Chapter 54 Posterior and Transforaminal Lumbar Interbody Fusion
History and Description
Dr. Ralph Cloward, who spent the majority of his professional life in Hawaii as one of the only practicing neurosurgeons during the period encompassing World War II, is credited as one of the original proponents of the posterior lumbar interbody fusion (PLIF). Although Mercer had theorized an interbody fusion as the “ideal” operation for spinal stabilization in 1936 and reportedly Jaslow performed the first PLIF in 1946, Cloward popularized the technique the following decade through presentations of his own cases at various meetings of organized neurosurgery.1–4 In his 1952 description of 321 patients treated with PLIF for ruptured intervertebral discs, Cloward mentions the unacceptably poor durability of symptomatic relief afforded by lumbar discectomy at the time as an impetus for reexamining the more conservative treatment of disc herniations. He implied a general lack of appreciation in the spine community of the primary etiology, namely instability resulting from a broken or damaged vertebral joint as the causative factor in the generation of lumbar disc disease. Cloward argued that effective treatment for the radicular symptoms as well as the mechanical symptoms of low back pain present in many of his patients required not only decompression but immobilization of the damaged joint. He advocated for intervertebral body fusion as opposed to fusion of the dorsal elements alone because this allowed for restoration of the intervertebral space (and thus indirectly the neural foramen and central canal) while incorporating the chief load-bearing elements into the fusion.1 As Cloward wrote in 1953, “The purpose of this procedure was to maintain the normal width of the intervertebral space and the intervertebra foramen. At the same time al1 false movement between the vertebra resulting from injury and collapse of the intervertebra disc was arrested.”2
Approximately 30 years after Cloward’s initial presentations regarding PLIF, Lin modified the original concept to include four central principles.5 Preservation of the posterior elements of the motion segment (by maintaining the supraspinous and interspinous ligaments and limiting bony decompression to a medial facetectomy) and total (80%) discectomy (thought to improve fusion rates) represented the first two principles. Partial decortication, but not complete removal of the bony end plates, and the concept of the “unigraft” represented the remaining two principles. The “unigraft” concept was analogous to the model of the interbody graft used in anterior cervical discectomy and fusion surgery, namely the packing of all remaining interbody space with autologous bone to achieve a single solid fusion mass.5
Lin also described the notion of “dynamic decompression,” which refers to the combining of two motion segments into one in a state of relative decompression. Widening of the neural foramen is achieved through both direct decompression and indirectly through the use of an interbody graft.5 This restoration of the normal anatomic relationship between the motion segment and neural structures achieved in part through maintenance of disc height and restriction of motion was thought to protect the nerve roots. Immobilization of the unstable degenerative area arrested further degeneration; some authorities thought that this helped to protect the nerve roots. Investigators have cited reestablishment of weight bearing to ventral structures, prevention of recurrent disc herniations (at that level), and placement of the anulus under tension as advantages of PLIF over more conservative decompressive procedures such as discectomy, laminectomy, and foraminotomy.6,7
The PLIF has seen many changes and procedural variations since its first description nearly 80 years ago.1,2,8 Modifications to the surgical technique have mainly focused on the expansion of available methods for achieving decompression, access to the disc space, and fusion. Unilateral versus bilateral exposures with unique methods of introducing grafts into the disc space and decompression strategies ranging from midline to far lateral, incorporating laminotomy, laminectomy, various extents of facetectomy, or foraminotomy have been devised. Additionally, the application of minimally invasive options for achieving interbody fusion has likely increased its attractiveness. An assortment of interbody grafts has been proposed, including various autologous sources such as morselized or structural elements of the posterior neural arch, bicortical or tricortical grafts, cancellous sources, or iliac crest.9 Allograft sources ranging from morselized cancellous bone chips to numerous shapes of structural cadaveric bone graft such as bone pegs, trapezoids, crescents, or other configurations designed in part to prevent retropulsion or graft migration have also been described. Titanium mesh cages, threaded cages (BAK cage, Ray cage), polymeric rectangular cages (Brantigan carbon cage, polyetheretherketone [PEEK] cage, bioabsorbable cages (Hydrosorb cage), and ceramic cages (hydroxyapatite blocks) have been developed, all with variable biomechanical and bioabsorptive properties and similar clinical outcome in most reviews.10–13 Facilitation of bony fusion and sound construction, applying concepts such as load sharing to complement the native spine successfully in its endurance of biomechanical forces, are among the fundamental properties used by these various grafts. Studies characterizing available interbody grafts generally describe variations such as sagittal contour, posterior disc height, fusion rates, clinical outcome, and biomechanical advantages in load sharing and the modulus of elasticity.9,14–19 One investigation found cylindrical cages to be associated with a higher rate of nerve root damage than wedge-shaped bone allografts.20 Some class III evidence supports general trends such as improvement in radiographic and functional outcome using artificial cages over bone chips. However, incorporating bilateral pedicle screw fixation may attenuate some of the differences described for the various interbody cages, and no consensus has been reached regarding efficacy among interbody grafts.21–25
Although some early pioneers of PLIF believed that a posterior fusion was redundant and perhaps unnecessary given the construction of a good anterior fusion, a description of supplementation with posterolateral bony fusion appeared early in the history of the PLIF.1,26 More recently, evidence has accumulated for the use of posterior instrumentation, which, among other advantages, provides immediate internal fixation and facilitates fusion, as well as helps prevent loss of disc space height, progressive kyphosis, and graft migration.13,27–30 The widespread use of transpedicular screw fixation for posterior spinal fusion began in the late 1980s and has generated necessary adaptations to the original concepts of PLIF as described by Mercer, Cloward, Lin, Jaslow, and others.1–6,31–33 This has spawned a plethora of outcomes studies describing new techniques.6,34,35
The use of PLIF was not widespread until the 1990s, when advances in instrumentation and surgical technique revitalized its popularity.36,37 Although the procedure is biomechanically sound conceptually and most reviews demonstrate significant improvement in various radiographic and functional outcomes when applied to spinal disorders commonly treated with decompression and fusion, comparison studies with other fusion techniques show mixed results.13,37–46 Spondylolisthesis, which is thought by many surgeons to require rigid internal fixation to better withstand inherent instability from factors such as shear forces, has varying results regarding need for interbody fusion.42,47–50 A recent international study comparing open pedicle screw instrumented fusion with and without posterior interbody fusion for spondylolisthesis found the PLIF group to have a better fusion rate and the other group to have a higher rate of hardware complications related to biomechanics; however, there were no significant differences in clinical outcome.7 Studies of degenerative spondylolisthesis suggest evaluation of preoperative segmental instability may be an important factor in determining whether to perform additional interbody fusion.47
More recently, the transforaminal approach to the intervertebral disc, known as transforaminal lumbar interbody fusion (TLIF), has gained popularity.13,19,51,52 Originally described by Harms in the late 1990s, the TLIF has arguably developed into the most commonly performed and efficacious posterior interbody fusion method in modern spine surgery.51,53,54 Although there are many variations on specific surgical techniques, the underlying concept is access to the intervertebral disc space from a more lateral trajectory; this is generally accomplished through unilateral exposure of the neural foramen and exiting nerve root using a greater degree of facetectomy.55 It should be noted, however, that although the “traditional” TLIF uses a less invasive unilateral approach to the disc space, whereas the “traditional” PLIF uses a more extensive bilateral exposure, both can be performed in a unilateral or bilateral fashion.55 The impetus for the development of the TLIF grew out of concern regarding damage to the cauda equina and lumbosacral nerve roots because the more midline exposure performed for the PLIF requires more retraction of the dura to achieve adequate operative exposure for the interbody fusion. Reflective of this concern is the limitation of PLIF to the levels of L3-S1 to avoid damage to the conus medullaris, whereas the TLIF may be performed at higher levels. Therefore, a major advantage to the TLIF is the potential for less damage to the dura or nerves.36,56 A recent prospective study evaluating TLIF for degenerative and isthmic spondylolisthesis found a median decrease in the Oswestry Disability Index (ODI) score of 10 points (23.5 to 13.5), with a fusion rate of 95%. There was a 7.6% serious postoperative complication rate requiring operative revision.57
