock, the risk of death remained elevated (HR, 2.99; 95% CI, 2.04 to 4.37; P < .001). The most common cause of death among patients who received any ICD shock was progressive heart failure. This suggests that regardless of the etiology of a shock, patients who receive shocks have a higher mortality rate that is more likely related to heart failure. Ongoing studies are evaluating whether shocks contribute to heart failure exacerbations or if they simply reflect the treatment of arrhythmias associated with worsening heart failure.
It is also likely that worsening heart failure may have been the result of frequent right ventricular pacing. The Dual Chamber and VVI Implantable Defibrillator (DAVID) trial suggested that in patients without indication for pacing, VVI pacing at a rate of 40 is superior to DDDR pacing at a rate of 70. A nonsignificant increase in mortality rate and hospitalization for heart failure was seen in the latter group (16.5% vs. 26.1%).46 Programmed device parameters were available for roughly three quarters of those in the ICD arm.44 The mean lower rate limit for pacing was 53 ± 12 beats/min, and in patients with dual-chamber devices the mean paced AV delay was 190 ± 43 ms. About 35% of the ICD cohort received right ventricular pacing 50% or more of the time, with 25% receiving right ventricular pacing for more than 90% of the time. The group that was paced more than 50% of the time also had an increased risk of VT or VF requiring ICD therapy (HR, 1.50; P = .02). A post hoc analysis of the Mode Selection Trial, a trial of pacemaker therapy for sick sinus syndrome, demonstrated that the cumulative percentage of right ventricular apical pacing was a strong predictor of hospitalization for heart failure.49 These findings suggest that right ventricular pacing was a likely contributor to increased hospitalization for heart failure despite a significant mortality reduction arising from aborted VT or VF.
Other retrospective analyses of the MADIT-II data were examined to determine particularities of ICD benefit in this patient population. Since this trial was performed in patients after MI, it was postulated that a differential benefit from ICD may exist depending on how remote the ICD was placed relative to the MI.50 The mortality benefit in remote MI was in those with MI occurring 18 months ago, with no benefit observed with more recent MI. In addition, the mortality benefit increased with time and remained substantial for up to at least 15 years. This contradicts older data, which suggested that the risk is greater in the first year after MI. This paradigm shift may be attributed to changes in current medical therapy, including acute revascularization and medical therapy targeting neurohormonal activation. In terms of revascularization, benefit was not appreciated until 6 months after the last coronary revascularization procedure, suggesting a benefit of anti-ischemic therapy on reducing SCD.51 Another randomized, controlled trial has shown no overall survival benefit from starting ICD therapy in the early weeks after an AMI in patients with clinical features of increased risk for arrhythmic events.52 In the Immediate Risk Stratification Improves Survival (IRIS) trial, which randomized almost 900 patients with AMI, overall mortality rate was unaffected by ICD therapy started within 1 month of the event in addition to medical therapy compared with medical therapy alone. Over a follow-up period of about 3 years, the ICD group’s HR for death compared with that of the control group was 1.04 (P = .78). Patients implanted with ICDs showed a 45% lower risk of SCD (P = .049), countered by an almost-doubled risk of non-SCD (P = .001), These results confirm the findings of the Defibrillator in Myocardial Infarction Trial (DINAMIT).
MADIT-II evaluated patients with prior MI 1 month or more after enrollment. As previously described, post hoc analysis of MADIT suggested that the benefit of ICD in ischemic cardiomyopathy may be appreciated long after the index infarction. The DINAMIT study investigated the use of ICDs in the primary prevention of SCD in the period 6 to 40 days after MI.53 This study randomized 332 patients to ICD and 342 to OMT, with recent MI, LVEF of 35% or less, and evidence of impaired autonomic cardiac function (manifested as depressed heart rate variability or elevated average 24-hour heart rate on Holter monitoring). The primary outcome was all-cause mortality, with death from arrhythmia as a prespecified secondary outcome. Key exclusion criteria were VT or VF, NYHA class IV heart failure, acute