Chapter 14 Preoperative and Therapeutic Endovascular Approaches for Spinal Tumors
NORMAL ANATOMY
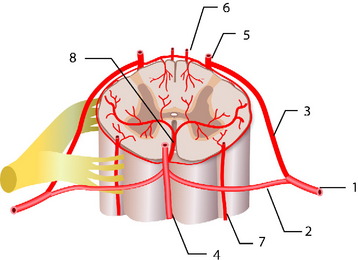
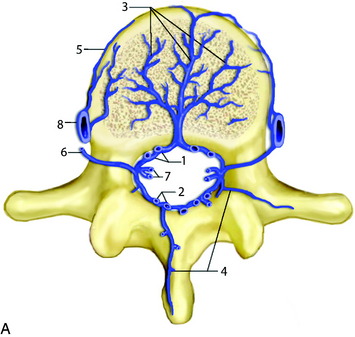
The normal arterial supply to the spinal cord consists of a single anterior spinal artery (ASA) and paired posterior spinal arteries (PSAs).1 The ASA is typically contiguous throughout its course and runs along the ventral surface of the cord. At its most cranial extent, the ASA is formed from paired arteries arising from the distal vertebral arteries. Additional contribution to ASA supply is provided by radiculomedullary arteries at various levels, the most prominent of which include the artery of the cervical enlargement, typically around C5–6, and the artery of Adamkiewicz. The PSAs are paired longitudinal arteries along the dorsal surface of the cord, supplied from multiple radiculopial arteries, with frequent communicating arteries between the two PSAs. There is a pial circumferential network on the surface of the cord connecting the ASA and PSA systems, but these small vessels typically are too small to provide sufficient collateral flow in the setting of occlusion. The ASA typically supplies the anterior two-thirds of the spinal cord, whereas the PSA territory is the remaining posterior third (Fig. 14-1).
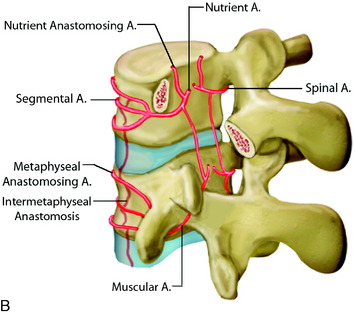
At each level in the spine, paired radicular arteries give supply to the vertebra, nerve root, and cord. These radicular arteries enter the thecal sac at the neural foramen and have dural branches as well as radiculomedullary arteries (supplying the ASA) and radiculopial branches (supplying the PSA). In the mid-cervical level, typically around C5–6, a prominent radicular branch supplies the ASA, known as the artery of the cervical enlargement.2 The ASA is larger here and in the lower thoracic regions because of increased metabolic needs, given the relatively higher amount of gray matter in the cord at these levels. In the cervical spine, supply also can arise from the ascending cervical and deep cervical arteries, which typically arise from the thyrocervical and costocervical trunks, respectively. Even though these branches typically supply the posterior musculature, and often the posterior elements, they also can supply the ASA and PSA.
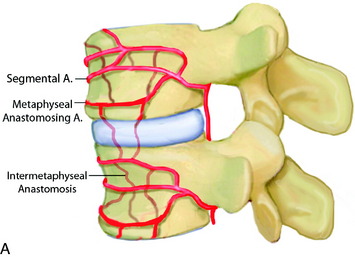
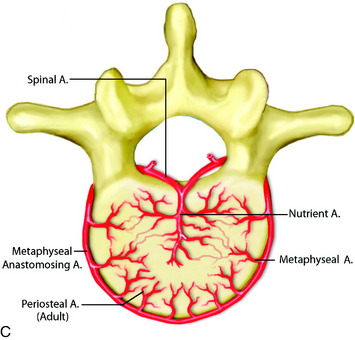
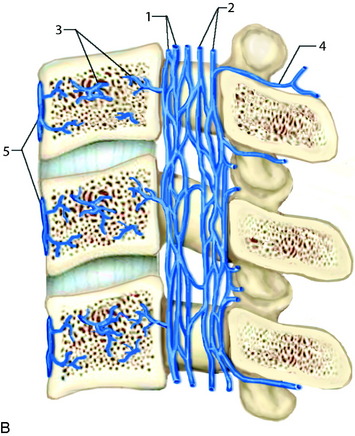
In the thoracic cord, radicular arteries of the upper thoracic spine arise from the supreme intercostal arteries, which arise directly from the costocervical trunk, a branch of the subclavian artery, and supply the T1–3 levels. From T4 downward, paired intercostal arteries arise from the thoracic aorta and a rich longitudinal anastomotic network is situated between them. Each intercostal branch gives rise to multiple, small, perforating arteries supplying the vertebral body and then gives off a dorsospinal branch, which further branches to dorsal muscular branches, and the radicular (spinal) artery for that level.3 The major supply to the vertebral body is from the nutrient artery, which is formed from paired spinal arteries along the dorsal surface of the vertebral body and then enters the body in its mid-portion (Fig. 14-2). At each vertebral body level, there are also multiple small, peripheral periosteal branches along the surface of the vertebral body, which supply the peripheral one-third of the lateral and anterior aspects of the vertebral bodies. In addition, there are smaller, metaphyseal arterial branches supplying the metaphyseal regions of the bodies.
