Chapter 40 Atrioventricular Block
In 1852, Stannius noted that placing a ligature between the atria and the ventricles could cause bradycardia in a frog’s heart.1 In the late 1800s, Tawara and His identified the atrioventricular (AV) node and His bundle as the normal conduction axis between the atria and the ventricles in humans, and Wenckebach suggested blocked AV conduction as a cause for slow and irregular pulses.2–4
Epidemiology of Atrioventricular Block
Transient AV block can be observed in children and young adults during sleep5; persistent AV block is unusual. This type of AV block is usually caused by increased vagal tone and is often a normal finding. In a continuous monitoring study of 100 healthy teenaged boys, transient first-degree AV block was observed in 12% and second-degree AV block in 11%.5 In young adults the incidence of transient AV block decreases to about 4% in women and 6% in men.6,7 In the normal older adult population, transient type I (Wenckebach) second-degree AV block is seen only rarely (1%), and higher-grade AV block is not observed.8
Persistent first-degree AV block is rarely seen in young adults. Review of more than 70,000 electrocardiograms (ECGs) from young men entering the Canadian and U.S. military demonstrated a prevalence of first-degree AV block of less than 1%.9,10 Electrocardiographic studies have shown increased P-R intervals and an increased incidence of first-degree AV block with aging.9–14 While approximately 2% of adults older than 20 years of age have first-degree AV block, the incidence increases to more than 5% in people older than 50 years of age.11,13 With increasing age, the development of AV conduction disorders is more common; in one epidemiologic study of 1500 patients older than 65 years of age, AV conduction and intraventricular conduction defects were identified in 30% of patients.14 Using high-resolution electrocardiographic techniques, the increased P-R interval associated with aging was found to be caused by delay in conduction in the AV node or the proximal portion of the His bundle.11 In people younger than 60 years of age, it is uncommon (4%) for persistent first-degree AV block to progress to second-degree or higher-grade AV block in the absence of associated disease.13
Acquired persistent second-degree and third-degree AV blocks are almost never observed in normal populations regardless of age. The incidence of symptomatic high-grade AV block is currently estimated to be 200 per million per year.15
Isolated congenital complete (third-degree) AV block is a well-described problem that occurs in approximately 1 in every 20,000 live births.16 Congenital complete AV block is the most common manifestation of neonatal lupus erythematosus and appears to be associated with the development of autoantibodies in the maternal circulation. Other hereditary conditions associated with AV block are the Kearns-Sayre syndrome (ophthalmoplegia, retinitis pigmentosa) and myotonic dystrophy.17
Anatomy
Anatomy and Blood Supply
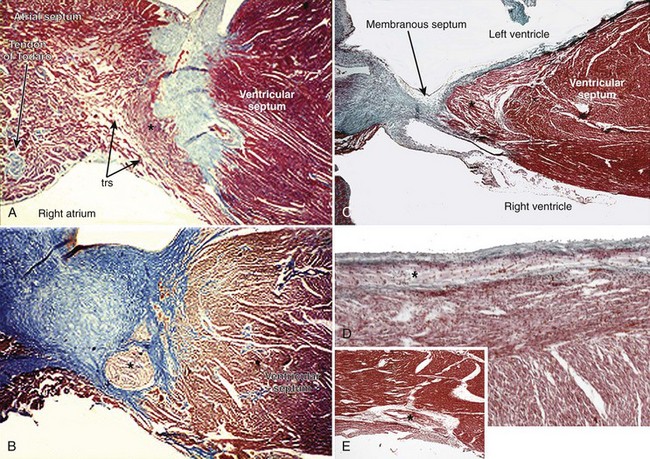
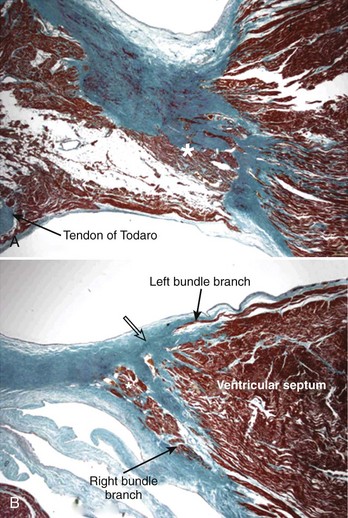
The AV system of specialized myocytes is the only muscular bridge through the insulating fibro-fatty tissue plane that separates the atrial myocardial masses from the ventricular myocardial masses. The AV system begins with the atrioventricular node at its atrial end. The compact node is recognizable, when seen in cross-sections, as a half-oval shaped structure adjacent to the central fibrous body in the young (Figure 40-1, A).18 With increasing age, the shape changes to become spindle-like.19 When traced inferiorly, toward the base of Koch’s triangle, the compact area diverges into two prongs, usually with the artery supplying the node running in between. The extent of the prongs varies from heart to heart; the right extension increases with age.20 Interposing between the compact node and the working atrial myocardium is a zone of transitional cells. These cells are histologically distinct from the cells of the compact node as well as the working cells but are not insulated from the surrounding myocardium. They approach the compact node superiorly, inferiorly, and from the left and right sides of the atrial septum. In the vestibular area of the right atrium, an overlay of ordinary myocytes is present in the subendocardium from the atrial wall in front of the oval fossa that streams over the zone of transitional cells. The transitional cells, therefore, provide the crucial proximal bridge between the ordinary and the specialized myocardium. Fibro-fatty tissue increases with age in this region, and the transitional cell zone becomes wider.19 When the conduction system is traced into the penetrating bundle of His, little difference is seen in the cellular composition of the two areas (see Figure 40-1, B). The specialized myocardial cells themselves, however, become aligned in a more parallel fashion distally into the AV conduction bundle. The AV node, therefore, is an integral part of the atrial musculature (see Figure 40-1, A), in contrast to the atrioventricular bundle, which, in passing through the central fibrous body, is insulated from the adjacent myocardium (see Figure 40-1, B). The His bundle, approximately 2 mm in cross-sectional diameter, is the only bridge of myocardial continuity between the atria and the ventricles. The common AV bundle and its continuation, the branching bundle, and bundle branches are also ensheathed in fibrous tissue. In the normal heart, the AV-branching bundle is sandwiched between the membranous septum and the muscular ventricular septum, usually a little to the left of the septal crest, giving origin to the left and right bundle branches (see Figures 40-1, C to E). The AV bundle lies in the subendocardium of the left ventricle beneath the commissure between the right-coronary and noncoronary leaflets of the aortic valve. In most hearts, the right bundle branch passes through the septal crest to emerge subendocardially in the septal aspect of the right ventricle (see Figure 40-1, E). Distally, the bundle branches divide into increasingly finer branches. Eventually, they ramify into the so-called Purkinje network and lose the fibrous sheaths at the interface with the ordinary ventricular myocardium.18
Most frequently, the arterial supply to the AV node is a branch from the right coronary artery, whereas it is a branch from the circumflex artery in nearly 20%. When the dominant right coronary artery extends beyond the cardiac crux, the nodal artery originates distal to the U loop of the dominant artery.20,21 In most hearts, the penetrating bundle and AV conduction bundle are supplied by a branch from the posterior descending coronary artery. The right bundle branch is supplied by the first septal perforating artery arising from the anterior descending coronary artery, which also supplies the AV conduction bundle in some hearts.22,23
Congenital Complete Atrioventricular Block
Congenital complete heart block can occur in congenitally malformed hearts or in otherwise normal hearts.24 Complete AV block associated with a cardiac defect is most frequently seen in the anomaly of congenitally corrected transposition, in isomeric arrangement of the atrial appendages, and in some AV septal defects. Although complete AV block may be present at birth, it is more usual for the arrhythmia to be progressive.
