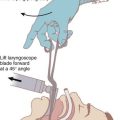
Chapter 13 Preoxygenation
I Historical Perspective
In 1948, Fowler and Comroe demonstrated that inhalation of 100% oxygen (O2) resulted in a very rapid increase of arterial oxyhemoglobin saturation (SaO2) to between 98% and 99%, but that attainment of the last 1% to 2% was a much slower process.1 They also observed that the rate of increase was attenuated in patients with pulmonary emphysema or pulmonary artherosclerosis.1 In 1955, Hamilton and Eastwood showed that “denitrogenation” was 95% complete within 2 to 3 minutes in subjects breathing normally from a circle anesthesia system with 5 L/min O2.2 Dillon and Darsi, in the same year, observed significant arterial oxyhemoglobin desaturation during apnea after anesthetic induction with sodium thiopental and recommended that induction of anesthesia and endoscopy should be preceded by O2 administration for 5 minutes.3 Six years later, Heller and Watson found that 3 to 4 minutes of O2 breathing was necessary in patients before anesthetic induction, whereas adequate oxygenation could be accomplished with the use of manual ventilation in 30 seconds.4 With the introduction of the rapid-sequence induction and intubation (RSI) technique in the 1950s in patients at risk for aspiration of gastric contents, preoxygenation became a component of the technique.5,6 Preoxygenation was emphasized by Sellick when he introduced cricoid pressure into clinical practice in 1961,7 and it was also recommended before RSI in pediatric patients in the 1970s.8
Preoxygenation before anesthetic induction and intubation became a widely accepted maneuver designed to increase O2 reserves and thereby delay the onset of arterial oxyhemoglobin desaturation during apnea. Various techniques and regimens have been advocated to ensure adequate preoxygenation. For many years, tidal volume breathing (TVB) of O2 for 3 to 5 minutes has been commonly practiced.2,3 Gold and colleagues challenged the need for 3 minutes of TVB by demonstrating that 4 deep breaths within 0.5 minutes (4 DB/0.5 min) and TVB for 5 minutes using a semiclosed circle absorber system were equally effective in increasing arterial oxygen tension (PaO2).9 Although some investigators corroborated their findings,10,11 others showed that TVB for 3 minutes provided better preoxygenation and longer protection against hypoxemia during apnea than 4 DB/0.5 min.12–15 Later investigations suggested that the use of extended deep breathing (8, 12, and 16 deep breaths in 1.0, 1.5, and 2.0 minutes, respectively) could produce maximal preoxygenation comparable to that achieved with TVB for 3 minutes and could also delay the onset of apnea-induced oxyhemoglobin desaturation.16,17
Regardless of the technique used, preoxygenation has become an integral component of the RSI technique, and it is particularly important if manual ventilation is not desirable, if difficulty with ventilation or endotracheal intubation is anticipated, and in patients with oxygen transport limitations. Because the “cannot intubate, cannot ventilate” (CICV) situation is largely unpredictable, the desirability of maximal preoxygenation is theoretically present for all patients.18
The original American Society of Anesthesiologists’ (ASA) difficult airway (DA) algorithm made no mention of preoxygenation. In an updated report by the ASA Task Force on Management of the DA (2003), the topic of “facemask preoxygenation before initiating management of the difficult airway” was added.19 “Routine” preoxygenation has become a new “minimum standard” of care, not only during induction of anesthesia, but also during emergence from anesthesia and tracheal extubation.20–22
II Body Oxygen Stores
Oxygen is carried in the blood in two forms: the greater portion is in reversible chemical combination with hemoglobin (Hb), and the smaller part is dissolved in plasma.23 The ability to carry large amounts of O2 in Hb is important, because without it, the amount carried in the plasma would be so small that the cardiac output would need to be increased more than 20 times to yield an adequate O2 flux.23 The amount of chemically bound O2 is directly related to the concentration of Hb and how saturated the Hb is with O2. Arterial O2 content (Cao2) can be calculated from the following equation:
1.36 = estimated mass volume of O2 that can be bound by 1 g of normal Hb
SaO2 = arterial oxyhemoglobin saturation (when fully saturated, SaO2 = 100%)
Hb uptake and release of O2 are regulated by a pattern demonstrated by the familiar oxyhemoglobin dissociation curve, which is a plot of SaO2 as a function of PaO2. The sigmoid shape of the curve reflects the fact that the four binding sites on a given Hb molecule interact with each other.23 When the first site has bound a molecule of O2, the binding of the next site is facilitated, and so forth. The result is a curve that is steep up to a PO2 of 60 mm Hg and becomes more shallow thereafter, approaching 100% saturation asymptotically. At a PO2 of 100 mm Hg, the normal arterial value, 97% of the hemes have bound O2; at 40 mm Hg, a typical value for (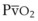 ) in a resting person, the saturation declines to about 75%. The shape of the oxyhemoglobin dissociation curve has important physiologic implications. The flatness of the curve above a PO2 of 80 mm Hg ensures a relatively constant SaO2 despite wide variations in alveolar O2 pressure. The steep portion of the curve between 20 to 60 mm Hg permits unloading of O2 from Hb at relatively high PO2 values, which favors the delivery of large amounts of O2 into the tissues by diffusion.
) in a resting person, the saturation declines to about 75%. The shape of the oxyhemoglobin dissociation curve has important physiologic implications. The flatness of the curve above a PO2 of 80 mm Hg ensures a relatively constant SaO2 despite wide variations in alveolar O2 pressure. The steep portion of the curve between 20 to 60 mm Hg permits unloading of O2 from Hb at relatively high PO2 values, which favors the delivery of large amounts of O2 into the tissues by diffusion.
The O2-binding properties of Hb are influenced by a number of factors, including pH, PCO2, and temperature.23 These factors cause shifts of the oxyhemoglobin dissociation curve to the right or left without changing the slope of the curve. For example, an increase in temperature or a decrease in pH, such as may occur in active tissues, decreases the affinity of Hb for O2 and shifts the oxyhemoglobin dissociation curve to the right. As a result, a higher PO2 is required to achieve a given saturation, which facilitates unloading of O2 at the tissue. To quantify the extent of a shift of the oxyhemoglobin dissociation curve, the so-called P50 is used—that is, the PO2 required for 50% saturation. The P50 of normal adult Hb at 37° C and normal pH and PCO2 is 26 to 27 mm Hg.
Despite its great importance, O2 is a very difficult gas to store in a biologic system. In subjects breathing air, O2 stores are small (Table 13-1).23,24 The relatively steep oxyhemoglobin dissociation curve and the small O2 stores imply that factors affecting PaO2 produce their full effects very quickly. This is in contrast to CO2, for which the large size of the stores buffers the body against rapid changes. Therefore, in a subject breathing air, a pulse oximeter probably gives an earlier indication of hypoventilation than does CO2 measurement. In contrast, in a subject breathing a high fraction of inspired O2 (FIO2), CO2 measurement gives an earlier indication of hypoventilation.23
Table 13-1 Body O2 Stores (in mL) during Room Air and 100% O2 Breathing
| Storage Site | Room Air | 100% O2 |
|---|---|---|
| In the lungs (FRC) | 450 | 3000 |
| In the blood | 850 | 950 |
| Dissolved in tissue fluids | 50 | 100 |
| In combination with myoglobin | 200? | 200 |
| Total | 1550 | 4250 |
FRC, Functional residual capacity; O2, oxygen.
From Nunn JF, editor: Nunn’s applied respiratory physiology, ed 4, Oxford, 1993, Butterworth-Heinemann, p 288.
The amounts of body O2 in the various storage sites of a person breathing air are increased with breathing 100% of O2 (Fig. 13-1; see also Table 13-1).23,24 The maximal increase of O2 stores occurs in the functional residual capacity (FRC). Storage of O2 in the tissue is rather difficult to assess, but assuming that Henry’s law applies and the partition coefficient for gases approximates the gas-water coefficients, breathing O2 for 3 minutes significantly increases tissue O2 stores.24
III Physiology of Apnea and Apneic Mass-Movement Oxygenation
During apnea, the total body O2 consumption (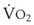 ) remains fairly constant at about 250 mL/min. Consequently, the alveolar O2 concentration (PAO2) decreases rapidly as a result of the depletion of the diminishing O2 stores in the lungs. If the airway becomes obstructed, O2 removal will generate a substantial and immediate negative pressure, contributing to a further decrease of PaO2. Although the PaO2 falls in direct relation to PAO2, SaO2 remains greater than 90% as long as the Hb can be reoxygenated in the lungs.25–28 SaO2 starts to decrease only after the lung O2 stores are depleted and the PaO2 is lower than 60 mm Hg. It is for this reason that oximetry is not the best “physiologic means,” compared with PaO2, for predicting the onset of hypoxemia. However, because it detects decreases in SaO2 before other clinical signs, oximetry is an invaluable clinical monitor that adds to the safety of anesthetic management.27 Critical oxyhemoglobin desaturation may be defined as SaO2 less than or equal to 80%; for patients with SaO2 less than 80%, the range in the rate of decrease is 20% to 40% per minute during apnea.29
) remains fairly constant at about 250 mL/min. Consequently, the alveolar O2 concentration (PAO2) decreases rapidly as a result of the depletion of the diminishing O2 stores in the lungs. If the airway becomes obstructed, O2 removal will generate a substantial and immediate negative pressure, contributing to a further decrease of PaO2. Although the PaO2 falls in direct relation to PAO2, SaO2 remains greater than 90% as long as the Hb can be reoxygenated in the lungs.25–28 SaO2 starts to decrease only after the lung O2 stores are depleted and the PaO2 is lower than 60 mm Hg. It is for this reason that oximetry is not the best “physiologic means,” compared with PaO2, for predicting the onset of hypoxemia. However, because it detects decreases in SaO2 before other clinical signs, oximetry is an invaluable clinical monitor that adds to the safety of anesthetic management.27 Critical oxyhemoglobin desaturation may be defined as SaO2 less than or equal to 80%; for patients with SaO2 less than 80%, the range in the rate of decrease is 20% to 40% per minute during apnea.29
Preoxygenation followed by O2 insufflation during subsequent apnea maintains SaO2 by apneic diffusion oxygenation.25,26 In the apneic adult, the 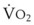 averages 230 mL/min, whereas the output of CO2 to the alveoli is limited to about 21 mL/min and the remaining CO2 production (approximately 90%) is buffered within the body tissues. The lung volume initially decreases by the net gas exchange ratio of 209 mL/min. Therefore, a pressure gradient is created between the upper airway and the alveoli, and if the airway is patent, this results in a mass movement of O2 down the trachea into the alveoli. Conversely, CO2 is not exhaled because of this mass movement of O2 down the trachea, and the alveolar CO2 concentration (PACO2) shows an initial rise of about 8 to 16 mm Hg during the first minute and a subsequent fairly linear rise of about 3 mm Hg/min.30
averages 230 mL/min, whereas the output of CO2 to the alveoli is limited to about 21 mL/min and the remaining CO2 production (approximately 90%) is buffered within the body tissues. The lung volume initially decreases by the net gas exchange ratio of 209 mL/min. Therefore, a pressure gradient is created between the upper airway and the alveoli, and if the airway is patent, this results in a mass movement of O2 down the trachea into the alveoli. Conversely, CO2 is not exhaled because of this mass movement of O2 down the trachea, and the alveolar CO2 concentration (PACO2) shows an initial rise of about 8 to 16 mm Hg during the first minute and a subsequent fairly linear rise of about 3 mm Hg/min.30
Fraioli and colleagues emphasized the importance of the ratio of FRC to body weight during apneic diffusion and demonstrated that patients with a low FRC/body weight ratio could not tolerate apnea for more than 4 minutes, whereas those with a high FRC/body weight ratio (>53.3 ± 7 mL/kg) maintained PaO2 at 90% of the control value.25 Some studies demonstrated that with a patent airway and an FIO2 of 1, SaO2 can be maintained at greater than 90% for up to 100 minutes with apneic oxygenation.25,26
The success of apneic mass-movement oxygenation depends on airway patency to allow O2 to move into the apneic lungs. In the presence of air obstruction, not only does the lung gas volume decrease rapidly, but the intrathoracic pressure also decreases at a rate that is dependent on 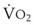 and thoracic compliance, subsequently leading to a marked fall in PaO2. When airway obstruction is relieved, rapid flow of O2 into the lungs occurs, and with high FIO2, rapid reoxygenation resumes.27
and thoracic compliance, subsequently leading to a marked fall in PaO2. When airway obstruction is relieved, rapid flow of O2 into the lungs occurs, and with high FIO2, rapid reoxygenation resumes.27
Apneic mass-movement oxygenation can be achieved by preoxygenation followed by insufflation of O2 through a nasopharyngeal or oropharyngeal cannula or through a needle inserted in the cricothyroid or cricotracheal membrane. This provides at least 10 minutes of adequate oxygenation in healthy apneic patients whose airways are unobstructed and therefore has many practical applications.31 In patients who are difficult to intubate or ventilate, pharyngeal O2 insufflation (or tracheal insufflation, in cases of upper airway obstruction) may allow additional time for laryngoscopy and endotracheal intubation.31–33 This can be advantageous in patients who have decreased O2 reserves, such as children, pregnant women, obese patients, and patients with adult respiratory distress syndrome (ARDS).30,32 The combination of preoxygenation and apneic diffusion oxygenation can be used during bronchoscopy and can provide the otolaryngologist with adequate time for glottic surgery unimpeded by the presence of the endotracheal tube (ETT) or by the patient’s respiratory movements.33
During apneic diffusion oxygenation via an open airway, the increase in time to Hb desaturation achieved by increasing the FIO2 from 0.9 to 1.0 is greater than that caused by increasing the FIO2 from 0.21 to 0.9. Increasing the FIO2 from 0.9 to 1.0 more than doubles the time to desaturation34 (Fig.13-2).
