Chapter 26 Postoperative Cardiac Recovery and Outcomes
FAST TRACK CARDIAC SURGERY CARE
Anesthetic Techniques
There have been few trials comparing inhalation agents for fast track cardiac anesthesia (FTCA). Several studies have examined the effectiveness of propofol versus inhalation agents, most demonstrated reductions in myocardial enzyme release (CK-MB, troponin I) and preservation of myocardial function in patients receiving inhalation agents.1 Although this endpoint is a surrogate for myocardial damage and does not show improved outcome per se, CK-MB release after CABG may be associated with poor outcome (Box 26-1).
The choice of muscle relaxant in FTCA is important to reduce the incidence of muscle weakness in the cardiac recovery area (CRA), which may delay tracheal extubation. Randomized trials have compared rocuronium (0.5 to 1 mg/kg) versus pancuronium (0.1 mg/kg) and found significant differences in residual paralysis in the ICU, and statistically significant delays were found in the time to extubation in the pancuronium group.2 None of the trials used reversal agents, so the use of pancuronium appears acceptable as long as neostigmine or edrophonium is administered to patients with residual neuromuscular weakness.
There have been several trials examining the use of different short-acting narcotic agents during FTCA. In these trials, fentanyl, remifentanil, and sufentanil were all found to be efficacious for early tracheal extubation. The anesthetic drugs and their suggested dosages are given in Table 26-1.
Table 26-1 Suggested Dosages for Fast Track Cardiac Anesthesia
| Induction |
| Narcotic |
MAC = minimal alveolar concentration.
From Mollhoff T, Herregods L, Moerman A, et al: Comparative efficacy and safety of remifentanil and fentanyl in “fast track” coronary artery bypass graft surgery: A randomized, double-blind study. Br J Anaesth 87: 718, 2001; Engoren M, Luther G, Fenn-Buderer N: A comparison of fentanyl, sufentanil, and remifentanil for fast-track cardiac anesthesia. Anesth Analg 93: 859, 2001; and Cheng DC, Newman MF, Duke P, et al: The efficacy and resource utilization of remifentanil and fentanyl in fast-tract coronary artery bypass graft surgery: A prospective randomized, double-blinded controlled, multi-center trial. Anesth Analg 92:1094, 2001.
Evidence Supporting Fast Track Cardiac Recovery
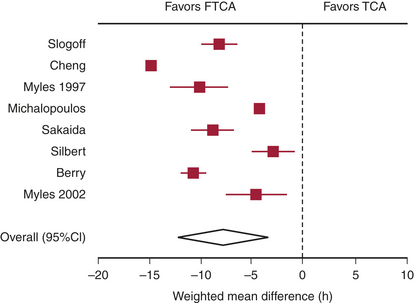
There are several randomized trials and a meta-analysis of randomized trials that have addressed the question of safety of FTCA.3 None of the trials was able to demonstrate differences in outcomes between the fast track group and the conventional anesthesia group, but meta-analysis of the randomized trials demonstrated a reduction in the duration of intubation by 8 hours (Fig. 26-1) and the ICU length of stay by 5 hours in favor of the fast track group. However, the length of hospital stay was not statistically different.
