Chapter 174 Postoperative Imaging
The management of chronic degenerative spinal conditions in the United States is estimated to cost nearly $85 billion annually.1 Spine surgery to treat degenerative disease represents a large portion of this expenditure, and procedures such as lumbar fusion have increased dramatically over the last 20 years.2 In the past, routine fusion procedures for common indications such as spondylolisthesis did not necessarily require postoperative imaging, especially with the widespread use of fluoroscopy for intraoperative placement of instrumentation. Imaging was reserved for those situations in which preoperative symptoms did not resolve or new symptoms arose postoperatively. However, the margin for error in instrumentation position is now much narrower, despite a lack of evidence of neurologic injury. In addition, determining the correct level of surgery is paramount, and when anatomic abnormalities such as transitional vertebral segments raise doubt about the precise level of pathology, imaging is increasingly utilized. Increasingly, 3D intraoperative imaging, with real-time navigation, is being used to confirm the correct level and instrumentation position.
Normal Postoperative Spine
Analysis of the postoperative image requires understanding not only the anatomy but also the surgical approach, the goals of surgery, and any potential complications. Imaging abnormalities in the spine related to surgical trauma persist typically through the first 6 to 8 weeks. The extent of bony removal at surgery greatly influences the postoperative imaging appearance. The removal of bone may allow dorsal expansion of the dura through defects, which can resemble a pseudomeningocele, seroma, or postoperative hematoma. Additionally, asymmetry in the paraspinal muscle-fat planes related to surgical dissection is a normal finding. Edema may obscure muscular margins. MRI T2-weighted images, particularly fast spin-echo sequences, provide excellent resolution of water content, allowing visualization of edema or fluid collections.3
When the intervertebral disc has been violated or partially excised, distinction between epidural tissue edema and the disrupted anulus fibrosus and recurrent disc herniation or residual disc material can be complex.4 Well-vascularized granulation tissue usually enhances homogeneously with gadolinium administration, whereas disc material typically enhances poorly or, at best, heterogeneously. Nonenhanced MRI is approximately 85% accurate in achieving this distinction,5 whereas enhanced MRI is 95% to 100% accurate.6 Both fibrosus and disc can be hyperintense relative to bone on T2-weighted images. An important technical point for these evaluations is the timing of imaging following contrast administration. If imaging is delayed more than several minutes beyond the administration of the contrast medium, gadolinium seeps across the disc, which is then seen to enhance more homogeneously. To avoid this phenomenon and to increase the specificity for detecting fibrosus, imaging should commence within 2 minutes of gadolinium injection.
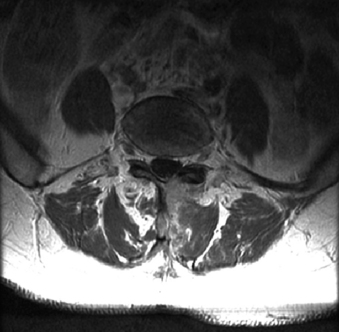
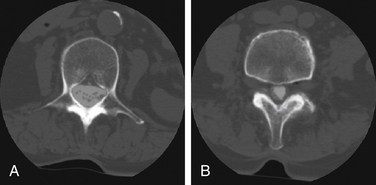
Epidural fibrosus is present in all patients following spine surgery to some degree. In some patients, formation of epidural granulation tissue is excessive and has been reported to contribute to residual pain and radiculopathy after surgery.7,8 However, other reports have contested this claim because granulation tissue is not thought to exert a compressive force.9 In the setting of recent discectomy and residual postoperative pain, enhancement of the epidural fibrosus, as well as the end plates and anulus fibrosus, may be easily mistaken for early signs of infection (Fig. 174-1).
Van Goethem et al. prospectively studied 34 patients with excellent outcomes following lumbar discectomy and obtained MRIs at 6 weeks and 6 months.10 Imaging findings in these asymptomatic patients mimicked diagnostic findings in patients with complications. Twenty percent of patients had recurrent disc herniation, and an additional 20% demonstrated nerve root enhancement at 6 weeks. At 6 months, contrast enhancement was seen along the surgical tract in all patients. Likewise, facet joint enhancement was also seen bilaterally and was attributed to mechanical stress during laminectomy.
A similar study reported MRI findings on 15 patients who had undergone anterior cervical discectomy and fusion (ACDF) immediately postoperatively, at 6 weeks, and at 6 months.11 Foraminal narrowing persisted in 66% of the first postoperative scans and did not resolve in the follow-up scans up to 6 months, despite symptomatic improvement. Nearly all cases demonstrated persistent edema, with high T2 signal, in the operative disc space at 6 weeks. In addition, all cases showed enhancement in this disc space at 6 weeks, and 50% persisted at 6 months.
Unresolved or Recurrent Symptoms
Recurrent Disc Herniation
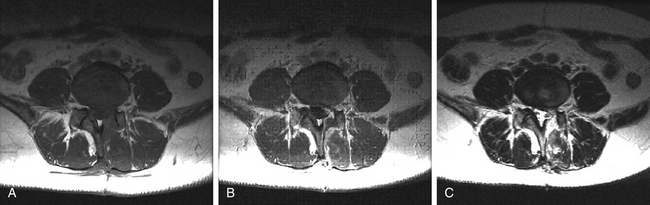
The incidence of recurrent disc herniation in the immediate postoperative period is unknown. In addition, for the reasons discussed earlier, small fragments of retained disc are difficult to identify on imaging. The appearances of recurrent or residual disc are low signal intensity on T1-weighted MRI without enhancement after the administration of IV gadolinium in contrast with epidural fibrosus that typically does enhance. A large sequestered disc may have central high signal intensity on T2-weighted images (Fig. 174-2). In contrast to scar, disc fragments tend to have a smooth margin.
