CHAPTER 18 Polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disturbance affecting women, and is a heterogeneous collection of signs and symptoms that, gathered together, form a spectrum of a disorder with a mild presentation in some women, and a severe disturbance of reproductive, endocrine and metabolic function in other women (Balen et al 1995). The pathophysiology of PCOS appears to be multifactorial. The definition of PCOS has been much debated. Key features include menstrual cycle disturbance, hyperandrogenism and obesity. There are many extraovarian aspects to the pathophysiology of PCOS, yet ovarian dysfunction is central. The international consensus definition of PCOS is the presence of two out of the following three criteria: (i) oligo-ovulation and/or anovulation; (ii) hyperandrogenism (clinical and/or biochemical); and (iii) polycystic ovaries, with the exclusion of other aetiologies of menstrual disturbance or hyperandrogenism (Fauser et al 2004). There is considerable heterogeneity of symptoms and signs amongst women with PCOS, and for an individual, these may change over time. Polycystic ovaries can exist without clinical signs of PCOS, expression of which may be precipitated by various factors, most predominantly an increase in body weight.
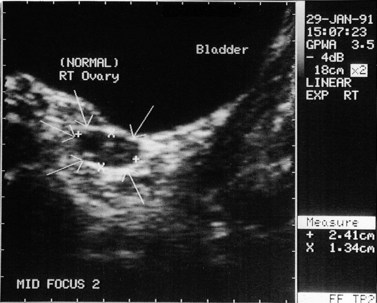
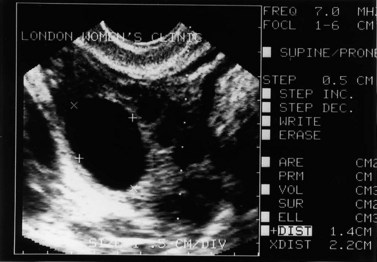
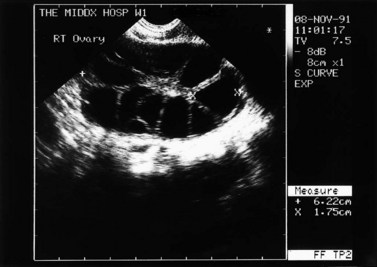
The morphology of the polycystic ovary is an ovary with 12 or more follicles measuring 2–9 mm in diameter and/or increased ovarian volume (>10 cm3) (Balen et al 2003) (Figures 18.1–18.5). Polycystic ovaries are commonly detected by pelvic ultrasound, with estimates of prevalence in the general population being in the order of 20–33% (Michelmore et al 1999). However, not all women with polycystic ovaries demonstrate the clinical and biochemical features which define PCOS. These include menstrual cycle disturbances, hirsutism, acne, alopecia and abnormalities of biochemical profiles, including elevated serum concentrations of luteinizing hormone (LH), testosterone and androstenedione. Obesity and hyperinsulinaemia are associated features, although only 40–50% of women with PCOS are overweight (Balen and Michelmore 2002). Presentation of PCOS is so varied that one, all or any combination of the above features may be present in association with an ultrasound picture of polycystic ovaries (Table 18.1).
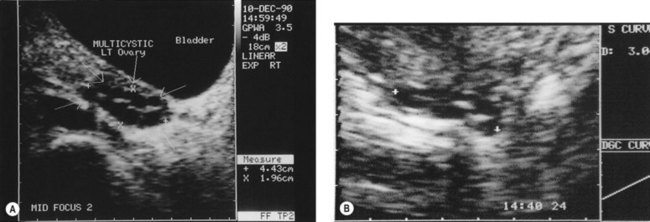
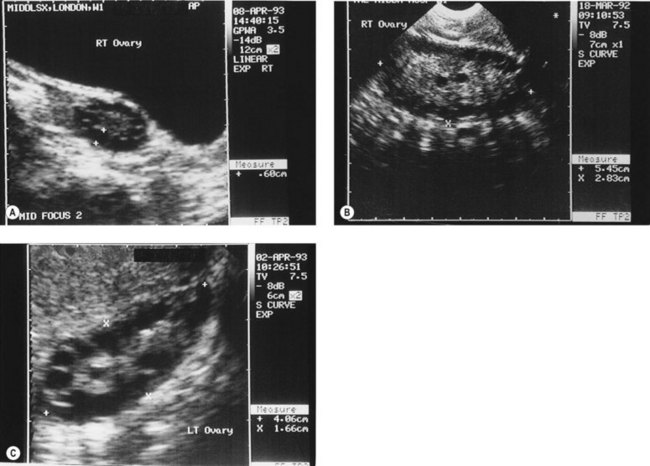
Figure 18.3 (A) Transabdominal scan of a multicystic ovary. (B) Transvaginal ultrasound scan of a multicystic ovary.
