Powders, granules and granulation
Michael E. Aulton and Malcolm P. Summers
Chapter contents
Powdered and granulated products as dosage forms
Powders and granules for oral administration
Powders for other routes of administration
Preparations requiring further treatment at time of dispensing
Granules used as an intermediate in tablet manufacture
Pharmaceutical technology of granule production
Pharmaceutical granulation processes
Mechanisms of granule formation
Key points
• Powders and granules are themselves dosage forms.
• Powders are also used for inhalation (pulmonary or nasal) and for external use (dusting powders).
Introduction
The scientific aspects of powders and powder technology have been discussed in Part 2 of this book. This chapter discusses the application of powders and granulated products in pharmaceutical dosage forms. Powders and granules are used as dosage forms in their own right, but by far the greatest use of granules in pharmaceutical manufacturing is as an intermediate during the manufacture of compressed tablets.
The term ‘powder’, when used in the context of a dosage form, describes a formulation in which a drug powder has normally been mixed with other powdered excipients to produce a final product. The function of the added excipients depends upon the intended use of the product. Colouring, flavouring and sweetening agents, for example, may be added to powders for oral use.
Granules that are used as a dosage form comprise powder particles that have been aggregated to form larger particles sufficiently robust to withstand handling.
Granulation is the process in which dry primary powder particles (i.e. single, discrete powder particles) are processed to adhere to form larger multi-particle entities called granules. Pharmaceutical granules typically have a size range between 0.2 and 4.0 mm, depending on the subsequent use of the granules. In the majority of cases, when granules will be made as an intermediate product, they have a size range towards the lower end of this spectrum – typical between 0.2 and 0.5 mm. When prepared for use as a dosage form in their own right, they are usually much larger (typically 1–4 mm).
After granulation, the granules will either be packaged (when used as a dosage form) or they may be mixed with other excipients prior to tablet compaction or capsule filling.
Reasons for granulation
The reasons why granulation is often necessary are as follows:
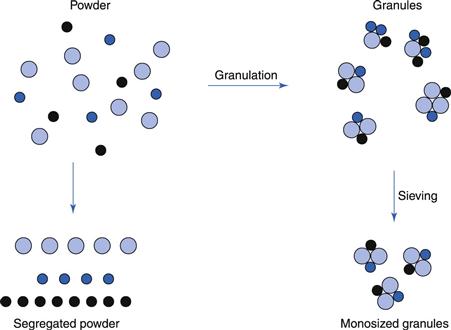
To prevent segregation of the constituents of the powder mix
Segregation (or demixing, discussed in Chapter 11) occurs primarily due to differences in the size and/or density of the components of the mix; the smaller particles and/or denser particles concentrate at the base of a container with the large particles and/or less dense above them. An ideal granulation will contain all the constituents of the mix in the correct proportion in each granule, and if this is achieved segregation of individual ingredients will not occur (Fig. 28.1).
It is also important to control the particle size distribution of the granules because, although the individual components may not segregate, if there is a wide size distribution of the granules themselves, the granules may segregate. If this occurs in the hoppers of sachet-filling machines, capsule-filling machines or tabletting machines, products having large weight variations will result. This is because these machines fill by volume rather than weight. If different regions in the hopper contain granules of different sizes (and hence different bulk density), a given volume from each region will contain a different weight of granules. This will lead to an unacceptable variability of the drug content within the batch of finished product even if the drug is evenly distributed, weight per weight, through individual granules.
To improve the flow properties of the mix
Many powders, because of their small size, irregular shape or surface characteristics, are cohesive and do not flow well. Poor powder flow will also often result in a wide weight variation within the final product due to variable fill of tablet dies, etc. The resulting granules produced from irregular particles will be larger and more isodiametric, both factors contributing to improved flow properties (discussed more fully in Chapter 12).
To improve the compaction characteristics of the mix
Some primary powder particles are difficult to compact into tablets even if a readily compactable adhesive is included in the blend. Granules of the same formulation are often more easily compacted and produce stronger tablets. This is associated with the method employed to produce the granule and its resulting structure. Often solute migration (see Chapter 29) may occur during the post-wet granulation drying stage and this can result in a binder-rich outer layer to the granules. This in turn leads to direct binder–binder bonding which assists the consolidation of weakly bonding materials.
Other reasons
The above are the primary reasons for the granulation of pharmaceutical products but there are others which may necessitate the granulation of powdered material:
Powdered and granulated products as dosage forms
Powdered and granulated products are dispensed in many forms and these are discussed below. The advantages of this type of preparation as a dosage form are as follows:
The disadvantages of powdered and granulated dosage forms are as follows:
1. Bulk powders or granules (i.e. where doses are not pre-divided into individual aliquots) are far less convenient for the patient to carry than a small container of tablets or capsules, and are as inconvenient to self-administer as liquid preparations, such as mixtures. Modern packaging methods for divided preparations, such as heat-sealable laminated sachets (Chapter 47), mean that individual doses can be carried conveniently.
Powders and granules for oral administration
Oral powders
Oral powders are preparations consisting of solid, loose, dry particles of varying degrees of fine particle size. They contain one or more active substances, with or without excipients and, if necessary, approved colouring matter and flavouring. They are generally administered in or with water or another suitable liquid, or they may also be swallowed directly. They are presented as single-dose or multidose preparations in suitable containers.
Multidose oral powders are packed into a suitable bulk container, such as a wide-mouthed glass jar. They require the provision of a measuring device capable of delivering the quantity prescribed. Because of the difficulty in precisely measuring single doses from this type of preparation the constituents are usually relatively non-toxic medicaments with a large dose. Relatively few proprietary examples exist, although many dietary/food supplements are packed in this way.
Each dose of a single-dose powder is enclosed in an individual container, for example a sachet or a vial. Traditionally, single doses were wrapped in paper. This was unsatisfactory for most products, particularly if the ingredients were hygroscopic, volatile or deliquescent. Modern packaging materials of foil and plastic laminates have replaced such paper wrappings; they offer superior protective qualities and are amenable to use on high-speed packing machines. However, some paper-wrapped powders continue in over-the-counter products.
In the manufacture of oral powders, effort is made to ensure a suitable particle size is used with regard to the intended use. Additionally during manufacture, packaging, storage and distribution of oral powders, suitable means must be taken to ensure microbial quality. All powders and granules should be stored in a dry place to prevent deterioration due to ingress of moisture. Even if hydrolytic decomposition of ingredients does not occur, the particles will adhere and cake, producing an inelegant, often unusable product.
Effervescent powders
Effervescent powders are presented as single-dose or multidose preparations and generally, in addition to the drug, contain acid substances and carbonates or hydrogen carbonates which react rapidly and effervesce when the patient adds the powder to water to produce a draught. Citric acid plus sodium bicarbonate is a common combination that releases carbon dioxide. The drug is quickly dissolved or dispersed in the water before administration.
It is preferred that effervescent powders are packed in individual dose units in airtight containers (laminated sachets are ideal, Chapter 47). It is important to protect the powder from the ingress of moisture during manufacture and on subsequent storage to prevent the reaction occurring prematurely.
Granules
One disadvantage of powders is that, because of particle size differences, the ingredients may segregate (see Chapter 11), either in the hoppers of packaging machines or on storage in the final bulk container. If this happens, the product will be non-uniform and the patient will not receive the same dose of the ingredients on each occasion. This can be minimized by efficient granulation of the mixed powders.
Granules are preparations consisting of solid, dry aggregates of powder particles sufficiently resistant to withstand handling. They are intended for oral administration. Some are swallowed as such, some are chewed and some are dissolved or dispersed in water or another suitable liquid before being administered.
