CHAPTER 41 Postoperative Infections of the Spine
Postoperative infections in patients undergoing spine surgery are unfortunate complications that significantly contribute to patient morbidity. Although infection in the general spine surgery population is relatively infrequent, with rates between 1% and 5.4% usually being reported,1–7 specific subpopulations, such as trauma or cancer patients, may have much higher infection rates.4,8–10 The cost of treating postoperative infections was estimated at $100,000 per patient in 1996 in the United States,11 but it is difficult to estimate the physical and social impact on the patient, who may be subjected to repeat washout and revision procedures and prolonged courses of intravenous antibiotic treatment. Modern surgical techniques, antisepsis, and antibiotic prophylaxis have made significant inroads into the problem of postoperative infection, but surgeons must be continually vigilant for this complication. Familiarity with current state-of-the art diagnostic tests, imaging evaluation, and treatment methods is essential.
Incidence
Noninstrumented Spinal Procedures
Lumbar diskectomy is one of the most common spinal procedures and is associated with highly successful clinical outcomes.12 Fortunately, these procedures have an extremely low rate of infection, with most large series reporting an incidence of 1% or less.13,14 Newer series of endoscopic minimally invasive diskectomy have achieved even lower rates of infection,15,16 with one study reporting no infections in the treatment of 262 patients.16 Infections after lumbar disk surgery can be manifested as superficial wound infections or as diskitis, with increasing back pain 2 weeks to several months postoperatively in conjunction with fever and laboratory and radiographic abnormalities.
Patients undergoing laminectomy without fusion may also enjoy a low incidence of infection, although slightly higher than that for patients undergoing diskectomy alone. Infection rates of approximately 2% are commonly reported in the literature for this procedure.17 Laminoplasty techniques have been associated with a higher rate of infection and are probably more appropriately considered instrumented procedures because most involve the placement of some type of implant.18
Noninstrumented posterior spinal fusion is associated with a higher rate of infection than is simple laminectomy or lumbar diskectomy,4,19 a factor attributable to longer operating times, more blood loss, greater soft tissue destruction, and placement of devascularized allograft.
Although most noninstrumented spinal surgeries involve a posterior approach, anterior cervical diskectomy and fusion procedures can be and often are performed without instrumentation, especially when only a single level is treated. Infection rates for the anterior cervical approach, however, are extremely low with and without the use of instrumention,20 thus making it difficult to discern any real difference between these two groups.
Finally, relatively limited interventional procedures such as chemonucleolysis and diskography are associated with an infection rate of up to 4% in the absence of preoperative antibiotics. Fortunately, this incidence can be dramatically decreased with the use of prophylactic antibiotics.21–24
Instrumented Spinal Procedures
The use of instrumentation in posterior spinal procedures increases the incidence of postoperative infection to approximately 3% to 7% in most series.2,25–28 Spinal instrumentation increases the risk for infection by acting as a locus minoris resistentiae for organisms rather than as a source of inoculation.29 In fact, one study demonstrated that 11 of 21 patients undergoing hardware removal for noninfectious reasons had positive bacterial growth on cultures.30 Although most infections occur in the immediate postoperative period, there are multiple reports of delayed infections occurring years after surgery.31–34 It is thus likely that colonization of implants is commonplace and that clinical infection occurs either when bacteria are sufficiently pathogenic or when host factors predispose to infection.
The type of instrumentation may affect the probability of clinical infection. Older steel implants tend to be uniquely implicated in the development of late spinal infections.34 Corrosion and fretting at cross-connector sites have been associated with foreign body reactions and the development of a local environment favorable for the growth of endogenous or low-virulence bacteria.32,34–36 This has not been reported with newer titanium implants, which are resistant to corrosion and thought to be relatively bacteria resistant.
Anterior instrumented spine surgeries are associated with extremely low rates of infection; when infections do occur, they tend to be superficial.20,37–39 The low incidence of infections with the anterior approach is probably attributable to the use of avascular planes in dissections and minimization of soft tissue trauma and necrosis. Although the anterior approach itself is associated with a low risk for infection, the highest rates of infection are encountered with combined anterior and posterior approaches to the spine,40 a finding probably attributable to the greater length and complexity of these cases.
An emerging category of spine surgery is minimally invasive surgery. The goal of minimally invasive spine surgery is to minimize soft tissue trauma and blood loss and thereby hasten patient recovery and decrease the risk for infection. Although several authors have realized good results with minimally invasive techniques,41,42 most series are small and no reduction in wound infection rates has been conclusively demonstrated.43 Further experience with these techniques will clarify the exact extent of the reduced infection risk with these methods.
Finally, the implantation of intrathecal drug delivery systems and spinal cord stimulators is associated with an approximately 5% risk for infection.44,45 Infection with these devices occurs in the pump or stimulator pocket in most cases, although infection of the intraspinal component can lead to meningitis or epidural abscess.44–46 These infections tend to occur early, usually within the first 2 postoperative months.
Infection Risk Factors
Although the type of surgery plays a large role in determining a patient’s risk for infection, numerous patient-, surgery-, and disease-specific factors have been elucidated (Table 41-1).
| TYPE OF FACTOR | CONDITION | INCREASED RISK |
|---|---|---|
| Patient specific | Age | >20 years |
| Diabetes mellitus | Glucose intolerance | |
| Malnutrition | Albumin <3.5 mg/dL Total lymphocyte count <1500/mL |
|
| Obesity | ||
| Alcoholism | ||
| Tobacco use | ||
| Urinary/fecal incontinence | ||
| Disease specific | Immunocompromised state | Steroid use Rheumatoid disease |
| Malignancy | ||
| Trauma | Spinal cord injury | |
| Surgery specific | Posterior approaches | Staged anterior-posterior procedures |
| Length of surgery | >5 hr | |
| Number of levels | ||
| Estimated blood loss | >1 L Blood transfusion |
|
| Postoperative stay in an intensive care unit | ||
| Preoperative hospital stay |
Patient Factors
Important among patient factors are medical comorbid conditions, including increasing age, obesity, diabetes, poor nutritional status, and alcohol and tobacco use.6,40,47–50 Other factors associated with an increased risk for postoperative infection include steroid use, rheumatoid disease, and an immunocompromised state.40,51,52
Obesity is a frequent comorbidity in the spine surgery population. Several studies have demonstrated an increased risk for infections in obese patients undergoing spine surgery.40,49,51,53,54 Obese patients are subject to longer operative times; greater amounts of retraction, which, in turn, causes increased soft tissue necrosis; greater amounts of poorly vascularized fatty tissue with decreased oxygen tension; decreased immune defense in adipose tissue; and poor tissue concentrations of prophylactic antibiotics.29,55–57 Finally, obesity predisposes the patient to diabetes.
Malnutrition is a well-known factor that predisposes patients to infection. It has been demonstrated to impair immune response and wound healing. Klein and coauthors reported that 25% of patients undergoing elective lumbar surgery had positive indices of malnutrition and that 11 of 13 infections occurred in these patients.47 Other authors have also reported a high rate of infection in malnourished patients undergoing spine surgery,58 as well as the development of malnutrition in some spine surgery patients during their hospital stay, a particular concern for those undergoing staged procedures.59 Commonly used indices of malnutrition are serum albumin level and the total lymphocyte count, with values of less than 3.5 mg/dL and 1500/mL, respectively, being considered abnormal.60 Other indices, including skinfold thickness, transferrin levels, arm muscle circumference, and weight-height ratio, can also be used to assess nutritional status.61 Malnutrition may be associated with malignancy and trauma, two conditions known to be related to high rates of infection.
