CHAPTER 40 Postoperative Infections of the Head and Brain
Before Lister’s 1867 introduction of surgical antisepsis, nearly 80% of operations were followed by infections at the surgical site and almost half of the patients died after operation.1 Despite considerable advances in our understanding of the pathogenesis of surgical infection, the introduction of rigorous aseptic practices within the operating room, and the use of prophylactic antibiotics for clean operations, infection after neurosurgical intervention remains an all too frequent occurrence. Although mortality rates have decreased markedly, postcraniotomy infections commonly require prolonged antibiotic treatment and additional surgical interventions for successful eradication and frequently result in significant morbidity, prolonged hospitalization, and increased health care expenses. The economic burden of postoperative infections is significant: the estimated average cost of a surgical site infection (SSI) attributable to methicillin-resistant Staphylococcus aureus (MRSA) is almost $100,000,2 and the overall cost of SSIs is believed to account for up to $10 billion annually in health care expenditures.3
Epidemiology and Etiology
Postoperative infections are typically categorized according to anatomic site. The Centers for Disease Control and Prevention (CDC) defines superficial incisional infections as those limited to the skin and subcutaneous tissue, whereas deep incisional infections may involve the subgaleal space and bone flap. Deep organ space infections include subdural empyema, brain abscess, and meningitis/ventriculitis. According to data from the CDC’s National Nosocomial Infection Surveillance (NNIS) program, superficial infections are responsible for 60% of SSIs after craniotomy. Meningitis is the most common deep organ space infection and represents 22% of postcraniotomy infections, whereas other intracranial infections, including subdural empyema and brain abscess, account for 14% of infections.4 Estimating the infection rate after craniotomy from the neurosurgical literature is difficult because of differences in definitions and methodology. Several large prospective studies have reported infection rates ranging from 1% to 8%.4–10 McClelland and Hall reviewed the postoperative courses of 1587 patients who underwent elective cranial operations over a 15-year period performed by a single surgeon and found an impressively low rate (0.8%) of postoperative infection.7
In accord with other studies,11,12 McClelland and Hall identified S. aureus as the causative agent for approximately half of the infections that develop after craniotomy.7 Data from the NNIS also demonstrated S. aureus to be the most common pathogen after craniotomy, followed by coagulase-negative staphylococci. Other bacteria frequently causing postcraniotomy infection included enterococci, Streptococcus spp., Pseudomonas aeruginosa, Acinetobacter spp., Citrobacter spp., Enterobacter spp., Klebsiella pneumoniae, Escherichia coli, miscellaneous other gram-negative bacilli, and yeast; each of these organisms accounted for less than 10% of episodes.13,14 Although direct spread from contiguous areas of infection is common, the causative agents tend to vary depending on the site of infection. Yang and colleagues retrospectively identified 31 patients with brain abscesses after neurosurgical procedures and found gram-negative bacilli and polymicrobial infections to be the most frequent pathogens isolated.15 Gram-negative bacilli are also the most common cause of postoperative meningitis and account for 29% to 38% of nosocomial episodes.16,17 Isolation of Propionibacterium acnes, an anaerobic gram-positive bacillus, from neurosurgical specimens has been dismissed as a contaminant because it is commensal scalp flora. However, the role of P. acnes as a causative agent of postcraniotomy infections is increasingly being recognized. Earlier reports probably underestimated its pathologic role because of its often indolent clinical manifestation, as well as difficulties associated with microbiologic isolation of the organism, specifically the need for anaerobic culture held for 10 days.18
Risk Factors for Infection and Preventive Strategies
Multiple factors combine to affect the risk for development of an SSI after craniotomy. Although it is unlikely that all postoperative infections can be completely prevented, many of the factors influencing the development of infection may be modifiable, including those attributable to the patient and those related to the surgical intervention itself. The majority of postsurgical infections are due to contamination of the wound with bacteria from the patient’s skin. Although the magnitude of contamination and the virulence of the contaminating organism certainly contribute to the rate of infection, all surgical wounds become inoculated with bacteria to some extent at the time of surgery, but in only a small percentage of patients does this contamination lead to clinical infection.19 Host defense mechanisms represent the primary barrier to establishment of infection, and these defenses may be impaired in patients undergoing craniotomy. Low levels of antibody and complement contribute to make the brain less efficient than other organs of the body at eradicating infection, and many of the underlying pathologies leading to neurosurgical intervention may significantly impair immune function. For example, patients with malignant gliomas express a variety of immune defects, including increased secretion of immunosuppressive cytokines and an increased fraction of regulatory T cells.20 Additionally, many of the adjunctive therapies used for treating brain tumors, such as corticosteroids, chemotherapy, and radiation, may result in immune compromise. Other frequent indications for craniotomy, such as trauma, have also been shown to be profoundly immunosuppressive.21 General surgical and infection control studies have identified other host factors that influence the risk for SSI, including advanced age, obesity, hypoalbuminemia, diabetes mellitus, and poor functional status.3,22–25 Gianotti and associates demonstrated the importance of nutritional status in oncologic surgery by showing that malnourished patients had improved resistance to infection after as little as 5 days of enteral nutrition.26 The increased rate of SSIs associated with these factors has been attributed to nonspecific deficits in host defense. Even though earlier reports suggested an increased rate of postoperative infection after general surgical procedures in patients infected with human immunodeficiency virus (HIV),27,28 several more recent retrospective studies performed since the advent of highly active antiretroviral therapy (HAART) have failed to demonstrate an association between SSI rates and HIV infection.29–31 Although the influence of these intrinsic factors on the rate of SSI after neurosurgical intervention has not been established in prospective studies, optimization of immune function through minimization of corticosteroid use, adequate nutritional support, and optimized perioperative glucose control may all be potentially helpful in the prevention of postcraniotomy infections.
Several factors specific to craniotomy have been identified as increasing the risk for postoperative infection. In a prospective multicenter trial, Korinek identified postoperative CSF leakage and early subsequent reoperation as independent risk factors for SSI, thus suggesting that careful attention to closure techniques and meticulous hemostasis may potentially result in lower rates of postoperative infection.5 Several other studies confirmed CSF leakage as a major risk factor for infection.8,32–35 Additionally, Korinek identified four independent predictors of postoperative infection after craniotomy: surgery lasting longer than 4 hours, emergency surgery, clean-contaminated and contaminated surgery, and neurosurgical intervention in the preceding month.5 Valentini and coworkers also observed an increased relative risk of 24.3 for postoperative infection in elective clean craniotomies lasting longer than 3 hours.36 The association between longer duration of surgery and infection has not been defined precisely, but plausible explanations include greater complexity of the surgery and prolonged exposure of the wound to bacterial contamination.37
A variety of other risk factors associated with infection after craniotomy have been less reliably demonstrated, including placement of drains or intracranial pressure monitors, poor neurological status, paranasal sinus entry, diabetes mellitus, and foreign body implantation (other than shunts).5,8,38 Synthetic dural substitutes are foreign bodies and might represent a potential risk factor for infection in comparison to autologous graft materials such as pericranium, temporalis fascia, or fascia lata. Actual evidence demonstrating increased rates of infection with their use, however, is limited. Malliti and coauthors reported a nonsignificantly increased incidence of deep wound infections after craniotomy with the use of a nonresorbable polyester urethane synthetic dural graft (Neuro-Patch, B. Braun, Boulogne, France).39 Postoperative CSF leaks were also significantly more frequent when using the synthetic dural substitute, thus limiting the ability of this study to determine whether use of the Neuro-Patch independently increased the risk for infection. The presence of nonresorbable dural substitutes may also impair the potential for an infected wound to be successfully treated because these grafts may become chronically colonized and could require removal to eradicate the infection.40 A variety of nonautologous, resorbable collagen dural substitutes are currently available, and their relationship to surgical infection has not been well explored. McCall and coworkers reported the uncomplicated use of several of these materials in a small number of patients in the setting of contaminated wounds, a finding suggesting that they may not impede clearance of infection.40 The use of Gliadel wafers (MGI Pharma, Inc., Bloomington, MN), which contain 1,3-bis-(2-chloroethyl)-1-nitrosurea, for the treatment of malignant gliomas has also been associated with an increased incidence of postoperative infection. McGovern and coauthors reported a 29% rate of infection in cases associated with insertion of Gliadel wafers between 1996 and 1999.41 Subsequent reports with larger patient populations, however, have not revealed statistically significant differences in the rate of infection with Gliadel use.41,42
Multiple prospective randomized clinical studies and a meta-analysis have validated the effectiveness of preoperative antibiotics in reducing the incidence of SSIs after craniotomy.11,33,34,43,44 Hugh Cairns described the first trial of a modern prophylactic antibiotic in neurosurgery in 1947 when he reported sprinkling a “light frosting” of penicillin powder directly onto the brain in 670 patients and thought that the results were superior to those of historical controls.45,46 In 1979, Malis demonstrated the ability of a prophylactic antibiotic regimen (vancomycin and an aminoglycoside) to reduce the incidence of SSI after craniotomy.47 Since these initial reports, a variety of antibiotic regimens have been used for effective surgical prophylaxis, and the choice of agent should be guided by individual institutional data on frequently recovered pathogens and their resistance profiles. In general, the antibiotic chosen for prophylaxis should be safe, provide an appropriate narrow spectrum of coverage against relevant bacteria, and be administered for a defined, brief course. The Surgical Infection Prevention (SIP) project has recommended three performance measures for monitoring appropriate antimicrobial prophylaxis use: selection of an appropriate antibiotic, administration within 1 hour before incision (2 hours is allowed for the administration of vancomycin and fluoroquinolones), and discontinuation of the antibiotic within 24 hours after surgery is completed.48 Antibiotics with short half-lives such as cefazolin should be readministered every 3 to 4 hours during surgery to ensure adequate drug levels throughout the operation, including the time of wound closure.49 Prolonged use of antibiotics beyond 24 hours postoperatively has not shown a greater benefit, may increase the risk for other nosocomial infections, and might encourage the emergence of multidrug-resistant pathogens.50,51 Despite wide acceptance of these basic measures, compliance with them continues to remain poor in the United States.52 Prophylactic antibiotics for craniotomy are covered in more detail elsewhere in this book.