Granules contain one or more active substances with or without excipients and, if necessary, suitable colouring and flavouring substances. They are mainly used for low-toxicity, high-dose drugs. Methylcellulose Granules, for example, are used as a bulk-forming laxative and have a dose of 1–4 g daily. Many proprietary preparations contain similar bulk-forming laxatives.
Granules are presented as single-dose or multidose preparations. Each dose of a multidose preparation is administered by means of a device suitable for measuring the quantity prescribed. For single-dose granules, each dose is enclosed in an individual container, for example a sachet or a vial. If the preparation contains volatile ingredients or the contents have to be protected, they should be stored in an airtight container. For example, Methylcellulose Granules should be kept in a wide-mouthed, airtight container.
There are several categories of granules:
Effervescent granules.
Effervescent granules are uncoated granules generally containing acid substances and carbonates or hydrogen carbonates which react rapidly in the presence of water to release carbon dioxide. They are intended to be dissolved or dispersed in water before administration. The effervescence and subsequent disintegration of the granules should be complete within 5 minutes at which time the granule ingredients should be either dissolved or dispersed in the water. Effervescent granules should be stored in an airtight container.
Coated granules.
Coated granules consist of granules coated with one or more layers of mixtures of various excipients. The substances used as coatings (generally polymers) are usually applied as a solution or suspension in conditions in which evaporation of the vehicle occurs leaving a film of coating (see Chapter 32). A suitable test should be carried out to demonstrate the appropriate release of the active substance(s), for example one of the tests described in Chapter 35.
Modified-release granules.
Modified-release granules are coated or uncoated granules that contain special excipients or which are prepared by special procedures, or both, designed to modify the rate, the place or the time at which the active substance or substances are released.
Modified-release granules may have prolonged-release or delayed-release properties. A suitable test must be carried out to demonstrate the appropriate kinetics and extent of the release of the active substance(s).
Gastro-resistant granules.
Gastro-resistant granules (also referred to as enteric-coated granules) are delayed-release granules that are intended to resist the gastric fluid and to release the active substance(s) in the intestine fluid. This is generally achieved by covering the granules with a gastro-resistant polymer (see Chapters 31 and 32). Again a suitable test should be carried out to demonstrate the appropriate release of the active substance(s).
Powders for other routes of administration
Powders for inhalation
The use of dry-powder systems for pulmonary drug delivery is now extensive. This dosage form has developed into one of the most effective methods of delivering active ingredients to the lung for the treatment of asthma and chronic obstructive pulmonary disease. Its popularity is reflected in the number of commercial preparations available in a number of sophisticated and increasingly precise delivery devices. Pulmonary delivery is discussed fully in Chapter 37.
Nasal powders
Nasal powders are medicated powders intended for inhalation into the nasal cavity by means of a suitable device. Some potent drugs are presented in this way because they are rapidly absorbed when administered as a fine powder via the nose (see Chapter 38 for a detailed discussion of the nasal route of administration). To enhance convenience and ensure that a uniform dose is delivered on each occasion, delivery devices have been developed. Sufficient drug for one dose may be presented in a hard gelatin capsule diluted with an inert, soluble diluent such as lactose. The capsule is placed in the body of the nasal delivery device and is broken when the device is assembled. The drug is inhaled, via the nose, by the patient as a fine powder. The size of the particles is such as to localize their deposition in the nasal cavity and is verified by adequate methods of particle-size determination.
Powders for external use
Powders for cutaneous application (topical powders)
Powders for cutaneous application are preparations consisting of solid, loose, dry particles of varying degrees of fineness. They contain one or more active substances, with or without excipients and, if necessary, appropriate colouring matter.
Powders for cutaneous application are presented as single-dose powders or multidose powders. They should be free from grittiness. Powders specifically intended for use on large open wounds or on severely injured skin must be sterile.
Multidose powders for cutaneous application may be dispensed in sifter-top containers, containers equipped with a mechanical spraying device or in pressurized containers.
In the manufacture of powders for cutaneous application, measures should be taken to ensure a suitable particle size (determined and controlled by sieving) with regard to the intended use. Additionally, suitable measures should be taken to ensure their microbial quality and if the label indicates that the preparation is sterile, it must comply with a test for sterility.
Sterile powders used in cutaneous application must be prepared using materials and methods designed to ensure sterility and to avoid the introduction of contaminants and the growth of micro-organisms.
Dusting powders
Dusting powders contain ingredients used for therapeutic, prophylactic or lubricant purposes and are intended for external use. Only sterile dusting powders should be applied to open wounds.
Dusting powders for lubricant purposes or superficial skin conditions need not be sterile but they should be free from pathogenic organisms. As minerals such as talc and kaolin may be contaminated at source with spores of organisms causing tetanus and gangrene, these should be sterilized before they are incorporated into the product. Talc Dusting Powder is a sterile cutaneous powder containing starch and purified talc in which the talc is sterilized before incorporation with the starch, or the final product is subject to a suitable terminal sterilization procedure.
Dusting powders are normally dispensed in glass or metal containers with a perforated lid. The powder must flow well from such a container, so that it can be dusted over the affected area. The active ingredients must therefore be diluted with materials having reasonably good flow properties, e.g. purified talc or maize starch.
Hexachlorophene Dusting Powder contains an antibacterial agent and Talc Dusting Powder is used as a lubricant to prevent chafing. Proprietary products are available, usually for the treatment of bacterial or fungal infections, e.g. Canesten® Powder (clotrimazole) is used as an antifungal agent.
Dusting powders are powders for cutaneous application which have a suitable fineness. An example is Talc Dusting Powder, which is a mix of 10% of starch and 90% of Purified Talc, where the particle size is controlled by size separation using, typically, a 250 µm sieve.
Ear powders
Powders containing active ingredients can also be administered to the ear. Ear powders normally have to comply with the pharmaceutical requirements for powders for cutaneous application. They are supplied in containers fitted with a suitable device for application.
Preparations requiring further treatment at time of dispensing
Some preparations for oral use are prepared from powders or granules to yield oral solutions or suspensions using a suitable vehicle. This may be performed at the dispensing stage or by the patient prior to administration. The vehicle for any preparations for oral use is chosen having regard to the nature of the active substance(s) and to provide organoleptic characteristics appropriate to the intended use of the preparation.
Several categories of preparations may be distinguished:
Powders and granules for solution or suspension
Powders and granules for the preparation of oral solutions or suspensions generally conform to the definitions in the normal pharmacopoeial standards for oral powders or granules as appropriate. They may contain excipients, in particular to facilitate dispersion or dissolution and to prevent caking. After dissolution or suspension, the resulting product should comply with the requirements for oral solutions or oral suspensions, as appropriate.
The label should explain the method of preparation of the solution or suspension from the powder or granules, and the conditions and the duration of storage after reconstitution.
Powders and granules for syrups
Syrups are aqueous preparations characterized by a sweet taste and a viscous consistency. They may contain sucrose at a concentration of at least 45%. The sweet taste can also be obtained by using other polyols or sweetening agents. Syrups usually contain aromatic or other flavouring agents.
All of the necessary ingredients for the syrup may be manufactured and stored in the dry powdered or granular state and then reconstituted (usually by the addition of water alone) at the time of dispensing or administration. After dissolution, the resulting syrup must comply with the normal pharmacopoeial requirements for syrups.
Antibiotic syrups.
