CHAPTER 38 Premalignant disease of the genital tract
The Cervix
In 1886, Sir John Williams described eight cases of cervical cancer, one of which was equivalent to carcinoma in situ:
The terminology of cervical premalignancy
The premise that cervical squamous premalignancy was a continuum underpinned the concept of cervical intraepithelial neoplasia (CIN), which was suggested by Richart (1967). The usefulness of this system was limited by significant interobserver variability in the diagnosis and grading of CIN, particularly in differentiating CIN1 from human papilloma virus (HPV) lesions, and separating CIN1 from CIN2 lesions. Furthermore, there is no clear evidence that CIN3 arises from earlier lesions.
Pathology of cervical premalignancy
Cervical intraepithelial neoplasia
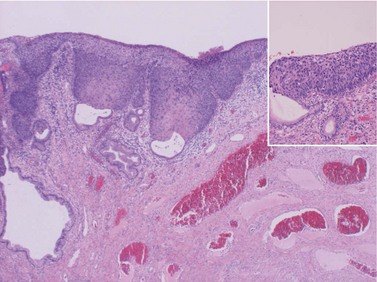
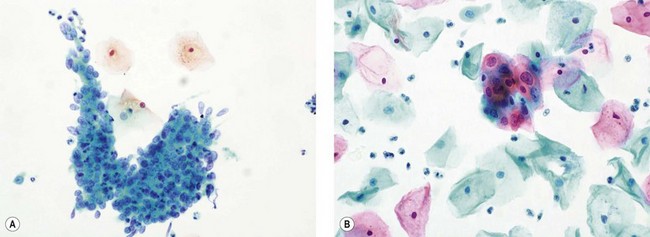
CIN may affect the gland crypts as well as the surface epithelium (Figure 38.1). It is recognized that the degree and depth of crypt involvement increases with the grade of CIN. Histological assessment of crypt involvement in women with CIN3 has shown a mean depth of 1–2 mm, with a maximum of 5.22 mm and a mean ±3 standard deviations (99.7%) of 3.8 mm (Anderson and Hartley 1980). These figures suggested that treatment of ectocervical lesions to a depth of 7 mm should be sufficient to eradicate most CIN.
Cervical glandular intraepithelial neoplasia
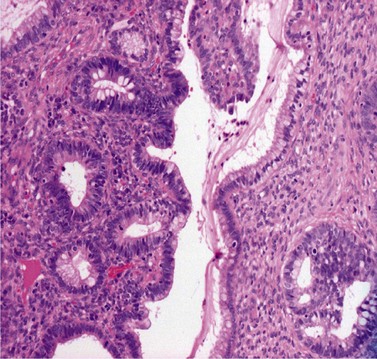
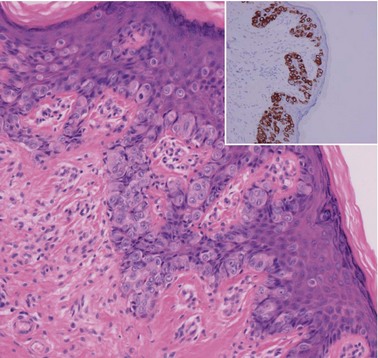
CGIN is characterized by columnar cells with hyperchromatic nuclei and stippled chromatin (Figure 38.2). The nuclei show increased stratification and abnormal mitotic figures with loss of normal mucin. In some cases, the whole of a gland may be involved, but the lesion often occurs as a sharply demarcated area. It may be multifocal. CGIN is often associated with goblet cells or intestinal metaplasia of the endocervical cells. In two-thirds of cases, there are associated squamous abnormalities, and CGIN is often serendipitously discovered in the management of these abnormalities.