Although both PLIF and TLIF are regarded as technically demanding, repeated studies have shown them to be safe and effective means of establishing circumferential fusion with similar clinical outcomes.58 Numerous outcomes studies evaluating the efficacy of PLIF and the plethora of technical modifications are varied, however.45 With regard to fusion, it is now widely accepted that interbody grafts result in higher fusion rates, often exceeding 90% to 94%.59 Multilevel PLIF cases appear safe and effective, and although mention is made regarding increased invasiveness, good clinical outcomes and lumbar lordosis have been described.60 However, although both techniques include positioning of an interbody graft under compression, maintenance of the posterior tension band, and to an extent correction of deformity through restoration of lumbar lordosis, recent reports have found additional benefits of TLIF over PLIF.13,52,61 These include better improvement in lumbar lordosis given placement of interbody graft within the anterior column, greater enlargement of the neural foramen, and the option for using an effective unilateral approach; all these preserve other aspects of the posterior column integrity such as the contralateral lamina, facet, and pars, which may also provide a greater surface area for bony arthrodesis.*13,48,57
Compared with TLIF, it would appear that the larger dural exposure required for PLIF may carry with it added risk of durotomy during dissection of scar tissue in revision surgery. Fusion rates, however, are similar between the two and range between 89% and 95% in most studies.13,48,57 Humphreys et al. performed a comparison of operative characteristics and complications between TLIF and PLIF.57Although for one-level surgeries, there was no significant difference in blood loss, operative time, or length of hospitalization, there was a statistically significant decrease in overall complications as well as blood loss for two-level surgeries in the TLIF group.56 Preservation of the interspinous ligaments and preservation of the contralateral laminar surface were cited as additional advantages of TLIF in this study.56 Biomechanical studies focusing on the destabilizing effects of surgical approach for TLIF suggest no significantly increased spinal flexibility with the exception of axial rotation at L4-5. However, this was corrected with bilateral (but not unilateral) pedicle screws.62 No consensus can be made on the effect of exposure on destabilization or the ability of pedicle screws to correct this effect between TLIF and PLIF, however, because other studies have demonstrated good results with unilateral PLIF surgery.63
Many additional variations to both TLIF and PLIF have been described. TLIFs incorporating more aggressive decompressions through total bilateral facetectomies and resection of the pars (in combination with instrumented fusion), enabling placement of bilateral interbody graft placement as in PLIF, have been reported.55 Alternatively, less invasive procedures (e.g., LI-PLIF), which advocate the use of percutaneous pedicle screws if needed and “preservation of posterior elements and avoidance of far lateral dissection over the transverse processes,” have been described.35
Although outcomes studies often cite a steep “learning curve” for the necessary acquisition of new skills and familiarity of equipment as limitations, minimally invasive techniques (described elsewhere) have several unique advantages that make them attractive alternatives to open surgery.35 Quicker and less painful postoperative recovery and less destruction of adjacent tissue are commonly mentioned advantages.64–66 Recent studies have also demonstrated reduced hospital charges and lower transfer rates to inpatient rehabilitation facilities.67 Investigators have described similar clinical results for spondylolisthesis, including similar reductions in listhesis when original slip is less than 50%, between open and minimally invasive PLIF.66 Researchers recently reported a minimally invasive method of interbody fusion for isthmic spondylolisthesis.68 This technique, called the extraforaminal lumbar interbody fusion (ELIF), uses a prone position and several small parasagittal incisions and fluoroscopic guidance to achieve decompression and percutaneous pedicle screw fixation. This approach appears to be limited to decompression of the exiting nerve root, however. Another recent series describes minimally invasive TLIFs using unilateral percutaneous pedicle screw fixation and demonstrates results similar to those of previously published open TLIF/PLIF procedures.64 Reports of other approaches such as minimally invasive presacral approaches for intervertebral discectomy and fusion at L5-S1 exist in the literature.69 Although exposure to radiation and operative times may be longer for minimally invasive PLIF or TLIF, reported differences in long-term clinical outcomes with open surgery are not consistent.70,71
Although new reports refining and validating old techniques and innovative descriptions of novel concepts continue to represent a majority of the available literature, one appreciates some commonly debated concepts. These include trends in the preservation of various posterior elements, trajectories for accessing the disc space, strategies for achieving fusion, less invasive techniques, and advancements in materials research.72–74
Indications for Surgery
Controversy regarding the indications for PLIF has existed since its inception.1,2,26,45,75 Cloward designated broad indications for PLIF that included essentially all symptomatic lumbar disc disease (low back pain with or without radiculopathy resultant from a pathologic disc). Current treatment guidelines consist mainly of class III evidence, because class I and II data are lacking. Outcomes studies have generated a more extensive and specific list of indications; however, in light of the current trend of cost-benefit analysis and increasing costs of health care, controversy remains.
Degenerative disc disease (generally associated with Modic changes), lumbar segmental instability (iatrogenic, degenerative, or other causes), spondylolisthesis, degenerative scoliosis, pseudarthrosis after previous fusion surgery, spinal stenosis, deformity, and recurrent disc herniations are common indications for PLIF or TLIF surgery.13,59,76–78 Cloward, in his review of 100 patients with 30 to 40 years of follow-up after treatment for spondylolisthesis, has described PLIF in combination with laminectomy of the entire separate neural arch as a “superior operation.”32 Citing poor durability of posterior decompressive surgery for symptomatic spinal stenosis (15–20% short-term failure and 50% long-term failure), Hutter combined the benefits of anterior fusion and posterior decompression afforded by the PLIF and applied them to the treatment of this disorder. In 142 patients with a minimum of 3 years of follow-up, he described good or excellent results in 78% and a fusion rate of 91%.79 In a relatively large, long-term (12-year), single-surgeon comparison study of PLIF versus standard laminotomy and discectomy for lumbar disc disease, Hackenberg described similar results in clinical outcome; however, he found PLIF to reduce the rate of revision surgery, and overall he thought that it represented an improvement in the management of lumbar disc disease.49 Although one-level disc disease (at L5-S1) was well treated with either technique, additional instrumentation and stabilization were recommended for spondylolisthesis and severe multilevel lumbar disc disease.49 Lumbar instability has been debated for some time, and clear evidence of instability is not always found. Some authors state that a difference of 10 degrees of angulation on lumbar flexion and extension films or spondylolisthesis of 4 mm or more may support this diagnosis, whereas other surgeons rely more heavily on intraoperative findings of instability such as “rocking of the vertebral bodies one on another with two Kocher clamps on adjacent spinous processes” (as described by Brown).80,81 Segmental instability as defined by Frymoyer et al. refers to a “loss of motion stiffness such that force application to that motion segment produces greater displacement than would be seen in a normal structure, resulting in a painful condition and the potential for progressive deformity.”82 In a prospective study comparing discectomy to PLIF for massive disc herniations and/or segmental instability, some superiority of PLIF over discectomy was demonstrated within 5 years of follow-up.75 PLIF and TLIF have been shown to be more effective at deformity correction than posterolateral fusion and more cost-effective than anterior-posterior lumbar interbody fusion with similar outcomes.39,41,44 Most proponents of PLIF cite protection from pain resultant from recurrent disc herniations (attributed to the complete discectomy performed in preparation for the interbody fusion) as a primary indication for fusion surgery over more conservative decompressive procedures.49,75,83 Data regarding the durability of lumbar disc surgery for controlling radiculopathy describe recurrence rates between 10% and 29%, and reduction in the recurrence of radicular symptoms subsequent to disc herniations has been demonstrated with PLIF.49 Controversy remains regarding how many conservative lumbar discectomies should be performed (if any) prior to PLIF.