Approximately 10% of discectomy patients experience recurrent lumbar disc herniation requiring revision discectomy. The mean time for this complication in one study was 10.5 months.12 Patients with a larger preoperative anular defect and with a smaller percentage of disc volume removed had a significantly greater chance of recurrent disc herniation. Common hemostatic agents used in lumbar discectomy, such as oxidized cellulose (Surgicel), have been reported to cause immediate postoperative radiculopathy.13 The increasing use of minimally invasive techniques, with less direct visualization of the disc and nerve roots, may eventually result in a higher incidence of retained disc fragments. However, at present, the literature suggests that the rate of complications is the same for minimally invasive techniques as for traditional open surgery.14
Postoperative Hematoma
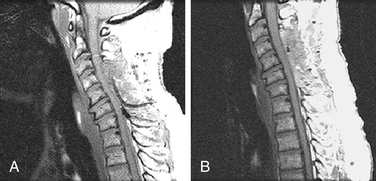
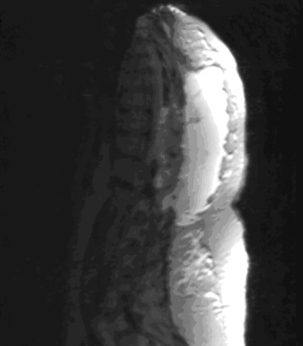
One of the most critical immediate postoperative complications to look for after spine surgery is a compressive hematoma, usually in an extradural location (Fig. 174-3). Fortunately, new onset of a major neurologic deficit in the lumbar spine is rare, due to canal space and mobility of nerve roots. However, in the cervical and thoracic spine, neural compression by a postoperative hematoma can have devastating neurologic consequences. Of nearly 12,000 adult spine operations performed over 10 years, the incidence of a major neurologic deficit immediately after surgery was 0.178%; in the cervical spine 0.293%, thoracic spine 0.488%, and lumbosacral spine 0.0745%.15 Epidural hematoma accounted for 38% of these complications, and it was the most common cause of immediate neurologic deficit. Other reasons for new deficit included inadequate decompression, presumed vascular compromise, graft or cage dislodgement, and presumed surgical trauma.
When a compressive hematoma is suspected, T2-weighted MRI is most sensitive for the detection of blood products in the spine.4 Instrumentation caused artifact on MRI and may obscure a subtle compressive lesion. It is sometimes necessary to also perform fine-cut CT with sagittal and coronal reconstructions to visualize hardware and bony anatomy. However, choice of imaging modality depends on the clinical scenario, since prompt reexploration is required with severe neurologic deficit.
The chances of a successful outcome following spine surgery depend on the initial indications, but generally are greater than 90% for common procedures such as microdiscectomy. However, a significant number of patients do not clinically improve following surgery, with estimates varying from 10% to 30%.16 In these instances, careful imaging of the operative site and spine is warranted.17 Several etiologies of continued pain and their imaging characteristics are discussed in the following sections.
Postoperative Infection
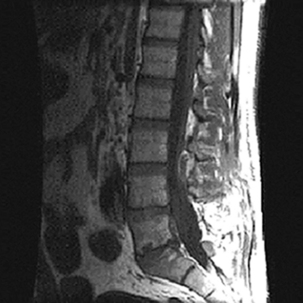
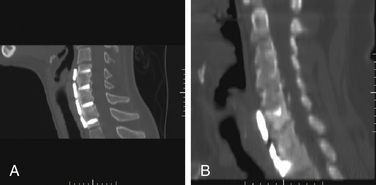
Radiographic diagnosis of postoperative spine infection is complicated—the time course of normal postoperative findings such as anular enhancement also mirrors when the same imaging characteristics represent pathologic infection. Postoperative infection may vary in its presentation, including spondylodiscitis, superficial cellulites or infected fluid collection, and paravertebral or epidural abscess (Fig. 174-4). Postprocedural discitis is relatively uncommon, with an incidence of less than 1%, although some studies reported an incidence as high as 3% of patients.18,19 Early in the infection, radiographs are not sensitive at detecting spondylodiscitis. CT may demonstrate destructive lesions and erosions in the vertebral end plates, as well as collapse of disc height. MRI is the best imaging modality for diagnosing discitis, with sensitivity and specificity of 93% and 97%, respectively.20 MRI may be supplemented with radionuclide scintigraphy, which has been reported to improve the specificity of MRI.21 The reported sensitivity and specificity of gallium scanning in detecting postoperative discitis is 89% and 85%, respectively.22 The MRI findings of postoperative spondylodiscitis include4,18
• Vertebral end-plate or marrow changes (low signal on T1- and high signal on T2-weighted images)
• Enhancing bone marrow adjacent to disc
• Enhancing spinal canal tissue
• Enhancing paravertebral soft tissue mass: rim (abscess) or homogeneous (phlegmon) enhancement
Although some of the aforementioned signal changes can occur normally after surgery (including disc enhancement), extensive contiguous enhancement of the disc and adjacent marrow is more consistent with infection. However, severe Modic changes from degenerative disease and aseptic discitis may appear indistinguishable from infection on MRI.23 Laboratory evaluations including serum white blood cell count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) are often more helpful in diagnosing infection than is imaging.24 Both ESR and CRP are nonspecific inflammatory markers and demonstrate a postoperative peak from the trauma of surgery. Nevertheless, a second rise or a persisting elevation of CRP levels after surgery has a sensitivity and specificity of 82% and 48%, respectively, for infectious complications.24 In addition, when following documented spine infection to ascertain clinical improvement after long-term antibiotic administration, ESR and CRP levels are crucial as measures of infection clearance and responsiveness to antibiotics. Administration of gadolinium in serial MRI has also been reported to assist in the conservative management of nonspecific spondylodiscitis.25
