Chapter 60 Postoperative Arrhythmias After Cardiac Surgery
Atrial Arrhythmias
Incidence and Predictors
Atrial arrhythmias occur frequently after most types of cardiac surgery, with a prevalence as high as 40% following coronary artery bypass grafting (CABG).1–4 Atrial fibrillation (AF) and flutter are the most common arrhythmias; atrial tachycardias (ATs), including multifocal AT, are also observed. Clinical variables that convey higher risk for the development of postoperative AF are described in Box 60-1.5–15
Box 60-1 Predictors of the Development of Post–Cardiac Surgery Atrial Arrhythmias
Passman and colleagues have proposed a series of nomograms to assess the degree of risk for postoperative AF based on multiple preoperative clinical and electrocardiogram (ECG) variables.11 These investigators performed a chart review of 229 consecutive patients who underwent CABG and found that independent predictors for postoperative AF included advanced age; left main or proximal right coronary artery stenoses; history of AF or heart failure; or preoperative ECG findings of a P-R interval 185 milliseconds (ms) or longer or a P-wave duration in lead V1 of 110 ms or longer. Univariate analysis indicated that in this cohort, chronic obstructive pulmonary disease (COPD), left main or proximal right coronary artery stenoses, frequent premature atrial contractions, and left atrial abnormality on ECG were not significant predictors for postoperative AF. Initial reports indicated that a minimally invasive approach to CABG (vs. a conventional sternotomy) does not lessen the incidence of postoperative AF, when corrected for disease severity.15,16 However, more recent investigations indicate that the incidence of postoperative AF is lessened with minimally invasive techniques for CABG and valvular surgery.17–19
CABG without the use of cardiopulmonary bypass (CPB) has been associated with a decreased incidence of postprocedural AF in situations of minimally invasive techniques, reoperative (“redo”) single-vessel revascularization, and octogenarian patients.20–22 Ascione and colleagues investigated, in a prospective, randomized trial, whether the use of CPB and cardioplegic arrest influenced the incidence of postoperative AF.10 Two hundred patients were randomized to CPB (with normothermic CPB vs. cardioplegic arrest CPB) and off-pump “beating heart” surgery. In this study, a risk factor for postoperative AF was observed to be CPB with cardioplegic arrest, not CPB in general. Other risk factors for postoperative AF described by this study included postoperative inotropic support, intubation time, chest infection, and length of hospital stay. In summary, similar to patients with postoperative AF observed after conventional CABG, patients with AF after minimally invasive techniques have a higher in-hospital morbidity rate, length of stay, and mortality rate compared with patients without AF; however, AF can occur with a lower frequency following minimally invasive cardiac surgery or after cardiac surgery without CPB or cardioplegia.
Risk stratification for postoperative atrial arrhythmias can be performed using clinical characteristics (see Box 60-1) or by laboratory methods. One example of a stratification method is based on P-wave duration, as calculated directly from the surface ECG or from signal-averaged data.23 An investigation by Buxton and Josephson assessed P-wave duration from standard electrocardiographic leads in 99 cardiac surgical patients and found that the mean total P-wave duration in patients who developed AF or atrial flutter was significantly longer than in patients who remained in sinus rhythm (mean total P-wave duration of 160 ms and 126 ms [P = .001], respectively).23 A significantly prolonged P-wave duration was observed to be a sensitive (83%) but not specific (43%) predictor of postoperative atrial arrhythmias. Prolonged P-wave duration in patients who develop postoperative atrial arrhythmias may be a reflection of underlying preoperative atrial disease.
Prognosis
Postoperative AF usually arises 1 to 5 days following surgery, with a peak incidence on day 2, and usually has a self-limited course.24–26 More than 90% of patients with AF following cardiac surgery who have no history of atrial arrhythmias are in sinus rhythm 6 to 8 weeks following their operation. Rubin and associates followed up postcardiac surgical patients for an average of 26 months and observed no differences in cardiovascular or cerebrovascular morbidity or mortality between patients with postoperative AF and patients without AF.27 However, other studies have shown an increased rate of early and late postoperative stroke in association with AF (see later section on postarrhythmia therapy).14,20,28,29 Postoperative atrial arrhythmias are usually considered to increase morbidity, length of stay in the intensive care unit (ICU), length of hospitalization, and medical costs. Almassi and coworkers, in a series of 3855 cardiac surgical patients, found that postoperative AF was associated with a longer ICU stay (3.6 vs. 2 days for patients without AF, P = .001), an increased rate of ICU re-admission (13% vs. 4%), a greater incidence of perioperative myocardial infarction (7.4% vs. 3.4%), more persistent congestive heart failure (4.6% vs. 1.4%), and a higher rate of re-intubation (10.6% vs. 2.5%).14 An investigation by Abreu and colleagues reported a longer hospital stay of 4.9 days because of postcardiac surgical AF, and it was calculated to increase medical costs by $10,000.17 It should be noted that, in slight distinction, a study by Kim and associates observed a shorter hospital stay (1 to 1.5 days in this study) attributed to postoperative AF.30 Villereal and colleagues found that the occurrence of AF after CABG identifies a subset of patients at an increased risk for both early and long-term cardiovascular events.31
Etiology
Many perioperative factors have been described in the pathogenesis of postoperative AF (Box 60-2), but no definitive data are available. The pathophysiology of postoperative AF is probably related to pre-existing age-related degenerative cardiac changes in many patients, coupled with perioperative abnormalities in several electrophysiological parameters such as dispersion of atrial refractoriness, atrial conduction velocity, and atrial transmembrane potential. Nonuniform atrial conduction is greatest on postoperative days 2 and 3, and the longest atrial conduction time is greatest on day 3.32 These abnormalities coincide with the time of greatest risk for AF, which has a peak incidence on days 2 and 3.33 AF after CABG has been associated with increased expression and heterogeneity in distribution of connexin40, an intercellular gap junction protein, unlike in patients who do not develop AF.34 These changes could result in differences in resistive properties and conduction velocity among spatially adjacent regions of the atrial myocardium. Postoperative pericarditis is generally felt to be an etiology, or at least a potentiating factor, for AF. Perioperative hypokalemia has been shown to be associated with atrial arrhythmias with an odds ratio of 1.7 (even after adjusting for confounding factors) in a multicenter trial that followed up over 2400 patients through cardiac surgery.35 Potential mechanisms whereby hypokalemia might alter atrial electrophysiology include increased phase 3 depolarization, increased automaticity, and decreased conduction velocity.
Prophylactic Therapy with Pharmacologic Agents
Given the high incidence of postoperative AF, it is strongly recommended that prophylactic treatment be considered, especially in the presence of the risk factors described in Box 60-1. The magnitude of benefit from β-blockers, sotalol, amiodarone, and pacing was evaluated in a 2004 meta-analysis of 58 randomized trials that included over 8500 patients and in which placebo or routine therapy was given to controls.36 Despite the significant reduction in AF, prophylactic drug therapy was associated with a nonsignificant reduction in stroke (odds ratio [OR], 0.76; 95% confidence interval [CI], 0.43 to 1.31), which may be attributed to a low rate of events (1.2% vs. 1.4%).36 The use of β-blockers, in the presence or absence of digitalis, has been demonstrated to decrease AF from 40% for CABG patients and 60% for valvular surgery patients to 20% and 30%, respectively.25,26,37 The effect of β-blockade in reducing postoperative AF, both alone or with digoxin, has been demonstrated in multiple meta-analyses.37,38 Even though a preventive strategy of β-blocker administration might save both medical resources and decrease the length of hospital stay, such benefits have not yet been demonstrated. Specifically, in the β-Blocker Length of Stay Study (BLOSS) trial, 1000 patients undergoing cardiac surgery were randomized in a double-blinded fashion to receive either metoprolol (possibly in an up-titrated dose) or placebo.12 In this trial, patients treated with β-blockers had a decreased incidence of atrial arrhythmias, but this did not translate into decreased length of hospital stay. Although investigators have found an association between postoperative AF and cardiovascular and other morbidities, broadly accepted therapies that reduce the incidence of AF have not yet been demonstrated to have long-term benefit or cost effectiveness.31
Digitalis given preoperatively or postoperatively has been shown only to be possibly helpful and not to the same extent or with the same reliability as β-blockers.38 Postoperative verapamil given to patients in sinus rhythm has been observed to slow the rate of AF if it occurs but not to alter the prevalence.39 Other antiarrhythmic agents such as procainamide have been studied in a prophylactic role but have been associated with varying benefits in different reports.40 No comprehensive data on the effectiveness of propafenone or flecainide are available.