Postcardiac Surgical Recovery Models
The failure of many randomized FTCA trials to show reductions in resource utilization likely stems from the traditional ICU models used by these centers during the study period. Even when trials were combined in a meta-analysis, the ICU length of stay was reduced only by 5 hours despite patients being extubated a mean of 8 hours earlier.3 Typically, patients who are extubated within the first 24 hours of ICU admission are transferred to the ward on postoperative day 1 in the morning or early afternoon. This allows the following daytime cardiac cases to have available ICU beds but prevents patient transfers during night-time hours. Two models have been proposed to deal with this issue: the parallel model and the integrated model. In the parallel model, patients are admitted directly to a CRA, where they are monitored with 1:1 nursing care until tracheal extubation. Following this, the level of care is reduced to reflect reduced nursing requirements with ratios of 1:2 or 1:3. Any patients requiring overnight ventilation are transferred to the ICU for continuation of care. The primary drawback with the parallel model is the physical separation of the CRA and ICU, which leads to two separate units and thus does not eliminate the requirement to transfer patients. The integrated model overcomes these limitations because all patients are admitted to the same physical area, but postoperative management such as nursing-to-patient ratio is variable based on patient requirements. Because nursing care accounts for 45% to 50% of ICU costs, reducing the nursing requirements where possible creates the greatest saving. Other cost savings from reductions in arterial blood gases measurement, use of sedative drugs, and ventilator maintenance are small. The goal is a postoperative unit that allows variable levels of monitoring and care based on patient need. Furthermore, FTCA has been demonstrated to be a safe and cost-effective practice that decreases resource utilization after patient discharge from the index hospitalization up to 1-year follow-up.4
INITIAL MANAGEMENT OF FAST TRACK CARDIAC ANESTHESIA PATIENTS: THE FIRST 24 HOURS
On arrival in the CRA, initial management of cardiac patients consists of ensuring an efficient transfer of care from operating room staff to CRA staff while at the same time maintaining stable patient vital signs. The anesthesiologist should relay important clinical parameters to the CRA team. To accomplish this, many centers have devised hand-off sheets to aid in the transfer of care. The patient’s temperature should be recorded, and, if it is low, active rewarming measures should be initiated with the goal of rewarming the patient to 36.5°C. Shivering may be treated with low intravenous doses of meperidine (12.5 to 25 mg). Hyperthermia, however, is common within the first 24 hours after cardiac surgery and may be associated with an increase in neurocognitive dysfunction, possibly as a result of hyperthermia exacerbating cardiopulmonary bypass (CPB)−induced neurologic injury (Box 26-2).
Ventilation Management: Admission to Tracheal Extubation
Ventilatory requirements should be managed with the goal of early tracheal extubation (Table 26-2). Arterial blood gas samples are initially drawn within 30 minutes after admission and then repeated as necessary. Patients should be awake and cooperative, hemodynamically stable, and have no active bleeding with coagulopathy. Respiratory strength should be assessed by hand grip or head lift to ensure complete reversal of neuromuscular blockade. The patient’s temperature should be above 36°C, preferably normothermic. When these conditions are met and arterial blood gas results are within the normal range, tracheal extubation may take place. Blood for arterial blood gas analysis should be drawn about 30 minutes after tracheal extubation to ensure adequate ventilation with maintenance of PaO2 and PaCO2. Inability to extubate patients as a result of respiratory failure, hemodynamic instability, or large amounts of mediastinal drainage will necessitate more complex weaning strategies. Some patients may arrive after extubation in the operating room. Careful attention should be paid to these patients. The patient’s respiratory rate should be monitored every 5 minutes during the first several hours. A blood sample should be drawn on admission and 30 minutes later to ensure the patient is not retaining carbon dioxide. If the patient’s respirations become compromised, ventilatory support should be provided. Simple measures such as reminders to breathe may be effective in the narcotized/anesthetized patient. Low intravenous doses of naloxone (0.04 mg) may also be beneficial. Trials of continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) may provide enough support to allow adequate ventilation. Reintubation should be avoided because it may delay recovery; however, it may become necessary if the just-mentioned measures fail, resulting in hypoxemia, hypercarbia, and a declining level of consciousness.
Table 26-2 Ventilation Management Goals during the Initial Trial of Weaning from Extubation
A/C = assist-controlled ventilation; PEEP = positive end-expiratory pressure.
Management of Bleeding
Chest tube drainage should be checked every 15 minutes after ICU admission to assess a patient’s coagulation status. Because blood loss is commonly divided into two types, surgical or medical, determining the cause of bleeding is often difficult. When bleeding exceeds 400 mL/hr during the first hour, 200 mL/hr for each of the first 2 hours, or 100 mL/hr over the first 4 hours, returning to the operating room for chest reexploration should be considered. The clinical situation must be individualized for each patient, however, and in the presence of a known coagulopathy, more liberal blood loss before chest reexploration may be acceptable. There are numerous medical causes for bleeding after cardiac surgery. Platelet dysfunction after cardiac surgery is common. Residual heparinization is common after cardiac surgery and frequently occurs when either heparinized pump blood is transfused after CPB or insufficient protamine is administered. Fibrinolysis is also very common after CPB, predominantly caused by a host of activated inflammatory and coagulation pathways. Coagulation factors may decrease from activation at air-blood interfaces or from dilution with the CPB pump priming solution. Hypothermia may also aggravate the coagulation cascade and lead to further bleeding. Conventional coagulation tests are helpful to identify the coagulation abnormality contributing to the bleeding. Common laboratory testing includes activated partial thromboplastin time, international normalized ratio, platelet count, fibrinogen level, and D-dimers. Unfortunately, with most conventional measures 20 to 40 minutes lapse before results are available (Box 26-3). The use of point-of-care testing such as thromboelastography has been demonstrated to reduce transfusion requirements without increasing blood loss and is commonly used, especially following difficult cardiac cases.