Attempts have been made to clarify the more subjective indications for PLIF. Evidence positively correlating preoperative level of disability (in the setting of degenerative disc disease) to functional recovery has been described.72 Acute disc herniations with significant and protracted pain, chronic mechanical axial low back pain (“discogenic” pain), central disc prolapse at the L4-5 level, high demand activity, the “failed back syndrome,” and chronic back pain following chemonucleolysis are all supported to some degree as indications for decompression and/or interbody fusion.26,36,81 Chemonucleosis, although currently not as commonly performed due to concerns regarding side effects such as allergic reactions and neurologic deficits, is proposed to lead to loss of intervertebral disc height and progression of degenerative disc disease similar to the standard model of degenerative lumbar disc disease.81
Contraindications to PLIF may include arachnoiditis, active infections, short life expectancy (<3 months), severe osteoporosis, severe subchondral sclerosis with failure to demonstrate viable bone marrow with MRI, and potentially significant epidural fibrosis.6,84
Techniques
The PLIF as proposed by Cloward in 1953 was described as a 2- to 3-hour surgery for an experienced surgeon. Recent studies have demonstrated similar operative times and have associated longer operative times with increased perioperative complications.85 Cloward described three separate surgical phases: harvest of the iliac crest bone graft, laminectomy and discectomy, and spinal fusion.1 Modern technical advances have generated some necessary adaptations to the original descriptions.
In contrast to most current positioning techniques, which maintain a more neutral position through slight hip extension and elevation in an attempt to preserve lumbar lordosis (i.e., Jackson spine frame which in addition facilitates use of 360-degree fluoroscopy), some surgeons may prefer the original position described by Cloward, which used a prone flexed position (knee-chest position) favoring an opened posterior lumbar spine. Although flexion of the hip reduces lumbar lordosis, it facilitates access to the spinal canal. Interestingly, however, a recent study failed to find a difference in the maintenance of lumbar lordosis after PLIF whether a Wilson frame or a Jackson table was used.86 An important aspect of either position is the elimination of pressure on the abdomen. A decrease in intra-abdominal pressure decreases epidural venous bleeding by reducing epidural venous plexus pressure. For the same reason, as well as to prevent damage from an overly distended bladder, most surgeons place a Foley catheter prior to positioning. Attention should also be given to properly supporting the chest to ensure adequate ventilation. Orbital pressure must be avoided to prevent visual loss through mechanical damage to the cornea or globe. A slightly reversed Trendelenburg position is used by many surgeons to help attenuate the increased intraocular pressure generated by the prone position. Although relatively controversial, it is thought to be especially important in possibly preventing visual deficits, including blindness, in patients with preexisting diseases that prevent proper ocular pressure regulation (i.e., glaucoma). Arms are positioned in such a way as to prevent brachial plexus injury, quickly assess an infiltrated intravenous line, eliminate pressure from superficial peripheral nerves, and support and protect all joints. This is accomplished with shoulder abduction and elbow flexion to 90 degrees and zealous use of padding. The feet should be slightly elevated to prevent venous stasis, and care should be given to protecting the lateral femoral cutaneous nerve because the anterior iliac crests often bear a large degree of the patient’s body weight and should be appropriately padded.87 Appropriate deep venous thrombosis prophylaxis is recommended.
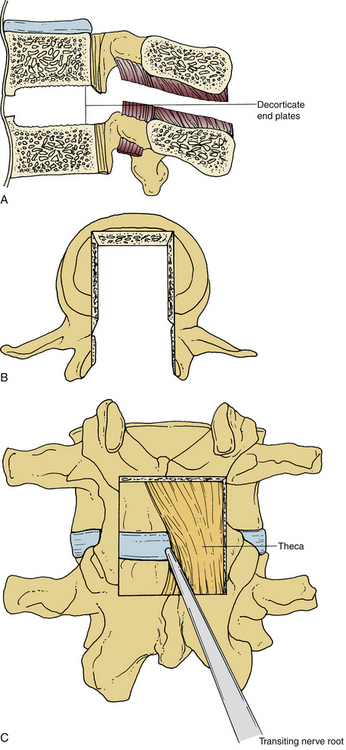
Facilitated by external landmarks, palpation of the spinous processes, and/or the use of preoperative fluoroscopy, a midline incision is planned over the levels to be fused. A standard approach to the posterior lumbar spine is performed.88 Dissection is carried through the subcutaneous tissue to the lumbosacral fascia. This may be done sharply using the scalpel or alternatively with electrocautery. Care is used with dissection through the fascia to maintain the supraspinous and infraspinous ligaments if so desired. This is thought to be especially critical if there are no plans for instrumented internal fixation. Once the correct levels are identified using intraoperative imaging studies, a subperiosteal technique is used to expose the laminae above and below the level to be fused (i.e., for the L4-5 disc, the L4 and L5 laminae are exposed). This is commonly performed by maintaining tension on the paraspinal musculature with the Cobb periosteal elevator while electrocautery or mechanical dissection with gauze sponges cleans the bone of the surrounding soft tissue. The laminectomy described by Cloward involved removal of only the laminae and spinous processes adjacent to the level to be fused (i.e., L4 and L5 laminae and spinous processes for L4-5 fusion). He stated that only a deep notch be made in the laminae and spinous processes rather than a complete laminectomy. Exposure was also facilitated by an instrument designed by Cloward called the vertebra spreader, although other methods are commonly used for distracting the adjacent segments (see later discussion). Cloward also advocated maintaining the attachment of the ligamentum flavum medially to the spinous process and preferred to superiorly reflect this ligament bilaterally after sharply dissecting it free from the facet laterally and laminae superiorly and inferiorly. Although a great deal of variation exists regarding the degree of bony resection, commonly it is carried laterally as far as the pedicle, removing at least half of the superior and inferior facets. Great care is taken not to damage the facet joint capsules, especially if a fusion is not to be performed. A greater degree of facetectomy improves visualization; however, controversy exists regarding its effect on the development of adjacent-level disease (ALD).89–92
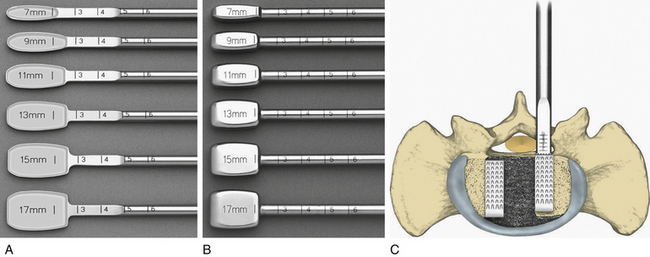
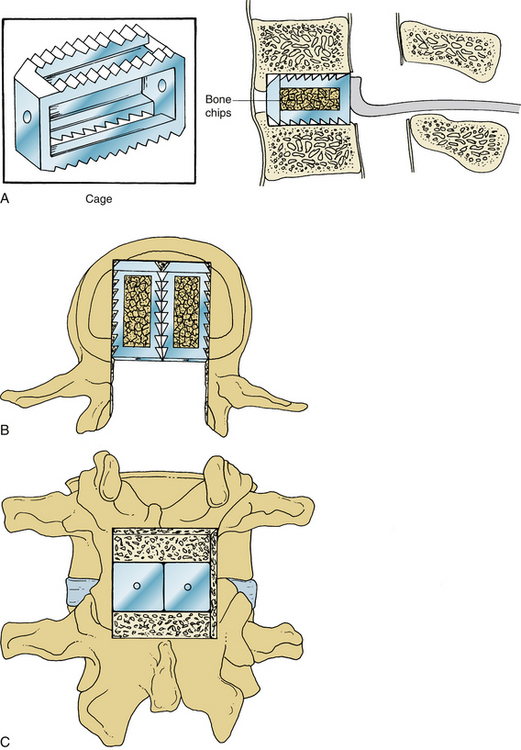