The primary route for venous drainage of the vertebral body is through the basivertebral veins, which coalesce at the dorsal mid-vertebral body to connect to the internal vertebral venous network.4 This valveless system has extensive longitudinal connections between the veins of adjacent levels and also connects with the cranial dural venous sinuses at the level of the foramen magnum. There also are connections anterior to the vertebral body along the anterior external vertebral venous network (Fig. 14-3).
ANGIOGRAPHIC EVALUATION
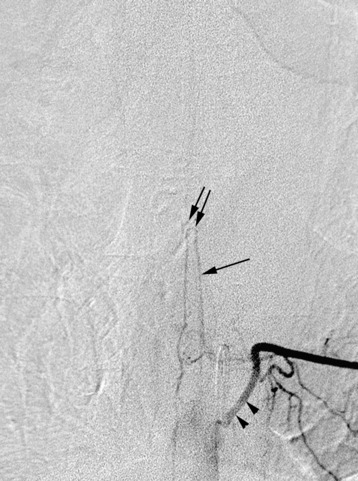
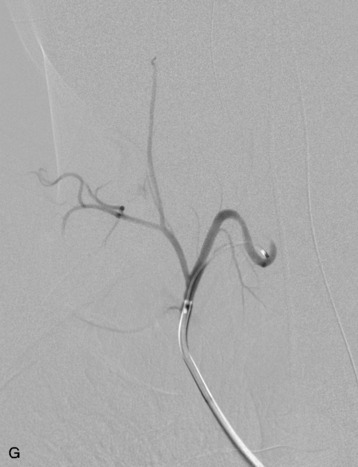
In evaluating thoracic or lumbar spinal tumors, one of the primary objectives of diagnostic angiography is to determine the level of origin of the artery of Adamkiewicz, also known as the artery radicularis magna, which provides the primary supply to the anterior spinal artery for the lower thoracic cord. This artery typically arises from between T9 and L2 and is more common on the left than the right.5 Angiography will typically reveal a characteristic hairpin turn in the course of the artery of Adamkiewicz (Fig. 14-4). It is very helpful for the surgeon to know whether the artery of Adamkiewicz arises from a radicular artery that is in the planned operative field. Selective injection of the intercostal arteries at least two levels above and below the affected levels should be performed because there is a rich collateral network among the intercostal arteries. The supreme intercostal artery also should be studied in all patients with upper thoracic lesions.