Pathogenesis of cervical premalignancy
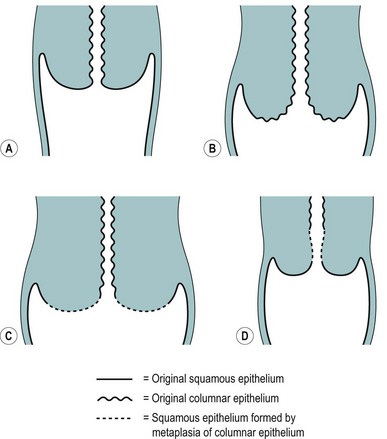
The position of the SCJ is influenced by the hormonal changes that occur during a woman’s life (Figure 38.3). With the onset of puberty, the uterus enlarges and the cervix swells with a resultant eversion, exposing columnar epithelium to the acid environment of the vagina. This induces metaplasia in the exposed columnar epithelium, resulting in the development of metaplastic squamous epithelium. This area of transformation is termed the ‘cervical transformation zone’ (cervical TZ), and it is within this area that preneoplastic changes can occur with the development of CIN. It is thought that these dysplastic changes occur at the time of metaplasia, indicating that this is the time when the cervix is most vulnerable to potential carcinogenic factors, such as HPV and other cofactors.
These considerations have important clinical significance with regards to detecting and treating CIN, as these changes occur within the cervical TZ and it is accessible. Furthermore, the recognition that the development of precancerous changes can occur early in a woman’s sexual and reproductive life provided some indication that these events were potentially associated with some form of environmental exposure related to sexual activity. In other words, most of the identified risk factors for CIN are thought to be largely surrogate markers of HPV infection (Table 38.1).
Table 38.1 Risk factors for cervical intraepithelial neoplasia
Cervical intraepithelial neoplasia
The malignant potential of CIN3 was shown by McIndoe et al (1984) in a crucial paper. This indicated that approximately 30% of women with CIN3 would develop invasive cancer over a 20-year period.
The authors’ rationale for treating CIN3 is based upon this paper, which implies that when CIN3 is discovered, it should be treated. However, there are caveats. Firstly, the patients in this study had large carcinoma in situ lesions, and can we be sure that the undoubted premalignant potential for the lesions managed in this paper are shared by patients with smaller lesions that have small foci of CIN3? For example, it has been estimated that perhaps one-third of cases with CIN3 regress (Östör 1993).
Aetiology of cervical premalignancy
The epidemiological risk factors for both squamous and glandular cervical premalignant lesions are similar and include young age at first intercourse and multiple sexual partners. It is now well established that infection with oncogenic high-risk HPV types is the central causal factor in the development of cervical neoplasia (Walboomers et al 1999).
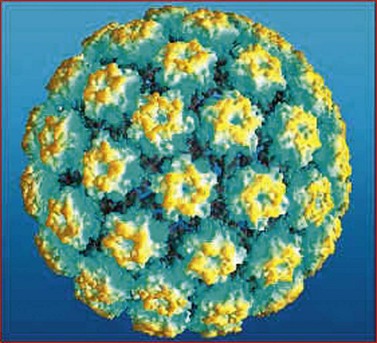
HPVs are small double-stranded DNA viruses which have an icosahedral protein capsid (Figure 38.4). They are typed according to the DNA sequence homology in particular genes, specifically L1 (which codes for the viral capsid) and E6 and E7 (which have important carcinogenic functions). Nearly 30 HPV types can infect the genital tract and can be classified into high-, intermediate- and low-risk oncogenic types. HPV types 16 and 18 are by far the most common high-risk types, accounting for 60% of HPV-positive invasive cervical cancers.
Prevention of cervical premalignancy
Primary prevention using immunization
Several phase III trials (Ault 2007) have shown that more than 90% of persistent HPV 16/18 infections can be prevented for up to 5 years after vaccination, and that more than 90% of precancerous lesions can be prevented in subjects who were HPV negative prior to vaccination. The long-term effects on cervical cancer incidence will require another 10–20 years of follow-up.
Detection of cervical premalignancy
Cervical cytology
The recognition that cervical cytology could be used to detect precancerous change led to the introduction of cervical cytology as a screening test (Figure 38.5). Early detection and treatment can prevent the development of 75% of cancers. Whilst cytology is used to detect women at risk of having cervical premalignancy, most abnormalities are not precancerous. Only a small proportion of women with abnormal smears would develop cancer, although these women are high risk compared with the normal population. There is therefore huge potential for overtreatment unless one can accurately select which lesions require treatment.