For patients who have difficulty taking capsules and tablets, e.g. young children, a liquid preparation of a drug offers a suitable alternative. Unfortunately, many antibiotics are physically or chemically unstable when formulated as a solution or suspension. The method used to overcome this instability problem is to manufacture the dry ingredients of the intended liquid preparation in a suitable container in the form of a powder or granules. When the product is dispensed, a given quantity of water is added to reconstitute the solution or suspension. This enables sufficient time for warehousing and distribution of the product and storage at the pharmacy without degradation. Once it is reconstituted, the patient must be warned of the short shelf-life. A shelf-life of 1–2 weeks for the reconstituted antibiotic syrup should not be a serious problem for the patient as the dosing would normally be complete by then. Examples are Amoxicillin Oral Suspension and Erythromycin Ethylsuccinate Oral Suspension.
Powders for oral drops
Oral drops are solutions, emulsions or suspensions that are administered in small volumes, such as in drops, by the means of a suitable device. Powders for the preparation of oral drops would have to conform to requirements of all other oral powders. They may contain excipients to facilitate dissolution or suspension in the prescribed liquid, or to prevent caking.
After dissolution or suspension, they comply with the specific pharmacopoeial requirements for pre-prepared oral drops. If the dose is measured in drops, the label should also state the number of drops per millilitre or per gram of preparation.
Powders for injection
Injections of medicaments that are unstable in aqueous solution must be made immediately prior to use. The ingredients are presented as sterile powders in ampoules or vials. Sufficient diluent, e.g. sterile Water for Injections, is added from a second container to produce the required drug concentration and the injection is used immediately. The powder may contain suitable excipients in addition to the drug, e.g. sufficient additive to produce an isotonic solution when the injection is reconstituted.
Powders for injection are most often manufactured by a freeze drying process (Chapter 29). The sterilization of these ‘lyophilized powders’ is described in Chapter 17 and their use as parenteral products is discussed in more detail in Chapter 36.
The label for powders for injection should state i) the amount of active ingredient contained in the sealed container, ii) the directions for preparing the injection or intravenous infusion from the powder and iii) that when dissolved or suspended, the preparation is intended for parenteral use.
Pharmacopoeial tests
The pharmacopoeial tests for assessing the quality of most powdered and granular dosage forms are very similar. The role of these tests is indicated by its title; details of procedures and standards can be found in the latest appropriate pharmacopeia.
Uniformity of dosage units.
Single-dose oral powders should comply with a pharmacopoeial test for uniformity of dosage units or, where justified and authorized, with the tests for uniformity of content and/or uniformity of mass.
Uniformity of mass.
Single-dose oral powders need to comply with a test for uniformity of mass of single-dose preparations. If the product complies with the uniformity of content test for all active substances, the test for uniformity of mass is not required.
Uniformity of content.
In the case where the oral powder contains a particularly active drug, single-dose oral powders must comply with a test for uniformity of content of active drug(s) in single-dose preparations. After shaking, empty each container as completely as possible; the test is carried out on the individual contents. As an example, the BP defines an active substance as one where a single-dose of powder or granules contains an amount of active substance less than 2 mg, or less than 2 per cent of the total mass of the single-dose preparation.
Uniformity of mass of delivered doses from multidose containers.
Oral powders and granules supplied in multidose containers must comply with this test.
Drug release.
Where appropriate (e.g. coated granules, modified-release granules, gastro-resistant granules) the rate and extent of release of the active drug(s) must be quantified and compared with the required specification.
Granules used as an intermediate in tablet manufacture
As indicated earlier in this chapter, by far the largest portion of pharmaceutical granules that are made will have a short lifetime before they are incorporated into tablets (mainly) or hard-gelatin capsule dosage forms. The methods of manufacture of granules are basically the same irrespective of the fate of the granules. However, in the context of manufacturing tablets, the mechanical properties of the granules and the way in which they deform and bond are critical in the tableting process. The remainder of this chapter discusses in more detail the way in which granules are formed and how their structure influences their compactability. Current manufacturing methods are also discussed.
Pharmaceutical technology of granule production
Pharmaceutical granulation processes
Granulation methods can be divided into two types: wet methods which utilize a liquid in the process and dry methods in which no liquid is used.
In a suitable formulation a number of different excipients will be needed in addition to the drug. The common types used are diluents, to produce a unit dose weight of suitable size, and disintegrating agents which are added to aid the break-up of the granule when it reaches a liquid medium, e.g. on ingestion by the patient. An adhesive (also known as a binder) in the form of a dry powder may also be added, particularly if dry granulation is employed. All ingredients will be mixed before granulation.
Dry granulation
In the dry methods of granulation, the primary powder particles are aggregated at high pressure. There are two main intermediate processes. Either the production of a large tablet (known as a ‘slug‘) in a heavy-duty tableting press (a process known as slugging) or the powder is squeezed between two rollers to produce a sheet or flakes of material (roller compaction). In both cases, the intermediate product is broken using a suitable milling technique to produce granular material which is usually sieved to separate the desired size fraction. The unused fine material may be reworked to avoid waste. This dry method may be used for drugs which do not compress well after wet granulation or those which are sensitive to moisture.
Wet granulation (involving wet massing)
Wet granulation involves the massing of a mix of dry primary powder particles using a granulating fluid. The granulating fluid contains a solvent that must be volatile, so that it can be removed by drying, and is non-toxic. Typical suitable liquids include water, ethanol and isopropanol either alone or in combination. The granulation liquid may be used alone or, more usually, as a solvent containing a dissolved adhesive (also referred to as a binder or binding agent) which is used to ensure particle adhesion once the granule is dry.
Water is commonly used for economic and ecological reasons. The disadvantages of water as a solvent are that it may adversely affect drug stability, causing hydrolysis of susceptible products, and it needs a longer drying time than organic solvents. This long drying time increases the duration of the process and again may affect chemical stability of the drug(s) because of the extended exposure to heat. The primary advantage of water is that it is non-flammable, which means that expensive safety precautions such as the use of flame-proof equipment need not be taken. Organic solvents are used as an alternative to dry granulation when water-sensitive drugs are processed, or when a rapid drying time is required.
In the traditional wet granulation method, the wet mass is forced through a sieve to produce wet granules which are then dried. A subsequent screening stage breaks agglomerates of granules and removes the fine material which can be recycled. Variations of this traditional method are dependent upon the equipment used but the general principle of initial particle aggregation using a liquid remains in all of the processes.
An alternative to the traditional wet-granulation process is melt granulation whereby thermosetting polymers are used to form the granules. This process is described below.
Effect of granulation method on granule structure
The type and capacity of granulating mixers significantly influence the work input and time necessary to produce a cohesive mass, adequate liquid distribution and intragranular porosity of the granular mass. The method and conditions of granulation affect intragranular pore structure by changing the degree of packing within individual granules. It has been shown that precompacted granules, consisting of drug and binder particles, are held together by simple bonding formed during consolidation. Granules prepared by wet massing consist of intact drug particles held together in a sponge-like matrix of binder. Fluidized-bed granules are similar to granules prepared by the wet massing process but possess greater porosity, and the granule surface is covered by a film of binding agent. With spray-dried systems, the granules consist of spherical particles composed of an outer shell with an inner core of particles or air. Thus, the properties of the granule are influenced by the manufacturing process.
Granulation mechanisms
Particle bonding mechanisms
To form granules, bonds must be formed between powder particles so that they adhere and these bonds must be sufficiently strong to prevent breakdown of the granule to powder in subsequent handling operations.
The five primary bonding mechanisms between particles are:
2. interfacial forces in mobile liquid films within the granules
3. the formation of solid bridges after solvent evaporation
Different types of mechanism have been identified in each group, and the ones discussed below are those which are of relevance to pharmaceutical granulations.