Preoperative embolization of tumors can significantly assist the surgeon in obtaining hemostasis. Among the particularly vascular lesions are metastatic renal cell carcinoma, thyroid carcinoma, and aggressive hemangiomas of the bone. Embolization also has been performed for aneurysmal bone cysts of the spinal column.6 Embolization for intradural lesions carries higher risk and should be used judiciously based on tumor characteristics and patient symptoms. Embolization of intramedullary lesions carries the highest risk and should only be considered for known hypervascular lesions, such as hemangioblastomas.7–9
CLINICAL STUDIES
Several series have examined the results of preoperative embolization for spinal tumors. Guzman et al10 studied 24 patients with hypervascular metastatic lesions, predominantly renal cell carcinoma (15 patients) and thyroid cancer (four patients). Mean intraoperative blood loss in the 22 patients in whom embolization was successfully performed was 1900 cc, compared with 5000 cc in the two patients in whom embolization was only partial. Chatziioannou et al11 examined 26 patients (28 surgical procedures) with renal cell metastases to the spinal column and found a significant difference in blood loss in patients in whom embolization was complete (10 cases, mean blood loss 535 cc) and those in whom it was incomplete (18 cases, mean blood loss 1247 cc). Berkefeld12 examined the intraoperative blood loss in 50 patients undergoing corpectomy, who underwent either proximal coil embolization (26 patients), particulate and coil embolization (24 patients), or just particulate embolization (nine patients), and compared these with a control group of 10 patients with no embolization. The most common histology was renal cell carcinoma (31 of 69 patients), and 65 of these 69 were either thoracic or lumbar in location. Mean blood loss for the groups were 4350 cc for no embolization, 2650 for coils alone, 1850 for particles and coils, and 1800 for particles alone. They concluded that proximal occlusion alone without distal tumor penetration was not sufficient to limit intraoperative blood loss. Vetter et al13 reviewed their experience with 38 patients with cervical tumors treated with preoperative embolization, including particulate embolization, coil embolization, or vessel sacrifice. The procedures were technically successful in all patients, and mean intraoperative blood loss was 2400 cc. Several other studies also have demonstrated a similar benefit to preoperative embolization.14–19
EMBOLIZATION TECHNIQUE