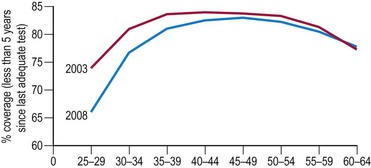
In the UK, the incidence of cervical cancers has halved since the National Health Service’s (NHS) cervical screening programme was introduced in 1988. The NHS cervical screening programme is highly organized. In the UK, women aged 25–65 years are invited for screening every 3 or 5 years. It is thought that screening under the age of 25 years may do more harm than good as cervical cancer is rare in this age group (Sasieni and Adams 1999). There are clear service guidelines, effective data collection systems using a number of mandatory returns from cytological laboratories, and internal and external quality assurance systems. Target population coverage is the key to success. The programme aims for coverage of over 80% of the target population, but there has been a worrying fall in levels in recent years, falling as low as 66% in women aged 25–30 years (Figure 38.6).
HPV detection assay
It is likely that the combination of prophylactic HPV vaccination and the use of HPV testing as a primary test will be the most cost-effective strategy. Assuming a protective effect of prophylactic HPV vaccination of 15–20 years, nationwide prepubertal vaccination may allow delaying the onset of the cervical screening programme to 30 years instead of 25 years as is the current guidance (Bulkmans et al 2007).
Colposcopy
Colposcopic examination
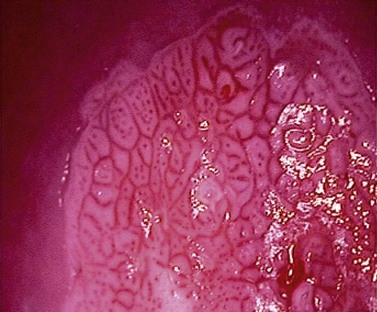
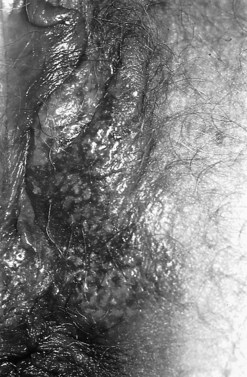
At the initial examination, obvious macroscopic abnormality is sought, including leukoplakia, viral condylomata and invasion. Invasion is associated with the surface of the cervix appearing raised or ulcerated (Figure 38.7). Atypical vessels seen on invasive lesions run a bizarre course and are often corkscrew- or comma-shaped (Figure 38.8). Condylomata are usually obvious from their regular frond-like surface (Figure 38.9).
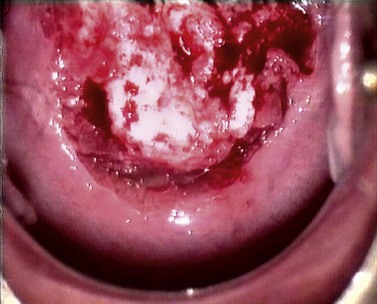
Figure 38.7 Colpophotograph of a stage Ib cervical cancer showing the irregular surface.
Image courtesy of Dr Lázló Szalay, Györ, Hungary.
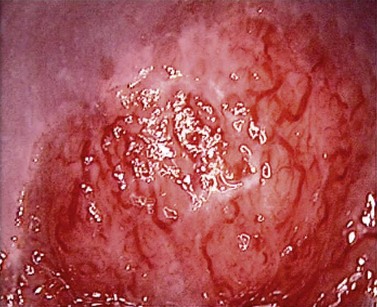
Figure 38.8 Colpophotograph showing abnormal vessels.
Image courtesy of Dr Lázló Szalay, Györ, Hungary.
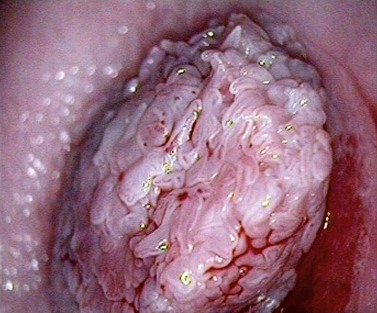
Figure 38.9 Colpophotograph of a cervical condylomata.
Image courtesy of Dr Lázló Szalay, Györ, Hungary.