Adhesion and cohesion forces in immobile films
If sufficient liquid is present in a powder to form a very thin, immobile layer, there will be an effective decrease in interparticulate distance and an increase in contact area between the particles. The bond strength between the particles will be increased because of this, as the van der Waals forces of attraction are proportional to the particle diameter and inversely proportional to the square of the distance of separation.
This situation will arise with adsorbed moisture and accounts for the cohesion of slightly damp powders. Although such films may be present as residual liquid after granules prepared by wet granulation have been dried, it is unlikely that they contribute significantly to the final granule strength. In dry granulation, however, the pressures used will increase the contact area between the adsorbed layers and decrease the interparticulate distance and this will contribute to the final granule strength.
Thin, immobile layers may also be formed by highly viscous solutions of adhesives. The resulting bond strength will be greater than that produced by the mobile films discussed below. The use of starch mucilage in pharmaceutical granulations may produce this type of film.
Interfacial forces in mobile liquid films
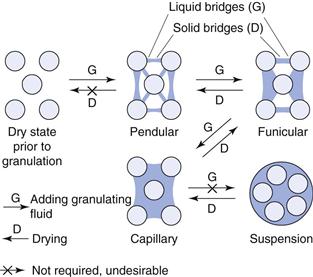
During wet granulation, liquid is added to the powder mix and will be distributed as a film around and between the particles. Sufficient liquid is usually added to exceed that necessary for an immobile layer and this produces a mobile film. The three states of water distribution between particles are illustrated in Figure 28.2.
At low moisture levels, termed the pendular state, the particles are held together by lens-shaped rings of liquid. These cause adhesion because of the surface tension forces of the liquid–air interface and the hydrostatic suction pressure in the liquid bridge. When all the air has been displaced from between the particles, the capillary state is reached, and the particles are held by capillary suction at the liquid–air interface, which is now only at the granule surface. The funicular state represents an intermediate stage between the pendular and capillary states. Moist granule tensile strength increases about three times from the pendular to capillary state.
It may appear that the state of the powder bed is dependent upon the total moisture content of the wetted powders but the capillary state may also be reached by decreasing the separation of the particles. In the massing process during wet granulation, continued kneading/mixing of material originally in the pendular state will densify the wet mass, decreasing the pore volume occupied by air and eventually producing the funicular or capillary state without further liquid addition.
In addition to these three states, a further state, the droplet, is illustrated in Figure 28.2. This will be important in the process of granulation by spray drying of a suspension. In this state, the strength of the droplet is dependent upon the surface tension of the liquid used.
These wet bridges are only temporary structures in wet granulation because the moist granules will be dried. They are, however, a prerequisite for the formation of solid bridges formed by adhesives present in the liquid, or by materials which dissolve in the granulating liquid.
Solid bridges
These can be formed by:
Partial melting.
Although not considered to be a predominant mechanism in pharmaceutical materials, it is possible that the pressures used in dry granulation methods may cause melting of low melting point materials where the particles touch and high pressures are developed. When the pressure is relieved, crystallization will take place, binding the particles together.
Hardening binders.
This is the common mechanism in pharmaceutical wet granulations when an adhesive is included in the granulating solvent. The liquid will form liquid bridges, as discussed above, and the adhesive will harden or crystallize on drying to form solid bridges to bind the particles. Adhesives such as polyvinylpyrrolidone, the cellulose derivatives (such as carboxymethylcellulose) and pregelatinized starch function in this way.
Crystallization of dissolved substances.
The solvent used to mass the powder during wet granulation may partially dissolve one of the powdered ingredients. When the granules are dried, crystallization of this material will take place and the dissolved substance then acts as a hardening binder. Any material soluble in the granulating liquid will function in this manner, e.g. lactose incorporated into powder blends granulated with water.
The size of the crystals produced in the bridge will be influenced by the rate of drying of the granules; the slower the drying time, the larger the particle size. It is therefore important that the drug does not dissolve in the granulating liquid and recrystallize because it may adversely affect the dissolution rate of the drug if crystals larger than that of the starting material are produced.
Attractive forces between solid particles
In the absence of liquids and solid bridges formed by binding agents, there are two types of attractive force which can operate between particles in pharmaceutical systems.
Electrostatic forces may be of importance in causing powder cohesion and the initial formation of agglomerates, e.g. during mixing. In general they do not contribute significantly to the final strength of the granule.
Van der Waals forces, however, are about four orders of magnitude greater than electrostatic forces and contribute significantly to the strength of granules produced by dry granulation. The magnitude of these forces will increase as the distance between adjacent surfaces decreases and in dry granulation this is achieved using pressure to force the particles together.
Mechanisms of granule formation
In the dry methods, adhesion of particles takes place because of applied pressure. A compact or sheet is produced which is larger than the granule size required and therefore the required size can be attained by milling and sieving.
In wet granulation methods, liquid added to dry powders has to be distributed through the powder by the mechanical agitation produced in the granulator. The particles adhere to each other because of liquid films and further agitation and/or liquid addition causes more particles to adhere. The precise mechanism by which a dry powder is transformed into a bed of granules varies for each type of granulation equipment but the mechanism discussed below serves as a useful broad generalization of the process.
The proposed granulation mechanism can be divided into three stages: nucleation, transition and ball growth.
Nucleation
Granulation starts with particle–particle contact and adhesion due to liquid bridges. A number of particles will join to form the pendular state illustrated in Figure 28.2. Further agitation densifies the pendular bodies to form the capillary state and these bodies act as nuclei for further granule growth.
Transition
Nuclei can grow by two possible mechanisms: either single particles can be added to the nuclei by pendular bridges or two or more nuclei may combine. The combined nuclei will be reshaped by the agitation of the bed.
This stage is characterized by the presence of a large number of small granules with a fairly wide size distribution. Providing that the size distribution is not excessively large, this point represents a suitable endpoint for granules used in capsule and tablet manufacture as relatively small granules will produce a uniform tablet die or capsule fill. Larger granules may give rise to problems in small-diameter dies due to bridging across the die and uneven fill.
Ball growth
Further granule growth produces large, spherical granules and the mean particle size of the granulating system will increase with time. If agitation is continued, granule coalescence will continue and produce an unusable, over-massed system, although this is dependent upon the amount of liquid added and the properties of the material being granulated.
Although ball growth produces granules which may be too large for pharmaceutical purposes, some degree of ball growth will occur in planetary mixers and it is an essential feature of some spheronizing equipment.
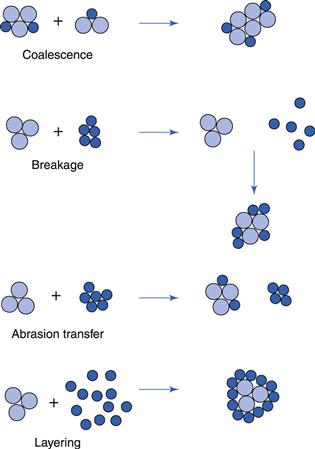
The four possible mechanisms of ball growth are illustrated in Figure 28.3.
Coalescence.
Two or more granules join to form a larger granule.
Breakage.
Granules break into fragments which adhere to other granules, forming a layer of material over the surviving granule.
Abrasion transfer.
Agitation of the granule bed leads to attrition of material from granules. This abraded material adheres to other granules, increasing their size.
Layering.
When a second batch of powder mix is added to a bed of granules, the powder will adhere to the granules, forming a layer over the surface and thus increasing the granule size. This mechanism is only of relevance to the production of layered granules using spheronizing equipment.
There will be some degree of overlap between these stages and it will be very difficult to identify a given stage by inspection of the granulating system. For end-product uniformity, it is desirable to finish every batch of a formulation at the same stage and this may be a major problem in pharmaceutical production.