Additional perioperative factors that may potentially reduce the risk for postoperative infection include maintenance of normothermia and supplemental oxygenation. Several prospective randomized trials evaluating active warming of patients during colorectal surgery to maintain normothermia have shown decreased rates of infectious complications; the proposed mechanism of action is support of adequate blood flow and tissue oxygenation at the surgical site.53–55 Supplemental administration of oxygen may also assist in preventing infection by increasing tissue oxygen levels and facilitating oxidative killing of bacteria by neutrophils. Belda and colleagues conducted a prospective trial to evaluate the postoperative infection rate in patients randomized to receive either 30% oxygen or 80% oxygen during elective colorectal surgery.56 The group receiving 80% oxygen had a 54% reduction in wound infections. Studies evaluating the role of supplemental oxygen or temperature control for neurosurgical interventions have yet to be performed.
Other perioperative risk reduction considerations include surgical site preparation and environmental control within the operating room. Although no evidence has been found that preoperative hair removal reduces the incidence of postoperative infection, any hair removal that is performed should be done as close to the time of surgery as possible and clippers used rather than a razor to minimize the number of bacteria that colonize the inevitable small cuts and abrasions that develop from shaving.57–60 Several antiseptic skin preparations have been used (chlorhexidine, iodophor compounds, alcohol), but no agent has been shown to be more effective than another.61 To provide effective antisepsis, these agents must remain on the skin until they dry naturally, with avoidance of any pooling. Theoretically, adhesive barrier drapes with antiseptic embedded within the adhesive may prevent regrowth of bacteria at the surgical site throughout the operative procedure; however, their ability to reduce the incidence of SSI has not been proved.62 Similarly, preoperative bathing or showering with an antiseptic skin product has no evidence in support of it.63 The operating room environment represents another important consideration in the reduction of SSIs. The number of health care workers within the operating room and traffic throughout the procedure should be kept to a minimum because bacterial shedding increases with activity and can potentially result in increased airborne contamination.58 Ensuring adequate ventilation minimizes the particulates and bacteria in the perioperative environment, and the use of high-efficiency particulate air (HEPA) filters has been shown to reduce the rate of SSI development after orthopedic implant surgery.52
Principles of Treatment
Immune defenses within the brain are rarely adequate to control infection once it has been established. Postoperative infections tend to be particularly difficult to resolve because of the complex anatomic changes resulting from craniotomy and the frequent involvement of virulent organisms. Early and decisive intervention is critical to limit morbidity, and the keystone of successful treatment is effective source control (i.e., drainage of abscesses and infected fluid collections and débridement of necrotic tissue).64 Once source control has been achieved, initiation of appropriate antibiotic therapy is necessary to eliminate any residual local infection.
The ability of antimicrobials to treat postcraniotomy infections successfully is a function of multiple factors. Selection of an antibiotic regimen should be based on the capacity of the antibiotic to penetrate the infected tissue effectively and to exhibit activity against the suspected pathogen. Bactericidal rather than bacteriostatic agents are generally preferred because of the inefficient opsonization and phagocytic capabilities within the brain.65 Maximal bactericidal activity is achieved only when the peak antibiotic concentration at the site of infection exceeds the minimal inhibitory concentration of the causative organism by at least 5- to 10-fold.66,67 Most antibiotic agents enter the CNS predominantly by passive diffusion down a concentration gradient, with physical barriers such as the blood-brain and blood-CSF barriers functioning as the primary determinants of drug distribution. Inflammation at the site of infection may facilitate entry of drugs across these barriers and into the brain, but not all postoperative infections are accompanied by marked inflammation, and concomitant treatment with corticosteroids may further impair drug entry.68 Other inherent physiochemical properties of the antimicrobial agent may affect its penetration into the CNS, including molecular weight, lipophilicity, protein binding, and ionization state. Optimal antibiotic administration and dosing rely on an understanding of the pharmacodynamic properties of the agent and the susceptibility profile of the microorganism. In the absence of data from prospective randomized clinical trials evaluating the success rates of specific antibacterial agents, recommendations for the treatment of postcraniotomy infections are based largely on the results of previous experience, along with consideration of the complex physiologic, bacteriologic, and pharmacologic factors involved.
Empirical treatment of postoperative infections should include broad coverage for the full spectrum of potential pathogens, including resistant gram-positive organisms (e.g., MRSA) and gram-negative bacilli (e.g., Pseudomonas and Acinetobacter spp.). Failure to include an antibacterial agent with activity against the responsible bacterium may result in severe neurological sequelae or death.65 Suitable empirical regimens for postcraniotomy infections typically include a combination of vancomycin and a second drug such as a third- or fourth-generation cephalosporin having antipseudomonal activity (e.g., ceftazidime, cefepime) or a carbapenem (e.g., meropenem). Antibiotic selection can be tailored once speciation and susceptibility testing from a microbiologic specimen are available.
Vancomycin has weaker activity against staphylococcal infections relative to β-lactams69 and decreased penetration into the CNS because of its large molecular weight (1449 daltons).65 Even in the presence of significant inflammation, concentrations of vancomycin may be critically low at the site of infection,70 and substitution of a β-lactamase–resistant penicillin (e.g., nafcillin, oxacillin) for vancomycin is appropriate, except in the setting of resistance or hypersensitivity. First-generation cephalosporins (e.g., cefazolin) have relatively poor CNS penetration and are not recommended for the treatment of deep wound infections.
Third-generation cephalosporins (specifically cefotaxime, ceftriaxone, and ceftazidime) are often used for the treatment of CNS and postcraniotomy infections because of their low toxicity, good CNS penetration, and excellent in vitro activity against many of the responsible bacterial pathogens. Administration of these agents in high doses achieves therapeutic concentrations within brain abscess cavities.71,72 Carbapenems such as imipenem (with cilastatin) and meropenem also cover a broad antimicrobial spectrum and have been used successfully for the treatment of bacterial brain abscesses.73–75 These agents, principally imipenem-cilastatin, are associated with an increased seizure risk, and their use in patients with postcraniotomy infections should be considered primarily for resistant pathogens. From a pharmacokinetic viewpoint, fluoroquinolones (levofloxacin, ciprofloxacin, moxifloxacin) are attractive agents for the treatment of CNS infection because of their lipophilicity and low molecular mass. The usefulness of these agents, however, is limited by a high rate of bacterial resistance, increased seizure potential (albeit modest), and a relative paucity of data regarding their clinical effectiveness for postoperative CNS infections.76
Newer agents that may prove useful for the treatment of resistant staphylococcal infections include linezolid and daptomycin. Linezolid has bacteriostatic activity against both MRSA and vancomycin-resistant enterococci and bactericidal activity against most streptococci. Linezolid may be administered intravenously or orally and has excellent bioavailability. Experience with this agent for the treatment of postcraniotomy infections is limited, and potential side effects include reversible myelosuppression and irreversible peripheral neuropathy.69 Daptomycin is a novel cyclic lipopeptide antibiotic that shows better in vitro microbicidal activity against MRSA than either vancomycin or linezolid and has been used primarily for the treatment of skin and soft tissue infections. Animal models of meningitis suggest that it may be an effective therapeutic agent in a setting of meningeal inflammation77–79; human studies to date are lacking.
