Porphyria cutanea tarda
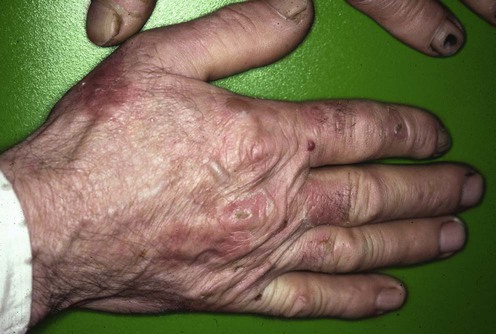
Specific investigations
First-line therapies
Second-line therapies
Third-line therapies
Removal of plasma porphyrins with high-flux hemodialysis in porphyria cutanea tarda associated with end stage renal disease.
Carson RW, Dunnigan EJ, DuBose TD Jr, Goeger DE, Anderson KE. J Am Soc Nephrol 1992; 2: 1445–50.
High-flux hemodialysis may remove porphyrins more effectively than conventional hemodialysis.








 Serial phlebotomies
Serial phlebotomies Chloroquine, hydroxychloroquine
Chloroquine, hydroxychloroquine Deferoxamine (desferrioxamine)
Deferoxamine (desferrioxamine) Erythropoietin
Erythropoietin Antiretroviral therapy
Antiretroviral therapy Interferon-α
Interferon-α Vitamins E and C
Vitamins E and C Plasmapheresis, plasma exchange
Plasmapheresis, plasma exchange High-flux hemodialysis
High-flux hemodialysis Enteric sorbents (cholestyramine, activated charcoal)
Enteric sorbents (cholestyramine, activated charcoal) Metabolic alkalinization by oral sodium bicarbonate
Metabolic alkalinization by oral sodium bicarbonate Cimetidine
Cimetidine Photothermolysis
Photothermolysis