DL–Sotalol has β-blocker and class III activities and may have a role in the prophylactic treatment of postoperative AF. Preliminary data indicate that oral sotalol may reduce the incidence of AF following cardiac surgery.41,42 Sotalol may be more effective than metoprolol, speaking to a potential incremental benefit of the activity of sotalol class III.43 Conversely, a relatively large study of 429 consecutive patients by Suttorp and associates demonstrated no dramatic difference in the benefit of low-dose sotalol versus high-dose sotalol compared with low-dose β-blockers versus high-dose β-blockers, which suggests that the potential benefit of DL-sotalol arises from its β-blocker effect.44
The potential prophylactic role of sotalol was further examined in a recent prospective, randomized, double-blinded, placebo-controlled study of 85 post–cardiac-surgery patients by Gomes and coworkers.45 In this group of patients, a significant reduction in postoperative AF was observed with sotalol treatment compared with either placebo or β-blocker treatment. Also, no increase in ventricular arrhythmias was detected, which suggests that the membrane effect of sotalol class III, when given in this study’s setting, was not a liability. However, this study excluded patients with heart failure or marked left ventricular dysfunction (characteristics that might predict a high risk for postoperative AF or for ventricular proarrhythmia), which suggests that although sotalol may be useful, it cannot be broadly applied.46
Amiodarone—both oral and parenteral formulations—have been evaluated for prophylaxis against perioperative atrial arrhythmias. Daoud and colleagues, in a placebo-controlled trial, assessed the potential benefit of preoperatively administered amiodarone in 124 patients undergoing cardiac surgery.47 Patients who received amiodarone, which had been initiated at least 7 days preoperatively, had a lower incidence of AF (25%) compared with patients who had received placebo (53%). Amiodarone administration was also associated with shorter duration of hospitalization and resultant decreased hospital costs. These data suggest a possible benefit of outpatient preoperative medication with oral amiodarone in decreasing the incidence of AF. However, despite a high rate of use of β-blockers—an approach shown to prevent at least 50% of AF in almost every trial in which it has been studied—a high incidence of atrial arrhythmias was observed in the control group, which suggests that these observations may not be broadly applicable. A more complete assessment of the effects of prophylactic amiodarone was provided by a 2005 meta-analysis of 10 trials.2,38,48 Amiodarone therapy was associated with a significant reduction in the rate of AF or atrial flutter (22% vs. 35%; relative risk [RR], 0.64; 95% CI, 0.55 to 0.75). Also, significant reductions in the much less frequent complications of ventricular tachycardia (VT) or ventricular fibrillation (3.6% vs. 9.6%; RR, 0.42; 95% CI, 0.28 to 0.63) and stroke (1.5% vs. 4.%; RR, 0.39; 95% CI, 0.21 to 0.76) were seen. Only 61% of patients in these trials were treated with β-blockers. A similar magnitude of benefit was seen in the largest randomized trial of amiodarone in relation to cardiac surgery (PAPABEAR).49 In this trial, which was published after the above-mentioned meta-analysis, 601 patients undergoing elective CABG or valve surgery were randomly assigned to oral amiodarone (10 mg/kg daily starting 6 days prior to surgery and continued until 6 days after surgery) or placebo. In the entire population, a 48% reduction was seen in perioperative atrial tachyarrhythmias. This benefit was consistent across a number of predefined subgroups, including patients 65 years of age or younger, those undergoing CABG or valve surgery, and those who were taking preoperative β-blockers, and those not taking β-blockers. Patients assigned to amiodarone who had an atrial tachyarrhythmia had a significantly lower average ventricular rate (105 vs. 131 beats/min with placebo). Patients randomly assigned to amiodarone had more adverse cardiac events compared with those taking placebo, including bradycardia requiring temporary pacing (5.7% vs. 2%) and QT prolongation (1.3% vs. 0%). Preoperative amiodarone treatment does have distinct disadvantages: the need to identify patients well in advance of their procedure, potential bradyarrhythmic hazards (especially in an outpatient setting and in older adults), and, although rare, a risk of perioperative pulmonary toxicity.50 The latter may be caused by a potentiated risk, from amiodarone, of CPB-associated adult respiratory distress syndrome (ARDS), which has a poor prognosis.
The Amiodarone Reduction in Coronary Heart (ARCH) trial investigated, in a placebo-controlled, double-blind study of 300 patients, whether postoperative administration of intravenous (IV) amiodarone reduced the incidence of AF.51 Results showed a significant decrease in the incidence of AF in patients given amiodarone (35%) compared with those given placebo (47%), without significant risk from the active agent. However, the size of the benefit did not result in shorter hospital stay in this study. The relatively modest benefit of IV amiodarone in this report would probably have been even smaller had a greater number of patients received β-blockers.51
β-Blockers have been shown to be the most effective prophylactic agents and carry a lower risk relative to other antiarrhythmic agents.2,38 On the basis of these data, the authors of this chapter believe that β-blocker prophylaxis should be widely applied. Use of other drugs for prophylaxis needs to be further investigated.
Prophylactic Therapy with Pacing
The potential role of nonpharmacologic therapy in the prevention of postoperative AF has been examined in several studies. Single-site and multiple-site atrial pacing has been shown to be helpful in some cases of non-perioperative paroxysmal AF.52 Investigations into the potential role of single-site and multiple-site atrial pacing in the prevention of postoperative AF have shown varying benefits.
Initial reports indicated that atrial pacing might not be beneficial for postoperative AF. An investigation of 86 post-CABG patients found that atrial pacing via single-site atrial epicardial wires, at a rate of at least 80 beats/min and always above the intrinsic sinus rate (“overdrive pacing”), was not associated with a different incidence of postoperative atrial arrhythmias when compared with the absence of pacing. A recent study of 100 post-CABG patients, randomized to no atrial pacing versus atrial pacing at 10 beats/min or more above the resting heart rate, indicated that atrial pacing significantly increased atrial ectopy and did not attenuate the rate of AF occurrence.53 The potential role of bi-atrial overdrive pacing in the prevention of postoperative AF has also been investigated in several studies. One prospective, randomized trial by Kurz and colleagues examined the effect of bi-atrial pacing in a group of post-CABG patients, assessing the incidence of AF and the possible proarrhythmic effects of pacing.54 Unfortunately, after only 21 of the planned 200 patients were randomized, the study was terminated because this study’s pacing protocol was observed to promote AF, a possible consequence of undersensing of atrial signals by the epicardial pacing system leading to asynchronous atrial pacing. An investigation by Gerstenfeld and coworkers studied 61 post-CABG patients who were randomized to right atrial pacing, left and right atrial pacing, or no pacing, using epicardial wires.55 No significant difference in the incidence of AF was observed among groups, although a trend toward less atrial arrhythmia in paced patients also receiving a β-blocker was seen.