When occurring in an otherwise structurally normal heart, the pattern of the cardiac conduction system can take one of three anatomic forms according to the location of the interruption: (1) atrial-axis discontinuity, (2) nodal-ventricular discontinuity, or (3) intraventricular discontinuity.25–27 The last form is extremely rare.28 The AV node is deficient or even absent in the most common form, which is described as atrial-axis discontinuity.25,29 The block is proximal to the His bundle. In the extreme form, the area that is normally occupied by the compact AV node and the zone of transitional cells is replaced by fibrous tissue or fibro-fatty tissue (Figure 40-2, A). In others, variable remnants of the nodal tissues appear as islands in the fibrous or fibro-fatty tissues.
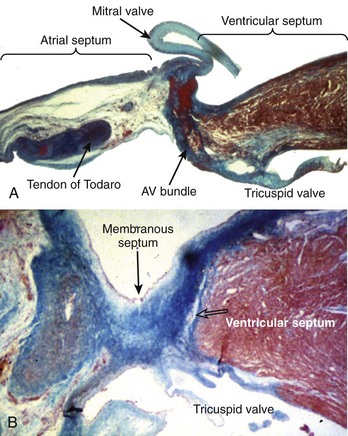
Figure 40-2 Histologic sections from two infants with congenital complete heart block cut in similar planes as those shown in Figure 40-1. A, An extreme case of atrial-axis discontinuity. The atrial septum totally lacks the myocardium in the region of the atrioventricular node and the node is missing. B, An example of intraventricular discontinuity. Only a small island of the branching bundle buried in the fibrous tissue remains (open arrow) (trichrome stain: myocardium in red; fibrous tissue in green-blue).
The rarest form, intraventricular discontinuity of the conduction bundle, may occur in families.28 The branching bundle or the proximal parts of the bundle branches are replaced by fat or fibrous tissue (see Figure 40-2, B).
The association of congenital complete heart block with maternal connective tissue disease is well documented.30–32 The presence of anti-Ro(SS-A) antigen in maternal serum is a marker for isolated congenital heart block.32 A study of hearts from 8 afflicted children has shown the lack of an AV node in 7, whose maternal sera were anti-Ro positive, and nodal-ventricular discontinuity in the remaining 1, whose maternal serum was anti-Ro negative.33 However, Chow et al described two cases with a combination of nodal-ventricular and intraventricular discontinuities demonstrating that morphologic patterns are not always clear cut.34 Furthermore, anti-Ro antibodies were not exclusively associated with atrial-axis discontinuity, since maternal serum was anti-Ro positive in one of these two cases.
Another study compared the anatomic substrate producing complete heart block in normally structured hearts and in hearts with isomerism of the atrial appendages.35 The pattern of AV block in the cases with left isomerism was nodal-ventricular discontinuity, whereas all but one of the normally structured hearts showed atrial-axis discontinuity with the anticipated nodal area filled with fibro-fatty tissue. The remaining normally structured heart showed the rarest substrate—intraventricular discontinuity. Interestingly, associated fibrosis of several of the sinus nodes was present, suggesting some sort of target mechanism or deficiency specific to the conduction tissues.
The association of complete AV block with congenitally corrected transposition of the great arteries is well recognized.36 The block may be congenital because of deficiency of the AV node or bundle. More commonly, AV block is progressive in adults. When congenitally corrected transposition occurs with usual arrangement of the atria, the AV conduction system is abnormally located. The AV node is displaced anteriorly, and the AV conduction bundle courses anterosuperiorly, in the trigonal region of fibrous continuity between the pulmonary and mitral valves, related to the anterocephalad border of the left ventricular outflow tract before entering the ventricular septum. The long bundle renders it particularly vulnerable to fibrotic changes with increasing age, eventually resulting in interruption.
Acquired Complete Atrioventricular Block
Complete AV block can be categorized into four major pathologic groups and a further group with miscellaneous conditions.37 An autopsy series of hearts from 200 patients who had AV block of more than 1-month duration found idiopathic bilateral bundle branch fibrosis in 38%, coronary artery disease in 17.5%, cardiomyopathy in 13%, calcific AV block in 11%, and other causes in 20.5%.37
Idiopathic Bilateral Bundle Branch Fibrosis
The crest of the muscular ventricular septum undergoes degenerative changes, including fibrosis, fatty infiltration, and focal microscopic dystrophic calcification with advancing age. The normal aging process begins in the fourth decade of life but may be accelerated by coronary artery disease, diabetes mellitus, and hypertensive heart disease. Since the branching AV bundle and the beginning of the main bundle branches are located in this area, they are susceptible to being disrupted (Figure 40-3). Two forms of bundle branch fibrosis, focal and diffuse—dubbed Lev disease and Lenègres disease, respectively—are recognized, but they probably represent extreme ends of a pathologic spectrum.38,39 In both groups the mean ages are reported to be in the sixth and seventh decades. Damage to the distal branches is minimal in the focal form, whereas damage in the diffuse form can extend to the middle and distal portions of both left and right bundle branches.
Coronary Artery Disease
Complete AV block can occur in acute or chronic coronary artery disease. Since different segments of the proximal portion of the AV conduction system are supplied by the right or the left coronary systems, the site of infarction related to coronary occlusion is important. When AV block complicates acute posteroseptal myocardial infarction, the occluded coronary artery is always the artery that ultimately gives origin to the AV nodal artery. Most cases reveal only small focal areas of necrosis in the node, the AV bundle, or both. Massive necrosis of the AV node and its approaches, inflammatory cell infiltration, or occlusion of the nodal artery is rare. In some cases, no obvious pathologic changes are seen in conduction tissues. By contrast, anteroseptal infarction associated with occlusion of the anterior descending coronary artery is more extensive and causes necrotic damage to the branching and bundle branches. The AV node may or may not be spared, although careful studies have shown no arterial lesions in the supply to the AV node.40
Chronic coronary insufficiency, with or without previous myocardial infarction, can affect various parts of the AV conduction system. The damage predominantly to the bundle branches may, over time, progress to cause complete AV block. The most common pattern in autopsy series is destruction of both bundle branches.39,41 Pathologic studies suggest that the majority of patients with chronic AV block do not have more coronary atherosclerosis than do normal patients without AV block.41,42 Some cases may be associated with small vessel disease.