Rifampin is a broad-spectrum antimicrobial that may have a role in the adjunctive treatment of infections associated with foreign body implantation or bone flap osteomyelitis. These types of infections are notoriously difficult to eradicate because of their resistance to host defense mechanisms and poor penetration of antimicrobials. Most foreign body infections are caused by staphylococci growing in biofilms consisting of bacteria clustered together in an extracellular matrix attached to the foreign body.80 Depletion of metabolic substances within the biofilm causes the microbes to enter a slowly growing (sessile) state, which renders them up to 1000 times more resistant to most antimicrobial agents than their free-living (planktonic) counterparts.69,81–84 Rifampin is one of just a few agents that can effectively penetrate biofilms and kill organisms in the sessile phase of growth. Because of the rapid emergence of resistance, rifampin must always be used in combination with a second active agent. In vitro data, experimental animal models, and several randomized clinical trials suggest that dual therapy that includes rifampin may be better than monotherapy for orthopedic hardware–related staphylococcal infections in terms of bone sterilization and cure rates.69,85,86 This experience makes adjunctive therapy with rifampin an attractive consideration for difficult postcraniotomy staphylococcal infections associated with retained hardware or osteitis. Caution must be used with rifampin therapy because of its very large number of drug interactions. Through cytochrome P-450 enzyme induction, rifampin increases the metabolism of many substrates, including antiseizure drugs, anticoagulants, and immunosuppressive and chemotherapeutic agents.87
Aminoglycosides have excellent activity against aerobic gram-negative bacilli, including P. aeruginosa, as well as synergistic activity with β-lactams against aerobic gram-positive cocci. Systemic use of aminoglycosides is limited by their toxicity profile and a narrow therapeutic window. Penetration into CSF and across the blood-brain barrier is poor.88 Polymyxins (e.g., colistin) also have activity against a broad array of gram-negative bacilli but fell out of favor because of nephrotoxicity.89 As a result of the retained activity of polymyxins against multidrug-resistant gram-negative bacilli, including P. aeruginosa and Acinetobacter baumannii, this class again plays a role in difficult to treat infections. Similar to the aminoglycosides, the distribution of systemically administered polymyxins to CSF is poor. Intraventricular antibiotic administration bypasses the blood-brain barrier, can achieve much higher CSF concentrations than with systemic administration, and has been used successfully in multiple case reports.90–92 Intraventricular antibiotic dosing has been associated with neurotoxicity, however, in experimental animal models and a small number of case reports.93,94 Currently, there are no well-established data to support adjunctive intraventricular administration when a systemically delivered antimicrobial can achieve adequate microbicidal concentrations in CSF.
Superficial Infections and Bone Flap Osteomyelitis
Clinical Manifestations
Superficial infections are the most frequent infectious complication after craniotomy. Although every surgical patient is at risk for postoperative infection, a variety of factors may contribute to create an environment that is suboptimal for wound healing and more favorable for infection, including repeat operative intervention, poor tissue quality, impaired vascular supply, radiation injury, nutritional deficiencies, and the presence of foreign bodies. The role of foreign material in facilitating infection was first reported by Elek and Conen, who demonstrated a 10,000-fold increased risk for skin abscesses in the presence of suture material.95,96 Continuous activation of granulocytes by foreign bodies may lead to local impairment of phagocytic ability, thereby reducing the amount of bacterial contamination needed to establish infection.18,66
Superficial infections are typically manifested as local erythema, swelling, and tenderness at the craniotomy site or as wound breakdown and suppurative drainage. With progressive infection, systemic signs such as malaise, fever, or chills may develop. The presence of neurological symptoms such as meningismus, altered mental status, or new focal deficits strongly suggests the coexistence of deep wound infection. The most common pathogenic agents of superficial wound infections are gram-positive cocci, including S. aureus, coagulase-negative staphylococci, and P. acnes.35,53,58,97 Infection of the bone flap most often results from either direct bacterial inoculation at the time of surgery or extension of infection from the adjacent subgaleal or epidural compartments.
Diagnostic Imaging and Laboratory Data
The presence of superficial wound infection is often clinically apparent; however, imaging studies can frequently assist in defining the anatomic extent of infection (especially extension through the dura), as well as possible precipitating factors, such as entry into the mastoid air cells or paranasal sinuses during craniotomy. Computed tomography (CT) or magnetic resonance imaging (MRI) may reveal fluid collections in the subgaleal or epidural spaces that require surgical evacuation or extension of infection beyond the dura and into the subdural space or brain parenchyma. Imaging studies may also show evidence of bone flap destruction suggestive of osteomyelitis. Unfortunately, diffusion-weighted imaging, which is very sensitive to spontaneous intracerebral abscesses, is frequently unreliable in diagnosing the presence of superficial infection after craniotomy.98
Measurement of the erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) level may provide some assistance in detecting infection and monitoring the response to therapy. These acute-phase reactants are normally elevated after craniotomy and return toward baseline levels by the fifth postoperative day.99 Although these markers are highly nonspecific, prolonged elevation or a secondary increase in their levels may indicate the development of bone flap infection. Failure of the ESR or CRP level to decline after institution of therapy may signify persistent infection and the need for prolonged antibiotic therapy or further diagnostic imaging to detect recurrence.
Treatment
Treatment of superficial wound infections depends on the extent of infection. Superficial cellulitis, a spreading infection of subcutaneous tissue without deeper infection of the subgaleal space or bone flap, is generally treated with oral or intravenous antibiotic therapy. Oral agents typically used to treat gram-positive bacteria include first-generation cephalosporins (e.g., cefazolin) or β-lactamase–resistant penicillins (e.g., dicloxacillin). In patients with rapidly spreading infection, prominent systemic symptoms, or significant comorbidity, initial antibiotic administration should be by the intravenous route until the symptoms improve and fever abates.100
Devitalization and devascularization of the bone flap at the time of craniotomy present a unique challenge in the treatment of infection because of impaired delivery of host defense mechanisms and antibacterial agents. Treatment options include antibiotic therapy alone, débridement with replacement of the bone flap, or surgical débridement with removal of the bone flap. Prolonged antibiotic therapy may control the clinical manifestations of infection but rarely leads to complete eradication, with frequent recrudescence after discontinuation of the antibio. Removal of the infected bone flap followed by delayed cranioplasty allows the best chance of clearing the initial infection; however, this treatment approach entails multiple surgical interventions and at least temporary cosmetic deformity while predisposing to the possibility of subsequent brain injury with a long-term risk for cranioplasty infection.13,101–103 Several small case series have reported clinical resolution of infection with preservation of the bone flap. Bruce and Bruce reported salvage of the bone flap in 11 of 13 patients simply by mechanical débridement of the bone flap to remove any necrotic or purulent debris and soaking the bone in antibiotic-containing solution and povidine.104 Closed suction antibiotic solution irrigation systems have also been used to treat bone flap osteomyelitis with varying degrees of success.102,105 In selecting a therapeutic approach for each patient, the risks associated with salvaging the bone flap must be weighed against the hazards of the infection itself.
Hyperbaric oxygen (HBO) therapy is sometimes used to treat complicated superficial infections, including those involving the bone flap. HBO therapy increases oxygen tension in infected tissues, thereby improving oxidative killing of aerobic bacteria by phagocytic cells and providing a direct bactericidal effect on anaerobic organisms such as P. acnes. Larsson and associates used HBO to treat postcraniotomy infections successfully without removing the bone flap in 15 of 19 patients and in 3 of 6 patients with acrylic cranioplasties.106 HBO therapy may also prove useful in the treatment of poorly healing, secondarily infected wounds such as those frequently associated with radiation injury.107 Irradiation may impair wound healing by multiple mechanisms, including microvascular injury and ischemic damage, fibroblast dysfunction, and alterations in the synthesis of growth factors.108 In addition to helping clear the infection, HBO therapy may also promote neoangiogenesis and reverse the vascular compromise present at the wound.6,109 Limitations of HBO therapy include the cost of treatment and the need for multiple sessions. The possibility of increased tumor growth with the use of HBO in patients with malignancy has been raised as a potential concern, although clinical and experimental evidence of a tumor stimulatory effect is lacking.110–114 The use of local rotational or pedicled flaps or vascularized myocutaneous free flaps represents another potential treatment option for chronic postoperative infections that cannot be eradicated with conventional surgical débridement and bone flap removal.115
Subdural Empyema
Clinical Manifestations
Although spontaneously occurring subdural empyemas are typically accompanied by fever and headache, followed by the rapid development of focal neurological deficits, altered mental status, and seizures, this fulminant manifestation is rarely seen in patients in whom subdural empyema develops after craniotomy.97,116 Hlavin and associates reviewed their experience in 27 patients with postoperative subdural and epidural empyemas and found that only a third were febrile and 85% were without headache.16 The most common findings were evidence of superficial wound infection and the presence of diffuse encephalopathy. In almost half of the patients, the subdural empyema occurred more than 1 month after the craniotomy. Seizures were present in 25% of patients with postoperative subdural empyema.
