38 Postoperative Pain Management for the Cardiac Patient
Adequate postoperative analgesia prevents unnecessary patient discomfort, may decrease morbidity, may decrease postoperative hospital length of stay, and thus may decrease cost. Because postoperative pain management has been deemed important, the American Society of Anesthesiologists has published practice guidelines regarding this topic.1 Furthermore, in recognition of the need for improved pain management, the Joint Commission on Accreditation of Healthcare Organizations has developed new standards for the assessment and management of pain in accredited hospitals and other health care settings.2 Patient satisfaction (no doubt linked to adequacy of postoperative analgesia) has become an essential element that influences clinical activity of not only anesthesiologists but all healthcare professionals.
Achieving optimal pain relief after cardiac surgery is often difficult. Pain may be associated with many interventions, including sternotomy, thoracotomy, leg-vein harvesting, pericardiotomy, and/or chest tube insertion, among others. Inadequate analgesia and/or an uninhibited stress response during the postoperative period may increase morbidity by causing adverse hemodynamic, metabolic, immunologic, and hemostatic alterations.3–5 Aggressive control of postoperative pain, associated with an attenuated stress response, may decrease morbidity and mortality in high-risk patients after noncardiac surgery6,7 and may also decrease morbidity and mortality in patients after cardiac surgery.8,9 Adequate postoperative analgesia may be attained via a wide variety of techniques (Table 38-1). Traditionally, analgesia after cardiac surgery has been obtained with intravenous opioids (specifically morphine). However, intravenous opioid use is associated with definite detrimental side effects (nausea/vomiting, pruritus, urinary retention, respiratory depression), and longer-acting opioids such as morphine may delay tracheal extubation during the immediate postoperative period via excessive sedation and/or respiratory depression. Thus, in the current era of early extubation (“fast-tracking”), cardiac anesthesiologists are exploring unique options other than traditional intravenous opioids for control of postoperative pain in patients after cardiac surgery.10,11 No single technique is clearly superior; each possesses distinct advantages and disadvantages. It is becoming increasingly clear that a multimodal approach/combined analgesic regimen (utilizing a variety of techniques) is likely the best way to approach postoperative pain (in all patients after surgery) to maximize analgesia and minimize side effects. When addressing postoperative analgesia in cardiac surgical patients, choice of technique (or techniques) is made only after a thorough analysis of the risk/benefit ratio of each technique in the specific patient in whom analgesia is desired.
TABLE 38-1 Techniques Available for Postoperative Analgesia
Pain and cardiac surgery
Surgical or traumatic injury initiates changes in the peripheral and central nervous systems (CNSs) that must be addressed therapeutically to promote postoperative analgesia and, it is hoped, positively influence clinical outcome (Boxes 38-1 and 38-2). The physical processes of incision, traction, and cutting of tissues stimulate free nerve endings and a wide variety of specific nociceptors. Receptor activation and activity are further modified by the local release of chemical mediators of inflammation and sympathetic amines released via the perioperative surgical stress response. The perioperative surgical stress response peaks during the immediate postoperative period and exerts major effects on many physiologic processes. The potential clinical benefits of attenuating the perioperative surgical stress response (above and beyond simply attaining adequate clinical analgesia) have received much attention during the 2000s and remain fairly controversial.12 However, it is clear that inadequate postoperative analgesia and/or an uninhibited perioperative surgical stress response has the potential to initiate pathophysiologic changes in all major organ systems, including the cardiovascular, pulmonary, gastrointestinal, renal, endocrine, immunologic, and/or CNSs, all of which may lead to substantial postoperative morbidity.
Pain after cardiac surgery may be intense and it originates from many sources, including the incision (sternotomy, thoracotomy, etc.), intraoperative tissue retraction and dissection, vascular cannulation sites, vein-harvesting sites, and chest tubes, among others.13,14 Patients in whom an internal mammary artery (IMA) is surgically exposed and used as a bypass graft may have substantially more postoperative pain.15
A prospective clinical investigation involving 200 consecutive patients undergoing cardiac surgery via median sternotomy assessed the location, distribution, and intensity of postoperative pain.13 All patients received 25 to 50 μg/kg intraoperative intravenous fentanyl, were subjected to routine cardiopulmonary bypass (CPB), had their arms positioned along their body on the operating table, had their sternum closed with five peristernal wires, and received mediastinal and thoracic drains passed through the rectus abdominis muscle just below the xiphoid. A subgroup (127 patients) also underwent long saphenous vein harvesting either from the calf (men) or thigh (women). All patients were extubated before the first postoperative morning. Postoperative analgesic management was standardized and included intravenous morphine, oral paracetamol, oral tramadol, and subcutaneous morphine. Pain location, distribution, and intensity were documented in the morning on the first, second, third, and seventh postoperative days using a standardized picture dividing the body into 32 anatomic areas. A numerical rating scale of 0 to 10 (with 0 representing no pain and 10 representing worst possible pain) was used to assess maximal pain intensity.
These investigators found that maximal pain intensity was highest on the first postoperative day and lowest on the third postoperative day. However, maximal pain intensity was only graded as “moderate” (mean pain score was approximately 3.8) and did not diminish during the first 2 postoperative days, yet started to decline between postoperative days 2 and 3. Pain distribution did not appear to vary throughout the postoperative period, yet location did (more shoulder pain observed on the seventh postoperative day). As time after surgery increased, pain usually moved from primarily incisional/epigastric to osteoarticular. Another source of postoperative pain in patients after cardiac surgery is thoracic cage rib fractures, which may be common.16,17 Furthermore, sternal retraction, causing posterior rib fracture, may lead to brachial plexus injury. In these patients, routine chest radiographs may be normal despite the presence of fracture. Thus, bone scans (better at detecting rib fractures than chest radiographs) are recommended whenever there is unexplained postoperative nonincisional pain in a patient who has undergone sternal retraction.17 Other studies have indicated that the most common source of pain in patients after cardiac surgery is the chest wall. Age also appears to impact pain intensity; patients younger than 60 often have greater pain intensity than patients older than 60. Although maximal pain intensity after cardiac surgery is usually only moderate, there remains ample room for clinical improvement in analgesic control to minimize pain intensity, especially during the first few postoperative days.
Persistent pain after cardiac surgery, although rare, can be problematic.18–20 The cause of persistent pain after sternotomy is multifactorial, yet tissue destruction, intercostal nerve trauma, scar formation, rib fractures, sternal infection, stainless-steel wire sutures, and/or costochondral separation may all play roles. Such chronic pain is often localized to the arms, shoulders, or legs. Postoperative brachial plexus neuropathies also may occur and have been attributed to rib fracture fragments, IMA dissection, suboptimal positioning of patients during surgery, and/or central venous catheter placement. Postoperative neuralgia of the saphenous nerve has also been reported after harvesting of saphenous veins for coronary artery bypass grafting (CABG). Younger patients appear to be at greater risk for development of chronic, long-lasting pain. The correlation of severity of acute postoperative pain and development of chronic pain syndromes has been suggested (patients requiring more postoperative analgesics may be more likely to develop chronic pain), yet the causative relation is still vague.
Ho and associates18 assessed via survey 244 patients after cardiac surgery and median sternotomy and found that persistent pain (defined as pain still present 2 or more months after surgery) was reported in almost 30% of patients. The incidence rate of persistent pain at any site was 29% (71 patients) and for sternotomy was 25% (61 patients). Other common locations of persistent pain reported to these investigators were the shoulders (17.4%), back (15.9%), and neck (5.8%). However, such persistent pain was usually reported as mild, with only 7% of patients reporting interference with daily living. The most common words used to describe the persistent pain were “annoying” (57%), “nagging” (33%), “dull” (30%), “sharp” (25%), “tiring” (22%), “tender” (22%), and “tight” (22%). The temporal nature of this pain was mostly reported as being brief/transient and periodic/intermittent. Twenty patients (8%) also described symptoms of numbness, burning pain, and tenderness over the IMA-harvesting site, symptoms suggestive of IMA syndrome. Thus, it was concluded that mild persistent pain after cardiac surgery and median sternotomy is common yet only infrequently substantially interferes with daily life.
Although the most common source of pain in patients after cardiac surgery remains the chest wall, leg pain from vein-graft harvesting can be problematic as well. Such pain may not become apparent until the late postoperative period, which may be related to the progression of patient mobilization, as well as the decreasing impact of sternotomy pain (unmasking leg incisional pain). The recent utilization of minimally invasive vein-graft harvesting techniques (endoscopic vein-graft harvesting) decreases postoperative leg pain intensity and duration compared with conventional open techniques.21 Although initial harvest times may be prolonged, harvest times become equivalent between the two techniques (endoscopic vs. conventional) once a short learning curve is overcome. Furthermore, leg morbidity (infection, dehiscence, etc.) may be less in patients undergoing endoscopic vein harvest compared with patients undergoing conventional open techniques because of different incisional lengths.
Patient satisfaction with quality of postoperative analgesia is as much related to the comparison between anticipated and experienced pain as it is to the actual level of pain experienced. Satisfaction is related to a situation that is better than predicted, dissatisfaction to one that is worse than expected. Patients undergoing cardiac surgery remain concerned regarding the adequacy of postoperative pain relief and tend to preoperatively expect a greater amount of postoperative pain than that which is actually experienced.14 Because of these unique preoperative expectations, patients after cardiac surgery who receive only moderate analgesia postoperatively will likely still be satisfied with their pain control. Thus, patients may experience pain of moderate intensity after cardiac surgery yet still express very high satisfaction levels.14,15
Potential clinical benefits of adequate postoperative analgesia
Inadequate analgesia (coupled with an uninhibited stress response) during the postoperative period may lead to many adverse hemodynamic (tachycardia, hypertension, vasoconstriction), metabolic (increased catabolism), immunologic (impaired immune response), and hemostatic (platelet activation) alterations. In patients undergoing cardiac surgery, perioperative myocardial ischemia (diagnosed by electrocardiography [ECG] and/or transesophageal echocardiography) is most commonly observed during the immediate postoperative period and appears to be related to outcome.22,23 Intraoperatively, initiation of CPB causes substantial increases in stress response hormones (norepinephrine, epinephrine, etc.) that persist into the immediate postoperative period and may contribute to myocardial ischemia observed during this time.24–26 Furthermore, postoperative myocardial ischemia may be aggravated by cardiac sympathetic nerve activation, which disrupts the balance between coronary blood flow and myocardial oxygen demand.27 Thus, during the pivotal immediate postoperative period after cardiac surgery, adequate analgesia (coupled with stress–response attenuation) may potentially decrease morbidity and enhance health-related quality of life.27,28
Evidence exists indicating that aggressive control of postoperative pain in patients after noncardiac surgery may beneficially affect outcome.6,7 In 1987, Yeager et al,7 in a small (n = 53 patients), randomized, controlled clinical trial involving patients undergoing major thoracic/vascular surgery, revealed that patients who were managed with more intense perioperative anesthesia and analgesia demonstrated decreased postoperative morbidity and improved operative outcome. In 1991, Tuman et al,6 in another small (n = 80 patients), randomized, controlled clinical trial involving patients undergoing lower extremity revascularization, revealed that patients who were managed with more intense perioperative anesthesia and analgesia demonstrated improved outcome compared with patients receiving routine on-demand narcotic analgesia.
Evidence also exists that aggressive control of postoperative pain in patients after cardiac surgery may beneficially affect outcome. Two intriguing clinical investigations published in 1992 hint at such possibilities.8,9 Mangano et al8 prospectively randomized 106 adult patients undergoing elective CABG to receive either standard postoperative analgesia or intensive analgesia during the immediate postoperative period. Standard-care patients received low-dose intermittent intravenous morphine for the first 18 postoperative hours, whereas intensive-analgesia patients received a continuous intravenous sufentanil infusion during the same time period. Patients receiving sufentanil demonstrated a lesser severity of myocardial ischemia episodes (detected by continuous ECG monitoring) during the immediate postoperative period. The authors postulated that the administration of intensive analgesia during the immediate postoperative period may have more completely suppressed sympathetic nervous system activation, thereby having numerous beneficial clinical effects, including beneficial alterations in sensitivity of platelets to epinephrine, beneficial alterations in fibrinolysis, enhanced regional left ventricular function, and decreased coronary artery vasoconstriction, all potentially leading to a reduced incidence/severity of myocardial ischemia. Anand and Hickey9 prospectively randomized 45 neonates undergoing elective cardiac surgery (mixed procedures) to receive either standard perioperative care or deep opioid anesthesia. Standard-care patients received a halothane/ketamine/morphine anesthetic with intermittent intravenous morphine for the first 24 postoperative hours, whereas deep-opioid patients received an intravenous sufentanil anesthetic with a continuous infusion of either intravenous fentanyl or intravenous sufentanil during the same postoperative time period. Neonates receiving continuous postoperative opioid infusions demonstrated a reduced perioperative stress response (assessed via multiple blood mediators), less perioperative morbidity (hyperglycemia, lactic acidemia, sepsis, metabolic acidosis, disseminated intravascular coagulation), and significantly fewer deaths than the control group (0/30 vs. 4/15, respectively; p < 0.01).
The accompanying editorial accurately summarizes this clinical investigation: “What Anand and Hickey have shown is that this reluctance to treat pain adequately is not a necessary evil. It markedly contributes to a bad outcome.”29 Unfortunately, aggressive control of postoperative pain in patients after cardiac surgery with relatively large amounts of intravenous opioids in this manner does not allow tracheal extubation in the immediate postoperative period (a goal of current practice).
Techniques available for postoperative analgesia
Although the mechanisms of postoperative pain and the pharmacology of analgesic drugs are relatively well understood, the delivery of effective postoperative analgesia remains far from universal. Many techniques are available (see Table 38-1). In general, the American Society of Anesthesiologists Task Force on Acute Pain Management in the Perioperative Setting reports that the existing literature supports the efficacy and safety of three techniques used by anesthesiologists for perioperative pain control: regional analgesic techniques (including but not limited to intercostal blocks, plexus blocks, and local anesthetic infiltration of incisions), patient-controlled analgesia (PCA) with systemic opioids, and intrathecal/epidural opioid analgesia.1 Regarding regional analgesic techniques, the existing literature supports the analgesic efficacy of peripheral nerve blocks and postincisional infiltration with local anesthetics for postoperative analgesia, yet is equivocal regarding the analgesic benefits of preincisional infiltration. Regarding PCA with systemic opioids, the existing literature supports its efficacy (compared with intramuscular techniques) for postoperative pain management, yet the existing literature is equivocal regarding the efficacy of PCA techniques compared with nurse- or staff-administered intravenous analgesia. In addition, the existing literature is equivocal regarding the comparative efficacy of epidural PCA versus intravenous PCA techniques.
Local anesthetic infiltration
Pain after cardiac surgery is often related to median sternotomy (peaking during the first 2 postoperative days). Because of problems associated with traditional intravenous opioid analgesia (nausea and vomiting, pruritus, urinary retention, respiratory depression) and the more recently introduced nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase (COX) inhibitors (gastrointestinal bleeding, renal dysfunction), alternative methods of achieving postoperative analgesia in cardiac surgical patients have been sought. One such alternative method that may hold promise is continuous infusion of local anesthetic (Box 38-3). In a prospective, randomized, placebo-controlled, double-blind clinical trial, White et al30 studied 36 patients undergoing cardiac surgery. Intraoperative management was standardized. All patients had two indwelling infusion catheters placed at the median sternotomy incision site at the end of surgery (one in the subfascial plane above the sternum, one above the fascia in the subcutaneous tissue). Patients received 0.25% bupivacaine (n = 12), 0.5% bupivacaine (n = 12), or normal saline (n = 12) via a constant-rate infusion through the catheter (4 mL/hr) for 48 hours after surgery. Average times to tracheal extubation were similar in the three groups (approximately 5 to 6 hours). Compared with the control group (normal saline), there was a statistically significant reduction in verbal rating scale pain scores and intravenous PCA morphine use in the 0.5% bupivacaine group. Patient satisfaction with their pain management was also improved in the 0.5% bupivacaine group (vs. control). However, there were no significant differences in PCA morphine use between the 0.25% bupivacaine and control groups. Although tracheal extubation time and the duration of the intensive care unit (ICU) stay (30 vs. 34 hours, respectively) were not significantly altered, time to ambulation (1 vs. 2 days, respectively) and duration of hospital stay (4.2 vs. 5.7 days, respectively) were lower in the 0.5% bupivacaine group than in the control group.
Another clinical investigation revealed the potential benefits of using a continuous infusion of local anesthetic in patients after cardiac surgery.31 In this prospective, randomized, placebo-controlled, double-blind clinical trial, Dowling et al31 studied 35 healthy patients undergoing cardiac surgery. Patients undergoing elective CABG via median sternotomy were randomized to either “ropivacaine” or “placebo” groups. At the end of the operation, before wound closure, bilateral intercostal nerve injections from T1 to T12 were performed using 20 mL of either 0.2% ropivacaine or normal saline. After sternal reapproximation with wires, two catheters with multiple side openings were placed anterior to the sternum (Figure 38-1). These catheters were connected to a pressurized elastomeric pump containing a flow regulator, which allowed for delivery of 0.2% ropivacaine or normal saline at approximately 4 mL/hr. The intraoperative anesthetic technique was standardized (short-acting anesthetics were used to minimize the presence of residual anesthetic agents in the postoperative period), as was postoperative pain management via intravenous PCA morphine (for 72 hours).
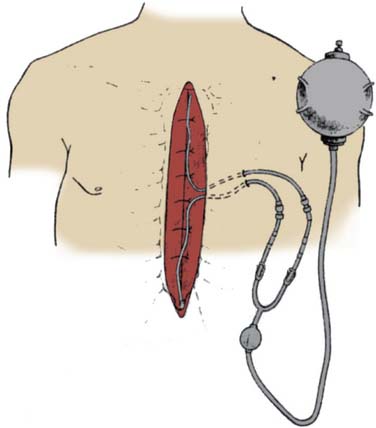
Figure 38-1 Intraoperative placement of the pressurized elastomeric pump and catheters.
(From Dowling R, Thielmeier K, Ghaly A, et al: Improved pain control after cardiac surgery: Results of a randomized, double-blind, clinical trial. J Thorac Cardiovasc Surg 126:1271, 2003.)
The management of postoperative pain with continuous direct infusion of local anesthetic into the surgical wound has been described after a wide variety of surgeries other than cardiac (inguinal hernia repair, upper abdominal surgery, laparoscopic nephrectomy, cholecystectomy, knee arthroplasty, shoulder surgery, and gynecologic operative laparoscopy).32 The infusion pump systems used for anesthetic wound perfusion are regulated by the U.S. Food and Drug Administration as medical devices. Thus, adverse events involving these infusion pump systems during direct local anesthetic infusion into surgical wounds are reported to this organization.
Complications encountered with these infusion pump systems reported to the U.S. Food and Drug Administration include tissue necrosis, surgical wound infection, and cellulitis after orthopedic, gastrointestinal, podiatric, and other surgeries. None of these reported adverse events has involved patients undergoing cardiac surgery. The most commonly reported complication is tissue necrosis, an adverse event almost never seen after normal surgical procedures. Furthermore, consequences of these reported adverse events were typically severe and required intervention and additional medical and/or surgical treatment. Although these initial reports may be isolated incidents, they may also represent an early warning that is representative of a problem that is widespread. Nevertheless, these reports provide a potentially important signal, suggesting the need for further investigation into the relation between use of these infusion pumps for direct continuous infusion of local anesthetics and other drugs into surgical wounds and tissue necrosis, serious infections, or cellulitis. Neither of the two clinical investigations involving local anesthetic infusion in patients after cardiac surgery with median sternotomy reported such wound complications.30,31 Regardless, these safety issues merit careful consideration because of the importance of sternal wound complications in this setting.
The anterior and posterior branches of the intercostal nerves innervate the sternum. Parasternal infiltration of local anesthetic, therefore, is a possible means of improving postoperative analgesia. Although the use of parasternal blocks has not been extensively investigated, one small, prospective, randomized, placebo-controlled, double-blind clinical study indicated that parasternal block and local anesthetic infiltration of the sternotomy wound and mediastinal tube sites with local anesthetic may be a useful analgesic adjunct for patients who are expected to undergo early tracheal extubation after cardiac surgery.33
Nerve blocks
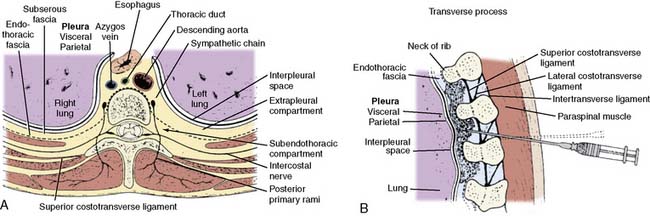
With the increasing popularity of minimally invasive cardiac surgery, which uses nonsternotomy incisions (minithoracotomy), the use of nerve blocks for the management of postoperative pain has increased as well34–39 (Box 38-4). Thoracotomy incisions (transverse anterolateral minithoracotomy, vertical anterolateral minithoracotomy), because of costal cartilage trauma tissue damage to ribs, muscles, or peripheral nerves, may induce more intense postoperative pain than that resulting from median sternotomy. Adequate analgesia after thoracotomy is important because pain is a key component in alteration of lung function after thoracic surgery. Uncontrolled pain causes a reduction in respiratory mechanics, reduced mobility, and increases in hormonal and metabolic activity. Perioperative deterioration in respiratory mechanics may lead to pulmonary complications and hypoxemia, which may, in turn, lead to myocardial ischemia/infarction, cerebrovascular accidents, thromboembolism, and delayed wound healing, leading to increased morbidity and prolonged hospital stay. Various analgesic techniques have been developed to treat postoperative thoracotomy pain. The most commonly used techniques include intercostal nerve blocks, intrapleural administration of local anesthetics, and thoracic paravertebral blocks. Intrathecal techniques and epidural techniques also are effective in controlling postthoracotomy pain and are covered in detail later in this chapter.
