Chapter 169 Posterior Lumbar Interbody Fusion
The introduction of novel fixation techniques and instrumentation has resulted in their ever increasing application for arthrodesis of the spine. The first successful report of a posterior lumbar interbody fusion (PLIF) dates to 1940, when Cloward performed this operation using a spinous process autograft.1 Since Cloward’s original report, many surgeons have modified this technique using various grafts.2–6 In recent years, two major improvements have resulted in greater application of PLIF or its variants: the application of pedicle screws to augment the rate of arthrodesis7,8 and the application of minimally invasive spine techniques to perform arthrodesis in a percutaneous fashion.9
The indications for performing a PLIF include recurrent disc herniation, failed back surgery syndrome, spondylolisthesis, bilateral midline disc herniation, segmental instability, and degenerative disc disease. Compared with classic posterolateral arthrodesis techniques, PLIF offers improved blood supply to the fusion/graft construct, balanced load sharing on the graft, complete decompression of the neural elements, a wide area of graft–intervertebral body contact, and restoration of the interbody and neural foraminal height.10–12 Herein, we describe our surgical technique for open and minimally invasive PLIF.
Open PLIF
After the patient is placed in the prone position, pressure points are padded to minimize pressure sores (Fig. 169-1). A Jackson table can be used to avoid compression and venous congestion, which may cause excessive bleeding during posterior approaches. Fluoroscopy should be used to identify the spinal level of interest. As in any posterior approach, the landmarks are the spinous processes, iliac crest, and posterior superior iliac spine.
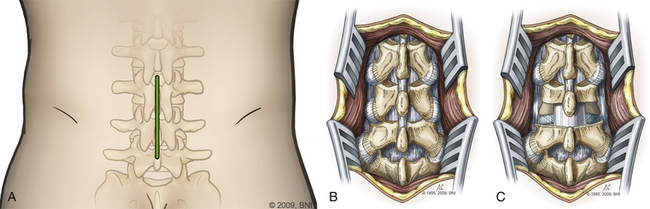
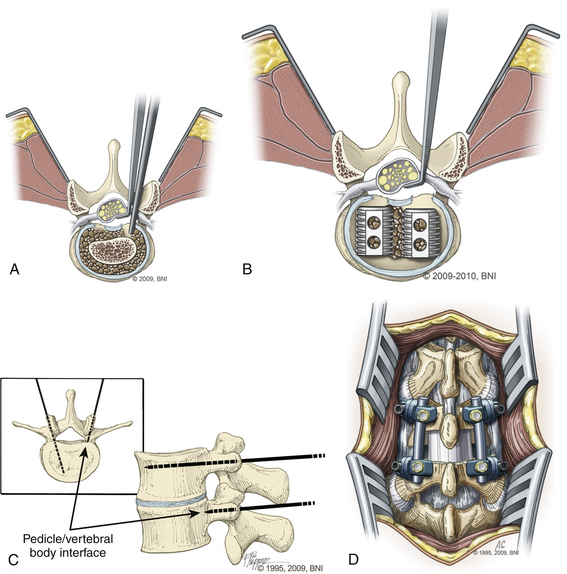
The skin is incised (Fig. 169-2A) in the midline above the spinous process, and the subcutaneous tissue is dissected. The thoracolumbar fascia is incised and dissection proceeds in a subperiosteal fashion, detaching the paraspinal muscles from the spinous process and lamina (Fig. 169-2B). For each level that is to be treated, the cranial lamina and ligamentum flavum are removed completely (Fig. 169-2C). The cranial aspect of the caudal lamina should also be removed to gain adequate access to the interbody space. To prevent traction on the spinal cord or nerve roots during placement of the interbody graft, a wide window is created by resecting the lower third of the inferior facet and the medial two thirds of the superior facet. Next, the nerve root and dural sac are retracted medially, creating maximal exposure of the interbody space for removal of any bony or disc material (Fig. 169-3A).
Following adequate decompression of the neural structures and removal of the disc material, the disc space is distracted using pedicle screws or disc space distractors. We prefer to use pedicle screws as distractors (Fig. 169-3B). Next, the end plates are prepared, using curettes to remove any remaining disc material while preserving the bony end plates to prevent graft subsidence. Adequate preparation of the graft site and preparation of the end plates are important steps for ensuring a proper fusion.
Next, a graft spacer or cage is placed into the interbody space under direct visualization of the cranial and caudal nerve roots (Fig. 169-3C). Bone graft packing is used to augment the fusion. The graft choice is highly surgeon dependent, with options such as threaded cages, titanium mesh cages, and auto- and allograft with or without the use of bone morphogenetic protein. The graft-filled interspace is stabilized with internal fixation using screws and rods or a plate construct to aid in fusion and immobilization (Fig. 169-3D).