volume overload, life expectancy less than 1 year, or coronary artery bypass grafting (or three-vessel percutaneous revascularization) since qualifying MI or planned within 4 weeks of enrollment. ICDs were single-lead systems programmed at VVI 40 beats/min, with VT zone with ATP at a rate of 175 beats/min, and a VF shock-only zone at 200 beats/min. All shock discharges were at maximal output. ATP was specified to deliver four bursts of 6 to 10 beats at 81% of the tachycardia cycle length with 10-ms decrements between ATP attempts. Both groups were similar, with a mean EF of 28%, and more than 85% of patients in both arms were taking β-blockers and ACE inhibitors. Two patients in the ICD arm died before device implantation. After a mean follow-up of 30 ± 13 months, no difference in overall mortality was observed between the two groups. Of the study patients, 62 in the ICD group and 58 in the OMT group died (HR for death, 1.08; 95% CI, 0.76 to 1.55; P = .66). A significant reduction occurred in death caused by arrhythmia in the ICD arm (12 vs. 29; HR, 0.42; CI, 0.22 to 0.83; P = .009). However, more significantly, more deaths occurred from nonarrhythmic causes in the ICD group (50 vs. 29, P = .02). Of the deaths from nonarrhythmic causes, 78% were cardiac in nature. No evidence of procedure-related deaths was present. The authors did not believe that the deaths were caused by excessive right ventricular pacing as in DAVID since patients were all programmed VVI 40.45 As also proposed in retrospective analysis of the MADIT-II data, the authors suggested that the excessive nonarrhythmic deaths were caused by the natural history of heart failure not otherwise ended prematurely by SCD. These data are consistent with MADIT-II, which showed no benefit of ICD early after MI. Other differences that may have contributed to different overall outcomes in these studies did exist. For instance, LVEF was lower in MADIT-II (23% vs. 28%). In addition, the tests of autonomic function may have not accurately selected the patients at highest risk for SCD, although these tests have previously been shown to predict patients at high risk of pump failure.54
The most recent and large scale clinical trial of ICDs and prevention of SCD was the SCD-HeFT.17 In this study, 2521 patient with an LVEF of 35% or less and NYHA class II to III were randomized to one of three arms: (1) OMT, (2) OMT plus amiodarone, or (3) a conservatively programmed single-lead ICD. The primary endpoint was all-cause mortality. The single-ventricular-lead devices with a single zone were programmed with a detection rate of 187 or more with no ATP. Devices were programmed VVI 35, with no rate response to prevent pacing. DFT testing was performed in all patients with a maximum of two inductions. Interestingly, the first shock was 20 J; if unsuccessful, the next induction was followed by a 30-J shock. If the DFT was 30 J, the device was nevertheless implanted without additional pharmacologic or mechanical intervention in an attempt to reduce DFTs or guarantee a 10-J safety margin. Recently published data from SCD-HeFT suggested there was no benefit from DFT testing in this particular patient population. In addition, baseline DFT testing did not predict long-term mortality or shock efficacy.55 Patients in the amiodarone arm were loaded with oral amiodarone and placed on a maintenance dose of 200 mg to 400 mg/day based on weight. The dose could be reduced in the event of symptomatic bradycardia. The use of β-blockers, statins, ACE inhibitors, aldosterone antagonists, and aspirin was required if clinically indicated.
A prespecified subgroup analysis of the SCD-HeFT participants was performed based on multiple characteristics. Interestingly, when stratified on the basis of symptomatology, patients with NYHA class II heart failure derived a marked benefit from ICD, whereas class III patients received no benefit from ICDs. A 46% relative reduction in death occurred in the class II patients, translating into an 11.9% absolute reduction in mortality rate at 5 years. This contradicts the findings in the MADIT-II and Defibrillators in Non-ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trials, which showed no differences on the basis of NYHA class and a greater benefit in NYHA class III patients, respectively.14,16 In addition, the trend in previous trials has been greater treatment benefit with ICDs in sicker patients. Subgroup analysis suggested a greater benefit in patients with an LVEF of 30% or less compared with those with an EF of 30% to 35% as well as patients with a wider QRS.