Diabetes impairs wound healing and predisposes to wound infection in spine and other surgeries.6,40,62–64 Postoperative wound infections have been reported to occur in up to 24% of diabetic patients undergoing spine surgery.40,63 Proposed mechanisms by which diabetes contributes to infection risk include increased glucose concentrations in wound fluids, the presence of dysfunctional polymorphonuclear neutrophils and macrophages, impaired lymphocyte chemotaxis, and delayed wound re-epithelialization.65–68 Impaired glucose tolerance without overt diabetes has additionally been correlated with this complication in the spine.6,51 Although studies of deep sternal surgical site infection in cardiothoracic procedures have demonstrated an ability to reduce this risk with strict perioperative glucose control, such a study in the spine is lacking.69,70
Tobacco use has been demonstrated to be a risk factor for wound infection in several studies.71–73 Hypothesized mechanisms include deprivation of oxygen to tissues and impaired wound healing and neutrophil defense.74–76
Surgical Factors
Several surgical variables other than those discussed earlier have been identified that may predispose patients to infection. Many of these variables appear to correlate with the magnitude of the surgery itself. It is therefore not surprising that the number of levels treated, length of the surgery, procedural complexity, and amount of blood loss have all been associated with an elevated risk for infection.2,25,49,62,77,78 Operative times longer than 5 hours have been associated with an increased rate of infection, as has blood loss of 1000 mL.62,77
The use of a cell saver system has been inconsistently correlated with infection risk. Although blood that has been processed by the cell saver system has been shown to be contaminated in 37% of various surgical procedures, no contamination was found in neurosurgical procedures.79 Additionally, even though use of the cell saver system was correlated with infection in series of spinal patients,31 no increased risk has been noted in other specialties.80 The use of blood transfusion, however, has been correlated with infection in numerous studies, and this risk may be independent of the amount of blood loss.31,80,81
Other surgical risk factors include revision surgery, the use of allograft material, and surgery extending to the sacral region, the latter of which may be attributable to urine and fecal contamination.25,82,83 Finally, the presence of two or more resident surgeons being involved in the procedure has been correlated with increased infectious risk in one study.51 Although not completely explored, this variable may be a reflection of the length and complexity of the procedure rather than a truly independent risk factor.
Disease-Specific Factors
Infection risk has repeatedly been demonstrated as significantly altered by the disease state of the patient. The presence of malignancy appears to be associated with the highest incidence of infection, reported to be higher than 20% in some series.4,8 This rate has been reported to be even higher in patients undergoing radiation therapy in conjunction with open surgery.84–87 The high rate of postoperative infection in this population is probably multifactorial, however, with poor nutritional status, the long and complex surgical procedures necessary for spine reconstruction, and use of adjunctive therapies such as corticosteroids all contributing to the dramatically elevated risk for infection.
Traumatic spinal injury is also associated with a significantly higher risk for infection, especially in the presence of a complete neurological injury.9,10 Again, the elevated risk in this group may be multifactorial, with prolonged stay in the intensive care unit, urinary or fecal incontinence, and large procedures all playing a role.6
Prolonged presurgical hospitalization and postoperative stay in the intensive care unit are also risk factors for wound infection. Blam and associates reported that patients staying in the intensive care unit for more than 1 day had a 6- to 13-fold greater risk for postoperative infection than did patients who did not stay in the intensive care unit.9 Wimmer and coworkers showed that extensive presurgical hospital stay was significantly associated with infection.62
Clinical Findings
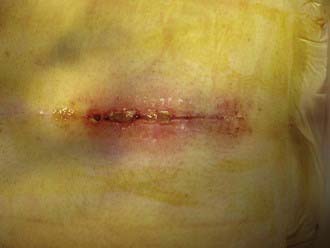
The signs and symptoms of spinal infection depend on whether the infection is superficial or deep. Superficial infections occur above the lumbodorsal fascia in the dermis and subcutaneous tissue and are usually manifested in the immediate postoperative period as erythema, purulent drainage, and local tenderness. Patients may have low-grade fever, and laboratory evaluation may reveal elevated erythrocyte sedimentation rate (ESR), elevated C-reactive protein (CRP) level, and leukocytosis. The presence of these indices is variable, however. For example, Levi and coauthors reported an average temperature of 37.5° C and a white blood cell (WBC) count of 10.2 × 106 cells/mL in 17 patients with postoperative infections.2 If the wound is open or purulence is expressible, Gram stain and cultures are often useful in revealing the pathogen and targeting treatment (Fig. 41-1).
Deep infections have a much more variable manifestation. They may develop in the immediate postoperative period, with some authors reporting most occurring 2 to 3 weeks postoperatively, or in a significantly delayed fashion several months to several years after surgery.32,34 Patients with an acute manifestation are often symptomatic with significant pain, fever, anorexia, and night sweats. The wound overlying a deep infection can appear completely normal or, if the infection tracks superficially, can be purulent. Patients with a delayed manifestation often have increasing back pain, wound drainage, and erythema but may lack fever altogether.34,88
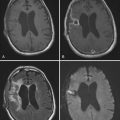
Spinal epidural abscess is a rare complication of spine surgery that may occur in an acute or delayed fashion and cause increased back pain, fever, and neurological deficit.89,90 Patients with spinal epidural abscess may have a rapid neurological decline, and the presence of any neurological deficit should raise concern for this process.91
Evaluation
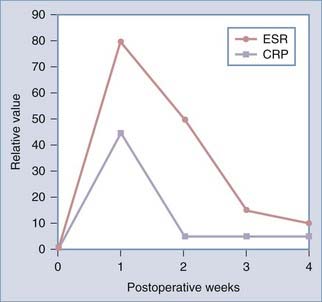
Both laboratory evaluation and imaging are important in the assessment of postoperative spine infections. Laboratory evaluation should include a WBC count, ESR, CRP levels, and cultures and Gram staining if there is purulent drainage or an open wound. The WBC evaluation may be and often is normal, especially in patients with a delayed manifestation, but it can useful if elevated.88,92 The ESR is reliably elevated in the setting of infection, but a high ESR can be difficult to interpret in the immediate postoperative period. ESR values normally rise to a maximal value of 102 mm/hr after spine fusion surgery and 75 mm/hr after disk surgery on postoperative day 4 before declining to normal levels 2 to 4 weeks postoperatively.92 Patients with infection have persistently elevated ESRs, usually more than 2 SD greater than the mean.92 Infections with low-grade pathogens such as Propionibacter may, however, be associated with low or normal ESR values.93 Obtaining serial ESRs can additionally be useful in tracking the response to treatment of infection. CRP values may be of added benefit in the diagnosis of infection, as well as in monitoring treatment response. A normal elevation of CRP is also seen in the immediate postoperative period; however, this elevation is more rapid and returns to baseline more quickly than does the rise in ESR, although complete normalization may take up to 2 weeks (Fig. 41-2).94,95 Additionally, CRP values are elevated more frequently than ESRs in the setting of infection with low-grade pathogens.93
Accurate diagnosis of bacterial pathogens is critical in the treatment of postoperative infections. Antibiotic therapy should be withheld until after specimens are taken for Gram staining and culture. These specimens are easily obtained from draining or open wounds, but care should be taken to prepare the skin carefully before specimen collection to prevent being misled by normal skin flora. If débridement is planned, specimens should be taken from both superficial and deep parts of the wound. Blood cultures can aid in the diagnosis of a pathogen when obtaining a direct specimen is difficult. Alternatively, computed tomography (CT)-guided or open biopsy of infected tissue may be helpful, with a diagnosis being obtained more than 50% of the time.96–98
Imaging Diagnosis
Plain Radiographs and Computed Tomography
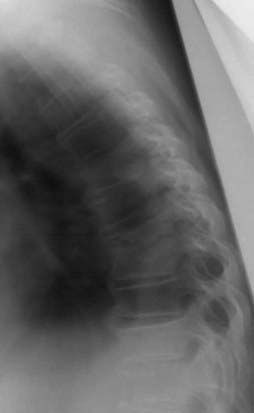
Plain radiographs are useful in the assessment of spine alignment, local soft tissue reaction to infection, and bony response to infection but have limited utility in the immediate postoperative period. Early bony changes in response to infection are manifested approximately 2 to 3 weeks postoperatively by evidence of disk space narrowing, bony destruction, and blurring of end plates. These findings may be followed by vertebral body collapse or sclerosis of end plates and bony ankylosis in the more chronic setting (Fig. 41-3).99 Increased swelling noted in soft tissues, especially in the retropharyngeal space after anterior spine surgery, may indicate the presence of an abscess. Finally, plain radiographs are useful for evaluation of the integrity of spine hardware, with lucency around screws being associated with loosening and failure.