Some studies, however, have shown a potential benefit from atrial pacing in the prevention of postoperative AF. Greenberg and associates performed an investigation of 154 patients following CABG or CABG plus aortic valve replacement.56 Patients were randomized to no pacing, right atrial pacing, left atrial pacing, or bi-atrial pacing for 72 hours postoperatively, and efforts were made to administer β-blocker medications. Any pacing modality reduced the incidence of AF from 37.5% to 17% and the length of hospitalization from 7.8 to 6.1 days. In this study, multivariate analysis indicated that the most effective sites of pacing were the right atrial, left atrial, and bi-atrial sites, in that order. Of note, patients in this study did not have significant left ventricular dysfunction (average ejection fraction 53 ± 10%). In contrast, an investigation by Blommaert and colleagues examined the course of 96 postoperative patients who had a wide range of left ventricular function.57 Patients were randomized to no pacing versus 24 hours of atrial pacing using a dynamic overdrive algorithm.57 Attention was paid to the use of β-blocker medication. Pacing was associated with a lower incidence of AF (10%) compared with no pacing (27%). Multivariate analyses showed that the beneficial effect of atrial pacing was observed particularly in patients with preserved left ventricular function and older patients.
Findings regarding the potential benefit of bi-atrial pacing in the prevention of postoperative AF have been varied. In contrast to the findings of Greenberg and coworkers, Fan and colleagues observed a greater benefit with bi-atrial versus single-site atrial pacing.56,58 Fan and associates studied 132 postoperative patients without a history of AF and randomized them to no pacing, bi-atrial pacing, left atrial pacing, or right atrial pacing. After overdrive atrial pacing for 5 days, the incidence of AF was 41.9%, 12.5%, 36.4%, and 33.3%, respectively. Reductions in the rates of postoperative AF translated into shorter hospital stays in this study. Also, patients who remained in sinus rhythm had significant reductions in P-wave duration and variability in P-wave duration following pacing therapy.
Other Prophylactic Agents
Angiotensin Inhibition
Although angiotensin converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have not previously been considered a specific therapy in patients with AF, a number of observations suggest their benefit in nonsurgical settings. A reduction in the incidence of postoperative AF with ACE inhibitors was also seen in a multicenter analysis of 4657 patients undergoing CABG.59 Postoperative AF occurred significantly less often in patients who were treated preoperatively and postoperatively with ACE inhibitors compared with those who were not (20% vs. 34%; OR, 0.62). Patients who had previously been taking ACE inhibitors and were withdrawn from therapy had an increase in risk (46%; OR, 1.69).
Statins
Statins may reduce the incidence of perioperative AF. This was illustrated in the ARMYDA-3 (Antiplatelet Therapy for Reduction of Myocardial Damage During Angioplasty Study–3) trial with 200 patients who underwent CABG without a prior history of statin treatment.60 Patients were randomly assigned to 40 mg of atorvastatin or placebo daily, starting 7 days prior to surgery. Atorvastatin significantly lowered the incidence of AF (35% vs. 57% with placebo). On the basis of the established benefits of statin therapy in patients with coronary heart disease, patients should be on a statin prior to elective CABG. The possible suppression of perioperative AF may be an added benefit, but the validity of this finding does not impact the recommendation for statin use in this setting.
Glucocorticoids
On the basis of the hypothesis that perioperative inflammation may contribute to the development of AF, glucocorticoids have been suggested as prophylactic therapy. In a multicenter trial, 241 patients undergoing CABG, valve surgery, or both were randomly assigned to 100 mg of hydrocortisone every 8 hours for 3 days after surgery or to placebo.61 During a follow-up period of 84 hours after surgery, the incidence of AF was significantly reduced with hydrocortisone therapy (30% vs. 48% with placebo). No difference in adverse events, including infections, was observed between the groups. Because of the wide range of the physiological effects of glucocorticoids, additional data on both the efficacy and safety of this approach are necessary before it can be considered for routine use.
Fish Oil
Consumption of fish that induce high plasma levels of n-3 polyunsaturated fatty acids (PUFAs) may be associated with a moderate reduction in the risk of AF.62 A randomized controlled trial of 160 patients assessed whether the administration of PUFAs (2 g per day), compared with placebo, would reduce the incidence of postoperative AF after CABG.63 The development of AF was significantly reduced in patients receiving PUFAs (15.2% vs. 33.3%).
Postarrhythmia Therapy
Rate Control Treatment for Postoperative Atrial Fibrillation
Given the self-limited course of postoperative AF in the vast majority of patients with no history of preoperative atrial arrhythmias, treatment to control the ventricular response rate in postoperative AF is a useful strategy. Rate control therapy with β-blockers should be the first-line choice, with the relative benefit partly attributable to treatment of the hyperadrenergic postoperative state and prevention of the well-demonstrated phenomenon of β-blocker withdrawal. Rapid administration of IV digoxin is occasionally mentioned as being helpful in restoring sinus rhythm, although the data are not supportive.64 AV-nodal blocking agents such as calcium channel blockers and digoxin have roles in the control of the ventricular rate in AF but are not more effective than β-blockers; calcium channel blockers or digoxin may be useful when β-blockers cannot be given (for instance, in the presence of bronchospasm). A randomized, double-blind investigation by Tisdale and associates, comparing parenteral diltiazem and digoxin in post-CABG patients with AF, indicated that this calcium channel blocker results in rate control of AF more rapidly than does digoxin; however, after 12 and 24 hours, no significant difference in effect or in length of hospital stay was observed.65
An investigation by Clemo and colleagues assessed the potential benefits of IV amiodarone in critically ill patients with atrial arrhythmias with rapid ventricular response rates, in some cases following cardiac surgery.66 The data were retrospectively obtained from 38 patients with atrial arrhythmias in an ICU setting who had AF with resultant hemodynamic destabilization despite previous use of conventional AV-nodal blocking agents for rate control. IV amiodarone administration was associated with improved rate control, peripheral blood pressure, cardiac filling pressures, and cardiac output. However, no significantly increased rate of spontaneous reversion to sinus rhythm after IV amiodarone treatment was observed. In summary, this investigation showed that IV amiodarone has a beneficial role in slowing ventricular rate in AF in critically ill patients, possibly including groups of post-CABG patients, particularly when previous AV-nodal blocking drugs have not been fully effective. Although sole treatment with IV amiodarone cannot be relied on for conversion from AF, it is likely to be quite helpful in maintaining sinus rhythm, and it (or some anti-arrhythmic agent) should be considered before electrical cardioversion.67
Electrical Cardioversion for Postoperative Atrial Fibrillation
Conversion from well-tolerated postoperative AF is generally not actively pursued because of the high recurrence rate as well as the self-limited course. For patients with symptoms, however, therapies are similar to those employed in non-postoperative circumstances; however, a greater emphasis should be placed on postconversion pharmacologic therapy because causative factors inevitably persist to cause a recurrence. If conversion is necessary, atrial defibrillation or, if atrial flutter or tachycardia is present, pace termination can be employed.68 In a case of AF that is difficult to convert by using the usual external techniques, consideration should be given to (1) internal defibrillation using transvenous coils if available; (2) a “double defibrillator” technique in which two pairs of orthogonally placed transthoracic, external patch electrodes are discharged simultaneously; or (3) pretreatment with IV ibutilide.69–71 Information is emerging on low-energy atrial defibrillation via operatively implanted temporary epicardial coils in animal models and in clinical studies; this may ultimately be an effective strategy for high-risk patients (see Box 60-1) who are unable to tolerate pharmacologic therapy or as an adjunct to prophylactic therapy.72,73
Pharmacologic Cardioversion for Postoperative Atrial Fibrillation
Pharmacologic measures for the conversion of AF should be considered, especially if the patient’s respiratory status makes anesthesia for an electrical conversion potentially hazardous or some other contraindication to general anesthesia exists. Medications that have been shown to be potentially useful include newer class III agents (such as ibutilide) and investigational agents such as tedisamil and tercetilide. A recent study by the Ibutilide Investigators compared the use of increasing doses of IV ibutilide with placebo in the treatment of postoperative atrial arrhythmias.74 Ibutilide was significantly more effective than placebo in a dose-responsive fashion. This IV type III agent was also observed to be more efficacious in atrial flutter than in AF, as is often the case with class III agents. Ibutilide carries a risk of ventricular proarrhythmia (which occurs most often as sustained or nonsustained torsades de pointes) in about 2% to 4% of patients; this is particularly associated with bradyarrhythmia, hypokalemia, hypomagnesemia, and female gender.74 However, when this medication is used carefully (with attention to the above risk factors and with telemetry observation during and following its administration), the risk of proarrhythmia can be mitigated. Indeed, the Ibutilide Investigators group observed that ibutilide-treated patients with higher heart rates had a lower incidence of ventricular arrhythmias than did rate-controlled patients, a likely consequence of bradyarrhythmia-induced torsades de pointes in the latter group.74
Anticoagulation for Postoperative Atrial Fibrillation
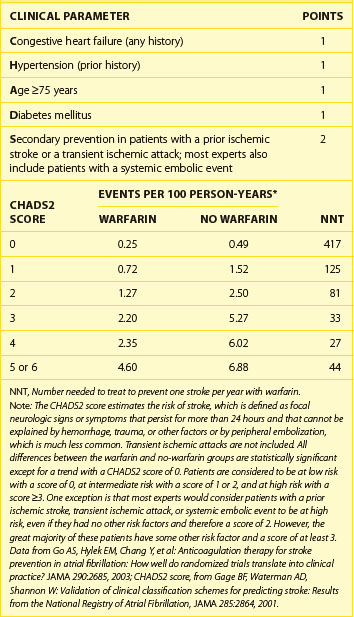
Although one might expect a relatively low risk for thromboembolic events in association with a limited course of postoperative AF, several studies have shown, with both prospective case series and case-controlled retrospective analyses, an increased rate of post-CABG stroke in association with postoperative AF, even after correction for comorbid risk factors.14,28,29 Given the potentially devastating consequences of a thromboembolic event, anticoagulation with warfarin (Coumadin) should be considered for postoperative AF, particularly for patients with mitral valvular disease or prosthesis, left atrial enlargement, marked left ventricular dysfunction, previous thromboembolic events, and age 65 years or older (Table 60-1). Because of the risk of bleeding in postoperative patients, anticoagulation must be performed carefully, and IV heparin is often not employed. If cardioversion is performed for postoperative AF, conventional recommendations for anticoagulation should be followed. A summary of therapeutic measures for post-CABG atrial arrhythmias, including treatments for prophylaxis and treatments for postoperatively occurring AF, are summarized in Box 60-3.