Once the bony removal is performed, the dura is retracted medially with either the use of handheld retractors or a self-retaining retractor as described by Cloward (Fig. 54-1). A standard discectomy using a scalpel to incise the anulus and a series of pituitary rongeurs to remove the majority of the disc is performed. A variety of instruments used to prepare the vertebral end plates, such as end-plate scrapers, shavers, curets, or rasps, have been developed since the use of the chisel in many of the first descriptions of the PLIF.1 After the discectomy is performed and the end plates are adequately prepared using a series of reamers, shavers, rasps, curets, and other available instruments, the grafts are ready to be placed (Fig. 54-2A).59 Careful attention should be paid to the insertion depth of all instruments while working within the disc space, however, because the average depth of the disc space often ranges from 25 mm under the facet to 35 mm in the center of the interspace.6 Original descriptions of the PLIF include bilateral exposures with bilateral graft placement, and although this is still commonplace, more recent descriptions of unilateral graft and/or pedicle screw placement exist.17 Many modern instrumentation sets contain trial grafts that are used to verify correct size of the interbody graft prior to its actual placement (Fig. 54-2B). Studies have demonstrated maximal biomechanical advantage with graft coverage representing greater than 30% of the intervertebral body surface area; a larger surface area of contact between cage and vertebral body has shown lower stress distribution patterns (Fig. 54-2C).13,93,94 A variety of cage materials and designs have been developed, including carbon fiber implants (Fig. 54-3).
When performing a TLIF (unilateral or bilateral), to gain access to the intervertebral disc, the pars interarticularis is identified and resected. Next, a hemifacetectomy of the superior and inferior facets of the levels to be fused is accomplished, often with an osteotome, high-speed drill, and a series of rongeurs.51,56 The inferior nerve root is retracted medially along with the thecal sac. In initial operations, the superior nerve root is easily visualized because it courses inferiorly to the pedicle of the superior vertebral body. Revision cases may require extensive dissection for complete exposure of this nerve root as it exits the neural foramen. Although this nerve rarely requires any retraction, exposure is critical for ensuring protection during cage placement. Discectomy is then performed in standard fashion, although with care to preserve the medial portion of the anulus. Distraction of the disc space to facilitate placement of the intervertebral graft may be accomplished with a variety of methods. A temporary rod may be placed in the contralateral pedicle screws under gentle distraction. Care must be taken at this step not to overdistract; this may cause screw pull-out, screw breakage, or pedicle fracture. Overdistraction at the L4-5 level has also been shown to increase rates of ALD.95 Once discectomy and preparation of the end plates have been accomplished, a trial may be used for verifying correct graft size. Whether structural allograft or an intervertebral cage is used, a combination of morselized autograft or allograft is often placed within the disc space. Positioning of the TLIF graft in the anterior versus middle column has not been shown to affect stability.27
Based on biomechanical studies, pedicle screw fixation is suggested for single-level TLIFs and PLIFs and felt to be necessary when two levels are fused.27 If pedicle screw instrumentation and/or posterolateral bony fusion is planned, additional exposure of the transverse processes of the levels to be fused is generally performed for appropriate localization of the pedicle and drilling of a small pilot hole or defect in the cortical bone using a high-speed drill or rongeurs. Pedicle screws are placed by introducing a pedicle finder through the pedicle into the vertebral body, followed by assessment of the trajectory for breach using a ball-tip probe. A tap is used to generate an appropriately sized trajectory, and a ball-tip probe is used once again to assess for evidence of a cortical breach, prior to final placement of the pedicle screws. Appropriate training in pedicle screw instrumentation is necessary, because misplaced or inappropriately placed screws may significantly increase surgical morbidity. Inadvertent durotomy, neurovascular injury, or compromise in construct biomechanics may result. Proper knowledge of the instrumentation set is critical in selecting instrumentation of appropriate caliber, length, and size in relation to the anatomy. Although the addition of a cross-link has been found through biomechanical studies to have measurable effects on added stability (mainly in axial rotation), general consensus regarding its clinical usefulness is lacking.96
If posterolateral bony fusion is to be performed, decortication of exposed bone, including the lamina and transverse processes, is achieved using a cutting bur on a high-speed drill. Morselized bone chips (autograft and/or allograft) are used to pack the lateral gutters between transverse processes and lateral to rods or plates if pedicle screw fusion is performed. Although bone morphogenetic protein (BMP)-soaked collagen sponges are not currently approved by the Food and Drug Administration (FDA) for posterior lumbar fusion, some surgeons choose to use them as an adjunct for augmenting fusion.36
Minimally invasive surgical techniques have been developed for posterior interbody fusion and instrumentation of the lumbar spine. Schwender et al. described a minimally invasive technique for TLIF97 and subsequently reported favorable outcomes using this technique in patients with spondylolisthesis at a minimum follow-up of 2 years.71 Although whether the minimally invasive approach for TLIF is superior to the traditional open techniques remains a controversial topic,98 several reports have documented potential benefits of the minimally invasive approach compared with the open approach. These include decreased infection rates, shorter hospital stays, reduced postoperative narcotic use, and faster return to work.99–102
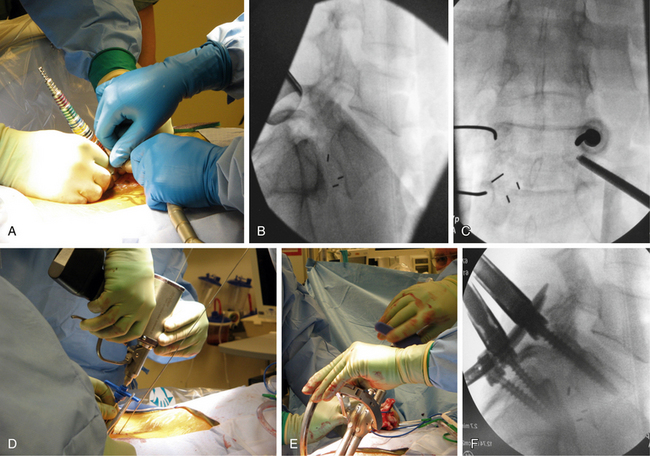
In brief, a standard approach for minimally invasive TLIF includes paramedian dilation to the underlying facet (Fig. 54-4A), followed by facetectomy, discectomy, and placement of an interbody spacer and graft material through the tubular retractor (Fig. 54-4B). Percutaneous pedicle screw placement can then be accomplished with alignment of a Jamshidi needle with the pedicle (Fig. 54-4C) and placement of a guidewire through the Jamshidi needle (Fig. 54-4D). Cannulated screws can then be placed over the guidewires, and rods can be passed and secured with minimally invasive techniques (Figs. 54-4E and F). Radiographically, the final appearance of the minimally invasive TLIF is very similar to that of the open approach (Fig. 54-5).