CT imaging reveals a sequence of bony changes similar to that seen in radiographs but provides improved anatomic detail and can better detect paraspinal masses and epidural collections.99 When performed with contrast enhancement, CT can accurately delineate the presence of an abscess and provide a useful aid in surgical planning or can be used to guide percutaneous biopsy of infected bone or soft tissues.100 CT myelography may be useful in aiding the diagnosis of epidural or subdural empyema when magnetic resonance imaging (MRI) is unavailable or contraindicated.101
Nuclear Imaging
Technetium 99m–labeled methylene diphosphonate bone scans can be used to help diagnose and localize an infection, but this technique suffers from limited sensitivity and specificity given that other conditions, such as trauma, tumor, or vascular insult, may have increased uptake and that uptake may be negative in photopenic areas where there is decreased blood flow or bone tissue.99,102 A three-phase bone scan may increase the sensitivity and specificity of this test.103 An indium 111– or technetium 99m–labeled leukocyte scan may help in diagnosing infection in settings where other factors, such as fracture, may cause false-positive results on bone scanning.103,104
Recent experience with 18F-fluorodeoxyglucose positron emission tomography (PET) shows some promise, with the major caveat being the inability to differentiate tumor from infection.105,106 PET may be particularly useful in the postoperative setting, however, where a negative PET scan can rule out infection.107
Magnetic Resonance Imaging
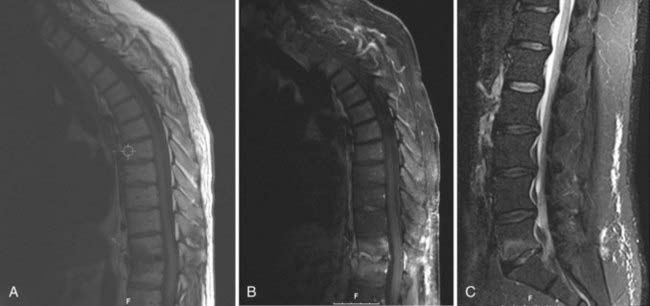
MRI is the imaging modality of choice when evaluating spine infections and enjoys a sensitivity and specificity of approximately 95% in the diagnosis of various spine infections, including osteomyelitis.108–110 Early MRI findings in the setting of infection include bone marrow edema signal manifested as hypointensity on T1-weighted sequences and hyperintensity on T2/STIR (short tau inversion recovery) signals.110,111 Although these findings are somewhat nonspecific, the observation of end-plate erosion with loss of the low signal intensity line, disk space narrowing, and disk space hyperintensity on T2-weighted sequences dramatically increases the sensitivity for infections.112 Loss of the normal low-intensity disk space cleft on T2-weighted sequences is another clue to the presence of infection.99 The addition of contrast enhancement is useful in confirming the presence of infection, delineating the extent of infection, and differentiating infection from solid granulation tissue, with the latter most often having a homogeneous pattern of enhancement (Fig. 41-4).111–114 Finally, MRI is also useful in detecting the presence of soft tissue masses associated with infection, such as paraspinal or epidural abscesses. Identification of such an associated mass increases the likelihood of infection being present to 98%.112
Some of these MRI findings, including vertebral body edema signal and contrast enhancement, may be present in the postoperative setting in the absence of infection.115,116 The presence of an adjacent soft tissue mass in this setting, however, strongly supports the diagnosis of infection. Moreover, absence of the MRI findings just listed strongly correlates with the absence of infection.116
Bacteriology
Skin flora are the most common causative organisms in postoperative spine infection, with staphylococcal species, particularly Staphylococcus aureus, being the most frequently detected.2,27,52,117 Methicillin-resistant S. aureus (MRSA), gram-negative organisms, and mixed flora are detected in sufficient frequency, however, to recommend withholding antibiotics until adequate culture specimens have been obtained and then providing treatment with broad-spectrum antibiotics until the culture results are available. Delayed infections are most frequently caused by bacteria of low virulence, with Propionibacterium and Staphylococcus epidermidis being common offenders.34,93,118 Isolation of Propionibacterium in culture specimens can take up to a week, and recent reports indicate that this bacterium might play a role in acute infections as well.93
Treatment
Nonoperative Treatment
Postoperative diskitis can most often be treated nonoperatively. The diagnosis of infecting pathogens should be made by blood cultures, which are positive in just more than half of patients,119 or by tissue biopsy before the institution of antibiotic therapy. Broad-spectrum antibiotics should be used until culture results are available to guide targeted antibiotic therapy. If culture results do not indicate the causative organism, broad-spectrum antibiotics with good staphylococcal coverage should be used.120 Although evidence-based recommendations for the duration of antibiotic therapy are lacking, a course of 6 weeks of intravenous antibiotics followed by 6 weeks of oral therapy is commonly used, along with an orthotic brace for comfort. Medical treatment is usually successful, although surgical treatment is indicated in patients with a poor clinical response to medical treatment, continued back pain, or instability. The surgical goal in such instances should be thorough débridement of infected tissue, including infected bone, and stabilization with bone graft and titanium instrumentation. Thoracic diskitis or osteomyelitis requiring débridement often requires an anterior approach, which may be difficult in patients with significant medical comorbid conditions. Thoracoscopic approaches are now used more often in this regard and have been reported to be effective in treating thoracic infections with acceptable morbidity.121,122
Surgical Débridement
Wounds that have broken down, have purulent drainage, or are fluctuant or otherwise concerning for deep extension usually require operative exploration with the goals of (1) diagnosis of the infective agent, (2) débridement of nonviable tissue, and (3) assurance of stabilization. Preoperative antibiotics are withheld until adequate culture specimens have been obtained and the entire length of the wound is opened. Preoperative imaging is helpful in determining the extent of débridement, with evidence of deep infection mandating opening of the lumbodorsal fascia. Although some authors recommend opening the fascia in all cases,29 we believe that it can be left closed when it is intact and only superficial infection and wound breakdown are likely based on preoperative imaging and intraoperative findings. Alternatively, it has been suggested that the subfascial compartment be aspirated with a needle and an intraoperative Gram stain obtained, with the results guiding the need to open this layer.25,78 If the lumbodorsal fascia is opened, it should be done only after thorough débridement and irrigation of the superficial compartment. Specimens for culture from the deep compartment should be taken and labeled separately.
The issue of instrumentation removal has been a significant controversy in the setting of infection, with some authors recommending removal in all or most cases1,33,34,93,117,118,123 and others reporting successful treatment with the instrumentation maintained.27,28,78,124–127 Some authors even purport that the added stability endowed by maintenance of instrumentation may help clear the infection.127 Recurrent infections with retention of hardware are often reported in the setting of delayed infections in the treatment of scoliosis in which old steel constructs that may predispose the patient to continued infection were used. Newer titanium implants are not associated with this problem and have been used with success in stabilizing the infected spine.126,128 Furthermore, removal of the hardware in an unstable spine is not a viable option, and we therefore recommend maintenance of the hardware in all cases in which spine stability or fusion maturation are in question.