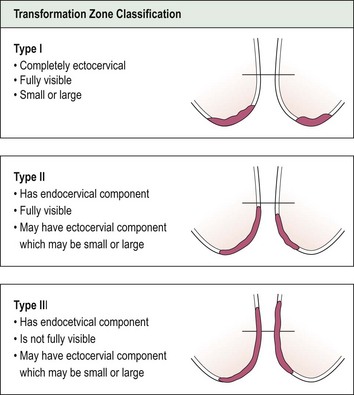
Having completed the initial inspection of the cervix, 5% acetic acid is applied liberally and gently. This turns abnormal epithelium white, producing the so-called ‘acetowhite’ changes of CIN. The position of the SCJ must then be ascertained to define the upper limits of the abnormality. On the basis of these findings, the cervical TZ can be classified into one of three types (Figure 38.10). In a type III TZ, the SCJ is not visible and the examination cannot be regarded as satisfactory. This has important management implications.
Colposcopic abnormalities
The key colposcopic features of suspected abnormalities are acetowhitening, abnormal vasculature and topography. Acetowhite change is the most important of all colposcopic features. Its actual mechanism is uncertain, but it is a reversible reaction which has an association with the activity of the epithelium and results in a temporarily increased reflection of light (Figure 38.11). These changes are non-specific. There is a spectrum of change from low- to high-density acetowhitening which corresponds with an increasing likelihood of high-grade CIN.
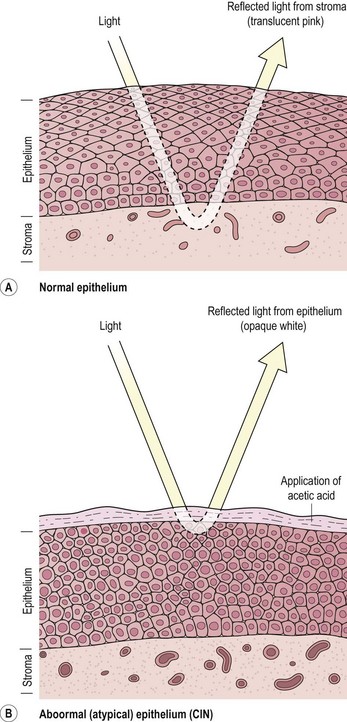
Figure 38.11 Tissue basis for colposcopy. (A) Normal epithelium, (B) abnormal (atypical) epithelium.
Source: Singer A, Monaghan J 2000. Lower Genital Tract Precancer. Blackwell Science, Oxford, p 10.
Abnormal vascular patterns comprise punctuation, mosaicism (Figure 38.12) and abnormal vessels. In general terms, the coarser the vascular pattern, the greater the likelihood of high-grade change. Abnormal vessels are typically irregular in size, shape and arrangement, and can suggest the presence of invasion.
Colposcopic diagnosis
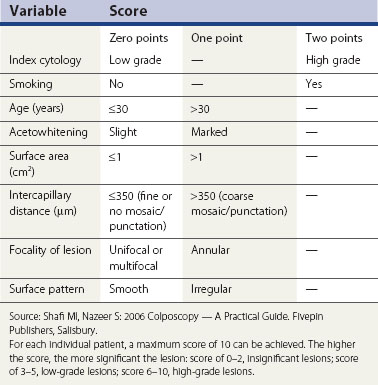
Colposcopic diagnosis is multifaceted. In part, it involves comparing the visual findings with the established patterns of disease as described above. These skills and abilities require training and experience. In addition, there are other important factors that a colposcopist uses to make a diagnostic decision, and these include the referring cytology, patient’s age and parity, and smoking history. These other factors are, on occasions, at least as important as the colposcopic image in making a diagnosis, especially when the colposcopic features are low grade or normal. This observation has led to the use of a number of scoring systems to facilitate diagnosis (Table 38.2), although these are not recommended for routine clinical use.
Colposcopic diagnostic performance might be improved using some technological innovations such as digital image enhancement (Figure 38.13), and reflective and impedance spectroscopy (Louwers et al 2008). Despite a number of promising initial reports, these newer techniques have not been adopted, principally because of expense, inconvenience and insufficient high-quality evidence.
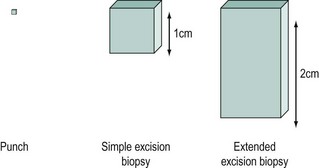
Colposcopic biopsy
Colposcopy is often viewed as a means of deciding whether or not to take a biopsy and where to take it from. Colposcopic biopsies can be classified as in Figure 38.14. An extended excisional biopsy aims to sample most of the endocervical canal and can be performed when the SCJ is not visible or a glandular lesion is suspected. It is accepted that excisional biopsy is the gold standard for histological diagnosis, but this is best avoided when low-grade lesions are suspected.