Although epidural abscess is a rare postoperative complication, the overall incidence of this condition has been increasing to 2 cases per 100,000 people annually.25 An infected epidural collection may either enhance homogeneously, if phlegmonous, or in a rim fashion, as with abscesses. An epidural collection may cause neurologic symptoms from mass effect or thrombosis of spinal draining veins, and in either case, emergent decompression is required. Recently, limited surgical approaches have been advocated to prevent the spread of infection to other anatomic structures such as vertebral bodies or discs.26
Postoperative Arachnoiditis
Chronic arachnoiditis is another potential cause of the failed back surgery syndrome (FBSS).27,28 The potential causes of arachnoiditis are protean, but not proven. The inflammatory response may be to blood, infection, trauma, contrast medium, or any other intrathecally injected substance. The incidence of arachnoiditis after spinal surgery is approximately 3%.29 Clinical findings of radiculopathy do not necessarily correlate with the severity of the arachnoiditis as appreciated on imaging.30
Myelography, with and without CT, and MRI (particularly T2-weighted fast-spin echo sequencing) both depict arachnoiditis with high accuracy, and classical findings on each correlate well with each other (Fig. 174-5).29 MRI can detect arachnoiditis with 92% sensitivity, 100% specificity, and 99% accuracy.31 There are three typical imaging patterns of arachnoiditis:32
1. Nerve roots are conglomerated in the center of the thecal sac, representing mild disease.
2. An “empty” thecal sac caused by adhesions of the nerve roots to the walls of the dura, representing moderate disease.
3. An intrathecal soft tissue mass with a broad dural base that may obstruct the cerebrospinal (CSF) pathway. The mass has intermediate signal intensity and may show varying degrees of enhancement.
Postoperative Radiculitis
Enhancement of nerve roots is a common finding in a normal postoperative spine but may also represent a source of ongoing symptoms. The enhancement is caused by disruption of the blood-nerve barrier, either after surgery or from chronic or severe compression by a herniated disc. Root enhancement can be seen in asymptomatic patients for 6 to 8 months postoperatively, after which it usually resolves.33 Thereafter, root enhancement correlates well with radiculopathy.34
A recent study correlated patient symptoms after lumbar discectomy with MRI findings.35 A total of 120 postoperative patients underwent MRI to evaluate their nerve roots for enhancement, thickening, and displacement. The incidence of nerve root enhancement was 65.7%, and this MRI finding was associated with symptoms in the offending nerve root distribution, with sensitivity of 91.7%, specificity of 73.2%, and a positive predictive value of 83.7%. When all three imaging findings were present (enhancement, thickening, and displacement), the positive predictive value increased to 94.1%.
Pseudomeningocele
A pseudomeningocele is a collection of CSF that is not lined by arachnoid or dura. Intraoperatively, a dural violation is either not recognized or is inadequately repaired, resulting in a persistent opening. Back pain or radiculopathy can ensue.36 Most commonly, the imaging characteristics of CSF are identified (Fig. 174-6); in the immediate postoperative period, blood products may be mixed in. It is difficult to distinguish between a normal postoperative fluid collection that is not in communication with the thecal sac and a true pseudomeningocele that requires surgical repair. Serial imaging may reveal continued expansion of the collection, indicating that a communication exists. Recently, digital subtraction myelography has been used successfully to detect small dural leaks associated with pseudomeningoceles.37,38
Instrumentation
In 2004, more than 300,000 spinal fusions were performed in the United States, accounting for more than $16 billion in hospital charges alone.39 The widespread use of instrumentation in spinal procedures raises unique challenges for imaging, both intraoperatively and postoperatively. Although implanted hardware is composed of a variety of composite materials, the vast majority are radiopaque, causing MRI artifact and signal scatter. Titanium has the advantage of being nonferromagnetic and therefore MRI compatible. However, a 360-degree spinal construct greatly diminishes the resolution of neural elements within the canal on MRI. Polyetheretherketone (PEEK) hardware is increasingly employed because of its radiolucent characteristics. Despite radiopaque markers, migration of PEEK cages is more difficult to detect on postoperative plain radiography.