On completion of débridement, the integrity of the fusion construct should be confirmed by visual inspection and manual manipulation. If the instrumentation is found to be loose, it must be removed and alternative means of fixation used. In the case of pedicle screw fixation, a larger diameter screw can be used in a rescue fashion. Structural allograft used in the initial construct may also be left in place, but loose chips of bone should be removed.126,129 If the infection has occurred in a delayed fashion and the fusion is solid, however, the surgeon can consider removing the instrumentation.7 Careful follow-up should ensue in these cases because loss of correction has been observed with removal of instrumentation despite solid bony fusion.34,118,123,130 It should also be noted that the presence of infection is associated with an increased rate of pseudarthrosis, and this complication, despite not strictly being correlated with a negative clinical outcome, should be watched for in follow-up examinations.78,131
Before closure, the wound should be thoroughly irrigated. We often use a low-pressure pulsatile irrigator with copious amounts (9 L) of antibiotic solution.132,133 Primary wound closure should be attained if possible. Although some authors recommend closing wounds in a delayed fashion or allowing healing to occur by secondary intention,125,134,135 this may mandate a return trip to the operating room and exposes the wound to a risk for secondary infection.28 The wound should be closed in layers around a drain to eliminate dead space. Some authors have additionally proposed the performance of a routine review procedure 2 to 3 days postoperatively.29 We have found this practice to be unnecessary in most patients and use clinical examination and laboratory findings to guide this decision.
Treatment of Intrathecal Pump and Spinal Cord Stimulator Infection
Treatment of intrathecal pump and spinal cord stimulator infections typically involves removal of the hardware and initial treatment with broad-spectrum antibiotics, with gradual narrowing of antibiotic coverage once culture results have been obtained.44 Removal of intrathecal drug delivery devices is, however, associated with a risk for drug withdrawal symptoms,44 and this has prompted the generation of several reports on the nonoperative treatment of such infections, even in the setting of meningitis.45,136–138 Although novel techniques such as injection and infusion of antibiotics into the drug delivery system are appealing in this setting, the efficacy of such measures has not been firmly established, and further experience is needed before routine nonsurgical treatment of neuroprosthetic devices can be recommended.
Adjuvant Surgical Techniques
Vacuum-Assisted Closure
Although there have been numerous reports of the efficacy of VAC in the general, orthopedic, and plastic surgery literature,139–141 the application of VAC in the closure of spinal wounds has only recently been reported. VAC is a technique in which negative pressure is applied to the wound, which aids in closure via mechanisms of edema removal, improvement of blood flow, stimulation of angiogenesis, stimulation of granulation tissue development, and reduced bacterial load.142–145 The VAC device is composed of a polyurethane sponge, a plastic sealant drape, and a negative-pressure suction device. The sponge should be placed within the confines of the wound bed, with care taken to ensure that it contacts all areas of the wound, thereby leaving no dead space, while at the same time avoiding skin contact. The sealant should cover an adequate amount of the surrounding area to prevent suction leak, and the device is then activated with approximately 125 mm Hg of suction. VAC dressings are then replaced every 2 to 4 days, as needed, by a skilled nurse or in the operating room. When the infection has been eradicated and the wound edges appear healthy, the patient can be returned to the operating room for delayed primary wound closure.
Although a randomized controlled trial has yet to prove the efficacy of this technique in spine surgery, potential advantages include more rapid resolution of infection, fewer trips to the operating room for wound washout, negation of the need for frequent dressing changes in open wounds, and decreased likelihood of reliance on closure by secondary intention.146 Potential complications include significant blood loss, hypoalbuminemia, toxic shock syndrome, and retained sponges.147
Irrigation-Suction Technique
Application of an irrigation-suction apparatus has also been reported sporadically in the literature.2,148 This technique involves closure of the wound over irrigation catheters and drains placed separately in both the deep and superficial compartments of the wound. An antibiotic solution, chosen according to Gram stain and culture results, is irrigated through the wound at a rate of up to 50 mL/hr, and the suction catheter is attached to a medium-pressure Hemovac. The drains are left in place for 5 to 7 days, at which point the irrigation drains are removed, with removal of the Hemovac a day later.
Other Techniques
Other surgical adjuncts for the treatment of infection are the use of antibiotic beads and muscle flaps. The use of antibiotic beads placed in the surgical bed has been reported in the treatment of spinal infections with promising results.78 High local concentrations of antibiotic are provided with this technique, which has also been shown to be effective in the treatment of open and contaminated fractures.149,150
In severe cases of wound breakdown, muscle flaps may be necessary to cover the wound and aid in wound healing by reducing dead space and enhancing antibiotic and oxygen delivery. Potential donor sites for spinal coverage include the latissimus, trapezius, gluteus, and paraspinal muscles. The assistance of a plastic surgeon is usually required for such reconstructions.151–153
Prevention
Preoperative optimization of controllable risk can aid in the reduction of infection, especially in elective cases. Preoperative smoking cessation has been linked to a decreased risk for surgical complications, although the length of cessation needed to have an effect is not clear.154 Because nutritional status is a major risk factor for the development of infection, consideration should be given to assessing indices of this variable preoperatively.47,48 Easy tests include serum albumin or the total lymphocyte count. Abnormal values may prompt a delay in elective surgery, whereas deficiencies are corrected with the aid of a nutritionist or internist.155–161 The presence of concomitant infections should be identified and treatment undertaken before surgery if possible. The presence of a common urinary tract or pulmonary infection may increase the risk for operative wound infection, especially in the setting of instrumentation.
The issue of hair shaving in skin preparation has received much attention in the cranial literature, and one recent randomized trial in the spine literature showed a significantly increased risk for infection in patients shaved preoperatively.162 Potential mechanisms by which skin shaving may increase infection risk include the loss of protective skin flora and the creation of microabrasions that may facilitate bacterial colonization.162,163 If the presence of hair will interfere with the surgical approach, it should be removed with clippers and not a razor to reduce microtrauma to the skin.
The use of prophylactic antibiotic medications in spine surgery has been considered a standard of care since the mid-1970s when Horwitz and Curtin reported a significant decrease in postoperative infection with the use of prophylactic antibiotics.19 Six subsequent randomized studies have examined the efficacy of antibiotics in preventing infection. Although none of them found a significant benefit alone, all have trended toward reduced infection with prophylaxis,164–169 and when collective data were pooled in a meta-analysis, a significant overall reduction in infection rate from 5.9% to 2.2% was obtained.170 Prophylactic antibiotics should cover skin flora and take into account local resistance patterns, as well as the history of patient reactions to antibiotics. Some authors additionally recommend screening even low-risk patients for MRSA preoperatively.171 First-generation cephalosporins, such as cefazolin, are typically used prophylactically because they are effective against gram-positive skin flora and more frequent gram-negative offenders such as Escherichia coli. The use of second- and third-generation cephalosporins should be reserved for the treatment of infection rather than prophylaxis.172 Patients who are MRSA positive or allergic to cephalosporins should be given a glycopeptide, such as vancomycin, and gentamicin. Antibiotics should be administered approximately 30 minutes to 1 hour before skin incision to ensure that adequate blood levels are achieved at skin incision and readministered at half the dose every 4 hours or with every 1500 mL of blood loss during surgery if cephalosporins are used or every 8 hours if a glycopeptide and gentamicin are given.51,171,173–175 Doses should take into account patient body habitus, with those weighing more than 80 kg, for example, receiving 2 g cefazolin preoperatively.51,173
Patients undergoing procedures involving the disk space, such as diskography or diskectomy, should also receive antibiotic prophylaxis, although the optimal choice of drug is unclear. Several animal and human studies indicate that β-lactam antibiotics and cephalosporins have poor penetration into the disk and nucleus, but clindamycin, aminoglycosides, and glycopeptides have been shown to have good penetration.24,155–161 Additionally, good results have been reported in retrospective studies in which antibiotics have been instilled directly into the disk space at the time of the procedure, either mixed with contrast material for diskography or delivered with a sponge at diskectomy.21,176 Although randomized trials have not been conducted to validate the efficacy of either method of prophylaxis, careful thought should be given to each method by the treating physician.