IV Efficacy and Efficiency of Preoxygenation
Studies of preoxygenation have focused on measurements of indices reflecting its efficacy and efficiency.18 Measurements of alveolar O2,14,35,36 alveolar N2,37 or PaO2 reflect the efficacy of preoxygenation, whereas the decline of SaO2 during apnea is indicative of its efficiency.9,16,18,37,38
The SaO2 may be misleading as a guide to alveolar denitrogenation. A saturation of 100% measured by pulse oximetry (SpO2) is most definitely not a reason to stop denitrogenation and may occur well before the lungs are adequately denitrogenated. Conversely, failure of SpO2 to increase substantially during denitrogenation does not necessarily indicate failure of preoxygenation or lack of its value; patients with substantial pulmonary shunting may achieve excellent pulmonary O2 reservoirs while remaining hypoxemic.39
A Efficacy of Preoxygenation
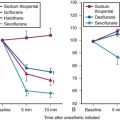
Preoxygenation increases the alveolar O2 and decreases the alveolar N2 in a parallel fashion (Fig. 13-3).37,40 It is the washout of N2 from the lungs that is the key to achieving preoxygenation.37,40 The terms preoxygenation and denitrogenation have been used synonymously to describe the same process, although a change in focus from preoxygenation to denitrogenation has been suggested.37
1. The anesthesia circuit is flushed by a high O2 flow.
2. A nonleaking face mask is used to avoid air entrainment.
3. An O2 flow of 5 L/min is required for TVB, and a flow of 10 L/min is necessary for the deep breathing.
The end points of maximal alveolar preoxygenation or denitrogenation have been defined as an end-tidal O2 concentration (EtO2) of approximately 90% and an end-tidal nitrogen concentration (EtN2) of 5%.15,35,36 In an adult with a normal FRC and VO2, an EtO2 greater than 90% means that the lungs contain more than 2000 mL of O2 (8 to 10 times the VO2).27 Because of the obligatory presence of CO2 and water vapor in the alveolar gas, an EtO2 greater than 97% cannot be easily achieved. Factors affecting the efficacy of preoxygenation include FIO2, duration of breathing, and the 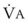 /FRC ratio (Box 13-1).
/FRC ratio (Box 13-1).
Box 13-1 Factors Affecting the Efficacy and Efficiency of Preoxygenation
1 Fraction of Inspired Oxygen
The main reasons for failure to achieve an FIO2 close to 1.0 are a leak under the face mask,14,18,41–43 rebreathing of exhaled gases, and the use of systems incapable of delivering a high O2 concentration, such as resuscitation bags.40 Even the presence of minor leaks may not be fully compensated for by increasing the fresh gas flow (FGF) or by increasing the duration of preoxygenation. Bearded patients, edentulous patients, patients with sunken cheeks, the presence of nasogastric tubes, use of a wrong face mask size, improper use of head straps, and use of systems allowing air entrainment under the face mask are all common factors causing leaks between the face mask and the patient’s head resulting in lower FIO2. Clinical end points indicative of a sealed system are movement of the reservoir bag in and out with inhalation and exhalation, presence of a normal capnogram and end-tidal CO2, (EtCO2), and measurements of inspired and EtCO2 values.18
There is reluctance among some anesthesiologists to use preoxygenation routinely because the mask presents discomfort to the patient and some patients find preoxygenation objectionable. There is clearly marked overestimation of the patient’s discomfort by the anesthesiologist. In fact, patients’ discomfort during preoxygenation is not more than the discomfort during other procedures such as the placement of intravenous lines.44,45 It is our experience that patients accept a tight-fitting mask if the procedure is explained beforehand and they are told that “it is important to fill the tank with oxygen.”
Although anesthetic circuits can deliver 100% O2 concentration, the FIO2 can be influenced by the type of breathing, the level of FGF, and the duration of breathing.17 In a study involving volunteers that compared preoxygenation techniques using a semiclosed circle absorber with varying FGF in the same subjects, it was found that with TVB, inspired O2 concentration was 95% with FGF of 5 L/min and increased to 98% with FGFs of 7 and 10 L/min. However, with deep breathing, the inspired O2 concentration was only 88% at 5 L/min, 91% at 7 L/min, and 95% at 10 L/min FGF (Fig. 13-4).17 These findings imply that increasing the FGF from 5 to 10 L/min has little impact on increasing FIO2 during TVB but has a noticeable effect during deep breathing.17 Because of the breathing characteristics of the circle system, the minute ventilation during deep breathing may exceed the FGF, resulting in rebreathing of exhaled gases (N2) and consequently decreasing the FIO2; in contrast, during TVB, rebreathing of exhaled gases is negligible, and increasing the FGF from 5 to 10 L/min would have only a slight effect on FIO2.12,17
2 Duration of Breathing, Functional Residual Capacity, and Alveolar Ventilation
Sufficient time is needed to accomplish maximal preoxygenation. With an FIO2 close to 1, most healthy adult patients can reach the target level of EtO2 greater than or equal to 90% (or EtN2 ≤ 5%) within 3 to 5 minutes of TVB. The half-time for exponential change in fraction of alveolar O2 concentration (FAO2) with a step change in FIO2 for a nonrebreathing system is given by the equation, FAO2 = 0.693 × VFRC/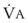 . (VFRC is volume of functional residual capacity). With a VFRC of 2.5 L, the half-times are 26 seconds when
. (VFRC is volume of functional residual capacity). With a VFRC of 2.5 L, the half-times are 26 seconds when 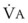 = 4 L/min and 13 seconds when
= 4 L/min and 13 seconds when 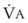 = 8 L/min.18 Therefore, most of the O2 that can be stored in the lungs may be brought in by hyperventilation with an FIO2 of 1.0 for a shorter period of time than that needed with TVB.18 This is the basis for the deep breathing techniques, which have been introduced as an alternative to TVB.9,18
= 8 L/min.18 Therefore, most of the O2 that can be stored in the lungs may be brought in by hyperventilation with an FIO2 of 1.0 for a shorter period of time than that needed with TVB.18 This is the basis for the deep breathing techniques, which have been introduced as an alternative to TVB.9,18
Changes in 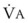 and FRC can have a marked effect on the rate of rise in EtO2 (and decrease in EtN2) during preoxygenation. Because of their increased
and FRC can have a marked effect on the rate of rise in EtO2 (and decrease in EtN2) during preoxygenation. Because of their increased 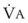 and decreased FRC, EtO2 rises faster in pregnant women than in nonpregnant women.14,46,47 Similarly, preoxygenation can be accomplished faster in infants and children than in adults.48,49
and decreased FRC, EtO2 rises faster in pregnant women than in nonpregnant women.14,46,47 Similarly, preoxygenation can be accomplished faster in infants and children than in adults.48,49
B Efficiency of Preoxygenation
Preoxygenation can markedly delay arterial oxyhemoglobin desaturation during apnea. In healthy individuals breathing air, desaturation to 70% can occur within 1 minute, whereas with adequate preoxygenation, desaturation occurs after 5 minutes. The delay in desaturation during apnea depends on the efficacy of preoxygenation, the capacity for O2 loading, and 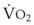 (see Box 13-1). Patients with a decreased capacity for O2 loading (decreased FRC, PaO2, CaO2, or cardiac output) or with increased
(see Box 13-1). Patients with a decreased capacity for O2 loading (decreased FRC, PaO2, CaO2, or cardiac output) or with increased 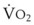 , or both, desaturate much faster during apnea than healthy patients do.18,29,42,50–52 The main difference in the rate of apnea-induced oxyhemoglobin desaturation after different preoxygenation techniques is observed between SaO2 levels of 100% and 99%.18,27,29,34,50 This range represents the flat portion of the oxyhemoglobin dissociation curve. When O2 reserves are depleted, rapid desaturation occurs regardless of the technique of preoxygenation and is similar to that observed in patients breathing air.
, or both, desaturate much faster during apnea than healthy patients do.18,29,42,50–52 The main difference in the rate of apnea-induced oxyhemoglobin desaturation after different preoxygenation techniques is observed between SaO2 levels of 100% and 99%.18,27,29,34,50 This range represents the flat portion of the oxyhemoglobin dissociation curve. When O2 reserves are depleted, rapid desaturation occurs regardless of the technique of preoxygenation and is similar to that observed in patients breathing air.