Cardiomyopathy
Hypertrophic cardiomyopathy is rarely associated with chronic AV block. Histologic investigations have shown interstitial fibrosis or necrosis in the conduction system, interruption of the His bundle, abnormally small intramural coronary arteries with thick walls, and narrow lumens.43–45
Miscellaneous
This heterogeneous group includes amyloidosis, hemochromatosis, connective tissue disease, myocarditis familial cardiomyopathy and skeletal myopathy, infective endocarditis, and tumors involving the conduction system. In amyloidosis, heavy deposits are found in the sinus node with relatively small amounts in the AV node and main conduction bundles. In patients with AV block, deposits are present in the ventricular myocardium, which also shows extensive scarring. Involvement of the small arteries suggest fibrosis and attenuation of the conduction bundles are more related to the scarring process.41 Myocarditis of any etiology, whether acute or chronic, may cause complete AV block. In the acute phase, inflammatory changes are predominantly in the ventricular myocardium affecting the distal bundle branches. Rarely, the conduction system is more involved than is the surrounding myocardium.41 When the conduction system is involved, the changes include degeneration, vacuolization, and necrosis of cells of the bundle branches. Infective endocarditis affecting the aortic valve, the mitral valve, or both may extend to the central fibrous body, membranous septum, and crest of the muscular ventricular septum to involve the AV bundles. Any type of tumor that metastasizes to the heart has the potential to affect the conduction system to varying degrees. Metastatic tumor deposits in the ventricular septum can destroy the bundle branches. One particular benign tumor, a mesothelioma, has an affinity for the AV node and its approaches. It is a slow-growing cystic mass located in this region of the atrial septum, hence also known as cystic tumor of the AV node. A female-to-male ratio of approximately 3 : 1 and a mean age of 38 years at presentation are seen. Some patients may die suddenly without any history of heart problems.46
Surgical and Interventional
Complete AV block may be a consequence of interventional procedures to correct congenital heart malformation. Damage may be caused by sutures or incisions into the musculature containing the conduction bundles, AV node, or their arterial supply. Although surgically induced iatrogenic block is rare nowadays, the shift toward trans-catheter device closure for ventricular septal defects has again put the spotlight on the potential risk to the AV conduction system.47,48 AV block may also be a complication following isolated myectomy or alcohol ablation for hypertrophic obstructive cardiomyopathy.49,50
Basic Electrophysiology
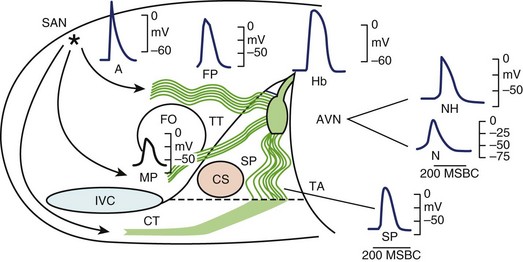
It is generally accepted that the AV junction consists of the AV node with its atrial connections of transitional fibers and the bundle of His.51 In electrophysiological considerations of AV block, it is useful to define the AV junction more broadly, including the atrial connections to the AV node, the AV node, the bundle of His, and the proximal portions of the bundle branches that are insulated from the ventricular myocardium by fibrous matrix. Heart block can occur from interruption of conduction in any of these components of the AV junction as defined broadly. In this array of components, great electrophysiological and histologic diversity is seen (Figure 40-4). The range of conduction velocities, resting and action potential (AP) amplitudes, AP durations, and intercellular conductivity observed within the AV junction is greater than in the remainder of the atrial and ventricular myocardium.
The Compact Atrioventricular Node
Within the central compact node are cells with distinct electrophysiological characteristics. These characteristics of the prototypical AV nodal cells manifested by intracellular recordings in multicellular preparations have been replicated in cells isolated from the AV node, allowing determination of the activities of specific currents, pumps, and exchangers.52,53 The amplitudes of resting potentials (–60 mV), APs (–85 mV), and upstroke velocities (<10 V/sec) are reduced. These cells manifest diastolic depolarization, a high total transmembrane (input) resistance, and relative insensitivity to changes in extracellular K+. They have post-repolarization refractoriness; the absolute refractory period outlasts the AP. The relative refractory period, during which relatively diminished, more slowly conducting APs are generated, is prolonged well into diastole. The amplitudes and upstroke velocities of APs and conduction velocity are strongly modulated by autonomic activity, increasing with adrenergic (sympathetic) stimulation and decreasing with cholinergic (vagal) stimulation. The AV junction is richly innervated with vagal and sympathetic fibers, but recent observations with high-resolution staining for neural tissues indicate that innervation of the compact node may be more sparse than that of the transitional fibers or the nodal extensions.53,54
AV nodal cells, like sinoatrial nodal cells, lack sodium (Na+) channels, which provide the excitatory current for atrial and ventricular cells; some cells may have channels that are inactivated at the low resting potentials.55,56 The excitatory current for AV nodal cells is ICaL, a calcium current that generates a slow upstroke because it is slower and less intense than the Na+ current. L-type calcium ion (Ca2+) channels require a relatively long period to recover from inactivation, hence the post-repolarization refractoriness of the AV node. The potent actions of autonomic stimulation on AV nodal conduction are largely due to the modulation of ICaL by adrenergic and cholinergic stimulation via specific receptors and transduction systems. This current is enhanced, and its kinetics of activation, inactivation, and recovery are accelerated by adrenergic stimulation; it is conversely affected by cholinergic stimulation.
T-type Ca2+ current (ICaT) has been shown recently to be important in AV conduction in mice. Genetically modified mice lacking these channels manifest slow conduction within the AV node as well as reduced rates of AV nodal pacemakers.57 A study has reported congenital heart block and sinus bradycardia in children exposed to maternal antibodies that reduce both L-type and T-type channels, indicating a possible role of T-type channels in human AV nodal conduction.58
Ca2+-activated K+ channels (KCa) have been demonstrated in the activation of KCa in human atrial myocytes and has been shown to increase the rate of AV nodal pacemakers and accelerate repolarization.59,60
Gap junctions appear to be sparse and smaller in the central AV node than in the atrial and ventricular myocardium.61 The gap junctions in the node are composed predominantly of connexin40 rather than connexin43, which is dominant in the ventricular myocardium. The relatively poor intercellular communication in the AV node is probably the result of these gap junction properties as well as a relative increase in the volume of extracellular space surrounding AV nodal cells, compared with the atrial and ventricular myocardium.
Transitional Atrio-Nodal Connections
The transitional fibers connecting the atrium to the AV node aggregate in zones that are functionally relatively discrete, though not insulated from the atrial myocardium. Observations indicate that anisotropy is a major determinant of directional conduction in these pathways.62 They extend from the node to and beyond the border of Koch’s triangle and connect to the node at relatively discrete sites along its margins.
Newer techniques for high-resolution integrated histologic and molecular mapping of the AV junction and its atrial connections in the rabbit heart have disclosed additional complexities of its structure and function.63 The compact node stains positive for neurofilament, which is expressed in the conduction system but not the atrial or ventricular myocardium, but connexin43 stains weakly in the node. The myocytes in the node are small and dispersed within fibrous tissue. The infero-posterior extension of the node toward the coronary sinus, thought to represent the slow pathway, is also positive for neurofilament and sparse in connexin43 with small dispersed cells like the compact node. Transitional tissue, which joins the node at its inferoposterior end near the coronary sinus and also more anteriorly near the compact node, has connexin43, lacks neurofilament, but has smaller dispersed cells, thus being intermediate between the node and the atrium. The anterior transitional fibers are thought to represent the fast pathway. The node is connected to the atrium at discrete sites with transitional fibers joined to nodal and atrial fibers. The infero-posterior extension may be the primary site for AV nodal pacemakers.64
Certain observations indicate that the atrial connections to the node are more complex.51 A mid-pathway with deep (in relation to the right endocardium) connections to the node in its superior aspect and connections to the atrium in the septum posterior to the fossa ovalis has been described. Left-sided connections have been described in humans.65 The functional inter-relationships of the pathways in normal AV conduction are not fully elucidated. It is clear that these pathways, the atria, and the AV node can form various configurations of re-entry circuits that cause AV nodal re-entrant tachycardia. Interruption of the atrial connections to the AV node can produce AV block.