Complications
There are specific concerns and limitations to PLIF.59 Most surgeons agree about the technically demanding nature of the surgery and have reported difficult intraoperative cases with poor outcomes.59,103 The necessary acquisition of autologous interbody graft and/or selection of appropriate interbody allograft or composite material are potential limitations. ALD, graft migration or subsidence, collapse of the intervertebral space with resultant neuroforaminal stenosis, and the potential for segmental instability or pseudarthrosis are among the complications that may be associated with this type of surgery.72,92,103–106 If not supplemented with pedicle screw fixation, immediate instability prior to bony arthrodesis is also of concern, especially with aggressive decompressions. Postoperative neurologic deficit, however, is arguably one of the most serious complications of this surgery, with rates ranging from 9.0% to 24.6%.13,85,90
Some authorities have reported complication rates as high as 37.5% for initial PLIF surgery, whereas others have quoted rates of new radiculopathy ranging from 13.0% to 16.4%.48,59,85,107 Postoperative neuralgia is a known complication after PLIF, and there are reports of rates in the order of 7% (no significant difference in subtotal vs. total facetectomy) in the literature.108 Nerve damage secondary to retraction with resultant endoneural fibrosis and chronic radiculopathy is possible, especially if the dorsal root ganglion is damaged. A recent study of 1680 patients with a mean follow-up of 5 years found a 13.2% rate of revision surgery after PLIF. No statistically significant difference occurred in the rate of revision for single-level versus multilevel PLIF, although a trend toward a greater rate of ALD was apparent in the multilevel group.109 Most authors agree that revision surgery for failed PLIF surgery is technically difficult, with limited surgical options; clinical outcomes are poor.110 In a recent outcome study of PLIF of elderly patients (≥70 years of age), although no obvious differences in the clinical results were observed, collapsed and delayed unions were more common and postoperative ALD was less frequent in the elderly patients, compared with younger patients (<70 years of age).111 ALD is a well-described complication after spinal fusion.92,112 Although preoperative individual anatomic factors (e.g., facet sagittalization or tropism and laminar inclination) have been linked with its development, studies have failed to demonstrate correlation of radiologic degeneration of adjacent levels and clinical outcome.92 Not all results have been statistically significant. However, in a recent comparison of the development of ALD among groups who have had PLIF, posterolateral fusion, and ligamentoplasty, PLIF appeared to develop ALD earlier and require revision surgery more often than in the other two groups.113 Attempts have been made to attenuate the development of ALD through innovations in surgical technique with some measurable results.114 Presumably a result from the more lateral trajectory of the graft placement for TLIF, anecdotal reports exist that suggest cage migration after TLIF may not cause neural compression as often as after PLIF and may not always necessitate revision surgery.115,116 Cage migration rates as high as 8% have been seen after uninstrumented PLIF surgery (and are also associated with total facetectomy) and in contrast to TLIF, often require revision surgery, which is more technically difficult.116 Cage type and positioning have also been found to influence rate of migration.117 Vascular complications may result from graft migration, and care should be taken in compacting graft material ventrally.106
Other complications not unique to PLIF/TLIF warrant mentioning. Increased blood loss, higher rates of durotomy (5.4–10.0%), and development of arachnoiditis are disadvantages compared with posterolateral fusion.36 Wound infection (0.2–7.0%), iliac crest bone harvest site pain, delayed wound healing, hematoma, screw misplacement, intraoperative pedicle fracture, postoperative urinary retention, pulmonary embolism, cerebellar infarction or hemorrhage, mass effect from epidural fat grafts, seroma, and epidural fibrosis/scar are among the reported complications.5,6,13,34,49,59,103,104 Although surgical debridement and prolonged antibiotics are often required for the treatment of wound infection after PLIF, the removal of the cage itself is not always warranted.118
Recombinant BMP has been demonstrated to facilitate bone fusion and is currently FDA-approved for use in “stand alone” anterior lumbar interbody fusion (ALIF) surgery. Off-label use is not uncommon, including in PLIF/TLIF surgery.119,120 Anecdotal reports of complications of its use exist. Theoretically, increased risk of cage migration and subsidence subsequent to loosening during the early resorptive phase have been postulated during the use of PEEK cages.121 Although previously thought to represent a benign radiologic finding, limited reports of symptomatic ectopic bone formation with the use of BMP exist.122,123
Future Directions
Advancements in surgical techniques will continue to be described. The use of radiofrequency ablation for end-plate preparation has recently been described.124 As minimally invasive techniques continue to be explored and incorporated by spine surgeons, its use will be expanded. Currently, surgeons are in need of longer follow-up for many early studies comparing minimally invasive and open techniques.125 Technologic advancements in spine navigation surgery may increase its attractiveness may provide safer instrumentation techniques. Materials development will continue to advance the repertoire of interbody grafts and instrumentation. Biomechanical studies will help guide the application of new technology.
Cloward R.B. Posterior lumbar interbody fusion updated. Clin Orthop Relat Res. 1985;193:16-19.
Dickerman R.D., Reynolds A., Bennett M., et al. Posterior lumbar interbody fusion. J Neurosurg Spine. 2007;6:194-195. author reply 195
Ekman P., Moller H., Tullberg T., et al. Posterior lumbar interbody fusion versus posterolateral fusion in adult isthmic spondylolisthesis. Spine (Phila Pa 1976). 2007;32:2178-2183.
Harms J., Jeszensky D. The unilateral, transforaminal approach for posterior lumbar interbody fusion. Orthop Traumatol. 1998;6:88-99.
Harris B.M., Hilibrand A.S., Savas P.E., et al. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine (Phila Pa 1976). 2004;29:E65-E70.
Humphreys S.C., Hodges S.D., Patwardhan A.G., et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976). 2001;26:567-571.
Okuda S., Miyauchi A., Oda T., et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine. 2006;4:304-309.
1. Cloward R.B. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10:154-168.
2. Cloward R.B. The treatment of ruptured lumbar intervertebral discs; criteria for spinal fusion. Am J Surg. 1953;86:145-151.
3. Jaslow I. Intercorporal bone graft in spinal fusion after disc removal. Surg Gynecol Obstet. 1946;82:215-218.
4. Mercer W. Spondylolisthesis with a description of a new method of operative treatment and notes on 10 cases. Edinburg Med J. 1936;43:545-572.
5. Lin P.M. Posterior lumbar interbody fusion (PLIF): past, present, and future. Clin Neurosurg. 2000;47:470-482.
6. Lin P.M., Cautilli R.A., Joyce M.F. Posterior lumbar interbody fusion. Clin Orthop Relat Res. 1983;180:154-168.
7. Cheng L., Nie L., Zhang L. Posterior lumbar interbody fusion versus posterolateral fusion in spondylolisthesis: a prospective controlled study in the Han nationality. Int Orthop. 2009;33:1043-1047.