Electrolyte Management
Hypokalemia is common after cardiac surgery, especially if diuretics were given intraoperatively. Hypokalemia contributes to increased automaticity and may lead to ventricular arrhythmias, ventricular tachycardia, or ventricular fibrillation. Treatment consists of potassium infusions (20 mEq of potassium in 50 mL of D5W infused over 1 hour) until the potassium exceeds 3.5 mEq/mL. In patients with frequent premature ventricular contractions caused by increased automaticity, a potassium level of 5.0 mEq/mL may be desirable. Hypomagnesemia contributes to ventricular preexcitation and may contribute to atrial fibrillation. Management consists of intermittent boluses of magnesium—1 to 2 g over 15 minutes. Hypocalcemia is also frequent during cardiac surgery and may reduce cardiac contractility. Intermittent boluses of calcium chloride or calcium gluconate (1 g) may be required (Table 26-3).
Table 26-3 Common Electrolyte Abnormalities and Possible Treatment Options
SSx = signs and symptoms, Rx = treatment.
Glucose Management
Diabetes is a common comorbidity (up to 30%) and is a known risk factor for adverse outcome in patients presenting for cardiac surgery. Hyperglycemia itself is common during CPB. The risk factors for hyperglycemia include diabetes, administration of corticosteroids before CPB, volume of glucose-containing solutions administered, and use of epinephrine infusions. Poor perioperative glucose control is associated with increases in mortality and morbidity, including an increased risk of infection and a prolonged duration of ventilation.5 In a large prospective, randomized, controlled trial of tight glucose control (blood glucose levels of 4.1 to 6.5 mmol/L) during postoperative ICU stay, reductions in mortality were shown by the authors compared with more liberal glucose control (blood glucose levels of 12 mmol/L). This trial enrolled both diabetic and nondiabetic hyperglycemic patients who underwent cardiothoracic surgery and demonstrates that tight management of glucose is beneficial in the CRA. Given all the associated risks of hyperglycemia, attempts should be made to maintain euglycemia throughout the perioperative setting in all patients presenting for surgery.
Pain Control
Pain control after cardiac surgery has become a concern as narcotic doses have been reduced to facilitate fast track protocols. Intravenous morphine is still the mainstay of treatment for post−cardiac surgery patients. The most common approach is patient-demanded, nurse-delivered intravenous morphine, and this treatment remains popular because of 1:1 to 1:2 nursing typically provided during cardiac recovery. However, with a change to more flexible nurse coverage and, therefore, higher nurse-to-patient ratios, patient-controlled analgesia (PCA) morphine is becoming increasingly popular (Table 26-4).
* May increase serious adverse events (one trial using cyclooxygenase-2–specific inhibitors)
Medications for Risk Reduction after Coronary Artery Bypass Graft Surgery
CABG surgery itself reduces the risk of mortality and angina recurrence, but several medical management issues may help maintain the long-term benefit after CABG surgery. Specifically, the use of aspirin, β-blockers, and lipid-lowering agents has been demonstrated to prolong survival and/or reduce graft restenosis (Box 26-4).
BOX 26-4 Medications for Risk Reduction after Coronary Artery Bypass Graft Surgery
Aspirin
Several studies have demonstrated the efficacy of aspirin use on graft patency and reductions in MI and mortality after CABG surgery.6 A large observational study showed a reduction in mortality of nearly 3% and a reduction in MI rate of 48% with the early use of aspirin postoperatively (within 48 hours). Dosages of aspirin have ranged from 100 mg daily to 325 mg three times a day orally. Both ticlopidine and clopidogrel may be suitable alternatives in patients who are allergic to aspirin. Clopidogrel, through reductions in all-cause mortality, stroke, and MI, may be superior to aspirin in patients who return with recurrent ischemic events after cardiac surgery.7 Ticlopidine, however, should be used with caution because it may cause neutropenia (necessitating white blood cell counts to be monitored during initial use). Clopidogrel has a lower incidence of adverse reactions compared with ticlopidine and is therefore preferred as a second-line agent when aspirin is contraindicated.