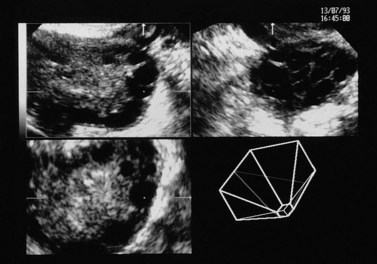
Figure 18.4 Three-dimensional transvaginal ultrasound scan of a polycystic ovary.
Courtesy of Dr A. Kyei-Mensah.
Table 18.1 The spectrum of clinical manifestations of polycystic ovary syndrome
| Symptoms | Serum endocrinology | Possible late sequelae |
|---|---|---|
| Obesity | ↑ Androgens (testosterone and androstenedione) |
Pathogenesis
The pathogenesis of polycystic ovaries and PCOS is still being elucidated, but the heterogeneity of presentation of PCOS suggests that a single cause is unlikely. Some genetic studies have identified a link between PCOS and disordered insulin metabolism, and indicate that PCOS may be the presentation of a complex genetic trait disorder (Franks et al 2001). PCOS runs in families, with approximately half of first-degree relatives (i.e. sisters, mothers and daughters) also being affected; incidentally, male relatives also show an increased rate of metabolic problems. The features of obesity, hyperinsulinaemia and hyperandrogenaemia, which are commonly seen in PCOS, are also known to be factors which confer an increased risk of cardiovascular disease and type 2 diabetes (Rajkowha et al 2000). There are studies which indicate that women with PCOS have an increased risk for these diseases which pose long-term risks for health.
PCOS appears to have its origins during adolescence and is thought to be associated with increased weight gain during puberty. However, the polycystic ovary gene(s) has not yet been identified, and the effects of environmental influences such as weight changes and circulating hormone concentrations, and the age at which these occur, are still being unravelled. Prior to puberty, there appears to be two periods of increased ovarian growth. The first is at adrenarche in response to increased concentrations of circulating androgens, and the second is just before and during puberty due to rising gonadotrophin levels, and the actions of growth hormone, insulin-like growth factor-1 (IGF-1) and insulin on the ovary. Sampaolo et al (1994) reported a study of 49 obese girls at different stages of puberty, comparing their pelvic ultrasound features and endocrine profiles with 35 age- and pubertal-stage-matched controls. They found that obesity was associated with a significant increase in uterine and ovarian volume. They also found that obese postmenarcheal girls with polycystic ovaries had larger uterine and ovarian volumes than obese postmenarcheal girls with normal ovaries. Sampaolo et al concluded that obesity leads to hyperinsulinism, which causes both hyperandrogenaemia and raised IGF-1 levels, which augments the ovarian response to gonadotrophins. This implies that obesity may be important in the pathogenesis of polycystic ovaries, but further study is required to evaluate this. It is known that obesity is not a prerequisite for PCOS. After menarche, it is common for the menstrual cycle to be erratic for several months. If the irregularity persists beyond 2 years, there is a high chance that the adolescent girl has PCOS.
Investigations
Endocrine profile
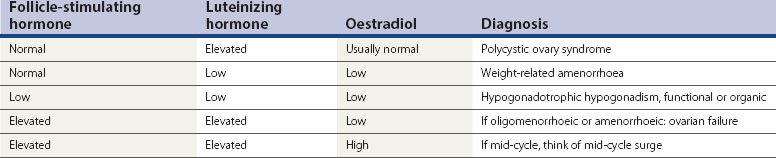
Women with PCOS usually have a normal serum FSH concentration (Table 18.2). LH is the second gonadotrophin which, like FSH, is released by the gonadotrophs in the anterior pituitary gland, under the influence of pulsatile release of gonadotrophin-releasing hormone (GnRH). The differential control of FSH and LH secretion relies upon the need for priming of the pituitary by oestradiol before it will become responsive to GnRH and release LH. FSH secretion, on the other hand, is under tonic inhibitory control by inhibin acting in a negative feedback loop from the ovaries. Therefore, in times of oestrogen deficiency, such as weight-related amenorrhoea, LH concentrations in the circulation are lower than FSH, whilst the mid-cycle surge that is primed by rising oestradiol secretion from the ovary results in a greater release of LH than FSH.