The technique for embolization initially involves identifying the normal arterial supply to the region, including selective injections of appropriate vessels (as discussed previously). Once the anatomy has been delineated, super-selective catheterization of the vessels supplying the lesion can be performed.7,10 After this, embolization is typically performed using particulate agents, such as polyvinyl alcohol (PVA) spheres. Again, care should be taken to ensure that there are no anastomoses seen between the vessel being embolized and supply to the anterior or posterior spinal arteries. Typically, initial particle size will be between 100–300 μm, with progressive increase in size as distal flow slows. The use of proximal occlusion with Gelfoam should be reserved until after the distal tumor bed has been embolized with small particles. Proximal occlusion alone carries the risk of recanalization and recruitment of new blood flow into the tumor bed and has been shown to be not as effective as particulate embolization.7,12 In patients in whom tumor supply is shared with supply to the ASA, extreme care should be taken in performing the embolization. In a recent series, Prabhu et al20 described their experience with embolizing spinal tumors, and in 29 of their 51 patients, tumor supply was shared with the radiculomedullary artery. In 80% of those patients, they were able to embolize more than 80% of the tumor with careful microcatheter placement beyond the origin of the radiculomedullary artery.
BALLOON TEST OCCLUSION AND PERMANENT VESSEL OCCLUSION
For patients with cervical spinal lesions, it may be beneficial to perform a balloon test occlusion (BTO) of the vertebral artery on the side of maximal tumor involvement to determine the safety of sacrificing this artery either surgically or endovascularly. When performing BTO, the patient typically is kept awake, and neurophysiological monitoring (including physical examination, as well as continuous electroencephalography (EEG), somatosensory evoked potentials (SSEPs), and brainstem auditory evoked potentials) is performed. Typically, the balloon is inflated in the vessel distal to the highest level of tumor, if possible, so as to simulate the level of vessel sacrifice at surgery, and kept inflated for 15–20 minutes.13
ILLUSTRATIVE CASES
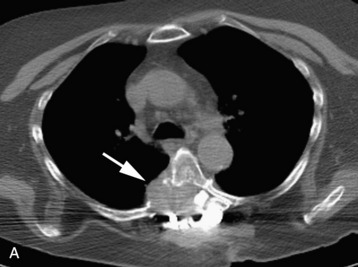
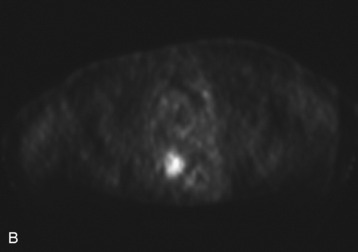
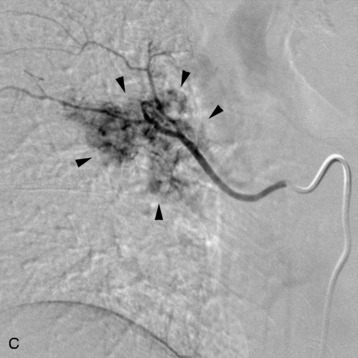
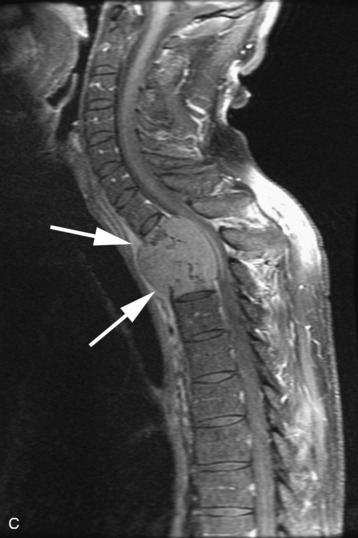
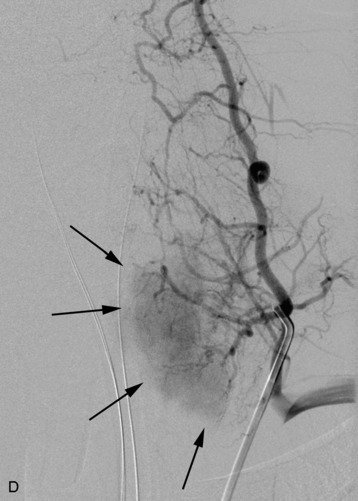
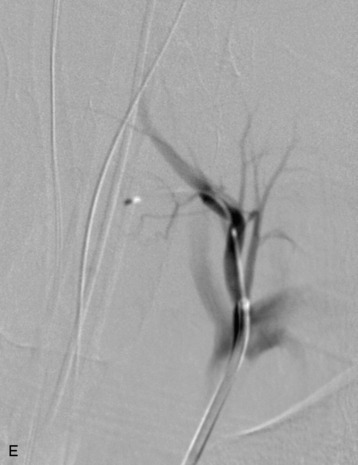
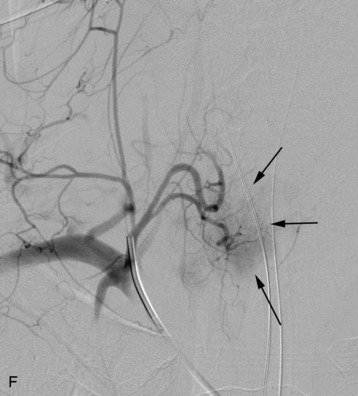
A 56-year-old woman had a history of recurrent lung carcinoma with a new lesion involving the upper thoracic spine. Non-contrast CT scan showed a focal destructive lesion involving the right pedicle of T5 (Fig. 14-5). Trans-axial image from 2-deoxy-2-[18F] fluoro-D-glucose (18FDG) positron emission tomography (PET) scan performed with attenuation correction demonstrated focal area of increased FDG activity corresponding to the lesion on the CT scan. A frontal projection image from a selective right T5 intercostal injection demonstrated a hypervascular mass with prominent tumor blush. This was successfully embolized with PVA particles, initially 100–300 μm and subsequently 300–500 μm. Surgical stabilization was performed without incident.