Primary Prevention of Sudden Cardiac Death in Nonischemic Cardiomyopathy
The overall rate of SCD in DEFINITE was lower than in previous trials. This may have been caused by more patients taking β-blockers and ACE inhibitors than in comparable trials. The incidence of SCD as well as the relatively small sample size may have resulted in the lack of significant reduction in all-cause mortality. Similar findings were published in two other smaller NICM primary prevention trials.56,57 The Cardiomyopathy Trial (CAT) enrolled 104 patients with NICM of recent onset (<9 months) and an EF of 30% or less and randomized patients to ICD or medical therapy. The trial was terminated prematurely because the observed annual mortality rate was 3.7% instead of 30% as predicted. No SCD occurred during the first and second years of follow-up.56 The Amiodarone Versus Implantable Cardioverter-Defibrillator (AMIOVIRT) study evaluated 103 patients with NICM, an LVEF of 35% or less, NYHA class I to III heart failure, and asymptomatic NSVT.57 Patients were randomized to receive either amiodarone or an ICD. The primary endpoint was all-cause mortality. In this study, 52% of the patients were taking β-blockers and 85% were taking ACE inhibitors. The mean EF was 23%, and 64% of patients were NYHA class II. The study was stopped when the prospective stopping rule for futility was reached. The percent of patients surviving at 1 year (90% vs. 96%) and 3 years (88% vs. 87%) in the amiodarone and ICD groups, respectively, was not statistically different (P = .8). The authors proposed that the lack of a control group, small sample size, and lower than predicted mortality rate explained the lack of difference between the two arms. In 2004, a meta-analysis of randomized clinical trials of patients with non-ischemic dilated cardiomyopathy (NIDCM) was performed.58 This study analyzed both primary prevention and secondary prevention trials. The primary prevention trials included in the analysis were CAT, AMIOVIRT, DEFINITE, SCD-HeFT, and Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION). The analysis included 1854 patients with NICM. Pooled analysis suggested a significant reduction in total mortality among patients randomized to ICD or CRT plus defibrillator (CRT-D) versus medical therapy (RR, 0.69; 95% CI, 0.55 to 0.87; P = .002) (Table 87-2). Mortality reduction remained significant even after elimination of CRT-D trials. The averaged annual mortality rate from these trials was 7%. Assuming a 31% RR reduction this would result in an absolute risk reduction in all-cause mortality of 2% per year. The NNT to prevent one death at 2 years would be 25. In comparison, on the basis of MADIT-II, the NNT to achieve the same primary prevention benefit in ischemic cardiomyopathy is 18 patients.14
Cardiac Resynchronization Therapy
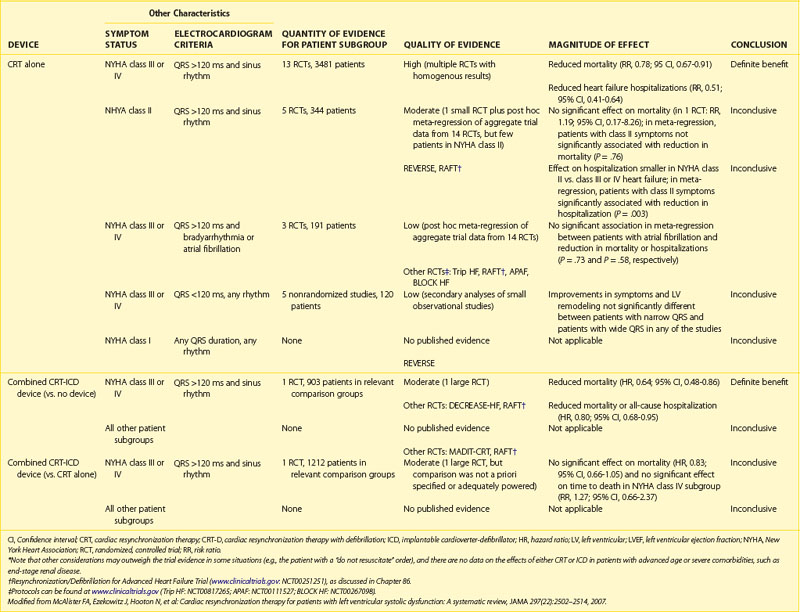
Certain patient populations with heart failure have an overall poorer prognosis. Within this group are patients with prolonged QRS duration and worse functional status. When the QRS is prolonged, delayed mechanical activation of the ventricular free wall and septum often occurs, resulting in dyssynchronous contraction of the left ventricle.59 Dyssynchronous contraction results in decreased systolic performance and efficiency, functional mitral regurgitation (primarily caused by prolonged delay to left ventricular activation), and increased residual end-systolic ventricular volume.60 Among patients with electrical dyssynchrony marked by QRS widening, bi-ventricular pacing restores ventricular synchrony by activating the left ventricular free wall and the left ventricular septum via pacing from the left ventricular free wall. The left ventricular lead can be placed transvenously via the coronary sinus or through thoracotomy by direct epicardial implantation. Early trials dating back more than a decade have shown significant improvement in NYHA functional class, exercise time, quality of life, and oxygen consumption during metabolic testing.18,19,21,61–68 These early trials monitored patients for up to 6 months in a blinded fashion. Noninvasive cardiac imaging in these patients showed reverse remodeling, as evidenced by an increase in EF and reductions in end-diastolic and end-systolic volumes. Because of low enrollment, early trials were underpowered to assess clinical endpoints such as total mortality, cardiovascular mortality, and hospitalization. The data supporting the use of CRT to alleviate heart failure symptoms is not reviewed here because it is outside the focus of this chapter. More recently, two large randomized trials evaluated CRT with defibrillator (CRT-D) or with pacing alone (CRT-P).18,19