Bundle of His and Bundle Branches
The bundle of His and bundle branches, which are insulated by fibrous matrix from the ventricular myocardium, constitute the ventricular aspect of the AV conduction axis. They function to rapidly disseminate activation to the ventricular myocardium in a pattern that optimizes ejection of blood by a synchronized and coordinated apex to base contraction.66 Conduction in these tissues is the most rapid of all conductions in cardiac tissue, promoting synchronization of activation of distal sites. The architecture of the bundle branches and their proximal insulation promote apex-to-base contraction and septal-free wall synchronization. The APs of these fibers in multicellular preparations manifest very rapid upstrokes, prolonged plateaus and repolarization, and automaticity.
Quantitative measurement of individual currents in isolated Purkinje cells has been relatively limited. The Na+ current is abundant, accounting, in part, for the fast upstroke and the rapid conduction velocity. The ionic basis for the prolonged plateau has not been determined, but IKs appears to be less active in Purkinje cells than in ventricular myocardial cells.67 The T-type Ca2+ current is present in Purkinje fibers and may contribute to automaticity and to the excitatory current.68
The basis for the automaticity of the His-Purkinje system is debated. Some favor the decay of a K+ current activated during the AP as the primary basis for automaticity, whereas others favor the current If.69 As in the sinoatrial node and the AV node, multiple ionic determinants of automaticity may be present. The automaticity of the His-Purkinje system is more prominent proximally than distally. Automatic firing of the distal Purkinje system is slow and erratic. As a result, heart block caused by degeneration and fibrosis of the bundle branches is more malignant than is heart block caused by the interruption of conduction in the AV junction proximal to the bundle of His.
Gap junctions are abundant in the Purkinje strands of the bundle branches and their distal ramifications. They are uniformly distributed along the lengths and ends of the fibers and promote good intracellular communication throughout the margins of the cells and rapid conduction. However, the bundle of His fibers appear relatively poorly connected in the transverse dimension, so dissociation within the bundle of His has been observed in experimental animals and in humans.70
Diagnostic Techniques
Since the prognosis and treatment for AV block differ, depending on whether block is within the AV node or is infranodal, determining the site of block is important. In many cases, this can be done noninvasively. The QRS duration, P-R intervals, and the ventricular rate on surface ECG can provide important clues in localizing the level of block. Several noninvasive interventions may also prove helpful, such as vagal maneuvers, exercise, or administration of intravenous atropine. These methods take advantage of the differences in autonomic innervation of the AV node and of the His-Purkinje system.71 While the AV node is richly innervated and highly responsive to both sympathetic and vagal stimuli, the His-Purkinje system is influenced minimally by the autonomic nervous system. Carotid sinus massage increases vagal tone and worsens AV nodal block. Exercise or atropine improves AV nodal conduction due to sympathetic stimulation. In contrast, carotid sinus massage improves infranodal block, while exercise and atropine worsen infranodal block because of the change in the rate of the impulses being conducted through the AV node.
An electrophysiological study is indicated in a patient with suspected high-grade AV block as the cause of syncope or presyncope when documentation cannot be obtained noninvasively.72,73 In patients with coronary artery disease, it may be unclear whether symptoms are caused by AV block or ventricular tachycardia, and an electrophysiological study can be useful in establishing the diagnosis. Some patients with known second-degree or third-degree block may benefit from an invasive study to localize the site of AV block and to determine therapy or assess prognosis. Once symptoms and AV block are correlated by electrocardiography, further documentation by invasive studies is not typically required. Asymptomatic patients with transient Wenckebach block associated with increased vagal tone should not undergo invasive electrophysiological investigation.
The electrophysiological study allows analysis of the bundle of His ECG as well as the performance of atrial and ventricular pacing to identify conduction abnormalities and inducible ventricular tachycardia. The atrio-His (A-H) and His-ventricle (H-V) intervals are measured from the bundle of His ECG.74 Atrial pacing techniques are used to define the site of block and assess AV nodal and His-Purkinje conduction. During decremental atrial pacing, the A-H interval normally will gradually lengthen until AV nodal Wenckebach block is noted. The H-V interval will normally remain consistent despite different pacing rates. Abnormal AV nodal conduction is defined as Wenckebach block occurring at slower atrial-paced rates than what is normally seen (i.e., >500 ms). To determine whether AV nodal disease is truly present or just under the influence of excessive vagal tone, atropine alone or autonomic blockade with atropine and propranolol can be given to differentiate inherently abnormal AV nodal conduction from vagally mediated abnormalities. Infranodal block (Mobitz type 2) is present when the atrial deflection is followed by the His ECG, but no ventricular depolarization is seen. Block below the His is abnormal, unless associated with very-short-paced cycle lengths (350 ms or less).75
While retrospective studies provide useful information that can often help generate a hypothesis, they frequently exaggerate the benefit of treatment. It is always preferable to base clinical decisions, if possible, on data from prospective randomized trials. However, no prospective randomized trials have evaluated the efficacy of pacing therapy in patients with AV block, as there are no alternatives to pacing therapy for the patient with symptomatic AV block (not because of reversible causes). In addition, it is difficult to assemble large groups of asymptomatic patients with specific types of AV block. Recommendations for permanent pacemaker implantation are based on observational studies on the natural history of AV block. In general, permanent pacemakers are implanted in patients with symptomatic AV block and in patients with asymptomatic AV block caused by His-Purkinje disease.76
Pacing Mode Choice
In the Pacemaker Selection in the Elderly (PASE) trial, 407 older adults (mean age 76 years) were randomized to either the rate-adaptive ventricular inhibited (VVIR) or rate-adaptive dual-chamber (AV) inhibited/triggered (DDDR) pacing mode.77 In the 201 patients who had pacing systems implanted for AV block, no reduction in mortality, stroke, or atrial fibrillation was observed. In contrast to the observation in patients with sinus node dysfunction, dual-chamber pacing was not associated with improvement in quality-of-life indices. In the Canadian Trial of Physiologic Pacing (CTOPP), 2568 patients with symptomatic bradycardia were randomized to single-chamber ventricular pacing or a “physiologic” pacing mode that preserved AV synchrony (single-chamber atrial pacing or dual-chamber pacing).78 AV block was the indication for pacing in approximately 60% of the patients. Physiological pacing was not associated with a reduction in stroke or death due to cardiovascular disease, which was the study’s primary endpoint. Interestingly, physiological pacing was associated with a reduction in the development of chronic atrial fibrillation even in those patients with pacing systems implanted for AV block.79 A large prospective study recently examined the importance of pacing mode selection in older adults with AV block.80 In the United Kingdom, in the Pacing and Clinical Events (UK-PACE) Trial, patients older than 70 years of age with AV block were randomized to the ventricular inhibited (VVI), VVIR, and DDD pacing modes. Patients were followed up for at least 3 years. The primary endpoint was all-cause mortality, although other secondary outcomes such as atrial fibrillation, heart failure, stroke/transient ischemic attack/thromboembolism, pacing system revision, cardiovascular events such as angina or myocardial infarction, exercise capacity, and quality of life were also evaluated. A composite endpoint of all outcomes was also examined. Patient recruitment occurred from 1995 to 1999, and the trial terminated in September 2002 with a median follow-up of 4 years.80 Of the patients, 504 were randomized to VVI pacing, 505 to VVIR pacing, and 1012 to DDD pacing. Some patients remained in the randomized mode: 96.6% of VVI patients, 96.4% of VVIR patients, and 87.7% of DDD patients. No difference was seen in all-cause mortality or time to atrial fibrillation or other cardiovascular events. An increased stroke risk with VVI pacing (hazard ratio, 1.58) was observed, but no differences were found in any other secondary endpoints or in the composite endpoint. This trial suggests that older patients derive limited and specific benefits and fail to obtain other favorable results compared with younger patients with DDD pacing. This may reflect more advanced atrial and cardiovascular disease status or other variables.