Table 60-1 CHADS2 Score, Thromboembolic Risk, and Effect of Warfarin in 11,526 Patients with Nonvalvular Atrial Fibrillation and No Contraindications to Warfarin Therapy
Box 60-3 Summary of Therapeutic Measures for Postcardiac Surgical Atrial Arrhythmias
Treatment (For Postoperative Atrial Arrhythmia)
For the prevention of postoperative AF, guidelines were published in 2006 by the American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) and in 2004 by the ACC/AHA.75,76 This is summarized in Box 60-4. β-Blockers are the most widely used and, if not contraindicated, should be given as soon as possible after cardiac surgery.36,48,75,76
Box 60-4 ACC/AHA/ESC Guideline Summary
Management of Postoperative Atrial Fibrillation
Data from Fuster, V, Ryden, LE, Cannom, DS, et al: ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation), J Am Coll Cardiol 48:e149, 2006.
Although nonselective β-blockers can cause bronchospasm in patients with COPD, β1 selective β-blockers (e.g., atenolol or metoprolol) appear to be safe even when a bronchospastic component is present.77 The optimal duration of therapy for the prevention of postoperative atrial arrhythmias is uncertain, but β-blockers are often continued until the first postoperative visit. However, many patients who undergo cardiac surgery have a clear indication for the continued use of β-blocker therapy (e.g., previous myocardial infarction [MI], heart failure, or hypertension).
Amiodarone and sotalol are also effective in the postoperative setting.36,48 With respect to amiodarone, the cost, the need for monitoring, and the transient nature of postoperative AF have limited its use. The authors of this chapter and the 2004 ACC/AHA guidelines suggest use of preoperative amiodarone in patients who have a contraindication to β-blockers and are at high risk for postoperative AF.48,75 High-risk features include previous AF and mitral valve surgery.33
Ventricular Arrhythmias
Incidence and Prognosis
Sustained and unsustained ventricular arrhythmias—both monomorphic VT and ventricular fibrillation (VF)—are observed after cardiac surgery. The reported incidence of de novo sustained and nonsustained ventricular arrhythmias is 0.7% to 3% and 36%, respectively.78,83 Much earlier observations have shown an incidence of postoperative sustained ventricular arrhythmias of up to 6%, consistent with the known development of cardiac surgical techniques and postoperative care since the 1960s.84 Nonetheless, the occurrence of ventricular arrhythmias predicts significant mortality. For example, Tam and colleagues observed de novo, sustained ventricular arrhythmias in only 16 of 2364 patients in the first week following cardiac surgery (most notably in patients with marked left ventricular dysfunction preoperatively).79 However, of those patients with ventricular arrhythmias, 75% had recurrences of sustained VT or VF, with a 19% mortality rate. Kron and coworkers, in studying 1251 postoperative patients, observed an in-hospital mortality rate of 44% in patients with unprecedented and sustained ventricular arrhythmias. Recurrences of VT beyond the immediate postoperative period have been observed in 40% of patients with early postoperative VT who are also inducible to VT at electrophysiological study (EPS).85
Etiologies
Etiologies of postoperative ventricular arrhythmias include structural heart disease, such as previous infarction, fibrosis and dilation, and hypertrophy. Potential acute precipitants of postoperative ventricular arrhythmias are also described in Box 60-5.78–82,86–88 Several electrolyte and metabolic abnormalities are associated with postoperative ventricular arrhythmias, most notably hypokalemia (but not low potassium levels intracellularly).89
Diagnostic Issues
In the postcardiac surgical patient with a wide-complex tachycardia, the diagnosis of VT is most often favored because of the high frequency of structural heart disease. In situations where the surface ECG recordings are ambiguous, a diagnosis of VT versus supraventricular tachycardia or rapidly conducted AF with aberrancy can often be made with the use of epicardial atrial wire recordings.90,91
Sustained Monomorphic Ventricular Tachycardia
A study by Steinberg and associates prospectively enrolled consecutive patients undergoing CABG and found that 3.1% of patients had at least one episode of sustained VT at a mean of 4.1 days following the operation; these patients had a 25% in-hospital mortality rate.78 Predictors of postoperative VT included previous MI, marked heart failure, significant left ventricular dysfunction (defined as an ejection fraction <0.40), and revascularized myocardium previously supplied by a noncollateralized native vessel. When the first three of these predictive factors were present, the incidence of VT increased to 30%.
Ventricular Fibrillation or Polymorphic Ventricular Tachycardia
An underlying arrhythmogenic substrate for monomorphic VT (e.g., myocardial scarring) is almost always present, whereas polymorphic VT is more likely related to transient perioperative abnormalities.81,82,92 Kron and colleagues observed that acute cardiac ischemia following heart surgery was more highly associated with primary VF than with VT.80 The perioperative factors that may contribute to polymorphic ventricular arrhythmias are described earlier and in Box 60-3. Whether polymorphic VT or VF events occurring immediately after CPB, in the absence of additional risk factors for ventricular arrhythmia, predict an increased risk for ventricular arrhythmic events in the long term is not supported by clear data.