Preoperative Use of Imaging
Preoperative imaging, CT or MRI, not only confirms the indication for placement of instrumentation, but also allows for important planning for hardware dimensions, trajectory, and fine bony anatomy. Although plain radiographs may provide some measurement of such details as pedicle diameter, fine-cut CT with sagittal and coronal reconstructions is increasingly being used for preoperative planning. The equivalent mineral density (EMD), calculated from quantitative CT, has been correlated with the ability of cancellous bone to hold screws.40 Recently, dual-energy x-ray absorptiometry (DEXA) is used to determine preoperative bone mineral density. Those patients with bone mineral density less than 0.45 g/cm2 required a significantly lower number of loading cycles to induce screw loosening.41
Fusion
Intraoperative Imaging for Fusion Procedures
Unfortunately, it is relatively common to detect misplaced instrumentation on postoperative imaging. The accuracy of screw placement varies widely, with some reporting a misplacement rate as high as 41%, even in the hands of experienced spine surgeons working with the benefits of intraoperative fluoroscopic guidance.42 The rate of misplacement differs depending on the type of radiography used to measure screw position, by plain film or CT, as well as the definition of misplacement.43–45 Using postoperative CT and defining accurate screw placement as “within 2 mm of the medial border of the pedicle,” one study reported a misplacement rate of 19%.46 Placement of pedicle screws in the thoracic spine is more technically difficult because of the small pedicle diameter and vital structures nearby. A recent review found a 20.3% rate of misplacement for upper-middle thoracic pedicle screws as determined by CT.47 It is now accepted that the rate of detection for misplaced screws is greater with high-resolution CT imaging, which allows excellent bony detail, compared with either plain radiographs or MRI.43,48–50
Detection of a misplaced screw postoperatively usually occurs in an asymptomatic patient. In 50 patients who underwent 360-degree lumbar fusion, 41% of pedicle screws breached.42 Of these, 32% breached medially and were associated with displacement of the dura. However, only one patient presented with symptoms related to a misplaced screw, in this case, an S1 radiculopathy. In addition, all patients in this study were reported to have achieved normal fusion. In the lumbar spine, a breach of 4 mm or less has been reported as safe, due to the large epidural space.51 Minimally invasive techniques, such as percutaneous pedicle screw placement, have a similar rate of instrumentation misplacement and clinical consequences. In a study of 51 patients, 27 screws (6.6%) were misplaced, and only 1 misplaced screw resulted in an injury to the L4 nerve root.52
As more fusion procedures are performed, surgeons routinely face the dilemma of whether to reoperate for an asymptomatic misplaced screw, especially if neurologic compromise could theoretically occur at a later time. In addition, the changing medicolegal climate has led to a policy among some spine surgeons requiring that all instrumentation be documented as correctly positioned. In this atmosphere, the role of intraoperative imaging is taking on new importance. The use of fine-cut CT and 3D guidance, such as the O-arm Imaging System (Medtronic, Minneapolis, MN), is increasingly being employed for routine elective degenerative spine cases. Using such navigation systems can improve the accuracy of even upper thoracic pedicle screws to greater than 90%.53 In surgeries to correct scoliosis deformity, use of intraoperative navigation for screw placement can decrease the breach rate to only 2%.54 The sensitivity and specificity of 3D guidance systems for placement of pedicle screws has been reported as 91.3% and 98.2%, respectively.55 Intraoperative evaluation of the reconstructed fine-cut CTs resulted in a revision rate of 2.7% of pedicle screws and lowered the secondary reoperation rate to 0.5%.55 After careful review of an intraoperative CT, many surgeons do not obtain additional postoperative imaging.
Postoperative Imaging after Fusion
Patients who develop new pain or neurologic deficits postoperatively should receive expeditious imaging in order to detect any reversible hardware-related complications. Depending on the urgency of the symptom, many surgeons still begin with conventional radiographs to evaluate the primary instrumentation. The use of at least two perpendicular planes (anteroposterior and lateral) greatly enhances the ability to detect complications such as bony fracture or misplaced screws. However, plain radiographs are reported to correlate with intraoperative findings in fusion cases less than 70% of the time.56,57
Fine-cut, high-resolution CT with coronal and sagittal reconstruction is generally accepted to provide the greatest level of instrumentation detail. Precise information regarding cortical bone is provided.58 Evidence of fusion, bridging bony trabeculation around cages, was detected in 90% of patients by CT compared with only 7% of patients by plain radiograph, as confirmed by surgical reexploration.59 CT also shows subtle lucencies surrounding instrumentation, either cages or pedicle screws, earlier and with a higher sensitivity and specificity than plain radiographs (Fig. 174-7).59–61 Finally, fine-cut CTs with reconstructions have a significantly greater degree of interobserver and intraobserver agreement regarding fusion status than do flexion-extension and anteroposterior plain radiographs.62
However, evaluation for a new neurologic symptom following a fusion procedure should not only include CT but also another modality to assess the nerve roots and spinal cord. As discussed earlier, MRI has its limitations due to artifact signal that may obscure detail within the spinal canal. Although more invasive and time intensive, CT myelography provides fine detail of both instrumentation and neural structures.63 A recent study compared CT-myelogram with MRI in patients who had previously undergone anterior cervical discectomy and fusion.64 Despite the presence of the ventral titanium plate, MRI was more useful for detecting disc abnormalities or nerve root compression. However, CT-myelogram provided better detail of foraminal stenosis and bony lesions in the cervical canal. CT-myelogram, particularly in the cervical spine, requires an experienced interventional radiologist for injection of contrast medium into the subarachnoid space. The risks inherent with the CT-myelogram procedure may reduce its utility; especially as new, less-invasive technology is developed. 3D MRI/CT fusion imaging allows for construction of colored images that combine both imaging modalities. This technique also shows promise for depicting lumbar instrumentation in relation to neural architecture.65