8. Blume H.G., Rojas C.H. Unilateral lumbar interbody fusion (posterior approach) utilizing dowel grafts: experience in over 200 patients. J Neurol Orthop Surg. 1981;2:171-178.
9. Groth A.T., Kuklo T.R., Klemme W.R., et al. Comparison of sagittal contour and posterior disc height following interbody fusion: threaded cylindrical cages versus structural allograft versus vertical cages. J Spinal Disord Tech. 2005;18:332-336.
10. Asazuma T., Masuoka K., Motosuneya T., et al. Posterior lumbar interbody fusion using dense hydroxyapatite blocks and autogenous iliac bone: clinical and radiographic examinations. J Spinal Disord Tech. 2005;18(Suppl):S41-S47.
11. Brantigan J.W., Neidre A., Toohey J.S. The Lumbar I/F Cage for posterior lumbar interbody fusion with the variable screw placement system: 10-year results of a Food and Drug Administration clinical trial. Spine J. 2004;4:681-688.
12. Couture D.E., Branch C.L.Jr. Posterior lumbar interbody fusion with bioabsorbable spacers and local autograft in a series of 27 patients. Neurosurg Focus. 2004;16:E8.
13. DiPaola C.P., Molinari R.W. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16:130-139.
14. Fogel G.R., Toohey J.S., Neidre A., et al. Outcomes of posterior lumbar interbody fusion with the 9-mm width lumbar I/F cage and the variable screw placement system. J Surg Orthop Adv. 2009;18:77-82.
15. Kanayamam C.B., Haggerty C.J., Abumi K., et al. In vitro biomechanical investigation of the stability and stressshielding effect of lumbar interbody fusion devices. J Neurosurg. 2000;2(Suppl):259-265.
16. Kwon Y.-M., Chin D.-K., Jin B.-H., et al. Long term efficacy of posterior lumbar interbody fusion with standard cages alone in lumbar disc diseases combined with modic changes. J Korean Neurosurg Soc. 2009;46:322-327.
17. Moreland D.B., Asch H.L., Czajka G.A., et al. Posterior lumbar interbody fusion: comparison of single intervertebral cage and single side pedicle screw fixation versus bilateral cages and screw fixation. Minim Invasive Neurosurg. 2009;52:132-136.
18. Suh K.T., Park W.W., Kim S.J., et al. Posterior lumbar interbody fusion for adult isthmic spondylolisthesis: a comparison of fusion with one or two cages. J Bone Joint Surg [Br]. 2008;90:1352-1356.
19. Xiao Y.X., Chen Q.X., Li F.C. Unilateral transforaminal lumbar interbody fusion: a review of the technique, indications and graft materials. J Int Med Res. 2009;37:908-917.
20. Barnes B., Rodts G.E.Jr., Haid R.W.Jr., et al. Allograft implants for posterior lumbar interbody fusion: results comparing cylindrical dowels and impacted wedges. Neurosurgery. 2002;51:1191-1198.
21. Bagby G.W. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11:931-934.
22. Leong J.C., Chow S.P., Yau A.C. Titanium-mesh block replacement of the intervertebral disk. Clin Orthop Relat Res. 1994;300:52-63.
23. Ray C.D. Threaded titanium cages for lumbar interbody fusions. Spine. 1997;22:667-679. discussion 679–680
24. Wang S.T.G.V., Fu C.Y., et al. Posterior instrumentation reduces differences in spine stability as a result of different cage orientations: an in vitro study. Spine. 2005;30:62-67.
25. Yu C.-H., Wang C.-T., Chen P.-Q. Instrumented posterior lumbar interbody fusion in adult spondylolisthesis. Clin Orthop Relat Res. 2008;466:3034-3043.
26. Cloward R.B. Posterior lumbar interbody fusion updated. Clin Orthop Relat Res. 1985;193:16-19.
27. Ames C.P., Acosta F.L.Jr., Chi J., et al. Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion performed at 1 and 2 levels. Spine (Phila Pa 1976). 2005;30:E562-E566.
28. Okuyama K., Kido T., Unoki E., Chiba M. PLIF with a titanium cage and excised facet joint bone for degenerative spondylolisthesis—in augmentation with a pedicle screw. J Spinal Disord Tech. 2007;20:53-59.
29. Pitzen T., Matthis D., Muller-Storz H., et al. Primary stability of 2 PLIF (posterior lumbar interbody fusion) techniques—a biomechanical and finite element analysis. Zentralbl Neurochir. 1999;60:114-120. [in German]
30. Wu C.-H., Wong C.-B., Chen L.-H., et al. Instrumented posterior lumbar interbody fusion for patients with degenerative lumbar scoliosis. J Spinal Disord Tech. 2008;21:310-315.
31. Cloward R.B. Lesions of the intervertebral disks and their treatment by interbody fusion methods. The painful disk. Clin Orthop Relat Res. 1963;27:51-77.
32. Cloward R.B. Spondylolisthesis: treatment by laminectomy and posterior interbody fusion. Clin Orthop Relat Res. 1981;154:74-82.
33. Steffee A.D., Sitkowski D.J. Posterior lumbar interbody fusion and plates. Clin Orthop Relat Res. 1988;227:99-102.
34. Branch C.L., Branch C.L.Jr. Posterior lumbar interbody fusion with the keystone graft: technique and results. Surg Neurol. 1987;27:449-454.
35. Dickerman R.D., East J.W., Winters K., et al. Anterior and posterior lumbar interbody fusion with percutaneous pedicle screws: comparison to muscle damage and minimally invasive techniques. Spine (Phila Pa 19760). 2009;34:E923-E925.
36. Kasis A.G., Marshman L.A., Krishna M., Bhatia C.K. Significantly improved outcomes with a less invasive posterior lumbar interbody fusion incorporating total facetectomy. Spine. 2009;34:572-577.
37. Cole C.D., McCall T.D., Schmidt M.H., Dailey A.T. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2:118-126.
38. Suk S.I., Lee C.K., Kim W.J., et al. Adding posterior lumbar interbody fusion to pedicle screw fixation and posterolateral fusion after decompression in spondylolytic spondylolisthesis. Spine (Phila Pa 1976). 1997;22:210-219. discussion 219–220
39. Branch C.L.Jr. The case for posterior lumbar interbody fusion. Clin Neurosurg. 1996;43:252-267.
40. Christensen F.B., Hansen E.S., Eiskjaer S.P. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine (Phila Pa 1976). 2002;27:2674-2683.
41. Dantas F.L.R., Prandini M.N., Ferreira M.A.T. Comparison between posterior lumbar fusion with pedicle screws and posterior lumbar interbody fusion with pedicle screws in adult spondylolisthesis. Arq Neuropsiquiatr. 2007;65:764-770.
42. Dehoux E., Fourati E., Madi K., et al. Posterolateral versus interbody fusion in isthmic spondylolisthesis: functional results in 52 cases with aminimum follow-up of 6 years. Acta Orthop Belg. 2004;70:578-582.
43. Ekman P., Moller H., Tullberg T., et al. Posterior lumbar interbody fusion versus posterolateral fusion in adult isthmic spondylolisthesis. Spine (Phila Pa 1976). 2007;32:2178-2183.