In summary, it is unlikely that any randomized trial will be performed to evaluate the efficacy of pacing therapy in patients with AV block. Although the evidence for the usefulness of dual-chamber pacing in patients with AV block is incomplete at present, most patients with AV block should receive a dual-chamber pacing system.76,81
Management of Atrioventricular Block
Temporary Pacing
In the hemodynamically unstable patient with AV block, the clinician must identify any rapidly reversible causes. Reversible causes include hyperkalemia, hypoxia, increased vagal tone, and ischemia. While treatment for reversible causes is initiated, it must be decided quickly whether temporary pacing will be required for the hemodynamically unstable patient. Temporary pacing is most quickly initiated by transcutaneous passage of current between two specially designed pads placed on the chest wall. Patch position is the most important factor for determining the effectiveness of transcutaneous pacing. The cathode should be placed on the left chest over the cardiac apex, and the anode placed on the back between the spine and the scapula or anteriorly just above the right nipple. Currents of 20 to 140 mA are usually required to capture and activate the ventricles. Using the correct technique, transcutaneous pacing is effective in more than 90% of cases and associated with very few complications.82,83 However, transcutaneous pacing cannot be used for prolonged periods because of patient discomfort and the unreliability of capture due to impedance changes.
If temporary pacing must be used for more than 30 minutes, transvenous pacing should be initiated, as it is far more stable and better tolerated. With transvenous pacing, intravascular access is obtained usually through the right internal jugular vein and a pacing catheter is positioned into the right ventricle. The pacing catheter is connected to a pulse generator; ventricular capture can usually be obtained with currents of 1 to 2 mA. Transvenous pacing can be used for long periods with minimal complications (<2% once venous access is achieved).82
Permanent Pacing
Once the patient is stabilized, the clinician must assess whether the AV block will be permanent. In several conditions, persistent AV block will gradually resolve. In approximately 10% to 15% of patients with inferior and posterior wall myocardial infarctions, transient second-degree, advanced, or complete AV block will be observed.84 In almost all cases, AV block will resolve, and permanent pacing is not required. Approximately 8% to 10% of patients with Lyme disease will have transient AV conduction abnormalities caused by myocarditis involving the AV nodal region. AV block usually resolves within several weeks, and permanent pacing is almost never required. Acute rheumatic fever can also present with AV block that is expected to resolve after several weeks.84
Indications for Permanent Pacing
Indications for permanent pacing in acquired AV block have been published by a Joint Committee of the American College of Cardiology (ACC) and the American Heart Association (AHA) in 1984, 1991, 1998, and 2002, and in 2008 in collaboration with The Heart Rhythm Society (HRS).76 The ACC/AHA/HRS 2008 guidelines use the standard three-group classification schema. Class I indications are conditions for which there is evidence or general agreement that a given procedure or treatment is beneficial, useful, and effective. Class II indications are conditions for which there is conflicting evidence or a divergence of opinion about the usefulness/efficacy of a procedure or treatment. Class II has been further divided into class IIa, where the weight of evidence/opinion is in favor of usefulness/efficacy, and class IIb, where usefulness/efficacy is less well established by evidence/opinion. Class III indications are conditions for which there is evidence or general agreement that a procedure/treatment is not useful/effective and, in some cases, may be harmful. Despite some shortcomings,76 the ACC/AHA/HRS guidelines provide a useful framework for management. The indications for permanent pacing in symptomatic patients with second-degree or third-degree AV block are often straightforward (Table 40-1). Controversial indications involve mostly asymptomatic patients.76,85 The decision to implant a pacemaker in asymptomatic patients is more difficult and requires knowledge of the pathophysiology and natural history of AV block.86 As a general rule, since escape rhythms from ventricular tissue are unreliable, a pacemaker should be implanted in asymptomatic patients if AV block occurs in His-Purkinje tissue.
Table 40-1 Indications for Permanent Pacing in Atrioventricular Block
| Permanent pacemaker implantation is indicated for third-degree and advanced second-degree atrioventricular (AV) block at any anatomic level associated with the following:
1. Bradycardia with symptoms (including heart failure) or ventricular arrhythmias presumed to be caused by AV block
2. Arrhythmias and other medical conditions that require drug therapy that results in symptomatic bradycardia, in awake, symptom-free patients in sinus rhythm, with documented periods of asystole ≥3.0 seconds) or any escape rate less than 40 beats/min
|
Second-Degree Atrioventricular Block
In general, type 1 second-degree AV block associated with a narrow QRS complex (<0.12 seconds) is due to block in the AV node, and the current published guidelines do not recommend pacemaker implantation in the asymptomatic patient. In a study of 56 patients with documented chronic second-degree AV block caused by AV nodal conduction delay, those without associated cardiac disease had a benign course, whereas those with associated cardiac disease had a poor prognosis because of progression of underlying cardiac disease rather than the development of sudden bradycardia.86 However, in another retrospective study of 214 patients with second-degree AV block, survival and requirement for pacing were not different among patients with type 1 and type 2 heart block, and the presence or absence of bundle branch block did not appear to aid in the prediction of survival.87 In view of these conflicting data, it is prudent to closely monitor patients with type 1 second-degree AV block and a narrow QRS complex for symptoms and for progression of conduction tissue disease (e.g., development of fascicular block or QRS widening). If type 1 second-degree AV block is associated with a wide QRS complex (>0.12 seconds) AV block will be located in the AV node in 30% to 40% of patients and in the His-Purkinje system in 60% to 70% of cases.88,89 In these cases, an invasive electrophysiological study is often required to identify the site of block. If intra-Hisian or infra-Hisian block is identified, a pacemaker should be implanted, as these conditions usually progress to complete heart block within 5 years.90
Type 2 second-degree AV block generally occurs in His-Purkinje tissue. Asymptomatic patients with type 2 AV block usually do develop symptoms and will require permanent pacing.79
In 2 : 1 second-degree AV block, every other P wave conducts, preventing comparison of consecutive P-R intervals. The QRS complex provides a clue as to the site of block: A narrow QRS complex is associated with His-Purkinje block 30% of the time, and a wide QRS complex is associated with His-Purkinje block approximately 80% of the time.17,91 In the asymptomatic patient with 2 : 1 block, maneuvers (such as exercise, atropine, or continuous ECG monitoring) to alter the conduction ratio between the atria and the ventricles may allow localization of the site of block. However, in some cases, electrophysiological evaluation to determine the site of block will be required.