Treatment of cervical premalignancy
A failure to appreciate the nature and behaviour of cervical premalignancy has bedevilled its treatment. In the past, CIN was treated by radical hysterectomy but it soon became evident that this was unnecessary, and simple hysterectomy became the method of choice. In time, it was realized that cone biopsy was just as effective, and hysterectomy is now reserved for women with difficult-to-treat recurrent disease or who have additional indications for hysterectomy. The introduction of colposcopy and a better appreciation of the limited location of CIN led to the introduction of more conservative methods of treatment (Soutter and Fletcher 1994).
With the exception of radical diathermy, ablative treatments were not associated with a significantly increased risk of serious adverse pregnancy outcomes. However, all excisional procedures used to treat CIN seem to be associated with adverse obstetric morbidity, but among these, only cold knife conization is associated with a significantly increased rate of severe outcomes (Kyrgiou et al 2006). The risk of serious obstetric morbidity has not been confirmed with the use of loop excision, but excisions that remove large amounts of cervical tissue probably have the same effect as knife cone biopsies. Most loop excisions in young women with fully visible transformation zones only need to be 1 cm deep, and this should protect against serious obstetric outcomes.
Types of treatment
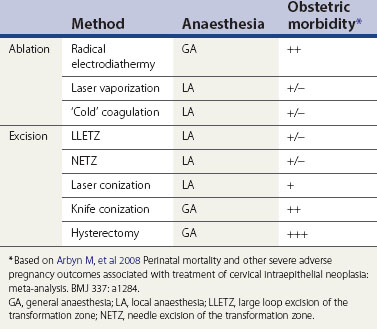
Ablation
A number of ablative methods are available (Table 38.3). The potential advantages of ablative treatments are that general anaesthesia is not usually required (with the exception of radical electrodiathermy) and the associated damage might be less. This might mean less obstetric morbidity. However, there are caveats. Firstly, the treatment method has to be fit for purpose; cryotherapy, for example, is easy to use but it destroys insufficient tissue to treat CIN adequately.
Secondly, ablative therapy can only be undertaken when the whole of the cervical TZ can be examined reliably, so the SCJ must be visible. When ablative treatment is contraindicated (Table 38.4), excisional therapy must be performed.
Excision
Excisional treatments range from local treatments to hysterectomy (Table 38.3). The extent of local excision depends on the context. When treating ectocervical lesions, the aim is to excise to a depth of approximately 10 mm. However, the depth of excision may need to be extended if the SCJ is not visible or if a glandular lesion is suspected.
The Vagina
Natural history of vaginal intraepithelial neoplasia
VAIN is seldom seen as an isolated vaginal lesion. It is more usual for it to be coexistent with CIN. In most cases, it is diagnosed colposcopically prior to any treatment during the investigation of an abnormal smear. However, it may not be recognized until after a hysterectomy has been performed. When this happens, abnormal epithelium is likely to be buried behind the sutures used to close the vault and cannot be assessed (Figure 38.15). Untreated or inadequately treated VAIN may progress to frank invasive cancer. Very rarely, VAIN may be seen many years after radiotherapy for cervical carcinoma, when it is probably a new lesion. Care must be taken in these women to ensure that postradiotherapy changes are not being misinterpreted as VAIN.
Treatment of vaginal intraepithelial neoplasia
In the past, a number of ablative techniques such as carbon dioxide laser, topical 5-fluorouracil and radiotherapy have been used, but surgical excision gives better results and is the only effective option available to patients previously treated with radiotherapy. Following hysterectomy, an abdominal or combined abdominal and vaginal approach is preferable to vaginal excision (Ireland and Monaghan 1988).
The Vulva
Treatment of vulvar intraepithelial neoplasia
Carbon dioxide laser
An alternative approach is to vaporize the abnormal epithelium with a carbon dioxide laser. Careful control of the depth of destruction is essential for good cosmetic results. Given the very irregular surface of the vulva, it is very difficult to achieve a uniform depth of destruction. Moreover, the depth of treatment required for VIN is still unclear. In some cases, hair follicles may be involved for several millimetres below the surface, but it may not always be necessary to destroy the whole depth of involved appendages. In any case, treatment of the whole vulva to such a depth would result in a third-degree burn which would need skin grafting. In practice, laser vaporization has proved to be disappointing in UK practice and is seldom used (Shafi et al 1989).