Diagnostic Imaging and Laboratory Data
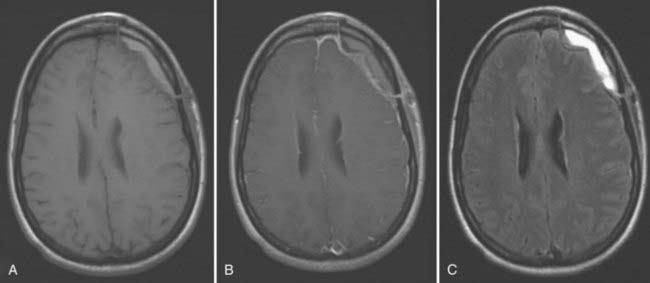
Sterile extra-axial fluid collections are commonly noted on postoperative imaging studies, and differentiation from infected purulent fluids (empyema) may be difficult in the absence of overt clinical signs. In subdural empyema, non–contrast-enhanced CT typically demonstrates a crescent-shaped fluid collection that is slightly more dense than CSF and located beneath the craniotomy flap or adjacent to the falx. Increased signal intensity is usually seen on T1-weighted and fluid-attenuated inversion recovery (FLAIR) MRI sequences because of the increased protein concentration of an empyema relative to CSF. Peripheral enhancement of the fluid collection is common (Fig. 40-1). Unfortunately, these imaging characteristics are nonspecific and may also be seen with postoperative hematomas or sterile effusions. The presence of restricted diffusion on MRI may be helpful, although the absence of restricted diffusion does not exclude the presence of infection; 29% of confirmed postoperative subdural infections did not demonstrate diffusion abnormalities in one study.98 Progressive enlargement of the fluid collection or unexplained edema in adjacent cerebral cortex may be helpful in identifying the existence of infection.
Laboratory data findings in patients with subdural empyema are typically nonspecific. Hlavin and colleagues found an elevated white blood cell count in 63% of their patients with postoperative infections, whereas ESR values were often within the normal range.16 CSF findings may be normal or show evidence of a parameningeal reaction but rarely yield definitive evidence of infection. Additionally, lumbar puncture is frequently contraindicated in the setting of subdural empyema because of the possibility of cerebral herniation. In one study of 280 patients with spontaneous subdural empyemas who underwent lumbar puncture, 33 were thought to have experienced neurological deterioration as a direct result of the procedure.117
Treatment
Although postoperative subdural empyemas tend to have a more insidious course than spontaneous infections, early diagnosis and aggressive treatment are necessary to prevent spread of infection intraparenchymally and to avoid complications such as thrombophlebitis and venous infarction. Surgical drainage is usually necessary because antimicrobial agents do not reliably sterilize the empyema.116 The goals of surgery are to evacuate the purulent collection completely and achieve adequate decompression of the brain when significant edema is associated with the infection. Although the optimal surgical approach (craniotomy versus bur-hole drainage) is debated, craniotomy is generally advocated because it ensures maximal drainage of the collection and allows inspection of adjacent anatomic areas and removal of the bone flap if necessary.
Empirical antibiotic therapy should be started as soon as material for culture has been obtained or earlier if surgical intervention must be delayed. The antibiotics chosen should be active against both skin flora and gram-negative bacilli because the latter have been shown to account for about half of subdural empyemas after craniotomy.16 Vancomycin plus a third-generation cephalosporin with good activity against Pseudomonas, such as ceftazidime, is a frequently used empirical regimen, with adjustment according to individual institutional profiles of resistance. The duration of antibiotic therapy is typically 4 to 6 weeks. The role of imaging, especially diffusion imaging, in the evaluation of response to therapy or duration of therapy has been only minimally explored.118,119
Brain Abscess
Clinical Manifestations
Localized intraparenchymal abscesses may develop after craniotomy as a result of direct bacterial seeding or by extension of more superficial infection through an incompetent dura. Although the development of a brain abscess after craniotomy is rare, it is likely that common postoperative sequelae, such as small fluid collections or compromised areas of contused or ischemic brain, serve as a nidus for abscess formation. Once infection has been established, abscess development begins as a localized area of cerebritis characterized by perivascular inflammation and edema formation. It then progresses to a discrete focus of necrotic, purulent material surrounded by a well-vascularized capsule composed of fibroblasts and reactive collagen.120
Clinically, the classic triad of headache, fever, and focal neurological deficit is rarely present, and the signs and symptoms of postoperative abscess are frequently nonspecific. Fever is present in only about half of affected patients, and its absence should not be used to exclude the diagnosis.15,72,121–123 Symptoms are often related to the presence of an expanding, irritative mass lesion and include altered level of consciousness, nausea, vomiting, and seizures. In a series of 31 patients with nosocomial brain abscess after neurosurgical intervention, Yang and coauthors reported that 17 had a disturbance in consciousness.15 The prognosis is much poorer in patients with significant alterations in mental status or rapid progression of symptoms, and a high degree of suspicion is necessary to recognize the existence of infection as early as possible.72,124 Seizures develop in about 20% of patients with spontaneous brain abscess and appear to occur at a similar rate with postoperative abscesses.15,122,125
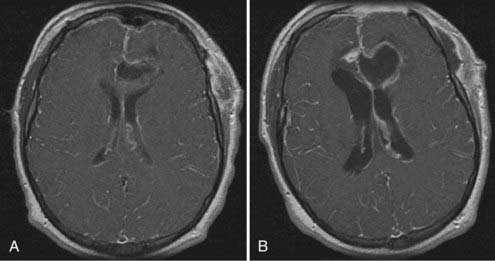
Abrupt worsening of preexisting headache accompanied by new onset of meningismus may indicate rupture of a brain abscess into the cerebral ventricle, a condition associated with a high mortality rate that may require more aggressive medical and surgical intervention. Intraventricular rupture of a brain abscess (IVROBA) may also be manifested clinically by sudden neurological deterioration with obtundation or coma (Fig. 40-2). The pathophysiology of this decline is probably multifactorial, including both the development of severe widespread meningoencephalitis and alterations in CSF flow causing an increase in intracranial pressure. Zeidman and colleagues, in an extensive review of the literature from 1950 to 1993, identified 129 reported cases of IVROBA with a combined mortality rate of 85%.126 Furthermore, although the overall mortality for brain abscesses decreased significantly over successive decades, the mortality rate for IVROBA remained consistent throughout this period. Lee and coauthors reported the most recent data on IVROBA outcome in a series of 62 patients treated between 1986 and 2005.127 Their mortality rate was 27%; however, the overall rate of poor neurological outcome, including severe disability, persistent vegetative state, or death, was nearly 50%. Clinical and radiographic prediction of patients at increased risk for intraventricular rupture should prompt more urgent surgical intervention and decrease the incidence of IVROBA and its sequelae. Neither the specific infecting organism nor abscess size is associated with risk for rupture, although multiloculated abscesses have been correlated with increased risk.127 Not surprisingly, decreased distance from the abscess capsule to the ventricular wall has also been demonstrated to correlate with the rate of intraventricular rupture.127,128 These data correspond to findings in pathologic studies revealing that abscess capsule formation tends to be more complete on the cortical side of a brain abscess than on the ventricular side, which probably contributes to the increased rate of intraventricular rupture with deep-seated abscesses.129 The presence of localized ventricular enhancement on CT has also been shown to herald impending intraventricular rupture and clinical deterioration.130 Once IVROBA has occurred, radiographic imaging often reveals diffuse ependymal and meningeal enhancement and the presence of debris within the ventricles (see Fig. 40-2). Hydrocephalus accompanies IVROBA in about 50% of cases, and septation of the ventricles may occur as a delayed complication of intraventricular rupture.127,131 Takeshita and colleagues found that all 20 of their patients with IVROBA reported prodromal symptoms of severe headache and meningeal irritation before the onset of their rapid clinical decline.130 Decreased morbidity seems to be associated with IVROBA in patients taking antibiotics or with sterile abscesses at the time of rupture, thus suggesting that antimicrobial therapy should be instituted rapidly in patients exhibiting prodromal symptoms or with abscesses adjacent to the ventricular system.128,130
Diagnostic Imaging and Laboratory Data
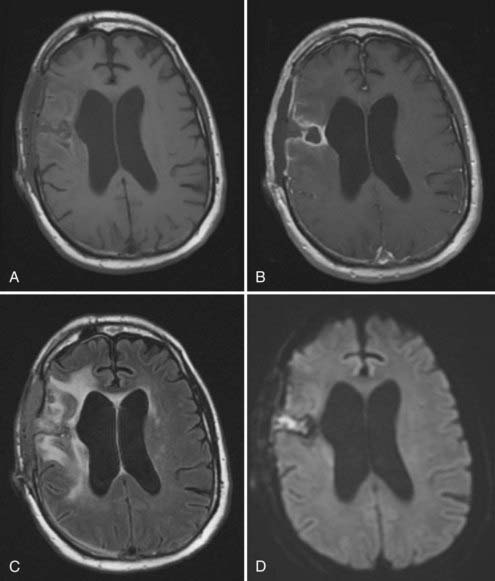