First-Degree Atrioventricular Block
If first-degree AV block is severe, atrial activation and contraction can occur while the ventricles are contracting in response to the previous atrial contraction, which leads to an inappropriate rise in atrial pressures and symptoms similar to pacemaker syndrome. In this situation, symptoms can be significant, even with exercise, as the P-R interval does not shorten appropriately with adrenergic stimulation. The current guidelines classify symptomatic first-degree AV block as a class IIa indication for pacemaker implantation.76 Asymptomatic first-degree AV block is a class III indication for pacing. The one exception to this recommendation is the asymptomatic patient with first-degree AV block, abnormal QRS axis caused by left anterior or left posterior fascicular block, and neuromuscular disease.76 Neuromuscular diseases such as myotonic dystrophy and Kearns-Sayre syndrome are associated with progressive AV block; since development of complete heart block can be unpredictable, implantation of a permanent pacemaker is justified.
Pacing Mode Choice
Three pacing modes (DDD, VDD, and VVI) can be used to prevent bradycardia in patients with AV block.
VVI and VVIR Pacing
In the VVI or VVIR pacing mode, bradycardia is prevented, and if rate adaption is programmed to be “on,” the heart rate will increase with exercise. However, AV synchrony not present in either of these pacing modes. The importance of AV synchrony is controversial in patients with AV block because the main contribution to increased cardiac output with exercise is heart rate rather than AV synchrony and the incidence of pacemaker syndrome is lower in patients with AV block compared with patients with sinus node dysfunction. However, it seems intuitively reasonable that in the presence of organized atrial activity, the VDD and DDD pacing modes are most appropriate. In fact, since the early 1990s, the British Pacing and Electrophysiology Group guidelines have stated that the only indication for the VVI and VVIR pacing modes is atrial fibrillation/flutter with AV block or slow ventricular response.81 The VVI and VVIR pacing modes may also be appropriate in patients who are incapacitated and inactive as well as in those with other medical problems associated with a short life expectancy.
DDD and DDDR Modes
The DDD pacing mode prevents bradycardia and provides AV synchrony in patients with AV block. Although less important for exercise-related increases in cardiac output, several studies have demonstrated that AV synchrony is associated with improved symptoms at baseline levels of activity. The report of a large randomized study (UK-PACE) that compared the VVI and DDD pacing modes in older patients (older than 70 years of age) with second-degree or third-degree AV block has been published.80 Although the DDD pacing mode is the most complex, most guidelines recommend this pacing mode in patients with AV block, as it maintains AV synchrony regardless of the cause of the bradycardia.
Key References
Abate E, Kusumoto F, Goldschlager N. Techniques for temporary pacing. In Kusumoto FM, Goldschlager N, editors: Cardiac pacing for the clinician, ed 2, New York: Springer, 2008.
Barold SS, Hayes DL. Second-degree atrioventricular block: A reappraisal. Mayo Clin Proc. 2001;76(1):44-57.
Bharati S, Rosen KM, Strasberg B, et al. Anatomic substrate for congenital atrioventricular block in middle aged adults. Pacing Clin Electrophysiol. 1982;5:860-869.
Connolly SJ, Kerr CR, Gent M, et al: Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes, N Engl J Med 342:1385–1391
Epstein AE, Dimarco JP, Ellenbogen KA, et al. American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons: ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive summary. Heart Rhythm. 2008;5(6):934-955.
Fleg JL, Kennedy HL. Cardiac arrhythmias in a healthy elderly population: Detection by 24-hour ambulatory electrocardiography. Chest. 1892;81:301-307.
Futami C, Tanuma K, Tanuma Y, Saito T. The arterial blood supply of the conducting system in normal human hearts. Surg Radiol Anat. 2003;25:42-49.
Ho SY, Esscher E, Anderson RH, Michaelsson M. Anatomy of congenital complete heart block and relation to maternal anti-Ro antibodies. Am J Cardiol. 1986;58:291-294.
Ho SY, McCarthy KP, Ansari A, et al. Anatomy of the atrioventricular node and atrioventricular conduction system. J Bifurcation Chaos. 2003;12:3665-3674.
James TN. Cardiac innervation: Anatomic and pharmacologic relations. Bull NY Acad Sci. 1967;43:1041-1086.
Johnson RL, Averill KH, Lamb LE. Electrocardiographic findings in 67,375 asymptomatic subjects. Am J Cardiol. 1960;6:153-157.
Lamas GA, Orav EJ, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. N Engl J Med. 1998;338:1097-1104.
Lazzara R, Scherlag BJ, Belardinelli L. Atrioventricular conduction. In: Spooner PM, Rosen MR, editors. Foundations of cardiac arrhythmias: Basic concepts and clinical approaches. New York: Marcel Dekker, 2001.
Petrecca K, Shrier A. Spatial distribution of ion channels, receptors, and innervation in the AV node. In: Mazgalev TN, Tchou PJ, editors. Atrial-AV nodal electrophysiology: A view from the millennium. Armonk, NY: Futura, 2000.
Rosen KM. The contribution of His bundle recording to the understanding of cardiac conduction in man. Circulation. 1971;43:961-966.
Scherlag BJ, Patterson E, Yamanashi W, et al. The AV conjunction: A concept based on ablation techniques in the normal heart. In: Mazgalev TN, Tchou PJ, editors. Atrial-AV nodal electrophysiology: A view from the millennium. Armonk, NY: Futura, 2000.
Scott JS, Maddison PJ, Taylor PV, et al. Connective tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983;4:209-212.
Skanes AC, Krahn AD, Yee R, et al. Progression to chronic atrial fibrillation after pacing: The Canadian Trial of Physiologic Pacing. J Am Coll Cardiol. 2001;38:167-172.
Toff WD, Camm AJ, Skehan JD, United Kingdom Pacing and Cardiovascular Events Trial Investigators. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. 2005;353(2):145-155.
Waki K, Kim JS, Becker AE. Morphology of the human atrioventricular node is age dependent: A feature of potential clinical significance. J Cardiovasc Electrophysiol. 2000;11:1144-1151.
1 Stannius HF. Zwei Reihen physiologischer Verusche. Arch Anat Physiol Wis Med. 1852;2:85-100.
2 His WJr. Die Tatigkeit des embryonalen Herzens und deren Bedeutung fur die Lehre von der Herzbewegung beim Erwachsenen. Arb Med Klin Leipzig. 1893;14:49.
3 Tawara S. Das Reizleitungssystem des Saugetierherzens. Eine anatomisch histologische Studie uber das Atrioventrikularbundel und ide Purkinjeschen Faden. Mit einem Vorwort von L, Aschoff (Marburg): Fischer, Jena; 1906.
4 Wenckebach KF. Beirage zur Kenntnis der menschlichen Herztatigleit. Arch Anat Physiol. 1906:297-354.
5 Dickinson DF, Scott O. Ambulatory electrocardiographic monitoring in 100 teenage boys. Br Heart J. 1984;51:179-183.
6 Sobotka PA, Mayer JH, Bauernfeind RA, et al. Arrhythmias documented by 24 hour continuous ambulatory electrocardiographic monitoring in young women without apparent heart disease. Am Heart J. 1981;101:753-759.
7 Brodsky M, Wu D, Denes P, et al. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 male medical students without apparent heart disease. Am J Cardiol. 1977;39:390-395.
8 Manning GW. Electrocardiography in the selection of Royal Canadian Air Force crew. Circulation. 1954;10:401-412.
9 Johnson RL, Averill KH, Lamb LE. Electrocardiographic findings in 67,375 asymptomatic subjects. Am J Cardiol. 1960;6:153-157.