Using the slower processes such as the planetary mixer, there is usually a sufficient length of time to stop the process before overmassing. In faster granulation equipment, the duration of granulation can only be used as a control parameter when the formulation is such that granule growth is slow and takes place at a fairly uniform rate. In many cases, however, the transition from a non-granulated to an overmassed system is very rapid and monitoring equipment is necessary to stop the granulation at a predetermined point. This is known as granulation endpoint control.
Pharmaceutical granulation equipment and processes
Wet granulators
There are three main types of granulator used in the pharmaceutical industry for wet granulation.
Shear granulators
The older shear granulators have largely disappeared and have been replaced by the much more efficient high-speed mixer/granulators. In the traditional shear (or planetary) granulation process, dry-powder blending usually has to be performed as a separate initial operation using different powder-mixing equipment. The older process suffered from a number of major disadvantages: its long duration, the need for several pieces of equipment and the high material losses which can be incurred because of the transfer stages between the different equipment. The process served the pharmaceutical industry well for many years but the advantages of modern mixer/granulators have proved too attractive for their continued use.
High-speed mixer/granulators
This type of granulator (e.g. Diosna) is used extensively for pharmaceutical granulation. They were developed from traditional planetary mixers in order to speed up the process and to reduce the number of pieces of equipment and separate process steps required. The machines have a stainless steel mixing bowl containing a three-bladed main impeller which revolves in the horizontal plane and a three-bladed auxiliary chopper (or breaker blade) which revolves in either the vertical or horizontal plane (Fig. 28.4). The main blade is designed to rotate at about 150–300 rpm and the high-speed chopper rotates at about 1500 and 3000 rpm.
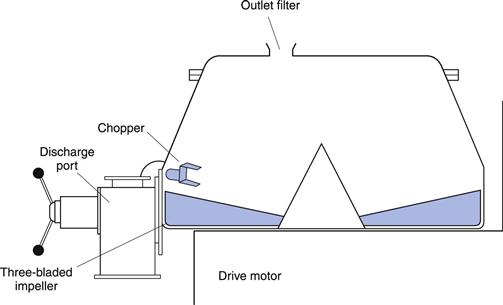
Fig. 28.4 High-speed mixer/granulator.
The unmixed dry powders are placed in the bowl and mixed by the rotating impeller for a few minutes. Granulating liquid is then added via a port in the lid of the granulator whilst the impeller is turning. The granulating fluid is mixed into the powders by the impeller. The chopper is usually switched on when the moist mass is formed as its function is to break up the wet mass to produce a bed of granular material. Once a satisfactory granule has been produced, the granular product is discharged, passing through a wire mesh, which breaks up any large aggregates, into the bowl of a fluidized-bed drier.
Like most modern process equipment, high-speed mixer/granulators are available in a wide range of sizes. These are often designed to have similar geometric and powder movement characteristics in an attempt to minimize scale-up problems when a product moves from development to production. Bowl volumes between 1 L and 1250 L are available. The weight of powder that each holds will depend on its bulk density and the optimum fill capacity (working volume) of each bowl. Bowls are manufactured from high-quality polished stainless steel.
The advantage of the process is that powder blending, wet massing and granulation are all performed in a few minutes in the same piece of equipment. The process needs to be controlled with care as the granulation progresses so rapidly that a usable granule can be transformed very quickly into an unusable, overmassed system. Thus it is often necessary to use a suitable monitoring system to indicate the end of the granulation process, i.e. when a granule of the desired properties has been attained. The process is also sensitive to variations in raw materials but this may be minimized by using a suitable granulation endpoint monitor.
A variation of the Diosna type of design is the Collette UltimaGral mixer (GEA Collette, Vanguard) (Fig. 28.5). This is based on the bowl and overhead drive of the planetary mixer but the single paddle of a planetary mixer is replaced with two mixing shafts. One of these carries three blade arms which rotate in the horizontal plane at the base of the bowl and the second carries smaller blades which act as the chopper and rotate rapidly in the upper regions of the granulating mass. Thus, the operating principle is similar to that of the Diosna type described above.
This design is available in sizes ranging from 10 L to 200 L volume (capable of processing about 3 kg to 80 kg batches, respectively). The main mixing blade rotates at 450–600 rpm in the 10 L model and at 150–200 rpm in the 200 L model. This rotational speed variation is to attempt to maintain the same linear velocity of movement of the blades, as this has been found to help scale-up. In all models the high-speed chopper rotates at 1500–3000 rpm.
An attractive feature of high-speed granulators is the fact that the product is usually granular and a separate step to granulate the wet mass is avoided (the granules being produced by the action of the high-speed chopper). Occasionally this is not fully satisfactory and the moist mass then has to be transferred to a granulator such as an oscillating granulator (Fig. 28.6). The rotor bars of the granulator oscillate at an adjustable rate between 60 and 100 rpm and force the moist mass through the sieve screen, the size of which determines the granule size. The mass should be sufficiently moist to form discrete granules when sieved. If excess liquid is added at the wet massing stage, strings of material will be formed and if the mix is too dry, the mass will be sieved to a powder, and granules will not be formed.
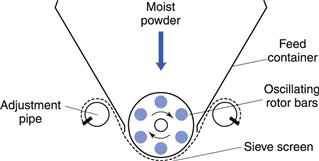
Fig. 28.6 Oscillating granulator.
This type of granulator can also deal with the size reduction of dry material. They are available in a range of sizes capable of dealing with 300–500 kg of wet mass per hour or 700–1200 kg/h of dry mass.
Fluidized-bed granulators
Fluidized-bed granulators (e.g. Aeromatic-Fielder, Glatt, Vanguard) have a similar design and operation to fluidized-bed driers, i.e. the powder particles are fluidized in a stream of air, but in addition granulation fluid is sprayed from a nozzle onto the bed of powders (Fig. 28.7).
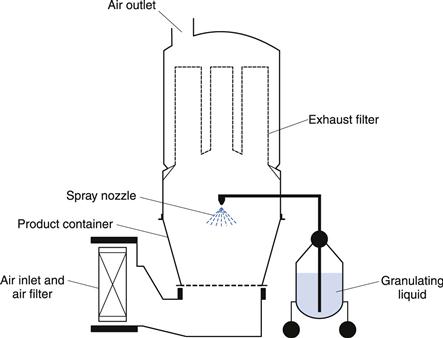
Fig. 28.7 Fluidized-bed granulator.
Heated and filtered air is blown or sucked through the bed of unmixed powders to fluidize the particles and mix the powders; fluidization is actually a very efficient mixing process. Granulating fluid is pumped from a reservoir through a spray nozzle or multiple nozzles positioned over the bed of particles. A variety of spray nozzles is available to cope with a wide range of products. The granulating fluid causes the primary powder particles to adhere when the droplets and powders collide. Escape of material from the granulation chamber is prevented by exhaust filters which are periodically agitated to reintroduce the collected material into the fluidized bed. Sufficient liquid is sprayed to produce granules of the required size at which point the spray is turned off – but the fluidizing air continued. The wet granulates are then dried in the heated fluidizing air stream.
Commercial apparatus ranges from laboratory models that, by changing the bowl, have a volume between 0.2 and 2 L, giving a capacity of a few grams up to about 1 kg, up to production machines. A wide range is available to cope with the wide variety of production volumes encountered in the pharmaceutical industry. They can be obtained in sizes suitable for batches between 5 kg and a massive 1550 kg, these calculations being based on an optimum 70% bowl fill and a powder/granule bulk density of 0.5 kg/L.
Advantages of fluidized-bed granulation.