Several intraoperative measures may play a role in reducing infectious risk. Maintenance of strict aseptic technique is critical, involves participation of the entire operating room staff, and requires minimization of operating room traffic. Periodic release of tissue retractors may reduce ischemic damage to the paraspinal musculature.177,178 This may, in turn, reduce tissue necrosis, which may contribute to wound-healing issues and infection. Double gloving is highly recommended in spine procedures, especially those requiring instrumentation. Yinusa and colleagues recorded glove puncture in 63.6% of spine operations, the highest for any subspecialty of orthopedic surgery.179 Glove puncture rates for the inner glove in those who double-gloved was significantly less than the rates for single gloves.
Intraoperative irrigation of the wound has frequently been cited as a prophylactic measure. The use of bacitracin irrigation has been shown to offer benefit over saline irrigation in a contaminated canine osseous tissue model.180 Antibiotic irrigation has demonstrated promising results in general and orthopedic surgical procedures and is used widely.181–184 The most robust evidence to date is that of Chang and associates,185 who performed a prospective randomized trial using dilute (0.35%) povidone-iodine (Betadine) solution to irrigate tissues before bone grafting. They reported a reduction in the rate of infection from 3.4% with saline irrigation to 0% with Betadine irrigation without any adverse effects. Although this trial has not been replicated, use of Betadine irrigation is simple, and the results of this trial are strong enough to recommend routine use in posterior spinal cases. Care should be taken, however, to irrigate the spinal wound before decortication because bacitracin and Betadine have been noted to have a cytotoxic effect on osteoblasts at higher concentrations.186
Several postoperative measures have been proposed for decreasing the risk for infection, although none have proved their efficacy in randomized controlled trials. Use of a drain is controversial, with the authors of some studies reporting a decrease in infection, others reporting an increased incidence of infection, and still others reporting no change.31,187 If a drain is used, prophylactic antibiotic use for the duration of drainage has not been shown to be beneficial.171 Other postoperative measures that may reduce the risk for infection are the application of a sterile dressing, strict glucose control,6,51,69 and aggressive maintenance of nutritional status.8,47
The use of postoperative antibiotics is also controversial. A recent retrospective study reported no difference in infection rates when postoperative antibiotics were continued for just the day of surgery versus continuation for 5 to 7 days. Infections occurring in the setting of prolonged administration were, however, more likely to involve resistant organisms.172
Chang FY, Chang MC, Wang ST, et al. Can povidone-iodine solution be used safely in a spinal surgery? Eur Spine J. 2006;15:1005.
Fraser RD, Osti OL, Vernon-Roberts B. Iatrogenic discitis: the role of intravenous antibiotics in prevention and treatment. An experimental study. Spine. 1989;14:1025.
James SL, Davies AM. Imaging of infectious spinal disorders in children and adults. Eur J Radiol. 2006;58:27.
Klein JD, Hey LA, Yu CS, et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine. 1996;21:2676.
Lee MC, Wang MY, Fessler RG, et al. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004;17(6):E7.
Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975.
Mangram A, Horan T, Pearson M, et al. Guideline for prevention of surgical site infection, 1999. Center for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:96.
Mehbod AA, Ogilvie JW, Pinto MR, et al. Postoperative deep wound infections in adults after spinal fusion: management with vacuum-assisted wound closure. J Spinal Disord Tech. 2005;18:14.
Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98:149.
Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90:62.
Rubinstein E, Findler G, Amit P, et al. Perioperative prophylactic cephazolin in spinal surgery: a double-blind placebo-controlled trial. J Bone Joint Surg Br. 1994;76:99.
Weinstein MA, McCabe JP, Cammisa FPJr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422.
Wimmer C, Gluch H. Management of postoperative wound infection in posterior spinal fusion with instrumentation. J Spinal Disord. 1996;9:505.
Wimmer C, Gluch H, Franzreb M, et al. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11:124.
1 Abbey DM, Turner DM, Warson JS, et al. Treatment of postoperative wound infections following spinal fusion with instrumentation. J Spinal Disord. 1995;8:278.
2 Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg. 1997;86:975.
3 Roberts FJ, Walsh A, Wing P, et al. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine. 1998;23:366.
4 Weinstein MA, McCabe JP, Cammisa FPJr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422.
5 Banco SP, Vaccaro AR, Blam O, et al. Spine infections: variations in incidence during the academic year. Spine. 2002;27:962.
6 Olsen MA, Mayfield J, Lauryssen C, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98:149.
7 Wimmer C, Gluch H. Management of postoperative wound infection in posterior spinal fusion with instrumentation. J Spinal Disord. 1996;9:505.
8 McPhee IB, Williams RP, Swanson CE. Factors influencing wound healing after surgery for metastatic disease of the spine. Spine. 1998;23:726.
9 Blam OG, Vaccaro AR, Vanichkachorn JS, et al. Risk factors for surgical site infection in the patient with spinal injury. Spine. 2003;28:1475.
10 Rechtine GR, Bono PL, Cahill D, et al. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15:566.
11 Calderone RR, Garland DE, Capen DA, et al. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27:171.
12 Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA. 2006;296:2451.
13 Bongartz EB, Ulrich P, Fidler M, et al. Reoperation in the management of post-operative disc space infection. Zentralbl Neurochir. 1994;55:120.
14 Mastronardi L, Rychlicki F, Tatta C, et al. Spondylodiscitis after lumbar microdiscectomy: effectiveness of two protocols of intraoperative antibiotic prophylaxis in 1167 cases. Neurosurg Rev. 2005;28:303.
15 Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine. 2002;27:722.
16 Hoogland T, van den Brekel-Dijkstra K, Schubert M, et al. Endoscopic transforaminal discectomy for recurrent lumbar disc herniation: a prospective, cohort evaluation of 262 consecutive cases. Spine. 2008;33:973.
17 Hansraj KK, Cammisa FPJr, O’Leary PF, et al. Decompressive surgery for typical lumbar spinal stenosis. Clin Orthop Relat Res. 2001;384:10.
18 Casha S, Engelbrecht HA, DuPlessis SJ, et al. Suspended laminoplasty for wide posterior cervical decompression and intradural access: results, advantages, and complications. J Neurosurg Spine. 2004;1:80.
19 Horwitz NH, Curtin JA. Prophylactic antibiotics and wound infections following laminectomy for lumber disc herniation. J Neurosurg. 1975;43:727.
20 Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine. 2007;32:2310.
21 Osti OL, Fraser RD, Vernon-Roberts B. Discitis after discography. The role of prophylactic antibiotics. J Bone Joint Surg Br. 1990;72:271.
22 Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br. 1987;69:26.
23 Fraser RD, Osti OL, Vernon-Roberts B. Discitis following chemonucleolysis. An experimental study. Spine. 1986;11:679.
24 Fraser RD, Osti OL, Vernon-Roberts B. Iatrogenic discitis: the role of intravenous antibiotics in prevention and treatment. An experimental study. Spine. 1989;14:1025.
25 Perry JW, Montgomerie JZ, Swank S, et al. Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis. 1997;24:558.
26 Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine. 2001;26:1990.
27 Mirovsky Y, Floman Y, Smorgick Y, et al. Management of deep wound infection after posterior lumbar interbody fusion with cages. J Spinal Disord Tech. 2007;20:127.
28 Picada R, Winter RB, Lonstein JE, et al. Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation: incidence and management. J Spinal Disord. 2000;13:42.