10 Perlman LV, Ostrander LD, Keller JB, Chiang BN. An epidemiologic study of first degree atrioventricular block in Tecumseh, Michigan. Chest. 1971;59:40-46.
11 Logue RB, Hanson JF. Heart block. A study of 100 cases with prolonged PR interval. Am J Med Sci. 1944;207:765-769.
12 Fleg JL, Kennedy HL. Cardiac arrhythmias in a healthy elderly population: Detection by 24-hour ambulatory electrocardiography. Chest. 1982;81:301-307.
13 Mymin D, Mathewson FAL, Tate RB, et al. The natural history of first-degree atrioventricular heart block. N Engl J Med. 1986;315:1183-1188.
14 Bhat PK, Watanabe K, Rao DB, Luisado AA. Conduction defects in the aging heart. J Am Geriatr Soc. 1974;22:517-520.
15 Morgensen L. Cardiac arrhythmias in the asymptomatic individual without overt cardiac disease. In: Kulbertus H, editor. Medical management of cardiac arrhythmias. Edinburgh: Churchill Livingstone, 1986.
16 McCue CM, Mantakas ME, Tingelstad JB, et al. Congenital heart block in newborns of mothers with connective tissue disease. Circulation. 1977;56:82-90.
17 Barold SS, Herweg B. Acquired AV block. In Kusumoto FM, Goldschlager N, editors: Cardiac pacing for the clinician, ed 2, New York: Springer, 2008.
18 Ho SY, McCarthy KP, Ansari A, et al. Anatomy of the atrioventricular node and atrioventricular conduction system. J Bifurcation Chaos. 2003;12:3665-3674.
19 Waki K, Kim JS, Becker AE. Morphology of the human atrioventricular node is age dependent: A feature of potential clinical significance. J Cardiovasc Electrophysiol. 2000;11:1144-1151.
20 Inoue S, Becker AE. Posterior extensions of the human compact atrioventricular node. A neglected anatomic feature of potential clinical significance. Circulation. 1998;97:188-193.
21 Kozlowski D, Kozluk E, Adamowicz M, et al. Histological examination of the topography of the atrioventricular nodal artery within the triangle of Koch. Pacing Clin Electrophysiol. 1998;21:163-167.
22 Sánchez-Quintana D, Ho SY, Cabrera JA, Farré J, Anderson RH. Topographic anatomy of the inferior pyramidal space: Relevance to radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2001;12:210-217.
23 Hosseinpour AR, Anderson RH, Ho SY. The anatomy of the septal perforating arteries in normal and congenitally malformed hearts. J Thorac Cardiovasc Surg. 2001;121:1046-1052.
24 Futami C, Tanuma K, Tanuma Y, Saito T. The arterial blood supply of the conducting system in normal human hearts. Surg Radiol Anat. 2003;25:42-49.
25 Lev M. Pathogenesis of congenital atrioventricular block. Prog Cardiovasc Dis. 1972;15:145-157.
26 Lev M, Silverman J, Fitzmaurice FM, et al. Lack of connection between the atria and the more peripheral conduction system in congenital atrioventricular block. Am J Cardiol. 1971;27:481-490.
27 Lev M, Cuadros H, Paul MH. Interruption of the atrioventricular bundle with congenital atrioventricular block. Circulation. 1971;43:703-710.
28 Lev M. Familial congenital bundle branch system disease. Am J Cardiol. 1973;32:365-369.
29 Bharati S, Rosen KM, Strasberg B, et al. Anatomic substrate for congenital atrioventricular block in middle aged adults. Pacing Clin Electrophysiol. 1982;5:860-869.
30 Hull D, Binns BAO, Joyce D. Congenital heart block with widespread fibrosis due to maternal lupus erythematosus. Arch Dis Child. 1966;41:688-690.
31 McCue CM, Mantakas ME, Tingelstad JB, Ruddy S. Congenital heart block in newborns of mothers with connective tissue disease. Circulation. 1977;56:82-89.
32 Scott JS, Maddison PJ, Taylor PV, et al. Connective tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983;4:209-212.
33 Ho SY, Esscher E, Anderson RH, Michaelsson M. Anatomy of congenital complete heart block and relation to maternal anti-Ro antibodies. Am J Cardiol. 1986;58:291-294.
34 Chow LTC, Cook AC, Ho SY, et al. Isolated congenitally complete heart block attributable to combined nodoventricular and intraventricular discontinuity. Hum Pathol. 1998;29:729-736.
35 Ho SY, Fagg N, Anderson RH, Cook A, Allan L. Disposition of the atrioventricular conduction tissues in the heart with isomerism of the atrial appendages: Its relation to congenital complete heart block. J Am Coll Cardiol. 1992;20:904-910.
36 Daliento L, Corrado D, Buja G, et al. Rhythm and conduction disturbances in isolated, congenitally corrected transposition of the great arteries. Am J Cardiol. 1986;58:314-318.
37 Davies MJ, Anderson RH, Becker AE. Permanent atrioventricular block. In: The conduction system of the heart. London: Butterworths; 1983.
38 Lev M. The pathology of complete atrioventricular block. Prog Cardiovasc Dis. 1964;37:742-748.
39 Lenègre J. Aetiology and pathology of bilateral bundle branch fibrosis in relation to complete heart block. Prog in Cardiovasc Dis. 1964;6:409-444.
40 Davies M, Harris A. Pathological basis of primary heart block. Brit Heart J. 1969;31:219-226.
41 Harris A, Davies M, Redwood D, et al. Aetiology of chronic heart block. A clinico-pathological correlation in 65 cases. Brit Heart J. 1969;31:206-218.
42 Begg FR, Magovern GJ, Cushing WJ, et al. Selective cine-angiography in patients with complete heart block. J Thorac Cardiovasc Surg. 1969;57:9-16.
43 Gavrilescu S, Gavrilescu M, Streian A, Luca C. Hypertrophic obstructive cardiomyopathy associated with complete heart block. Pathologic correlations in a case studied with His bundle electrography. Acta Cardiol. 1974;29:241-249.
44 Maron BJ, Savage DD, Wolfson JK, Epstein SE. Prognostic significance of 24 hour ambulatory electrographic monitoring in patients with hypertrophic cardiomyopathy: A prospective study. Am J Cardiol. 1981;11:147-153.
45 Bharati S, McAnulty JH, Lev M, Rahimtoola SH. Idiopathic hypertrophic subaortic stenosis with split His bundle potentials. Electrophysiologic and pathologic correlations. Circulation. 1980;62:1373-1380.
46 Burke AP, Anderson PG, Virmani R, et al. Tumours of the atrioventricular nodal region. A clinical and immunohistochemical study. Arch Pathol Lab med. 1990;114:1057-1062.
47 Carminati M, Butera G, Chessa M, et al. Investigators of the European VSD Registry. Transcatheter closure of congenital ventricular septal defects: Results of the European Registry. Eur Heart J. 2007;28:2361-2368.
48 Predescu D, Chaturvedi RR, Friedberg MK, et al. Complete heart block associated with device closure of perimembranous ventricular septal defect. J Thorac Cardiovasc Surg. 2008;136:1223-1228.
49 Smedira NG, Lytle BW, Lever HM, et al. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008;85:127-133.
50 Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol. 2006;19:319-327.