44. Fraser R.D. Interbody, posterior, and combined lumbar fusions. Spine (Phila Pa 1976). 1995;20:S167-S177.
45. Hee H.T., Castro F.P.Jr., Majd M.E., et al. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord. 2001;14:533-540.
46. Kim K.-T., Lee S.-H., Lee Y.-H., et al. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine (Phila Pa 1976). 2006;31:1351-1357. discussion 1358
47. Periasamy K., Shah K., Wheelwright E.F. Posterior lumbar interbody fusion using cages, combined with instrumented posterolateral fusion: a study of 75 cases. Acta Orthop Belg. 2008;74:240-248.
48. Ha K.-Y., Na K.-H., Shin J.-H., Kim K.-W. Comparison of posterolateral fusion with and without additional posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis. J Spinal Disord Tech. 2008;21:229-234.
49. Hackenberg L., Halm H., Bullmann V., et al. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J. 2005;14:551-558.
50. Rish B.L. A critique of posterior lumbar interbody fusion: 12 years’ experience with 250 patients. Surg Neurol. 1989;31:281-289.
51. Schlegel K.F., Pon A. The biomechanics of posterior lumbar interbody fusion (PLIF) in spondylolisthesis. Clin Orthop Relat Res. 1985;193:115-119.
52. Harms J., Rolinger H. A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author’s transl). Z Orthop Ihre Grenzgeb. 1982;120:343-347.
53. Yan D.-L., Pei F.-X., Li J., Soo C.-L. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17:1311-1316.
54. Faundez A.A. Re: Ames CP, Acosta FL Jr, Chi J, et al. Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion performed at 1 and 2 levels. Spine 2005;30:E562–6. Spine (Phila Pa 1976). 2006;31:504.
55. Harms J., Jeszensky D. The unilateral, transforaminal approach for posterior lumbar interbody fusion. Orthop Traumatol. 1998;6:88-99.
56. Heary R.F., Kumar S., Karimi R.J. Dorsal lumbar interbody fusion for chronic axial, mechanical low back pain: a modification of two established techniques. Neurosurgery. 2008;63:ONS102-ONS106. discussion ONS106–ONS107
57. Humphreys S.C., Hodges S.D., Patwardhan A.G., et al. Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine (Phila Pa 1976). 2001;26:567-571.
58. Lauber S., Schulte T.L., Liljenqvist U., et al. Clinical and radiologic 2-4-year results of transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Spine (Phila Pa 1976). 2006;31:1693-1698.
59. Rosenberg W.S., Mummaneni P.V. Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery. 2001;48:569-574. discussion 574–575
60. Hutter C.G. Posterior intervertebral body fusion. A 25-year study. Clin Orthop Relat Res. 1983;179:86-96.
61. Hioki A., Miyamoto K., Kodama H., et al. Two-level posterior lumbar interbody fusion for degenerative disc disease: improved clinical outcome with restoration of lumbar lordosis. Spine J. 2005;5:600-607.
62. Salehi S.A., Tawk R., Ganju A., et al. Transforaminal lumbar interbody fusion: surgical technique and results in 24 patients. Neurosurgery. 2004;54:368-374. discussion 374
63. Harris B.M., Hilibrand A.S., Savas P.E., et al. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine (Phila Pa 1976). 2004;29:E65-E70.
64. Shin H.C., Yi S., Kim K.N., et al. Posterior lumbar interbody fusion via a unilateral approach. Yonsei Med J. 2006;47:319-325.
65. Deutsch H., Musacchio M.J.Jr. Minimally invasive transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Neurosurg Focus. 2006;20:E10.
66. Ghahreman A., Ferch R.D., Rao P.J. Bogduk N: Minimal access versus open posterior lumbar interbody fusion in the treatment of spondylolisthesis. Neurosurgery. 2010;66:296-304. discussion 304
67. Wang M.Y., Cummock M.D., Yu Y., Trivedi R.A. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12:694-699.
68. Baek O.K., Lee S.-H. Extraforaminal lumbar interbody fusion for the treatment of isthmic spondylolisthesis. J Spinal Disord Tech. 2009;22:219-227.
69. Marotta N., Cosar M., Pimenta L., Khoo L.T. A novel minimally invasive presacral approach and instrumentation technique for anterior L5-S1 intervertebral discectomy and fusion: technical description and case presentations. Neurosurg Focus. 2006;20:E9.
70. Ntoukas V., Muller A. Minimally invasive approach versus traditional open approach for one level posterior lumbar interbody fusion. Minim Invasive Neurosurg. 2010;53:21-24.
71. Park Y., Ha J.W. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976). 2007;32:537-543.
72. Mofidi A., Sedhom M., O’Shea K., et al. Is high level of disability an indication for spinal fusion? Analysis of long-term outcome after posterior lumbar interbody fusion using carbon fiber cages. J Spinal Disord Tech. 2005;18:479-484.
73. Sears W. Posterior lumbar interbody fusion for degenerative spondylolisthesis: restoration of sagittal balance using insert-and-rotate interbody spacers. Spine J. 2005;5:170-179.
74. Sears W. Posterior lumbar interbody fusion for lytic spondylolisthesis: restoration of sagittal balance using insert-and-rotate interbody spacers. Spine J. 2005;5:161-169.
75. Satoh I., Yonenobu K., Hosono N., et al. Indication of posterior lumbar interbody fusion for lumbar disc herniation. J Spinal Disord Tech. 2006;19:104-108.
76. Kai Y., Oyama M., Morooka M. Posterior lumbar interbody fusion using local facet joint autograft and pedicle screw fixation. Spine (Phila Pa 1976). 2004;29:41-46.
77. McAfee P.C., DeVine J.G., Chaput C.D., et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976). 2005;30:S60-S65.
78. McLaughlin M.R., Haid R.W.Jr., Rodts G.E.Jr., Subach B.R. Posterior lumbar interbody fusion: indications, techniques, and results. Clin Neurosurg. 2000;47:514-527.
79. Hutter C.G. Spinal stenosis and posterior lumbar interbody fusion. Clin Orthop Relat Res. 1985;193:103-114.
80. Brown M., Finneson B., editor. Low back pain, ed 2. Philadelphia: JB Lippincott. 1980:387-388.
81. Sepulveda R., Kant A.P. Chemonucleolysis failures treated by PLIF. Clin Orthop Relat Res. 1985;193:68-74.
82. Frymoyer J.W., Newburg A.A., Pope M.H., et al. Spine radiographs in patients with low back pain. J Bone Joint Surg [Am]. 1984;66:1048-1055.
83. Rish B.L. A comparative evaluation of posterior lumbar interbody fusion for disc disease. Spine (Phila Pa 1976). 1985;10:855-857.
84. Jones D.S., Wray S.D., Branch C.L.Jr. Posterior lumbar interbody fusion and transpedicular screw fixation. In: Fessler R.G., Sekhar L., editors. Atlas of neurosurgical techniques: spine and peripheral nerves. New York: Thieme; 2006:681-689.
85. Hosono N., Namekata M., Makino T., et al. Perioperative complications of primary posterior lumbar interbody fusion for nonisthmic spondylolisthesis: analysis of risk factors. J Neurosurg Spine. 2008;9:403-407.
86. Lee J.H., Lee J.-H., Yoon K.-S., et al. Effect of intraoperative position used in posterior lumbar interbody fusion on the maintenance of lumbar lordosis. J Neurosurg Spine. 2008;8:263-270.