Assessment of Fusion
The chief goal of any stabilizing spinal procedure—with or without instrumentation—is fusion of bony elements. Although successful fusion does not necessarily lead to good outcome, documentation of arthrodesis is an essential component of patient follow-up.57,66,67 It is well-established that pseudarthrosis, a leading complication associated with thoracolumbar spinal arthrodesis, can contribute greatly to persistent postoperative pain.68,69
Successful fusion depends on the establishment of a continuous mass of bone across a motion segment, preventing movement in any plane across that segment. A spinal pseudarthrosis is defined as an absence of bridging bone between adjacent vertebrae.68 Assessment of fusion depends on the time of postoperative imaging and the imaging modality. Either iliac crest bone graft or cadaveric bone, placed into the dorsolateral gutters during spinal fusion, shows high-density characteristics on immediate postoperative CT but exhibits decreased density at 6-week follow-up. The physiologic process of bone fusion, with extensive remodeling and surrounding tissue edema, obscures the presence of the dorsolateral bone. In addition, before solid fusion occurs, the bone is a trabecular network bridging different spinal levels. High-density cortical bone is not typically visible until at least 3 months after surgery and thereafter continues to increase in density beyond 1 year. Radiolucent zones around pedicle screws often indicate delayed union or pseudarthrosis. However, these radiolucent zones may be present in up to 40% of postoperative patients as evidenced by plain radiograph at 6 months.70 At 3 years, 15% of patients still showed radiolucent zones by plain radiograph, but only 53% of these patients actually had pseudarthrosis on reexploration.70
Many imaging characteristics have been studied to determine whether the evidence of radiographic fusion truly equates with solid bone fusion in an actual patient. Using fine-cut CT, one study reported that evidence of bone fusion in dorsolateral gutters represented actual fusion in 89% of cases, compared with only 74% of cases when both facets were fused.71 When both facets and dorsolateral gutters were fused on CT, the probability of a solid fusion on exploration is 96%. Another study specified that determination of fusion involves a multistep approach.60 First, spinal alignment should be assessed over a 6-month period, by plain radiographs in the standing position. Progressive subsidence or change in spinal alignment represents a fusion failure. Second, dynamic flexion-extension radiographs should demonstrate no significant motion across the fusion segment. Third, close evaluation of the host bone reaction to instrumentation, as shown by CT, is required. Cystic or sclerotic changes within subchondral bone of the vertebral end plates and progressive radiographic lucencies around pedicle screws are suggestive of pseudarthrosis. Fourth, radiographic evidence of the formation of new bone, in dorsolateral gutters and across the disc space, is the most reliable finding of solid fusion.
There are several published grading systems for fusion. Brantigan describes radiographic criteria for fusion:72
1. Radiographic pseudarthrosis: Collapse of construct, vertebral slip, broken screws, resorption of bone graft, or major lucency or gap visible in the fusion area.
2. Uncertain fusion: Bone graft visible, but it has not increased in density, or a small lucency or gap is visible in the fusion area.
3. Radiographic fusion: Graft shows a higher density, and there is no interface between graft bone and vertebral bone. Mature bony trabeculae bridge the fusion area, ventral vertebral traction spurs are minimal, facet joints are fused, and there is ventral progression of the graft within the disc space.
Molinari et al. specified five categories of fusion:73
1. Definite—Solid trabeculated transverse process and facet fusion
2. Probable—Thick fusion mass on one side, difficult to visualize on other side
3. Probably not—Possible lucency or defect in the fusion mass
4. Not fused—Definite resorption of graft with fatigue of instrumentation
Glassman et al. performed a prospective, randomized study of iliac crest bone graft versus recombinant human bone morphogenetic protein-2 in dorsolateral instrumented fusion.74 Using CT at 6 and 12 months, they employed a five-part grading system to evaluate the fusion mass:
1. No fusion—evidence of radiolucencies around instrumentation and no evidence of bone graft cortication
2. Partial or limited unilateral fusion—Some bone formation, although with possible lucencies or sclerotic margins
3. Partial or limited bilateral fusion—The same imaging characteristics bilaterally
4. Solid unilateral fusion—Corticated bone spanning the disc space, dorsolateral gutter, and facet
5. Solid bilateral fusion—The same imaging characteristics bilaterally
For pseudarthrosis of Glassman grade I/II or Molinari grade III/IV, plain radiographs may suffice for diagnosis and surgical decision to reoperate. A recent study demonstrated a sensitivity of 100%, specificity of almost 90%, and negative predictive value of 100% for healed fusion, for plain x-ray films.75
Postoperative Imaging for Tumor
In most instances, postoperative imaging after spinal tumor resection is done to confirm the extent of resection and serve as a baseline for subsequent follow-up studies. In benign intradural extramedullary tumors such as meningioma or schwannoma, gross total excision is often achieved. However, yearly follow-up imaging is generally recommended to identify recurrence prior to the onset of symptoms. The recurrence rate for spinal meningioma is approximately 7.5% at 10 years and 9.3% at 20 years.76
Carreon L.Y., Djurasovic M., Glassman S.D., et al. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine (Phila Pa 1976). 2007;32(8):892-895.
Jinkins J.R., Van Goethem J.W. The postsurgical lumbosacral spine. Magnetic resonance imaging evaluation following intervertebral disk surgery, surgical decompression, intervertebral bony fusion, and spinal instrumentation. Radiol Clin North Am. 2001;39(1):1-29.