29 Singh K, Rechtine G, Heller J. Postoperative Spine Infections. In: Herkowitz H, Garfon S, Eismont I, et al, editors. Rothman-Simeone The Spine. 5th ed. Philadelphia: WB Saunders; 2006:1496.
30 Moussa FW, Anglen JO, Gehrke JC, et al. The significance of positive cultures from orthopedic fixation devices in the absence of clinical infection. Am J Orthop. 1997;26:617.
31 Ho C, Sucato DJ, Richards BS. Risk factors for the development of delayed infections following posterior spinal fusion and instrumentation in adolescent idiopathic scoliosis patients. Spine. 2007;32:2272.
32 Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine. 1999;24:1909.
33 Richards BS. Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg Am. 1995;77:524.
34 Muschik M, Luck W, Schlenzka D. Implant removal for late-developing infection after instrumented posterior spinal fusion for scoliosis: reinstrumentation reduces loss of correction. A retrospective analysis of 45 cases. Eur Spine J. 2004;13:645.
35 Wimmer C, Gluch H. Aseptic loosening after CD instrumentation in the treatment of scoliosis: a report about eight cases. J Spinal Disord. 1998;11:440.
36 Gaine WJ, Andrew SM, Chadwick P, et al. Late operative site pain with Isola posterior instrumentation requiring implant removal: infection or metal reaction? Spine. 2001;26:583.
37 Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565.
38 Rauzzino MJ, Shaffrey CI, Nockels RP, et al. Anterior lumbar fusion with titanium threaded and mesh interbody cages. Neurosurg Focus. 1999;7(6):e7.
39 Huang TJ, Hsu RW, Sum CW, et al. Complications in thoracoscopic spinal surgery: a study of 90 consecutive patients. Surg Endosc. 1999;13:346.
40 Fang A, Hu SS, Endres N, et al. Risk factors for infection after spinal surgery. Spine. 2005;30:1460.
41 Deutsch H, Musacchio MJJr. Minimally invasive transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Neurosurg Focus. 2006;20(3):E10.
42 Ikuta K, Tono O, Tanaka T, et al. Surgical complications of microendoscopic procedures for lumbar spinal stenosis. Minim Invasive Neurosurg. 2007;50:145.
43 Eck JC, Hodges S, Humphreys SC. Minimally invasive lumbar spinal fusion. J Am Acad Orthop Surg. 2007;15:321.
44 Follett KA, Boortz-Marx RL, Drake JM, et al. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology. 2004;100:1582.
45 Vender JR, Hester S, Waller JL, et al. Identification and management of intrathecal baclofen pump complications: a comparison of pediatric and adult patients. J Neurosurg. 2006;104:9.
46 Rauchwerger JJ, Zoarski GH, Waghmarae R, et al. Epidural abscess due to spinal cord stimulator trial. Pain Pract. 2008;8:324.
47 Klein JD, Hey LA, Yu CS, et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine. 1996;21:2676.
48 Klein JD, Garfin SR. Nutritional status in the patient with spinal infection. Orthop Clin North Am. 1996;27:33.
49 Capen DA, Calderone RR, Green A. Perioperative risk factors for wound infections after lower back fusions. Orthop Clin North Am. 1996;27:83.
50 Carreon LY, Puno RM, Dimar JR2nd, et al. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2003;85:2089.
51 Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90:62.
52 Beiner JM, Grauer J, Kwon BK, et al. Postoperative wound infections of the spine. Neurosurg Focus. 2003;15(3):E14.
53 Patel N, Bagan B, Vadera S, et al. Obesity and spine surgery: relation to perioperative complications. J Neurosurg Spine. 2007;6:291.
54 Cheng J, Schmidt M, Muller W, et al. The obese patient. In: Benzel E, editor. Spine Surgery: Techniques, Complication Avoidance, and Management. ed 2. Philadelphia: Churchill Livingstone; 2004:1320.
55 Anaya DA, Dellinger EP. The obese surgical patient: a susceptible host for infection. Surg Infect (Larchmt). 2006;7:473.
56 Kabon B, Nagele A, Reddy D, et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274.
57 Edmiston CE, Krepel C, Kelly H, et al. Perioperative antibiotic prophylaxis in the gastric bypass patient: do we achieve therapeutic levels? Surgery. 2004;136:738.
58 Stambough JL, Beringer D. Postoperative wound infections complicating adult spine surgery. J Spinal Disord. 1992;5:277.
59 Mandelbaum BR, Tolo VT, McAfee PC, et al. Nutritional deficiencies after staged anterior and posterior spinal reconstructive surgery. Clin Orthop Relat Res. 1988;234:5.
60 Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66:71.
61 Guarnieri G, Faccini L, Lipartiti T, et al. Simple methods for nutritional assessment in hemodialyzed patients. Am J Clin Nutr. 1980;33:1598.
62 Wimmer C, Gluch H, Franzreb M, et al. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11:124.
63 Simpson JM, Silveri CP, Balderston RA, et al. The results of operations on the lumbar spine in patients who have diabetes mellitus. J Bone Joint Surg Am. 1993;75:1823.
64 Kanafani ZA, Dakdouki GK, El-Dbouni O, et al. Surgical site infections following spinal surgery at a tertiary care center in Lebanon: incidence, microbiology, and risk factors. Scand J Infect Dis. 2006;38:589.
65 Delamaire M, Maugendre D, Moreno M, et al. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29.
66 Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med. 2006;23:594.
67 Spravchikov N, Sizyakov G, Gartsbein M, et al. Glucose effects on skin keratinocytes: implications for diabetes skin complications. Diabetes. 2001;50:1627.
68 Hirsch T, Spielmann M, Zuhaili B, et al. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surg. 2008;8:5.
69 Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352.
70 Hruska LA, Smith JM, Hendy MP, et al. Continuous insulin infusion reduces infectious complications in diabetics following coronary surgery. J Card Surg. 2005;20:403.
71 Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209.
72 Lind J, Kramhoft M, Bodtker S. The influence of smoking on complications after primary amputations of the lower extremity. Clin Orthop Relat Res. 1991;267:211.
73 Sorensen LT, Horby J, Friis E, et al. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol. 2002;28:815.
74 Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238:1.
75 Kiens B, Jorgensen I, Lewis S, et al. Increased plasma HDL-cholesterol and apo A-1 in sedentary middle-aged men after physical conditioning. Eur J Clin Invest. 1980;10:203.
76 Jorgensen LN, Kallehave F, Christensen E, et al. Less collagen production in smokers. Surgery. 1998;123:450.
77 Simchen E, Stein H, Sacks TG, et al. Multivariate analysis of determinants of postoperative wound infection in orthopaedic patients. J Hosp Infect. 1984;5:137.
78 Glassman SD, Dimar JR, Puno RM, et al. Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine. 1996;21:2163.
79 Wehner M, Konig F. [Microbiological contamination of intraoperatively collected erythrocyte concentrate in mechanical autotransfusion in tumor surgery.]. Anaesthesiol Reanim. 2001;26:11.
80 Verwaal VJ, Wobbes T, Koopman-van Gemert AW, et al. Effect of perioperative blood transfusion and cell saver on the incidence of postoperative infective complications in patients with an aneurysm of the abdominal aorta. Eur J Surg. 1992;158:477.
81 Christodoulou AG, Givissis P, Symeonidis PD, et al. Reduction of postoperative spinal infections based on an etiologic protocol. Clin Orthop Relat Res. 2006;444:107.
82 Lonstein JE, Akbarnia A. Operative treatment of spinal deformities in patients with cerebral palsy or mental retardation. An analysis of one hundred and seven cases. J Bone Joint Surg Am. 1983;65:43.
83 Sponseller PD, LaPorte DM, Hungerford MW, et al. Deep wound infections after neuromuscular scoliosis surgery: a multicenter study of risk factors and treatment outcomes. Spine. 2000;25:2461.