Acute Management
No comprehensive or controlled studies of the acute treatment for postoperative VT have been conducted so far. However, commonly accepted and consensus treatments include ACLS-based algorithms, including synchronized DC cardioversion and pace termination (facilitated by the presence of epicardial pacing wires). Prevention of additional episodes can be aided by attention to abnormalities such as ischemia, fluid overload, electrolyte disturbances, and polypharmacy with potentially proarrhythmic cardiac or other agents.93 Effective acute treatment can also be achieved with antiarrhythmic agents such as parenteral amiodarone, β-blockers, sotalol, magnesium sulfate (specifically for torsades de pointes), and procainamide.40,94–98 Recurrent VT can also be prevented or treated with the use of overdrive ventricular pacing or burst ventricular pacing, respectively.99 Mechanical supportive measures such as intra-aortic balloon counterpulsation, intrathoracic ventricular-assist devices, or external cardiopulmonary support have been used.100–104 A case of simultaneous intra-aortic balloon and external cardiopulmonary therapy allowing for high-dose β-blocker infusion in a patient with refractory postoperative VT has been reported, although this technique is not widely applied.105
Postoperative Risk Stratification and Treatment
Longer-term treatment for patients who have manifested monomorphic VT postoperatively may involve risk stratification with an assessment of left ventricular function, EPS, or both.81,85 Post–hospital discharge recurrences of VT have been noted in 40% of patients with postoperative VT who were also inducible to VT at postoperative EPS, which suggests the value of long-term treatment (e.g., with an implantable cardioverter-defibrillator [ICD]), although data showing a direct benefit in terms of mortality rates or other measures are lacking.85 Of note, a discordance has been observed at EPS in inducibility of VT by programmed electrical stimulation (PES) performed via single-site epicardial pacing and that performed via dual-site endocardial pacing. Sheppard and colleagues performed EPS on 26 post-CABG patients using both operatively implanted epicardial pacing wires and endocardial electrophysiological catheters.106 Despite similar effective and functional refractory periods that were epicardially and endocardially measured, concordant results of inducibility to VT between the techniques were obtained in only 70% of patients; 40% of patients inducible with dual-site endocardial PES had been uninducible via single-site epicardial PES, which suggests that single-site PES from epicardial wires may not fully assess the postoperative risk for VT.
The presence of late potentials on postoperative signal-averaged ECG has been reported as an independent predictor for VT early after CABG, but no comprehensive data on longer-term VT occurrences or outcomes in patients with an abnormal signal-averaged ECG are available.107 The modality of exercise testing is also employed, although no direct evidence has shown its utility in predicting the risk of postoperative VT. The timing of postoperative risk stratification is important, with some investigators suggesting a waiting period of at least 1 week to allow for both healing and equilibration of substrate to occur.83,108
Lifelong treatment with antiarrhythmic medication, ICD placement, or both is likely useful for patients who are found to be at high risk for ventricular arrhythmia, such as those who have manifested postoperative ventricular arrhythmias and who also have significant left ventricular dysfunction, inducible monomorphic ventricular arrhythmias at EPS, or both. The significant rate of subsequent clinical ventricular arrhythmic events in patients with postoperative VT who are also inducible to VT at postoperative EPS indicates the value of long-term treatment, although direct data showing a benefit in mortality or other measures for patients with VT that emerges postoperatively are lacking.85
Bradyarrhythmias
Incidence and Prognosis
Cardiac conduction abnormalities and sinus bradyarrhythmias are reported in 17% to 34% of postoperative patients, depending on a multitude of factors (Box 60-6); however, a significant rate of reversibility does exist.109,110 Persistent bradyarrhythmias requiring permanent pacing occur in 0.8% to 4% of postcardiac surgical patients.111–116 The need for permanent pacemaker implantation following cardiac surgery is significant following valvular surgery, in the presence of pre-existing conduction system disease, and in several other situations, as described in Box 60-4.111–114,117–120 Bradyarrhythmias necessitating permanent pacing have been associated with longer postoperative courses in the ICU and in the hospital, as well as with inevitably increased postoperative economic costs, although no direct data on cost exist.16
Box 60-6 Risk Factors for the Need for Permanent Pacemaker Implantation Following Cardiac Surgery
It is likely that recovery from postoperative bradyarrhythmias occurs in a significant proportion of patients. An investigation by Glikson and colleagues on long-term pacemaker dependency in a group of 120 patients who had received a permanent pacemaker following cardiac surgery indicated that 41% of patients eventually became pacemaker nondependent.117 Patients with postoperative complete heart block (CHB) as the indication for pacemaker implantation were more likely to remain pacemaker dependent in this study. In contrast, in a smaller study of 93 consecutive post-CABG patients by Baerman and Morady, all three patients who underwent pacemaker implantation because of third-degree heart block were no longer in heart block after 2 months.112 The issue of eventual use of a permanent pacemaker implanted after cardiac surgery was further explored in a study by Gordon and coworkers.114 This investigation, which involved prospective data collection from 10,421 consecutive patients who had cardiac operations, included a logistic regression analysis of independent and multivariate predictors of permanent pacing following cardiac surgery (with predictors including the risk factors listed in Box 60-4). The investigators also found that their predictive model had a high correlation with eventual pacemaker use, which suggests that the presence of a greater number of risk factors for pacemaker implantation following cardiac surgery predicts a higher rate of actual pacemaker use.
Risk Stratification and Management Strategies
The decision to implant a permanent pacemaker in a post-CABG patient with high-grade atrioventricular (AV) block and a poor escape rhythm who is otherwise ready for hospital discharge is relatively straightforward. However, no clear data on the optimal waiting time in more equivocal situations exist. For complete AV block, some authors have recommended that a decision regarding permanent pacemaker implantation be made no later than day 6 and day 9 following surgery for wide-complex and narrow-complex escape rhythms, respectively.117 In a study of pediatric patients with CHB following operation for congenital heart disease (with most cases involving the closure of a ventricular septal defect), Bonatti and associates recommend permanent pacing after at least a 2-week observation period with temporary pacing in that population.115
Any decision regarding timing of implantation of a permanent pacemaker will be impacted by the stability of the temporary pacing system. Epicardial electrode temporary pacing systems frequently display increasing pacing thresholds with time, and endocardial pacing catheters can have similar problems plus infective and vascular complications. A prospective study of 30 post-CABG patients by Kosmas and colleagues found that effective atrial, ventricular, and dual-chamber pacing via epicardial wires could not be performed by the fifth postoperative day in 39%, 38%, and 61% of patients, respectively.121
In cases of resolved or resolving heart block, the assessment of persistent risk of bradyarrhythmias and the need for permanent pacemaker implantation can be influenced by several factors. First, consideration should be given to determining the need for concomitant antiarrhythmic medication (which would be expected to exacerbate even borderline bradyarrhythmias). Second, consideration should be given to performing an EPS (an abbreviated but effective form of which can be performed at the bedside with atrial pacing via operatively placed epicardial wires) to help detect significant AV conduction, sinus node, or both. EPS results suggesting that permanent pacing is needed are similar to generally applied criteria, including an H-V interval greater than 75 ms, infra-Hisian block occurring spontaneously or at an atrial paced cycle length of 400 ms or less (300 ms in a pediatric population), or a corrected sinus node recovery time of 550 ms or longer.115 Third, an exercise study can be performed to assess chronotropic competence and AV conduction at elevated heart rates.
Acknowledgment
The authors thank Rose Marie Wells for her expert assistance with the preparation of this chapter.
Key References
Andrews TC, Reimold SC, Berlin JA, Antman EM. Prevention of supraventricular arrhythmias after coronary artery bypass surgery: A meta-analysis of randomized control trials. Circulation. 1991;84:III236-III244.
Calo L, Bianconi L, Colivicchi F, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723-1728.
Crystal E, Connolly SJ, Sleik K, et al. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: A meta-analysis. Circulation. 2002;106:75-80.
Daudon P, Corcos T, Gandjbakhch I, et al. Prevention of atrial fibrillation or flutter by acebutolol after coronary bypass grafting. Am J Cardiol. 1986;58:933-936.
Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:e340-e437.
Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651-745.
Kowey PR, Levine JH, Herre JM, et al. Randomized, double-blind comparison of intravenous amiodarone and bretylium in the treatment of patients with recurrent, hemodynamically destabilizing ventricular tachycardia or fibrillation. The Intravenous Amiodarone Multicenter Investigators Group. Circulation. 1995;92:3255-3263.
Kowey PR, Taylor JE, Rials SJ, Marinchak RA. Meta-analysis of the effectiveness of prophylactic drug therapy in preventing supraventricular arrhythmia early after coronary artery bypass grafting. Am J Cardiol. 1992;69:963-965.