Lee Y.S., Choi E.S., Song C.J. Symptomatic nerve root changes on contrast-enhanced MR imaging after surgery for lumbar disk herniation. AJNR Am J Neuroradiol. 2009;30(5):1062-1067.
McGirt M.J., Eustacchio S., Varga P., et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976). 2009;34(19):2044-2051.
Molinari R.W., Bridwell K.H., Klepps S.J., et al. Minimum 5-year follow-up of anterior column structural allografts in the thoracic and lumbar spine. Spine (Phila Pa 1976). 1999;24(10):967-972.
Rajasekaran S., Vidyadhara S., Ramesh P., Shetty A.P. Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine (Phila Pa 1976). 2007;32(2):E56-E64.
Van Goethem J.W., Parizel P.M., van den Hauwe L., De Schepper A.M. Imaging findings in patients with failed back surgery syndrome. J Belge Radiol. 1997;80(2):81-84.
1. Martin B.I., Deyo R.A., Mirza S.K., et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656-664.
2. Weinstein J.N., Lurie J.D., Olson P.R., et al. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine (Phila Pa 1976). 2006;31(23):2707-2714.
3. Shafaie F.F.B.C., Jinkins J.R. Post-therapeutic neurodiagnostic imaging. Philadelphia: Lippincott-Raven; 1997.
4. Jinkins J.R., Van Goethem J.W. The postsurgical lumbosacral spine. Magnetic resonance imaging evaluation following intervertebral disk surgery, surgical decompression, intervertebral bony fusion, and spinal instrumentation. Radiol Clin North Am. 2001;39(1):1-29.
5. Bundschuh C.V., Modic M.T., Ross J.S., et al. Epidural fibrosis and recurrent disk herniation in the lumbar spine: MR imaging assessment. AJR Am J Roentgenol. 1988;150(4):923-932.
6. Hueftle M.G., Modic M.T., Ross J.S., et al. Lumbar spine: postoperative MR imaging with Gd-DTPA. Radiology. 1988;167(3):817-824.
7. Ross J.S., Robertson J.T., Frederickson R.C., et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38(4):855-861. discussion 861–863
8. Epter R.S., Helm S.2nd, Hayek S.M., et al. Systematic review of percutaneous adhesiolysis and management of chronic low back pain in post lumbar surgery syndrome. Pain Physician. 2009;12(2):361-378.
9. Harrington J.F., Messier A.A., Hoffman L., et al. Physiological and behavioral evidence for focal nociception induced by epidural glutamate infusion in rats. Spine (Phila Pa 1976). 2005;30(6):606-612.
10. Van Goethem J.W., Van de Kelft E., Biltjes I.G., et al. MRI after successful lumbar discectomy. Neuroradiology. 1996;38(Suppl 1):S90-S96.
11. Bommireddy R., Kamat A., Smith E.T., et al. Magnetic resonance image findings in the early post-operative period after anterior cervical discectomy. Eur Spine J. 2007;16(1):27-31.
12. McGirt M.J., Eustacchio S., Varga P., et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976). 2009;34(19):2044-2051.
13. Partheni M., Kalogheropoulou C., Karageorgos N., et al. Radiculopathy after lumbar discectomy due to intraspinal retained Surgicel: clinical and magnetic resonance imaging evaluation. Spine J. 2006;6(4):455-458.
14. German J.W., Adamo M.A., Hoppenot R.G., et al. Perioperative results following lumbar discectomy: comparison of minimally invasive discectomy and standard microdiscectomy. Neurosurg Focus. 2008;25(2):E20.
15. Cramer D.E., Maher P.C., Pettigrew D.B., Kuntz C. 4th: Major neurologic deficit immediately after adult spinal surgery: incidence and etiology over 10 years at a single training institution. J Spinal Disord Tech. 2009;22(8):565-570.
16. Shamim M.S., Enam S.A., Qidwai U. Fuzzy logic in neurosurgery: predicting poor outcomes after lumbar disk surgery in 501 consecutive patients. Surg Neurol. 2009;72(6):565-572. discussion 572
17. Van Goethem J.W., Parizel P.M., van den Hauwe L., De Schepper A.M. Imaging findings in patients with failed back surgery syndrome. J Belge Radiol. 1997;80(2):81-84.
18. Silber J.S., Anderson D.G., Vaccaro A.R., et al. Management of postprocedural discitis. Spine J. 2002;2(4):279-287.
19. Fernand R., Lee C.K. Postlaminectomy disc space infection. A review of the literature and a report of three cases. Clin Orthop Relat Res. 1986;209:215-218.
20. Szypryt E.P., Hardy J.G., Hinton C.E., et al. A comparison between magnetic resonance imaging and scintigraphic bone imaging in the diagnosis of disc space infection in an animal model. Spine (Phila Pa 1976). 1988;13(9):1042-1048.
21. Modic M.T., Feiglin D.H., Piraino D.W., et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157(1):157-166.
22. Bruschwein D.A., Brown M.L., McLeod R.A. Gallium scintigraphy in the evaluation of disk-space infections: concise communication. J Nucl Med. 1980;21(10):925-927.
23. Grane P., Josephsson A., Seferlis A., Tullberg T. Septic and aseptic post-operative discitis in the lumbar spine—evaluation by MR imaging. Acta Radiol. 1998;39(2):108-115.