Farmery and Roe developed a computer model describing the rate of oxyhemoglobin desaturation during apnea.50 This model was found to agree reasonably well with actual data from patients whose weight and degree of normalcy and preoxygenation were reliably known (Fig. 13-5).29,50 Because it would be dangerous to obtain data in time to marked oxyhemoglobin desaturation in humans, this model is uniquely useful for analysis of oxyhemoglobin desaturation below 90%.29,50 As the preapnea FAO2 is progressively decreased from 0.87 to 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, and 0.13 (air) in a healthy 70-kg patient, the apnea time to 60% SaO2 is progressively decreased from 9.9 to 9.31, 8.38, 7.30, 6.37, 5.40, 4.40, 3.55, and 2.8 minutes, respectively.29,50 Figure 13-5 shows that for a healthy 70-kg adult, a moderately ill 70-kg adult, a healthy 10-kg child, and an obese 127-kg adult, 80% SaO2 is reached after 8.7, 5.5, 3.7, and 3.1 minutes, respectively, whereas 60% SaO2 is reached at 9.9, 6.2, 4.23, and 3.8 minutes, respectively.18,29
V Techniques of Preoxygenation
Two main techniques are used: TVB and deep breathing (Box 13-2).
A Tidal Volume Breathing
The traditional TVB has proved to be an effective preoxygenation technique. To ensure maximal preoxygenation, the duration of TVB should be 3 minutes or longer in adults, and an FIO2 near 1 should be maintained (see Box 13-2 and Fig. 13-3). Various anesthetic systems (circle absorber,9–12 Mapleson A,14,53–55 Mapleson D,13–15,54,55 and nonrebreathing systems) and FGFs ranging from 5 to 35 L/min have been used successfully.2,14,15,43,54,55 However, the circle absorber system (the system most commonly used in the operating room) with an FGF as low as 5 L/min is just as effective.40 Increasing the FGF from 5 to 10 L/min has little effect in enhancing preoxygenation during TVB in normal subjects (Fig. 13-6).40
B Deep Breathing Techniques
Based on the assumption that alveolar denitrogenation can be achieved rapidly by deep breathing, Gold and colleagues introduced the 4 DB/0.5 min method of preoxygenation.9 They showed that the PaO2 after 4 DB/0.5 min was no different from the PaO2 after TVB for 3 minutes. Although a few studies corroborated their findings,10,11 other investigations showed that 3 minutes of TVB provided better preoxygenation (Fig.13-6) and longer protection against hypoxemia during apnea than the 4 DB/0.5 min method did, particularly in pregnant women, patients with morbid obesity (MO), and elderly patients.12–15
There are two main reasons why the 4 DB/0.5 min method is inferior to TVB. First, if the ventilation in 0.5 minutes is much greater than the O2 inflow rate, rebreathing of exhaled nitrogen must occur, which decreases FIO2. Nimmagadda and colleagues confirmed that 4 DB/0.5 min provided suboptimal preoxygenation in volunteers; no subject achieved an EtO2 value of 90% or better (see Fig. 13-6).17 Another possible reason why patients preoxygenated with 4 DB/0.5 min desaturate faster is that the tissue and venous compartments need longer than 0.5 minutes to fill with O2.18,24 Probably, these compartments have the capability of storing additional O2 above that contained while breathing room air.17,18 Such stored O2 increases in an exponential fashion. It is possible that the 4 DB/0.5 min technique leads to rapid arterial oxygenation without substantial increase in the tissue O2 stores and hence results in more rapid desaturation during subsequent apnea than would a longer period of preoxygenation with TVB.18 Because the 4 DB/0.5 min technique yields submaximal preoxygenation, it should be reserved for emergency situations when time is limited.17
To optimize the deep breathing method of preoxygenation, investigators have focused on (1) extending the duration of deep breathing to 1, 1.5, and 2 minutes (to allow 8, 12, and 16 deep breaths, respectively) and (2) using high FGF (≥10 L/min).16,17 These maneuvers result in maximal preoxygenation (evidenced by higher EtO2, PaO2, and lower EtN2) and improved efficiency (delayed onset of oxyhemoglobin desaturation during apnea) compared with the original 4 DB/0.5 min method.16,17 An investigation suggested that preoxygenation using 8 deep breaths in 1.0 minute at an FGF of 10 L/min is associated with slower oxyhemoglobin desaturation than that using 4 DB/0.5 min or TVB for 3 min (Fig. 13-7).16 Several explanations were given for this finding, including leftward shift of the oxyhemoglobin dissociation curve secondary to hyperventilation-induced reduction in PaCO2 (Fig. 13-8) and the occurrence of several extra deep breaths during anesthetic induction.18,56
The use of maximal exhalation before any preoxygenation maneuver has been suggested.32,57 In a healthy subject with an FRC of 3 L, forced exhalation to the residual volume decreases the lung volume to approximately 1.5 L. A 50% reduction of the FRC leads to 50% reduction in the time constant of the O2 washin (N2 washout) curve.58 The influence of prior maximal exhalation on preoxygenation using TVB or deep breathing has been studied.59 TVB after maximal exhalation resulted in a more rapid rise in EtO2 during the first minute than did TVB without prior maximal exhalation. However, the time required to reach maximal preoxygenation (EtO2 ≥90%) was the same (2.5 minutes) with or without maximal exhalation before TVB (Fig. 13-9).59 During deep breathing, the time courses of preoxygenation with and without prior maximal exhalation were identical. Apparently, the decrease in FRC resulting from maximal exhalation is minor in comparison with the level of 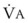 associated with deep breathing. Regardless of whether prior maximal exhalation was used, 1.5 minutes of deep breathing was still required to reach an EtO2 of 90% or greater. Therefore, prior maximal exhalation confers little or no additional practical benefit to preoxygenation.59
associated with deep breathing. Regardless of whether prior maximal exhalation was used, 1.5 minutes of deep breathing was still required to reach an EtO2 of 90% or greater. Therefore, prior maximal exhalation confers little or no additional practical benefit to preoxygenation.59
It has been demonstrated that preoxygenation using the single vital capacity breath (SVCB) technique can provide within 30 seconds a PaO2 comparable to that achieved by TVB for 3 minutes (Fig. 13-10).60 This technique is basically a triphasic process. The first phase consists of forced exhalation to the residual volume, which minimizes lung N2 content and the subsequent dilution of incoming O2. The second phase is deep inspiration to expand the lungs to their total capacity, with a consequent maximal increase in PaO2. The third phase consists of holding the chest in full inspiratory position, which may allow gas movement from the compliant alveoli to the less compliant alveoli (pendelluft maximization), because the time constants of filling between alveoli are not uniform.60 The SVCB technique can optimize preoxygenation, especially when it is used for fast induction of inhalation anesthesia.60
VI Breathing Systems for Preoxygenation
Mapleson suggested that the degree of rebreathing during spontaneous respiration is less with the Mapleson A system than with the Mapleson D system.61 Such difference of rebreathing between the anesthesia systems can affect the efficacy of preoxygenation. When using the Mapleson A or the circle system for preoxygenation by TVB, an O2 flow of 5 L/min can adequately preoxygenate the patient within 3 minutes, whereas an O2 flow of 10 L/min is required to achieve a similar EtO2 with the Mapleson D system.54,55 In contrast, when deep breathing is used, an O2 flow of 10 L/min is required irrespective of the anesthesia circuit.55
A system designed specifically for preoxygenation has gained some popularity in Europe (NasOral; LogoMed GambH, Windhagen, Germany).62,63 The NasOral system is a nonrebreathing system that delivers O2 from a 3.3-L reservoir bag to a small nasal mask, with exhalation occurring by the oral route using a mouthpiece.40,62,63 One-way valves in the nasal mask and the mouthpiece ensure unidirectional flow (Fig. 13-11). The FGF is adjusted to the individual ventilation to maintain an inflated reservoir bag.62,63 Investigations have shown that the semiclosed circle absorber and NasOral systems are equally effective in achieving oxygenation despite their different characteristics (see Fig. 13-3).40 Accordingly, there is little justification for the additional cost of this device for use in the operating room, where the circle absorber can be used for preoxygenation and the administration of anesthesia.40
In the critical care setting, resuscitation bags are commonly used for preoxygenation. However, their effectiveness differs markedly during spontaneous respiration.40 Some, because of their design, cannot deliver high FIO2 despite an FGF of 15 L/min or greater (Fig. 13-12).40 Resuscitation bags can be categorized into two groups depending on the type of valve mechanism. Disk-type valve systems use single or multiple disks to allow fresh gas to flow to the subject (and seal the exhalation port) during inspiration. The disk returns to its former position and opens the exhalation port during exhalation (Fig. 13-13). Because the disk valve function is not dependent on compression of the reservoir bag, this type of resuscitation bag functions equally well during manual and spontaneous ventilation and therefore can be used for preoxygenation.40,64,65
Resuscitation bags using duckbill inspiratory valves function differently during manual and spontaneous ventilation.40,65 During manual ventilation, gas is forced through the valve base, opening the duckbill valve and delivering fresh gas to the patient’s lungs. The force generated also seals the valve base to the exhalation port. During exhalation, the valve base returns to its former position, and exhaled gases are vented to the exhalation port (Fig. 13-14). Mills and colleagues found that duckbill-type resuscitation bags, without one-way exhalation valves to prevent air entrainment, showed variability in delivered O2 concentration during spontaneous respiration.65 These findings were confirmed by Nimmagadda and associates, who showed that some duckbill resuscitation bags cannot deliver a high FIO2 during spontaneous ventilation even if a high FGF is used.40 In the absence of a one-way valve on the exhalation port, generation of sufficient negative pressure to open the duckbill valve becomes impossible. During inspiration, the unsealed valve base allows room air to enter through the exhalation port and mix with O2 from a partially open duckbill valve (see Fig. 13-14). With the addition of a one-way valve on the exhalation port, duckbill-type resuscitation bags (Laerdal and Kirk) can reliably deliver an FIO2 greater than 0.9 with an FGF of 15 L/min. This valve seals the exhalation port during inspiration and allows the patient to generate sufficient negative pressure to open the duckbill valve, permitting O2 to flow without dilution40 (see Fig. 13-14).
The NasOral system has several advantages over resuscitation bags when used for preoxygenation: (1) It provides better preoxygenation than all available resuscitation bags because of a higher FIO2 and reduced apparatus dead space (see Fig. 13-12), (2) It is economical when O2 tanks are used because it requires an FGF comparable to the subject’s minute ventilation; (3) It can provide apneic oxygenation through the nasal mask during laryngoscopy and orotracheal intubation, if needed. Disadvantages of the NasOral system are that it requires the patient’s cooperation to use the mouthpiece and that the system cannot be used for positive-pressure ventilation.40
The inability of a resuscitation bag to deliver a high FIO2 may have serious consequences during RSI in the critical care setting or during transport of the spontaneously breathing, critically ill patient.40 Clinicians should ascertain that the resuscitation bags used for preoxygenation in their institutions are capable of delivering a high FIO2 during spontaneous ventilation.40
VII Special Situations
Investigations have highlighted risk factors for the rapid development of hypoxemia during apnea. These factors are additive and include a reduced FRC, inadequate denitrogenation, hypoventilation prior to apnea, increased 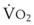 , and airway obstruction.34,39 Patients with a combination of these risk factors should be considered to be at high risk for hypoxemia during apnea.
, and airway obstruction.34,39 Patients with a combination of these risk factors should be considered to be at high risk for hypoxemia during apnea.