51 Scherlag BJ, Patterson E, Yamanashi W, et al. The AV conjunction: A concept based on ablation techniques in the normal heart. In: Mazgalev TN, Tchou PJ, editors. Atrial-AV nodal electrophysiology: A view from the millennium. Armonk, NY: Futura, 2000.
52 Lazzara R, Scherlag BJ, Belardinelli L. Atrioventricular conduction. In: Spooner PM, Rosen MR, editors. Foundations of cardiac arrhythmias: Basic concepts and clinical approaches. New York: Marcel Dekker, 2001.
53 Petrecca K, Shrier A. Spatial distribution of ion channels, receptors, and innervation in the AV node. In: Mazgalev TN, Tchou PJ, editors. Atrial-AV nodal electrophysiology: A view from the millennium. Armonk, NY: Futura, 2000.
54 Hucker WJ, Nikolski VP, Efimov IR. Autonomic control and innervation of the atrioventricular junctional pacemaker. Heart Rhythm. 2007;4(10):1326-1337.
55 Petrecca K, Amellal F, Laird DW, et al. Sodium channel distribution within the rabbit atrioventricular node as analyzed by confocal microscopy. J Physiol (Lond). 1997;501:263-274.
56 Zipes DP, Mendez C. Action of manganese ions and tetrodotoxin on atrioventricular nodal transmembrane potentials in isolated rabbit hearts. Circ Res. 1973;32:447-454.
57 Mangoni ME, Traboulsie A, Leoni AL, et al. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/α1G T-type calcium channels. Circ Res. 2006;98:1422-1430.
58 Hu K, Qu Y, Yue Y, Boutjdir M. Functional basis of sinus bradycardia in congenital heart block. Circ Res. 2004;94:e32-e38.
59 Xu Y, Tuteja D, Zhang Z, et al. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278(49):49085-49094.
60 Zhang Q, Timofeyev V, Lu L, et al. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circ Res. 2008;102:465-471.
61 Saffitz JE, Yamada KA, Schuessler RB. Distribution and function of gap junction proteins in atrial-AV nodal conduction. In: Mazgalev TN, Tchou PJ, editors. Atrial-AV nodal electrophysiology: A view from the millennium. Armonk, NY: Futura, 2000.
62 Hocini M, Loh P, Siew Y, et al. Anisotropic conduction in the triangle of Koch of mammalian hearts: Electrophysiologic and anatomic correlations. J Am Coll Cardiol. 1989;31:629-636.
63 Li J, Greener ID, Inada S, et al. Computer three-dimensional reconstruction of the atrioventricular node. Circ Res. 2008;102:975-985.
64 Dobrzynski H, Nikolski VP, Sambelashvili AT, et al. Site of origin and molecular substrate of atrioventricular junctional rhythm in the rabbit heart. Circ Res. 2003;93:1102-1110.
65 Lockwood D, Otomo K, Wang Z, et al. Electrophysiological characteristics of atrioventricular nodal reentrant tachycardia: Implications of the reentrant circuits. In Zipes D, Jalife J, editors: Cardiac electrophysiology; From cell to bedside, ed 4, Philadelphia: Saunders, 2004.
66 Lazzara R. Electrophysiology of the specialized conduction system: Selected aspects relevant to clinical bradyarrhythmias. In Samet P, El-Sherif N, editors: Cardiac pacing, ed 2, New York: Grune & Stratton, 1980.
67 Han W, Wang Z, Nattel S. Slow delayed rectifier current and repolarization in canine cardiac Purkinje cells. Am J Physiol Heart Circ Physiol. 2001;280:H1075-H1080.
68 Rosati B, Dun W, Hirose M, et al. Molecular basis of the T- and L-type Ca2+ currents in canine Purkinje fibres. J Physiol. 2007;579(2):465-471.
69 Vasalle M. The vicissitudes of the pacemaker current IKdd of cardiac Purkinje fibers. J of Biomedical Science. 2007;14:699-716.
70 Narula OS. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation. 1977;56:996-1006.
71 James TN. Cardiac innervation: Anatomic and pharmacologic relations. Bull NY Acad Sci. 1967;43:1041-1086.
72 Damato AN, Lau SH, Helfant R, et al. A study of heart block in man using His bundle recordings. Circulation. 1969;39:297-305.
73 Rosen KM. The contribution of His bundle recording to the understanding of cardiac conduction in man. Circulation. 1971;43:961-966.
74 Scheinman MM, Peters RW, Sauve MJ, et al. Value of the H-Q interval in patients with bundle branch block and the role of prophylactic permanent pacing. Am J Cardiol. 1984;50:1316-1322.
75 Dhingra RC, Wyndham C, Bauernfeind R, et al. Significance of block distal to the His bundle induced by atrial pacing in patients with chronic bifascicular block. Circulation. 1979;60:1455-1464.
76 Epstein AE, Dimarco JP, Ellenbogen KA, et al. American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons: ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive summary. Heart Rhythm. 2008;5(6):934-955.
77 Lamas GA, Orav EJ, Stambler BS, et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. N Engl J Med. 1998;338:1097-1104.
78 Connolly SJ, Kerr CR, Gent M, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. N Engl J Med. 2000;342:1385-1391.
79 Skanes AC, Krahn AD, Yee R, et al. Progression to chronic atrial fibrillation after pacing: The Canadian Trial of Physiologic Pacing. J Am Coll Cardiol. 2001;38:167-172.
80 Toff WD, Camm AJ, Skehan JD, United Kingdom Pacing and Cardiovascular Events Trial Investigators. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. 2005;353(2):145-155.
81 Clarke M, Sutton R, Ward D, et al. Recommendations for pacemaker prescription for symptomatic bradycardia. Report of a working party of the British Pacing and Electrophysiology Group. Br Heart J. 1991;66:185-191.
82 Abate E, Kusumoto F, Goldschlager N. Techniques for temporary pacing. In Kusumoto FM, Goldschlager N, editors: Cardiac pacing for the clinician, ed 2, New York: Springer, 2008.
83 Zoll PM. Noninvasive cardiac stimulation revisited. PACE. 1990;13:2014.
84 Barold SS, Sischy D, Punzi K, et al. Advanced atrioventricular block in a 39-year-old man with acute rheumatic fever. PACE. 1998;21:2025-2028.
85 Barold SS, Hayes DL. Second-degree atrioventricular block: A reappraisal. Mayo Clin Proc. 2001;76(1):44-57.
86 Shaw DB, Kekwick CA, Veale D, et al. Survival in second-degree atrioventricular block. Br Heart J. 1985;53:587-593.
87 Narula OS. Atrioventricular block. In: Narula OS, editor. Cardiac arrhythmias. Electrophysiology, diagnosis, and management. Baltimore: Williams & Wilkins, 1979.
88 Peuch P, Wainwright RJ. Clinical electrophysiology of atrioventricular block. Cardiol Clin. 1983;1:209-224.
89 Barold SS, Hayes DL. Second-degree atrioventricular block: A reappraisal. Mayo Clin Proc. 2001;76:44-57.
90 Sumiyoshi M, Nakata Y, Yasuda M, et al. Changes in conductivity in patients with second or third degree atrioventricular block after pacemaker implantation. Jpn Circ J. 1995;59:284-291.
91 Dhingra RC, Denes P, Wu D, et al. The significance of second-degree atrioventricular block and bundle branch block: Observations regarding site and type of block. Circulation. 1974;49:638-646.