Landymore RW, Howell F. Recurrent atrial arrhythmias following treatment for postoperative atrial fibrillation after coronary bypass operations. Eur J Cardiothorac Surg. 1991;5:436-439.
Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061-1073.
Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720-1729.
Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368-373.
Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: Results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455-1461.
Rubin DA, Nieminski KE, Reed GE, Herman MV. Predictors, prevention, and long-term prognosis of atrial fibrillation after coronary artery bypass graft operations. J Thorac Cardiovasc Surg. 1987;94:331-335.
Stephenson LW, MacVaugh HIII, Tomasello DN, Josephson ME. Propranolol for prevention of postoperative cardiac arrhythmias: A randomized study. Ann Thorac Surg. 1980;29:113-116.
1 Daudon P, Corcos T, Gandjbakhch I, et al. Prevention of atrial fibrillation or flutter by acebutolol after coronary bypass grafting. Am J Cardiol. 1986;58:933-936.
2 Andrews TC, Reimold SC, Berlin JA, Antman EM. Prevention of supraventricular arrhythmias after coronary artery bypass surgery: A meta-analysis of randomized control trials. Circulation. 1991;84:III236-III244.
3 Asher CR, Miller DP, Grimm RA, et al. Analysis of risk factors for development of atrial fibrillation early after cardiac valvular surgery. Am J Cardiol. 1998;82:892-895.
4 Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539-549.
5 Dixon FE, Genton E, Vacek JL, et al. Factors predisposing to supraventricular tachyarrhythmias after coronary artery bypass grafting. Am J Cardiol. 1986;58:476-478.
6 Leitch JW, Thomson D, Baird DK, Harris PJ. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;100:338-342.
7 Fuller JA, Adams GG, Buxton B. Atrial fibrillation after coronary artery bypass grafting. Is it a disorder of the elderly? J Thorac Cardiovasc Surg. 1989;97:821-825.
8 Roffman JA, Fieldman A. Digoxin and propranolol in the prophylaxis of supraventricular tachydysrhythmias after coronary artery bypass surgery. Ann Thorac Surg. 1981;31:496-501.
9 Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390-397.
10 Ascione R, Caputo M, Calori G, et al. Predictors of atrial fibrillation after conventional and beating heart coronary surgery: A prospective, randomized study. Circulation. 2000;102:1530-1535.
11 Passman R, Beshai J, Pavri B, Kimmel S. Predicting post-coronary bypass surgery atrial arrhythmias from the preoperative electrocardiogram. Am Heart J. 2001;142:806-810.
12 Connolly SJ, Cybulsky I, Lamy A, et al. Double-blind, placebo-controlled, randomized trial of prophylactic metoprolol for reduction of hospital length of stay after heart surgery: The beta-Blocker Length Of Stay (BLOS) study. Am Heart J. 2003;145:226-232.
13 Hattori Y, Yang Z, Sugimura S, et al. Terminal warm blood cardioplegia improves the recovery of myocardial electrical activity. A retrospective and comparative study. Jpn J Thorac Cardiovasc Surg. 2000;48:1-8.
14 Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: A major morbid event? Ann Surg. 1997;226:501-511.
15 Tamis JE, Steinberg JS. Atrial fibrillation independently prolongs hospital stay after coronary artery bypass surgery. Clin Cardiol. 2000;23:155-159.
16 Cohn WE, Sirois CA, Johnson RG. Atrial fibrillation after minimally invasive coronary artery bypass grafting: A retrospective, matched study. J Thorac Cardiovasc Surg. 1999;117:298-301.
17 Abreu JE, Reilly J, Salzano RP, et al. Comparison of frequencies of atrial fibrillation after coronary artery bypass grafting with and without the use of cardiopulmonary bypass. Am J Cardiol. 1999;83:775-776. A9
18 Asher CR, DiMengo JM, Arheart KL, et al. Atrial fibrillation early postoperatively following minimally invasive cardiac valvular surgery. Am J Cardiol. 1999;84:744-747. A9
19 Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg. 1997;226:421-426.
20 Stamou SC, Dangas G, Hill PC, et al. Atrial fibrillation after beating heart surgery. Am J Cardiol. 2000;86:64-67.
21 Stamou SC, Pfister AJ, Dangas G, et al. Beating heart versus conventional single-vessel reoperative coronary artery bypass. Ann Thorac Surg. 2000;69:1383-1387.
22 Stamou SC, Dangas G, Dullum MK, et al. Beating heart surgery in octogenarians: Perioperative outcome and comparison with younger age groups. Ann Thorac Surg. 2000;69:1140-1145.
23 Buxton AE, Josephson ME. The role of P wave duration as a predictor of postoperative atrial arrhythmias. Chest. 1981;80:68-73.
24 Landymore RW, Howell F. Recurrent atrial arrhythmias following treatment for postoperative atrial fibrillation after coronary bypass operations. Eur J Cardiothorac Surg. 1991;5:436-439.
25 Matangi MF, Neutze JM, Graham KJ, et al. Arrhythmia prophylaxis after aorta-coronary bypass. The effect of minidose propranolol. J Thorac Cardiovasc Surg. 1985;89:439-443.
26 Stephenson LW, MacVaugh HIII, Tomasello DN, Josephson ME. Propranolol for prevention of postoperative cardiac arrhythmias: A randomized study. Ann Thorac Surg. 1980;29:113-116.
27 Rubin DA, Nieminski KE, Reed GE, Herman MV. Predictors, prevention, and long-term prognosis of atrial fibrillation after coronary artery bypass graft operations. J Thorac Cardiovasc Surg. 1987;94:331-335.
28 Reed GLIII, Singer DE, Picard EH, DeSanctis RW. Stroke following coronary-artery bypass surgery. A case-control estimate of the risk from carotid bruits. N Engl J Med. 1988;319:1246-1250.
29 Taylor GJ, Malik SA, Colliver JA, et al. Usefulness of atrial fibrillation as a predictor of stroke after isolated coronary artery bypass grafting. Am J Cardiol. 1987;60:905-907.
30 Kim MH, Deeb GM, Morady F, et al. Effect of postoperative atrial fibrillation on length of stay after cardiac surgery (The Postoperative Atrial Fibrillation in Cardiac Surgery study [PACS(2)]. Am J Cardiol. 2001;87:881-885.
31 Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742-748.
32 Tsikouris JP, Kluger J, Song J, White CM. Changes in P-wave dispersion and P-wave duration after open heart surgery are associated with the peak incidence of atrial fibrillation. Heart Lung. 2001;30:466-471.
33 Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061-1073.
34 Dupont E, Ko Y, Rothery S, et al. The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation. 2001;103:842-849.
35 Wahr JA, Parks R, Boisvert D, et al. Preoperative serum potassium levels and perioperative outcomes in cardiac surgery patients. Multicenter Study of Perioperative Ischemia Research Group. JAMA. 1999;281:2203-2210.
36 Crystal E, Connolly SJ, Sleik K, et al. Interventions on prevention of postoperative atrial fibrillation in patients undergoing heart surgery: A meta-analysis. Circulation. 2002;106:75-80.
37 Andrews TC, Reimold SC, Berlin JA, Antman EM. Prevention of supraventricular arrhythmias after coronary artery bypass surgery. A meta-analysis of randomized control trials. Circulation. 1991;84:III236-III244.
38 Kowey PR, Taylor JE, Rials SJ, Marinchak RA. Meta-analysis of the effectiveness of prophylactic drug therapy in preventing supraventricular arrhythmia early after coronary artery bypass grafting. Am J Cardiol. 1992;69:963-965.
39 Davison R, Hartz R, Kaplan K, et al. Prophylaxis of supraventricular tachyarrhythmia after coronary bypass surgery with oral verapamil: A randomized, double-blind trial. Ann Thorac Surg. 1985;39:336-339.
40 Gold MR, O’Gara PT, Buckley MJ, DeSanctis RW. Efficacy and safety of procainamide in preventing arrhythmias after coronary artery bypass surgery. Am J Cardiol. 1996;78:975-979.