Paget’s disease
This is an uncommon condition and is similar to that found in the breast. It is characterized by large malignant cells arranged singly and in clusters. The typical Paget’s cell has a vesicular nucleus and abundant cytoplasm which contains demonstrable mucin (Figure 38.16). The cells of primary Paget’s disease are characteristically positive with cytokeratin 7, carcinoembryonic antigen, CAM 5.2, androgen receptor, gross cystic disease fluid protein 15 and human epidermal growth factor receptor 2 (HER 2) (Goldblum and Hart 1997).
Pruritis is the presenting complaint and it often presents as a red, crusted plaque with sharp edges (Figure 38.17), sometimes with multiple erosions. The diagnosis is made histologically on biopsy.
The Uterine Corpus
Investigation of endometrial hyperplasia
In the UK, the cancer waiting time and 18-week waiting time initiatives have increasingly focused attention on one-stop assessment and diagnosis. Women over the age of 45 years with irregular bleeding or postmenopausal women are referred on the cancer pathway and seen within 14 days. Transvaginal ultrasound (TVS) is the primary investigation of choice in postmenopausal women as it can assess the endometrium and detect other relevant pelvic pathologies. The endometrial thickness is measured and, using a cut-off of more than 5 mm, has a high sensitivity and specificity for detecting endometrial pathology, as well as a true negative predictive value approaching 100% (Smith-Bindman et al 1998). If the endometrial thickness exceeds 5 mm, endometrial assessment is required. Whilst the diagnostic gold standard is hysteroscopy, blind endometrial sampling using tests such as Pipelle (Punimar, Wilton, Connecticut, USA) are frequently used and are cost-effective. When outpatient sampling is not feasible or is unsatisfactory, or in the context of the patient being on tamoxifen or in whom symptoms have persisted despite negative findings on initial assessment, hysteroscopy should be undertaken. Hysteroscopy allows the whole surface of the uterine cavity to be inspected and facilitates targeted biopsy or curettage. Hysteroscopy with biopsy has an excellent sensitivity and specificity for detecting endometrial pathology in women aged less than 45 years who have symptoms of irregular and/or heavy bleeding that is non-responsive to first-line management. In this clinical scenario, an endometrial sample should be the primary test, as TVS is less specific. Notwithstanding the above, TVS should always be considered when clinical findings are abnormal or examination is suboptimal.
Management of endometrial hyperplasia
Non-atypical endometrial hyperplasia (simple or complex hyperplasia)
Given the low risk of progression to carcinoma, there is no indication for hysterectomy or for progestin therapy in asymptomatic women, and subsequent management can probably be decided on the basis of further symptoms. Available data suggest that persistent or progressive disease will occur in one-third of conservatively managed cases (Clark et al 2006). Progestin therapy may result in its complete disappearance. Progestogens can be delivered systemically, either alone or in combination with oestrogen (as in the oral contraceptive pill or hormone replacement therapy), or locally in the form of the levonorgestrol intrauterine device (Mirena, Schering Health). Both systemic and local administration of progestogens show a 75–100% conversion rate to normal endometrium.
Atypical hyperplasia
Most women with atypical hyperplasia should have a hysterectomy and bilateral salpingo-oophorectomy because of the high risk of coexistent carcinoma. However, younger women who wish to preserve their fertility may be managed with medical therapy and repeated endometrial sampling. One study reported a 94% success rate with 3–18 months of therapy, allowing five women to become pregnant, delivering at full term (Randall and Kurman 1997). However, these encouraging results have to be weighed against those where a 25% risk of progression to carcinoma was described (Ferenczy and Gelfand 1989). Most data relate to the use of various progestins given for short-term courses or as continuous therapy for many years. Most of the studies only include carefully selected cases, and the results of the different studies are not really comparable because of the selection criteria applied. It is clear that these results should be regarded with considerable caution, and that long-term follow-up is essential because recurrences may not appear for many years (Ferenczy and Gelfand 1989).