Intercostal nerve blocks have been used extensively for analgesia after thoracic surgery.34–36 They can be performed either intraoperatively or postoperatively and usually provide sufficient analgesia lasting approximately 6 to 12 hours (depending on amount and type of local anesthetic used) and may need to be repeated if additional analgesia is required. Local anesthetics may be administered as a single treatment under direct vision, before chest closure, as a single preoperative percutaneous injection, as multiple percutaneous serial injections, or via an indwelling intercostal catheter. Blockade of intercostal nerves interrupts C-fiber afferent transmission of impulses to the spinal cord. A single intercostal injection of a long-acting local anesthetic can provide pain relief and improve pulmonary function in patients after thoracic surgery for up to 6 hours. A continuous extrapleural intercostal nerve block technique may be used in which a catheter is placed percutaneously into an extrapleural pocket by the surgeon to achieve longer duration of analgesia. A continuous intercostal catheter allows frequent dosing or infusions of local anesthetic agents and avoids multiple needle injections. Various clinical studies have confirmed the analgesic efficacy of this technique, and the technique compares favorably with thoracic epidural analgesic techniques.34 A major concern associated with intercostal nerve block is the potentially high amount of local anesthetic systemic absorption, yet multiple clinical studies involving patients undergoing thoracic surgery have documented safe blood levels with standard techniques. Clinical investigations involving patients undergoing thoracic surgery indicate that intercostal nerve blockade by intermittent or continuous infusion of 0.5% bupivacaine with epinephrine is an effective method, as is continuous infusion of 0.25% bupivacaine through indwelling intercostal catheters for supplementing systemic intravenous opioid analgesia for postthoracotomy pain. The value of single preclosure injections remains doubtful.
Intrapleural administration of local anesthetics initiates analgesia via mechanisms that remain incompletely understood. However, the mechanism of action of extrapleural regional anesthesia seems to depend primarily on diffusion of the local anesthetic into the paravertebral region. Local anesthetic agents then affect not only the ventral nerve root but also afferent fibers of the posterior primary ramus. Posterior ligaments of the posterior primary ramus innervate posterior spinal muscles and skin and are traumatized during posterolateral thoracotomy. Intrapleural administration of local anesthetic agent to this region through a catheter inserted in the extrapleural space thus creates an anesthetic region in the skin. The depth and width of the anesthetic region depend on diffusion of the local anesthetic agent in the extrapleural space. With this technique, local anesthetics may be administered via an indwelling intrapleural catheter placed between the parietal and visceral pleura by intermittent or continuous infusion regimens. Concerns regarding systemic absorption of local anesthetic and toxicity are always a concern with this technique, yet have not been substantiated in clinical studies that assayed plasma levels. A handful of clinical investigations involving patients undergoing thoracic surgery via thoracotomy incision suggests that 0.25% to 0.5% bupivacaine may improve analgesia in patients after thoracic surgery, yet its true efficacy as a postoperative analgesic in this patient population remains somewhat controversial.37 The analgesic benefits are of short duration and there does not appear to be a significant overall opioid-sparing effect. Furthermore, the optimum concentration and duration regimen remains to be defined. However, a prospective, randomized, clinical study involving 50 patients undergoing minimally invasive direct CABG (via minithoracotomy) indicated that an intrapleural analgesic technique (with 0.25% bupivacaine) is safe, effective, and compares favorably (provided superior postoperative analgesia) with a conventional thoracic epidural technique.38 These investigators noted, however, that careful catheter positioning, chest tube clamping, and anchoring of the catheter are mandatory for postoperative intrapleural analgesia to be effective. A major factor implicated in lack of efficacy regarding intrapleural techniques is loss of local anesthetic solution through intercostal chest drainage tubes. Although clamping the chest tubes during the postoperative period will increase analgesic efficacy, it may not be safe to clamp chest tubes because they provide important drainage of hemorrhage and air and allow for enhanced lung patency and expansion. Apart from proper catheter positioning (insertion of catheter under direct vision and anchoring catheter to skin are essential), effective analgesia with this technique also appears to depend on whether lung surgery is performed or whether the pleural anatomy and physiology are relatively intact.
Thoracic paravertebral block involves injection of local anesthetic adjacent to the thoracic vertebrae close to where the spinal nerves emerge from the intervertebral foramina (Figure 38-2). Thoracic paravertebral block, compared with thoracic epidural analgesic techniques, appears to provide equivalent analgesia, is technically easier, and may harbor less risk. Several different techniques exist for successful thoracic paravertebral block and recently have been extensively reviewed.35 The classic technique, most commonly used, involves eliciting loss of resistance. Injection of local anesthetic results in ipsilateral somatic and sympathetic nerve blockade in multiple contiguous thoracic dermatomes above and below the site of injection (together with possible suppression of the neuroendocrine stress response to surgery). These blocks may be effective in alleviating acute and chronic pain of unilateral origin from the chest, abdomen, or both. Bilateral use of thoracic paravertebral block also has been described. Continuous thoracic paravertebral infusion of local anesthetic via a catheter placed under direct vision at thoracotomy is also a safe, simple, and effective method of providing analgesia after thoracotomy. It is usually used in conjunction with adjunct intravenous medications (opioid or other analgesics) to provide optimum relief after thoracotomy.
Although supplemental intravenous analgesics are usually required, opioid requirements are substantially reduced. Unilateral paravertebral block is useful for attaining post-thoracotomy analgesia because pain after lateral thoracotomy is essentially always unilateral. The role of bilateral thoracic paravertebral block remains to be defined. The benefits of unilateral paravertebral blockade are a lesser incidence of adverse events (hypotension, urinary retention) and a decreased risk for systemic local anesthetic toxicity because less local anesthetic is used. Few clinical investigations involve unilateral paravertebral block in patients undergoing thoracic surgery. Therefore, it is not possible to determine from the available literature whether the technique of paravertebral blockade (single injection) is truly useful in the postoperative analgesic management of patients after thoracotomy. However, continuous thoracic paravertebral block, as part of a balanced analgesic regimen, may provide effective pain relief with few adverse effects after thoracotomy and appears to be comparable with thoracic epidural analgesia.35
For a wide variety of reasons (increased use of small thoracic incisions by cardiac surgeons, etc.), the last decade has seen a resurgence of nerve blocks (usually catheter-based techniques) in patients undergoing cardiac surgery. Specifically, recent clinical studies using intercostal catheters,40 intrapleural catheters,41,42 and paravertebral blockade43,44 indicate that these techniques may have unique advantages, even when compared with traditional intrathecal/epidural techniques.45–47
Opioids
Beginning in the 1960s (and continuing for essentially 30 years), large doses of intravenous opioids (starting with morphine) have been administered to patients undergoing cardiac surgery48,49 (Box 38-5). Because even very large amounts of intravenous opioids do not initiate “complete anesthesia” (unconsciousness, muscle relaxation, suppression of reflex responses to noxious surgical stimuli), other intravenous/inhalation agents must be administered during the intraoperative period.50 Analgesia is the best known and most extensively investigated opioid effect, yet opioids also are involved in a diverse array of other physiologic functions, including control of pituitary and adrenal medulla hormone release and activity, control of cardiovascular and gastrointestinal function, and in the regulation of respiration, mood, appetite, thirst, cell growth, and the immune system.51 A number of well-known and potential side effects of opioids (nausea and vomiting, pruritus, urinary retention, respiratory depression) may limit postoperative recovery when they are used for postoperative analgesia.
Fentanyl is rapidly transferred across the blood–brain barrier, resulting in a rapid onset of action after intravenous injection. The relative potential for entering the CNS is approximately 150 times greater for fentanyl than for morphine. However, the large quantities of fentanyl taken up by adipose tissues may act as a reservoir (depending on dosage amounts) that slowly releases fentanyl back into the circulation when plasma concentrations decline to less than that in fat. This slow reentry may serve to maintain the plasma concentration and is one factor in the relatively long plasma terminal elimination half-life of fentanyl. Fentanyl is rapidly and extensively metabolized by the liver to inactive metabolites. After bolus intravenous injection, plasma fentanyl concentrations decrease rapidly because of distribution from the plasma to tissues, so that after moderate (10 μg/kg) doses, fentanyl has a short duration of action (see Chapter 9).
The popularity of fentanyl as an intraoperative analgesic agent relates directly to the cardiovascular stability it provides, even in critically ill patients. Also, its analgesic efficacy relative to the intensity of side effects has prompted much interest in its use as an analgesic after surgery and/or in critically ill patients.52 Fentanyl (as well as any opioid) can be administered intravenously for postoperative analgesia in many ways: using a loading dose with a continuous fixed or variable infusion, a fixed background infusion with PCA, or PCA alone. An intravenous bolus of 1 to 2 μg/kg usually is administered before initiating an infusion. If variable, the infusion rate is usually 1 to 2 μg/kg/hr and may be adjusted upward or downward as required by fluctuations in analgesic requirements or appearance of side effects. Before the infusion rate is increased, small intravenous bolus doses of fentanyl may be administered. Infusion rates of 1.5 to 2.5 μg/kg/hr usually provide good-to-excellent postoperative analgesia. At rest, the quality of analgesia remains stable; however, with movement, analgesia may not be sufficient, even with greater infusion rates.
The performance of a patient-demand, target-controlled alfentanil infusion system has compared favorably with traditional morphine PCA in patients after cardiac surgery.53 Checketts et al53 prospectively randomized 120 patients undergoing elective cardiac surgery to receive either morphine PCA or alfentanil PCA for postoperative analgesia (nonblinded). All patients received a similar standardized intraoperative anesthetic technique and were extubated during the immediate postoperative period. Overall median visual analog pain scores were significantly lower in patients receiving alfentanil, yet both alfentanil and morphine delivered high-quality postoperative analgesia (Figure 38-3). Although the clinical impression by these investigators was that alfentanil patients were less sedated in the immediate postoperative period, this clinical observation was not substantiated after statistical analysis of sedation scores. The two groups did not differ with respect to overall sedation scores, frequency of nausea and vomiting, hemodynamic instability, myocardial ischemia, or hypoxemia during the immediate postoperative period.
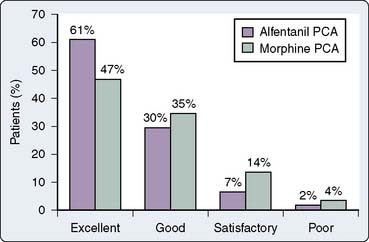
Figure 38-3 Overall patient satisfaction with postoperative analgesia.
(From Checketts MR, Gilhooly CJ, Kenny GNC: Patient-maintained analgesia with target-controlled alfentanil infusion after cardiac surgery: A comparison with morphine PCA. Br J Anaesth 80:748, 1998 ©The Board of Management and Trustees of the British Journal of Anaesthesia, by permission of Oxford University Press/British Journal of Anaesthesia.)
In 1996, Bowdle et al54 evaluated the use of a remifentanil infusion to provide postoperative analgesia during recovery from total intravenous anesthesia with remifentanil and propofol from a wide variety of noncardiac surgeries (abdominal, spine, joint replacement, thoracic surgery). This multi-institutional study involving 157 patients had a detailed protocol that specified doses and method of administration of all anesthetic drugs. In essence, total intraoperative intravenous anesthesia consisted of midazolam (premedication only), remifentanil, propofol, and vecuronium. Propofol was stopped immediately before intraoperative extubation, and the remifentanil infusion was continued for postoperative analgesia. During the immediate postoperative period, intravenous morphine was administered during tapering of remifentanil infusion. Adverse respiratory events (oxygen saturation via pulse oximetry < 90%, respiratory rate less than 12 per minute, apnea) affected 45 patients (29%, 2 required naloxone). Apnea occurred in 11 patients (7% treated with mask ventilation and downward titration of remifentanil infusion; 1 required naloxone). The administration of a bolus of remifentanil preceded the onset of adverse respiratory events in 19 of 45 cases and in 9 of 11 cases of apnea.
These data suggest that remifentanil boluses plus an infusion are particularly likely to produce clinically significant adverse respiratory events. The authors of this open, dose-ranging study concluded that although remifentanil certainly initiates analgesia, its use in the immediate postoperative period may pose dangers. Additional studies are needed to investigate the transition from remifentanil to longer-lasting analgesics and to refine strategies that minimize respiratory depression whereas optimizing pain control. The administration of a potent, rapidly acting opioid such as remifentanil by continuous infusion for postoperative analgesia must be performed with meticulous attention to detail and constant vigilance. Extreme caution should be exercised in the postoperative administration of bolus doses of remifentanil because substantial respiratory depression (including apnea) may develop. Furthermore, the remifentanil infusion should be inserted into the intravenous line as close as possible to the patient to minimize dead space, and the rate of the main intravenous infusion should be controlled at a rate that is high enough to continuously flush remifentanil from the tubing. A more dilute remifentanil solution that would run at greater rates (on a volume per time basis) would help to minimize the effect of variations in flow rate of the main intravenous tubing on delivery of remifentanil to the patient. Remifentanil also may possess detrimental cardiovascular effects via bradycardia and decreases in systemic vascular resistance, leading to decreased cardiac output and hypotension.55 Such changes may occur during clinically utilized doses for cardiac surgery (0.1 to 1.0 μg/kg/min), inducing significant cardiovascular disturbances that are potentially deleterious to patients with cardiac disease.55
Patient-controlled analgesia
When intravenous opioids are used for controlling postoperative pain (most commonly morphine and fentanyl), PCA technology generally is used. Essentials in the successful use of PCA technology include “loading” the patient with intravenous opioids to the point of patient comfort before initiating PCA, ensuring that the patient wants to control analgesic treatment, using an appropriate PCA dose and lockout interval, and considering the use of a basal rate infusion. Focused guidance of PCA dosing by a dedicated acute pain service, compared with surgeon-directed PCA, may result in more effective analgesia with fewer adverse effects. Patient-controlled epidural analgesic techniques, with opioids and/or local anesthetics, also have been proved reliable, effective, and safe.56,57
Although PCA is a well-established technique (used for more than two decades) and offers potential unique benefits (reliable analgesic effect, improved patient autonomy, flexible adjustment to individual needs, etc.), whether it truly offers significant clinical advantages (compared with traditional nurse-administrated analgesic techniques) to patients immediately after cardiac surgery remains to be determined.58–63 A clinical investigation by Gust et al59 indicated that PCA techniques provide a higher quality of postoperative analgesia, which may lead to a reduction in postoperative respiratory complications. In this prospective, randomized, clinical investigation involving 120 healthy patients after extubation after elective CABG, patients received either intravenous PCA piritramide, intravenous PCA piritramide plus rectal indomethacin, or conventional nurse-controlled analgesia with intravenous piritramide and/or rectal indomethacin for 3 days. Postoperative assessment included daily visual analog pain scoring and chest radiographs graded for the extent of atelectasis by a radiologist blinded to treatment. Perioperative management (surgical treatment, intraoperative anesthetic management) was standardized. Although chest radiography atelectasis scores and visual analog pain score values were similar among the three groups on the first and second postoperative days, on the third postoperative day, chest radiography atelectasis scores and visual analog pain score values were significantly better in the two PCA groups compared with the control (nurse-controlled analgesia) group.
At the end of the study, all patients retrospectively graded their postoperative pain management on average as good, but significantly more patients in the two PCA groups assessed their pain management as excellent compared with the control group. These investigators concluded that treatment with PCA may reduce respiratory complications in patients after CABG. However, no difference was observed regarding perioperative oxygenation values among the three groups during the entire study period, and not a single patient in any group met prospectively defined criteria for diagnosis of pneumonia. Other clinical investigations also have indicated that PCA techniques, compared with standard nurse-based pain therapy, may provide higher quality analgesia leading to reduced cardiopulmonary morbidity after cardiac surgery.60,63
Despite the popularity of PCA techniques and the results of the earlier quoted studies, other clinical investigations demonstrated no major benefits offered.58,61,62 Tsang and Brush58 prospectively evaluated 69 patients after cardiac surgery via median sternotomy. Thirty-nine were randomized to receive PCA morphine after surgery, whereas 30 were randomized to receive nurse-administered morphine after surgery. Perioperative care was standardized, visual analog pain scores were used for pain assessment, and pulmonary function tests were performed before surgery and every 6 hours after surgery until discharge from the ICU. These clinical investigators found no difference between the two groups regarding postoperative morphine consumption (Figure 38-4), postoperative visual analog pain scores, postoperative sedation scores, and postoperative pulmonary function (Figure 38-5). These investigators concluded that there are no significant advantages achieved when using PCA routinely in patients after cardiac surgery. Interestingly, in this study, opinions expressed by the nursing staff on the use of PCA were not as positive as expected (repetition of PCA instruction to patients was often required during the study period). Potential reasons for required repeated instructions on PCA include poor retention of preoperative learning because of anxiety after hospital admission, incomplete recovery of higher cognitive function after prolonged general anesthesia and CPB, and/or ICU-induced disorientation.
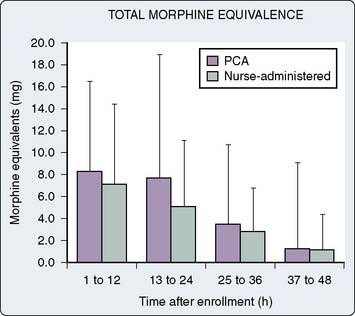
Figure 38-4 Total morphine equivalents.
(From Tsang J, Brush B: Patient-controlled analgesia in postoperative cardiac surgery. Anaesth Intensive Care 27:464, 1999.)
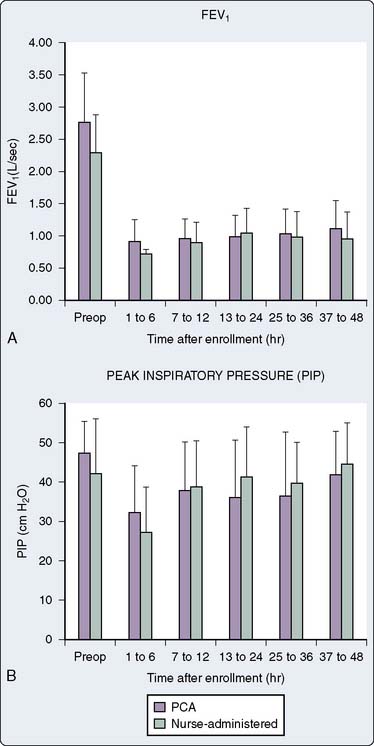
Figure 38-5 Postoperative pulmonary function.
(From Tsang J, Brush B: Patient-controlled analgesia in postoperative cardiac surgery. Anaesth Intensive Care 27:464, 1999.)
These results suggest that there may be additional patient limitations to the effective use of PCA immediately after cardiac surgery even though patients can obey simple commands and acknowledge discomfort. Munro and associates,61 when comparing intravenous PCA morphine and nurse-administered subcutaneous morphine, detailed similar findings. They prospectively randomized 92 patients undergoing elective cardiac surgery to receive either intravenous PCA morphine or nurse-administered subcutaneous morphine during the postoperative period. They found no differences between the two groups regarding many postoperative variables, including total postoperative morphine requirements, postoperative visual analog pain scores at rest and with movement, daily verbal pain relief scores, side effect profiles, and physiotherapist’s evaluation of effectiveness of analgesia for chest physiotherapy. Subcutaneous techniques are attractive because they have low equipment and disposable costs, eliminate the need for bulky pumps in ambulating patients, and may be more effective for the elderly or mildly confused postoperative patient.
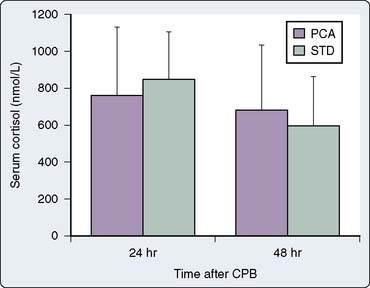
Myles et al62 also were unable to find any specific clinical benefits during use of PCA techniques in patients after cardiac surgery. In their prospective clinical investigation, 72 patients undergoing elective cardiac surgery were randomized to receive either intravenous PCA morphine or intravenous nurse-titrated morphine during the immediate postoperative period. They found no differences between the two groups regarding many postoperative variables, including postoperative morphine consumption, postoperative pain scores, postoperative nausea scores, and postoperative serum cortisol levels (Figure 38-6). Much like Munro and associates, they noted that patients also had variable ability and understanding of the requirements of PCA, particularly in the early postoperative period when they were confused or too weak to operate the demand button. These investigators also noted that overall pain management in their patients was optimized by receiving experienced one-to-one nursing care (other studies evaluating PCA also have found that nurse-administered techniques may provide the highest quality analgesia). It could, therefore, be argued that these studies support increased staff education and involvement to optimize postoperative analgesia. The nurses in these clinical investigations all raised concerns regarding the time required for PCA setup and the inability of patients to cope with the demands of PCA in the early stages of their recovery, particularly if elderly, confused, or both. However, PCA was well received later in the recovery process and was found to be less demanding on nursing time.
Nonsteroidal anti-inflammatory agents
NSAIDs, in contrast with the opioids’ CNS mechanism of action, mainly exert their analgesic, antipyretic, and anti-inflammatory effects peripherally by interfering with prostaglandin synthesis after tissue injury64,65 (Box 38-6). NSAIDs inhibit COX, the enzyme responsible for the conversion of arachidonic acid to prostaglandin (Figure 38-7). Combining NSAIDs with traditional intravenous opioids may allow a patient to achieve an adequate level of analgesia with fewer side effects than if a similar level of analgesia was obtained with intravenous opioids alone. Numerous clinical investigations reveal the potential value (opioid-sparing effects) of NSAIDs when combined with traditional intravenous opioids during the postoperative period after noncardiac surgery. In fact, the administration of NSAIDs is one of the most common nonopioid analgesic techniques currently used for postoperative pain management. The efficacy of NSAIDs for postoperative pain has been demonstrated repeatedly in many analgesic clinical trials. Unlike opioids, which preferentially reduce spontaneous postoperative pain, NSAIDs have comparable efficacy for both spontaneous and movement-evoked pain, the latter of which may be more important in causing postoperative physiologic impairment. Certainly, NSAIDs reduce postoperative opioid consumption, accelerate postoperative recovery, and represent an integral component of balanced postoperative analgesic regimens after noncardiac surgery. However, little is known regarding NSAID use in the management of pain after cardiac surgery. It is likely that concerns regarding NSAID side effects, including alterations in the gastric mucosal barrier, renal tubular function, and inhibition of platelet aggregation, have made clinicians reluctant to use NSAIDs in patients undergoing cardiac surgery. Other rare adverse effects of NSAIDs (from COX inhibition) include hepatocellular injury, asthma exacerbation, anaphylactoid reactions, tinnitus, and urticaria. Despite these fears, a small number of clinical investigations seem to indicate that NSAIDs may provide analgesia in patients after cardiac surgery without untoward effects (gastrointestinal ulceration, renal dysfunction, excessive bleeding). Although NSAIDs have been associated with reports of increased postoperative blood loss, other studies have failed to corroborate this.