41 Nystrom U, Edvardsson N, Berggren H, et al. Oral sotalol reduces the incidence of atrial fibrillation after coronary artery bypass surgery. Thorac Cardiovasc Surg. 1993;41:34-37.
42 Suttorp MJ, Kingma JH, Peels HO, et al. Effectiveness of sotalol in preventing supraventricular tachyarrhythmias shortly after coronary artery bypass grafting. Am J Cardiol. 1991;68:1163-1169.
43 Parikka H, Toivonen L, Heikkila L, et al. Comparison of sotalol and metoprolol in the prevention of atrial fibrillation after coronary artery bypass surgery. J Cardiovasc Pharmacol. 1998;31:67-73.
44 Suttorp MJ, Kingma JH, Tjon Joe Gin RM, et al. Efficacy and safety of low- and high-dose sotalol versus propranolol in the prevention of supraventricular tachyarrhythmias early after coronary artery bypass operations. J Thorac Cardiovasc Surg. 1990;100:921-926.
45 Gomes JA, Ip J, Santoni-Rugiu F, et al. Oral d,l sotalol reduces the incidence of postoperative atrial fibrillation in coronary artery bypass surgery patients: A randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 1999;34:334-339.
46 Podrid PJ. Prevention of postoperative atrial fibrillation: What is the best approach? J Am Coll Cardiol. 1999;34:340-342.
47 Daoud EG, Strickberger SA, Man KC, et al. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. N Engl J Med. 1997;337:1785-1791.
48 Aasbo JD, Lawrence AT, Krishnan K, et al. Amiodarone prophylaxis reduces major cardiovascular morbidity and length of stay after cardiac surgery: A meta-analysis. Ann Intern Med. 2005;143:327-336.
49 Mitchell LB, Exner DV, Wyse DG, et al. Prophylactic Oral Amiodarone for the Prevention of Arrhythmias that Begin Early After Revascularization, Valve Replacement, or Repair: PAPABEAR: A randomized controlled trial. JAMA. 2005;294:3093-3100.
50 Greenspon AJ, Kidwell GA, Hurley W, Mannion J. Amiodarone-related postoperative adult respiratory distress syndrome. Circulation. 1991;84:III407-III415.
51 Guarnieri T, Nolan S, Gottlieb SO, et al. Intravenous amiodarone for the prevention of atrial fibrillation after open heart surgery: The Amiodarone Reduction in Coronary Heart (ARCH) trial. J Am Coll Cardiol. 1999;34:343-347.
52 Delfaut P, Saksena S, Prakash A, Krol RB. Long-term outcome of patients with drug-refractory atrial flutter and fibrillation after single- and dual-site right atrial pacing for arrhythmia prevention. J Am Coll Cardiol. 1998;32:1900-1908.
53 Chung MK, Augostini RS, Asher CR, et al. Ineffectiveness and potential proarrhythmia of atrial pacing for atrial fibrillation prevention after coronary artery bypass grafting. Ann Thorac Surg. 2000;69:1057-1063.
54 Kurz DJ, Naegeli B, Kunz M, et al. Epicardial, biatrial synchronous pacing for prevention of atrial fibrillation after cardiac surgery. Pacing Clin Electrophysiol. 1999;22:721-726.
55 Gerstenfeld EP, Hill MR, French SN, et al. Evaluation of right atrial and biatrial temporary pacing for the prevention of atrial fibrillation after coronary artery bypass surgery. J Am Coll Cardiol. 1999;33:1981-1988.
56 Greenberg MD, Katz NM, Iuliano S, et al. Atrial pacing for the prevention of atrial fibrillation after cardiovascular surgery. J Am Coll Cardiol. 2000;35:1416-1422.
57 Blommaert D, Gonzalez M, Mucumbitsi J, et al. Effective prevention of atrial fibrillation by continuous atrial overdrive pacing after coronary artery bypass surgery. J Am Coll Cardiol. 2000;35:1411-1415.
58 Fan K, Lee KL, Chiu CS, et al. Effects of biatrial pacing in prevention of postoperative atrial fibrillation after coronary artery bypass surgery. Circulation. 2000;102:755-760.
59 Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720-1729.
60 Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: Results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455-1461.
61 Halonen J, Halonen P, Jarvinen O, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: A randomized controlled trial. JAMA. 2007;297:1562-1567.
62 Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368-373.
63 Calo L, Bianconi L, Colivicchi F, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723-1728.
64 Weiner P, Bassan MM, Jarchovsky J, et al. Clinical course of acute atrial fibrillation treated with rapid digitalization. Am Heart J. 1983;105:223-227.
65 Tisdale JE, Padhi ID, Goldberg AD, et al. A randomized, double-blind comparison of intravenous diltiazem and digoxin for atrial fibrillation after coronary artery bypass surgery. Am Heart J. 1998;135:739-747.
66 Clemo HF, Wood MA, Gilligan DM, Ellenbogen KA. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
67 Galve E, Rius T, Ballester R, et al. Intravenous amiodarone in treatment of recent-onset atrial fibrillation: Results of a randomized, controlled study. J Am Coll Cardiol. 1996;27:1079-1082.
68 Waldo AL, MacLean WA, Cooper TB, et al. Use of temporarily placed epicardial atrial wire electrodes for the diagnosis and treatment of cardiac arrhythmias following open-heart surgery. J Thorac Cardiovasc Surg. 1978;76:500-505.
69 Saliba W, Juratli N, Chung MK, et al. Higher energy synchronized external direct current cardioversion for refractory atrial fibrillation. J Am Coll Cardiol. 1999;34:2031-2034.
70 Bjerregaard P, El Shafei A, Janosik DL, et al. Double external direct-current shocks for refractory atrial fibrillation. Am J Cardiol. 1999;83:972-974. A10
71 Oral H, Souza JJ, Michaud GF, et al. Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med. 1999;340:1849-1854.
72 Mehmanesh H, Bauernschmitt R, Lange R, Hagl S. Adjustable atrial and ventricular temporary electrode for low-energy termination of tachyarrhythmias early after cardiac surgery. Pacing Clin Electrophysiol. 1999;22:1802-1807.
73 Liebold A, Wahba A, Birnbaum DE. Low-energy cardioversion with epicardial wire electrodes: New treatment of atrial fibrillation after open heart surgery. Circulation. 1998;98:883-886.
74 VanderLugt JT, Mattioni T, Denker S, et al. Efficacy and safety of ibutilide fumarate for the conversion of atrial arrhythmias after cardiac surgery. Circulation. 1999;100:369-375.
75 Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:e340-e437.
76 Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651-745.
77 Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective beta-blockers in patients with reactive airway disease: A meta-analysis. Ann Intern Med. 2002;137:715-725.
78 Steinberg JS, Gaur A, Sciacca R, Tan E. New-onset sustained ventricular tachycardia after cardiac surgery. Circulation. 1999;99:903-908.
79 Tam SK, Miller JM, Edmunds LHJr. Unexpected, sustained ventricular tachyarrhythmia after cardiac operations. J Thorac Cardiovasc Surg. 1991;102:883-889.
80 Kron IL, DiMarco JP, Harman PK, et al. Unanticipated postoperative ventricular tachyarrhythmias. Ann Thorac Surg. 1984;38:317-322.
81 Topol EJ, Lerman BB, Baughman KL, Platia EV, Griffith LS. De novo refractory ventricular tachyarrhythmias after coronary revascularization. Am J Cardiol. 1986;57:57-59.
82 Pires LA, Wagshal AB, Lancey R, Huang SK. Arrhythmias and conduction disturbances after coronary artery bypass graft surgery: Epidemiology, management, and prognosis. Am Heart J. 1995;129:799-808.
83 Carlson MD, Biblo LA, Waldo AL. Post open heart surgery ventricular arrhythmias. Cardiovasc Clin. 1992;22:241-253.
84 Favaloro RG, Effler DB, Groves LK, et al. Direct myocardial revascularization with saphenous vein autograft. Clinical experience in 100 cases. Dis Chest. 1969;56:279-283.