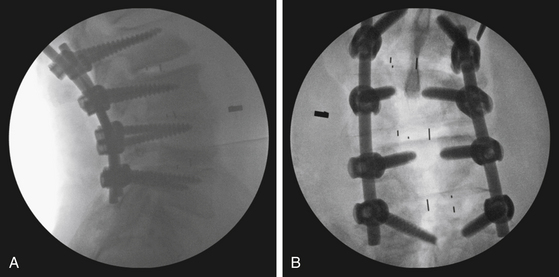
PLIF is an effective procedure and results in excellent fusion (Fig. 169-4) with rates in the range of 80% to 98%.13,14 PLIF also has been shown to be effective for reducing pain and disability.13
Minimally Invasive Spine PLIF
Minimally invasive PLIF is an alternative to the open technique described earlier. As is the case for open PLIF, the patient is positioned prone (see Fig. 169-1). Using fluoroscopic guidance, the correct level is identified. At the Barrow Neurological Institute, we use the METRx microdiscectomy system of endoscopic and microscopic tubular dilators, retractors, and a bed-mounted flexible arm. Once the correct level is identified, a small 2- to 4-cm incision is placed off midline (Fig. 169-5) at the appropriate level and a Steinmann pin is passed until it reaches the medial border of the facet joint. Next, the skin incision is extended 2 to 3 cm above and below the Steinmann pin, and serial dilators are used for muscle splitting (Fig. 169-6). Once adequate muscle splitting and visualization are achieved, the final channel is fixed to the flexible-arm retractor mounted to the table side rail and the sequential dilators are removed. Exposure, laminotomy, ligamentum flavum removal, and discectomy are then performed as described previously.
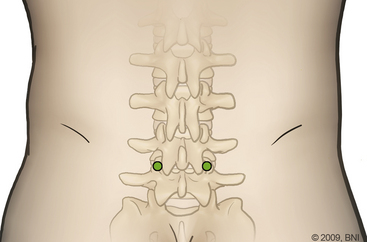
FIGURE 169-5 Incision used in the minimally invasive PLIF centered over the thoracolumbar spine.
(Used with permission from Barrow Neurological Institute.)
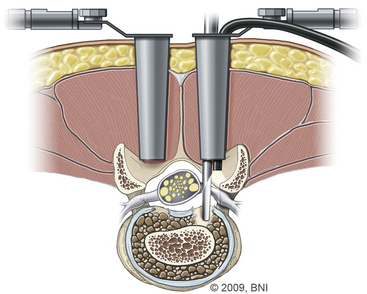
FIGURE 169-6 Dilator setup used for the muscle-splitting technique.
(Used with permission from Barrow Neurological Institute.)
Next, a No. 11 Jamshidi needle is used to identify and cannulate the lumbar pedicles. After adequate visualization of the pedicle using fluoroscopy, the Jamshidi needle is used to perforate the dorsolumbar fascia down to the level of the pedicle. The needle is tapped into place and gradually advanced through the pedicles beyond the posterior margin of the main vertebral body. After the location of the needle is confirmed, the obturator is removed and a long K wire is exchanged through it.
Next, using sequential dilators, the screw path is defined, and a tap is passed coaxially over the K wire through the pedicle into the cancellous portion of the vertebral body. Multiaxial pedicle screws are attached to the screw extender sleeves and passed over the K wire through the previously tapped screw pathway. Precontoured rods are passed and lodged to the screws. Locking bolts on the screw heads are used to secure the rods in place. The locking bolts are tightened and released from the inserter.
Complications of PLIF
PLIF is a technically challenging and demanding procedure and consequently is associated with complications. Two of the primary complications of PLIF are nerve root injury and incidental durotomy related to the significant traction that must be placed on the thecal sac and nerve roots to gain access to the interbody space.15 The nerve root that exits at the level above the disc space often lies near the interbody graft as it is being placed and can easily be injured.One study reported an incidence of nerve root injury as high as 13%.16 Angled nerve root retractors and direct visualization of the nerve roots at all times can help prevent neurologic injury during the procedure. A more aggressive facetectomy can provide a more lateral approach, offering an excellent window for graft placement while minimizing retraction of the thecal sac. Dural tears can usually be repaired primarily but can be more difficult to repair when using the minimally invasive technique.
As is the case with any surgical operation, infection, bleeding, stroke, and death are other possible complications. Other PLIF and back surgery–specific complications include wrong-level surgery, adjacent-level disease, graft retropulsion, and pseudarthrosis in the case of instrumentation with PLIF.17 Frequent causes of graft retropulsion include inappropriate sizing of the graft, resulting in graft mobility; delayed fusion; and instability of motion segments, however, the rate of retropulsion is less than 2%.18
Arnold P.M., Robbins S., Paullus W., et al. Clinical outcomes of lumbar degenerative disc disease treated with posterior lumbar interbody fusion allograft spacer: a prospective, multicenter trial with 2-year follow-up. Am J Orthop. 2009;38(7):E115-E122.
Barnes B., Rodts G.E.Jr., Haid R.W.Jr., et al. Allograft implants for posterior lumbar interbody fusion: results comparing cylindrical dowels and impacted wedges. Neurosurgery. 2002;51(5):1191-1198.