A Pregnant Patients
Because pregnant women are at high risk for pulmonary aspiration, RSI is desirable whenever general anesthesia is administered to these patients. Maximal preoxygenation can be achieved faster in pregnant than in nonpregnant women because of the increased 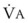 and decreased FRC.11,14,47 However, during apnea, pregnant women become hypoxemic more rapidly because of the limited O2 stores in their small FRC and increased
and decreased FRC.11,14,47 However, during apnea, pregnant women become hypoxemic more rapidly because of the limited O2 stores in their small FRC and increased 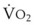 .46 This can be further compounded by pregnancy-associated airway changes which can add to delay in securing the airway.27 From the fifth month of pregnancy, the FRC decreases to 80% of that in the nonpregnant state, while the
.46 This can be further compounded by pregnancy-associated airway changes which can add to delay in securing the airway.27 From the fifth month of pregnancy, the FRC decreases to 80% of that in the nonpregnant state, while the 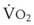 increases by 30% to 40%. In pregnant women, preoxygenation can be accomplished by TVB for 3 minutes or by deep breathing for 1 minute or longer before anesthetic induction. A combination of both techniques may also be used. Because of the increased
increases by 30% to 40%. In pregnant women, preoxygenation can be accomplished by TVB for 3 minutes or by deep breathing for 1 minute or longer before anesthetic induction. A combination of both techniques may also be used. Because of the increased 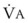 during pregnancy, an O2 flow of 10 L/min or higher is necessary (using the circle absorber) during TVB or deep breathing.66 However, the 4 DB/0.5 min technique should be used only in emergency situations when time is limited.
during pregnancy, an O2 flow of 10 L/min or higher is necessary (using the circle absorber) during TVB or deep breathing.66 However, the 4 DB/0.5 min technique should be used only in emergency situations when time is limited.
The influence of preoxygenation in the supine position versus the 45-degree head-up position on the duration of apnea leading to a decrease in SpO2 to 95% has been investigated.46 The average time for SpO2 to reach 95% was shorter in pregnant than in nonpregnant patients (173 versus 243 seconds) in the supine position.46 Use of the head-up position resulted in an increase in desaturation time in nonpregnant patients but had no effect in pregnant patients.46 It is possible that the gravid uterus prevents descent of the diaphragm in the upright position and therefore does not allow the expected increase in FRC.
B Morbidly Obese Patients
MO is associated with a more rapid decrease in SpO2 during apnea after induction of anesthesia.10,53,67–70 This is particularly hazardous because MO complicated by obstructive sleep apnea may be associated with an increased risk of difficult intubation (DI) and difficult mask ventilation (DMV).
The more rapid hemoglobin desaturation in the patient with MO is attributed to increased 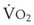 associated with a decreased FRC (Fig. 13-15). The supine position further decreases the FRC due to the cephalad displacement of the diaphragm by the abdominal contents. Anesthetic induction results in an additional reduction of the FRC. Whereas the FRC of nonobese patients decreases by 20% after induction of anesthesia, it decreases by 50% in MO patients. The tidal volume of the patient with MO may fall within the closing capacity, resulting in atelectasis, with a subsequent increase in intrapulmonary shunting.
associated with a decreased FRC (Fig. 13-15). The supine position further decreases the FRC due to the cephalad displacement of the diaphragm by the abdominal contents. Anesthetic induction results in an additional reduction of the FRC. Whereas the FRC of nonobese patients decreases by 20% after induction of anesthesia, it decreases by 50% in MO patients. The tidal volume of the patient with MO may fall within the closing capacity, resulting in atelectasis, with a subsequent increase in intrapulmonary shunting.
In the patient with MO, the time required for SpO2 to fall to 90% during apnea after preoxygenation with TVB for 3 minutes is significantly reduced compared to the time in nonobese patients (Fig. 13-16).29,70 In one study, the time to desaturation (SaO2 = 90%) for patients of normal weight was 6 minutes after preoxygenation, but for those with MO it was 2.7 minutes.67 A significant negative correlation between body mass index (BMI) and time to oxyhemoglobin desaturation has been described (see Fig. 13-15).70 The head-up position (25 degrees) during preoxygenation in MO patients has been shown to prolong the time of desaturation by about 50 seconds.69 The application of continuous positive airway pressure (CPAP) has been suggested to optimize preoxygenation in these patients, based on the assumption that CPAP will increase the FRC. 71 However, clinical observations showed that it did not delay the onset of desaturation, because the FRC returns to pre-CPAP levels once the patient is anesthetized and the CPAP mask is removed.71
It has been shown that nasopharyngeal O2 insufflation after preoxygenation in patients with MO delays the onset of oxyhemoglobin desaturation during the subsequent apnea by apneic diffusion oxygenation.70 In the critically ill MO patient with respiratory failure, traditional preoxygenation without or even with subsequent nasopharyngeal O2 insufflation may not increase the FRC O2 store and improve SaO2 before, during, or after endotracheal intubation. This may be attributed to atelectasis and decreased FRC with marked intrapulmonary shunting. In this situation, the use of noninvasive bi-level positive airway pressure (BiPAP) can improve alveolar recruitment with a consequent decrease in intrapulmonary shunting.72 BiPAP preoxygenation can achieve a notable increase in SaO2 associated with less hypercarbia, compared with the traditional technique of preoxygenation.72
C Elderly Patients
Several changes during aging may influence the period of preoxygenation needed in elderly patients and the subsequent time to oxyhemoglobin desaturation.13,15,36 On one hand, the basal 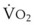 declines with age (from 143 mL/min/m2 in a male aged 20 years to 124 mL/min/m2 in a male aged 60 years), so the demand for O2 is less; on the other hand, changes in lung function make O2 uptake less efficient. The closing volume increases with age, leading to less efficient denitrogenation and a longer period of preoxygenation. The reduction of O2 demand in elderly patients does not compensate fully for the less efficient O2 uptake, because the mean times to 93% desaturation in elderly patients are approximately one half of those reported for equivalent preoxygenation periods in young patients. Reliable preoxygenation can be achieved with extended TVB (>3 minutes) or deep breathing for longer than 1 minute.
declines with age (from 143 mL/min/m2 in a male aged 20 years to 124 mL/min/m2 in a male aged 60 years), so the demand for O2 is less; on the other hand, changes in lung function make O2 uptake less efficient. The closing volume increases with age, leading to less efficient denitrogenation and a longer period of preoxygenation. The reduction of O2 demand in elderly patients does not compensate fully for the less efficient O2 uptake, because the mean times to 93% desaturation in elderly patients are approximately one half of those reported for equivalent preoxygenation periods in young patients. Reliable preoxygenation can be achieved with extended TVB (>3 minutes) or deep breathing for longer than 1 minute.
D Patients with Lung Disease
Patients with ARDS have decreased FRC, increased intrapulmonary shunting, and increased 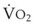 ; therefore, interruption of ventilation can result in rapid oxyhemoglobin desaturation. During thoracic surgery, the time to desaturation after one-lung ventilation is almost one half the time after two-lung ventilation (Fig. 13-17).73 This can be attributed to collapse of the nonventilated lung with a consequent decrease of the FRC O2 store; the rate of oxyhemoglobin desaturation may be further exaggerated by the associated intrapulmonary shunting.73 A similar decrease of the safety margin may occur in patients with lung disease characterized by decreased FRC or increased intrapulmonary shunting or both. In these patients, maximal preoxygenation should precede interruption of ventilation or tracheobronchial suction.
; therefore, interruption of ventilation can result in rapid oxyhemoglobin desaturation. During thoracic surgery, the time to desaturation after one-lung ventilation is almost one half the time after two-lung ventilation (Fig. 13-17).73 This can be attributed to collapse of the nonventilated lung with a consequent decrease of the FRC O2 store; the rate of oxyhemoglobin desaturation may be further exaggerated by the associated intrapulmonary shunting.73 A similar decrease of the safety margin may occur in patients with lung disease characterized by decreased FRC or increased intrapulmonary shunting or both. In these patients, maximal preoxygenation should precede interruption of ventilation or tracheobronchial suction.
E Pediatric Patients
Because of their smaller FRC and increased metabolic requirements, children are at increased risk for developing faster oxyhemoglobin desaturation than in adults whenever there is an interruption of their O2 delivery.48,74–80 The younger the child, the faster is the onset of hypoxemia.45,74–80 With a satisfactory mask fit, the efficacy of preoxygenation depends on the age and the duration and type of breathing.48,80,81 Studies in children have demonstrated that maximal preoxygenation can be reached faster than in adults. With TVB, an EtO2 of 90% or greater is reached within 60 to 100 seconds in almost all children.48,49,80 Among those in the first year of life, it is reached in 36 seconds (range, 20 to 60 seconds); between 3 and 5 years age, it is reached in 50 seconds (range, 30 to 90 seconds), and in those older than 5 years, it is reached in 68 seconds (range, 30 to 100 seconds).80 Deep breathing in children results in faster preoxygenation than TVB and also faster preoxygenation than deep breathing in adults (Fig. 13-18).49 Optimal preoxygenation can be accomplished in children with the use of deep breathing for 30 seconds.49
Several factors affect the onset of apnea-induced oxyhemoglobin desaturation after preoxygenation in children. These include the efficacy of preoxygenation, the child’s age (or weight), the presence of disease, and the composition of gases in the lungs. Some studies have examined the time required for SpO2 to decrease from 100% to 95% (Fig. 13-19)76,77; others have targeted a level as low as 90%.75,78,81 In a comparison of three groups of children who breathed 100% O2 at TVB for 1, 2, and 3 minutes before apnea, the times needed for SpO2 to decrease from 100% to 98%, 95%, and 90% were shorter in those who had breathed O2 for 1 minute.81 On the basis of these findings, 2 minutes of preoxygenation with TVB in children seems to provide maximum benefit and allows at least 2 minutes of safe apnea.81 In a 1-month-old infant, the rate of decline in PaO2 during apnea is three times more rapid than in an adult.82 The times for SpO2 to decrease from 100% to 95% and to 90% are shorter in younger children than in older children. Most infants reach an SpO2 of 90% in 70 to 90 seconds (see Fig. 13-19).75 This duration is shortened in the presence of upper respiratory infection.76 The benefit of preoxygenation is greater in the older child than that in the infant.82 In an 8-year-old, the time after the onset of apnea to 90% SaO2 is 0.47 minutes without preoxygenation but slightly longer than 5 minutes with preoxygenation.82
Although the time for desaturation is mainly dependent on the O2 content of the lungs at the start of apnea, other gas components may play a role. If 60% nitrous oxide (N2O) in O2 is used, the time for SpO2 to decrease to 95% is shortened to approximately one third,77 but it is still longer than the time observed after air-O2 breathing and maintains the same delivered O2 concentration.77 This can be explained by the second-gas effect. Compared with N2 in case of air-O2 breathing, N2O continues to dissolve into the blood and is carried away from the lungs, resulting in an increase in the PaO2 and hence delaying the onset of desaturation.77
It should be emphasized that the apnea-desaturation studies were performed in healthy children with a patent airway. The presence of cardiac or respiratory disease or airway obstruction could lead to faster desaturation during apnea.77 Premature infants are usually given a low FIO2 using an air-O2 mixture because of fear of retinopathy. Oxyhemoglobin desaturation occurs very quickly in these infants even after a short period of apnea. Transiently increasing the FIO2, limiting apnea to very short periods, and close monitoring are important considerations.83 The respiratory rate in preterm infants is 30 to 60 breaths/min. Rapid respirations tend to maintain their FRC by not allowing time to complete exhalation. Slow respirations and apnea result in marked decrease of FRC and fast desaturation after apnea.83
F Tracheobronchial Suctioning
Tracheobronchial suctioning to remove secretions is a commonly practiced maneuver in anesthetized and critically ill patients. Although the effects of suctioning are usually mild, they on occasion can have serious consequences.84–93 Cardiovascular collapse and even death as a consequence of severe hypoxemia associated with suctioning were recognized as early as the 1950s.84 Traditionally, tracheobronchial suctioning requires disconnection of the ETT from the anesthesia machine or the ventilator. A catheter is then introduced inside the ETT or tracheostomy tube, and suction is performed. This common method is referred to as open endotracheal suctioning (OES).94 The mechanisms of acute hypoxemia after OES are twofold.90,92,94 First, disconnection dilutes the FIO2 with air and induces massive loss of FRC, especially in patients with acute lung injury.90,92,94 Second, the high negative pressure required for removal of secretions contributes to further dilution of FIO2 and decrease in FRC, which results in atelectasis, fall in lung compliance, and intrapulmonary shunting.86,93
The effects of OES can be severe in patients with limited O2 reserves, when large suction catheters are used, with prolonged suctioning, and when selective bronchial suctioning is performed.86,89–95 Especially vulnerable is the infant, because a suction catheter may occupy a large part of the lumen of a small ETT.86,95 Studies in infants have consistently demonstrated a sharp fall in pulmonary compliance with prolonged suctioning and when large catheters are used.86 Infants ventilated at constant volumes had increases in transpulmonary pressure after tracheal suctioning ranging from 15% to 60%, whereas in infants ventilated with pressure-preset ventilators, tidal volumes fell by 25% to 70% (Fig. 13-20).86 Failure of tidal volumes to return to the pre-suctioning level was observed for periods as long as 30 minutes86 (Fig. 13-21).