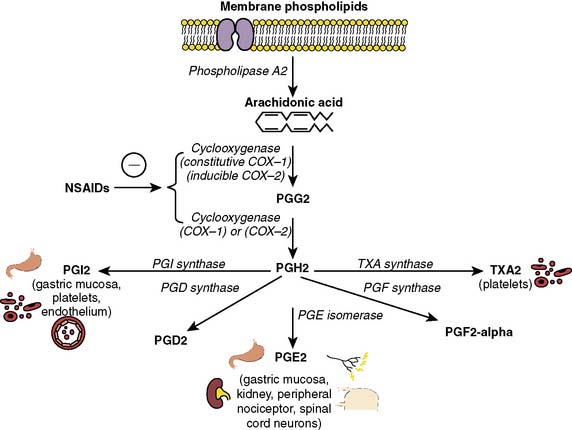
Figure 38-7 The role of cyclooxygenase in prostaglandin (PG) synthesis.
(From Gilron I, Milne B, Hong M: Cyclooxygenase-2 inhibitors in postoperative pain management: Current evidence and future directions [review article]. Anesthesiology 99:1198, 2003.)
NSAIDs are not a homogenous group and vary considerably in analgesic efficacy as a result of differences in pharmacodynamic and pharmacokinetic parameters. NSAIDs are nonspecific inhibitors of COX, which is the rate-limiting enzyme involved in the synthesis of prostaglandins. A major scientific discovery revealed that COX exists in multiple forms. Most important, a constitutive form is present in normal conditions in healthy cells (COX-1) and an inducible form (COX-2) exists, which is the major isozyme induced by and associated with inflammation. Simplistically, COX-1 is ubiquitously and constitutively expressed, and has a homeostatic role in platelet aggregation, gastrointestinal mucosal integrity, and renal function, whereas COX-2 is inducible and expressed mainly at sites of injury (and kidney and brain) and mediates pain and inflammation. NSAIDs are nonspecific inhibitors of both forms of COX, yet vary in their ratio of COX-1 to COX-2 inhibition. Recent molecular studies distinguishing between constitutive COX-1 and inflammation-inducible COX-2 enzymes have led to the exciting hypothesis that the therapeutic and adverse effects of NSAIDs could be uncoupled66–71 (Figure 38-8). Subsequently, over the past ten years, clinicians have witnessed an exponential increase in publications and the growing use of COX-2 inhibitors in the perioperative period after noncardiac surgery. A compelling body of evidence now exists that COX-2 inhibitors, like their predecessors the nonselective NSAIDs, in general provide postoperative analgesia, decrease intravenous opioid requirements, and provide greater patient satisfaction compared with placebo. There is also some evidence that opioid sparing by COX-2 inhibitors also spares opioid side effects. The primary advantage of COX-2 inhibitors, compared with NSAIDs, is their lack of effect on platelet function and bleeding, hence the opportunity for perioperative administration.
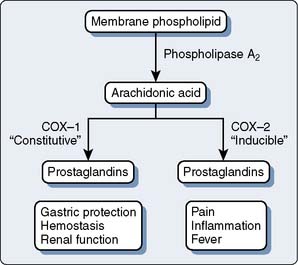
Figure 38-8 Cyclooxygenase (COX) pathways.
(From Gajraj NM: Cyclooxygenase-2 inhibitors [review article]. Anesth Analg 96:1720, 2003.)
Only a handful of clinical studies have investigated the potential of NSAIDs in the management of postoperative pain after cardiac surgery.72–75 One well-designed clinical investigation showed that a combination of NSAID and intravenous opioid may provide superior analgesia after cardiac surgery without untoward effects. Rapanos et al74 prospectively randomized 57 patients to receive either indomethacin suppositories or placebo suppositories in a double-blinded fashion during the immediate postoperative period after elective CABG. Patients receiving indomethacin suppositories demonstrated significantly less (P = 0.019) morphine consumption (assessed via PCA morphine) and significantly lower (P = 0.006) pain scores (assessed via visual analog pain scores) during the immediate postoperative period compared with controls. Postoperative morphine use during the first 24 postoperative hours was 22.40 ± 12.55 mg in the indomethacin group and 35.99 ± 25.84 mg in the placebo group. There were no differences between groups regarding tracheal extubation time or postoperative blood loss (assessed via chest tube output). None of the study subjects (either group) developed postoperative renal dysfunction. In fact, a moderate reduction in serum creatinine concentration was observed in both groups. These investigators concluded that the combination of indomethacin suppositories with morphine after cardiac surgery results in reduced postoperative pain scores and opioid consumption without an increase in side effects.
However, two well-designed clinical investigations demonstrated that the use of NSAIDs or NSAID-like drugs (acetaminophen) in patients after cardiac surgery may not offer any substantial clinical benefits.72,75 Hynninen et al,72 in a prospective, double-blind, placebo-controlled study, randomized patients to receive either diclofenac (n = 28 patients), ketoprofen (n = 28 patients), indomethacin (n = 27 patients), or placebo (n = 31 patients) for postoperative analgesia after elective CABG via a median sternotomy. All patients received standardized fast-track cardiac anesthesia and standardized postoperative analgesia treatment. Mean morphine consumption in the immediate postoperative period was significantly reduced only in the diclofenac group when compared with placebo (12.4 vs. 19.0 mg, respectively; P < 0.05). Total analgesic consumption calculated as morphine equivalents was also significantly lower only in the diclofenac group compared with placebo (18.1 vs. 26.5 mg, respectively; P ≤ 0.05). No additional important differences were observed when doses of other analgesics were compared. The visual analog pain scores at rest were comparable among the four groups at all times. Also, there were no postoperative differences among the four groups regarding creatinine concentration, percentage of patients with 20% and greater increases in creatinine level after surgery, and 24-hour blood loss. These findings indicate that although some NSAIDs may offer opioid-sparing effects, others may not.
Lahtinen et al,75 in a prospective, double-blind, placebo-controlled study, randomized patients to receive either propacetamol, a prodrug of acetaminophen (n = 40 patients), or placebo (n = 39 patients) for postoperative analgesia after elective CABG via a median sternotomy. Acetaminophen (not an NSAID) might be a safer nonopioid analgesic in cardiac surgery because it does not depress platelet function or renal function as much as traditional NSAIDs. The mechanism behind the analgesic action of acetaminophen remains unclear. Acetaminophen has only a weak inhibitory influence on peripheral COXs and has no substantial anti-inflammatory activity. Acetaminophen-induced analgesia may be partially centrally mediated, and the peak cerebrospinal fluid concentrations may reflect analgesic actions. Intravenous propacetamol is quickly hydrolyzed to acetaminophen in the bloodstream. In the clinical investigation by Lahtinen et al,75 a standardized intraoperative anesthetic technique was used for all patients, and extubation times were identical between the two groups (approximately 5.3 hours). From the time of extubation, all patients had access to PCA oxycodone using a standardized protocol. The variation of oxycodone consumption was large in both groups, and although postoperative cumulative oxycodone consumption (combined amount administered via PCA and given as rescue doses) was less in the propacetamol group compared with the placebo group, the difference was not statistically significant (123.5 ± 51.3 mg vs. 141.8 ± 57.5 mg, respectively; P = 0.15). Postoperative visual analog pain scores (obtained at rest and during a deep breath) were similar, as well as patients’ satisfaction with analgesia, between the two groups. Furthermore, no differences existed between the two groups regarding postoperative pulmonary function tests (forced expiratory volume in 1 second, peak expiratory volume, forced vital capacity), blood gas analysis, bleeding, renal function tests, and liver function tests. Postoperative nausea and vomiting were the most common adverse events, which occurred with identical frequency in both groups. These investigators concluded that propacetamol neither enhances postoperative opioid-based analgesia in patients after CABG, nor does it decrease cumulative opioid consumption or reduce adverse effects.
One prospective, randomized clinical study investigated the potential advantages and disadvantages of using COX inhibitors in patients after cardiac surgery.76 Immer et al76 prospectively randomized 69 patients scheduled for elective CABG with conventional sternotomy to receive either a COX-2 inhibitor (etodolac), a nonselective COX inhibitor (diclofenac), or a weak opioid (tramadol) for postoperative analgesia. Postoperative pain was assessed via a visual analog scale, perioperative blood samples were obtained for serum creatinine and urea levels, and creatinine clearance was determined on the first postoperative day (before starting study medication) and on the fourth postoperative day (after receiving study medication). In patients with insufficient postoperative analgesia (defined via predetermined visual analog scale score), supplemental subcutaneous morphine was administered. Total morphine consumption and occurrence of nausea were recorded daily. At the doses analyzed by these investigators, etodolac and diclofenac produced slightly better postoperative analgesia (assessed via visual analog scale scores and morphine consumption) with fewer adverse effects (assessed via antiemetic therapy) than tramadol. However, a short-lasting impairment of renal function was found in patients treated with etodolac and diclofenac (assessed via serum creatinine and urea levels; Figures 38-9 and 38-10). However, at hospital discharge, no significant differences existed among the three groups regarding serum creatinine and urea levels (see Figures 38-9 and 38-10). Furthermore, all three groups experienced similar decreases in postoperative creatinine clearance.
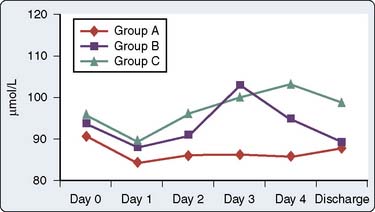
Figure 38-9 Serum creatinine values.
(From Immer FF, Immer-Bansi AS, Trachsel N, et al: Pain treatment with a COX-2 inhibitor after coronary artery bypass operation: A randomized trial. Ann Thorac Surg 75:490, 2003.)
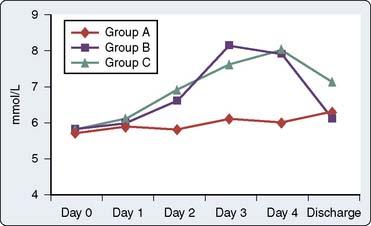
Figure 38-10 Serum urea values.
(From Immer FF, Immer-Bansi AS, Trachsel N, et al: Pain treatment with a COX-2 inhibitor after coronary artery bypass operation: A randomized trial. Ann Thorac Surg 75:490, 2003.)
Another clinical investigation in CABG patients suggested a proportionately, but not significantly, greater incidence of serious cardiac and cerebrovascular adverse events in patients taking COX-2 inhibitors.77 In this multicenter (58 institutions), prospective, randomized, double-blind, parallel-group trial performed by Ott et al,77 462 patients undergoing CABG were allocated at a ratio of 2:1 to parecoxib/valdecoxib (311 patients) or standard care (151 patients; control) groups, respectively. Patients in the parecoxib/valdecoxib group required significantly less morphine or morphine equivalents than patients in the control group during the postoperative period (up to 6 days). Both patients and physicians evaluated the study medication (parecoxib/valdecoxib) as significantly better than control therapy. Pain questionnaires detected significant improvements in the parecoxib/valdecoxib group beginning on day 4 and continuing for at least 4 days. However, although there were no differences between the groups in overall adverse events, serious adverse events occurred twice as frequently in parecoxib/valdecoxib-treated patients than in control patients (19.0% vs. 9.9%, respectively; P = 0.015).
Such thrombosis-mediated complications also merit careful consideration. In cardiac surgery patients exposed to CPB, the delicate balance among platelets, endothelial cells, and serum clotting factors is disturbed, with consequent thrombosis and clot lysis occurring disparately and unpredictably throughout the vascular system. Given that COX-2 inhibitors are platelet sparing, they might tip the balance toward thrombosis during periods of platelet activation. In addition, because COX-1 is unaffected, consequent release of thromboxane A2 may further promote platelet activation and thrombosis. Of note, some analyses addressing these issues in chronically treated patients with arthritis indicate a potential association between COX-2 inhibition and thrombogenic events (myocardial infarction, stroke, vascular death).78 Ott et al77 concluded that, in patients undergoing CABG, the COX-2 inhibitor combination of parecoxib/valdecoxib is effective in controlling postoperative analgesia. However, the treatment regimen may be associated with an increased incidence of serious adverse events overall and sternal wound infections in particular. Their study, therefore, raises important concerns requiring a comprehensive evaluation of the potential link between this class of drugs and perioperative complications in a large-scale clinical trial before the COX-2 inhibitors are routinely used in patients undergoing cardiac surgery.
Over the next decade, much more will be learned about COX-2 inhibitors. Their analgesic (opioid-sparing) effects and lack of deleterious effects on coagulation (in contrast with nonselective NSAIDs) certainly are desirable. The evidence to date does not suggest that COX-2 inhibitors provide major advantages over traditional NSAIDs. It is possible that their continued development will lead to specific drugs with a superior therapeutic profile. Many important questions regarding their safety remain to be answered, such as effects on CNS sensitization, perioperative renal function, preemptive analgesia, clinically significant blood loss, the gastrointestinal system, the cardiovascular system, chronic postsurgical pain, bone/wound healing, blood pressure, and peripheral edema, among others. Specifically regarding patients undergoing cardiac surgery, the potential links between this class of drugs and sternal wound infections and thromboembolic complications need to be fully evaluated. Lastly, the recent and unprecedented retraction of more than 20 peer-reviewed articles and abstracts (spanning 15 years) published by a leading investigator in the perioperative use of NSAIDs and COX-2 inhibitors raises important questions as to the potential adverse impact of this investigator’s fraudulent work on the practice of acute postoperative pain management.79 Simply put, such unprecedented retraction forces clinicians to question all that was previously “known” (assumed true) of the advantages/disadvantages of using NSAIDs and COX-2 inhibitors, prompting the need for future clinical analysis studies to address these important issues.
α2-Adrenergic agonists
The α2-adrenergic agonists provide analgesia, sedation, and sympatholysis (Box 38-7). The initial impetus for the use of α2-agonists in anesthesia resulted from astute clinical observations made in patients during intraoperative anesthesia who were receiving clonidine therapy. Soon thereafter, investigators revealed that clonidine substantially reduced anesthetic requirements (minimal alveolar concentration). More recently, dexmedetomidine has undergone extensive clinical evaluation for perioperative use. Dexmedetomidine exerts profound effects on cardiovascular parameters and thus appears to affect its own pharmacokinetics. At high doses, there is marked vasoconstriction, which probably reduces the drug’s volume of distribution. The elimination half-life of dexmedetomidine is 2 to 3 hours.
α2-Adrenergic agonists produce clinically sedative effects via stimulation of α2 receptors in the locus ceruleus and clinically analgesic effects via stimulation of α2 receptors within the locus ceruleus and the spinal cord.80 Evidence exists indicating that α2-agonists enhance the analgesic effects of the opioids via an unknown mechanism of action. Several mechanisms of action have been postulated for the analgesia noted with α2-adrenergic agonists, including supraspinal, ganglionic, spinal, and peripheral mechanisms. Clinically, systemic administration of these agents produces antinociception and sedation, whereas intrathecal administration usually produces only antinociception. Like other adrenergic receptors, the α2-adrenergic agonists demonstrate tolerance after prolonged administration.
As with all clinically used analgesics, the α2-adrenergic agonists possess clinically important side effects that may limit their usefulness. The effects of dexmedetomidine on the respiratory system include a decrease in tidal volume, minimal changes in respiratory rate, and a rightward shift and depression of slope of the carbon dioxide response curve (all of which may cause hypercarbia). However, respiratory depression associated with the drug is usually clinically unimportant even during profound levels of sedation. The effects of dexmedetomidine on the cardiovascular system are many, and in contrast with the respiratory effects, may become clinically important. Physiologic changes include decreased heart rate, decreased systemic vascular resistance, and possibly indirectly decreased myocardial contractility, all potentially leading to decreased cardiac output and decreased blood pressure in susceptible patients. By developing more highly selective α2-adrenergic agonists, it is hoped that these detrimental cardiovascular effects will be minimized while maximizing desired analgesic and sedative properties. Currently, the clinical role of these drugs includes preoperative sedation, an intraoperative adjuvant during anesthesia to reduce sedative and analgesic requirements, and postoperative sedation and analgesia. The potential ability of the α2-adrenergic agonists to reduce and/or prevent perioperative myocardial ischemia, although intriguing, remains to be determined.81
The potential perioperative analgesic benefits of α2-agonists, when administered to patients undergoing cardiac surgery, were demonstrated almost 20 years ago.82 In 1987, Flacke et al,82 in prospective, nonblinded fashion, randomized patients undergoing elective CABG to receive either perioperative oral clonidine supplementation (10 patients) or serve as control patients (10 patients). Outside of oral clonidine supplementation, management of the two study groups was identical. Patients receiving oral clonidine required significantly less preinduction diazepam and significantly less intraoperative sufentanil (Figure 38-11) and isoflurane to maintain intraoperative normotension (clearly establishing clonidine’s sedative/analgesic properties). Furthermore, patients receiving oral clonidine were extubated earlier during the postoperative period compared with control patients (approximately 11 vs. 16 hours, respectively; P < 0.05). However, 4 of 10 patients receiving oral clonidine required atropine for treatment of intraoperative bradycardia. Unfortunately, postoperative analgesia was not assessed in this clinical investigation.
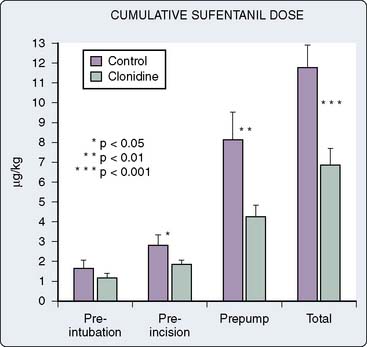
Figure 38-11 Cumulative sufentanil dose.
(From Flacke JW, Bloor BC, Flacke WE, et al: Reduced narcotic requirement by clonidine with improved hemodynamic and adrenergic stability in patients undergoing coronary bypass surgery. Anesthesiology 67:11, 1987.)
Although the analgesic properties of α2-adrenergic agonists are undisputed, most of the clinical investigations regarding perioperative use of this class of drugs remain focused on exploiting the sedative effects and beneficial cardiovascular effects (decreasing hypertension and tachycardia) associated with their use.83–86 α2-Adrenergic agonists have been used perioperatively in patients undergoing cardiac surgery, yet the focus of such clinical investigations has been on the intraoperative period and the potential for enhanced postoperative hemodynamic stability, potentially leading to reduced postoperative myocardial ischemia (not specifically to enhanced postoperative analgesia).87–90 Taken together, these clinical investigations indicated that perioperative administration of α2-adrenergic agonists to patients undergoing cardiac surgery decreases intraoperative anesthetic requirements, may enhance perioperative hemodynamic stability, and may decrease perioperative myocardial ischemia, yet may cause excessive postoperative sedation and aggravate postoperative hemodynamic instability via bradycardia and/or decreased systemic vascular resistance (leading to hypotension and increased pacing requirements in susceptible patients). The potential ability of this class of drugs to initiate reliable postoperative analgesia awaits definitive investigation.
Intrathecal and epidural techniques
It is clear from numerous clinical investigations that intrathecal or epidural techniques, or both (using opioids and/or local anesthetics), initiate reliable postoperative analgesia in patients after cardiac surgery91 (Boxes 38-8 and 38-9). Additional potential advantages of using intrathecal or epidural techniques, or both, in patients undergoing cardiac surgery include stress-response attenuation and thoracic cardiac sympathectomy.
An uninhibited stress response during the postoperative period may lead to many adverse hemodynamic (tachycardia, hypertension, vasoconstriction), metabolic (increased catabolism), immunologic (impaired immune response), and hemostatic (platelet activation) alterations. Intrathecal or epidural anesthesia and analgesia (with local anesthetics or opioids) can effectively inhibit the stress response associated with surgical procedures.27 Local anesthetics appear to possess greater efficacy than opioids in perioperative stress-response attenuation, perhaps because of their unique mechanism of action. Although still a matter of some debate, perioperative stress-response attenuation with epidural local anesthetics and/or opioids in high-risk patients after major noncardiac surgery may decrease morbidity and mortality.6,7,27 In patients undergoing cardiac surgery, initiation of CPB causes significant increases in stress-response hormones that persist into the immediate postoperative period.24–26 Attenuation of this component of the perioperative stress response with intravenous opioids also may decrease morbidity and mortality in these patients.8,9 Unfortunately, perioperative stress-response attenuation in patients undergoing cardiac surgery with intravenous opioids in this manner does not allow tracheal extubation to occur in the immediate postoperative period. Intrathecal or epidural anesthesia and analgesia techniques (particularly with local anesthetics) are attractive alternatives to intravenous opioids in this setting for their potential to attenuate the perioperative stress response, yet still allow tracheal extubation to occur in the immediate postoperative period.