24. Mok J.M., Pekmezci M., Piper S.L., et al. Use of C-reactive protein after spinal surgery: comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine (Phila Pa 1976). 2008;33(4):415-421.
25. Bettini N., Girardo M., Dema E., Cervellati S. Evaluation of conservative treatment of non specific spondylodiscitis. Eur Spine J. 2009;18(Suppl 1):143-150.
26. Lohr M., Reithmeier T., Ernestus R.I., et al. Spinal epidural abscess: prognostic factors and comparison of different surgical treatment strategies. Acta Neurochir (Wien). 2005;147(2):159-166. discussion 166
27. Burton C.V. Causes of failure of surgery on the lumbar spine: ten-year follow-up. Mt Sinai J Med. 1991;58(2):183-187.
28. Burton C.V., Kirkaldy-Willis W.H., Yong-Hing K., Heithoff K.B. Causes of failure of surgery on the lumbar spine. Clin Orthop Relat Res. 1981;157:191-199.
29. Fitt G.J., Stevens J.M. Postoperative arachnoiditis diagnosed by high resolution fast spin-echo MRI of the lumbar spine. Neuroradiology. 1995;37(2):139-145.
30. Burton C.V. Lumbosacral arachnoiditis. Spine (Phila Pa 1976). 1978;3(1):24-30.
31. Firooznia H., Kricheff II, Rafii M., Golimbu C. Lumbar spine after surgery: examination with intravenous contrast-enhanced CT. Radiology.. 1987;163(1):221-226.
32. Babar S., Saifuddin A. MRI of the post-discectomy lumbar spine. Clin Radiol. 2002;57(11):969-981.
33. Boden S.D., Davis D.O., Dina T.S., et al. Postoperative diskitis: distinguishing early MR imaging findings from normal postoperative disk space changes. Radiology. 1992;184(3):765-771.
34. Jinkins J.R., Osborn A.G., Garrett D.Jr., et al. Spinal nerve enhancement with Gd-DTPA: MR correlation with the postoperative lumbosacral spine. AJNR Am J Neuroradiol. 1993;14(2):383-394.
35. Lee Y.S., Choi E.S., Song C.J. Symptomatic nerve root changes on contrast-enhanced MR imaging after surgery for lumbar disk herniation. AJNR Am J Neuroradiol. 2009;30(5):1062-1067.
36. Lee K.S., Hardy I.M.2nd. Postlaminectomy lumbar pseudomeningocele: report of four cases. Neurosurgery. 1992;30(1):111-114.
37. Phillips C.D., Kaptain G.J., Razack N. Depiction of a postoperative pseudomeningocele with digital subtraction myelography. AJNR Am J Neuroradiol. 2002;23(2):337-338.
38. Hoxworth J.M., Patel A.C., Bosch E.P., Nelson K.D. Localization of a rapid CSF leak with digital subtraction myelography. AJNR Am J Neuroradiol. 2009;30(3):516-519.
39. Deyo R.A. Back surgery—who needs it? N Engl J Med. 2007;356(22):2239-2243.
40. Wittenberg R.H., Shea M., Swartz D.E., et al. Importance of bone mineral density in instrumented spine fusions. Spine (Phila Pa 1976). 1991;16(6):647-652.
41. Lim T.H., An H.S., Hasegawa T., et al. Prediction of fatigue screw loosening in anterior spinal fixation using dual energy x-ray absorptiometry. Spine (Phila Pa 1976). 1995;20(23):2565-2568. discussion 2569
42. Schulze C.J., Munzinger E., Weber U. Clinical relevance of accuracy of pedicle screw placement. A computed tomographic-supported analysis. Spine (Phila Pa 1976). 1998;23(20):2215-2220. discussion 2220–2211
43. Ferrick M.R., Kowalski J.M., Simmons E.D.Jr. Reliability of roentgenogram evaluation of pedicle screw position. Spine (Phila Pa 1976). 1997;22(11):1249-1252. discussion 1253
44. Yoo J.U., Ghanayem A., Petersilge C., Lewin J. Accuracy of using computed tomography to identify pedicle screw placement in cadaveric human lumbar spine. Spine (Phila Pa 1976). 1997;22(22):2668-2671.
45. Youkilis A.S., Quint D.J., McGillicuddy J.E., Papadopoulos S.M. Stereotactic navigation for placement of pedicle screws in the thoracic spine. Neurosurgery. 2001;48(4):771-778. discussion 778–779
46. Gertzbein S.D., Robbins S.E. Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976). 1990;15(1):11-14.
47. Guzey F.K., Emel E., Hakan Seyithanoglu M., et al. Accuracy of pedicle screw placement for upper and middle thoracic pathologies without coronal plane spinal deformity using conventional methods. J Spinal Disord Tech. 2006;19(6):436-441.
48. Weinstein J.N., Spratt K.F., Spengler D., et al. Spinal pedicle fixation: reliability and validity of roentgenogram-based assessment and surgical factors on successful screw placement. (Phila Pa 1976). 1988;13(9):1012-1018.
49. Castro W.H., Halm H., Jerosch J., et al. Accuracy of pedicle screw placement in lumbar vertebrae. Spine (Phila Pa 1976). 1996;21(11):1320-1324.
50. Laine T., Makitalo K., Schlenzka D., et al. Accuracy of pedicle screw insertion: a prospective CT study in 30 low back patients. Eur Spine J. 1997;6(6):402-405.