In adult patients with acute lung injury, OES induces an 18% decrease in PaO2 and an increase in PaCO2 of 8%94 (Fig. 13-22). The maximum impairment appears within 1 minute after suctioning is begun and persists for 15 minutes after the end of suctioning. A 70% reduction in PaO2 has been observed in some patients.94 Decreases in 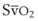 are commonly observed during OES in critically ill patients.91 The rise in PaCO2 may explain why OES is frequently associated with an increase in intracranial pressure in patients with severe head injury.94,96 A collapsed lung, as a consequence of suctioning, may remain collapsed for hours or even days, unless high pressures or high volumes are used to reinflate the lungs.86 Collapsed alveoli begin to open at an airway pressure of 18 to 23 cm H2O, and frequently an inflation pressure of 25 cm H2O or higher is desirable.86 Impairment in oxygenation after OES can be attenuated by prior maximal preoxygenation and by using a postsuctioning maneuver consisting of a multiple number of high tidal volumes.
are commonly observed during OES in critically ill patients.91 The rise in PaCO2 may explain why OES is frequently associated with an increase in intracranial pressure in patients with severe head injury.94,96 A collapsed lung, as a consequence of suctioning, may remain collapsed for hours or even days, unless high pressures or high volumes are used to reinflate the lungs.86 Collapsed alveoli begin to open at an airway pressure of 18 to 23 cm H2O, and frequently an inflation pressure of 25 cm H2O or higher is desirable.86 Impairment in oxygenation after OES can be attenuated by prior maximal preoxygenation and by using a postsuctioning maneuver consisting of a multiple number of high tidal volumes.
In the critical care setting, closed-circuit endotracheal suctioning (CES) has been advocated to prevent hypoxemia resulting from ventilator disconnection.94,97–99 Two methods are used. In one, suctioning is performed via a swivel adapter positioned at the proximal tip of the ETT; and in the other, total CES is done with a catheter continuously placed between the ETT and the Y-piece of the ventilator circuit.93,94 Because neither method requires disconnection, the loss of lung volume and hypoxemia are much less compared with OES, and the effect of suctioning becomes dependent only on the negative suction pressure.93
There is evidence that OES is more efficient than CES for secretion removal.94 Several mechanisms contribute to this difference. First, during OES, the airway pressure is zero, whereas in CES it is positive, especially if positive end-expiratory pressure (PEEP) is used. Second, during CES, the positive pressure from the ventilator fills the suction catheter with gas and tends to blow secretions distally. Third, disconnection from the ventilator during OES induces a sudden decrease in expiratory lung volume, and bronchial secretions may be entrained proximally with the expiratory gas flow, thereby facilitating secretion removal. The problem of decreased secretion removal with CES can be corrected by increasing the negative suction pressure from −200 cm H2O to −400 cm H2O, which enhances suctioning efficiency without further impairment of gas exchange.94
1 Guidelines for Suctioning
a Open Endotracheal Suctioning
1. The Presuctioning Stage: Before suctioning, the patient’s cardiovascular and respiratory status are optimized, and appropriate monitoring is continued. An additional dose of a muscle relaxant, opioid, or intravenous lidocaine may be given. The patient’s oxygenation should be maximized by using an FIO2 of 1 for several minutes. An indication of the efficacy of oxygenation is an EtO2 close to 90%. Other respiratory parameters may be adjusted to enhance O2 delivery. Suction equipment and sterile supplies should be checked. The choice of appropriate suction catheter size is important. The outer diameter (OD) of the catheter should be approximately 60% of the inner diameter (ID) of the ETT (e.g., 16 F for an 8.0-mm ETT, 14 F or for a 7.5-mm ETT). In infants, a slightly larger suction catheter with an OD 70% of the ID of the ETT may be used.
2. The Suctioning Procedure: After disconnection from the anesthesia machine or the ventilator, the suction catheter is introduced into the ETT and advanced until resistance is felt and is then withdrawn about 2 cm. Negative pressure of −50 to −200 cm H2O is applied intermittently for 2 to 4 seconds by occluding the side hole located at the proximal end of the catheter with the operator’s thumb. Intermittent suctioning may be continued (up to 20 seconds), during which the catheter is gently rotated and withdrawn. If additional suctioning is required, a recruitment maneuver (with high FIO2) should be performed before suctioning is repeated.
3. Postsuctioning Maneuvers: After suctioning, the patient is reconnected to the ventilator and a recruitment maneuver is performed while administering high FIO2 immediately.92,94 The most common maneuver is tidal volumes set at twice the baseline value, provided that the airway pressure does not exceed 25 to 50 cm H2O (depending on the existing lung pathology). The numbers of these high tidal volumes can vary from 2 to 20 breaths. In anesthetized healthy patients, 3 large TVBs are adequate. A study in ARDS patients showed that 2 consecutive sustained inflations lasting 20 seconds with an interval of 1 minute in between reversed the OES-induced decrease in PaO2 and lung volume.100
G “Routine” Preoxygenation Before Induction, During Recovery from Anesthesia, and in Critically Ill Patients
Preoxygenation is mandatory in the context of RSI, and it is arguably so in an additional range of scenarios. Imperative preoxygenation is indicated in those patients who cannot tolerate a fall in PO2, such as those with ischemic heart disease, and in patients with low PaO2, such as those with lung disease or right-to-left shunt. Because conditions such as the DI-DMV scenarios are unpredictable and many adverse reactions (e.g., drug-induced anaphylaxis, hypotension, hypoventilation) can occur, “routine” preoxygenation offers identifiable safety benefits during anesthetic induction.20–22 However, “routine” preoxygenation must be used as an adjunct rather than an alternative to a sequence of fundamental preoperative precautions that minimize adverse sequelae.21
Recovery from anesthesia and extubation have received limited critical safety measures compared with attention to the identification and management of potential DI, despite the observation that airway complications are more likely to be associated with extubation than intubation.101–103 The ASA Task Force on Management of the DA recommended that each anesthesiologist must have a preformulated strategy for extubation of the DA and an airway management plan for dealing with postextubation hypoventilation.19 Even routine discontinuation of anesthesia and tracheal extubation can be complicated with hypoxemia, hypoventilation, and loss of airway patency because of the residual effects of anesthetics and incomplete reversal of neuromuscular block.104,105 These effects can result in decreased functional activity of the pharyngeal muscles, leading to upper airway obstruction and a fivefold increase in the risk of aspiration; and they can affect the contractions of the respiratory muscles, resulting in hypoventilation and inability to cough effectively.104,105 They can also obtund the hypoxic drive by the peripheral chemoreceptors.105 In addition, adequate oxygenation before reversal of neuromuscular block is essential to ensure safe reversal and to minimize neostigmine-induced cardiac arrhythmias.106 Accordingly, “routine” preoxygenation before reversal of neuromuscular blockade and extubation is recommended to improve the margin of safety, given the potential of unpredictable airway and ventilation problems.
In critically ill, hemodynamically unstable patients, preoxygenation is less effective in raising PaO2. Whereas in a healthy patient, preoxygenation (FIO2 = 1) typically raises the PaO2 from 80 to 400 mm Hg, the increase in hemodynamically unstable patients is from 67 to 107 mm Hg only.107 Multiple factors limit the rise in PaO2, including inability to provide high FIO2, increased VO2, blood loss, anemia, stress, hypoventilation, acidemia, hypermetabolic state, older age, cardiopulmonary disease, and airway obstruction.107 This limitation implies that the time available to safely perform airway management maneuvers is rather limited in critically ill patients. Nevertheless, preoxygenation enhances the safety of these patients requiring emergency intubation. Findings from a recent swine hemorrhagic shock model confirm that FIO2 does influence the rate of apneic desaturation.108 A fivefold increase in time until critical desaturation can be achieved with preoxygenation compared with breathing of room air.108
IX Clinical Pearls
• The principal stores of body O2 are increased with breathing 100% O2; the maximal increase occurs in the functional residual capacity (FRC).
• The end points of maximal preoxygenation or denitrogenation have been defined as EtO2 of 90% or higher and EtN2 5% or lower.
• An O2 saturation of 100% on pulse oximetry (SpO2) should not be a reason to stop preoxygenation; conversely, failure of SpO2 to increase substantially does not necessarily indicate failure of preoxygenation.
• During apneic diffusion oxygenation via an open airway, the increase in time to hemoglobin desaturation achieved by increasing the fraction of inspired oxygen (FIO2) from 0.9 to 1.0 is greater than that caused by increasing the FIO2 from 0.21 to 0.9.
• In most patients, maximal preoxygenation can be accomplished by using tidal volume breathing (TVB) for 3 minutes or longer with a fresh gas flow (FGF) of 5 L/min or deep breathing for 1.5 minutes (FGF of 10 L/min) with the circle absorber.
• In the critical care setting, clinicians should ascertain that the resuscitation bags used for preoxygenation before rapid-sequence intubation (RSI) are capable of delivering high FIO2 during spontaneous ventilation.
• Patients with decreased O2 stores or increased 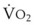 or both—including morbidly obese patients, children, pregnant women, critically ill patients, patients with ARDS, patients undergoing one-lung ventilation, and those patients with airway obstruction—develop faster arterial oxyhemoglobin desaturation during apnea.
or both—including morbidly obese patients, children, pregnant women, critically ill patients, patients with ARDS, patients undergoing one-lung ventilation, and those patients with airway obstruction—develop faster arterial oxyhemoglobin desaturation during apnea.