The first large CRT clinical trial to include all-cause mortality as the primary endpoint was the COMPANION trial published in 2004.18 This trial was designed to determine if CRT with or without an ICD would reduce the risk of death and hospitalization from any cause. Patients enrolled in the trial had an LVEF of 35% or less, QRS duration of 120 ms or greater, P-R interval greater than 150 ms, sinus rhythm, and NYHA class III or IV heart failure, with a hospital admission for heart failure in the previous year. Notable exclusion criteria were a clinical indication for a pacemaker or defibrillator and AF. The use of β-blockers, ACE inhibitors or ARBs, spironolactone, and statins was required if clinically indicated. In the trial, 1520 patients were randomized in a 1 : 2 : 2 fashion to OMT, CRT-P, or CRT-D, respectively. Both devices were programmed VDD, with a lower rate well below the patient’s lowest intrinsic heart rate to maintain atrial tracking at rest. No differences in baseline patient characteristics or mandated background medical therapy were noted among the three groups. The population was roughly divided between ischemic and nonischemic cardiomyopathies. The mean LVEF was 21%, with QRS duration of 160 ms. Of the patients receiving devices, 71% had an LBBB pattern, and 86% had NYHA class III heart failure. Device implantation was successful in 91% of patients. Five procedure-related deaths (0.8%) occurred in the CRT-P arm, with three procedure-related deaths (0.5%) in the CRT-D arm. Left ventricular lead dislodgement occurred in 2% and 2.5% of the CRT and CRT-D groups, respectively. Specific crossover to the ICD arm was not reported in COMPANION; rather, the total “withdrawal” from the medical therapy arm was presented. This occurred in 26% of those assigned to OMT. The mean follow-up period was only 16 months in the device arms and 12 months in the OMT arm. When compared with OMT, CRT-P reduced the primary composite endpoint of all-cause mortality and hospitalization by 19% (HR, 0.81; P = .014), as did CRT-D (HR, 0.80; P = .01). Heart failure–related deaths or hospitalizations were reduced by 34% in the CRT-P group (P < .002) and by 40% in the CRT-D group (P < .001). The secondary endpoint of death from any cause was reduced by 24% (P = .059) in the CRT-P group and by 36% (P = .003) in the CRT-D arm.
One year after COMPANION was published, the Cardiac Resynchronization-Heart Failure (CARE-HF) study was published.19 In this study, inclusion criteria were NYHA class III to IV heart failure for at least 6 weeks, LVEF of 35% or less, left ventricular end-diastolic dimension of 30 mm indexed to height in meters, QRS duration of at least 120 ms, and treatment with OMT. Those with QRS duration between 120 and 149 ms were required to meet two of three echocardiographic criteria for dyssynchrony: (1) an aortic pre-ejection delay of more than 140 ms, (2) an interventricular mechanical delay of more than 40 ms, or (3) delayed activation of the posterolateral left ventricular wall. Key exclusion criteria were conventional indications for a pacemaker or defibrillator, requirement for continuous inotropic therapy, and chronic atrial arrhythmias. The devices had bi-ventricular pacing capability without defibrillator capacity. The CRT-P devices were programmed with backup atrial pacing at 60 beats/min, an interventricular delay of 0, and a programmed AV delay that was echocardiographically optimized. Every attempt was made to ensure that the left ventricular lead was placed along the posterolateral left ventricular wall during implantation guided by fluoroscopy. The primary endpoint was a composite of all-cause mortality or an unplanned hospitalization for a major cardiovascular event. Heart transplantation was counted as a death. The principal secondary outcome was death from any cause. In the study, 813 patients were randomized to OMT or to OMT plus CRT-P. Of the 409 patients, 390 (95%) assigned to CRT-P had a device implanted and 8 had an ICD lead placed in the right ventricle. Device implantation was attempted in 67 patients in the OMT arm, including 43 with CRT-P and 23 with CRT-D systems. Devices were activated in 50 of 404 patients in the OMT arm. The mean follow-up was 29.4 months, with survival status known in all patients. Baseline characteristics were similar between both groups. The mean LVEF was 25% and mean QRS 160 ms, and more than 60% of patients had NICM. More than 90% of patients in both arms had NYHA class III functional status at baseline. More than half the patients were taking β-blockers and spironolactone, with more than 90% taking ACE inhibitors or ARBs. The primary endpoint of death or hospitalization for heart failure was met in 159 patients in the CRT group versus 224 in the OMT group (39% vs. 55%; HR, 0.63; 95% CI, 0.51 to 0.77; P < .0001). In the CRT group, 82 deaths (20%) occurred versus 120 (30%) in the OMT group (HR, 0.64; 95% CI, 0.48 to 0.85; P < .002). The principal cause of death was cardiovascular in 83%, noncardiovascular in 17%, and not classifiable in 0.5%. In the medical therapy arm, 47% of deaths were attributed to progressive heart failure and 32% to SCD. In the CRT cohort, death was attributed to worsening heart failure in 40% and SCD in 35%. The 1-year all-cause mortality rate was 12.6% and 9.7%, respectively, in