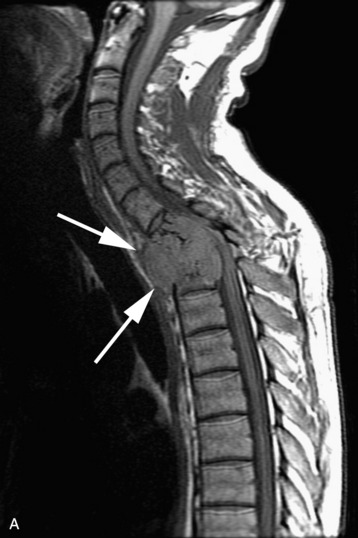
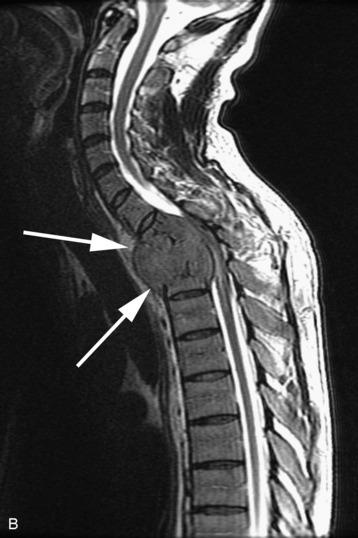
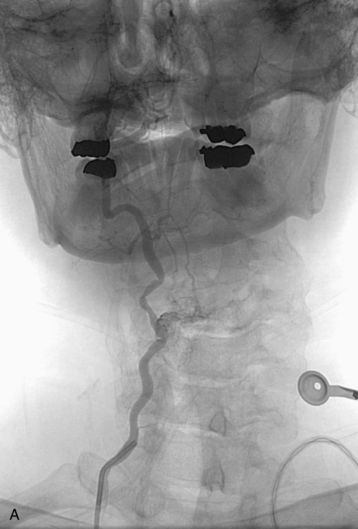
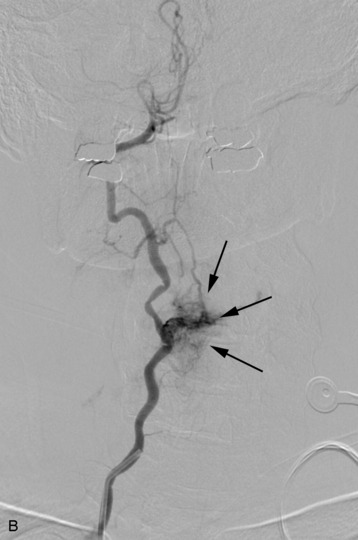
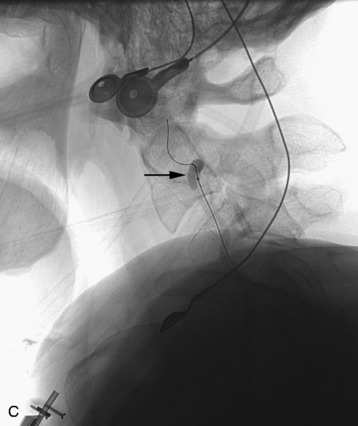
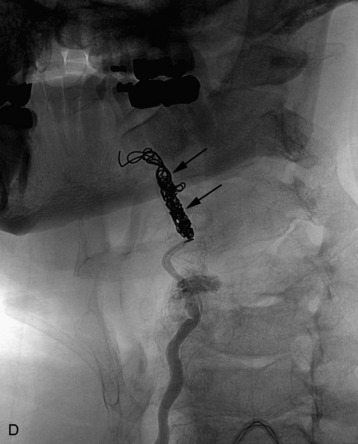
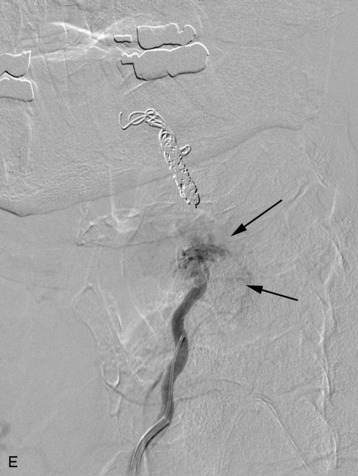
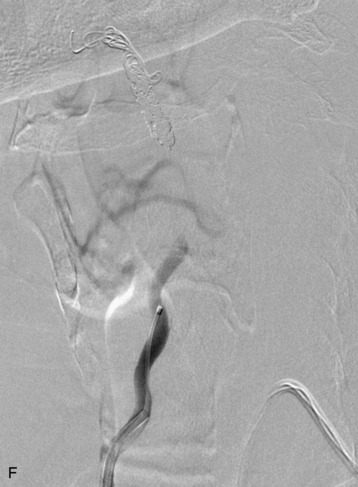
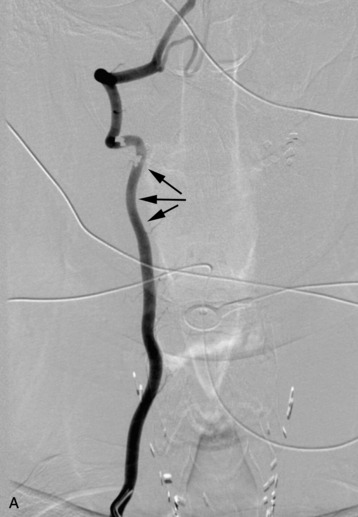
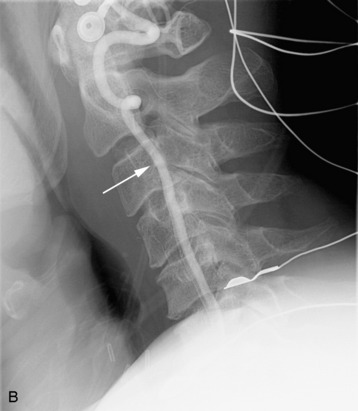
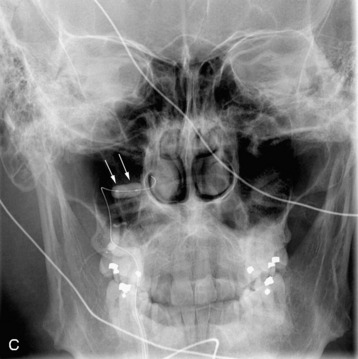
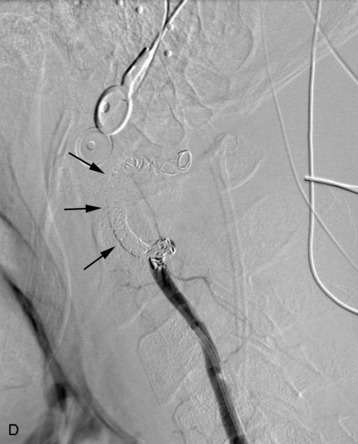
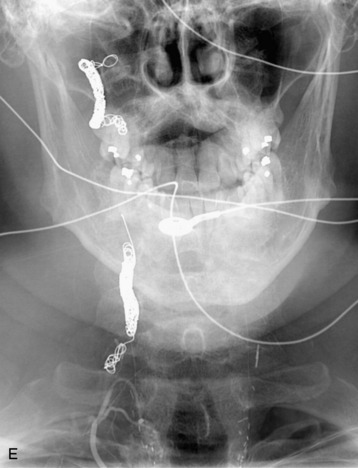
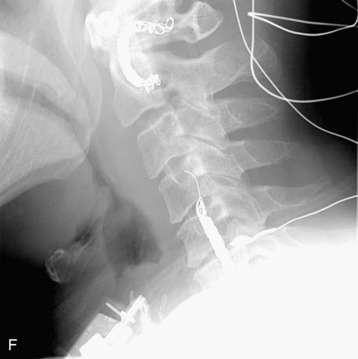
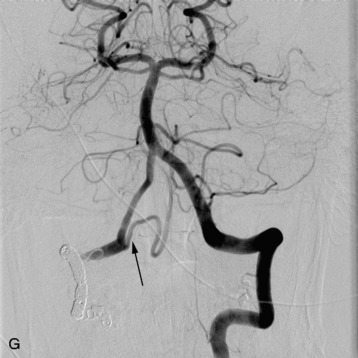
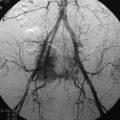
completely. Surgical plan included resection of tumor with vessel sacrifice. Right vertebral artery angiogram demonstrate no tumor blush, as is typical of chordoma. There is, however, mass effect on the vertebral artery from the mass. BTO was performed distal to the affected segment, with the balloon placed in the distal V3 segment of the vertebral artery just proximal to the intradural segment. After passing the BTO, distal vessel sacrifice was then performed, with the coil mass placed above the level of tumor and above the planned operative bed. Subsequently the proximal vertebral artery was also sacrificed. A left vertebral artery angiogram after right vertebral vessel sacrifice showed retrograde flow to the origin of the right posterior inferior cerebellar artery.