• Preoxygenation in the head-up position in morbidly obese patients prolongs the time of oxyhemoglobin desaturation.
• “Routine” preoxygenation is recommended, not only before anesthetic induction, but also before and during reversal of neuromuscular blockade, emergence from anesthesia, and extubation. It is also recommended before interruption of ventilation and tracheobronchial suctioning and before emergency intubation in the critically ill.
• Routine preoxygenation must be used as an adjunct rather than an alternative to a sequence of fundamental precautions that minimize adverse sequelae.
All references can be found online at expertconsult.com.
17 Nimmagadda U, Chiravuri SD, Salem MR, et al. Preoxygenation with tidal volume and deep breathing techniques: The impact of duration of breathing and fresh gas flow. Anesth Analg. 2001;92:1337–1341.
18 Benumof JL. Preoxygenation: Best method for both efficacy and efficiency [editorial]. Anesthesiology. 1999;91:603–605.
24 Campbell IT, Beatty PCW. Monitoring preoxygenation. Br J Anaesth. 1994;72:3–4.
29 Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to unparalyzed state from 1 mg/kg succinylcholine. Anesthesiology. 1997;87:979–982.
34 McNamara MJ, Hardman JG. Hypoxaemia during open-airway apnoea: A computational modeling analysis. Anaesthesia. 2005;60:741–746.
40 Nimmagadda U, Salem MR, Joseph NJ, et al. Efficacy of preoxygenation with tidal volume breathing: Comparison of breathing systems. Anesthesiology. 2000;93:1–7.
42 Drummond GB, Park GR. Arterial oxygen saturation before intubation of the trachea. Br J Anaesth. 1984;54:987–992.
46 Baraka AS, Hanna MT, Jabbour SI, et al. Preoxygenation of pregnant and nonpregnant women in the head-up versus supine position. Anesth Analg. 1992;75:757–759.
50 Farmery AD, Roe PG. A model to describe the rate of oxyhemoglobin desaturation during apnoea. Br J Anaesth. 1996;76:284–291.
69 Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25° head-up position than in the supine position in severely obese patients. Anesthesiology. 2005;102:1110–1115.
1 Fowler WS, Comroe JH. Lung function studies: 1. The rate of increase of arterial oxygen saturation during the inhalation of 100% oxygen. J Clin Invest. 1948;27:327–334.
2 Hamilton WK, Eastwood DW. A study of denitrogenation with some inhalation anesthetic systems. Anesthesiology. 1955;16:861–867.
3 Dillon JB, Darsi ML. Oxygen for acute respiratory depression due to administration of thiopental sodium. JAMA. 1955;159:1114–1116.
4 Heller ML, Watson TR, Jr. Polarographic study of arterial oxygenation during apnea in man. N Engl J Med. 1961;264:326–330.
5 Snow RG, Nunn JF. Induction of anaesthesia in the foot-down position for patients with a full stomach. Br J Anaesth. 1959;31:493–497.
6 Wylie WD. The use of muscle relaxants in the induction of anaesthesia of patients with a full stomach. Br J Anaesth. 1963;35:168–173.
7 Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404–406.
8 Salem MR, Wong AY, Collins VJ. The pediatric patient with a full stomach. Anesthesiology. 1973;39:435–440.
9 Gold MI, Duarte I, Muravchik S. Arterial oxygenation in conscious patients after 5 minutes and after 30 seconds of oxygen breathing. Anesth Analg. 1981;60:313–315.
10 Goldberg ME, Norris MC. Larijani GE, et al. Preoxygenation in the morbidly obese: A comparison of two techniques. Anesth Analg. 1989;68:520–522.
11 Norris MC, Dewan DM. Preoxygenation for cesarean section: A comparison of two techniques. Anesthesiology. 1985;62:827–829.
12 Gambee AM, Hertzka RE, Fisher DM. Preoxygenation techniques: Comparison of three minutes and four breaths. Anesth Analg. 1987;66:468–470.
13 McCarthy G, Elliott P, Mirakhur RK, McLoughlin C. A comparison of different pre-oxygenation techniques in the elderly. Anaesthesia. 1991;46:824–827.
14 Russell GN, Smith CL, Snowdon SL, Bryson THL. Pre-oxygenation and the parturient patient. Anaesthesia. 1987;42:346–351.
15 Valentine SJ, Marjot R, Monk CR. Preoxygenation in the elderly: A comparison of the four-maximal-breath and the three-minute techniques. Anesth Analg. 1990;71:516–519.
16 Baraka AS, Taha SK, Aouad MT, et al. Preoxygenation: Comparison of maximal breathing and tidal volume breathing techniques. Anesthesiology. 1999;91:612–615.
17 Nimmagadda U, Chiravuri SD, Salem MR, et al. Preoxygenation with tidal volume and deep breathing techniques: The impact of duration of breathing and fresh gas flow. Anesth Analg. 2001;92:1337–1341.
18 Benumof JL. Preoxygenation: Best method for both efficacy and efficiency [editorial]. Anesthesiology. 1999;91:603–605.
19 Practice guidelines for management of the difficult airway. An update report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
20 Baraka A. Routine preoxygenation. Anaesthesia. 2006;61:612–613.
21 Bell MDD. Routine preoxygenation: A new “minimum standard” of care. Anaesthesia. 2004;59:943–945.
22 Kung MC, Hung CT, Ng KP, et al. Arterial desaturation during induction in healthy adults: Should preoxygenation be a routine? Anaesth Intensive Care. 1991;19:192–196.
23 Nunn JF. Oxygen. In: Nunn JF, ed. Nunn’s applied respiratory physiology. ed 4. Oxford: Butterworth-Heinemann; 1993:247–305.
24 Campbell IT, Beatty PCW. Monitoring preoxygenation. Br J Anaesth. 1994;72:3–4.
25 Fraioli RL, Sheffer LA, Steffenson JL. Pulmonary and cardiovascular effects of apneic oxygenation in man. Anesthesiology. 1973;39:588–596.
26 Fruman MJ, Epstein RM, Cohen G. Apneic oxygenation in man. Anesthesiology. 1959;20:789–798.
27 Sirian R, Wills J. Physiology of apnoea and the benefits of preoxygenation. Contin Educ Anaesth Crit Care Pain. 2009;9:105–108.
28 Hardman JG, Wills JS, Aitkenhead AR. Factors determining the onset and course of hypoxemia during apnea: An investigation using physiological modeling. Anesth Analg. 2000;90:619–624.
29 Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to unparalyzed state from 1 mg/kg succinylcholine. Anesthesiology. 1997;87:979–982.
30 Eger EJ, II., Severinghaus JW. The rate of rise of PACO2 in the apneic anesthetized patient. Anesthesiology. 1961;22:419–425.
31 Teller LE, Alexander GM, Fruman MJ, Gross JB. Pharyngeal insufflation of oxygen prevents arterial desaturation during apnea. Anesthesiology. 1988;69:980–982.
32 Baraka A, Salem MR, Joseph NJ. Critical hemoglobin desaturation can be delayed by apneic diffusion oxygenation. Anesthesiology. 1999;90:332–333.
33 Holmdahl MH. Pulmonary uptake of oxygen, acid-base metabolism, and circulation during prolonged apnoea. Acta Chir Scand. 1956;212(Suppl):1–128.
34 McNamara MJ, Hardman JG. Hypoxaemia during open-airway apnoea: A computational modeling analysis. Anaesthesia. 2005;60:741–746.
35 Berry CB, Miles PS. Preoxygenation in healthy volunteers: A graph of oxygen “washing” using end-tidal oxygraphy. Br J Anaesth. 1994;72:116–118.
36 Bhatia PK, Bhandari SC, Tulsioni KL, Kumar Y. End-tidal oxygraphy and safe duration of apnea in young adults and elderly patients. Anaesthesia. 1997;52:175–178.
37 Carmichael FJ, Cruise CJE, Crago RR, Paluck S. Preoxygenation: A study of denitrogenation. Anesth Analg. 1989;68:406–409.
38 Archer GW, Marx GF. Arterial oxygen tension during apnoea in parturient women. Br J Anaesth. 1974;46:358–360.
39 McCahon RA, Hardman JG. Fighting for breath: Apnoea, vs the anaesthetist. Anaesthesia. 2007;62:105–108.
40 Nimmagadda U, Salem MR, Joseph NJ, et al. Efficacy of preoxygenation with tidal volume breathing: Comparison of breathing systems. Anesthesiology. 2000;93:1–7.
41 Berthoud M, Read DH, Norman J. Pre-oxygenation: How long? Anaesthesia. 1983;38:96–102.
42 Drummond GB, Park GR. Arterial oxygen saturation before intubation of the trachea. Br J Anaesth. 1984;54:987–992.
43 McGowan P, Skinner A. Preoxygenation: The importance of a good face mask seal. Br J Anaesth. 1995;75:777–778.
44 Schlack W, Heck Z, Lorenz C. Mask tolerance and preoxygenation: A problem for anesthesiologists but not for patients [letter]. Anesthesiology. 2001;94:546.
45 Machlin HA, Myles PS, Berry CB, et al. End-tidal oxygen measurement compared with patient factor assessment for determining preoxygenation time. Anaesth Intensive Care. 1993;21:409–412.
46 Baraka AS, Hanna MT, Jabbour SI, et al. Preoxygenation of pregnant and nonpregnant women in the head-up versus supine position. Anesth Analg. 1992;75:757–759.
47 Byrne F, Oduro-Dominah A, Kipling R. The effect of pregnancy on pulmonary nitrogen washout: A study of pre-oxygenation. Anaesthesia. 1987;42:148–150.
48 Butler PJ, Munro HM, Kenny MB. Preoxygenation in children using expired oxygraphy. Br J Anaesth. 1996;77:333–334.
49 Salem MR, Joseph NJ, Villa EM, et al. Preoxygenation in children: Comparison of tidal volume and deep breathing techniques [abstract]. Anesthesiology. 2001;95:A1247.
50 Farmery AD, Roe PG. A model to describe the rate of oxyhemoglobin desaturation during apnoea. Br J Anaesth. 1996;76:284–291.
51 Hayes AH, Breslin DS, Mirakhur RK, et al. Frequency of haemoglobin desaturation with the use of succinylcholine during rapid sequence induction of anesthesia. Acta Anaesthesiol Scand. 2001;45:746–749.
52 Heier T, Feiner JR, Lin J, et al. Hemoglobin desaturation after succinylcholine-induced apnea: A study of the recovery of spontaneous ventilation in healthy volunteers. Anesthesiology. 2001;94:754–759.
53 Berthoud MC, Peacock JE, Reilly CS. Effectiveness of preoxygenation in morbidly obese patients. Br J Anaesth. 1991;67:464–466.
54 Taha S, El-Khatib M, Siddik-Sayyid SM, et al. Preoxygenation with the Mapleson D system requires higher oxygen flows than Mapleson A or circle systems. Can J Anesth. 2007;54:141–145.