the OMT and CRT arms. The 2-year all-cause mortality rate followed a similar trend, with 25.1% in the OMT arm and 18% in the CRT group. As in previous trials of CRT, patients in the CRT arm had significant improvements in subjective and objective measures of heart failure symptomatology. The benefit seemed to be similar in both ischemic and NICM. Patients in the CRT group had significant and continual improvements in the hemodynamic and echocardiographic parameters. At 18 months, the CRT group had a 6.3 mm Hg increase in systolic blood pressure (P < .001), 6.9% improvement in EF (P < .001), and significant reductions in mitral regurgitation. The estimates of reduction in mortality rate should be interpreted in the setting of previously published literature. This was the first and only trial, thus far, that demonstrated an improvement in all-cause mortality with a pacing strategy alone compared with OMT. On the basis of these data, for every 9 patients implanted with CRT, one death and three hospitalizations for heart failure are prevented. This reduction in mortality rate is similar to that attributed to β-blocker treatment in heart failure versus placebo.69 Despite multiple trials showing the benefits of CRT in terms of symptom improvement, hemodynamic benefit, and overall mortality, many questions still remain.
Since the approval of CRT devices, observational reports have suggested that bi-ventricular pacing may increase the risk of ventricular arrhythmias in some patients.70 However, other studies have suggested that bi-ventricular pacing should reduce the risk of ventricular arrhythmias. Bi-ventricular pacing and left ventricular pacing have been shown to better reduce sympathetic nerve activation compared with right ventricular pacing.71 A substudy of 50 patients from the MIRACLE trial showed that control patients had 25% less heart rate variability compared with those randomized to CRT.72 Additional studies have shown that bi-ventricular pacing reduces QT dispersion and ventricular premature beats.73,74 Studies of appropriate ICD therapy in trials of CRT-D have been neutral.75
Although both bundle branch patterns were included in many of the CRT trials, the primary benefit of CRT has been realized in patients with left bundle branch pattern on ECG. Nonetheless, 20% to 30% of CRT recipients do not receive any benefit from device implantation. Accordingly, identifying clinical responders is an important goal. Echocardiographic measures of dyssynchrony did not prove clinically useful in a large multi-center study of CRT.76,77 At present, the magnitude of QRS prolongation in LBBB remains the best predictor of hemodynamic and clinical responses. Consistent with these observations, the Cardiac Resynchronization Therapy in Heart Failure and Narrow QRS Complexes (RETHINQ) study showed no benefit of CRT among patients with advanced heart failure and narrow QRS durations (≤130 ms) despite mechanical dyssynchrony.78 Another important group to evaluate are those with mild heart failure. Investigators sought to determine if early implantation of CRT could prevent progression of heart failure events in this patient population. In this regard, REVERSE showed significant reverse remodeling and a reduction of heart failure hospitalizations or mortality rate among patients with NYHA class I to II CHF, LVEF of 40% or less, and QRS duration of 120 ms or greater.79 Subsequently, the findings of MADIT-CRT were released.80 In this study, patients with an LVEF of 30% or less, QRS durations of 130 ms or greater, and NYHA class I or II were randomized in a 3 : 2 ratio to CRT-D or conventional ICD. The primary endpoint was death from any cause or a nonfatal heart failure event. With a mean follow-up of 28 months, 17.2% in the CRT-D group and 25.3% in the ICD only group reached the primary endpoint. This benefit was driven primarily by a 41% reduction in heart failure–related events. Neither REVERSE nor MADIT-CRT showed a reduction in SCD with ICD therapy. However, the study population in both these trials was at very low risk of SCD; the study was therefore underpowered to show a statistically significant reduction in SCD. It is likely that the findings of recent CRT trials will result in indications for CRT in those with more mild heart failure symptoms who otherwise meet the inclusion criteria for REVERSE and MADIT-CRT, except for possibly the exclusion of patients without LBBB.
Key References
Abraham WT. Cardiac resynchronization therapy for heart failure: biventricular pacing and beyond. Curr Opin Cardiol. 2002;17:346-352.
The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576-1583.
Bakker P, Meijburg H, deVries J, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Cardiovasc Electrophysiol. 2000:4395-4404.
Bardy G, Lee KL, Mark D, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461-2471.
Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure, for the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. N Engl J Med. 2004;350(21):2140-2150.
Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical electrocardiographic syndrome: A multicenter report. J Am Coll Cardiol. 1992;20:1391-1396.
Buxton A, Lee K, Fisher J, et al. A randomized study of the prevention of sudden cardiac death in patients with coronary artery disease, for the Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882-1890.
Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001:344873-344880.
Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure, Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. N Engl J Med. 2005;352(15):1539-1549.
Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT-II: frequency, mechanisms, predictors, and survival impact, for the MADIT-II Investigators. J Am Coll Cardiol. 2008;51(14):1357-1365.
Domanski M, Sakseena S, Epstein A, et al. Relative effectiveness of the implantable cardioverter-defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. J Am Coll Cardiol. 1999;34:1090-1095.
Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo: The (CAST) Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781-788.
Goldenberg I, Moss AJ, McNitt S, et al. Relationship among renal function, risk of sudden cardiac death, and benefit of implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98(4):485-490.
Hohnloser SH, Kick KH, Dorian P, et al. Prophylactic use of implantable cardioverter-defibrillator after myocardial infarction, on behalf of the DINAMIT Investigators. N Eng J Med. 2004;351:2481-2488.
Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy, for the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. N Engl J Med. 2004;350:2151-2158.
Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: The metoprolol CR/XL Randomized Intervention Trial In Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338.
Moss A, Hall W, Cannom D, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia, for the Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940.
Moss A, Zareba W, Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction, for the MADIT-II Investigators. N Engl J Med. 2002;346:877-883.
Sakseena S, Madan N, Lewis C. Implantable cardioverter-defibrillator are preferable to drugs as primary therapy in sustained ventricular tachyarrhythmias. Prog Cardiovasc Dis. 1996;38:445-454.
Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator: Randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707-1712.
1 American Cancer Society: Surveillance Research, Cancer Facts and Figures, Atlanta, GA, 2001, American Cancer Society.
2 Pell JP. Presentation, management and outcome of out-of-hospital cardiopulmonary arrest: Comparison by underlying etiology. Heart. 2003;89:839-842.
3 Larsen MP, Eisenberg MS, Cummins RO, Hallstrom AP. Predicting survival from out-of-hospital cardiac arrest: A graphic model. Ann Emerg Med. 1993;22:1652-1658.
4 Hazinski MF, Idris AH, Kerber RE, et al. Lay rescuer automated external defibrillator (“public access defibrillation”) programs: Lessons learned from an international multicenter trial. Circulation. 2005;111(24):3336-3340.
5 Merit-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: The metoprolol CR/XL Randomized Intervention Trial In Congestive Heart Failure (MERIT-HF). Lancet. 1999;353(9169):2001-2007.
6 Beck C, Pritchard W, Heil H. Ventricular fibrillation of long duration abolished by electrical shock. JAMA. 1947;135:985-986.
7 Mirowski M, Reid P, Mower M, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303:322-324.
8 The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576-1583.
9 Connolly S, Gent M, Roberts R, et al. Canadian Implantable Defibrillator Study (CIDS): A randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297-1307.
10 Kuck K, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: The Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748-754.
11 Nademanee K, Veerakul G, Mower M, et al. Defibrillator versus beta-blockers for unexplained death in Thailand (DEBUT): A randomized clinical trial. Circulation. 2003;107(17):2221-2226.
12 Lau EW, Griffith MJ, Pathmanathan RK, et al. The Midlands Trial of Amiodarone Versus Electrophysiologic-guided Interventions and Implantable Cardioverter-Defibrillators (MAVERIC): A multicentre prospective randomized clinical trial on the secondary prevention of sudden cardiac death. Europace. 2004;6(4):257-266.
13 Moss A, Hall W, Cannom D, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia, for the Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933-1940.
14 Moss A, Zareba W, Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction, for the MADIT-II Investigators. N Engl J Med. 2002;346:877-883.
15 Buxton A, Lee K, Fisher J, et al. A randomized study of the prevention of sudden cardiac death in patients with coronary artery disease, for the Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882-1890.
16 Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy, for the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. N Engl J Med. 2004;350:2151-2158.
17 Bardy G, Lee KL, Mark D, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
18 Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure, for the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. N Engl J Med. 2004;350(21):2140-2150.
19 Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure, Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. N Engl J Med. 2005;352(15):1539-1549.
20 Freemantle N, Tharmanathan P, Calvert MJ, et al. Cardiac resynchronisation for patients with heart failure due to left ventricular systolic dysfunction—a systematic review and meta-analysis. Eur J Heart Fail. 2006;8(4):433-440.
21 Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial, for the Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. JAMA. 2003;289:2685-2694.