84 Ghogawala Z, Mansfield FL, Borges LF. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine. 2001;26:818.
85 Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645.
86 Heller JG, Zdeblick TA, Kunz DA, et al. Spinal instrumentation for metastatic disease: in vitro biomechanical analysis. J Spinal Disord. 1993;6:17.
87 Martenson JAJr, Evans RG, Lie MR, et al. Treatment outcome and complications in patients treated for malignant epidural spinal cord compression (SCC). J Neurooncol. 1985;3:77.
88 Viola RW, King HA, Adler SM, et al. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine. 1997;22:2444.
89 Carmouche JJ, Molinari RW. Epidural abscess and discitis complicating instrumented posterior lumbar interbody fusion: a case report. Spine. 2004;29:E542.
90 Choma T, Burke M, Kim C, et al. Epidural abscess as a delayed complication of spinal instrumentation in scoliosis surgery: a case of progressive neurologic dysfunction with complete recovery. Spine. 2008;33:E76.
91 Rigamonti D, Liem L, Sampath P, et al. Spinal epidural abscess: contemporary trends in etiology, evaluation, and management. Surg Neurol. 1999;52:189.
92 Jonsson B, Soderholm R, Stromqvist B. Erythrocyte sedimentation rate after lumbar spine surgery. Spine. 1991;16:1049.
93 Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J. 2008;17:445.
94 Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine. 1992;17:400.
95 Fouquet B, Goupille P, Jattiot F, et al. Discitis after lumbar disc surgery. Features of “aseptic” and “septic” forms. Spine. 1992;17:356.
96 Hansen SE, Gutschik E, Karle A, et al. [Spontaneous and postoperative spondylodiscitis. A material concerning 23 patients.]. Ugeskr Laeger. 1998;160:5935.
97 Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218:211.
98 Wirtz DC, Genius I, Wildberger JE, et al. Diagnostic and therapeutic management of lumbar and thoracic spondylodiscitis—an evaluation of 59 cases. Arch Orthop Trauma Surg. 2000;120:245.
99 James SL, Davies AM. Imaging of infectious spinal disorders in children and adults. Eur J Radiol. 2006;58:27.
100 Golimbu C, Firooznia H, Rafii M. CT of osteomyelitis of the spine. AJR Am J Roentgenol. 1984;142:159.
101 Knudsen LL, Voldby B, Stagaard M. Computed tomographic myelography in spinal subdural empyema. Neuroradiology. 1987;29:99.
102 Alazraki NP. Radionuclide imaging in the evaluation of infections and inflammatory disease. Radiol Clin North Am. 1993;31:783.
103 Schauwecker DS. The scintigraphic diagnosis of osteomyelitis. AJR Am J Roentgenol. 1992;158:9.
104 Sutter CW, Shelton DK. Three-phase bone scan in osteomyelitis and other musculoskeletal disorders. Am Fam Physician. 1996;54:1639.
105 Stumpe KD, Zanetti M, Weishaupt D, et al. FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. AJR Am J Roentgenol. 2002;179:1151.
106 Schmitz A, Risse JH, Grunwald F, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography findings in spondylodiscitis: preliminary results. Eur Spine J. 2001;10:534.
107 De Winter F, Gemmel F, Van De Wiele C, et al. 18-Fluorine fluorodeoxyglucose positron emission tomography for the diagnosis of infection in the postoperative spine. Spine. 2003;28:1314.
108 Tins BJ, Cassar-Pullicino VN. MR imaging of spinal infection. Semin Musculoskelet Radiol. 2004;8:215.
109 Vaccaro AR, Shah SH, Schweitzer ME, et al. MRI description of vertebral osteomyelitis, neoplasm, and compression fracture. Orthopedics. 1999;22:67.
110 Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157.
111 Dagirmanjian A, Schils J, McHenry M, et al. MR imaging of vertebral osteomyelitis revisited. AJR Am J Roentgenol. 1996;167:1539.
112 Ledermann HP, Schweitzer ME, Morrison WB, et al. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228:506.
113 Longo M, Granata F, Ricciardi K, et al. Contrast-enhanced MR imaging with fat suppression in adult-onset septic spondylodiscitis. Eur Radiol. 2003;13:626.
114 Sandhu FS, Dillon WP. Spinal epidural abscess: evaluation with contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 1991;12:1087.
115 Grane P, Josephsson A, Seferlis A, et al. Septic and aseptic post-operative discitis in the lumbar spine—evaluation by MR imaging. Acta Radiol. 1998;39:108.
116 Van Goethem JW, Parizel PM, van den Hauwe L, et al. The value of MRI in the diagnosis of postoperative spondylodiscitis. Neuroradiology. 2000;42:580.
117 Ha KY, Kim YH. Postoperative spondylitis after posterior lumbar interbody fusion using cages. Eur Spine J. 2004;13:419.
118 Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J. 2005;14:783.
119 Jimenez-Mejias ME, de Dios Colmenero J, Sanchez-Lora FJ, et al. Postoperative spondylodiskitis: etiology, clinical findings, prognosis, and comparison with nonoperative pyogenic spondylodiskitis. Clin Infect Dis. 1999;29:339.
120 Rawlings CE3rd, Wilkins RH, Gallis HA, et al. Postoperative intervertebral disc space infection. Neurosurgery. 1983;13:371.
121 Amini A, Beisse R, Schmidt MH. Thoracoscopic debridement and stabilization of pyogenic vertebral osteomyelitis. Surg Laparosc Endosc Percutan Tech. 2007;17:354.
122 Muckley T, Schutz T, Schmidt MH, et al. The role of thoracoscopic spinal surgery in the management of pyogenic vertebral osteomyelitis. Spine. 2004;29:E227.
123 Ho C, Skaggs DL, Weiss JM, et al. Management of infection after instrumented posterior spine fusion in pediatric scoliosis. Spine. 2007;32:2739.
124 Keller RB, Pappas AM. Infection after spinal fusion using internal fixation instrumentation. Orthop Clin North Am. 1972;3:99.
125 Wenger DR, Mubarak SJ, Leach J. Managing complications of posterior spinal instrumentation and fusion. Clin Orthop Relat Res. 1992;284:24.
126 Lee MC, Wang MY, Fessler RG, et al. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004;17(6):E7.
127 Carragee EJ. Instrumentation of the infected and unstable spine: a review of 17 cases from the thoracic and lumbar spine with pyogenic infections. J Spinal Disord. 1997;10:317.
128 Sheehan E, McKenna J, Mulhall KJ, et al. Adhesion of Staphylococcus to orthopaedic metals, an in vivo study. J Orthop Res. 2004;22:39.
129 Dietze DDJr, Fessler RG, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg. 1997;86:981.
130 Deckey JE, Court C, Bradford DS. Loss of sagittal plane correction after removal of spinal implants. Spine. 2000;25:2453.
131 Weiss L, Vaccaro AR, Scuderi G, et al. Pseudoarthrosis after postoperative wound infection in the lumbar spine. J Spinal Disord. 1997;10:482.
132 Bhandari M, Adili A, Schemitsch EH. The efficacy of low-pressure lavage with different irrigating solutions to remove adherent bacteria from bone. J Bone Joint Surg Am. 2001;83:412.
133 Owens BD, Wenke JC. Early wound irrigation improves the ability to remove bacteria. J Bone Joint Surg Am. 2007;89:1723.
134 Szoke G, Lipton G, Miller F, et al. Wound infection after spinal fusion in children with cerebral palsy. J Pediatr Orthop. 1998;18:727.
135 Banta J. Combined anterior and posterior fusion for spinal deformity in myelomeningocele. Spine. 1990;15:946.
136 Boviatsis EJ, Kouyialis AT, Boutsikakis I, et al. Infected CNS infusion pumps. Is there a chance for treatment without removal? Acta Neurochir (Wien). 2004;146:463.