55 Taha SK, El-Khatib MF, Siddik-Sayyid SM. Preoxygenation by 9 deep breaths in 60 seconds using the Mapleson A (Magill), the circle system or the Mapleson D system. J Clin Anesth. 2009;21:574–578.
56 Salem MR, Joseph NJ, Crystal GJ, Nimmagadda U. Preoxygenation: Comparison of maximal breathing and tidal volume techniques [letter]. Anesthesiology. 2000;92:1845–1847.
57 McCrory JW, Matthews JNS. Comparison of four methods of preoxygenation. Br J Anaesth. 1990;64:571–574.
58 Baraka A, Taha SK, EL-Khatib MF, et al. Oxygenation using tidal volume breathing after maximal exhalation. Anesth Analg. 2003;97:1533–1535.
59 Nimmagadda U, Salem MR, Joseph NJ, Miko I. Efficacy of Preoxygenation using tidal volume breathing and deep breathing techniques with and without prior maximal exhalation. Can J Anesth. 2007;54:448–452.
60 Baraka A, Haroun-Bizri S, Khoury S, Chehab IR. Single vital capacity breath for preoxygenation. Can J Anaesth. 2000;47:1144–1146.
61 Mapleson WW. The elimination of rebreathing in various semi-closed anaesthetic systems. Br J Anaesth. 1954;26:323–332.
62 Mertzlufft F, Zander R. Intrapulmonary O2 storage with the NasOral system. Anathesiol Intensivmed Notfallmed Schmerzther. 1994;29:235–237.
63 Mertzlufft F, Zander R. Optimal preoxygenation: The NasOral system. Adv Exp Med Biol. 1994;345:45–50.
64 Hess D, Hirsch C, Marquis-D’Amico C, Macmarek RM. Imposed work and oxygen delivery during spontaneous breathing with adult disposable manual ventilators. Anesthesiology. 1994;81:1256–1263.
65 Mills PJ, Baptiste J, Preston J, Barnas GM. Manual resuscitators and spontaneous ventilation: An evaluation. Crit Care Med. 1991;19:1425–1431.
66 Russel EC, Wrench J, Feast M, Mohammed F. Preoxygenation in pregnancy: The effect of fresh gas flow rates within a circle breathing system. Anaesthesia. 2008;63:833–836.
67 Jense HG, Dubin SA, Silverstein PJ, O’Leary-Escolas U. Effect of obesity on safe duration of apnea in anesthetized humans. Anesth Analg. 1991;72:89–93.
68 Cressy DM, Berthand MC, Reilly CS. Effectiveness of continuous positive airway pressure to enhance preoxygenation in the morbidly obese woman. Anaesthesia. 2001;56:670–689.
69 Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25° head-up position than in the supine position in severely obese patients. Anesthesiology. 2005;102:1110–1115.
70 Baraka AS, Taha SK, Siddik SM, et al. Supplementation of preoxygenation in morbidly obese patients using nasopharyngeal oxygen insufflation. Anaesthesia. 2007;62:769–773.
71 Cresey DM, Berthoud MC, Reilly CS. Effectiveness of continuous positive airway pressure to enhance preoxygenation in morbidly obese women. Anaesthesia. 2001;56:680–684.
72 El-Khatib MF, Kanazi G, Baraka AS. Noninvasive bi-level positive airway pressure for preoxygenation of the critically ill morbidly obese patient. Can J Anesth. 2007;54:744–747.
73 Baraka A, Aouad M, Taha S, et al. Apnea-induced hemoglobin desaturation during one-lung vs two-lung ventilation. Can J Anaesth. 2000;47:58–61.
74 Laycock GJA, McNicol LR. Hypoxemia during induction of anesthesia: An audit of children who underwent general anaesthesia for routine elective surgery. Anaesthesia. 1988;43:981–984.
75 Dupeyrat A, Dubreuil M, Ecoffey C. Preoxygenation in children [letter]. Anesth Analg. 1994;79:1027.
76 Kinouchi K, Tanigami H, Tashiro C, et al. Duration of apnea in anesthetized infants and children required for desaturation of hemoglobin to 95%: Influence of upper respiratory infection. Anesthesiology. 1992;77:1105–1107.
77 Kinouchi K, Fukumitsu K, Tashiro C, et al. Duration of apnoea in anaesthetized children required for desaturation of haemoglobin to 95%: Comparison of three different breathing gases. Paediatr Anaesth. 1995;5:115–119.
78 Videira RLR, Neto PPR, Gomide RV, et al. Preoxygenation in children: For how long? Acta Anaesthesiol Scand. 1992;36:109–111.
79 Patel R, Lenczyck M, Hannallah RS, McGill WA. Age and onset of desaturation in apnoeic children. Can J Anaesth. 1994;41:771–774.
80 Morrison JE, Collier E, Friesen RH, Logan L. Preoxygenation before laryngoscopy in children: How long is enough? Paediatr Anaesth. 1998;8:293–298.
81 Xue F-S, Tong S-Y, Wang X-L, et al. Study of the optimal duration of preoxygenation in children. J Clin Anesth. 1995;7:93–96.
82 Hardman JG, Wills JS. The development of hypoxaemia during apnoea in children: A computational modeling investigation. Br J Anaesth. 2006;97:564–570.
83 Gregory GA. Respiratory care of the child. Crit Care Med. 1980;8:582–587.
84 Boba A, Cincotti JJ, Piazza TE, Landmesser CM. The effects of apnea, endotracheal suction, and oxygen insufflations, alone and in combination, upon arterial oxygen saturation in anesthetized patients. J Lab Clin Med. 1959;53:680–685.
85 Boutros AR. Arterial blood oxygenation during and after endotracheal suctioning in the apneic patient. Anesthesiology. 1970;32:114–118.
86 Brandstater B, Muallem M. Atelectasis following tracheal suction in infants. Anesthesiology. 1969;31:468–473.
87 Shim C, Fine N, Fernandez R, Williams MH, Jr. Cardiac arrhythmias resulting from tracheal suctioning. Ann Intern Med. 1969;71:1149–1153.
88 Urban BJ, Weitzner SW. Avoidance of hypoxemia during endotracheal suctioning. Anesthesiology. 1969;31:473–475.
89 Brown SE, Stansbury DW, Merrill EJ, et al. Prevention of suctioning-related arterial oxygen desaturation: Comparison of off-ventilator and on-ventilator suctioning. Chest. 1983;83:621–627.
90 Brochard L, Mion G, Isabey D, et al. Constant-flow insufflation prevents arterial oxygen desaturation during endotracheal suctioning. Am Rev Respir Dis. 1991;144:395–400.
91 Clark AP, Winslow EH, Tyler DO, White KM. Effects of endotracheal suctioning on mixed venous oxygen saturation and heart rate in critically ill adults. Heart Lung. 1990;19:552–557.
92 Lu Q, Capderou A, Cluzel P, et al. A computed tomographic scan assessment of endotracheal suctioning-induced bronchoconstriction in ventilated sheep. Am J Respir Crit Care Med. 2000;162:1898–1904.
93 Maggiore SM, Lellouche F, Pigeot J, et al. Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med. 2003;167:1215–1224.
94 Lasocki S, Lu Q, Sartorius A, et al. Open and closed-circuit endotracheal suctioning in acute lung injury: Efficiency and effects on gas exchange. Anesthesiology. 2006;104:39–47.
95 Salem MR, Wong AY, Mathrubhutham M, et al. Evaluation of selective bronchial suctioning techniques used for infants and children. Anesthesiology. 1978;48:379–380.
96 Kerr ME, Weber BB, Sereika SM, et al. Effect of endobronchial suctioning on cerebral oxygenation in traumatic brain-injured patients. Crit Care Med. 1999;27:2776–2781.
97 Harshbarger SA, Hoffman LA, Zullo TG, Pinsky MR. Effects of a closed-tracheal suction system on ventilator and cardiovascular parameters. Am J Crit Care. 1992;1:57–61.
98 Fernandez MD, Piacentini E, Blanch L, Fernandez R. Changes in lung volume with three systems of endotracheal suctioning with and without preoxygenation in patients with mild-to-moderate lung failure. Intensive Care Med. 2004;30:2210–2215.
99 Carlon GC, Fox SJ, Ackerman NJ. Evaluation of a closed-tracheal suction system. Crit Care Med. 1987;15:522–525.
100 Dyhr T, Band J, Larson A. Lung recruitment manoeuvres are effective in regaining lung volume and oxygenation after open endotracheal suctioning in acute respiratory distress syndrome. Crit Care. 2003;7:55–62.
101 Caplan RA, Posner KL, Ward RJ, et al. Adverse respiratory events in anesthesia: A closed claim analysis. Anesthesiology. 1990;72:828–833.
102 Miller KA, Harkin CO, Bailey PL. Postoperative tracheal extubation. Anesth Analg. 1995;80:149–172.
103 Asai T, Koga K, Voughan RS. Respiratory complications associated with tracheal intubation and extubation. Br J Anaesth. 1998;80:767–775.
104 Eriksson LI, Sundman E, Olsson R, et al. Functional assessment at rest and during swallowing in partially paralyzed humans: Simultaneous videomanometry and mechanography of awake human volunteers. Anesthesiology. 1997;87:1035–1043.
105 Eriksson LI. The effects of residual neuromuscular blockade and volatile anesthetics on the control of ventilation. Anesth Analg. 1999;89:243–251.
106 Baraka A. Safe reversal (2) atropine-neostigmine mixture: An electrocardiographic study. Br J Anaesth. 1968;40:30–36.
107 Mort TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med. 2005;33:2672–2675.
108 Pehbock D, Wenzel V, Voelckel W, et al. Effects of preoxygenation on desaturation time during hemorrhagic shock in pigs. Anesthesiology. 2010;113:593–599.

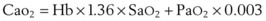
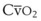 ) when there is a mixed venous O2 tension (
) when there is a mixed venous O2 tension (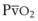 ) of 40 mm Hg and mixed venous oxyhemoglobin saturation (
) of 40 mm Hg and mixed venous oxyhemoglobin saturation (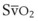 ) of 75% can be calculated accordingly.
) of 75% can be calculated accordingly.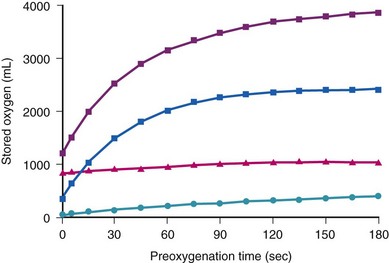
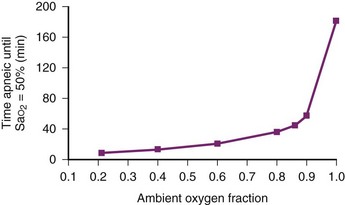
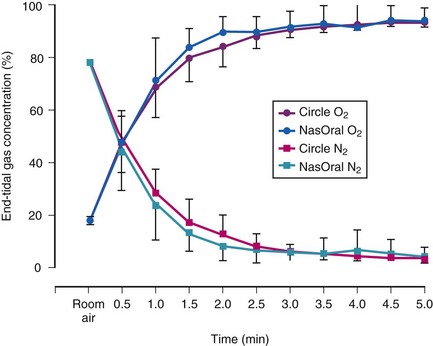
 ) to FRC. Because the O2 flow used for
) to FRC. Because the O2 flow used for  is delivered via an anesthesia circuit, preoxygenation occurs in two sequential stages according to the time constant, which is the time required for a flow through a container (volume) to equal the capacity. These two stages are
is delivered via an anesthesia circuit, preoxygenation occurs in two sequential stages according to the time constant, which is the time required for a flow through a container (volume) to equal the capacity. These two stages are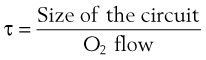
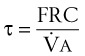
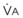 /FRC
/FRC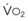
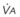 FRC, ratio of alveolar ventilation to functional residual capacity;
FRC, ratio of alveolar ventilation to functional residual capacity; 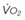 , O2 consumption.