The myocardium and coronary vasculature are densely innervated by sympathetic nerve fibers that arise from T1 to T5 and profoundly influence total coronary blood flow and distribution.92 Cardiac sympathetic nerve activation initiates coronary artery vasoconstriction93 and paradoxic coronary vasoconstriction in response to intrinsic vasodilators.94 In patients with CAD, cardiac sympathetic nerve activation disrupts the normal matching of coronary blood flow and myocardial oxygen demand.95,96 Animal models have revealed an intense poststenotic coronary vasoconstrictive mechanism mediated by cardiac sympathetic nerve activation that attenuates local metabolic coronary vasodilation in response to myocardial ischemia.97,98 Furthermore, myocardial ischemia initiates a cardiocardiac reflex mediated by sympathetic nerve fibers, which augments the ischemic process.99 Cardiac sympathetic nerve activation likely plays a central role in initiating postoperative myocardial ischemia by decreasing myocardial oxygen supply via the mechanisms listed earlier.27,100
Thoracic epidural anesthesia (TEA) with local anesthetics effectively blocks cardiac sympathetic nerve afferent and efferent fibers.27 Opioids, administered similarly, are unable to effectively block such cardiac sympathetic nerve activity.27 Patients with symptomatic CAD benefit clinically from cardiac sympathectomy, and the application of thoracic sympathetic blockade in the management of angina pectoris was described as early as 1965.101 TEA with local anesthetics increases the diameter of stenotic epicardial coronary artery segments without causing dilation of coronary arterioles,95 decreases determinants of myocardial oxygen demand,96 improves left ventricular function,102 and decreases anginal symptoms.96,103 Furthermore, cardiac sympathectomy increases the endocardial-to-epicardial blood flow ratio,104,105 beneficially affects collateral blood flow during myocardial ischemia,105 decreases poststenotic coronary vasoconstriction,98 and attenuates the myocardial ischemia-induced cardiocardiac reflex.98 In an animal model, TEA with local anesthetics actually decreased myocardial infarct size after coronary artery occlusion.104 Of note, these beneficial effects are not caused by systemic absorption of the local anesthetic.104 In short, TEA with local anesthetics may benefit patients undergoing cardiac surgery by effectively blocking cardiac sympathetic nerve activity and improving the myocardial oxygen supply/demand balance.
Intrathecal Techniques
Application of intrathecal analgesia to patients undergoing cardiac surgery was initially reported by Mathews and Abrams in 1980.106 They described the administration of intrathecal morphine (1.5 to 4.0 mg) to 40 adults after the induction of general anesthesia for cardiac surgery. Somewhat remarkably, all 40 patients awakened pain free at the end of surgery (before leaving the operating room), and 36 patients were tracheally extubated before transfer to the ICU. After surgery, all 40 patients were entirely pain free for the first 27.5 postoperative hours, and 17 did not require any supplemental analgesics before discharge from the hospital. Of the 17 patients who received 4.0 mg intrathecal morphine, 11 did not require any postoperative analgesic drugs. Mathews and Abrams106 summarized: “The benefits of recovering from surgery free from pain have been impressive. This has been particularly appreciated by patients who have had previous operations with conventional anesthesia and postoperative analgesic drugs. The patients have been remarkably comfortable, able to move more easily in bed, and more cooperative, thus greatly helping their nursing care.” After this impressive clinical display, other investigators have subsequently applied intrathecal anesthesia and analgesia techniques to patients undergoing cardiac surgery.107–132
Most clinical investigators have used intrathecal morphine in hopes of providing prolonged postoperative analgesia. Some clinical investigators have used intrathecal fentanyl, sufentanil, and/or local anesthetics for intraoperative anesthesia and analgesia (with stress–response attenuation) and/or thoracic cardiac sympathectomy. An anonymous survey of members of the Society of Cardiovascular Anesthesiologists indicated that almost 8% of practicing anesthesiologists incorporate intrathecal techniques into their anesthetic management of adults undergoing cardiac surgery.133 Of these anesthesiologists, 75% practice in the United States, 72% perform the intrathecal injection before induction of anesthesia, 97% use morphine, 13% use fentanyl, 2% use sufentanil, 10% use lidocaine, and 3% use tetracaine.133
Two randomized, blinded, placebo-controlled clinical studies revealed the ability of intrathecal morphine to induce significant postoperative analgesia after cardiac surgery.119,126 In 1988, Vanstrum et al126 prospectively randomized 30 patients to receive either intrathecal morphine (0.5 mg) or intrathecal placebo before induction of anesthesia. Intraoperative anesthetic management was standardized, and after surgery all patients received only intravenous morphine administered by a nurse who attempted to keep the linear analog pain score at less than 4 (a score of 1 represented no pain, 10 represented the worst pain imaginable; the scale was 25 cm long). Although pain scores between groups were not significantly different at any postoperative time interval tested, patients who received intrathecal morphine required significantly less intravenous morphine than placebo controls (2.4 vs. 8.3 mg, respectively; P < 0.02) during the initial 30 hours after intrathecal injection (Figure 38-12). Associated with this enhanced analgesia in patients receiving intrathecal morphine was a substantially decreased need for antihypertensive medications (sodium nitroprusside, nitroglycerin, hydralazine) during the immediate postoperative period. Time to tracheal extubation (approximately 20 hours) and postoperative arterial blood gas tensions after anesthesia were not significantly affected by the use of intrathecal morphine. In 1996, Chaney and associates119 prospectively randomized 60 patients to receive either intrathecal morphine (4.0 mg) or intrathecal placebo before induction of anesthesia for elective CABG. Intraoperative anesthetic management was standardized, and after tracheal extubation, all patients received intravenous morphine via PCA exclusively. The mean time from ICU arrival to tracheal extubation was similar in all patients (approximately 20 hours). However, patients who received intrathecal morphine required significantly less intravenous morphine than placebo controls (33.2 vs. 51.1 mg, respectively; P < 0.05) during the initial postoperative period (Table 38-2). Despite enhanced analgesia, no clinical differences between groups existed regarding postoperative morbidity (pruritus, nausea, vomiting, urinary retention, prolonged somnolence, atrial fibrillation, ventricular tachycardia, myocardial infarction, cerebral infarction), mortality, or duration of postoperative hospital stay (approximately 9 days in each group).
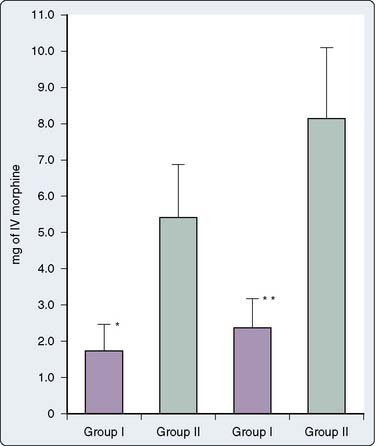
Figure 38-12 Postoperative supplemental intravenous (IV) morphine requirements.
(From Vanstrum GS, Bjornson KM, Ilko R: Postoperative effects of intrathecal morphine in coronary artery bypass surgery. Anesth Analg 67:261, 1988.)
TABLE 38-2 Postoperative Supplemental Intravenous Midazolam and Morphine Requirements
| Group MS (n = 27) | Group NS (n = 29) | |
|---|---|---|
| Midazolam use from ICU arrival to extubation, mg (range) | 8.7 ± 15.8 (0–80) | 8.3 ± 15.4 (0–66) |
| Morphine use from ICU arrival to 8:00 am POD 2, mg (range) | 33.2 ± 15.8 (4–74) | 51.1 ± 45.7 (4–254) |
| Morphine use from 8:00 am POD 2 to 8:00 am POD 3, mg (range) | 14.2 ± 16.4 (0–68) | 12.1 ± 12.6 (0–42) |
Patients receiving intrathecal morphine (group MS) required significantly less supplemental intravenous morphine during the immediate postoperative period compared with patients receiving intrathecal placebo (group NS; 33.2 vs. 51.1 mg, respectively; P < 0.05).
ICU, intensive care unit; POD, postoperative day.
From Chaney MA, Smith KR, Barclay JC, Slogoff S: Large-dose intrathecal morphine for coronary artery bypass grafting. Anesth Analg 83:215, 1996.
The mid-1990s saw the emergence of fast-track cardiac surgery, with the goal being tracheal extubation in the immediate postoperative period. Chaney and associates in 1997118 were the first to study the potential clinical benefits of intrathecal morphine when used in patients undergoing cardiac surgery and early tracheal extubation. They prospectively randomized 40 patients to receive either intrathecal morphine (10 μg/kg) or intrathecal placebo before induction of anesthesia for elective CABG. Intraoperative anesthetic management was standardized (intravenous fentanyl, 20 μg/kg, and intravenous midazolam, 10 mg), and after surgery all patients received intravenous morphine via PCA exclusively. Of the patients who were tracheally extubated during the immediate postoperative period, the mean time from ICU arrival to tracheal extubation was significantly (P = 0.02) prolonged in patients who received intrathecal morphine (10.9 ± 4.4 hours) compared with placebo controls (7.6 ± 2.5 hours). Three patients who received intrathecal morphine had tracheal extubation substantially delayed (12 to 24 hours) because of prolonged ventilatory depression (likely secondary to intrathecal morphine). Although the mean postoperative intravenous morphine use for 48 hours was less in patients who received intrathecal morphine (42.8 mg) compared with patients who received intrathecal placebo (55.0 mg), the difference between groups was not statistically significant. No clinical differences existed between groups regarding postoperative morbidity, mortality, or duration of postoperative hospital stay (approximately 9 days in each group).
These somewhat discouraging findings (absence of enhanced analgesia, prolongation of tracheal extubation time) stimulated the same group of investigators in 1999 to try again, this time decreasing the amount of intraoperative intravenous fentanyl patients received (hoping to decrease the effect of fentanyl on augmenting postoperative respiratory depression associated with intrathecal morphine).116 Forty patients were prospectively randomized to receive either intrathecal morphine (10 μg/kg) or intrathecal placebo before induction of anesthesia for elective CABG. Intraoperative anesthetic management was standardized (intravenous fentanyl, 10 μg/kg, and intravenous midazolam, 200 μg/kg), and after surgery all patients received intravenous morphine exclusively via PCA. Of the patients tracheally extubated during the immediate postoperative period, mean time to tracheal extubation was similar in patients who received intrathecal morphine (6.8 ± 2.8 hours) compared with intrathecal placebo patients (6.5 ± 3.2 hours). However, once again, four patients who received intrathecal morphine had tracheal extubation substantially delayed (14, 14, 18, and 19 hours) because of prolonged respiratory depression (likely secondary to intrathecal morphine). The mean postoperative intravenous morphine use during the immediate postoperative period was actually greater in patients receiving intrathecal morphine (49.8 mg) compared with patients receiving intrathecal placebo (36.2 mg), yet the difference between groups was not statistically significant. No clinical differences existed between groups regarding postoperative morbidity, mortality, or duration of postoperative hospital stay (approximately 6 days in each group). Thus, Chaney and associates, from their three prospective, randomized, double-blind, placebo-controlled, clinical investigations in the late 1990s involving 140 healthy adults undergoing elective CABG, concluded that although intrathecal morphine certainly can initiate reliable postoperative analgesia, its use in the setting of fast-track cardiac surgery and early tracheal extubation may be detrimental by potentially delaying tracheal extubation in the immediate postoperative period.116,118,119
Since this time, however, other clinical investigators have revealed that certain combinations of intraoperative anesthetic techniques, coupled with appropriate doses of intrathecal morphine, will allow tracheal extubation after cardiac surgery within the immediate postoperative period together with enhanced analgesia. Alhashemi et al108 prospectively randomized 50 adults undergoing elective CABG to receive either one of two doses of intrathecal morphine (250 μg or 500 μg) or intrathecal placebo. Intraoperative anesthetic management was standardized (fentanyl, midazolam), and all patients received intermittent morphine by a blinded practitioner during the postoperative period. Tracheal extubation times were similar in the placebo group, 250 μg intrathecal morphine group, and 500 μg intrathecal morphine group (7.3, 5.4, and 6.8 hours, respectively; P = 0.270). However, postoperative morphine requirements in the placebo group (21.3 ± 6.2 mg), 250 μg intrathecal morphine group (13.6 ± 7.8 mg), and the 500 μg intrathecal morphine group (11.7 ± 7.4 mg) were substantially different. There was at least a 36% reduction in postoperative intravenous morphine requirements among those patients who received intrathecal morphine. Although there were no differences in postoperative intravenous morphine requirements between patients randomized to receive either 250 or 500 μg intrathecal morphine, both groups required significantly less intravenous morphine during the immediate postoperative period compared with control patients (p = 0.001). However, despite enhanced analgesia, there were no differences among the study groups in regard to midazolam, nitroglycerin, and sodium nitroprusside requirements in the postoperative period (Table 38-3). Furthermore, postextubation blood gas analysis, use of supplemental inspired oxygen, and ICU length of stay (approximately 22 hours in all groups) were comparable among the three groups.
TABLE 38-3 Analysis of Outcome Measures in Patients Receiving Either Placebo, 250 μg Intrathecal Morphine, or 500 μg Intrathecal Morphine
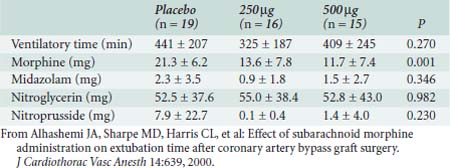
Many other suboptimally designed clinical investigations (retrospective, observational, etc.) attest to the ability of intrathecal morphine to induce substantial postoperative analgesia in patients after cardiac surgery (Table 38-4). Intrathecal doses of 0.5 to 10.0 mg administered before CPB initiate reliable postoperative analgesia, the quality of which depends not only on the intrathecal dose administered but on the type and amount of intravenous analgesics and sedatives used for the intraoperative baseline anesthetic. The optimal dose of intrathecal morphine for achieving the maximum postoperative analgesia with minimum undesirable drug effects is uncertain. Naturally, when larger doses of intrathecal morphine are used, more intense and prolonged postoperative analgesia is obtained at the expense of more undesirable drug effects (nausea and vomiting, pruritus, urinary retention, respiratory depression).
Because of morphine’s low lipid solubility, analgesic effects after intrathecal injection are delayed. Thus, even large doses of intrathecal morphine administered to patients before cardiac surgery will not initiate reliable intraoperative analgesia126–128,131 and, therefore, would not be expected to potentially attenuate the intraoperative stress response associated with CPB. Only an extremely large dose of intrathecal morphine (10.0 mg) may initiate reliable intraoperative analgesia in this setting.130 Only one clinical investigation has examined the ability of intrathecal morphine to potentially attenuate the intraoperative stress response associated with CPB as measured by blood catecholamine levels.119 In Chaney and associates’ clinical investigation,119 patients were prospectively randomized to receive either intrathecal morphine (4.0 mg) or intrathecal placebo before induction of anesthesia for elective CABG with CPB. Intraoperative anesthetic management was standardized, and multiple arterial blood samples were obtained perioperatively to ascertain norepinephrine and epinephrine levels. Patients who were administered intrathecal morphine experienced similar perioperative increases in blood catecholamine levels when compared with placebo controls. Thus, it appears that intrathecal morphine (even in relatively large doses) is unable to reliably attenuate the perioperative stress response associated with cardiac surgery and CPB.
Although intrathecal morphine cannot reliably prevent the perioperative stress response associated with CPB, it may (by initiating postoperative analgesia) potentially attenuate the stress response during the immediate postoperative period.126 Vanstrum et al126 revealed that patients who were administered 0.5 mg intrathecal morphine before the induction of anesthesia not only required significantly less intravenous morphine after surgery compared with placebo control patients, but also required significantly less intravenous nitroprusside (58.1 vs. 89.1 mg, respectively; p < 0.05) during the initial 24 postoperative hours to control hypertension, which suggests partial postoperative stress-response attenuation.
Some clinical investigators have used intrathecal fentanyl, sufentanil, and/or local anesthetics for patients undergoing cardiac surgery, hoping to provide intraoperative anesthesia and analgesia (and stress–response attenuation), with mixed results (see Table 38-4). Administration of intrathecal local anesthetics to patients after the induction of anesthesia for cardiac surgery may help promote intraoperative hemodynamic stability,120,122 whereas intrathecal sufentanil (50 μg) administered before the induction of anesthesia for cardiac surgery can reduce volatile anesthetic requirements during mediastinal dissection but is unable to reliably block intraoperative hemodynamic responses to laryngoscopy and intubation.123
Most clinical attempts at inducing thoracic cardiac sympathectomy in patients undergoing cardiac surgery have used TEA with local anesthetics. However, a small number of clinical investigators have attempted cardiac sympathectomy in this setting with an intrathecal injection of local anesthetic. In 1994, as reviewed retrospectively, 18 adult patients were administered lumbar intrathecal hyperbaric bupivacaine (23 to 30 mg) and/or hyperbaric lidocaine (150 mg) mixed with morphine (0.5 to 1.0 mg) after the induction of anesthesia.122 In an attempt to produce a “total spinal” and, thus, thoracic cardiac sympathectomy, Trendelenburg position was maintained for at least 10 minutes after intrathecal injection. Heart rate decreased significantly (baseline mean 67 beats/min to postinjection mean 52 beats/min) after the intrathecal injection (indicating cardiac sympathectomy was obtained), and not a single patient exhibited ECG evidence of myocardial ischemia before CPB. Although these authors reported that the technique provided stable perioperative hemodynamics, 17 of 18 patients required intravenous phenylephrine at some time intraoperatively to increase blood pressure. In 1996, the same group of investigators reported similar hemodynamic changes in a case report involving a 10-year-old child with Kawasaki disease who underwent CABG and received intrathecal hyperbaric bupivacaine mixed with morphine via a lumbar puncture after induction of anesthesia.120 Although Kowalewski’s group reported that these patients experienced enhanced postoperative analgesia, definite conclusions cannot be reached regarding this technique because of study design formats (retrospective review, case report).
A small (n = 38 patients), prospective, randomized, blinded clinical investigation showed that large doses of intrathecal bupivacaine (37.5 mg) administered to patients immediately before induction of general anesthesia (19 patients received intrathecal bupivacaine, 19 patients served as controls) for elective CABG may potentially initiate intraoperative stress–response attenuation (assessed via serum mediator levels, hemodynamics, and qualitative/quantitative alterations in myocardial β receptors).134 However, no effect on clinical outcome parameters (tracheal extubation times, respiratory function, perioperative spirometry, etc.) was observed. Mean tracheal extubation times (measured from the time of sternotomy dressing application) were extremely short in both groups (11 to 19 minutes). Specifically regarding postoperative analgesia, postoperative pain scores and morphine use via PCA did not differ between the two groups. Not surprisingly, phenylephrine use was more common in patients who received intrathecal bupivacaine compared with control patients.
The many clinical investigations involving the use of intrathecal analgesic techniques in patients undergoing cardiac surgery indicate that the administration of intrathecal morphine to patients before CPB initiates reliable postoperative analgesia after cardiac surgery. Intrathecal opioids or local anesthetics cannot reliably attenuate the perioperative stress response associated with CPB that persists during the immediate postoperative period. Although intrathecal local anesthetics (not opioids) may induce perioperative thoracic cardiac sympathectomy, the hemodynamic changes associated with a “total spinal” make the technique unpalatable in patients with cardiac disease. Indeed, a recently published meta-analysis of randomized, controlled trials (25 randomized trials, 1106 patients) concluded that spinal analgesia does not improve clinically relevant outcomes in patients undergoing cardiac surgery.135
Epidural Techniques
The initial description of TEA and analgesia applied to a cardiac surgical patient occurred in 1954, during the formative years of CPB.136 Clowes et al136 described their presurgical anesthetic technique in a 55-year-old man with severe cardiac failure: “An endotracheal tube was passed with topical anesthesia. Under extradural block of the upper thorax, hypotension developed but responded to the administration of a vasopressor drug. At this time the patient became comatose” (Figure 38-13). The patient eventually died. Application of TEA to patients undergoing cardiac surgery during the modern surgical era was initially reported by Hoar et al in 1976.137 They described the intraoperative insertion of thoracic epidural catheters in 12 patients after CABG (after intravenous protamine, before transfer to ICU). The epidural catheters were injected with lidocaine and bupivacaine during the immediate postoperative period to promote analgesia and effectively control hypertension. Administration of epidural local anesthetics to these patients significantly decreased postoperative blood pressure in hypertensive and normotensive patients, and not a single patient required cardiac or peripheral vascular stimulants during the immediate postoperative study period. The 1987 report by El-Baz and Goldin138 was the first to describe the insertion of thoracic epidural catheters in patients before performance of cardiac surgery. In prospective, randomized fashion, patients undergoing elective CABG received either routine treatment for postoperative pain (n = 30 patients, intravenous morphine) or a continuous infusion of morphine (0.1 mg/hr) via a thoracic epidural catheter (n = 30 patients). Thoracic epidural catheters were inserted at T3-4 immediately before induction of anesthesia on the day of surgery. Intraoperative anesthetic technique was standardized and mean postoperative tracheal extubation time was significantly shorter in patients receiving TEA compared with control patients (9 ± 3 hours vs. 18 ± 5 hours, respectively; P < 0.01).
Figure 38-13 Graph of clinical course of initial cardiac patient receiving presurgical epidural anesthesia in 1954.
(From Clowes GHA, Neville WE, Hopkins A, et al: Factors contributing to success or failure in the use of a pump oxygenator for complete by-pass of the heart and lung: Experimental and clinical. Surgery 36:557, 1954.)