An elevated serum concentration of LH in the follicular phase of the cycle suggests that the patient has PCOS, usually associated with a concentration of more than 10 IU/l in the early to mid-follicular phase of the cycle. In a series of over 1700 women with PCOS, approximately 40% of patients were found to have an elevated serum concentration of LH, which was associated with a significantly higher risk of infertility than in those with normal LH levels (Balen et al 1995). Other causes of an elevated LH serum concentration are the mid-cycle surge and ovarian failure (Table 18.2).
Hyperinsulinaemia
The association between insulin resistance, compensatory hyperinsulinaemia and hyperandrogenism has provided insight into the pathogenesis of PCOS. The cellular and molecular mechanisms of insulin resistance in PCOS have been investigated extensively, and it is evident that the major defect is a decrease in insulin sensitivity secondary to a postbinding abnormality in insulin-receptor-mediated signal transduction, with a less substantial, but significant, decrease in insulin responsiveness (Dunaif 1997). It appears that decreased insulin sensitivity in PCOS is potentially an intrinsic defect in genetically susceptible women, since it is independent of obesity, metabolic abnormalities, body fat topography and sex hormone levels.
Although the insulin resistance may occur irrespective of body mass index (BMI), the common association between PCOS and obesity has a synergistic deleterious impact on glucose homeostasis, and can worsen both hyperandrogenism and anovulation. An assessment of BMI alone is not thought to provide a reliable prediction of cardiovascular risk. It has been reported that the association between BMI and coronary heart disease almost disappeared after correction for dyslipidaemia, hyperglycaemia and hypertension. Some women have profound metabolic abnormalities in the presence of a normal BMI, and others have few risk factors with an elevated BMI. It has been suggested that rather than BMI itself, it is the distribution of fat that is important, with android obesity being more of a risk factor than gynaecoid obesity. Hence the value of measuring the waist:hip ratio or waist circumference, which detect abdominal visceral fat rather than subcutaneous fat. It is the visceral fat which is metabolically active and, when increased, results in increased rates of insulin resistance, type 2 diabetes, dyslipidaemia, hypertension and left ventricular enlargement. There is a closer link between waist circumference and visceral fat mass, as assessed by computer tomography, than waist:hip ratio or BMI (Lord and Wilkin 2002). Waist circumference should ideally be less than 79 cm, whilst a measurement of greater than 87 cm carries a significant risk. Exercise has a significant effect on reducing visceral fat and reducing cardiovascular risk; indeed, a 10% reduction in body weight may equate to a 30% reduction in visceral fat.
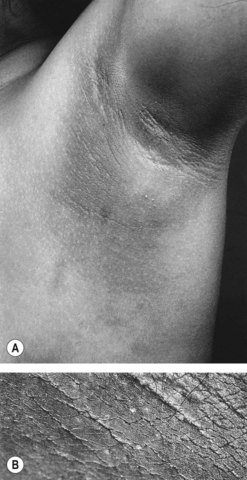
Insulin acts through multiple sites to increase endogenous androgen levels. Increased peripheral insulin resistance results in a higher serum insulin concentration. Excess insulin binds to IGF-1 receptors which enhances androgen production by theca cells in response to LH stimulation. Hyperinsulinaemia also decreases the synthesis of SHBG by the liver. Therefore, there is an increase in the serum free testosterone concentration, and consequent peripheral androgen action. Intraovarian androgen excess is responsible for anovulation by acting directly on the ovary, promoting the process of follicular atresia. This latter process is characterized by apoptosis of granulosa cells. As a consequence, there is an increasingly larger stromal compartment, which retains LH responsiveness and continues to secrete androgens. Hyperinsulinaemia also stimulates trophic changes in the skin that results in acanthosis nigricans in the skin creases (Figure 18.6).
Insulin resistance can be measured by a number of expensive and complex tests, but it is not necessary to measure it routinely in clinical practice; it is more important to check for impaired glucose tolerance. Simple screening tests for risk of impaired glucose tolerance (IGT) include an assessment of BMI and waist circumference. If the fasting blood glucose is less than 5.2 mmmol/l, the risk of impaired glucose tolerance is low. The 2-h standard 75 g oral glucose tolerance test may be conducted in those at high risk (BMI >30 kg/m2 in Caucasian women and >25 kg/m2 in women from South Asia, who have a greater degree of insulin resistance at a lower body weight) (Table 18.3).