Fluidized-bed granulation has many advantages over conventional wet massing. All the granulation processes, which normally need separate equipment in the conventional method, are performed in one unit, saving labour costs, transfer losses and time. Another advantage is that automation of the process can be achieved once the conditions affecting the granulation have been optimized and validated.
Disadvantages of fluidized-bed granulation.
On the downside, the equipment is initially expensive and optimization of process (and product) parameters affecting granulation needs extensive development work, not only during initial formulation work but also during scale-up from development to production scale. Similar development work for the traditional process is not as extensive as that for high-speed granulators.
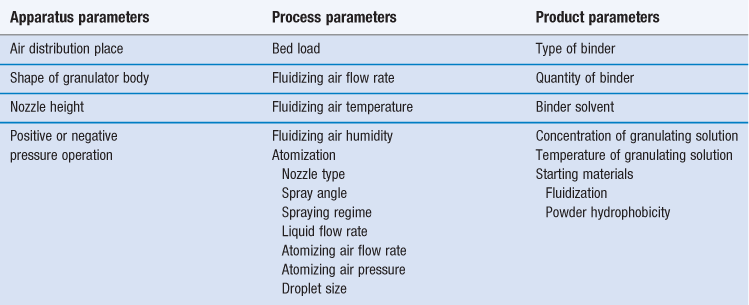
This long and very product-specific development process has proved to be a serious problem with fluidized-bed granulation in the pharmaceutical industry. There are numerous apparatus, process and product parameters which affect the quality of the final granule; these are listed in Table 28.1. The extent of this list, coupled with the fact that each formulation presents its own individual development problems, has led to fluidized-bed granulation not fulfilling its full potential in pharmaceutical production. This is exacerbated by the reality that most pharmaceutical companies have a wide range of products made at relatively small batch size, unlike other industries (fertilizers, herbicides, foodstuffs) where fluidized-bed granulation is used successfully and extensively. Intelligent computer control of the whole process is available but requires careful setting up to cope with the sensitivity to small changes in formulation and process variables.
Spray driers
These differ from the method discussed above in that a dry, granular product is made from a solution or a suspension rather than from dry primary powder particles. The solution or suspension may be of drug alone, a single excipient or a complete formulation.
The process of spray drying is discussed more fully in Chapter 29. The resultant granules are free-flowing hollow spheres and the distribution of the binder in such granules (at the periphery following solute migration during drying) results in good compaction properties.
This process can be used to make tablet granules although it is probably economically justified for this purpose only when suitable granules cannot be produced by the other methods. Spray drying can convert hard elastic materials into more ductile materials. Spray-dried lactose is the classic example and its advantages over α-lactose monohydrate crystals when compacted are discussed in Chapter 30.
The primary advantages of the process are the short drying time and the minimal exposure of the product to heat due to the short residence time in the drying chamber. This means that little deterioration of heat-sensitive materials takes place and it may be the only process suitable for this type of product.
Spheronizers/pelletizers
For some applications it may be desirable to have a dense, spherical pellet of the type difficult to produce with the equipment described above. Such pellets are used for controlled drug release products following coating with a suitable polymer coat and filling into hard gelatin capsules. Capsule filling with a mixture of coated and non-coated drug-containing pellets would give some degree of programmed drug release after the capsule shell dissolves.
A commonly used process involves the separate processes of wet massing, followed by extrusion of this wet mass into rod-shaped granules and subsequent spheronization of these granules. Because this process is used so frequently to produce modified-release multiparticulates, this process will be discussed in some detail.
Extrusion/spheronization
Extrusion/spheronization is a multi-step process used to make uniformly sized spherical particles. It is primarily used as a method to produce multiparticulates for controlled drug release applications. The major advantage over other methods of producing drug-loaded spheres or pellets is the ability to incorporate high levels of active ingredients without producing excessively large particles (i.e. minimal excipients are necessary).
The main steps of the process are:
• dry mixing of ingredients to achieve a homogeneous powder dispersion
• wet massing to produce a sufficiently plastic wet mass
• extrusion to form rod-shaped particles of uniform diameter
• spheronization to round off these rods into spherical particles
• drying to achieve the desired final moisture content
• screening (optional) to achieve the desired narrow size distribution.
Applications of extrusion/spheronization
Potential applications are many but relate mainly to controlled drug release and improved processing.
Controlled drug release.
Both immediate-release and controlled-release pellets can be formed. In turn, these pellets can either be filled into hard gelatin capsule shells or compacted with suitable excipients into tablets to form unit dosage forms. Pellets can contain two or more ingredients in the same individual unit or incompatible ingredients can be manufactured in separate pellets.
Pellets can be coated in sub-batches to give, say, rapid-, intermediate- and prolonged-release pellets in the same capsule shell. Dense multiparticulates disperse evenly within the gastrointestinal tract and have less variable gastric emptying and intestinal transit times than single units, such as coated monolithic tablets.
Processing.
The process of extrusion/spheronization can be used to increase the bulk density, improve flow properties and reduce the problems of dust usually encountered with low-density, finely divided active and excipient powders.
Extrusion/spheronization is a more labour-intensive process than other forms of granulation and therefore should only be considered when other methods of granulation are either not satisfactory for that particular formulation or are inappropriate (i.e. when spheres are required).
Desirable properties of pellets
Uncoated pellets have:
and once coated:
Process
Dry mixing of ingredients.
This uses normal powder mixing equipment.
Wet massing.
This stage also employs normal equipment and processes as used in wet granulation. There are two major differences in the granulation step compared with granulation for compaction:
The amount of fluid needed to achieve spheres of uniform size and sphericity is likely to be greater than that for a similar tablet granulation. Poor liquid dispersion will produce a poor-quality product.
Extrusion.
Extrusion produces rod-shaped particles of uniform diameter from the wet mass. The wet mass is forced through dies and shaped into small cylindrical particles with uniform diameter. The extrudate particles break at similar lengths under their own weight. Thus, the extrudate must have enough plasticity to deform but not so much that the extruded particles adhere to other particles when collected or rolled in the spheronizer.
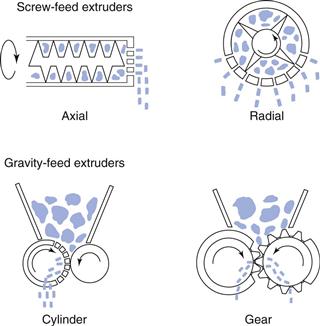
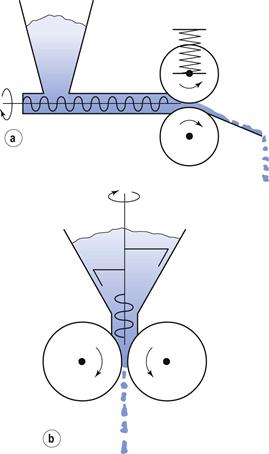
There are many designs of extruder but generally they can be divided into three classes, based on their feed mechanism:
• screw-feed extruders (axial or endplate, dome and radial)
The first two categories (shown in Fig. 28.8) are used for both development and production but the latter is only used for experimental development work as it is easy to add instrumentation.
The primary extrusion process variables are:
Spheronization.
The function of the fourth step in the process (i.e. spheronization) is to round off the rods produced by extrusion into spherical particles.
This process is carried out in a relatively simple piece of apparatus (Fig. 28.9). The working part consists of a bowl having fixed side walls, with a rapidly rotating bottom plate or disc. The rounding of the extrudate into spheres is dependent on frictional forces generated by particle–particle and particle–equipment collisions.
The bottom disc has a grooved surface to increase these forces. Two geometric patterns are generally used:
• a cross-hatched pattern with grooves running at right angles to one another
• a radial pattern with grooves running radially from the centre of the disc.