85 Costeas XF, Schoenfeld MH. Usefulness of electrophysiologic studies for new-onset sustained ventricular tachyarrhythmias shortly after coronary artery bypass grafting. Am J Cardiol. 1993;72:1291-1294.
86 Ducceschi V, D’Andrea A, Liccardo B, et al. Perioperative correlates of malignant ventricular tachyarrhythmias complicating coronary surgery. Heart Vessels. 1999;14:90-95.
87 Ducceschi V, D’Andrea A, Liccardo B, et al. Ventricular tachyarrhythmias following coronary surgery: Predisposing factors. Int J Cardiol. 2000;73:43-48.
88 Preisman S, Cheng DC. Life-threatening ventricular dysrhythmias with inadvertent asynchronous temporary pacing after cardiac surgery. Anesthesiology. 1999;91:880-883.
89 Johnson RG, Shafique T, Sirois C, et al. Potassium concentrations and ventricular ectopy: A prospective, observational study in post-cardiac surgery patients. Crit Care Med. 1999;27:2430-2434.
90 Malcolm ID, Cherry DA, Morin JE. The use of temporary atrial electrodes to improve diagnostic capabilities with Holter monitoring after cardiac surgery. Ann Thorac Surg. 1986;41:103-105.
91 Waldo AL, Henthorn RW, Epstein AE, Plumb VJ. Diagnosis and treatment of arrhythmias during and following open heart surgery. Med Clin North Am. 1984;68:1153-1169.
92 Azar RR, Berns E, Seecharran B, et al. De novo monomorphic and polymorphic ventricular tachycardia following coronary artery bypass grafting. Am J Cardiol. 1997;80:76-78.
93 Perrault LP, Denault AY, Carrier M, et al. Torsades de pointes secondary to intravenous haloperidol after coronary bypass grafting surgery. Can J Anaesth. 2000;47:251-254.
94 Kowey PR, Levine JH, Herre JM, et al. Randomized, double-blind comparison of intravenous amiodarone and bretylium in the treatment of patients with recurrent, hemodynamically destabilizing ventricular tachycardia or fibrillation. The Intravenous Amiodarone Multicenter Investigators Group. Circulation. 1995;92:3255-3263.
95 Perry JC, Knilans TK, Marlow D, et al. Intravenous amiodarone for life-threatening tachyarrhythmias in children and young adults. J Am Coll Cardiol. 1993;22:95-98.
96 Evrard P, Gonzalez M, Jamart J, et al. Prophylaxis of supraventricular and ventricular arrhythmias after coronary artery bypass grafting with low-dose sotalol. Ann Thorac Surg. 2000;70:151-156.
97 Speziale G, Ruvolo G, Fattouch K, et al. Arrhythmia prophylaxis after coronary artery bypass grafting: Regimens of magnesium sulfate administration. Thorac Cardiovasc Surg. 2000;48:22-26.
98 Thompson LD, Cohen AJ, Bellasis RM. Polymorphous ventricular tachycardia following cardiopulmonary bypass. J Natl Med Assoc. 1996;88:49-51.
99 Fisher JD, Mehra R, Furman S. Termination of ventricular tachycardia with bursts of rapid ventricular pacing. Am J Cardiol. 1978;41:94-102.
100 Kaplan JA, Craver JM, Jones EL, Sumpter R. The role of the intra-aortic balloon in cardiac anesthesia and surgery. Am Heart J. 1979;98:580-586.
101 Craver JM, Kaplan JA, Jones EL, et al. What role should the intra-aortic balloon have in cardiac surgery. Ann Surg. 1979;189:769-776.
102 Fotopoulos GD, Mason MJ, Walker S, et al. Stabilisation of medically refractory ventricular arrhythmia by intra-aortic balloon counterpulsation. Heart. 1999;82:96-100.
103 Swartz MT, Lowdermilk GA, McBride LR. Refractory ventricular tachycardia as an indication for ventricular assist device support. J Thorac Cardiovasc Surg. 1999;118:1119-1120.
104 Hamid BA, Azuma SS, Hong RA, Lau JM. Persistent postoperative ventricular tachycardia treatment by using external cardiopulmonary support. Hawaii Med J. 1994;53:10-11.
105 Kurose M, Okamoto K, Sato T, et al. Successful treatment of life-threatening ventricular tachycardia with high-dose propranolol under extracorporeal life support and intraaortic balloon pumping. Jpn Circ J. 1993;57:1106-1110.
106 Sheppard RC, Nydegger CC, Kutalek SP, Hessen SE. Comparison of epicardial and endocardial programmed stimulation in patients at risk for ventricular arrhythmias after cardiac surgery. Pacing Clin Electrophysiol. 1993;16:1822-1832.
107 Elami A, Merin G, Flugelman MY, et al. Usefulness of late potentials on the immediate postoperative signal-averaged electrocardiogram in predicting ventricular tachyarrhythmias early after isolated coronary artery bypass grafting. Am J Cardiol. 1994;74:33-37.
108 Bhandari AK, Au PK, Rose JS, et al. Decline in inducibility of sustained ventricular tachycardia from two to twenty weeks after acute myocardial infarction. Am J Cardiol. 1987;59:284-290.
109 Wexelman W, Lichstein E, Cunningham JN, et al. Etiology and clinical significance of new fascicular conduction defects following coronary bypass surgery. Am Heart J. 1986;111:923-927.
110 Chu A, Califf RM, Pryor DB, et al. Prognostic effect of bundle branch block related to coronary artery bypass grafting. Am J Cardiol. 1987;59:798-803.
111 Emlein G, Huang SK, Pires LA, et al. Prolonged bradyarrhythmias after isolated coronary artery bypass graft surgery. Am Heart J. 1993;126:1084-1090.
112 Baerman JM, Kirsh MM, de Buitleir M, et al. Natural history and determinants of conduction defects following coronary artery bypass surgery. Ann Thorac Surg. 1987;44:150-153.
113 Gundry SR, Sequeira A, Coughlin TR, Mclaughlin JS. As originally published in 1989: Postoperative conduction disturbances: A comparison of blood and crystalloid cardioplegia. Updated in 1997. Ann Thorac Surg. 1997;63:901-902.
114 Gordon RS, Ivanov J, Cohen G, Ralph-Edwards AL. Permanent cardiac pacing after a cardiac operation: Predicting the use of permanent pacemakers. Ann Thorac Surg. 1998;66:1698-1704.
115 Bonatti V, Agnetti A, Squarcia U. Early and late postoperative complete heart block in pediatric patients submitted to open-heart surgery for congenital heart disease. Pediatr Med Chir. 1998;20:181-186.
116 Del Rizzo DF, Menkis AH, Pflugfelder PW, et al. The role of donor age and ischemic time on survival following orthotopic heart transplantation. J Heart Lung Transplant. 1999;18:310-319.
117 Glikson M, Dearani JA, Hyberger LK, et al. Indications, effectiveness, and long-term dependency in permanent pacing after cardiac surgery. Am J Cardiol. 1997;80:1309-1313.
118 O’Connell JB, Wallis D, Johnson SA, et al. Transient bundle branch block following use of hypothermic cardioplegia in coronary artery bypass surgery: High incidence without perioperative myocardial infarction. Am Heart J. 1982;103:85-91.
119 Ellis RJ, Mavroudis C, Gardner C, et al. Relationship between atrioventricular arrhythmias and the concentration of K+ ion in cardioplegic solution. J Thorac Cardiovasc Surg. 1980;80:517-526.
120 Moore SL, Wilkoff BL. Rhythm disturbances after cardiac surgery. Semin Thorac Cardiovasc Surg. 1991;3:24-28.
121 Kosmas CE, Ryder RG, Poon MJ, et al. Time-limited efficacy of pacing electrodes following open heart surgery. Indian Heart J. 1996;48:681-684.