22 . Defibrillators (AVID)—rationale, design, and methods, Antiarrhythmics versus Implantable. Am J Cardiol. 1995;75:470-475.
23 Domanski M, Sakseena S, Epstein A, et al. Relative effectiveness of the implantable cardioverter-defibrillator and antiarrhythmic drugs in patients with varying degrees of left ventricular dysfunction who have survived malignant ventricular arrhythmias. J Am Coll Cardiol. 1999;34:1090-1095.
24 Cairns J, Connoly S, Roberts R, Gent M. Randomized trial of outcome after myocardial infarction in patients with ventricular premature depolarizations, for the Canadian Amiodarone Myocardial Infarction Arrhythmia Trial (CAMIAT) Investigators. Lancet. 1997;349:675-682.
25 Julian D, Camm A, Francis G, et al. Randomized trial of the effect of amiodarone on mortality in patients with left ventricular dysfunction after recent myocardial infarction, for the European Myocardial Infarct Amiodarone Trial (EMIAT) Investigators. Lancet. 1997;349:667-674.
26 Sakseena S, Madan N, Lewis C. Implantable cardioverter-defibrillator are preferable to drugs as primary therapy in sustained ventricular tachyarrhythmias. Prog Cardiovasc Dis. 1996;38:445-454.
27 Spielman S, Schwartz S, McCarthy D, et al. Predictors of the success or failure of medical therapy in patients with chronic recurrent sustained ventricular tachycardia: A discriminant analysis. J Am Coll Cardiol. 1983;1:401-408.
28 Domanski MJ, Epstein A, Hallstrom A, et al. Survival of antiarrhythmic or implantable cardioverter defibrillator-treated patients with varying degrees of left ventricular dysfunction who survived malignant ventricular arrhythmias. J Cardiovasc Electrophysiol. 2002;13(6):580-583.
29 Hallstrom AP, McAnulty JH, Wilkoff BL, et al. Patients at lower risk of arrhythmia recurrence: a subgroup in whom implantable defibrillators may not offer benefit. Antiarrhythmics Versus Implantable Defibrillator (AVID) trial investigators. J Am Coll Cardiol. 2001;37(4):1093-1099.
30 Raitt MH, Renfroe EG, Epstein AE, et al. Stable ventricular tachycardia is not a benign rhythm: Insights from the AVID registry. Circulation. 2001;103(2):244-252.
31 Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo: The (CAST) Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781-788.
32 Prystowsky EN. Prevention of sudden cardiac death. Clin Cardiol. 2005;28(11 Suppl 1):I12-I18.
33 Bardy GH, Hofer B, Johnson G, et al. Implantable transvenous cardioverter-defibrillators. Circulation. 1993;87:1152-1168.
34 Alter P, Waldhans S, Plachta E, et al. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28(9):926-932.
35 Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical electrocardiographic syndrome: A multicenter report. J Am Coll Cardiol. 1992;20:1391-1396.
36 Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials: AVID, CASH, and CIDS studies. Eur Heart J. 2000;21:2071-2078.
37 Lee D, Green L, Liu P, et al. Effectiveness of implantable defibrillators for preventing arrhythmic events and death: A meta-analysis. J Am Coll Cardiol. 2003;41(9):1573-1582.
38 Oseroff O, Retyk E, Bochoeyer A. Subanalysis of secondary prevention trials: AVID, CIDS, and CASH. Curr Opin Cardiol. 2004;19(1):26-30.
39 Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009-1017.
40 Singh SN, Fletcher RD, Fisher SG, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmias. N Engl J Med. 1995;333:77-82.
41 Buxton A, Lee K, DiCarlo L, et al. Multicenter Unsustained Tachycardia Trial Investigators: Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. N Engl J Med. 2000;342:1937-1945.
42 Buxton AE, Lee KL, Hafley GE, et al. Relation of ejection fraction and inducible ventricular tachycardia to mode of death in patients with coronary artery disease. An analysis of patients enrolled in the multicenter unsustained tachycardia trial. Circulation. 2002;106(19):2466-2472.
43 Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease. Lessons from the MUSTT study. J Am Coll Cardiol. 2007;50(12):1150-1157.
44 Steinberg JS, Fischer A, Wang P, et al. The clinical implications of cumulative right ventricular pacing in the Multicenter Automatic Defibrillator Trial II, for the MADIT-II Investigators. J Cardiovascular Electrophysiology. 2005;16(4):359-365.
45 Goldenberg I, Moss AJ, McNitt S, et al. Relationship among renal function, risk of sudden cardiac death, and benefit of implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98(4):485-490.
46 Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular back-up pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) trial. JAMA. 2002;288:3115-3123.