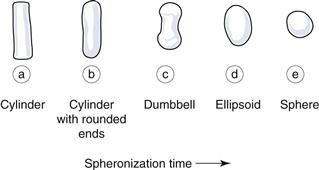
The transition from rods to spheres during spheronization occurs in various stages. These are best described by examining the diagrams in Figure 28.10.
If the moistened mass is too dry, spheres will not be formed; the rods will only transform as far as dumbbells.
Drying.
A drying stage is required in order to achieve the desired moisture content. Drying is often the final step in the process. Drying of the pellets can be accomplished in any dryer that can be used for conventional wet granulations, including tray dryers and fluidized-bed dryers. Both are used successfully for extrusion/spheronization. If solute migration (see Chapter 29) occurs during drying of the wet spheres, this may result in:
Screening (optional).
Screening may be necessary in order to achieve the desired narrow size distribution. Normal sieves are used. If all the previous stages are performed efficiently and with careful development of process and formulation conditions, this step may not be necessary.
Formulation variables
The composition of the wet mass is critical in determining the properties of the particles produced. During the granulation step, a wet mass is produced which must be plastic, deform when extruded and break off to form uniformly sized cylindrical particles which are easily deformed into spherical particles. Thus the process has a complex set of requirements that are strongly influenced by the ingredients of the pellet formulation.
Summary
Extrusion/spheronization is a versatile process capable of producing spherical granules having very useful properties. Because it is more labour intensive than more common wet massing techniques, its use should be limited to those applications where a sphere is required and other granulation techniques are unsuitable.
The most common application of the process is to produce spherical pellets for controlled drug release.
Care must be taken to understand the required properties of the pellets and the manner in which the process and formulation influence the ability to achieve these aims.
Rotorgranulation
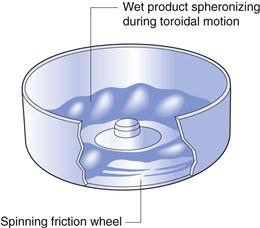
This process allows the direct manufacture of spheres suitable for controlled-release solid dosage forms from a dry powder in one process. The powder mix is added to the bowl and wetted with granulating liquid from a spray or multiple sprays (Fig. 28.11). The base plate rotates at high speed and centrifugal force keeps the moist mass at the edges of the rotor. Here the velocity difference between the rotor and static walls, combined with the upward flow of air around the rotor plate, causes the mass to move in a toroidal motion, resulting in the formation of discrete spherical pellets. This is an almost identical motion to that occurring in spheronizers, as shown in Figure 28.10. The resulting spheres (actually, of course, wet granules) are dried by the heated inlet air from the air chamber.
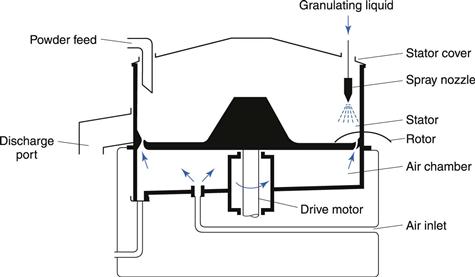
Fig. 28.11 Rotorgranulator.
Using this technique, it is possible to continue the process and coat the pellets by subsequently spraying coating solution onto the rotating dried pellets. Additionally, layered pellets can be produced by using uncoated pellets as nuclei in a second granulation with a powder mix of a second ingredient or ingredients.
Rotorgranulators (e.g. Freund, Vanguard) are manufactured in size ranges between 45 L and 450 L capacity. Again, the corresponding fill weights will depend upon the bulk density of the formulation and the optimum operating capacity of each bowl. This range requires corresponding bowl and rotor disc diameters between 300 and 1400 mm. Discs with different surface roughness patterns are available to cope with a wide range of materials. They also come with an adjustable air gap around the plate to assist in the manipulation of resulting granule size. Powder charging and discharging are made easy and there is precise liquid feeding. Intelligent computer control of the whole process is available.
Melt granulation
Introduction
Melt granulation is a size enlargement process in which a thermosetting material (hot-melt binder) is used to bind the primary powder particles into granules. It is a water-free alternative to wet granulation. The binder/granulating agent is a semi-solid or solid hydrophilic polymer or a hydrophobic wax. There are two variants to the technique:
In either case, liquid bridges are formed by the molten binder and powder agglomeration (i.e. granulation) takes place. The mechanism of granulation is analogous to wet granulation described above. The initial particle-particle bonds are formed by the surface tension of a liquid (this time the molten hot-melt binder). On subsequent cooling, the molten binder solidifies forming solid bridges that permanently bind the particles together.
Hot-melt binders
Hot-melt binders can be either hydrophilic/water soluble or hydrophobic/water insoluble. The most commonly used hydrophilic water-soluble binders are the polyethylene glycols (PEGs). Polyethylene glycol is the ideal hot-melt binder for granules intended for immediate release products. Grades between PEG 2000 and PEG 6000 can be used with PEG 3000 being well suited (melting point 48–54 °C).
Hydrophobic water-insoluble binders are particularly useful in producing controlled-release dosage forms. Many different hydrophobic waxes have been found to be suitable. These include: carnauba wax, hydrogenated castor oil, hydrogenated cottonseed oil, stearic acid and a wide variety of fatty acid derivatives (glyceryl behanate, glyceryl monostearate, glyceryl trilaurate, glyceryl trimyristate, glyceryl tripalmitate, glyceryl tristearate, hexadecyl palmitate, octadecyl stearate and sorbitan monostearate). Most of these are miscible when molten and can be used in combination.
Hot-melt processes
The heating procedure can be performed in a mixing vessel that is jacketed with hot water. In some formulations, the temperature rise can be generated by friction alone during agitation/mixing. High-speed mixers can sometimes generate a sufficient temperature rise in an acceptable processing time. Experimentation has shown that 60 °C can be achieved in as little as 10 minutes and that this can be reduced to 5 minutes with additional jacket heating.
There is also a variant of the standard extrusion/spheronization process in which low melting point waxes and heating are used in the extrusion process. Spheronization and cooling are carried out simultaneously. This is hot-melt extrusion/speronization.
The main factors influencing the efficiency of the hot-melt granulation process are the amount and melting point of the binder, its viscosity when molten, impellor rotation speed, massing time and temperature achieved.
The amount of binder needed varies widely. Indeed manipulation of the amount, and the creation of mixtures, of hydrophobic hot-melt binders can be part of the formulation development when designing modified-release products and adjusting the drug release versus time profile. For immediate-release systems, PEG 3000 25–30% by weight has been found to be a good starting point.
Following cooling of the melt-granulation granules or pellets back to room temperature, they follow the same fate as other granules, i.e. following an optional size reduction and screening procedure, they are either used in their own right as a dosage form, filled into hard gelatin capsule shells or compacted into tablets together with other excipients.
Advantages and limitations
The advantages of using a hot-melt granulation compared with aqueous wet granulation is that the use of water is avoided and so damage to hydrolytic drug molecules is minimized. Melt granulation also avoids the use of organic solvents that are sometimes employed as an alternative in such cases. However, thermal degradation can still be a problem. A limitation in the use of melt granulation for immediately-release products is that at the moment there is little alternative to the use of polyethylene glycols.
Dry granulators
Dry granulation converts primary powder particles into granules using the application of pressure without the intermediate use of a liquid. It therefore avoids heat/temperature combinations which may degrade the product.
Two pieces of equipment are necessary for dry granulation: first, a machine for compressing the dry powders into compacts or flakes and second, a mill for breaking up these intermediate products into granules.