87. Cho K.-T., Lee H.J. Prone position-related meralgia paresthetica after lumbar spinal surgery: a case report and review of the literature. J Korean Neurosurg Soc. 2008;44:392-395.
88. Osenbach R. Approach to the lumbar spine. In: Fessler R.G., Sekhar L., editors. Atlas of neurosurgical techniques: spine and peripheral nerves. New York: Thieme; 2006:612-617.
89. Dickerman R.D., Reynolds A., Bennett M., et al. Posterior lumbar interbody fusion. J Neurosurg Spine. 2007;6:194-195. author reply 195
90. Okuda S., Miyauchi A., Oda T., et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine. 2006;4:304-309.
91. Okuda S., Oda T., Miyauchi A., et al. Lamina horizontalization and facet tropism as the risk factors for adjacent segment degeneration after PLIF. Spine (Phila Pa 1976). 2008;33:2754-2758.
92. Okuda S.y., Iwasaki M., Miyauchi A., et al. Risk factors for adjacent segment degeneration after PLIF. Spine (Phila Pa 1976). 2004;29:1535-1540.
93. Closkey R.F.P.J., Lee C.K., et al. Mechanics of interbody spinal fusion: analysis of critical bone graft area. Spine (Phila Pa 1976). 1993;18:1011-1015.
94. Kumar N., Judith M.R., Kumar A., et al. Analysis of stress distribution in lumbar interbody fusion. Spine (Phila Pa 1976). 2005;30:1731-1735.
95. Kaito T., Hosono N., Mukai Y., et al. Induction of early degeneration of the adjacent segment after posterior lumbar interbody fusion by excessive distraction of lumbar disc space. J Neurosurg Spine. 2010;12:671-679.
96. Chutkan N.B., Zhou H., Akins J.P., Wenger K.H. Effects of facetectomy and crosslink augmentation on motion segment flexibility in posterior lumbar interbody fusion. Spine (Phila Pa 1976). 2008;33:E828-E835.
97. Schwender J.D., Holly L.T., Rouben D.P., Foley K.T. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1-S6.
98. Payer M. “Minimally invasive” lumbar spine surgery: a critical review. Acta Neurochir (Wien). 2011;153:1455-1459.
99. Adogwa O., Parker S.L., Bydon A., et al. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24:479-484.
100. McGirt M.J., Parker S.L., Lerner J., et al. Comparative analysis of perioperative surgical site infection after minimally invasive versus open posterior/transforaminal lumbar interbody fusion: analysis of hospital billing and discharge data from 5170 patients. J Neurosurg Spine. 2011;14:771-778.
101. Parker S.L., Adogwa O., Witham T.F., et al. Post-operative infection after minimally invasive versus open transforaminal lumbar interbody fusion (TLIF): literature review and cost analysis. Minim Invasive Neurosurg. 2011;54:33-37.
102. Smith J.S., Shaffrey C.I., Sansur C.A., et al. Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine (Phila Pa 1976). 2011;36:556-563.
103. Collis J.S. Total disc replacement: a modified posterior lumbar interbody fusion. Report of 750 cases. Clin Orthop Relat Res. 1985;193:64-67.
104. Khong P., Jerry Day M. Spontaneous cerebellar haemorrhage following lumbar fusion. J Clin Neurosci. 2009;16:1673-1675.
105. Tokuhashi Y., Ajiro Y. Umezawa N: Subsidence of metal interbody cage after posterior lumbar interbody fusion with pedicle screw fixation. Orthopedics. 32(4), 2009.
106. Yoshimoto H., Sato S., Nakagawa I., et al. Deep vein thrombosis due to migrated graft bone after posterior lumbosacral interbody fusion. Case report. J Neurosurg Spine. 2007;6:47-51.
107. Elias W.J.S.N., Kaptain G.J., Chadduck J.B., Whitehill R. Complications of posterior lumbar interbody fusion when using a titanium threaded cage device. J Neurosurg. 2000;93(Suppl 1):45-52.
108. Krishna M., Pollock R.D., Bhatia C. Incidence, etiology, classification, and management of neuralgia after posterior lumbar interbody fusion surgery in 226 patients. Spine J. 2008;8:374-379.
109. Greiner-Perth R., Boehm H., Allam Y., et al. Reoperation rate after instrumented posterior lumbar interbody fusion: a report on 1680 cases. Spine (Phila Pa 1976). 2004;29:2516-2520.
110. Wetzel F.T., LaRocca H. The failed posterior lumbar interbody fusion. Spine J. 1991;16:839-845.
111. Okuda S., Oda T., Miyauchi A., et al. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. Surgical technique. J Bone Joint Surg [Am]. 2007;89(Suppl 2):310-320.
112. Chosa E., Goto K., Totoribe K., Tajima N. Analysis of the effect of lumbar spine fusion on the superior adjacent intervertebral disk in the presence of disk degeneration, using the three-dimensional finite element method. J Spinal Disord Tech. 2004;17:134-139.
113. Kanayama M., Togawa D., Hashimoto T., et al. Motion-preserving surgery can prevent early breakdown of adjacent segments: comparison of posterior dynamic stabilization with spinal fusion. J Spinal Disord Tech. 2009;22:463-467.
114. Imagama S., Kawakami N., Matsubara Y., et al. Preventive effect of artificial ligamentous stabilization on the upper adjacent segment impairment following posterior lumbar interbody fusion. Spine (Phila Pa 1976). 2009;34:2775-2781.
115. Aoki Y., Yamagata M., Nakajima F., et al. Posterior migration of fusion cages in degenerative lumbar disease treated with transforaminal lumbar interbody fusion: a report of three patients. Spine (Phila Pa 1976). 2009;34:E54-E58.
116. Chen L., Yang H., Tang T. Cage migration in spondylolisthesis treated with posterior lumbar interbody fusion using BAK cages. Spine (Phila Pa 1976). 2005;30:2171-2175.
117. Abbushi A., Cabraja M., Thomale U.-W., et al. The influence of cage positioning and cage type on cage migration and fusion rates in patients with monosegmental posterior lumbar interbody fusion and posterior fixation. Eur Spine J. 2009;18:1621-1628.
118. Mirovsky Y., Floman Y., Smorgick Y., et al. Management of deep wound infection after posterior lumbar interbody fusion with cages. J Spinal Disord Tech. 2007;20:127-131.
119. Haid R.W.Jr., Branch C.L.Jr., Alexander J.T., Burkus J.K. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J. 2004;4:527-538. discussion 538–539
120. Meisel H.J., Schnoring M., Hohaus C., et al. Posterior lumbar interbody fusion using rhBMP-2. Eur Spine J. 2008;17:1735-1744.
121. Vaidya R., Sethi A., Bartol S., et al. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21:557-562.
122. Chen N-F., Smith Z.A., Stiner E., et al. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12:40-46.
123. Wong D.A., Kumar A., Jatana S., et al. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J. 2008;8:1011-1018.
124. Aryan H.E., Ames C.P., Szandera B., et al. Coblation of spinal endplates in preparation for interbody spinal fusion. J Clin Neurosci. 2006;13:349-352.
125. Tsutsumimoto T., Shimogata M., Ohta H., Misawa H. Mini-open versus conventional open posterior lumbar interbody fusion for the treatment of lumbar degenerative spondylolisthesis: comparison of paraspinal muscle damage and slip reduction. Spine (Phila Pa 1976). 2009;34:1923-1928.