Because of the often nonspecific clinical symptoms associated with brain abscess and the frequent absence of fever, neuroimaging typically plays a dominant role in the diagnosis of postoperative intraparenchymal infection. Imaging studies can also help define the anatomic extent of infection to guide surgical intervention and assist in evaluating the response to therapy. The radiographic features of brain abscess depend on its stage of progression. During the cerebritis stage, CT reveals a poorly defined area of low attenuation with a mass effect and significant edema. As a capsule begins to form around the infection, peripheral enhancement increases and the center of the lesion becomes progressively hypodense. Cerebritis typically appears on MRI as an area of high T2-weighted signal with patchy enhancement.132 Subsequent capsule development is characterized on T1-weighted images as a ring of gadolinium enhancement surrounding a necrotic cavity of low signal intensity (Fig. 40-3). Concurrent treatment with corticosteroids, radiation therapy, and chemotherapy may alter the radiographic progression of abscess development.133 Corticosteroids have been shown to reduce the thickness of the abscess capsule and the extent of contrast enhancement on both CT and MRI.76 Additionally, the presence of peripheral enhancement around a resection cavity is often nonspecific in the postcraniotomy setting and may reflect residual or recurrent tumor, treatment effect, infarction, or resolving hematoma. Diffusion-weighted MRI has demonstrated a high degree of specificity and sensitivity in differentiating spontaneous abscess from other ring-enhancing lesions, and its application to the diagnosis of postoperative brain abscess may prove useful. In a retrospective analysis we reviewed the diffusion-weighted findings in 50 patients with microbiologically confirmed postoperative infections and found evidence of abnormally restricted diffusion in all patients with intraparenchymal infection; much higher false-negative rates on diffusion-weighted imaging were found with more superficial infections such as epidural or subgaleal abscesses.98 Importantly, the presence of restricted diffusion is not specific for infection, and correlation with apparent diffusion coefficient and T2-weighted MRI sequences or CT is necessary to evaluate for blood products that may cause a “T2 shine-through effect” in which the infection appears bright on diffusion-weighted images. The role of other advanced MRI sequences potentially useful for the diagnosis of infection, such as spectroscopy or perfusion imaging, has largely been unexplored in the postoperative setting.134
Similar to the nonspecific clinical and radiographic manifestations of postcraniotomy brain abscess, there are no laboratory findings that definitively establish the diagnosis. Peripheral leukocytosis is frequently absent, and although the ESR and CRP level are usually elevated, normal values may occur in patients with proven infection.135,136 Blood cultures seldom yield a causative organism but should be performed to assess for a possible hematogenous source of infection. CSF analysis is rarely helpful and typically reveals only a nonspecific elevation in protein level and cell count, and lumbar puncture is frequently contraindicated because of increased intracranial pressure and risk for cerebral herniation.123
Treatment
The approach to treatment of postoperative brain abscesses is similar to that for spontaneous abscesses, although the increased frequency of multiantibiotic-resistant bacterial pathogens and the extension of infection into adjacent anatomic compartments in the postoperative setting may complicate treatment. The general goals of treatment are to relieve the mass effect, improve clinical symptoms, and fully resolve the infection. In most cases, a combination of surgical drainage and a prolonged course of intravenous antibiotics is required. Surgical options include open operative drainage or excision of the lesion and stereotactic aspiration. Both options have been used successfully in the treatment of postcraniotomy abscess, although stereotactic aspiration of brain abscesses is associated with a higher incidence of recurrence and the need for repeat surgical intervention.109,137 Additionally, postcraniotomy abscesses are often multiloculated, which predicts a higher chance of recurrence after initial drainage.138 Open surgical excision also allows débridement of any associated parameningeal infection and removal of necrotic debris or foreign bodies.
Once specimens have been obtained for culture, empirical antibiotic therapy should be started based on Gram stain results and institutional data regarding the probable causative agents and their antibiotic resistance patterns. Typically, vancomycin and a third- or fourth-generation cephalosporin with antipseudomonal activity (e.g., ceftazidime, cefepime) are appropriate initial choices, although studies comparing the relative efficacy of various treatment regimens have not been performed. Metronidazole may be added to the empirical regimen for coverage of anaerobic organisms if an otic, paranasal sinus, or mastoid source of infection is suspected based on the surgical intervention performed. In critically ill patients in whom urgent surgical drainage is not possible, initiation of broad-spectrum antibiotic therapy before culture results are available may be necessary. Mampalam and coworkers reported, however, that 30% of patients who received antibiotics preoperatively had sterile cultures, thus potentially resulting in inappropriate medical treatment or the need for prolonged therapy with multiple antibiotics.139
High-dose intravenous antibiotics have conventionally been administered for 6 to 8 weeks in patients with brain abscesses. Frequently, this is followed by a course of oral antibiotic therapy if a suitable agent is available, although the efficacy and necessity of this approach have not been established.72 Shorter course therapies have also been reported to be effective,137,140 and length of treatment should be guided by the virulence of the causative organism, clinical therapeutic response, and serial neuroimaging findings. Progressive enlargement of the abscess or failure of the abscess to become smaller despite treatment of a susceptible organism with an appropriate antibiotic should prompt repeat surgical drainage and microbiologic reassessment. Several reports have also advocated placement of drains into the abscess for postsurgical drainage and intracavitary administration of antibiotics for difficult to treat infections72,141,142; however, this form of therapy should be used with caution given the minimal evidence in support of its efficacy and the potential for neurotoxicity, including seizures.13 Brain abscesses associated with intraventricular rupture may also require more aggressive treatment to reduce morbidity and mortality. Intraventricular administration of antibiotics may be considered for the treatment of bacterial agents that are susceptible only to antibacterials with poor blood-CSF penetration. Multiple reports have demonstrated increased CSF antibiotic concentrations, successful clearance of ventricular infection, and minimal toxicity after intraventricular administration of antibiotics, most commonly vancomycin, aminoglycosides, and colistin.90,143–147
Bacterial Meningitis
Clinical Manifestations
Bacterial meningitis is relatively uncommon after neurosurgical procedures and complicates less than 1% of craniotomies.7,12 Although the clinical course of nosocomial meningitis tends to be less fulminant than that of community-acquired meningitis, rapid diagnosis and implementation of antimicrobial therapy are critical because the mortality rate may exceed 20% if treatment is delayed.68,148 The typical symptoms of meningitis tend to be present in patients with postoperative bacterial meningitis, including fever, headache, and neck stiffness; however, these symptoms may also occur in patients without infection after craniotomy, especially of the posterior fossa.
Complicating the diagnosis of postoperative meningitis is the clinically similar condition of a sterile postoperative meningitis presumed to be due to chemical irritation, as first described by Cushing and Bailey in 1928.149 Subsequent authors have shown that aseptic (chemical) meningitis is responsible for 60% to 75% of all cases of postoperative clinical meningitis and that it occurs most frequently in children and after posterior fossa surgery.148,150 Despite its frequent occurrence, the etiology of aseptic meningitis remains incompletely understood, but it is presumed to be caused by irritation from blood breakdown products or from factors released by surgical materials such as dural substitutes. Diagnosis of aseptic meningitis requires negative CSF Gram staining and cultures, and the patient must recover fully without the administration of antibiotics. Corticosteroids typically provide symptomatic relief in patients with aseptic chemical meningitis.
Diagnostic Imaging and Laboratory Data
Unfortunately, differentiation between chemical and bacterial meningitis is frequently problematic, and no single clinical sign or diagnostic test distinguishes between the two entities with certainty. Neuroimaging studies rarely assist in the diagnosis of postoperative meningitis because the characteristic imaging sign of meningeal enhancement can also be seen in up to 80% of postcraniotomy patients who do not have infections.151 CT or MRI may, however, reveal secondary complications of meningitis, including hydrocephalus, parameningeal abscess, or ischemia/infarction related to vasculitis and thrombosis of superficial vessels.
CSF culture data remain the “gold standard” for diagnosis of postoperative bacterial meningitis, although the definition of nosocomial meningitis used by the U.S. Centers for Disease Control and Prevention does allow the diagnosis of meningitis to be made without and Prevention positive CSF cultures under certain circumstances only if a physician institutes “appropriate antimicrobial therapy.”152 CSF Gram staining is highly insensitive for infection. Several studies have shown that Gram staining is positive in only 25% to 50% of cases of culture-confirmed bacterial meningitis.17,153 CSF hypoglycorrhachia and pleocytosis with neutrophilic predominance are common findings in both aseptic and bacterial meningitis, although one study found that CSF white blood cell counts greater than 7500 cells/µL and glucose concentrations lower than 10 mg/dL were not present in any patient with aseptic meningitis.153 Unfortunately, laboratory findings within this range were not very common in patients who did have confirmed bacterial infections.