, O2 consumption.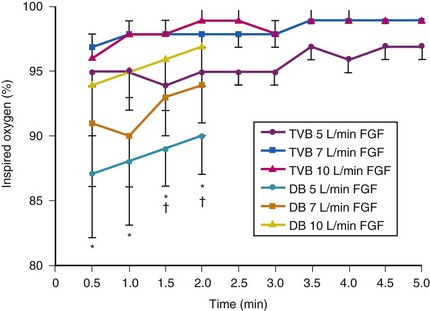
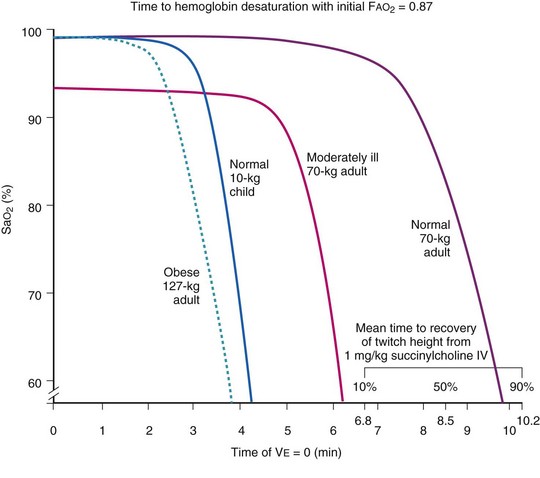
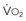 ]), and in a moderately ill adult, compared with a healthy adult. F
]), and in a moderately ill adult, compared with a healthy adult. F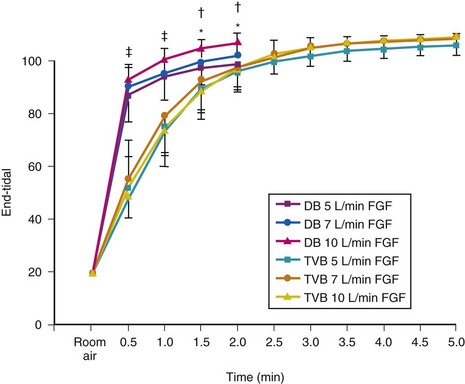
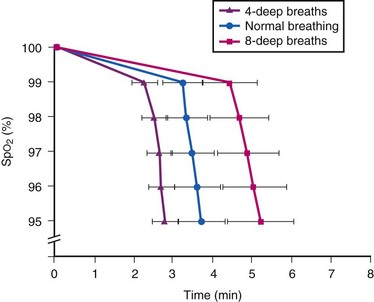
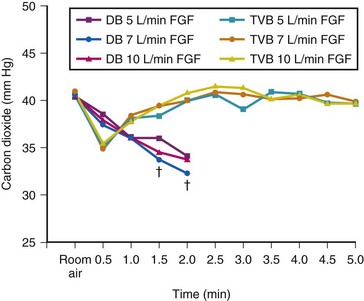
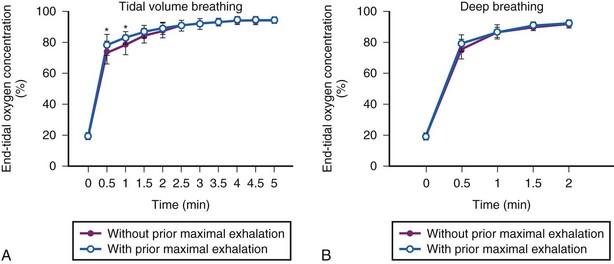
 ) versus TVB without prior maximal exhalation (purple •). *Statistically significant difference (P < .05) between techniques (TVB with or without prior maximal exhalation) at that time period. B, Comparison of end-tidal O2 concentration values (mean ± SD) over 2-minute periods during simulated preoxygenation using deep breathing technique after maximal exhalation (blue
) versus TVB without prior maximal exhalation (purple •). *Statistically significant difference (P < .05) between techniques (TVB with or without prior maximal exhalation) at that time period. B, Comparison of end-tidal O2 concentration values (mean ± SD) over 2-minute periods during simulated preoxygenation using deep breathing technique after maximal exhalation (blue  ) versus deep breathing without prior maximal exhalation (purple •).
) versus deep breathing without prior maximal exhalation (purple •).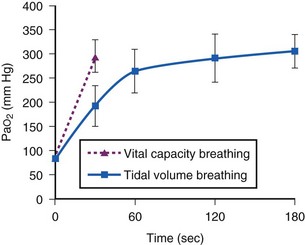
 ) compared with that achieved by the traditional preoxygenation technique (
) compared with that achieved by the traditional preoxygenation technique ( ).
).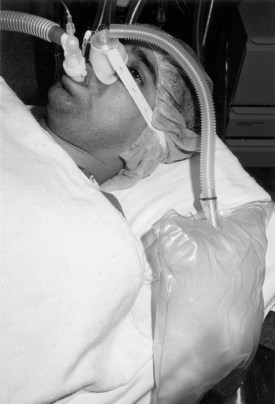
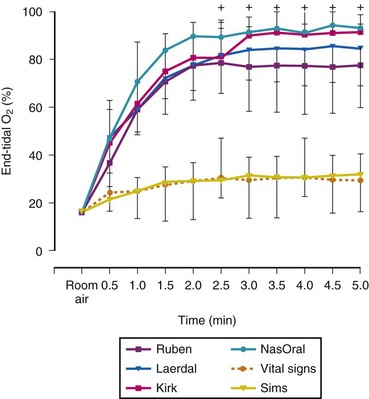
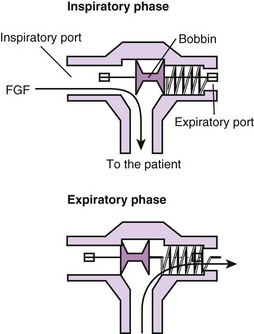
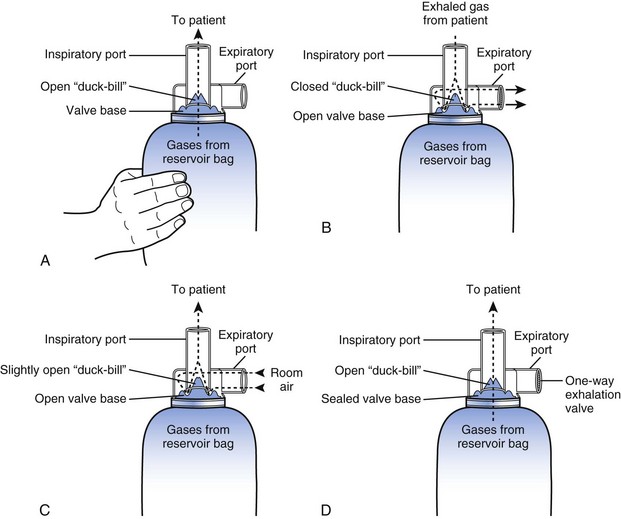
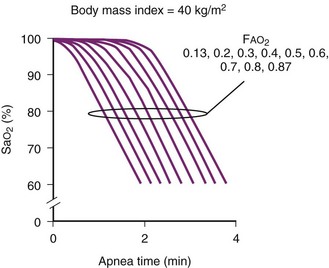
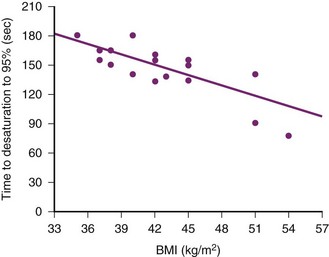
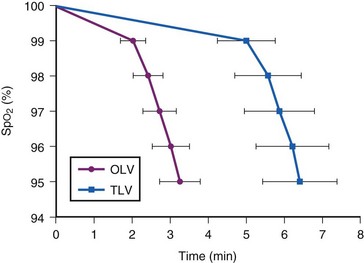
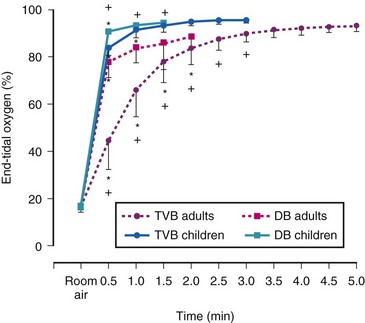
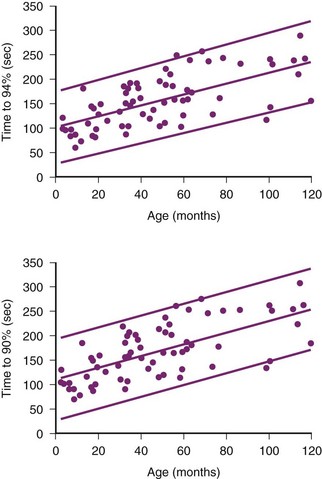
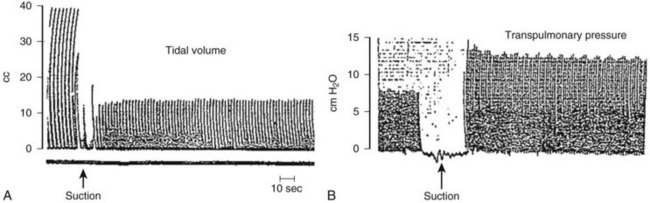
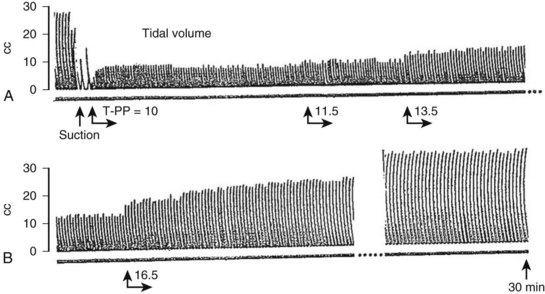
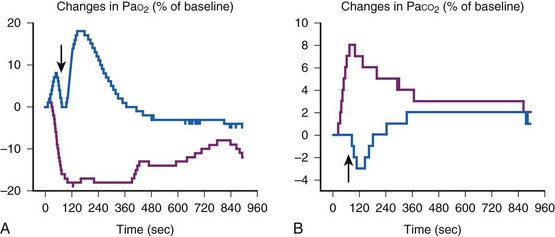
 or both. Preoxygenation should be considered when maneuvers that deplete the O2 reserves, such as suctioning, one-lung ventilation, or apneic oxygenation, are performed.
or both. Preoxygenation should be considered when maneuvers that deplete the O2 reserves, such as suctioning, one-lung ventilation, or apneic oxygenation, are performed.