Continuous thoracic epidural infusion of morphine also achieved better postoperative pain relief in patients than intravenous morphine (significantly better pain scores, significantly less supplemental intravenous morphine). Furthermore, in a subgroup of 20 patients (10 per group), postoperative “stress” was assessed via serum cortisol and β-endorphin levels. Patients receiving TEA had significantly lower postoperative levels of these mediators compared with control patients, indicating potential postoperative stress-response attenuation. Continuous thoracic epidural infusion of morphine (compared with controls) also was associated with a lower incidence of opioid-related side effects during the immediate postoperative period. The insertion of the thoracic epidural catheter immediately before systemic heparin administration was not associated with any neurologic problems. Since this initial impressive display of potential benefits (reliable postoperative analgesia, stress-response attenuation, facilitation of early tracheal extubation), other clinical investigators have subsequently applied TEA to patients undergoing cardiac surgery.139–174 Most clinical investigators have used thoracic epidural local anesthetics in hopes of providing perioperative stress-response attenuation and/or perioperative thoracic cardiac sympathectomy. Some clinical investigators have used thoracic epidural opioids to provide intraoperative and/or postoperative analgesia. An anonymous survey of members of the Society of Cardiovascular Anesthesiologists indicated that 7% of practicing anesthesiologists incorporate thoracic epidural techniques into their anesthetic management of adults undergoing cardiac surgery.133 Of these anesthesiologists, 58% practice in the United States. Regarding the timing of epidural instrumentation, 40% perform instrumentation before induction of general anesthesia, 12% perform instrumentation after induction of general anesthesia, 33% perform instrumentation at the end of surgery, and 15% perform instrumentation on the first postoperative day.133
TEA and analgesia with local anesthetics and/or opioids induce significant postoperative analgesia in patients after cardiac surgery. Patients randomized to receive a continuous thoracic epidural morphine infusion (0.1 mg/hr) after cardiac surgery required significantly less postoperative supplemental intravenous morphine compared with patients without thoracic epidural catheters (5 vs. 18 mg/day per patient, respectively; p < 0.05) during the initial 3 postoperative days.138 Children (aged 2 to 12 years) randomized to receive caudal epidural morphine (75 μg/kg) intraoperatively after cardiac surgery required significantly less postoperative supplemental intravenous morphine compared with patients who did not receive epidural morphine (0.32 vs. 0.71 mg/kg, respectively; p < 0.01) during the initial 24 postoperative hours.158 Numerous additional clinical studies further attest to the ability of TEA with local anesthetics and/or opioids to induce substantial postoperative analgesia in patients after cardiac surgery (Table 38-5).
One unique clinical investigation directly compared TEA and intravenous clonidine in patients undergoing cardiac surgery. Loick et al142 prospectively randomized 70 patients undergoing elective CABG to receive TEA supplementation (bupivacaine, sufentanil continuous infusion) perioperatively to general anesthesia (n = 25 patients), to receive intravenous clonidine supplementation (continuous infusion) perioperatively to general anesthesia (n = 24 patients), or to receive only general anesthesia (n = 21 patients, controls). Hemodynamics, plasma epinephrine and norepinephrine levels, plasma cortisol levels, the myocardium-specific contractile protein troponin T levels, and other plasma cardiac enzymes were assessed perioperatively. Both the TEA and intravenous clonidine groups experienced postoperative decreases in heart rate compared with the control group (without jeopardizing cardiac output or perfusion pressure). The effects on stress-response mediators were unpredictable and variable. Electrocardiographic evidence of ischemia (ST-segment elevation, ST-segment depression) occurred in 70% of control patients, 50% of TEA patients, and 40% of intravenous clonidine patients. The release of troponin T was attenuated (compared with control patients) in the TEA group only (no effect in the intravenous clonidine group). Interestingly enough, the highest quality of postoperative analgesia was found in the patients receiving intravenous clonidine. In the intravenous clonidine group, visual analog pain scores were nearly halved when compared with the two other groups. Sedation scores were similar among the three groups, with the exception of the 24-hour value in the intravenous clonidine group, which was greater than that in the TEA group. The postoperative comfort scores (rated between excellent and good) did not differ among the three groups.
Many clinical investigations have proved that TEA with local anesthetics also significantly attenuates the perioperative stress response in patients undergoing cardiac surgery. Patients randomized to receive intermittent boluses of thoracic epidural bupivacaine intraoperatively followed by continuous infusion postoperatively exhibited significantly decreased blood levels of norepinephrine and epinephrine perioperatively when compared with patients managed similarly without thoracic epidural catheters.152 Furthermore, increased blood catecholamine levels in these patients were associated with increased systemic vascular resistance.152 Patients randomized to receive continuous thoracic epidural bupivacaine infusion perioperatively exhibited significantly decreased blood levels of norepinephrine and cortisol perioperatively when compared with patients managed similarly without thoracic epidural catheters.149 Patients randomized to receive a continuous thoracic epidural bupivacaine and sufentanil infusion perioperatively exhibited significantly decreased blood levels of norepinephrine after sternotomy when compared with patients managed similarly without thoracic epidural catheters.155
Other clinical studies further attest to the ability of TEA with local anesthetics to promote perioperative hemodynamic stability in patients undergoing cardiac surgery, which suggests perioperative stress-response attenuation.137,151,152,155 Although most clinical attempts at stress-response attenuation involve thoracic epidural administration of local anesthetics, one investigation indicated that TEA with opioids may significantly attenuate the perioperative stress response in patients undergoing cardiac surgery.138 In this clinical investigation, patients randomized to receive a continuous thoracic epidural morphine infusion after surgery exhibited significantly decreased blood levels of cortisol and β-endorphin after surgery when compared with patients managed similarly without thoracic epidural catheters.138
Two provocative clinical studies demonstrated the ability of TEA to induce significant thoracic cardiac sympathectomy in patients undergoing cardiac surgery.150,151 In the first study, patients undergoing CABG were evaluated with reverse thermodilution catheters that had been inserted into the midcoronary sinus under fluoroscopic guidance before the induction of anesthesia.150 Intraoperative anesthetic management was standardized. Coronary sinus blood flow was measured by a constant-infusion technique, and coronary vascular resistance was calculated using coronary perfusion pressure (arterial diastolic pressure minus pulmonary capillary wedge pressure) and coronary sinus blood flow. Patients who had been randomized to receive intermittent boluses of thoracic epidural bupivacaine intraoperatively followed by a continuous infusion after surgery exhibited significant decreases in coronary vascular resistance after CPB when compared with pre-CPB values, whereas patients managed similarly without thoracic epidural catheters exhibited significant increases in coronary vascular resistance after CPB. In the second study, patients undergoing CABG were evaluated with catheters that had been inserted into the coronary sinus under fluoroscopic guidance and continuous pressure monitoring before the induction of anesthesia.151 Intraoperative anesthetic management was standardized and all patients received a continuous intravenous infusion of tritiated norepinephrine (allowed assessment of cardiac norepinephrine spillover to plasma via isotope dilution technique). Blood samples were obtained from the coronary sinus and radial artery and the rate of norepinephrine spillover from the heart was calculated according to the Fick principle to assess cardiac sympathetic activity.
Perioperative cardiac sympathectomy induced via TEA with local anesthetics may clinically benefit patients undergoing cardiac surgery by increasing myocardial oxygen supply.95,104,105 However, such a cardiac sympathectomy may offer additional benefits to patients undergoing cardiac surgery. Multiple clinical studies demonstrated that TEA with local anesthetics significantly decreases heart rate before155 and after149,155 initiation of CPB, and significantly decreases the need to administer β-blockers after CPB.152 Multiple clinical studies also demonstrated that TEA with local anesthetics significantly decreases systemic vascular resistance before151,152 and after155,159 initiation of CPB. Furthermore, patients undergoing cardiac surgery who receive TEA with local anesthetics not only exhibit significant decreases in postoperative heart rate and systemic vascular resistance but also significant decreases in postoperative ECG evidence of myocardial ischemia when compared with patients managed similarly without thoracic epidural catheters.155
A relatively large clinical investigation highlighted the potential clinical benefits of TEA in cardiac surgical patients.140 Scott et al140 prospectively randomized (nonblinded) 420 patients undergoing elective CABG to receive either TEA (bupivacaine/clonidine) and general anesthesia or general anesthesia alone (control group). The two groups received similar intraoperative anesthetic techniques. In TEA patients, the thoracic epidural infusion was continued for 96 hours after surgery (titrated according to need). In control patients, target-controlled infusion alfentanil was used for the first 24 postoperative hours, then followed by PCA morphine for the next 48 hours. After surgery, striking clinical differences were observed between the two groups (Table 38-6). Postoperative incidence of supraventricular arrhythmia, lower respiratory tract infection, renal failure, and acute confusion all were significantly lower in patients receiving TEA compared with control patients. However, data from this clinical investigation must be viewed with caution. The clinical protocol dictated that β-adrenergic blocker therapy could not be used intraoperatively or postoperatively for the 5 days of the study period (except in those patients who developed a new arrhythmia requiring additional therapy). Because approximately 90% of this study’s patients were taking β-adrenergic blockers before surgery, this unique perioperative management clouds interpretation of postoperative supraventricular arrhythmia data.
| Outcome | TEA (N = 206), n (%) | GA (N = 202), n (%) |
|---|---|---|
| Supraventricular arrhythmia | 21 (10.2) | 45 (22.3) |
| Lower respiratory tract infection | 31 (15.3) | 59 (29.2) |
| Renal failure | 4 (2.0) | 14 (6.9) |
| Cerebrovascular accident | 2 (1.0) | 6 (3.0) |
| Acute confusion | 3 (1.5) | 11 (5.5) |
| Significant bleeding | 35 | 23 |
| Any complications | 84 | 108 |
Significant (unadjusted) differences existed between groups regarding supraventricular arrhythmia (P = 0.0012), lower respiratory tract infection (P = 0.0007), renal failure (P = 0.016), acute confusion (P = 0.031), and any complications (P = 0.011).
GA, general anesthesia; TEA = thoracic epidural analgesia.
From Scott NB, Turfrey DJ, Ray DAA, et al: A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg 93:528, 2001.
Despite prospective randomization, substantially fewer patients receiving TEA were current active smokers before surgery compared with control patients (5.8% vs. 13.4%, respectively), which clouds interpretation of postoperative lower respiratory tract infection data. These investigators also found that postoperative pre-extubation maximal expiratory lung volumes were increased in TEA patients (compared with control patients), and postoperative tracheal extubation was facilitated via TEA as well (yet TEA patients and control patients were managed somewhat differently during the immediate postoperative period). Although postoperative analgesia was not definitively assessed in this clinical investigation, 11.9% of control patients were converted to TEA during the first 24 postoperative hours because of suboptimal postoperative analgesia, whereas only 2.9% of TEA patients were converted to target-controlled infusion alfentanil or PCA morphine because of suboptimal postoperative analgesia. The results of this clinical investigation are certainly intriguing, but definitive conclusions regarding the use of thoracic epidural techniques in patients undergoing cardiac surgery cannot be drawn because of the study’s substantial limitations, highlighted by an accompanying editorial175 and three subsequent letters to the editor.176–178
In contrast with the encouraging findings of Scott et al’s140 clinical investigation, two prospective, randomized, nonblinded clinical investigations revealed that using TEA techniques in patients undergoing cardiac surgery may not offer substantial clinical benefits.179,180 In 2002, Priestley et al180 prospectively randomized 100 patients undergoing elective CABG to receive either TEA (ropivacaine/fentanyl) and general anesthesia or general anesthesia alone (control group). The two groups received quite different intraoperative anesthetic techniques. Before surgery, TEA patients received epidural ropivacaine/fentanyl for 48 hours (supplemental analgesics available if needed), whereas control patients received nurse-administered intravenous morphine, followed by PCA morphine. Patients receiving TEA were extubated sooner than control patients (3.2 vs. 6.7 hours, respectively; P < 0.001), yet this difference may have been secondary to the different amounts of intraoperative intravenous opioid administered to the two groups (intraoperative intravenous anesthetic technique not standardized).
Postoperative pain scores at rest were significantly lower in patients receiving TEA only on postoperative days 0 and 1 (equivalent on days 2 and 3). Postoperative pain scores during coughing were significantly lower in patients receiving TEA only on postoperative day 0 (equivalent on days 1, 2, and 3; Figure 38-14). There were no significant differences between the two groups in postoperative oxygen saturation on room air, chest radiograph changes, or spirometry (Table 38-7). Furthermore, no clinical differences were detected between the two groups regarding postoperative mobilization goals, atrial fibrillation, postoperative hospital discharge eligibility, or actual postoperative hospital discharge. In short, this clinical investigation revealed that TEA may provide enhanced postoperative analgesia (though brief) and enhance early postoperative tracheal extubation, yet has no effect on important clinical parameters (morbidity, hospital length of stay, etc.). In 2003, Royse and associates179 prospectively randomized 80 patients undergoing elective CABG to receive either TEA (ropivacaine/fentanyl) and general anesthesia or general anesthesia alone (control group). The two groups received very different intraoperative anesthetic techniques. After surgery, TEA patients received epidural ropivacaine/fentanyl until the third postoperative day, whereas control patients received nurse-administered intravenous morphine followed by PCA morphine. Patients receiving TEA were tracheally extubated sooner during the immediate postoperative period than control subjects (2.6 vs. 5.4 hours, respectively; P < 0.001); yet, this difference may have been secondary to the different amounts of intraoperative intravenous anesthetics administered (intraoperative anesthetic technique not standardized). Postoperative pain scores at rest and with cough were significantly lower in patients receiving TEA on postoperative days 1 and 2 only (equivalent on postoperative day 3; Table 38-8). Much like Priestley et al’s180 investigation, there were no substantial differences between the two groups regarding important postoperative clinical parameters (respiratory function, renal function, atrial fibrillation, ICU length of stay, hospital length of stay).
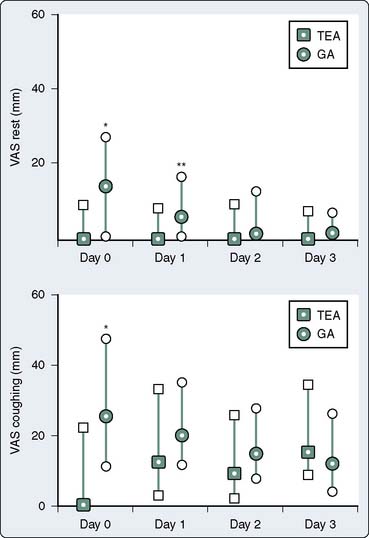
Figure 38-14 Visual analog scale (VAS) scores.
(From Priestley MC, Cope L, Halliwell R, et al: Thoracic epidural anesthesia for cardiac surgery: The effects on tracheal intubation time and length of hospital stay. Anesth Analg 94:275, 2002.)
TABLE 38-8 Visual Analog Scale Scores
| Pain Score | High Thoracic Epidural Analgesia (mean ± SD) | Control (mean ± SD) |
|---|---|---|
| Rest day 1 | 0.02 ± 0.2 | 0.8 ± 1.8 |
| Cough day 1 | 1.2 ± 1.7 | 4.4 ± 3.1 |
| Rest day 2 | 0.1 ± 0.4 | 1.2 ± 2.7 |
| Cough day 2 | 1.5 ± 2.0 | 3.6 ± 3.1 |
| Rest day 3 | 0.2 ± 1.0 | 0.3 ± 1.1 |
| Cough day 3 | 1.7 ± 2.3 | 2.7 ± 3.0 |
Mean pain scores at rest and with cough for days 1, 2, and 3. Significant differences (P < 0.05) existed between groups on days 1 and 2 (at rest and with cough) yet not on day 3.
From Royse C, Royse A, Soeding P, et al: Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Ann Thorac Surg 75:93, 2003.
Most recently, in 2006, Hansdottir et al (via the best-designed study to date) provided additional evidence that thoracic epidural techniques offer no real clinical benefits to patients undergoing cardiac surgery.181 This relatively large (113 patients) prospective trial randomized patients undergoing elective cardiac surgery to receive either patient-controlled TEA (catheter inserted the day before surgery; using bupivacaine, fentanyl, and epinephrine) or patient-controlled intravenous morphine analgesia during the immediate postoperative period. Perioperative care was standardized (all patients underwent general anesthesia and received a median sternotomy). When the two groups were compared, the only difference was a shorter time to postoperative tracheal extubation in patients receiving TEA (2.3 vs. 7.3 hours). Absolutely no differences were observed regarding postoperative analgesia (at rest and during cough), degree of sedation, lung volumes (forced vital capacity, forced vital capacity at 1 second, peak expiratory flow), degree of ambulation, global quality of recovery score (including all five domains studied), cardiac morbidity (myocardial infarction, atrial fibrillation, etc.), renal morbidity (peak serum creatinine), neurologic outcome (stroke, confusion), ICU stay, or hospital length of stay. Furthermore, this group of experienced investigators reported a very high (17%) “failure” rate for the use of thoracic epidural catheters in these patients.
Despite enhanced postoperative analgesia offered via thoracic epidural techniques, such analgesia does not appear to decrease the incidence of persistent pain after cardiac surgery. Ho and associates18 assessed via survey 244 patients after cardiac surgery via median sternotomy. One hundred fifty patients received perioperative supplementation of general anesthesia with TEA (ropivacaine/fentanyl infusion initiated before induction of anesthesia and continued after surgery for 2 to 3 days), and 94 patients received general anesthesia and routine postoperative nurse-controlled intravenous morphine infusion for analgesia (together with intraoperative wound infiltration with ropivacaine at chest wall closure). Persistent pain (defined as pain still present 2 or more months after surgery) was similar in the two cohorts (reported in almost 30% of patients). However, persistent pain reported by these patients was mild in most cases, infrequently interfering with daily life.
The quality of analgesia obtained with TEA techniques is sufficient to allow cardiac surgery to be performed in awake patients without general endotracheal anesthesia. The initial report of awake cardiac surgery was published in the Annals of Thoracic Surgery in 2000. Karagoz and associates,182 from Turkey, described the perioperative course of five patients who underwent elective off-pump single-vessel CABG via minithoracotomy with only TEA (spontaneous ventilation throughout). All five patients did well, and none had to be converted to general endotracheal anesthesia. Soon thereafter, a group of investigators from Germany described the perioperative course of 12 patients who underwent elective off-pump multivessel CABG via complete sternotomy with only TEA.183 All patients did well, yet two patients required conversion to general endotracheal anesthesia (one for incomplete analgesia, one for pneumothorax). Also in 2002, investigators from Brazil revealed that “outpatient” CABG was possible (discharge to home within 24 hours of hospital admission) in a small (n = 20) group of patients undergoing cardiac surgery solely via TEA.184 Since these initial small clinical reports appeared, larger series of patients have been published, demonstrating that “awake” cardiac surgery is feasible and safe.185–195 In 2003, the first case report of awake cardiac surgery requiring CPB was published.196 In this astonishing case report from Austria, a 70-year-old man with aortic stenosis underwent aortic valve replacement with assistance of normothermic CPB (total time: 123 minutes; cross-clamp time: 82 minutes) solely via TEA. Verbal communication with the patient was possible on demand throughout CPB. The patient did well and experienced an unremarkable postoperative course.
The many clinical investigations involving the use of epidural analgesic techniques in patients undergoing cardiac surgery indicate that administration of thoracic epidural opioids or local anesthetics before and/or after CPB initiates reliable postoperative analgesia after cardiac surgery. Administration of thoracic epidural local anesthetics (not opioids) can both reliably attenuate the perioperative stress response associated with CPB (that persists during the immediate postoperative period) and induce perioperative thoracic cardiac sympathectomy. Enhanced postoperative analgesia likely facilitates early tracheal extubation after cardiac surgery, yet patients may be extubated after cardiac surgery (with or without CPB) in the operating room without assistance of thoracic epidural techniques.197
All clinical reports involving utilization of intrathecal anesthesia and TEA and analgesia techniques for cardiac surgery involve small numbers of patients, and few (if any) are well designed (see Tables 38-4 and 38-5). Only a handful of clinical studies involving intrathecal analgesia are prospective, randomized, blinded, and placebo-controlled (see Table 38-4). There are no blinded, placebo-controlled clinical studies involving epidural techniques (see Table 38-5). Furthermore, none of the existing clinical studies involving intrathecal anesthesia and TEA and analgesia techniques for cardiac surgery uses clinical outcome as a primary end point. Thus, there are clear deficiencies in the literature that prohibit definitive analysis of the risk/benefit ratio of intrathecal anesthesia and TEA and analgesia techniques as applied to patients undergoing cardiac surgery.
A 2004 meta-analysis by Liu et al198 assessed effects of perioperative central neuraxial analgesia on outcome after CABG. These authors, via MEDLINE and other databases, searched for randomized, controlled trials in patients undergoing CABG with CPB. Fifteen trials enrolling 1178 patients were included for TEA analysis, and 17 trials enrolling 668 patients were included for intrathecal analysis. Thoracic epidural techniques did not affect the incidences of mortality or myocardial infarction, yet reduced risk for arrhythmias (atrial fibrillation and tachycardia), reduced risk for pulmonary complications (pneumonia and atelectasis), reduced time to tracheal extubation, and reduced analog pain scores. Intrathecal techniques did not affect incidences of mortality, myocardial infarction, arrhythmias, or time to tracheal extubation, and only modestly decreased systemic morphine utilization and pain scores (while increasing incidence of pruritus). These authors concluded that central neuraxial analgesia does not affect rates of mortality or myocardial infarction after CABG yet is associated with improvements in faster time to tracheal extubation, decreased pulmonary complications and cardiac arrhythmias, and reduced pain scores. However, the authors also noted that the majority of potential clinical benefits offered by central neuraxial analgesia (earlier extubation, decreased arrhythmias, enhanced analgesia) may be reduced and/or eliminated with changing cardiac anesthesia practice using fast-track techniques, use of β-adrenergic blockers or amiodarone, and/or use of NSAIDs or COX-2 inhibitors. These authors also noted that the risk for spinal hematoma (addressed later in this chapter) because of central neuraxial analgesia in patients undergoing full anticoagulation for CPB remains uncertain.
The use of intrathecal and/or epidural techniques in patients undergoing thoracotomy incisions (rare during cardiac surgery, yet sometimes used in certain circumstances) deserves brief mention.199 Many factors are involved in the occurrence of pulmonary dysfunction after thoracotomy. Postoperative changes in pulmonary function result from lung resection, atelectasis, and/or volume loss caused by pneumothorax and inspiratory muscle dysfunction. Pain after thoracotomy can be intense, which may produce pulmonary complications after surgery. Somewhat surprisingly, patients undergoing a “clamshell” incision (transverse thoracosternotomy) for bilateral lung transplantation do not experience more postoperative pain than patients undergoing a standard thoracotomy for single-lung transplantation, and lung transplant recipients undergoing thoracotomy have a lower incidence of adequate pain relief than patients undergoing thoracotomy for other indications.200 These clinical observations emphasize that the condition of the patient may play a major role (together with type of incision) regarding adequacy of postoperative pain control.200 Clearly, compared with standard thoracotomy incisions, patients receiving minithoracotomy incisions experience less postoperative pain and consume fewer supplemental analgesics during the immediate postoperative period. Furthermore, up to half of all patients undergoing thoracotomy incision will experience chronic pain related to the surgical site.