The Ovary
Introduction
As a result, attention has focused on the detection of small early-stage cancers in certain situations, such as the need for careful examination of ovaries and fallopian tubes removed at prophylactic surgery in patients with BRCA1 or BRCA2 mutations since small areas of malignant change can easily be missed. Similarly, it is recognized that endometriosis can be coexistent in up to 25–40% of cases of endometrioid and clear cell ovarian cancers (Fukunaga et al 1997), and consequentially all endometriotic cysts must be sampled optimally, especially to include any solid or suspicious areas. Only a small percentage of women with endometriosis will develop an ovarian cancer, and the mechanisms responsible for the malignant transformation have still to be elucidated. However, evidence is emerging of genetic mutations common to both ovarian endometriosis and endometriosis-associated ovarian cancer (Prowse et al 2006).
Ovarian carcinogenesis
Based on morphological and molecular genetic studies, a dualistic model has been suggested (Shih and Kurman 2004) in which ovarian cancers can be divided into two broad categories: type I and II tumours (Figure 38.18). The type I pathway resembles the adenoma–carcinoma pathway in colorectal cancer and is characterized by clearly recognized precursor lesions, namely cystadenoma and borderline lesions. Type I tumours evolve slowly in a stepwise fashion and are associated with distinct molecular changes that are not shared by the more common type II tumours. Type II tumours comprise high-grade tumours, such as poorly differentiated serous lesions and mixed mesodermal tumours, which arise and metastasize early.
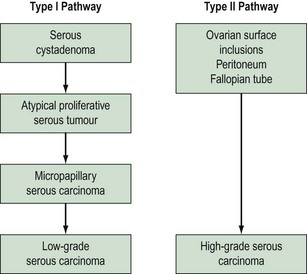
Figure 38.18 The dualistic model of ovarian cancer pathogenesis.
Adapted from Kurman RJ, Shih IM 2008 International Journal of Gynecological Pathology 27: 151–160.
The pathological evidence for the progression of type I tumours arises from studies which have shown the frequent occurrence of a transition or coexistence between malignant and benign areas in mucinous ovarian cancers, and between low-grade serous cancers and areas of borderline change (Malpica et al 2004). Also, borderline serous tumours typically recur as low-grade serous cancers (Crispens et al 2002).
Prevention of premalignancy of the ovary
Chemoprevention has particularly focused on the use of the oral contraceptive pill, which has been shown to reduce the risk of ovarian cancer in both low- and high-risk populations (Hankinson et al 1992, McLaughlin et al 2007). The use of oral contraceptives in BRCA mutation carriers is currently not advised due to an apparent increase in the rate of breast cancer, although the data on this are limited.
Bilateral salpingo-oophorectomy has also been shown to reduce the risk of ovarian and fallopian tube cancer in BRCA1 or BRCA2 mutation carriers (Rebbeck et al 2009). The timing of surgery is dependent on the individual, their fertility requirements, the potential surgical morbidity and long-term hormonal sequelae. The current advice is that surgery should be considered once a woman reaches 35 years of age. Prophylactic surgery, however, does not completely remove the risk of malignancy since women in some high-risk populations are still at risk of developing primary peritoneal carcinoma (Finch et al 2006).
KEY POINTS
Anderson MC, Hartley RB. Cervical crypt involvement by intraepithelial neoplasia. Obstetrics and Gynecology. 1980;55:546-550.
Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.
Ault KA, The Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a comparison of four randomised clinical trials. The Lancet. 2007;369:1861-1868.
Bulkmans NW, Berkhof J, Rozendaal L, van Kemendade FJ, Snijders PJ, Meijer CJ. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5 year follow-up of a randomised controlled implementation trial. The Lancet. 2007;370:1764-1772.
Clark TJ, Neelakantan D, Guptal JK. The management of endometrial hyperplasia. An evaluation of current practice. European Journal of Obstetrics, Gynecology and Reproductive Biology. 2006;125:259-264.
Crispens MA, Bodurka D, Deavers M, Lu K, Silva EG, Gershenson DM. Response and survival in patients with progressive or recurrent serous ovarian tumors of low malignant potential. Obstetrics and Gynecology. 2002;99:3-10.