47 Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT-II: frequency, mechanisms, predictors, and survival impact, for the MADIT-II Investigators. J Am Coll Cardiol. 2008;51(14):1357-1365.
48 Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009-1017.
49 Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction, and Mode Selection Trial Investigators. Circulation. 2003;107:2932-2937.
50 Wilber DJ, Zareba W, Hall WJ, et al. Time dependence of mortality risk and defibrillator benefit after myocardial infarction. Circulation. 2004;109(9):1082-1084.
51 Goldenberg I, Moss AJ, McNitt S, et al. Time dependence of defibrillator benefit after coronary revascularization in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47(9):1811-1817.
52 Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427-1436.
53 Hohnloser SH, Kick KH, Dorian P, et al. Prophylactic use of implantable cardioverter-defibrillator after myocardial infarction, on behalf of the DINAMIT Investigators. N Eng J Med. 2004;351:2481-2488.
54 Nolan J, Batin PD, Andrews R, et al. Prospective study of heart rate variability and mortality in chronic heart failure: Results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-Heart). Circulation. 1998;98(15):1510-1516.
55 Blatt JA, Poole JE, Johnson GW, et al. No benefit from defibrillation threshold testing in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial), for the SCD-HeFT Investigators. J Am Coll Cardiol. 2008;52(7):551-556.
56 Bansch D, Antz M, Boczor S, et al. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: The Cardiomyopathy Trial (CAT). Circulation. 2002;105:1453-1458.
57 Strickberger SA, Hummel JD, Bartlett TG, et al. Amiodarone versus implantable cardioverter-defibrillator: Randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J Am Coll Cardiol. 2003;41:1707-1712.
58 Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: A meta-analysis of randomized controlled trials. JAMA. 2004;292(23):2874-2879.
59 Abraham WT. Cardiac resynchronization therapy for heart failure: biventricular pacing and beyond. Curr Opin Cardiol. 2002;17:346-352.
60 Nelson GS, Berger RD, Fetics BJ, et al. Left ventricular or biventricular pacing improves cardiac function at diminished energy cost in patients with dilated cardiomyopathy and left bundle-branch block. Circulation. 2000;102:3053-3059.
61 Cazeau S, Ritter P, Bakdach S, et al. Four-chamber pacing in dilated cardiomyopathy. Pacing Clin Electrophysiol. 1994;17:1974-1979.
62 Cazeau S, Ritter P, Lazarus A, et al. Multisite pacing for end-stage heart failure: Early experience. Pacing Clin Electrophysiol. 1996;19:1748-1757.
63 Bakker P, Meijburg H, deVries J, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Cardiovasc Electrophysiol. 2000:4395-4404.
64 Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001:344873-344880.
65 Auricchio A, Stellbrink S, Sack S, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:1895-1898.
66 Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002:3461845-3461853.
67 Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: Results from the Multisite Stimulation in Cardiomyopathy (MUSTIC) Study. J Am Coll Cardiol. 2002;40:111-118.
68 Gras D, Leclercq C, Tang ASL, et al. Cardiac resynchronization therapy in advanced heart failure: The Multicenter InSync clinical study. Eur J Heart Fail. 2002;4:311-320.
69 CIBIS II Investigators. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet. 1999;353:9-13.
70 Shukla G, Chaudhry GM, Orlov M, et al. Potential proarrhythmic effect of biventricular pacing: Fact or myth? Heart Rhythm. 2005;2(9):951-956.
71 Hamdan MH, Zagrodzky JD, Joglar JA, et al. Biventricular pacing decreases sympathetic activity compared with right ventricular pacing in patients with depressed ejection fraction. Circulation. 2000;102:1027-1032.
72 Adamson PB, Kleckner KJ, VanHout WL, et al. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation. 2003;108:266-269.
73 Berger T, Hanser F, Hintringer F, et al. Effects of cardiac resynchronization therapy on ventricular repolarization in patients with congestive heart failure. J Cardiovasc Electrophysiol. 2005;16:611-617.
74 Walker S, Levy TM, Rex S, et al. Usefulness of suppression of ventricular arrhythmia by biventricular pacing in severe congestive cardiac failure. Am J Cardiol. 2000;86:231-233.
75 McSwain RL, Schwartz RA, DeLurgio DB, et al. The impact of cardiac resynchronization therapy on ventricular tachycardia/fibrillation: An analysis from the combined Contak-CD and InSync-ICD studies. J Cardiovasc Electrophysiol. 2005;16:1168-1171.
76 Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608-2616.
77 Higgins SL, Hummel JD, Niazi IK, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454-1459.
78 Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834-1843.
79 Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461-2471.
80 Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338.