Anticoagulation for Valve Surgery
Anticoagulation should be started in the early postoperative period for patients who have undergone valve replacement with either a mechanical or bioprosthesis and should also be considered when atrial fibrillation complicates the postoperative course. The recommended prophylactic regimens for patients with both mechanical and bioprosthetic heart valves are shown in Table 26-5.
Table 26-5 Suggested Antithrombotic Therapy for Heart Valve Prophylaxis
| Drug | Class | |
|---|---|---|
AVR = aortic valve replacement, MVR = mitral valve replacement.
* Risk factors: atrial fibrillation, left ventricular dysfunction, previous thromboembolism.
Modified from Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease): ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. J Am Coll Cardiol 32:1486-1588, 1998.
MANAGEMENT OF COMPLICATIONS
Complications are frequent after cardiac surgery. Although many are short-lived, some complications, such as stroke, are long-term catastrophic events that seriously affect a patient’s functional status.8 The incidence and predisposing risk factors are well studied for many of the complications (Table 26-6). Many of these complications have specific management issues, which may improve recovery after surgery (Box 26-5).
| Complication | Incidence | Risk Factors |
|---|---|---|
| Stroke | 2% to 4% | Age |
| Previous stroke/transient ischemic attack | ||
| Peripheral vascular disease | ||
| Diabetes | ||
| Unstable angina | ||
| Delirium | 8% to 15% | Age |
| Previous stroke | ||
| Duration of surgery | ||
| Duration of aortic cross-clamp | ||
| Atrial fibrillation | ||
| Blood transfusion | ||
| Atrial fibrillation | Up to 35% | Age |
| Male gender | ||
| Previous atrial fibrillation | ||
| Mitral valve surgery | ||
| Previous congestive heart failure | ||
| Renal failure | 1% | Low postoperative cardiac output |
| Repeat cardiac surgery | ||
| Valve surgery | ||
| Age | ||
| Diabetes |
Delirium
Delirium is defined generally as an acute transient neurologic condition with impairment of cognitive function, attention abnormalities, and altered psychomotor activity. It often includes a disorder with the sleep-wake cycle. It is fairly common after cardiac surgery with a prevalence of 8% to 15%.9 Risk factors associated with delirium include age, previous history of stroke, duration of surgery, duration of aortic cross-clamp, atrial fibrillation, and blood transfusion. Interestingly, delirium is self-limited and does not adversely affect patient outcome or hospital length of stay. Treatment is supportive, involving close observation of patients, and sedatives (midazolam, diazepam) or antipsychotics (haloperidol) as required.
Atrial Fibrillation
Atrial fibrillation after cardiac surgery is common and occurs in up to 35% of patients. While the cause is not completely understood, it is associated with an increase in mortality, stroke, and prolonged hospital stay. Known risk factors include age, male gender, previous episode, mitral valve surgery, and a history of congestive heart failure.10 Prevention and treatment of atrial fibrillation can be achieved effectively with amiodarone, sotalol, magnesium, or β-blockers. Biatrial pacing may also be effective prophylaxis. Management of atrial fibrillation consists of rate control with conversion to sinus rhythm or anticoagulation. Several studies conducted to determine which strategy was superior were unable to find a difference between treatment strategies (AFFIRM and RACE trials).11,12
Left Ventricular Dysfunction
Patients who have an unstable intraoperative course should have pulmonary artery filling pressures correlated to TEE findings and the results then passed to the recovery unit to allow for optimal initial management in the recovery unit. If the patient remains unstable in the ICU, then TEE can be used and cardiac function reassessed. When hypovolemia is thought to be the underlying cause of hypotension/low cardiac output, then colloids are initially used to optimize filling, because third spacing of fluids is common after CPB. The intravascular hypovolemia is best treated with the use of small intermittent boluses of colloid with continuous reassessment of central venous pressure, pulmonary artery pressure, wedge pressure, systemic pressures, or left ventricular end-diastolic area.13
If ventricular dysfunction is the main cause of hypotension/low cardiac output state, then inotropes and vasopressors should be added. Epinephrine (0.02 to 0.04 μg/kg/min) or dopamine (3 to 5 μg/kg/min) is commonly used to support patients coming off CPB and is usually continued into the ICU. If systolic pressure remains low, then the epinephrine infusion is usually increased to allow for greater α-receptor action (vasoconstriction). For patients with a low cardiac output or poor myocardial function on TEE, milrinone is commonly used (with or without a full loading dose). When volume and medical strategies are insufficient, especially in the presence of ischemic heart disease, mechanical support is used. IABPs are used in approximately 3% of cardiac surgical patients.14
POSTOPERATIVE RISK AND OUTCOME
One question that every patient asks and every anesthesiologist answers concerns the risks from this operation and the recovery course. Although seemingly a straightforward question, it gets to the heart of current medical practice, namely, assessing risk, weighing potential treatment options, determining best evidence for each patient, and then prescribing a course of treatment with the ultimate goal of providing the best possible care. It is important to know risk factors associated with cardiac surgery and to review treatment options for patients with specific reference to outcomes. This should all be placed within the context of cost and resource utilization, especially as medicine increasingly involves economic realities.15–18
SUMMARY
1. De Hert S.G., Cromheecke S., ten Broecke P.W., et al. Effects of propofol, desflurane, and sevoflurane on recovery of myocardial function after coronary surgery in elderly high-risk patients. Anesthesiology. 2003;99:314.