A variety of alternative diagnostic tests have been investigated to better distinguish between these two entities, with several retrospective studies identifying CSF lactate concentrations greater than 4 mmol/L and IL-1β levels greater than 90 ng/L predicting the presence of bacterial meningitis with good sensitivity and specificity in postsurgical patients.154,155 Elevated CSF lactate probably results from a combination of bacterial production, anaerobic glycolysis, and metabolism by CSF leukocytes,156 whereas IL-1β is a key inflammatory mediator in the response to meningeal infection.157 Although data regarding the clinical utility of these newer assays are promising, until they have been well validated in prospective studies, the recommendation remains that all patients with clinical and laboratory features consistent with postoperative meningitis receive empirical antibiotic treatment until CSF culture results are confirmed to be sterile.13,158
Treatment
The choice of empirical coverage depends on local bacterial infection and resistance patterns; however, typically the combination of vancomycin and a third-generation cephalosporin with antipseudomonal activity (e.g., ceftazidime) is appropriate. If the patient is not deteriorating clinically, CSF culture results remain sterile, and the treating clinician believes the original clinical syndrome to have been consistent with aseptic chemical meningitis, antibiotics may be discontinued after several days, provided that antibiotic therapy had not been started before CSF was obtained for culture. Using this algorithm, Zarrouck and colleagues demonstrated that the duration of antibiotic treatment of aseptic meningitis could be decreased from a mean of 11 days to 3.5 days, with no cases of diagnosed aseptic meningitis later proving to be misdiagnosed bacterial meningitis.148 However, the possibility of misdiagnosis suggests that the patient should be kept under close clinical observation for a time after treatment with antibiotics is stopped.
Alexander JW. The contributions of infection control to a century of surgical progress. Ann Surg. 1985;201:423.
Barker FG2nd. Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery. 1994;35:484.
Bruce JN, Bruce SS. Preservation of bone flaps in patients with postcraniotomy infections. J Neurosurg. 2003;98:1203.
Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422.
Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21.
Farrell CJ, Hoh BL, Pisculli ML, et al. Limitations of diffusion-weighted imaging in the diagnosis of postoperative infections. Neurosurgery. 2008;62:577.
Forgacs P, Geyer CA, Freidberg SR. Characterization of chemical meningitis after neurological surgery. Clin Infect Dis. 2001;32:179.
Hlavin ML, Kaminski HJ, Fenstermaker RA, et al. Intracranial suppuration: a modern decade of postoperative subdural empyema and epidural abscess. Neurosurgery. 1994;34:974.
Korinek AM. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidemiologie Hygiene et Prevention. Neurosurgery. 1997;41:1073.
Korinek AM, Golmard JL, Elcheick A, et al. Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg. 2005;19:155.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209.
Larsson A, Engstrom M, Uusijarvi J, et al. Hyperbaric oxygen treatment of postoperative neurosurgical infections. Neurosurgery. 2002;50:287.
Lee TH, Chang WN, Su TM, et al. Clinical features and predictive factors of intraventricular rupture in patients who have bacterial brain abscesses. J Neurol Neurosurg Psychiatry. 2007;78:303.
Lutsar I, Friedland IR. Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin Pharmacokinet. 2000;39:335.
Malis LI. Prevention of neurosurgical infection by intraoperative antibiotics. Neurosurgery. 1979;5:339.
Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250.
Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763.
McClelland S3rd, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45:55.
Nau R, Sorgel F, Prange HW. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin Pharmacokinet. 1998;35:223.
Seydoux C, Francioli P. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394.
Takeshita M, Kagawa M, Izawa M, et al. Current treatment strategies and factors influencing outcome in patients with bacterial brain abscess. Acta Neurochir (Wien). 1998;140:1263.
Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;3:CD004122.
Zarrouk V, Vassor I, Bert F, et al. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007;44:1555.
Zeidman SM, Geisler FH, Olivi A. Intraventricular rupture of a purulent brain abscess: case report. Neurosurgery. 1995;36:189.
1 Alexander JW. The contributions of infection control to a century of surgical progress. Ann Surg. 1985;201:423.
2 Engemann JJ, Carmeli Y, Cosgrove SE, et al. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis. 2003;36:592.
3 Anderson DJ, Chen LF, Schmader KE, et al. Poor functional status as a risk factor for surgical site infection due to methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2008;29:832.
4 Gantz NM. Nosocomial central nervous system infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:415-439.
5 Korinek AM. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidemiologie Hygiene et Prevention. Neurosurgery. 1997;41:1073.
6 Korinek AM, Baugnon T, Golmard JL, et al. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2008;62(Suppl 2):532.
7 McClelland S3rd, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45:55.
8 Mollman HD, Haines SJ. Risk factors for postoperative neurosurgical wound infection. A case-control study. J Neurosurg. 1986;64:902.
9 Raggueneau JL, Cophignon J, Kind A, et al. [Analysis of infectious sequelae of 1000 neurosurgical operations. Effects of prophylactic antibiotherapy.]. Neurochirurgie. 1983;29:229.
10 Lietard C, Thebaud V, Besson G, et al. Risk factors for neurosurgical site infections: an 18-month prospective survey. J Neurosurg. 2008;109:729.
11 van Ek B, Dijkmans BA, van Dulken H, et al. Antibiotic prophylaxis in craniotomy: a prospective double-blind placebo-controlled study. Scand J Infect Dis. 1988;20:633.
12 Wang KW, Chang WN, Huang CR, et al. Post-neurosurgical nosocomial bacterial meningitis in adults: microbiology, clinical features, and outcomes. J Clin Neurosci. 2005;12:647.
13 Kastenbauer S, Pfister H, Wispelwey B, et al. Brain Abscess. In: Scheld WM, Whitley RJ, Marra CM, editors. Infections of the Central Nervous System. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:479-507.
14 Moore CC, Farr BM, Scheld WM. Central nervous system infections. In: Bennett JV, Jarvis WR, Brachman PS, editors. Bennett & Brachman’s Hospital Infections. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007:573-581.
15 Yang KY, Chang WN, Ho JT, et al. Postneurosurgical nosocomial bacterial brain abscess in adults. Infection. 2006;34:247.
16 Hlavin ML, Kaminski HJ, Fenstermaker RA, et al. Intracranial suppuration: a modern decade of postoperative subdural empyema and epidural abscess. Neurosurgery. 1994;34:974.
17 Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21.
18 Nisbet M, Briggs S, Ellis-Pegler R, et al. Propionibacterium acnes: an under-appreciated cause of post-neurosurgical infection. J Antimicrob Chemother. 2007;60:1097.
19 Burke JF. Identification of the sources of staphylococci contaminating the surgical wound during operation. Ann Surg. 1963;158:898.
20 Vega EA, Graner MW, Sampson JH. Combating immunosuppression in glioma. Future Oncol. 2008;4:433.
21 Barie PS, Eachempati SR. Surgical site infections. Surg Clin North Am. 2005;85:1115.
22 Harbarth S, Huttner B, Gervaz P, et al. Risk factors for methicillin-resistant Staphylococcus aureus surgical site infection. Infect Control Hosp Epidemiol. 2008;29:890.
23 Haridas M, Malangoni MA. Predictive factors for surgical site infection in general surgery. Surgery. 2008;144:496.
24 Howard JM, Ehrlich E, Spitzer JJ, et al. Hyperlipemia in patients with acute pancreatitis. Ann Surg. 1964;160:210.
25 Neumayer L, Hosokawa P, Itani K, et al. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178.
26 Gianotti L, Braga M, Nespoli L, et al. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122:1763.
27 Albaran RG, Webber J, Steffes CP. CD4 cell counts as a prognostic factor of major abdominal surgery in patients infected with the human immunodeficiency virus. Arch Surg. 1998;133:626.
28 Robinson G, Wilson SE, Williams RA. Surgery in patients with acquired immunodeficiency syndrome. Arch Surg. 1987;122:170.
29 Horberg MA, Hurley LB, Klein DB, et al. Surgical outcomes in human immunodeficiency virus–infected patients in the era of highly active antiretroviral therapy. Arch Surg. 2006;141:1238.
30 Habermann B, Eberhardt C, Kurth AA. Total joint replacement in HIV positive patients. J Infect. 2008;57:41.
31 Friedman M, Landsberg R, Tanyeri H, et al. Endoscopic sinus surgery in patients infected with HIV. Laryngoscope. 2000;110:1613.
32 Korinek AM, Golmard JL, Elcheick A, et al. Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg. 2005;19:155.
33 Barker FG2nd. Efficacy of prophylactic antibiotics for craniotomy: a meta-analysis. Neurosurgery. 1994;35:484.
34 Bullock R, van Dellen JR, Ketelbey W, et al. A double-blind placebo-controlled trial of perioperative prophylactic antibiotics for elective neurosurgery. J Neurosurg. 1988;69:687.
35 Dempsey R, Rapp RP, Young B, et al. Prophylactic parenteral antibiotics in clean neurosurgical procedures: a review. J Neurosurg. 1988;69:52.
36 Valentini LG, Casali C, Chatenoud L, et al. Surgical site infections after elective neurosurgery: a survey of 1747 patients. Neurosurgery. 2008;62:88.
37 Dellinger EP, Ehrenkranz NJ, Jarvis WR. Surgical site infections. In: Bennett JV, Jarvis WR, Brachman PS, editors. Bennett & Brachman’s Hospital Infections. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007:583-598.
38 Erman T, Demirhindi H, Gocer AI, et al. Risk factors for surgical site infections in neurosurgery patients with antibiotic prophylaxis. Surg Neurol. 2005;63:107.
39 Malliti M, Page P, Gury C, et al. Comparison of deep wound infection rates using a synthetic dural substitute (Neuro-Patch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery. 2004;54:599.