Evidence exists that indicates adequate postoperative pain control after thoracotomy may help prevent the development of chronic postoperative thoracotomy pain. Therefore, an effective postoperative analgesic plan must be developed for these patients. In contrast with median sternotomy incisions and minithoracotomy incisions, there appears to be some clinical evidence indicating that use of regional anesthetic techniques may decrease postoperative complications after thoracotomy incisions. Specifically, Ballantyne et al201 and Licker et al202 provide ample evidence that postoperative pain control with epidural techniques after thoracotomy incision may reduce pulmonary morbidity and overall patient mortality. However, although ample evidence exists suggesting that TEA (superiority of thoracic over lumbar routes has been recently called into question) offers superior postoperative analgesia, not all clinical studies have shown that such techniques truly improve postoperative pulmonary function and reduce postoperative pulmonary complications.
Side Effects of Intrathecal and Epidural Local Anesthetics
The most troubling and undesirable drug effect of intrathecal and epidural local anesthetics is hypotension. Spinal anesthesia to upper thoracic dermatomes produces a decrease in mean arterial blood pressure that is accompanied by a parallel decrease in coronary blood flow.203,204 Exactly what percentage of blood pressure decrease is acceptable remains speculative, especially in patients with CAD. Disturbances in myocardial oxygenation appear to occur in patients with CAD if coronary perfusion pressure is allowed to decrease by more than 50% during induction of TEA with local anesthetics.205 Furthermore, if α-adrenergic agonists are used to increase blood pressure during this time, there may be detrimental effects (vasoconstriction) on the native coronary arteries and bypass grafts.206,207 Of the 19 patients who received intrathecal local anesthetics to produce a “total spinal” for cardiac surgery, 18 required intravenous phenylephrine intraoperatively to increase blood pressure, indicating that hypotension is a substantial problem with this technique.120,122 Hypotension also appears to be relatively common when thoracic epidural local anesthetics are used in this setting. Volume replacement, β-adrenergic agonists, and/or α-adrenergic agonists are required in a fair proportion of patients, and coronary perfusion pressure may decrease in susceptible patients after CPB.
After epidural administration, local anesthetics can produce blood concentrations of drug that may initiate detrimental cardiac electrophysiologic effects and myocardial depression.208 In fact, myocardial depression has been detected in patients receiving TEA with bupivacaine, a clinical effect at least partially caused by increased blood concentrations of the drug.209 Concomitant use of β-adrenergic blockers may further decrease myocardial contractility in this setting.210,211 Patients undergoing cardiac surgery who were randomized to receive intermittent boluses of thoracic epidural bupivacaine intraoperatively, followed by continuous infusion after surgery, exhibited significantly increased pulmonary capillary wedge pressures after CPB when compared with patients managed similarly without epidural catheters (10.8 vs. 6.4 mm Hg, respectively; P < 0.001), which suggests myocardial depression.152
Two case reports also indicated that the use of epidural anesthesia and analgesia may either mask myocardial ischemia or initiate myocardial ischemia.212,213 Oden and Karagianes213 described the perioperative course of an elderly patient who had a history of exertional angina and underwent uneventful cholecystectomy. After surgery, analgesia was achieved with continuous lumbar epidural fentanyl. On the second postoperative day, with continuous lumbar epidural fentanyl being administered, ST-segment depression was noted on the electrocardiogram. The patient was awake, alert, and did not experience angina. Initiation of intravenous nitroglycerin at this time resulted in normalization of ischemic ECG changes. It was thought by these authors that epidural fentanyl-induced analgesia masked the patient’s typical anginal pain. Easley et al212 described the perioperative course of a middle-aged patient without cardiovascular symptoms (“borderline” hypertension) who was scheduled for exploratory laparotomy. Before surgery, a low thoracic epidural catheter was inserted and local anesthetic was administered (sensory level peaked by pinprick at T2). The patient at this time began complaining of left-sided jaw pain, and substantial (2.7 mm) ST-segment depression was noted on the electrocardiogram. Surgery was canceled and the patient was treated with aspirin and nitroglycerin. The ECG normalized, yet based on ECG changes, troponin levels, and creatine kinase-MB fractions, the patient was diagnosed with a non–Q-wave myocardial infarction. Coronary angiography on the following day was unremarkable, and a presumptive diagnosis of coronary artery spasm was made. It was thought by these authors that low thoracic epidural-induced sympathectomy led to alterations in the sympathetic–parasympathetic balance (i.e., vasoconstriction above level of block) leading to coronary artery spasm.
Side Effects of Intrathecal and Epidural Opioids
Although many have been described, the four clinically relevant undesirable drug effects of intrathecal and epidural opioids are pruritus, nausea and vomiting, urinary retention, and respiratory depression.214 After administration of intrathecal or epidural opioids, the most common side effect is pruritus. The incidence rate varies widely (from 0% to 100%) and is often identified only after direct questioning of patients. Severe pruritus is rare, occurring in only approximately 1% of patients. The incidence of nausea and vomiting is approximately 30%. The incidence of urinary retention varies widely (from 0% to 80%) and occurs most frequently in young male patients. When intrathecal or epidural opioids are used in patients undergoing cardiac surgery, the incidences of pruritus, nausea and vomiting, and urinary retention are similar to that described earlier. Of note, if a large dose (4.0 mg) of intrathecal morphine is administered, prolonged postoperative urinary retention may occur.119
The most important undesirable drug effect of intrathecal and epidural opioids is respiratory depression. Only 4 months after the initial use of intrathecal215 and epidural216 opioids in humans, life-threatening respiratory depression was reported.217–219 The incidence of respiratory depression that requires intervention after conventional doses of intrathecal and epidural opioids is approximately 1%, the same as that after conventional doses of intramuscular and intravenous opioids. Early respiratory depression occurs within minutes of opioid injection and is associated with administration of intrathecal or epidural fentanyl or sufentanil. Delayed respiratory depression occurs hours after opioid injection and is associated with administration of intrathecal or epidural morphine. Delayed respiratory depression results from cephalad migration of morphine in cerebrospinal fluid and the subsequent stimulation of opioid receptors located in the ventral medulla.220 Factors that increase the risk for respiratory depression include large and/or repeated doses of opioids, intrathecal utilization, advanced age, and concomitant use of intravenous sedatives.214 The magnitude of postoperative respiratory depression is profoundly influenced by the dose of intrathecal or epidural morphine administered, and the type and amount of intravenous analgesics and amnestics used for the intraoperative baseline anesthetic. Prolonged postoperative respiratory depression may delay tracheal extubation, and naloxone may be required in some patients.
Children may be more susceptible to developing postoperative respiratory depression when intrathecal morphine is used in this setting. Of 56 children (aged 1 to 17 years) administered either 20 or 30 μg/kg intrathecal morphine before surgical incision for cardiac surgery, 3 of 29 who received 20 μg/kg and 6 of 27 who received 30 μg/kg required naloxone after surgery for respiratory depression.131
One clinical study indicated that administration of intrathecal morphine to patients undergoing cardiac surgery may be contraindicated if early extubation is planned.118 Patients were randomized to receive either intrathecal morphine (10 μg/kg) or intrathecal placebo before the induction of anesthesia. Intraoperative anesthetic management was standardized and consisted of intravenous fentanyl (20 μg/kg) and intravenous midazolam (10 mg total) together with inhaled isoflurane and/or intravenous nitroglycerin, if required. Regarding patients extubated during the immediate postoperative period, the mean time from ICU arrival to extubation was significantly increased in those who received intrathecal morphine compared with those who received intrathecal placebo (10.9 vs. 7.6 hours, respectively; P = 0.02). However, other clinical studies indicated that intrathecal or epidural morphine may yet prove to be a useful adjunct for cardiac surgery and early extubation. The optimal dose of intrathecal or epidural morphine in this setting, together with the optimal intraoperative baseline anesthetic that will provide significant postoperative analgesia yet not delay tracheal extubation in the immediate postoperative period, remains to be elucidated. In contrast with intrathecal and epidural opioids, epidural local anesthetics (which initiate no respiratory depression) should not delay tracheal extubation in the immediate postoperative period.
Risk for Hematoma Formation
Intrathecal or epidural instrumentation entails risk, the most feared complication being epidural hematoma formation. The estimated incidence of hematoma formation is approximately 1:220,000 after intrathecal instrumentation.221 Hematoma formation is more common (approximately 1:150,000) after epidural instrumentation because larger needles are used, catheters are inserted, and the venous plexus in the epidural space is prominent.221 Furthermore, hematoma formation does not occur exclusively during epidural catheter insertion; almost half of all cases develop after catheter removal.221
Although spontaneous hematomas can occur in the absence of intrathecal or epidural instrumentation,222 most occur when instrumentation is performed in a patient with a coagulopathy (from any cause) or when instrumentation is difficult or traumatic.221 Paradoxically, intrathecal or epidural instrumentation has been performed safely in patients with known clinical coagulopathy.223,224 Of 1000 epidural catheterizations performed in 950 patients receiving oral anticoagulants at time of catheter insertion, none experienced signs or symptoms of hematoma formation.224 Of 336 epidural injections performed in 36 patients with chronic cancer pain either fully anticoagulated (oral anticoagulants or intravenous heparin) or profoundly thrombocytopenic (platelet count < 50,000/mm3) at the time of instrumentation, none had signs or symptoms of hematoma formation.223
Risk is increased when intrathecal or epidural instrumentation is performed before systemic heparinization, and hematoma formation has occurred in patients when diagnostic or therapeutic lumbar puncture has been followed by systemic heparinization.225–228 When lumbar puncture is followed by systemic heparinization, concurrent use of aspirin, difficult or traumatic instrumentation, and administration of intravenous heparin within 1 hour of instrumentation increase the risk for hematoma formation.227 However, by observing certain precautions, intrathecal or epidural instrumentation can be performed safely in patients who will subsequently receive intravenous heparin.229,230 By delaying surgery 24 hours in the event of a traumatic tap, by delaying heparinization 60 minutes after catheter insertion, and by maintaining tight perioperative control of anticoagulation, more than 4000 intrathecal or epidural catheterizations were performed safely in patients undergoing peripheral vascular surgery who received intravenous heparin after catheter insertion.230 A retrospective review involving 912 patients further indicates that epidural catheterization before systemic heparinization for peripheral vascular surgery is safe.229 However, the magnitude of anticoagulation in these two studies (activated partial thromboplastin time of approximately 100 seconds229 and activated coagulation time approximately twice the baseline value230) involving patients undergoing peripheral vascular surgery was substantially less than the degree of anticoagulation required in patients subjected to CPB.
Although most investigators agree that risk for hematoma is likely increased when intrathecal or epidural instrumentation is performed in patients before systemic heparinization required for CPB, the absolute degree of increased risk is somewhat controversial; some believe the risk rate may be as high as 0.35%.225 An extensive mathematical analysis by Ho et al231 of the approximately 10,840 intrathecal injections in patients subjected to systemic heparinization required for CPB (without a single episode of hematoma formation) reported in the literature as of 2000 estimated that the minimum risk for hematoma formation was 1:220,000, and the maximum risk for hematoma formation was 1:3600 (95% confidence level); however, the maximum risk may be as high as 1:2400 (99% confidence level). Similarly, of the approximately 4583 epidural instrumentations in patients subjected to systemic heparinization required for CPB (without a single episode of hematoma formation) reported in the literature as of 2000, the minimum risk for hematoma formation was 1:150,000 and the maximum risk for hematoma formation was 1:1500 (95% confidence level); however, the maximum risk may be as high as 1:1000 (99% confidence level).231
Certain precautions, however, may decrease the risk.221,225 The technique should not be used in a patient with known coagulopathy from any cause. Surgery should be delayed 24 hours in the event of a traumatic tap, and time from instrumentation to systemic heparinization should exceed 60 minutes. In addition, systemic heparin effect and reversal should be tightly controlled (smallest amount of heparin used for the shortest duration compatible with therapeutic objectives), and patients should be closely monitored after surgery for signs and symptoms of hematoma formation. An obvious economic disadvantage of intrathecal or epidural instrumentation in patients before cardiac surgery is the possible delay in surgery in the event of a traumatic tap. However, one study involving more than 4000 intrathecal or epidural catheterizations via a 17-gauge Tuohy needle indicated that the incidence of traumatic tap (blood freely aspirated) is rare (< 0.10%).230
In 2004, the first case report of an epidural hematoma associated with a thoracic epidural catheter inserted in a patient before cardiac surgery was published.232 This 18-year-old man had a thoracic (T9-10) epidural catheter uneventfully inserted after induction of general anesthesia (the patient had intense fear of needles) immediately before initiation of CPB for aortic valve replacement surgery. Three hours elapsed from instrumentation to systemic heparinization. The entire intraoperative course and immediate postoperative course were uneventful (tracheally extubated soon after surgery, ambulating without difficulty on the first postoperative day). At 49 hours after surgery, intravenous heparin therapy was initiated (prosthetic valve thromboprophylaxis). At 53 hours after surgery, alteplase (thrombolytic drug) was used to flush a dysfunctional intravenous catheter. Within 2 hours of intravenous alteplase administration, the patient reported intense back pain while ambulating. At this point, the epidural catheter was removed. The activated partial thromboplastin time assessed at this time (during catheter removal) was 87.4 seconds (reference range, 24.8 to 37.3 seconds). The patient also was thrombocytopenic at this time. On catheter removal, the patient experienced sudden onset of numbness and weakness distal to T9. Intravenous heparin was discontinued, a computed tomographic scan was inconclusive, requiring a magnetic resonance imaging scan, which revealed an epidural hematoma. Five hours from the onset of neurologic symptoms, the patient underwent surgical evacuation of the hematoma (which extended from the T8 to T11 levels). Intraoperatively, intravenous methylprednisolone (30 mg/kg) was administered, followed by an infusion (5.4mg/kg/hr), which was continued for 72 hours. Twenty-four hours after laminectomy, the patient demonstrated mild residual lower extremity motor and sensory deficits. Six weeks later, his neurologic examination had returned to normal. The authors noted the factors affecting coagulation in this patient (heparin, alteplase, thrombocytopenia) that likely led to hematoma formation and theorized that removing the catheter may have increased bleeding, further compounding the problem.
Since 2004, numerous such reports (with catastrophic consequences, such as permanent paralysis) have appeared in the literature.233–235 In addition, thromboembolic complications (neurologic, stroke) may occur during the postoperative period when normalization of coagulation parameters (in a patient requiring anticoagulation) is achieved to safely remove the epidural catheter.236 Thus, bleeding and/or thromboembolic complications associated with these techniques in this setting are very real and potentially catastrophic.
Use of regional anesthetic techniques in patients undergoing cardiac surgery, although seemingly increasing in popularity, remains extremely controversial, prompting numerous editorials by recognized experts in the field of cardiac anesthesia.237–240 One of the main reasons such controversy exists (and likely will continue for some time) is that the numerous clinical investigations regarding this topic are suboptimally designed and use a wide array of disparate techniques, preventing clinically useful conclusions on which all can agree.241,242
Multimodal analgesia
The possibility of synergism between analgesic drugs is a concept that is nearly a century old.243,244 Although subsequent research has demonstrated the difference between additivity and synergy, the fundamental strategy behind such combinations (“multimodal” or “balanced” analgesia) remains unchanged: enhanced analgesia with minimization of adverse physiologic effects. Use of analgesic combinations during the postoperative period, specifically the combination of traditional intravenous opioids with other analgesics (NSAIDs, COX-2 inhibitors, ketamine, etc.), has been proved clinically effective in noncardiac patients for decades. Early clinical investigations simply reported analgesic efficacy, whereas more recent clinical investigations have additionally evaluated and described specific opioid-sparing effects (which should lead to a reduction in side effects). For example, in the late 1980s, initial clinical studies involving ketorolac (the first parenteral NSAID available in the United States) revealed significant opioid-sparing effects (analgesia) together with a reduction in respiratory depression. Subsequently, substantial clinical research has clearly established the perioperative analgesic efficacy and opioid-sparing effects of NSAIDs (together with reduction of side effects).
The American Society of Anesthesiologists Task Force on Acute Pain Management in the Perioperative Setting reported that the literature supports the administration of two analgesic agents that act by different mechanisms via a single route for providing superior analgesic efficacy with equivalent or reduced adverse effects.1 Potential examples include epidural opioids administered in combination with epidural local anesthetics or clonidine and intravenous opioids in combination with ketorolac or ketamine. Dose-dependent adverse effects reported with administration of a medication occur whether it is given alone or in combination with other medications (opioids may cause nausea, vomiting, pruritus, or urinary retention, and local anesthetics may produce motor block). The literature is insufficient to evaluate the postoperative analgesic effects of oral opioids combined with NSAIDs, COX-2 inhibitors, or acetaminophen compared with oral opioids alone. The Task Force believed that NSAIDs, COX-2 inhibitors, or acetaminophen administration has a dose-sparing effect for systemically administered opioids. The literature also suggests that two routes of administration, when compared with a single route, may be more effective in providing perioperative analgesia. Examples include intrathecal or epidural opioids combined with intravenous, intramuscular, oral, transdermal, or subcutaneous analgesics versus intrathecal or epidural opioids alone. Another example is intravenous opioids combined with oral NSAIDs, COX-2 inhibitors, or acetaminophen versus intravenous opioids alone. The literature is insufficient to evaluate the efficacy of pharmacologic pain management combined with nonpharmacologic, alternative, or complementary pain management compared with pharmacologic pain management alone.
How important is postoperative pain after cardiac surgery?
Cardiac surgery is unique, and because of this, it involves unique risks not routinely associated with noncardiac surgery.245 Furthermore, as all are aware, for a wide variety of reasons, patients presenting for cardiac surgery continue to get older and “sicker” (more comorbidities: neurologic dysfunction, myocardial dysfunction, renal dysfunction, etc.). Multiple factors interact in a complicated manner during the perioperative period that affect outcome and quality of life after cardiac surgery, including type and quality of surgical intervention, extent of postoperative neurologic dysfunction, extent of postoperative myocardial dysfunction, extent of postoperative pulmonary dysfunction, extent of postoperative renal dysfunction, extent of postoperative coagulation abnormalities, extent of systemic inflammatory response, and quality of postoperative analgesia. Obviously, depending on specific clinical situations, certain factors will be more important than others. It is extremely difficult (if not impossible) to determine exactly how important attaining adequate or “high-quality” postoperative analgesia truly is in relation to all these important clinical factors surrounding a patient undergoing cardiac surgery. For example, how important is it to obtain “high-quality” postoperative analgesia in an 80-year-old patient with preoperative myocardial dysfunction, renal dysfunction, and a heavily calcified aorta after double-valve replacement? It could be argued that factors other than quality of postoperative analgesia will determine clinical outcome in this patient. On the other hand, how important is it to obtain “high-quality” postoperative analgesia in an otherwise healthy 50-year-old patient after routine CABG? It is likely that this patient’s clinical outcome will be satisfactory even if postoperative analgesia is suboptimal. In essence, for cardiac and noncardiac surgery patients, there is insufficient evidence to confirm or deny the ability of postoperative analgesic techniques to affect postoperative morbidity or mortality.246,247
Conclusions
Multiple factors are important during the perioperative period that potentially affect outcome and quality of life after cardiac surgery, including type and quality of surgical intervention, extent of postoperative neurologic dysfunction, myocardial dysfunction, pulmonary dysfunction, renal dysfunction, coagulation abnormalities, quality of postoperative analgesia and/or extent of systemic inflammatory response, among others248 (Table 38-9). This list of factors is presented in no particular order; obviously, depending on specific clinical situations (surgical procedure, patient comorbidity, etc.), certain factors will be more important than others. It is extremely difficult (if not impossible) to determine exactly how important attaining adequate postoperative analgesia truly is in relation to all of these clinical factors surrounding a patient undergoing cardiac surgery. A clear link between “adequate” or “high-quality” postoperative analgesia and outcome in patients after cardiac surgery has yet to be established.249–251
However, despite the absence of substantiating scientific evidence, most clinicians intuitively believe that attaining high-quality postoperative analgesia is important because it may prevent adverse hemodynamic, metabolic, immunologic, and hemostatic alterations, all of which may potentially increase postoperative morbidity. Although many analgesic techniques are available, intravenous systemic opioids form the cornerstone of postcardiac surgery analgesia. Opioids have been used for many years in the treatment of postoperative pain in patients after cardiac surgery, with good results. Although NSAIDs (specifically COX-2 inhibitors) have received much recent attention, important clinical issues regarding their safety (gastrointestinal effects, renal effects, hemostatic effects, immunologic effects) need to be resolved. Although PCA techniques are commonly used, their clear superiority over traditional nurse-controlled analgesic techniques remains unproved. As a general rule, it is likely best to avoid intense, single-modality therapy for the treatment of acute postoperative pain. Clinicians should strive for an approach that uses a number of different therapies (multimodal therapy), each counteracting pain via different mechanisms. Preemptive analgesia, although intriguing, needs further study to determine its role in affecting postoperative analgesia and outcome.252–255
Finally, the American Society of Anesthesiologists Task Force on Acute Pain Management in the Perioperative Setting offered sound advice.1 It recommends that anesthesiologists who manage perioperative pain use analgesic therapeutic options only after thoughtfully considering the risks and benefits for the individual patient. The therapy (or therapies) selected should reflect the individual anesthesiologist’s expertise, as well as the capacity for safe application of the chosen modality in each practice setting. This includes the ability to recognize and treat adverse effects that emerge after initiation of therapy. Whenever possible, anesthesiologists should use multimodal pain management therapy. Dosing regimens should be administered to optimize efficacy while minimizing the risk for adverse events. The choice of medication, dose, route, and duration of therapy always should be individualized.
1 American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2004;100:1573.
2 Joint Commission on Accreditation of Healthcare Organizations, Pain assessment and management—an organizational approach; 2000; Available at: http://www.jcaho.org. Accessed October 15, 2005
3 Weissman C. The metabolic response to stress: An overview and update. Anesthesiology. 1990;73:308.
4 Kehlet H. Surgical stress: The role of pain and analgesia. Br J Anaesth. 1989;63:189.
5 Roizen M.F. Should we all have a sympathectomy at birth? Or at least preoperatively [Editorial]? Anesthesiology. 1988;68:482.