Slugging
The dry powders can be compacted using a conventional tablet machine or, more usually, a large heavy-duty rotary press can be used. This process is often known as slugging, the compacts made in the process (typically 25 mm diameter by about 10–15 mm thick) being termed slugs. A hammer mill is suitable for breaking the slugs. This is an old process that is being replaced by the more modern, and better, roller compaction process. Many pharmaceutical tableting materials suffer from a property known as work hardening which results in poor recompaction of these already compacted granules.
Roller compaction
Roller compaction is an alternative gentler method, the powder mix being squeezed between two counter-rotating rollers to form a compressed sheet (Fig. 28.12). The roller rotation speeds can be adjusted to allow variation in the compression time as the material passes between the rollers. Additionally, roller pressure can be adjusted and is maintained constant during a run by means of a hydraulic control system to yield granules of constant crushing strength. Even rollers with different surface grooves are available if a particular product is troublesome.
The sheet so formed is normally weak and brittle and breaks immediately into flakes that can be somewhat similar to cornflakes in their geometry. These flakes need gentler treatment to break them into granules. An oscillating granulator of the type shown in Figure 28.6 can be used with care, but often the conversion of these flakes to granules can be achieved by screening alone. Separation of fines (small powder particles) formed during the size reduction stage is often necessary. With suitable documentation, these may be recycled.
Advantages of the roller compaction process
• The process is easily scaled up.
• The product has uniform properties with respect to its mechanical strength.
Again, like most modern pharmaceutical process machinery, a wide range of sizes is available capable of operating with a wide range of throughputs and compaction forces (e.g. Alexanderwerk, Powtec). In the case of roller compactors, this range is enormous, being available for throughputs between 10 and 2000 kg/h and compaction forces between 16 and 64 kN/cm of roller length. This is achieved by the choice of machines with roller diameters of between 100 mm and 450 mm and roller lengths of 30 mm to 115 mm. This allows a wide range of APIs (active pharmaceutical ingredients, i.e. drugs) and excipients to be processed (or co-processed) and enables easy scale-up from a few hundred grams in development to very large-scale production of a successful product.
The Protec or Hutt type (Fig. 28.12b) has a vertical screw feeder in the hopper which produces an even flow of the material. Due to its design, it also has a precompacting and de-aerating effect on the powder charge. The speed of the screw, and that of the rollers, can be adjusted to provide control to the process.
Bibliography
1. Alvarez L, Concheiro A, Gomez-Amoza JL, Souto C, Martinez-Pacheco R. Effect of microcrystalline cellulose grade and process variables on pellets prepared by extrusion-spheronization. Drug Development and Industrial Pharmacy. 2002;28:451–456.
2. Baert L. Correlation of extrusion forces, raw materials and sphere characteristics. Journal of Pharmacy and Pharmacology. 1992;44:676–678.
3. Banker GS, Rhodes CT. Modern Pharmaceutics. 4th edn NY, USA: Marcel Dekker; 2002.
4. am Ende DJ. Wet granulation processes Chap 39. In: am Ende DJ, ed. Chemical Engineering in the Pharmaceutical Industry: R&D to manufacture. New Jersey, USA: John Wiley & Sons (in conjunction with AIChE); 2010.
5. am Ende DJ. Achieving hot melt extrusion Chap 42. In: am Ende DJ, ed. Chemical Engineering in the Pharmaceutical Industry: R&D to manufacture. New Jersey, USA: John Wiley & Sons (in conjunction with AIChE); 2010.
6. Erkoboni, D.F. (2003) Extrusion/spheronization. In: Ghebre-Sellassie, I. and Martin, C. (eds) Drugs and the Pharmaceutical Sciences Series, Marcel Dekker, 133, 277–322.
7. Farag Badawy SI. Effect of process parameters on compressibility of granulation manufactured in a higher-shear mixer. International Journal of Pharmaceutics. 2000;198(1):51–61.
8. Faure A, Grimsey IM, Rowe RC, York P, Cliff MJ. Applicability of a scale-up methodology for the wet granulation process in Collette Gral high shear mixer granulators. European Journal of Pharmaceutical Sciences. 1999;8:85–93.
9. Gandhi R, Kaul CL, Panchagnula R. Extrusion and spheronization in the development of oral controlled-release dosage forms. Pharmaceutical Science and Technology Today. 1999;2(4):160–181.
10. Ghebre-Sellassie I, Martin C, eds. Pharmaceutical Extrusion Technology. NY and Basel: Marcel Dekker (CRC Press); 2003.
11. Keleb EI, Vermeire A, Vervaet C, Remon JP. Extrusion granulation and high shear granulation of lactose and highly dosed drugs: a comparative study. Drug Development and Industrial Pharmacy. 2004;30(6):679–691.
12. Landin M, York P, Cliff MJ, Rowe RC. Scale up of a pharmaceutical granulation in planetary mixers. Pharmaceutical Development and Technology. 1999;4(2):145–150.
13. Law MF, Deasy PB, McLaughlin JP, Gabriel S. Comparison of two commercial brands of microcrystalline cellulose for extrusion-spheronization. Journal of Microencapsulation. 1997;14(6):713–723.
14. Lodaya, M., Mollan, M., Ghebre-Sellassie, I. et al. (2003) Twin-screw wet granulation. In: Ghebre-Sellassie, I. and Martin, C. (eds) Drugs and the Pharmaceutical Sciences Series, Marcel Dekker, 133, 323–343.
15. Maejima T, et al. Application of tumbling melt granulation (TMG) method to prepare controlled-release fine granules. Chemical and Pharmaceutical Bulletin. 1998;46(3):534–536.
16. Nurnberg E, Wunderlich J. Manufacturing pellets by extrusion and spheronization (Part I). Pharmaceutical Technology Europe. 1998;11(2):41–47 Part II 11(3).
17. Ogawa S, Kamijima T, Miyanoto Y, et al. A new attempt to solve the scale-up problem for granulation using response surface methodology. Journal of Pharmaceutical Sciences. 1994;83(3):439–443.
18. Parikh DM. Handbook of Pharmaceutical Granulation Technology. 3rd edn New York: Marcel Dekker; 2009.
19. Rubino OR. Fluid-bed technology: overview and criteria for process selection. Pharmaceutical Technology. 1999;6:104–113.
20. Shah RD, Kabadi M, Pope DG, Ausburger LL. Physico-mechanical characterization of the extrusion-spheronization process Part II: Rheological determinants for successful extrusion and spheronization. Pharmaceutical Research. 1995;12(4):496–507.
21. Sprockel OL, Stamato HJ. Design and Scale up of dry granulation processes Chap 38. In: am Ende DJ, ed. Chemical Engineering in the Pharmaceutical Industry: R&D to manufacture. New Jersey, USA: John Wiley & Sons (in conjunction with AIChE); 2010.
22. Thoma K, Ziegler I. Investigations on the influence of the type of extruder for pelletization by extrusion-spheronization I Extrusion behavior of formulations. Drug Development and Industrial Pharmacy. 1998;24(5):401–411 II Sphere characteristics. Drug Development and Industrial Pharmacy, 24(5), 413–422.
23. Troy DB, ed. Remington: The Science and Practice of Pharmacy. 21st edn Maryland, USA: Lippincott, Williams and Wilkins; 2006.
24. Vilhelmsen T, Kristensen J, Schaefer T. Melt pelletization with polyethylene glycol in a rotary processor. International Journal of Pharmaceutics. 2004;275:141–153.
25. Wørts O. Wet granulation – fluidized bed and high shear techniques compared. Pharmaceutical Technology Europe. 1998;10(11):27–30.
26. Zhang F, McGinty JM. Properties of sustained-release tablets prepared by hot-melt extrusion. Pharmaceutical Development and Technology. 1999;4(2):241–250.