40 McCall TD, Fults DW, Schmidt RH. Use of resorbable collagen dural substitutes in the presence of cranial and spinal infections—report of 3 cases. Surg Neurol. 2008;70:92.
41 McGovern PC, Lautenbach E, Brennan PJ, et al. Risk factors for postcraniotomy surgical site infection after 1,3-bis (2-chloroethyl)-1-nitrosourea (Gliadel) wafer placement. Clin Infect Dis. 2003;36:759.
42 Attenello FJ, Mukherjee D, Datoo G, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15:2887.
43 Blomstedt GC, Kytta J. Results of a randomized trial of vancomycin prophylaxis in craniotomy. J Neurosurg. 1988;69:216.
44 Young RF, Lawner PM. Perioperative antibiotic prophylaxis for prevention of postoperative neurosurgical infections. A randomized clinical trial. J Neurosurg. 1987;66:701.
45 Pennybacker JB, Taylor M, Cairns H. Penicillin in the prevention of infection during operations on the brain and spinal cord. Lancet. 1947;250:159.
46 Cairns H. Penicillin; Principles of Application in Surgical Infections. P V Expo Discuss Congr Soc Int Chir, 12 Congr. 1947;1:229.
47 Malis LI. Prevention of neurosurgical infection by intraoperative antibiotics. Neurosurgery. 1979;5:339.
48 Fry DE. Basic aspects of and general problems in surgical infections. Surg Infect (Larchmt). 2001;2(Suppl 1):S3.
49 Zanetti G, Giardina R, Platt R. Intraoperative redosing of cefazolin and risk for surgical site infection in cardiac surgery. Emerg Infect Dis. 2001;7:828.
50 Fukatsu K, Saito H, Matsuda T, et al. Influences of type and duration of antimicrobial prophylaxis on an outbreak of methicillin-resistant Staphylococcus aureus and on the incidence of wound infection. Arch Surg. 1997;132:1320.
51 Namias N, Harvill S, Ball S, et al. Cost and morbidity associated with antibiotic prophylaxis in the ICU. J Am Coll Surg. 1999;188:225.
52 Yoshida M, Nabeshima T, Gomi H, et al. Technology and the prevention of surgical site infections. J Surg Educ. 2007;64:302.
53 Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209.
54 Melling AC, Ali B, Scott EM, et al. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358:876.
55 Wong PF, Kumar S, Bohra A, et al. Randomized clinical trial of perioperative systemic warming in major elective abdominal surgery. Br J Surg. 2007;94:421.
56 Belda FJ, Aguilera L, Garcia de la Asuncion J, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA. 2005;294:2035.
57 Tang K, Yeh JS, Sgouros S. The Influence of hair shave on the infection rate in neurosurgery. A prospective study. Pediatr Neurosurg. 2001;35:13.
58 Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250.
59 Tanner J, Woodings D, Moncaster K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2006;3:CD004122.
60 Miller JJ, Weber PC, Patel S, et al. Intracranial surgery: to shave or not to shave? Otol Neurotol. 2001;22:908.
61 Edwards PS, Lipp A, Holmes A. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2004;3:CD003949.
62 Webster J, Alghamdi AA. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database Syst Rev. 2007;4:CD006353.
63 Webster J, Osborne S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. 2007;2:CD004985.
64 Schein M, Marshall J. Source control for surgical infections. World J Surg. 2004;28:638.
65 Nau R, Sorgel F, Prange HW. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin Pharmacokinet. 1998;35:223.
66 Tauber MG, Doroshow CA, Hackbarth CJ, et al. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J Infect Dis. 1984;149:568.
67 Lutsar I, Friedland IR. Pharmacokinetics and pharmacodynamics of cephalosporins in cerebrospinal fluid. Clin Pharmacokinet. 2000;39:335.
68 Kourtopoulos H, Holm SE, Norrby SR. The influence of steroids on the penetration of antibiotics into brain tissue and brain abscesses. An experimental study in rats. J Antimicrob Chemother. 1983;11:245.
69 Trampuz A, Zimmerli W. Antimicrobial agents in orthopaedic surgery: prophylaxis and treatment. Drugs. 2006;66:1089.
70 Viladrich PF, Gudiol F, Linares J, et al. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother. 1991;35:2467.
71 Green HT, O’Donoghue MA, Shaw MD, et al. Penetration of ceftazidime into intracranial abscess. J Antimicrob Chemother. 1989;24:431.
72 Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763.
73 Asensi V, Carton JA, Maradona JA, et al. Imipenem therapy of brain abscesses. Eur J Clin Microbiol Infect Dis. 1996;15:653.
74 Meis JF, Groot-Loonen J, Hoogkamp-Korstanje JA. A brain abscess due to multiply-resistant Enterobacter cloacae successfully treated with meropenem. Clin Infect Dis. 1995;20:1567.
75 Wong VK, Wright HTJr, Ross LA, et al. Imipenem/cilastatin treatment of bacterial meningitis in children. Pediatr Infect Dis J. 1991;10:122.
76 Tunkel AR. Brain abscess. In: Mandell GL, Douglas RG, Bennett JE, et al, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005:1150-1164.
77 Cottagnoud P, Pfister M, Acosta F, et al. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob Agents Chemother. 2004;48:3928.
78 Gerber P, Stucki A, Acosta F, et al. Daptomycin is more efficacious than vancomycin against a methicillin-susceptible Staphylococcus aureus in experimental meningitis. J Antimicrob Chemother. 2006;57:720.
79 Grandgirard D, Schurch C, Cottagnoud P, et al. Prevention of brain injury by the nonbacteriolytic antibiotic daptomycin in experimental pneumococcal meningitis. Antimicrob Agents Chemother. 2007;51:2173.
80 Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422.
81 Costerton JW. Introduction to biofilm. Int J Antimicrob Agents. 1999;11:217.
82 Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135.
83 Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167.
84 Zimmerli W, Waldvogel FA, Vaudaux P, et al. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487.
85 Perlroth J, Kuo M, Tan J, et al. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med. 2008;168:805.
86 Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant–related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537.
87 Baciewicz AM, Chrisman CR, Finch CK, et al. Update on rifampin and rifabutin drug interactions. Am J Med Sci. 2008;335:126.
88 Rahal JJJr, Hyams PJ, Simberkoff MS, et al. Combined intrathecal and intramuscular gentamicin for gram-negative meningitis. Pharmacologic study of 21 patients. N Engl J Med. 1974;290:1394.
89 Kaye KS, Kaye D. Polymyxins (polymyxin B and colistin). In: Mandell GL, Douglas RG, Bennett JE, et al, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005:435-440.
90 Kasiakou SK, Rafailidis PI, Liaropoulos K, et al. Cure of post-traumatic recurrent multiresistant gram-negative rod meningitis with intraventricular colistin. J Infect. 2005;50:348.
91 Ng J, Gosbell IB, Kelly JA, et al. Cure of multiresistant Acinetobacter baumannii central nervous system infections with intraventricular or intrathecal colistin: case series and literature review. J Antimicrob Chemother. 2006;58:1078.
92 Quinn AL, Parada JP, Belmares J, et al. Intrathecal colistin and sterilization of resistant Pseudomonas aeruginosa shunt infection. Ann Pharmacother. 2005;39:949.
93 Parrish KL, Tachibana M, Domer FR, et al. Ototoxicity of intracisternal gentamicin in cats. Ann Otol Rhinol Laryngol. 1981;90:255.
94 Watanabe I, Hodges GR, Dworzack DL, et al. Neurotoxicity of intrathecal gentamicin: a case report and experimental study. Ann Neurol. 1978;4:564.
95 Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man; a study of the problems of wound infection. Br J Exp Pathol. 1957;38:573.
96 Borges LF. Infections in neurologic surgery. Host defenses. Neurosurg Clin N Am. 1992;3:275.
97 Post EM, Modesti LM. “Subacute” postoperative subdural empyema. J Neurosurg. 1981;55:761.
98 Farrell CJ, Hoh BL, Pisculli ML, et al. Limitations of diffusion-weighted imaging in the diagnosis of postoperative infections. Neurosurgery. 2008;62:577.
99 Mirzayan MJ, Gharabaghi A, Samii M, et al. Response of C-reactive protein after craniotomy for microsurgery of intracranial tumors. Neurosurgery. 2007;60:621.
100 Swartz MN, Pasternack MS. Cellulitis and subcutaneous tissue infections. In: Mandell GL, Douglas RG, Bennett JE, et al, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005:1172-1194.
101 Blomstedt GC. Considerations of infections after craniotomy. In: Schmidek HH, Roberts DW, editors. Schmidek & Sweet Operative Neurosurgical Techniques: Indications, Methods, and Results. 5th ed. Philadelphia: Saunders; 2006:1600-1606.
102 Auguste KI, McDermott MW. Salvage of infected craniotomy bone flaps with the wash-in, wash-out indwelling antibiotic irrigation system. Technical note and case series of 12 patients. J Neurosurg. 2006;105:640.
