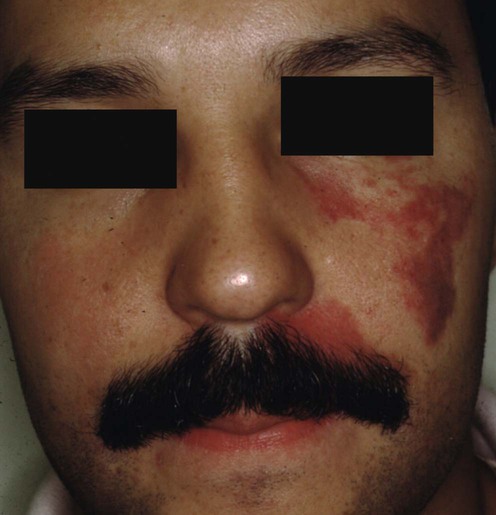
Port wine stain (‘nevus flammeus’)
Specific investigations
First-line therapies
Facial port wine stains in childhood: prediction of the rate of improvement as a function of the age of the patient, size and location of the port wine stain and the number of treatments with the pulsed dye (585 nm) laser.
Nguyen CM, Yohn JJ, Huff C, Weston WL, Morelli JG. Br J Dermatol 1998; 138: 821–5.
Pain relief measures and cooling devices
Second-line therapies
Third-line therapies



 Pulsed dye laser
Pulsed dye laser Intense pulsed light source
Intense pulsed light source Alexandrite 755 nm laser
Alexandrite 755 nm laser Neodymium:yttrium–aluminum–garnet (Nd:YAG) laser
Neodymium:yttrium–aluminum–garnet (Nd:YAG) laser Potassium titanyl phosphate laser
Potassium titanyl phosphate laser Imiquimod (as anti-angiogenic therapy)
Imiquimod (as anti-angiogenic therapy) Photodynamic therapy
Photodynamic therapy Rapamycin (as anti-angiogenic therapy)
Rapamycin (as anti-angiogenic therapy)