51. Sjostrom L., Jacobsson O., Karlstrom G., et al. CT analysis of pedicles and screw tracts after implant removal in thoracolumbar fractures. J Spinal Disord. 1993;6(3):225-231.
52. Wiesner L., Kothe R., Schulitz K.P., Ruther W. Clinical evaluation and computed tomography scan analysis of screw tracts after percutaneous insertion of pedicle screws in the lumbar spine. Spine (Phila Pa 1976). 2000;25(5):615-621.
53. Bledsoe J.M., Fenton D., Fogelson J.L., Nottmeier E.W. Accuracy of upper thoracic pedicle screw placement using three-dimensional image guidance. Spine J. 2009;9(10):817-821.
54. Rajasekaran S., Vidyadhara S., Ramesh P., Shetty A.P. Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine (Phila Pa 1976). 2007;32(2):E56-E64.
55. Beck M., Mittlmeier T., Gierer P., et al. Benefit and accuracy of intraoperative 3D-imaging after pedicle screw placement: a prospective study in stabilizing thoracolumbar fractures. Eur Spine J. 2009;18(10):1469-1477.
56. Brodsky A.E., Kovalsky E.S., Khalil M.A. Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine (Phila Pa 1976). 1991;16(Suppl 6):S261-S265.
57. Blumenthal S.L., Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine (Phila Pa 1976). 1993;18(9):1186-1189.
58. Kaech D.L.M.G. Principles, imaging, and complications of spinal instrumentation. Philadelphia: Lippincott-Raven; 1997.
59. Shah R.R., Mohammed S., Saifuddin A., Taylor B.A. Comparison of plain radiographs with CT scan to evaluate interbody fusion following the use of titanium interbody cages and transpedicular instrumentation. Eur Spine J. 2003;12(4):378-385.
60. Burkus J.K., Foley K., Haid R.W., LeHuec J.C. Surgical Interbody Research Group–radiographic assessment of interbody fusion devices: fusion criteria for anterior lumbar interbody surgery. Neurosurg Focus. 2001;10(4):E11.
61. Siambanes D., Mather S. Comparison of plain radiographs and CT scans in instrumented posterior lumbar interbody fusion. Orthopedics. 1998;21(2):165-167.
62. Carreon L.Y., Glassman S.D., Djurasovic M. Reliability and agreement between fine-cut CT scans and plain radiography in the evaluation of posterolateral fusions. Spine J. 2007;7(1):39-43.
63. Hsu C.J., Chou W.Y., Chang W.N., Wong C.Y. Clinical follow up after instrumentation-augmented lumbar spinal surgery in patients with unsatisfactory outcomes. J Neurosurg Spine. 2006;5(4):281-286.
64. Song K.J., Choi B.W., Kim G.H., Kim J.R. Clinical usefulness of CT-myelogram comparing with the MRI in degenerative cervical spinal disorders: is CTM still useful for primary diagnostic tool? J Spinal Disord Tech. 2009;22(5):353-357.
65. Yamanaka Y., Kamogawa J., Katagi R., et al. 3-D MRI/CT fusion imaging of the lumbar spine. Skeletal Radiol. 2010;39(3):285-288.
66. Bailey S.I., Bartolozzi P., Bertagnoli R., et al. The BWM spinal fixator system. A preliminary report of a 2-year prospective, international multicenter study in a range of indications requiring surgical intervention for bone grafting and pedicle screw fixation. Spine (Phila Pa 1976). 1996;21(17):2006-2015.
67. Dickman C.A., Fessler R.G., MacMillan M., Haid R.W. Transpedicular screw-rod fixation of the lumbar spine: operative technique and outcome in 104 cases. J Neurosurg. 1992;77(6):860-870.
68. Raiszadeh R., Heggeness M., Esses S.I. Thoracolumbar pseudarthrosis. Am J Orthop (Belle Mead NJ). 2000;29(7):513-520.
69. Slizofski W.J., Collier B.D., Flatley T.J., et al. Painful pseudarthrosis following lumbar spinal fusion: detection by combined SPECT and planar bone scintigraphy. Skeletal Radiol. 1987;16(2):136-141.
70. Tokuhashi Y., Matsuzaki H., Oda H., Uei H. Clinical course and significance of the clear zone around the pedicle screws in the lumbar degenerative disease. Spine (Phila Pa 1976). 2008;33(8):903-908.
71. Carreon L.Y., Djurasovic M., Glassman S.D., Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine (Phila Pa 1976). 2007;32(8):892-895.
72. Brantigan J.W. Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation. Spine (Phila Pa 1976). 1994;19(11):1271-1279. discussion 1280
73. Molinari R.W., Bridwell K.H., Klepps S.J., Baldus C. Minimum 5-year follow-up of anterior column structural allografts in the thoracic and lumbar spine. Spine (Phila Pa 1976). 1999;24(10):967-972.
74. Glassman S.D., Dimar J.R., Carreon L.Y., et al. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine (Phila Pa 1976). 2005;30(15):1694-1698.
75. Fogel G.R., Toohey J.S., Neidre A., Brantigan J.W. Fusion assessment of posterior lumbar interbody fusion using radiolucent cages: X-ray films and helical computed tomography scans compared with surgical exploration of fusion. Spine J. 2008;8(4):570-577.
76. Nakasu S., Fukami T., Jito J., Nozaki K. Recurrence and regrowth of benign meningiomas. Brain Tumor Pathol. 2009;26(2):69-72.