103 Bullitt E, Lehman RA. Osteomyelitis of the skull. Surg Neurol. 1979;11:163.
104 Bruce JN, Bruce SS. Preservation of bone flaps in patients with postcraniotomy infections. J Neurosurg. 2003;98:1203.
105 Chou SN, Erickson DL. Craniotomy infections. Clin Neurosurg. 1976;23:357.
106 Larsson A, Engstrom M, Uusijarvi J, et al. Hyperbaric oxygen treatment of postoperative neurosurgical infections. Neurosurgery. 2002;50:287.
107 Neovius EB, Lind MG, Lind FG. Hyperbaric oxygen therapy for wound complications after surgery in the irradiated head and neck: a review of the literature and a report of 15 consecutive patients. Head Neck. 1997;19:315.
108 Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol. 1997;42:99.
109 Hall WA, Truwit CL. The surgical management of infections involving the cerebrum. Neurosurgery. 2008;62(Suppl 2):519.
110 Daruwalla J, Christophi C. The effect of hyperbaric oxygen therapy on tumour growth in a mouse model of colorectal cancer liver metastases. Eur J Cancer. 2006;42:3304.
111 Feldmeier JJ, Heimbach RD, Davolt DA, et al. Does hyperbaric oxygen have a cancer-causing or -promoting effect? A review of the pertinent literature. Undersea Hyperb Med. 1994;21:467.
112 Schonmeyr BH, Wong AK, Reid VJ, et al. The effect of hyperbaric oxygen treatment on squamous cell cancer growth and tumor hypoxia. Ann Plast Surg. 2008;60:81.
113 Shi Y, Lee CS, Wu J, et al. Effects of hyperbaric oxygen exposure on experimental head and neck tumor growth, oxygenation, and vasculature. Head Neck. 2005;27:362.
114 Tang H, Sun Y, Xu C, et al. Effects of hyperbaric oxygen therapy on tumor growth in murine model of PC-3 prostate cancer cell line. Urology. 2009;73:205.
115 Mikami T, Minamida Y, Sugino T, et al. Free flap transfer for the treatment of intractable postcraniotomy subdural empyemas and epidural abscesses. Neurosurgery. 2007;60:ONS83.
116 Tunkel AR. Subdural empyema, epidural abscess, and suppurative intracranial thrombophlebitis. In: Mandell GL, Douglas RG, Bennett JE, et al, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005:1164-1171.
117 Nathoo N, Nadvi SS, van Dellen JR, et al. Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery. 1999;44:529.
118 Fanning NF, Laffan EE, Shroff MM. Serial diffusion-weighted MRI correlates with clinical course and treatment response in children with intracranial pus collections. Pediatr Radiol. 2006;36:26.
119 Wong AM, Zimmerman RA, Simon EM, et al. Diffusion-weighted MR imaging of subdural empyemas in children. AJNR Am J Neuroradiol. 2004;25:1016.
120 Kastrup O, Wanke I, Maschke M. Neuroimaging of infections. NeuroRx. 2005;2:324.
121 Wispelwey B, Scheld WM. Brain abscess. Semin Neurol. 1992;12:273.
122 Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1.
123 Chun CH, Johnson JD, Hofstetter M, et al. Brain abscess. A study of 45 consecutive cases. Medicine (Baltimore). 1986;65:415.
124 Seydoux C, Francioli P. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394.
125 Tseng JH, Tseng MY. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006;65:557.
126 Zeidman SM, Geisler FH, Olivi A. Intraventricular rupture of a purulent brain abscess: case report. Neurosurgery. 1995;36:189.
127 Lee TH, Chang WN, Su TM, et al. Clinical features and predictive factors of intraventricular rupture in patients who have bacterial brain abscesses. J Neurol Neurosurg Psychiatry. 2007;78:303.
128 Takeshita M, Kagawa M, Izawa M, et al. Current treatment strategies and factors influencing outcome in patients with bacterial brain abscess. Acta Neurochir (Wien). 1998;140:1263.
129 Waggener JD. The pathophysiology of bacterial meningitis and cerebral abscesses: an anatomical interpretation. Adv Neurol. 1974;6:1.
130 Takeshita M, Kawamata T, Izawa M, et al. Prodromal signs and clinical factors influencing outcome in patients with intraventricular rupture of purulent brain abscess. Neurosurgery. 2001;48:310.
131 Isono M, Wakabayashi Y, Nakano T, et al. Treatment of brain abscess associated with ventricular rupture—three case reports. Neurol Med Chir (Tokyo). 1997;37:630.
132 Foerster BR, Thurnher MM, Malani PN, et al. Intracranial infections: clinical and imaging characteristics. Acta Radiol. 2007;48:875.
133 Fischer SA, Harris AA. Brain abscess. In: Gorbach SL, Bartlett JG, Blacklow NR, editors. Infectious Diseases. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:1308-1320.
134 Ferreira NP, Otta GM, do Amaral LL, et al. Imaging aspects of pyogenic infections of the central nervous system. Top Magn Reson Imaging. 2005;16:145.
135 Grimstad IA, Hirschberg H, Rootwelt K. 99mTc-hexamethylpropyleneamine oxime leukocyte scintigraphy and C-reactive protein levels in the differential diagnosis of brain abscesses. J Neurosurg. 1992;77:732.
136 Vogelsang JP, Wehe A, Markakis E. Postoperative intracranial abscess—clinical aspects in the differential diagnosis to early recurrence of malignant glioma. Clin Neurol Neurosurg. 1998;100:11.
137 Cavusoglu H, Kaya RA, Turkmenoglu ON, et al. Brain abscess: analysis of results in a series of 51 patients with a combined surgical and medical approach during an 11-year period. Neurosurg Focus. 2008;24(6):E9.
138 Su TM, Lan CM, Tsai YD, et al. Multiloculated pyogenic brain abscess: experience in 25 patients. Neurosurgery. 2003;52:1075.
139 Mampalam TJ, Rosenblum ML. Trends in the management of bacterial brain abscesses: a review of 102 cases over 17 years. Neurosurgery. 1988;23:451.
140 Sjolin J, Eriksson N, Arneborn P, et al. Penetration of cefotaxime and desacetylcefotaxime into brain abscesses in humans. Antimicrob Agents Chemother. 1991;35:2606.
141 Broggi G, Franzini A, Peluchetti D, et al. Treatment of deep brain abscesses by stereotactic implantation of an intracavitary device for evacuation and local application of antibiotics. Acta Neurochir (Wien). 1985;76:94.
142 Mahmood A, Abad RM, Updegrove JH. Brain abscess treated by continuous antibiotic perfusion: technical note. Neurol Res. 1993;15:63.
143 Pfausler B, Haring HP, Kampfl A, et al. Cerebrospinal fluid (CSF) pharmacokinetics of intraventricular vancomycin in patients with staphylococcal ventriculitis associated with external CSF drainage. Clin Infect Dis. 1997;25:733.
144 Pfausler B, Spiss H, Beer R, et al. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J Neurosurg. 2003;98:1040.
145 Grabb PA, Albright AL. Intraventricular vancomycin-induced cerebrospinal fluid eosinophilia: report of two patients. Neurosurgery. 1992;30:630.
146 Wirt TC, McGee ZA, Oldfield EH, et al. Intraventricular administration of amikacin for complicated gram-negative meningitis and ventriculitis. J Neurosurg. 1979;50:95.
147 Gump WC, Walsh JW. Intrathecal colistin for treatment of highly resistant Pseudomonas ventriculitis. Case report and review of the literature. J Neurosurg. 2005;102:915.
148 Zarrouk V, Vassor I, Bert F, et al. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007;44:1555.
149 Cushing H, Bailey P. Tumors arising from the Blood-Vessels of the Brain; Angiomatous Malformation and Hemangioblastomas. Springfield, IL: C. C. Thomas; 1928. x, 219, 11
150 Carmel PW, Greif LK. The aseptic meningitis syndrome: a complication of posterior fossa surgery. Pediatr Neurosurg. 1993;19:276.
151 Burke JW, Podrasky AE, Bradley WGJr. Meninges: benign postoperative enhancement on MR images. Radiology. 1990;174:99.
152 Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004:1659-1702.
153 Forgacs P, Geyer CA, Freidberg SR. Characterization of chemical meningitis after neurological surgery. Clin Infect Dis. 2001;32:179.
154 Leib SL, Boscacci R, Gratzl O, et al. Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Dis. 1999;29:69.
155 Lopez-Cortes LF, Marquez-Arbizu R, Jimenez-Jimenez LM, et al. Cerebrospinal fluid tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and interleukin-8 as diagnostic markers of cerebrospinal fluid infection in neurosurgical patients. Crit Care Med. 2000;28:215.
156 Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267.
157 Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864.
158 Working Party of the British Society for Antimicrobial Chemotherapy. The management of neurosurgical patients with postoperative bacterial or aseptic meningitis or external ventricular drain-associated ventriculitis. Infection in Neurosurgery. Br J Neurosurg. 2000;14:7.