6 Tuman K.J., McCarthy R.J., March R.J., et al. Effects of epidural anesthesia and analgesia on coagulation and outcome after major vascular surgery. Anesth Analg. 1991;73:696.
7 Yeager M.P., Glass D.D., Neff R.K., et al. Epidural anesthesia and analgesia in high-risk surgical patients. Anesthesiology. 1987;66:729.
8 Mangano D.T., Siliciano D., Hollenberg M., et al. Postoperative myocardial ischemia: Therapeutic trials using intensive analgesia following surgery. Anesthesiology. 1992;76:342.
9 Anand K.J.S., Hickey P.R. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326:1.
10 Wallace A.W. Is it time to get on the fast track or stay on the slow track [Editorial]? Anesthesiology. 2003;99:774.
11 Myles P.S., Daly D.J., Djaiani G., et al. A systematic review of the safety and effectiveness of fast-track cardiac anesthesia. Anesthesiology. 2003;99:982.
12 Royston D., Kovesi T., Marczin N. The unwanted response to cardiac surgery: Time for a reappraisal [Editorial]? J Thorac Cardiovasc Surg. 2003;125:32.
13 Mueller X.M., Tinguely F., Tevaearai H.T., et al. Pain location, distribution, and intensity after cardiac surgery. Chest. 2000;118:391.
14 Nay P.G., Elliott S.M., Harrop-Griffiths A.W. Postoperative pain. Expectation and experience after coronary artery bypass grafting. Anaesthesia. 1996;51:741.
15 Meehan D.A., McRae M.E., Rourke D.A., et al. Analgesic administration, pain intensity, and patient satisfaction in cardiac surgical patients. Am J Crit Care. 1995;4:435.
16 Moore R., Follette D.M., Berkoff H.A. Poststernotomy fractures and pain management in open cardiac surgery. Chest. 1994;106:1339.
17 Greenwald L.V., Baisden C.E., Symbas P.N. Rib fractures in coronary bypass patients: Radionuclide detection. Radiology. 1983;148:553.
18 Ho S.C., Royse C.F., Royse A.G., et al. Persistent pain after cardiac surgery: An audit of high thoracic epidural and primary opioid analgesia therapies. Anesth Analg. 2002;95:820.
19 Kalso E., Mennander S., Tasmuth T., et al. Chronic post-sternotomy pain. Acta Anaesth Scand. 2001;45:935.
20 Chaney M.A., Morales M., Bakhos M. Severe incisional pain and long thoracic nerve injury after port-access minimally invasive mitral valve surgery. Anesth Analg. 2000;91:288.
21 Davis Z., Jacobs H.K., Zhang M., et al. Endoscopic vein harvest for coronary artery bypass grafting: Technique and outcomes. J Thorac Cardiovasc Surg. 1998;116:228.
22 Smith R.C., Leung J.M., Mangano D.T., SPI Research Group. Postoperative myocardial ischemia in patients undergoing coronary artery bypass graft surgery. Anesthesiology. 1991;74:464.
23 Leung J.M., O’Kelly B., Browner W.S., et al. Prognostic importance of postbypass regional wall-motion abnormalities in patients undergoing coronary artery bypass graft surgery. Anesthesiology. 1989;71:16.
24 Philbin D.M., Rosow C.E., Schneider R.C., et al. Fentanyl and sufentanil anesthesia revisited: How much is enough? Anesthesiology. 1990;73:5.
25 Reves J.G., Karp R.B., Buttner E.E., et al. Neuronal and adrenomedullary catecholamine release in response to CPB in man. Circulation. 1982;66:49.
26 Roberts A.J., Niarchos A.P., Subramanian V.A., et al. Systemic hypertension associated with coronary artery bypass surgery: Predisposing factors, hemodynamic characteristics, humoral profile, and treatment. J Thorac Cardiovasc Surg. 1977;74:846.
27 Liu S., Carpenter R.L., Neal M.J. Epidural anesthesia and analgesia: their role in postoperative outcome. Anesthesiology. 1995;82:1474.
28 Wu C.L., Naqibuddin M., Rowlingson A.J., et al. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97:1078.
29 Rogers M.C. Do the right thing. Pain relief in infants and children [Editorial]. N Engl J Med. 1992;326:55.
30 White P.F., Rawal S., Latham P., et al. Use of a continuous local anesthetic infusion for pain management after median sternotomy. Anesthesiology. 2003;99:918.
31 Dowling R., Thielmeier K., Ghaly A., et al. Improved pain control after cardiac surgery: Results of a randomized, double-blind, clinical trial. J Thorac Cardiovasc Surg. 2003;126:1271.
32 Brown S.L., Morrison A.E. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology. 2004;100:1305.
33 McDonald S.B., Jacobsohn E., Kopacz D.J., et al. Parastenal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: The effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg. 2005;100:75.
34 Soto R.G., Fu E.S. Acute pain management for patients undergoing thoracotomy. Ann Thorac Surg. 2003;75:1349.
35 Karmakar M.K. Thoracic paravertebral block. Anesthesiology. 2001;95:771.
36 Kavanagh B.P., Katz J., Sandler A.N. Pain control after thoracic surgery. A review of current techniques. Anesthesiology. 1994;81:737-759.
37 Bilgin M., Akcali Y., Oguzkaya F. Extrapleural regional versus systemic analgesia for relieving postthoracotomy pain: A clinical study of bupivacaine compared with metamizol. J Thorac Cardiovasc Surg. 2003;126:1580.
38 Mehta Y., Swaminathan M., Mishra Y., et al. A comparative evaluation of intrapleural and thoracic epidural analgesia for postoperative pain relief after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1998;12:162.
39 Riedel B.J. Regional anesthesia for major cardiac and noncardiac surgery: More than just a strategy for effective analgesia [editorial]? J Cardiothorac Vasc Anesth. 2001;15:279.
40 Allen M.S., Halgren L., Nichols F.C., et al. A randomized controlled trial of bupivacaine through intracostal catheters for pain management after thoracotomy. Ann Thorac Surg. 2009;88:903.
41 Maurer K., Blumenthal S., Rentsch K.M., et al. Continuous extrapleural infusion of ropivacaine 0.2% after cardiovascular surgery via the lateral thoracotomy approach. J Cardiothorac Vasc Anesth. 2008;22:249.
42 Wheatley G.H., Rosenbaum D.H., Paul M.C., et al. Improved pain management outcomes with continuous infusion of a local anesthetic after thoracotomy. J Thorac Cardiovasc Surg. 2005;130:464.
43 Myles P.A., Bain C. Underutilization of paravertibral block in thoracic surgery. J Cardiothorac Vasc Anesth. 2006;20:635.
44 Marret E., Bazelly B., Taylor G., et al. Paravertebral block with ropivacaine 0.5% versus systematic analgesia for pain relief after thoracotomy. Ann Thorac Surg. 2005;79:2109.
45 Joshi G.P., Bonnet F., Shah R., et al. A systematic review of randomized trials evaluating regional techniques for post thoracotomy analgesia. Anesth Analg. 2008;107:126.
46 Gottschalk A., Cohen S.P., Yang S., et al. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006;104:594.
47 Detterbeck F.C. Efficacy of methods of intercoastal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg. 2005;80:1550.
48 Raja S.N., Lowenstein E. The birth of opioid anesthesia (classic papers revisited). Anesthesiology. 2004;100:1013.
49 Bovill J.G., Sebel P.S., Stanley T.H. Opioid analgesics in anesthesia: With special reference to their use in cardiovascular anesthesia. Anesthesiology. 1984;61:731.
50 Hug C.C. Does opioid “anesthesia” exist [editorial]? Anesthesiology. 1990;73:1.
51 Kehlet H., Rung G.W., Callesen T. Postoperative opioid analgesia: Time for a reconsideration? J Clin Anesth. 1996;8:441.
52 Peng P.W.H., Sandler A.N. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology. 1999;90:576.
53 Checketts M.R., Gilhooly C.J., Kenny G.N.C. Patient-maintained analgesia with target-controlled alfentanil infusion after cardiac surgery: A comparison with morphine PCA. Br J Anaesth. 1998;80:748.
54 Bowdle T.A., Camporesi E.M., Maysick L., et al. A multicenter evaluation of remifentanil for early postoperative analgesia. Anesth Analg. 1996;83:1292.
55 Ouattara A., Boccara G., Kockler U., et al. Remifentanil induces systemic arterial vasodilation in humans with a total artificial heart. Anesthesiology. 2004;100:602.
56 Liu S.S., Allen H.W., Olsson G.L. Patient-controlled epidural analgesia with bupivacaine and fentanyl on hospital wards: Prospective experience with 1,030 surgical patients. Anesthesiology. 1998;88:688.
57 Boylan J.F., Katz J., Kavanagh B.P., et al. Epidural bupivacaine-morphine analgesia versus patient-controlled analgesia following abdominal aortic surgery: Analgesic, respiratory, and myocardial effects. Anesthesiology. 1998;89:585.
58 Tsang J., Brush B. Patient-controlled analgesia in postoperative cardiac surgery. Anaesth Intensive Care. 1999;27:464.
59 Gust R., Pecher S., Gust A., et al. Effect of patient-controlled analgesia on pulmonary complications after coronary artery bypass grafting. Crit Care Med. 1999;27:2218.
60 Boldt J., Thaler E., Lehmann A., et al. Pain management in cardiac surgery patients: Comparison between standard therapy and patient-controlled analgesia regimen. J Cardiothorac Vasc Anesth. 1998;12:654.
61 Munro A.J., Long G.T., Sleigh J.W. Nurse-administered subcutaneous morphine is a satisfactory alternative to intravenous patient-controlled analgesia morphine after cardiac surgery. Anesth Analg. 1998;87:11.
62 Myles P.S., Buckland M.R., Cannon G.B., et al. Comparison of patient-controlled analgesia and nurse-controlled infusion analgesia after cardiac surgery. Anaesth Intensive Care. 1994;22:672.
63 Searle N.R., Roy M., Bergeron G., et al. Hydromorphone patient-controlled analgesia (PCA) after coronary artery bypass surgery. Can J Anaesth. 1994;41:198.
64 Ralley F.E., Day F.J., Cheng D.C.H. Pro: Nonsteroidal anti-inflammatory drugs should be routinely administered for postoperative analgesia after cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14:731.
65 Griffin M. Con: Nonsteroidal anti-inflammatory drugs should not be routinely administered for postoperative analgesia after cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14:735.
66 Kharasch E.D. Perioperative COX-2 inhibitors: Knowledge and challenges [Editorial]. Anesth Analg. 2004;98:1.
67 Gilron I., Milne B., Hong M. Cyclooxygenase-2 inhibitors in postoperative pain management: Current evidence and future directions. Anesthesiology. 2003;99:1198.
68 Gajraj N.M. Cyclooxygenase-2 inhibitors. Anesth Analg. 2003;96:1720.
69 McCrory C.R., Lindahl S.G.E. Cyclooxygenase inhibition for postoperative analgesia. Anesth Analg. 2002;95:169.
70 FitzGerald G.A., Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433.
71 Crofford L.J. Rational use of analgesic and antiinflammatory drugs [Editorial]. N Engl J Med. 2001;345:1844.
72 Hynninen M.S., Cheng D.C.H., Hossain I., et al. Non-steroidal antiinflammatory drugs in treatment of postoperative pain after cardiac surgery. Can J Anesth. 2000;47:1182.
73 Lin J.C., Szwerc M.F., Magovern J.A. Nonsteroidal anti-inflammatory drug-based pain control for minimally invasive direct coronary artery bypass surgery. Heart Surg Forum. 1999;2:169.
74 Rapanos T., Murphy P., Szalai J.P., et al. Rectal indomethacin reduces postoperative pain and morphine use after cardiac surgery. Can J Anesth. 1999;46:725.
75 Lahtinen P., Kokki H., Hendolin H., et al. Propacetamol as adjunctive treatment for postoperative pain after cardiac surgery. Anesth Analg. 2002;95:813.
76 Immer F.F., Immer-Bansi A.S., Trachsel N., et al. Pain treatment with a COX-2 inhibitor after coronary artery bypass operation: A randomized trial. Ann Thorac Surg. 2003;75:490.
77 Ott E., Nussmeier N.A., Duke P.C., et al. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125:1481.
78 Mukherjee D., Nissen S.E., Topol E.J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954.
79 White P.F., Kehlet H., Liu S. Perioperative analgesia: What do we still know? Anesth Analg. 2009;108:1364.
80 Guo T.Z., Jiang J.Y., Buttermann A.E., et al. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873.
81 Nishina K., Mikawa K., Uesugi T., et al. Efficacy of clonidine for prevention of perioperative myocardial ischemia: A critical appraisal and meta-analysis of the literature. Anesthesiology. 2002;96:323.
82 Flacke J.W., Bloor B.C., Flacke W.E., et al. Reduced narcotic requirement by clonidine with improved hemodynamic and adrenergic stability in patients undergoing coronary bypass surgery. Anesthesiology. 1987;67:11.
83 Ebert T.J., Hall J.E., Barney J.A., et al. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382.
84 Multz A.S. Prolonged dexmedetomidine infusion as an adjunct in treating sedation-induced withdrawal. Anesth Analg. 2003;96:1054.
85 Arain S.R., Ebert T.J. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461.
86 Triltsch A.E., Welte M., von Homeyer P., et al. Bispectral index-guided sedation with dexmedetomidine in intensive care: A prospective, randomized, double-blind, placebo-controlled phase II study. Crit Care Med. 2002;30:1007.
87 Myles P.S., Hunt J.O., Holdgaard H.O., et al. Clonidine and cardiac surgery: Haemodynamic and metabolic effects, myocardial ischaemia and recovery. Anaesth Intensive Care. 1999;27:137-147.
88 Boldt J., Rothe G., Schindler E., et al. Can clonidine, enoximone, and enalaprilat help to protect the myocardium against ischaemia in cardiac surgery? Heart. 1996;76:207.
89 Abi-Jaoude F., Brusset A., Ceddaha A., et al. Clonidine premedication for coronary artery bypass grafting under high-dose alfentanil anesthesia: Intraoperative and postoperative hemodynamic study. J Cardiothorac Vasc Anesth. 1993;7:35.
90 Dorman B.H., Zucker J.R., Verrier E.D., et al. Clonidine improves perioperative myocardial ischemia, reduces anesthetic requirement, and alters hemodynamic parameters in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1993;7:386.
91 Chaney M.A. Intrathecal and epidural anesthesia and analgesia for cardiac surgery. Anesth Analg. 1997;84:1211.
92 Feigl E. Coronary physiology. Physiol Rev. 1983;63:1.
93 Lee D.D.P., Kimura S., DeQuattro V. Noradrenergic activity and silent ischaemia in hypertensive patients with stable angina: Effect of metoprolol. Lancet. 1989;1:403.
94 Vanhoutte P.M., Shimokawa H. Endothelium-derived relaxing factor and coronary vasospasm. Circulation. 1989;80:1.
95 Blomberg S., Emanuelsson H., Kvist H., et al. Effects of thoracic epidural anesthesia on coronary arteries and arterioles in patients with coronary artery disease. Anesthesiology. 1990;73:840.
96 Blomberg S., Curelaru I., Emanuelsson H., et al. Thoracic epidural anaesthesia in patients with unstable angina pectoris. Eur Heart J. 1989;10:437-444.
97 Heusch G., Deussen A., Thamer V. Cardiac sympathetic nerve activity and progressive vasoconstriction distal to coronary stenosis: Feed-back aggravation of myocardial ischemia. J Auton Nerv Syst. 1985;13:311.
98 Heusch G., Deussen A. The effects of cardiac sympathetic nerve stimulation on perfusion of stenotic coronary arteries in the dog. Circ Res. 1983;53:8.
99 Uchida Y., Murao S. Excitation of afferent cardiac sympathetic nerve fibers during coronary occlusion. Am J Physiol. 1974;226:1094.
100 Mangano D.T. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153.
101 Birkett D.A., Apthorp G.H., Chamberlain D.A., et al. Bilateral upper thoracic sympathectomy in angina pectoris: Results in 52 cases. Br Med J. 1965;2:187.
102 Kock M., Blomberg S., Emanuelsson H., et al. Thoracic epidural anesthesia improves global and regional left ventricular function during stress-induced myocardial ischemia in patients with coronary artery disease. Anesth Analg. 1990;71:625.
103 Blomberg S.G. Long-term home self-treatment with high thoracic epidural anesthesia in patients with severe coronary artery disease. Anesth Analg. 1994;79:413.
104 Davis R.F., DeBoer L.W.V., Maroko P.R. Thoracic epidural anesthesia reduces myocardial infarct size after coronary artery occlusion in dogs. Anesth Analg. 1986;65:711.
105 Klassen G.A., Bramwell R.S., Bromage P.R., et al. Effect of acute sympathectomy by epidural anesthesia on the canine coronary circulation. Anesthesiology. 1980;52:8.
106 Mathews E.T., Abrams L.D. Intrathecal morphine in open heart surgery [Correspondence]. Lancet. 1980;2:543.
107 Bowler I., Djaiani G., Abel R., et al. A combination of intrathecal morphine and remifentanil anesthesia for fast-track cardiac anesthesia and surgery. J Cardiothorac Vasc Anesth. 2002;16:709.
108 Alhashemi J.A., Sharpe M.D., Harris C.L., et al. Effect of subarachnoid morphine administration on extubation time after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2000;14:639.
109 Latham P., Zarate E., White P.F., et al. Fast-track cardiac anesthesia: A comparison of remifentanil plus intrathecal morphine with sufentanil in a desflurane-based anesthetic. J Cardiothorac Vasc Anesth. 2000;14:645.
110 Zarate E., Latham P., White P.F., et al. Fast-track cardiac anesthesia: Use of remifentanil combined with intrathecal morphine as an alternative to sufentanil during desflurane anesthesia. Anesth Analg. 2000;91:283.
111 Bowler I., Djaiani G., Hall J., et al. Intravenous remifentanil combined with intrathecal morphine decreases extubation times after elective coronary artery bypass graft (CABG) surgery [Abstract]. Anesth Analg. 2000;90:S33.
112 Lee T.W.R., Jacobsohn E., Maniate J.M., et al. High spinal anesthesia in cardiac surgery: Effects on hemodynamics, perioperative stress response, and atrial β-receptor function [Abstract]. Anesth Analg. 2000;90:SCA90.
113 Peterson K.L., DeCampli W.M., Pike N.A., et al. A report of two hundred twenty cases of regional anesthesia in pediatric cardiac surgery. Anesth Analg. 2000;90:1014.
114 Hammer G.B., Ngo K., Macario A. A retrospective examination of regional plus general anesthesia in children undergoing open heart surgery. Anesth Analg. 2000;90:1020.
115 Djaiani G., Bowler I., Hall J., et al. A combination of remifentanil and intrathecal morphine improves pulmonary function following CABG surgery [Abstract]. Anesth Analg. 2000;90:SCA64.
116 Chaney M.A., Nikolov M.P., Blakeman B.P., et al. Intrathecal morphine for coronary artery bypass graft procedure and early extubation revisited. J Cardiothorac Vasc Anesth. 1999;13:574.
117 Shroff A., Rooke G.A., Bishop M.J. Effects of intrathecal opioid on extubation time, analgesia, and ICU stay following coronary artery bypass grafting. J Clin Anesth. 1997;9:415.
118 Chaney M.A., Furry P.A., Fluder E.M., et al. Intrathecal morphine for coronary artery bypass grafting and early extubation. Anesth Analg. 1997;84:241.
119 Chaney M.A., Smith K.R., Barclay J.C., et al. Large-dose intrathecal morphine for coronary artery bypass grafting. Anesth Analg. 1996;83:215.
120 Kowalewski R., MacAdams C., Froelich J., et al. Anesthesia supplemented with subarachnoid bupivacaine and morphine for coronary artery bypass surgery in a child with Kawasaki disease. J Cardiothorac Vasc Anesth. 1996;10:243.
121 Taylor A., Healy M., McCarroll M., et al. Intrathecal morphine: One year’s experience in cardiac surgical patients. J Cardiothorac Vasc Anesth. 1996;10:225.
122 Kowalewski R.J., MacAdams C.L., Eagle C.J., et al. Anaesthesia for coronary artery bypass surgery supplemented with subarachnoid bupivacaine and morphine: A report of 18 cases. Can J Anaesth. 1994;41:1189.
123 Swenson J.D., Hullander R.M., Wingler K., et al. Early extubation after cardiac surgery using combined intrathecal sufentanil and morphine. J Cardiothorac Vasc Anesth. 1994;8:509.
124 Shroff A.B., Bishop M.J. Intrathecal morphine analgesia speeds extubation and shortens ICU stay following coronary artery bypass grafting (CABG) [Abstract]. Anesthesiology. 1994;81:A129.
125 Fitzpatrick G.J., Moriarty D.C. Intrathecal morphine in the management of pain following cardiac surgery. A comparison with morphine i.v. Br J Anaesth. 1988;60:639.
126 Vanstrum G.S., Bjornson K.M., Ilko R. Postoperative effects of intrathecal morphine in coronary artery bypass surgery. Anesth Analg. 1988;67:261.
127 Casey W.F., Wynands J.E., Ralley F.E., et al. The role of intrathecal morphine in the anesthetic management of patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1987;1:510.
128 Cheun J.K. Intraspinal narcotic anesthesia in open heart surgery. J Kor Med Sci. 1987;2:225.
129 Aun C., Thomas D., John-Jones L., et al. Intrathecal morphine in cardiac surgery. Eur J Anaesth. 1985;2:419.
130 Vincenty C., Malone B., Mathru M., et al. Comparison of intrathecal and intravenous morphine in post coronary bypass surgery [Abstract]. Crit Care Med. 1985;13:308.
131 Jones S.E.F., Beasley J.M., Macfarlane D.W.R., et al. Intrathecal morphine for postoperative pain relief in children. Br J Anaesth. 1984;56:137.