Boult M., Fraser R.D., Jones N., et al. Percutaneous endoscopic laser discectomy. Aust N Z J Surg. 2000;70(7):475-479.
Branch C.L.Jr. The case for posterior lumbar interbody fusion. Clin Neurosurg. 1996;43:252-267.
Brantigan J.W., Steffee A.D. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18(14):2106-2107.
Brodke D.S., Dick J.C., Kunz D.N., et al. Posterior lumbar interbody fusion. A biomechanical comparison, including a new threaded cage. Spine. 1997;22(1):26-31.
Cloward R. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154-168.
Cole C.D., McCall T.D., Schmidt M.H., Dailey A.T. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2(2):118-126.
Evans J.H.. Biomechanics of lumbar fusion. Clin Orthop Relat Res, 1985;193:38-46
Gill K., Blumenthal S.L. Posterior lumbar interbody fusion. A 2-year follow-up of 238 patients. Acta Orthop Scand Suppl. 1993;251:108-110.
Hutter C.G.. Spinal stenosis and posterior lumbar interbody fusion. Clin Orthop Relat Res, 1985;193:103-114
Khoo L.T., Palmer S., Laich D.T., Fessler R.G. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51(Suppl 5):S166-1.
Lin P.M.. Posterior lumbar interbody fusion technique: complications and pitfalls. Clin Orthop Relat Res, 1985;193:90-102
Lin P.M. Technique and complications of posterior lumbar interbody fusion. In: Lin P.M., Gill K. Lumbar Interbody Fusion. Rockville, MD: Aspen; 1989:171-199.
Lin P.M., Cautilli R.A., Joyce M.F.. Posterior lumbar interbody fusion. Clin Orthop Relat Res, 1983;180:154-168
Onesti S.T., Ashkenazi E. The Ray Threaded Fusion Cage for posterior lumbar interbody fusion. Neurosurgery. 1998;42(1):200-204.
Steffee A.D., Sitkowski D.J. Posterior lumbar interbody fusion and plates. Clin Orthop Relat Res. 1988 February;227:99-102.
Wiltse L.L.. Surgery for intervertebral disk disease of the lumbar spine. Clin Orthop Relat Res, 1977;129:22-45
1. Cloward R. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154-168.
2. Boult M., Fraser R.D., Jones N., et al. Percutaneous endoscopic laser discectomy. Aust N Z J Surg. 2000;70(7):475-479.
3. Brodke D.S., Dick J.C., Kunz D.N., et al. Posterior lumbar interbody fusion. A biomechanical comparison, including a new threaded cage. Spine. 1997;22(1):26-31.
4. Hutter C.G.. Spinal stenosis and posterior lumbar interbody fusion. Clin Orthop Relat Res, 1985;193:103-114
5. Lin P.M.. Posterior lumbar interbody fusion technique: complications and pitfalls. Clin Orthop Relat Res, 1985;193:90-102
6. Onesti S.T., Ashkenazi E. The Ray Threaded Fusion Cage for posterior lumbar interbody fusion. Neurosurgery. 1998;42(1):200-204.
7. Steffee A.D., Sitkowski D.J. Posterior lumbar interbody fusion and plates. Clin Orthop Relat Res. 1988;227:99-102.
8. Wiltse L.L.. Surgery for intervertebral disk disease of the lumbar spine. Clin Orthop Relat Res, 1977;129:22-45
9. Khoo L.T., Palmer S., Laich D.T., Fessler R.G. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51(Suppl 5):S166-1.
10. Branch C.L.Jr. The case for posterior lumbar interbody fusion. Clin Neurosurg. 1996;43:252-267.
11. Evans J.H.. Biomechanics of lumbar fusion. Clin Orthop Relat Res, 1985;193:38-46
12. Lin P.M., Cautilli R.A., Joyce M.F.. Posterior lumbar interbody fusion. Clin Orthop Relat Res, 1983;180:154-168
13. Arnold P.M., Robbins S., Paullus W., et al. Clinical outcomes of lumbar degenerative disc disease treated with posterior lumbar interbody fusion allograft spacer: a prospective, multicenter trial with 2-year follow-up. Am J Orthop. 2009;38(7):E115-E122.
14. Gill K., Blumenthal S.L. Posterior lumbar interbody fusion. A 2-year follow-up of 238 patients. Acta Orthop Scand Suppl. 1993;251:108-110.
15. Cole C.D., McCall T.D., Schmidt M.H., Dailey A.T. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2(2):118-126.
16. Barnes B., Rodts G.E.Jr., Haid R.W.Jr., et al. Allograft implants for posterior lumbar interbody fusion: results comparing cylindrical dowels and impacted wedges. Neurosurgery. 2002;51(5):1191-1198.
17. Brantigan J.W., Steffee A.D. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18(14):2106-2107.
18. Lin P.M. Technique and complications of posterior lumbar interbody fusion. In: Lin P.M., Gill K. Lumbar Interbody Fusion. Rockville, MD: Aspen; 1989:171-199.