137 Kallweit U, Harzheim M, Marklein G, et al. Successful treatment of methicillin-resistant Staphylococcus aureus meningitis using linezolid without removal of intrathecal infusion pump. Case report. J Neurosurg. 2007;107:651.
138 Zed PJ, Stiver HG, Devonshire V, et al. Continuous intrathecal pump infusion of baclofen with antibiotic drugs for treatment of pump-associated meningitis. Case report. J Neurosurg. 2000;92:347.
139 DeFranzo AJ, Argenta LC, Marks MW, et al. The use of vacuum-assisted closure therapy for the treatment of lower-extremity wounds with exposed bone. Plast Reconstr Surg. 2001;108:1184.
140 Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563.
141 Fuchs U, Zittermann A, Stuettgen B, et al. Clinical outcome of patients with deep sternal wound infection managed by vacuum-assisted closure compared to conventional therapy with open packing: a retrospective analysis. Ann Thorac Surg. 2005;79:526.
142 Banwell PE. Topical negative pressure therapy in wound care. J Wound Care. 1999;8:79.
143 Venturi ML, Attinger CE, Mesbahi AN, et al. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) device: a review. Am J Clin Dermatol. 2005;6:185.
144 Saxena V, Hwang CW, Huang S, et al. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086.
145 Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553.
146 Mehbod AA, Ogilvie JW, Pinto MR, et al. Postoperative deep wound infections in adults after spinal fusion: management with vacuum-assisted wound closure. J Spinal Disord Tech. 2005;18:14.
147 Jones GA, Butler J, Lieberman I, et al. Negative-pressure wound therapy in the treatment of complex postoperative spinal wound infections: complications and lessons learned using vacuum-assisted closure. J Neurosurg Spine. 2007;6:407.
148 Garrido E, Rosenwasser RH. Experience with the suction-irrigation technique in the management of spinal epidural infection. Neurosurgery. 1983;12:678.
149 Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br. 1995;77:93.
150 Seligson D, Mehta S, Voos K, et al. The use of antibiotic-impregnated polymethylmethacrylate beads to prevent the evolution of localized infection. J Orthop Trauma. 1992;6:401.
151 Meiners T, Flieger R, Jungclaus M. Use of the reverse latissimus muscle flap for closure of complex back wounds in patients with spinal cord injury. Spine. 2003;28:1893.
152 Frank CJ, Brantigan J, Cronan J. Bilateral interconnected latissimus dorsi–gluteus maximus muscular cutaneous flaps for closure of subfascial infections in lumbar spinal surgery. A technical note. Spine. 1997;22:564.
153 Mitra A, Mitra A, Harlin S. Treatment of massive thoracolumbar wounds and vertebral osteomyelitis following scoliosis surgery. Plast Reconstr Surg. 2004;113:206.
154 Theadom A, Cropley M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systematic review. Tob Control. 2006;15:352.
155 Scuderi GJ, Greenberg SS, Banovac K, et al. Penetration of glycopeptide antibiotics in nucleus pulposus. Spine. 1993;18:2039.
156 Riley LH3rd, Banovac K, Martinez OV, et al. Tissue distribution of antibiotics in the intervertebral disc. Spine. 1994;19:2619.
157 Gibson MJ, Karpinski MR, Slack RC, et al. The penetration of antibiotics into the normal intervertebral disc. J Bone Joint Surg Br. 1987;69:784.
158 Boscardin JB, Ringus JC, Feingold DJ, et al. Human intradiscal levels with cefazolin. Spine. 1992;17:S145.
159 Rhoten RL, Murphy MA, Kalfas IH, et al. Antibiotic penetration into cervical discs. Neurosurgery. 1995;37:418.
160 Vaverka M, Petrzelova J. [Penetration of antibiotics into the intervertebral disk.]. Acta Chir Orthop Traumatol Cech. 1991;58:98.
161 Currier BL, Banovac K, Eismont FJ. Gentamicin penetration into normal rabbit nucleus pulposus. Spine. 1994;19:2614.
162 Celik SE, Kara A. Does shaving the incision site increase the infection rate after spinal surgery? Spine. 2007;32:1575.
163 Kretschmer T, Braun V, Richter HP. Neurosurgery without shaving: indications and results. Br J Neurosurg. 2000;14:341.
164 Bullock R, vanDellen JR, Ketelbey W, et al. A double-blind placebo-controlled trial of perioperative prophylactic antibiotics for elective neurosurgery. J Neurosurg. 1988;69:687.
165 Djindjian M, Lepresle E, Homs J-B. Antibiotic prophylaxis during prolonged clean neurosurgery. J Neurosurg. 1990;73:383.
166 Geraghty J, Feely M. Antibiotic prophylaxis in neurosurgery. J Neurosurg. 1984;60:724.
167 Young RF, Lawner PM. Perioperative antibiotic prophylaxis for prevention of postoperative neurosurgical infections. J Neurosurg. 1987;66:701.
168 Rubinstein E, Findler G, Amit P, et al. Perioperative prophylactic cephazolin in spinal surgery: a double-blind placebo-controlled trial. J Bone Joint Surgery Br. 1994;76:99.
169 Pavel A, Smith R, Ballard A, et al. Prophylactic antibiotics in elective orthopedic surgery: a prospective study of 1,591 cases. South Med J. 1977;70:50.
170 Barker FG. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery. 2002;51:391.
171 Brown EM, Pople IK, de Louvois J, et al. Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine. 2004;29:938.
172 Kanayama M, Hashimoto T, Shigenobu K, et al. Effective prevention of surgical site infection using a Centers for Disease Control and Prevention guideline–based antimicrobial prophylaxis in lumbar spine surgery. J Neurosurg Spine. 2007;6:327.
173 Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395.
174 Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281.
175 Mangram A, Horan T, Pearson M, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:96.
176 Rohde V, Meyer B, Schaller C, et al. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine. 1998;23:615.
177 Datta G, Gnanalingham KK, Peterson D, et al. Back pain and disability after lumbar laminectomy: is there a relationship to muscle retraction? Neurosurgery. 2004;54:1413.
178 Kawaguchi Y, Matsui H, Gejo R, et al. Preventive measures of back muscle injury after posterior lumbar spine surgery in rats. Spine. 1998;23:2282.
179 Yinusa W, Li YH, Chow W, et al. Glove punctures in orthopaedic surgery. Int Orthop. 2004;28:36.
180 Rosenstein BD, Wilson FC, Funderburk CH. The use of bacitracin irrigation to prevent infection in postoperative skeletal wounds. An experimental study. J Bone Joint Surg Am. 1989;71:427.
181 Hargrove R, Ridgeway S, Russell R, et al. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62:446.
182 Lord JWJr. Intraoperative antibiotic wound irrigation. Surg Gynecol Obstet. 1983;157:357.
183 Lord JW, Rossi G, Daliana M. Intraoperative antibiotic wound lavage: an attempt to eliminate postoperative infection in arterial and clean general surgical procedures. Ann Surg. 1977;185:634.
184 Lord JWJr, LaRaja RD, Daliana M, et al. Prophylactic antibiotic wound irrigation in gastric, biliary, and colonic surgery. Am J Surg. 1983;145:209.
185 Chang FY, Chang MC, Wang ST, et al. Can povidone-iodine solution be used safely in a spinal surgery? Eur Spine J. 2006;15:1005.
186 Kaysinger KK, Nicholson NC, Ramp WK, et al. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J Orthop Trauma. 1995;9:303.
187 Sasso RC, Williams JI, Dimasi N, et al. Postoperative drains at the donor sites of iliac-crest bone grafts. A prospective, randomized study of morbidity at the donor site in patients who had a traumatic injury of the spine. J Bone Joint Surg Am. 1998;80:631.