Ferenczy A, Gelfand M. The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. American Journal of Obstetrics and Gynecology. 1989;160:126-131.
Finch A, Beiner M, Lubinski J, et alHereditary Ovarian Cancer Clinical Study Group. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 mutation. Journal of the American Medical Association. 2006;296:185-192.
Fukunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30(3):249-255.
Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. American Journal of Surgical Pathology. 1997;21:1178-1187.
Hankinson SE, Colditz GA, Hunter DJ, Spencer TL, Rosner B, Stampfer MJ. A quantitative assessment of oral contraceptive use and risk of ovarian cancer. Obstetrics and Gynecology. 1992;80:708-714.
Health and Social Care Information Centre, 2009. Cervical Screening Programme 2007/2008. Health and Social Care Information Centre, Sheffield.
Ireland D, Monaghan JM. The management of the patient with abnormal vaginal cytology following hysterectomy. BJOG: an International Journal of Obstetrics and Gynaecology. 1988;95:973-975.
Jimbo H, Yoshikawa H, Onda T, Yasugi T, Sakamoto A, Taketani Y. Prevalence of ovarian endometriosis in epithelial ovarian cancer. International Journal of Gynaecology and Obstetrics. 1997;59:245-250.
Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. The Lancet. 2006;367:489-498.
Louwers JA, Kocken M, ter Harmsel WA, Verheijen RHM. Digital colposcopy: ready for use? An overview of the literature. BJOG: an International Journal of Obstetrics and Gynaecology. 2008;116:220-229.
Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. American Journal of Surgical Pathology. 2004;28:496-504.
McIndoe WA, McLean MR, Jones RW, Mullins PR. The invasive potential of carcinoma in situ of the cervix. Obstetrics and Gynecology. 1984;64:451-458.
McLaughlin JR, Risch HA, Lubinski J, et alHereditary Ovarian Cancer Clinical Study Group. Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case–control study. The Lancet Oncology. 2007;8:26-34.
Mutter GL, Baak JPA, Crum CP, Richart RM, Ferenczy A, Faquin WC. Endometrial precancer diagnosis by histopathology, clonal analysis and computerized morphometry. Journal of Pathology. 2000;190:462-469.
Östör AG. Natural history of cervical intraepithelial neoplasia: a critical review. International Journal of Gynecologic Pathology. 1993;12:186-192.
Prowse AH, Manek S, Varma R, et al. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. International Journal of Cancer. 2006;119:556-562.
Randall TC, Kurman RJ. Progestin treatment of atypical endometrial hyperplasia and well-differentiated carcinoma of the endometrium in women under the age of 40. Obstetrics and Gynecology. 1997;40:434-440.
Rebbeck TR, Kauf ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. Journal of the National Cancer Institute. 2009;101:80-87.
Richart RM. Natural history of cervical intraepithelial neoplasia. Clinics in Obstetrics and Gynecology. 1967;10:748-784.
Sasieni P, Adams J. Effect of screening on cervical cancer mortality in England and Wales: analysis of trends with an age cohort model. BMJ (Clinical Research Ed.). 1999;318:1244-1245.
Shafi MI, Luesley DM, Byrne P, et al. Vulval intraepithelial neoplasia — management and outcome. BJOG: an International Journal of Obstetrics and Gynaecology. 1989;96:1339-1344.
Shafi MI, Nazeer S. Colposcopy — A Practical Guide. Salisbury: Fivepin Publishers; 2006.
Shih IEM, Kurman RJ. A proposed model based on morphological and molecular genetic analysis. American Journal of Pathology. 2004;164:1511-1518.
Singer A, Monaghan J 2000. Lower Genital Tract Precancer. Blackwell Science, Oxford, p 10.
Smith-Bindman R, Kerlickoske K, Feldstein V, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial pathologies. Journal of the American Medical Association. 1998;280:1510-1517.
Soutter WP, Fletcher A. Invasive cancer of the cervix in women with mild dyskaryosis followed cytologically. BMJ (Clinical Research Ed.). 1994;308:1421-1423.
Shih IM, Kurman RJ. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. International Journal of Gynecological Pathology. 2009;27:151-160.
Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer. Journal of Pathology. 1999;189:12-19.
Williams J. Cancer of the Uterus. Harverian Lectures for 1886. London: HK Lewis; 1886.