132 Bettex D.A., Schmidlin D., Chassot P.G., et al. Intrathecal sufentanil-morphine shortens the duration of intubation and improves analgesia in fast-track cardiac surgery. Can J Anesth. 2002;49:711.
133 Goldstein S., Dean D., Kim S.J., et al. A survey of spinal and epidural techniques in adult cardiac surgery. J Cardiothorac Vasc Anesth. 2001;15:158.
134 Lee T.W.R., Grocott H.P., Schwinn D., et al. High spinal anesthesia for cardiac surgery: Effects on β-adrenergic receptor function, stress response, and hemodynamics. Anesthesiology. 2003;98:499.
135 Zangrillo A., Bignami E., Biondi-Zuccai G.G.L., et al. Spinal analgesia in cardiac surgery: A meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2009;23:813.
136 Clowes G.H.A., Neville W.E., Hopkins A., et al. Factors contributing to success or failure in the use of a pump oxygenator for complete by-pass of the heart and lung, experimental and clinical. Surgery. 1954;36:557.
137 Hoar P.F., Hickey R.F., Ullyot D.J. Systemic hypertension following myocardial revascularization. A method of treatment using epidural anesthesia. J Thorac Cardiovasc Surg. 1976;71:859.
138 El-Baz N., Goldin M. Continuous epidural infusion of morphine for pain relief after cardiac operations. J Thorac Cardiovasc Surg. 1987;93:878.
139 Jideus L., Joachimsson P.O., Stridsberg M., et al. Thoracic epidural anesthesia does not influence the occurrence of postoperative sustained atrial fibrillation. Ann Thorac Surg. 2001;72:65.
140 Scott N.B., Turfrey D.J., Ray D.A.A., et al. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg. 2001;93:528.
141 Warters D., Knight W., Koch S.M., et al. Thoracic epidurals in coronary artery bypass surgery. Anesth Analg. 2000;90:767.
142 Loick H.M., Schmidt C., Van Aken H., et al. High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undergoing coronary artery bypass grafting. Anesth Analg. 1999;88:701.
143 Tenling A., Joachimsson P.O., Tyden H., et al. Thoracic epidural anesthesia as an adjunct to general anesthesia for cardiac surgery: Effects on ventilation-perfusion relationships. J Cardiothorac Vasc Anesth. 1999;13:258.
144 Sanchez R., Nygard E. Epidural anesthesia in cardiac surgery: Is there an increased risk? J Cardiothorac Vasc Anesth. 1998;12:170.
145 Loick H.M., Mollhoff T., Erren M., et al. Thoracic epidural anesthesia lowers catecholamine and TNFa release after CABG in humans [Abstract]. Anesth Analg. 1998;86:S81.
146 Warters R.D., Koch S.M., Luehr S.L., et al. Thoracic epidural anesthesia in CABG surgery [Abstract]. Anesth Analg. 1998;86:S116.
147 Shayevitz J.R., Merkel S., O’Kelly S.W., et al. Lumbar epidural morphine infusions for children undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:217.
148 Frank R.S., Boltz M.G., Sentivany S.K., et al. Combined epidural-general anesthesia for the repair of atrial septal defects in children results in shorter ICU stays [Abstract]. Anesthesiology. 1995;83:A1176.
149 Moore C.M., Cross M.H., Desborough J.P., et al. Hormonal effects of thoracic extradural analgesia for cardiac surgery. Br J Anaesth. 1995;75:387.
150 Stenseth R., Berg E.M., Bjella L., et al. Effects of thoracic epidural analgesia on coronary hemodynamics and myocardial metabolism in coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1995;9:503.
151 Kirno K., Friberg P., Grzegorczyk A., et al. Thoracic epidural anesthesia during coronary artery bypass surgery: Effects on cardiac sympathetic activity, myocardial blood flow and metabolism, and central hemodynamics. Anesth Analg. 1994;79:1075.
152 Stenseth R., Bjella L., Berg E.M., et al. Thoracic epidural analgesia in aortocoronary bypass surgery, I: Haemodynamic effects. Acta Anaesthesiol Scand. 1994;38:826.
153 Stenseth R., Bjella L., Berg E.M., et al. Thoracic epidural analgesia in aortocoronary bypass surgery, II: Effects on the endocrine metabolic response. Acta Anaesthesiol Scand. 1994;38:834.
154 Shapiro J.H., Wolman R.L., Lofland G.K. Epidural morphine as an adjunct for early extubation following congenital cardiac surgery [Abstract]. Anesth Analg. 1994;78:S385.
155 Liem T.H., Booij L.H.D.J., Hasenbos M.A.W.M., et al. Coronary artery bypass grafting using two different anesthetic techniques: Part I: Hemodynamic results. J Cardiothorac Vasc Anesth. 1992;6:148.
156 Liem T.H., Hasenbos M.A.W.M., Booij L.H.D.J., et al. Coronary artery bypass grafting using two different anesthetic techniques: Part 2: Postoperative outcome. J Cardiothorac Vasc Anesth. 1992;6:156.
157 Liem T.H., Booij L.H., Gielen M.J., et al. Coronary artery bypass grafting using two different anesthetic techniques: Part 3: Adrenergic responses. J Cardiothorac Vasc Anesth. 1992;6:162.
158 Rosen K.R., Rosen D.A. Caudal epidural morphine for control of pain following open heart surgery in children. Anesthesiology. 1989;70:418.
159 Joachimsson P.O., Nystrom S.O., Tyden H. Early extubation after coronary artery surgery in efficiently rewarmed patients: A postoperative comparison of opioid anesthesia versus inhalational anesthesia and thoracic epidural analgesia. J Cardiothorac Anesth. 1989;3:444.
160 Robinson R.J.S., Brister S., Jones E., et al. Epidural meperidine analgesia after cardiac surgery. Can Anaesth Soc J. 1986;33:550.
161 Pastor M.C., Sanchez M.J., Casas M.A., et al. Thoracic epidural analgesia in coronary artery bypass graft surgery: Seven years’ experience. J Cardiothorac Vasc Anesth. 2003;17:154.
162 Vlachtsis H., Vohra A. High thoracic epidural with general anesthesia for combined off-pump coronary artery and aortic aneurysm surgery. J Cardiothorac Vasc Anesth. 2003;17:226.
163 Sisillo E., Salvi L., Juliano G., et al. Thoracic epidural anesthesia as a bridge to redo coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2003;17:629.
164 Varadarajan B., Whitaker D.K., Vohra A., et al. Case 2-2002. Thoracic epidural anesthesia in patients with ankylosing spondylitis undergoing coronary artery surgery. J Cardiothorac Vasc Anesth. 2002;16:240.
165 de Vries A.J., Mariani M.A., van der Maaten J.M., et al. To ventilate or not after minimally invasive direct coronary artery bypass surgery: The role of epidural anesthesia. J Cardiothorac Vasc Anesth. 2002;16:21.
166 Canto M., Casas A., Sanchez M.J., et al. Thoracic epidurals in heart valve surgery: Neurologic risk evaluation. J Cardiothorac Vasc Anesth. 2002;16:723.
167 Fillinger M.P., Yeager M.P., Dodds T.M., et al. Epidural anesthesia and analgesia: Effects on recovery from cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:15.
168 Dhole S., Mehta Y., Saxena H., et al. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001;15:288.
169 Djaiani G.N., Ali M., Heinrich L., et al. Ultra-fast-track anesthetic technique facilitates operating room extubation in patients undergoing off-pump coronary revascularization surgery. J Cardiothorac Vasc Anesth. 2001;15:152.
170 Visser W.A., Liem T.H., Brouwer R.M. High thoracic epidural anesthesia for coronary artery bypass graft surgery in a patient with severe obstructive lung disease. J Cardiothorac Vasc Anesth. 2001;15:758.
171 Liem T.H., Williams J.P., Hensens A.G., et al. Minimally invasive direct coronary artery bypass procedure using a high thoracic epidural plus general anesthetic technique. J Cardiothorac Vasc Anesth. 1998;12:668.
172 Fawcett W.J., Edwards R.E., Quinn A.C., et al. Thoracic epidural analgesia started after CPB. Adrenergic, cardiovascular and respiratory sequelae. Anaesthesia. 1997;52:294.
173 Turfrey D.J., Ray D.A.A., Sutcliffe N.P., et al. Thoracic epidural anaesthesia for coronary artery bypass graft surgery. Effects on postoperative complications. Anaesthesia. 1997;52:1090.
174 Stenseth R., Bjella L., Berg E.M., et al. Effects of thoracic epidural analgesia on pulmonary function after coronary artery bypass surgery. Eur J Cardiothorac Surg. 1996;10:859-865.
175 O’Connor C.J., Tuman K.J. Epidural anesthesia and analgesia for coronary artery bypass graft surgery: Still forbidden territory [editorial]? Anesth Analg. 2001;93:523.
176 Amar D. Beta-adrenergic blocker withdrawal confounds the benefits of epidural analgesia with sympathectomy on supraventricular arrhythmias after cardiac surgery [Correspondence]. Anesth Analg. 2002;95:1119.
177 Riedel B.J., Shaw A.D. Thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass surgery [Correspondence]. Anesth Analg. 2002;94:1365.
178 Alston R.P. Thoracic epidurals and coronary artery bypass grafting surgery [Correspondence]. Anesth Analg. 2002;94:1365.
179 Royse C., Royse A., Soeding P., et al. Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Ann Thorac Surg. 2003;75:93.
180 Priestley M.C., Cope L., Halliwell R., et al. Thoracic epidural anesthesia for cardiac surgery: The effects on tracheal intubation time and length of hospital stay. Anesth Analg. 2002;94:275.
181 Hansdottir V., Philip J., Olsen M.F., et al. Thoracic epidural versus intravenous patient-controlled analgesia after cardiac surgery. Anesthesiology. 2006;104:142.
182 Karagoz H.Y., Sonmez B., Bakkaloglu B., et al. Coronary artery bypass grafting in the conscious patient without endotracheal general anesthesia. Ann Thorac Surg. 2000;70:91.
183 Aybek T., Dogan S., Neidhart G., et al. Coronary artery bypass grafting through complete sternotomy in conscious patients. Heart Surg Forum. 2002;5:17.
184 Souto G.L.L., Junior C.S.C., de Souza J.B.S., et al. Coronary artery bypass in the ambulatory patient. J Thorac Cardiovasc Surg. 2002;123:1008.
185 Aybek T., Kessler P., Dogan S., et al. Awake coronary artery bypass grafting: Utopia or reality? Ann Thorac Surg. 2003;75:1165.
186 Aybek T., Kessler P., Khan M.F., et al. Operative techniques in awake coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1394.
187 Karagoz H.Y., Kurtoglu M., Bakkaloglu B., et al. Coronary artery bypass grafting in the awake patient: Three years’ experience in 137 patients. J Thorac Cardiovasc Surg. 2003;125:1401.
188 Chakravarthy M., Jawali V., Patil T.A., et al. High thoracic epidural anesthesia as the sole anesthetic for redo off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2003;17:84.
189 Chakravarthy M.R., Jawali V., Patil T.A., et al. High thoracic epidural anaesthesia as the sole anaesthetic technique for minimally invasive direct coronary artery bypass in a high-risk patient. Ann Cardiac Anaesth. 2003;6:62.
190 Kessler P., Neidhart G., Bremerich D.H., et al. High thoracic epidural anesthesia for coronary artery bypass grafting using two different surgical approaches in conscious patients. Anesth Analg. 2002;95:791.
191 Vanek T., Straka Z., Brucek P., et al. Thoracic epidural anesthesia for off-pump coronary artery bypass without intubation. Eur J Cardiothorac Surg. 2001;20:858.
192 Anderson M.B., Kwong K.F., Furst A.J., et al. Thoracic epidural anesthesia for coronary bypass via left anterior thoracotomy in the conscious patient. Eur J Cardiothorac Surg. 2001;20:415.
193 Paiste J., Bjerke R.J., Williams J.P., et al. Minimally invasive direct coronary artery bypass surgery under high thoracic epidural. Anesth Analg. 2001;93:1486.
194 Zenati M.A., Paiste J., Williams J.P., et al. Minimally invasive coronary bypass without general endotracheal anesthesia. Ann Thorac Surg. 2001;72:1380.
195 Chakravarthy M., Jawali V., Patil T.A., et al. High thoracic epidural anesthesia as the sole anesthetic for performing multiple grafts in off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2003;17:160.
196 Schachner T., Bonatti J., Balogh D., et al. Aortic valve replacement in the conscious patient under regional anesthesia without endotracheal intubation. J Thorac Cardiovasc Surg. 2003;125:1526.
197 Straka Z., Brucek P., Vanek T., et al. Routine immediate extubation for off-pump coronary artery bypass grafting without thoracic epidural analgesia. Ann Thorac Surg. 2002;74:1544.
198 Liu S.S., Block B.M., Wu C.L. Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: A meta-analysis. Anesthesiology. 2004;101:153.
199 Ochroch E.A., Gottschalk A., Augostides J., et al. Long-term pain and activity during recovery from major thoracotomy using thoracic epidural analgesia. Anesthesiology. 2002;97:1234.
200 Richard C., Girard F., Ferraro P., et al. Acute postoperative pain in lung transplant recipients. Ann Thorac Surg. 2004;77:1951-1955.
201 Ballantyne J.C., Carr D.B., deFerranti S., et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: Cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86:598.
202 Licker M., de Perrot M., Hohn L., et al. Perioperative mortality and major cardiopulmonary complications after lung surgery for non-small-cell carcinoma. Eur J Cardiothorac Surg. 1999;15:314.
203 Sivarajan M., Amory D.W., Lindbloom L.E., et al. Systemic and regional blood-flow changes during spinal anesthesia in the rhesus monkey. Anesthesiology. 1975;43:78.
204 Hackel D.B., Sancetta S.M., Kleinerman J. Effect of hypotension due to spinal anesthesia on coronary blood flow and myocardial metabolism in man. Circulation. 1956;13:92.
205 Reiz S., Nath S., Rais O. Effects of thoracic epidural block and prenalterol on coronary vascular resistance and myocardial metabolism in patients with coronary artery disease. Acta Anaesth Scand. 1980;24:11.
206 DiNardo J.A., Bert A., Schwartz M.J., et al. Effects of vasoactive drugs on flows through left internal mammary artery and saphenous vein grafts in man. J Thorac Cardiovasc Surg. 1991;102:730.
207 Heusch G. α-Adrenergic mechanisms in myocardial ischemia. Circulation. 1990;81:1.
208 Reiz S., Nath S. Cardiotoxicity of local anaesthetic agents. Br J Anaesth. 1986;58:736.
209 Wattwil M., Sundberg A., Arvill A., et al. Circulatory changes during high thoracic epidural anaesthesia—influence of sympathetic block and of systemic effect of the local anaesthetic. Acta Anaesth Scand. 1985;29:849.
210 Blomberg S., Ricksten S.E. Effects of thoracic epidural anaesthesia on central haemodynamics compared to cardiac beta-adrenoceptor blockade in conscious rats with acute myocardial infarction. Acta Anaesth Scand. 1990;34:1.
211 Hotvedt R., Refsum H., Platou E.S. Cardiac electrophysiological and hemodynamic effects of β-adrenoceptor blockade and thoracic epidural analgesia in the dog. Anesth Analg. 1984;63:817.
212 Easley R.B., Rosen R.E., Lindeman K.S. Coronary artery spasm during initiation of epidural anesthesia. Anesthesiology. 2003;99:1015.
213 Oden R.V., Karagianes T.G. Postoperative myocardial ischemia possibly masked by epidural fentanyl analgesia. Anesthesiology. 1991;74:941.
214 Chaney M.A. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42:891.
215 Wang J.K., Nauss L.A., Thomas J.E. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149.
216 Behar M., Magora F., Olshwang D., et al. Epidural morphine in treatment of pain. Lancet. 1979;1:527.
217 Glynn C.J., Mather L.E., Cousins M.J., et al. Spinal narcotics and respiratory depression [Correspondence]. Lancet. 1979;2:356.
218 Liolios A., Andersen F.H. Selective spinal analgesia [Correspondence]. Lancet. 1979;2:357.
219 Scott D.B., McClure J. Selective epidural analgesia [Correspondence]. Lancet. 1979;1:1410.
220 Shook J.E., Watkins W.D., Camporesi E.M. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am Rev Respir Dis. 1990;142:895.
221 Vandermeulen E.P., Van Aken H., Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165.
222 Markham J.W., Lynge H.N., Stahlman E.B. The syndrome of spontaneous spinal epidural hematoma. Report of three cases. J Neurosurg. 1967;26:334.
223 Waldman S.D., Feldstein G.S., Waldman H.J., et al. Caudal administration of morphine sulfate in anticoagulated and thrombocytopenic patients. Anesth Analg. 1987;66:267.
224 Odoom J.A., Sih I.L. Epidural analgesia and anticoagulant therapy. Experience with one thousand cases of continuous epidurals. Anaesthesia. 1983;38:254.
225 Owens E.L., Kasten G.W., Hessel E.A. Spinal subarachnoid hematoma after lumbar puncture and heparinization: A case report, review of the literature, and discussion of anesthetic implications. Anesth Analg. 1986;65:1201.
226 Brem S.S., Hafler D.A., Van Uitert R.L., et al. Spinal subarachnoid hematoma: A hazard of lumbar puncture resulting in reversible paraplegia. N Engl J Med. 1981;303:1020.
227 Ruff R.L., Dougherty J.H. Complications of lumbar puncture followed by anticoagulation. Stroke. 1981;12:879.
228 Varkey G.P., Brindle G.F. Peridural anaesthesia and anticoagulant therapy. Can Anaesth Soc J. 1974;21:106.
229 Baron H.C., LaRaja R.D., Rossi G., et al. Continuous epidural analgesia in the heparinized vascular surgical patient: A retrospective review of 912 patients. J Vasc Surg. 1987;6:144.
230 Rao T.L.K., El-Etr A.A. Anticoagulation following placement of epidural and subarachnoid catheters: An evaluation of neurologic sequelae. Anesthesiology. 1981;55:618.
231 Ho A.M.H., Chung D.C., Joynt G.M. Neuraxial blockade and hematoma in cardiac surgery: Estimating the risk of a rare adverse event that has not (yet) occurred. Chest. 2000;117:551.
232 Rosen D.A., Hawkinberry D.W., Rosen K.R., et al. An epidural hematoma in an adolescent patient after cardiac surgery. Anesth Analg. 2004;98:966.
233 Chaney M.A. Thoracic epidural anaesthesia in cardiac surgery—the current standing. Ann Cardiac Anaesth. 2009;12:1.
234 Chaney M.A. Intrathecal and epidural anesthesia and analgesia for cardiac surgery. Anesth Analg. 2006;102:45.
235 Ho A.M., Li P.T., Kasmakar M.K. Risk of hematoma after epidural anesthesia and analgesia for cardiac surgery. Anesth Analg. 2006;103:1327.
236 Chaney M.A., Labovsky J.K. Case report of surgery: Balancing postoperative risks associated with hematoma formation and thromboembolic phenomenon. J Cardiothorac Vasc Anesth. 2005;19:798.
237 Mora Mangano C.T. Risky business [Editorial]. J Thorac Cardiovasc Surg. 2003;125:1204.
238 Castellano J.M., Durbin C.G. Epidural analgesia and cardiac surgery: Worth the risk [Editorial]? Chest. 2000;117:305.
239 Schwann N.M., Chaney M.A. No pain, much gain [Editorial]? J Thorac Cardiovasc Surg. 2003;126:1261.
240 Gravlee G.P. Epidural analgesia and coronary artery bypass grafting: The controversy continues [Editorial]. J Cardiothorac Vasc Anesth. 2003;17:151.
241 de Leon-Casasola O.A. When it comes to outcome, we need to define what a perioperative epidural technique is [Editorial]. Anesth Analg. 2003;96:315.
242 Rosenquist R.W., Birnbach D.J. Epidural insertion in anesthetized adults: Will your patients thank you [Editorial]? Anesth Analg. 2003;96:1545.
243 White P.F. The role of non-opioid analgesic techniques in the management of pain after ambulatory surgery. Anesth Analg. 2002;94:577.
244 Kehlet H., Dahl J.B. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048.
245 Chaney M.A. How important is postoperative pain after cardiac surgery? J Cardiothorac Vasc Anesth. 2005;19:705.
246 Liu S.S., Wu C.L. Effect of postoperative analgesia on major postoperative complications: A systematic update of the evidence. Anesth Analg. 2007;104:689.
247 White P.F., Kehlet H.K. Postoperative pain management and patient outcome: Time to return to work!. Anesth Analg. 2007;104:487.
248 Myles P.S., Hunt J.O., Fletcher H., et al. Relation between quality of recovery in hospital and quality of life at 3 months after cardiac surgery. Anesthesiology. 2001;95:862.
249 Fleron M.H., Weiskopf R.B., Bertrand M., et al. A comparison of intrathecal opioid and intravenous analgesia for the incidence of cardiovascular, respiratory, and renal complications after abdominal aortic surgery. Anesth Analg. 2003;97:2.
250 Beattie W.S., Badner N.H., Choi P. Epidural analgesia reduces postoperative myocardial infarction: A meta-analysis. Anesth Analg. 2001;93:853.
251 Wu C.L., Raja S.N. Optimizing postoperative analgesia: The use of global outcome measures [Editorial]. Anesthesiology. 2002;97:533.
252 Gottschalk A., Ochroch E.A. Preemptive analgesia: What do we do now [Correspondence]? Anesthesiology. 2003;98:280.
253 Hogan Q.H. No preemptive analgesia: Is that so bad [Editorial]? Anesthesiology. 2002;96:526.
254 Moiniche S., Kehlet H., Dahl J.B. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology. 2002;96:725.
255 Katz J., Cohen L., Schmid R., et al. Postoperative morphine use and hyperalgesia are reduced by preoperative but not intraoperative epidural analgesia: Implications for preemptive analgesia and the prevention of central sensitization. Anesthesiology. 2003;98:1449.