2. Murphy G.S., Szokol J.W., Marymont J.H., et al. Recovery of neuromuscular function after cardiac surgery: pancuronium versus rocuronium. Anesth Analg. 2003;96:1301.
3. Myles P.S., Daly D.J., Djaiani G., et al. A systematic review of the safety and effectiveness of fast-track cardiac anesthesia. Anesthesiology. 2003;99:982.
4. Cheng D.C., Wall C., Djaiani G., et al. Randomized assessment of resource use in fast-track cardiac surgery 1 year after hospital discharge. Anesthesiology. 2003;98:651.
5. Goldberg P.A., Sakharova O.V., Barrett P.W., et al. Improving glycemic control in the cardiothoracic intensive care unit: Clinical experience in two hospital settings. J Cardiothorac Vasc Anesth. 2004;18:690.
6. Mangano D.T. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002;347:1309.
7. Bhatt D.L., Chew D.P., Hirsch A.T., et al. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation. 2001;103:363.
8. Lahtinen J., Biancari F., Salmela E., et al. Postoperative atrial fibrillation is a major cause of stroke after on-pump coronary artery bypass surgery. Ann Thorac Surg. 2004;77:1241.
9. Bucerius J., Gummert J.F., Borger M.A., et al. Predictors of delirium after cardiac surgery delirium: Effect of beating-heart (off-pump) surgery. J Thorac Cardiovasc Surg. 2004;127:57.
10. Mathew J.P., Fontes M.L., Tudor I.C., et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720.
11. Hagens V.E., Ranchor A.V., Van Sonderen E., et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004;43:241.
12. Olshansky B., Rosenfeld L.E., Warner A.L., et al. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: Approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201.
13. Swenson J.D., Bull D., Stringham J. Subjective assessment of left ventricular preload using transesophageal echocardiography: Corresponding pulmonary artery occlusion pressures. J Cardiothorac Vasc Anesth. 2001;15:580.
14. Marra C., De Santo L.S., Amarelli C., et al. Coronary artery bypass grafting in patients with severe left ventricular dysfunction: A prospective randomized study on the timing of perioperative intraaortic balloon pump support. Int J Artif Organs. 2002;25:141.
15. Legrand V.M., Serruys P.W., Unger F., et al. Three-year outcome after coronary stenting versus bypass surgery for the treatment of multivessel disease. Circulation. 2004;109:1114.
16. Khan N.E., De Souza A., Mister R., et al. A randomized comparison of off-pump and on-pump multivessel coronary artery bypass surgery. N Engl J Med. 2004;350:21.
17. Cheng D., Bainbridge D., Martin J., et al. Does off-pump coronary artery bypass reduce mortality, morbidity and resource utilization when compared to conventional coronary artery bypass? A meta-analysis of randomized trials. Anesthesiology. 2005;102:188.
18. Puskas J.D., Williams W.H., Mahoney E.M., et al. Off-pump vs conventional coronary artery bypass grafting: Early and 1-year graft patency, cost, and quality-of-life outcomes: A randomized trial. JAMA. 2004;291:1841.