Heterogeneity of PCOS
A few years ago, the author reported a large series of women with polycystic ovaries detected by ultrasound scan (Balen et al 1995). All of the 1871 patients had at least one symptom of PCOS. Thirty-eight percent of the women were overweight (BMI >25 kg/m2). Obesity was significantly associated with an increased risk of hirsutism, menstrual cycle disturbance and an elevated serum testosterone concentration. Obesity was also associated with an increased rate of infertility. Twenty-six percent of patients with primary infertility and 14% of patients with secondary infertility had a BMI of more than 30 kg/m2. Approximately 30% of the patients had a regular menstrual cycle, 50% had oligomenorrhoea and 20% had amenorrhoea. In this study, the classical endocrine features of raised serum LH and testosterone were found in 40% and 30% of patients, respectively. Ovarian volume was significantly correlated with serum LH and testosterone concentrations. Other studies have reported correlation between markers of insulin resistance and ovarian volume and ovarian stromal echogenicity, which in turn have been correlated with androgen production.
National and racial differences in expression
Approximately 75–80% of women with polycystic ovaries have signs or symptoms of PCOS. Thus, in the UK, where it has been reported that up to 33% of women have polycystic ovaries (Michelmore et al 1999), 20–25% of women may have a degree of PCOS, albeit mild in many cases. There are large national and ethnic variations in the expression of PCOS, with women from the Far East having little in the way of hirsutism, whilst those with dark hair from Mediterranean and Middle Eastern or South Asian countries have a greater degree of expression. The highest reported prevalence of polycystic ovaries has been 52% among South Asian immigrants in Britain, of whom 49% had menstrual irregularity (Rodin et al 1998). It was also shown that South Asian women with polycystic ovaries had a comparable degree of insulin resistance to controls with established type 2 diabetes. Generally, there has been a paucity of data of the prevalence of PCOS among women of South Asian origin, both among migrant and native groups. Type 2 diabetes and insulin resistance have a high prevalence among indigenous populations in South Asia, with a rising prevalence among women. Insulin resistance and hyperinsulinaemia are common antecedents of type 2 diabetes, with a high prevalence in South Asians. Type 2 diabetes also has a familial basis, inherited as a complex genetic trait that interacts with environmental factors, chiefly nutrition, commencing from fetal life. It has been shown that ethnic variations in the overt features of PCOS (symptoms of hyperandrogenism, menstrual irregularity and obesity) in women of South Asian descent are linked to the higher prevalence and degree of insulin resistance in South Asians. It has also been shown that South Asians with anovulatory PCOS have greater insulin resistance and more severe symptoms of PCOS than anovular White Caucasians with PCOS (Wijeyaratne et al 2002).
The question remains as to whether differences in expression of PCOS are due to dietary and lifestyle factors or to genetic variations in hormone actions, such as polymorphisms in gonadotrophin subunits or receptor function (affecting the expression of androgens, gonadotrophins or insulin). A full discussion of the genetics of PCOS is beyond the scope of this chapter, and there are a number of candidate genes that have been proposed (see Franks et al 2001). It may be that some families or racial groups have genetic differences that affect the expression or presentation of PCOS.
Health Consequences
Insulin resistance
In a large retrospective study, Pierpoint et al (1998) reported the mortality rate in 1028 women diagnosed with PCOS between 1930 and 1979. All of the women were over 45 years of age and 770 women had been treated by wedge resection of the ovaries. In total, 786 women were traced; the mean age at diagnosis was 26.4 years and the average duration of follow-up was 30 years. There were 59 deaths, of which 15 were from circulatory disease. Of these 15 deaths, 13 were from IHD. There were six deaths from diabetes as an underlying or contributory cause, compared with the expected 1.7 deaths. The standardized mortality rate (SMR), both overall and for cardiovascular disease, was not higher in the women with PCOS compared with the national mortality rates in women, although the observed proportion of women with diabetes as a contributory or underlying factor leading to death was significantly higher than expected [odds ratio 3.6, 95% confidence interval (CI) 1.5–8.4]. Thus, despite surrogate markers for cardiovascular disease, Pierpoint et al (1998) found no increased rate of death from cardiovascular disease. A follow-up report from the same study, however, did demonstrate an increased, although non-significant, risk of death due to diabetes [after adjustment for BMI, the odds ratio was 2.2 (95% CI 0.9–5.2) for diabetes (Wild et al 2000)]. There was still no increase in long-term coronary heart disease mortality in the PCOS group, although there was evidence of increased stroke-related mortality, even after adjustment for BMI.
Homocysteine
A moderately increased total plasma homocysteine (Hcy) concentration is associated with an increased risk of atherosclerosis. Hcy is an essential intermediate in the transfer of activated methyl groups from tetrahydrofolate to S-adenosylmethionine, in the synthesis of cysteine from methionine, and in the production of homocysteine thiolactone. An abnormal elevation of Hcy in plasma and urine is caused by an imbalance between Hcy production and metabolism, which can be of demographic, genetic, nutritional or metabolic aetiology, and is associated with premature vascular disease. Mild hyperhomocysteinaemia has been demonstrated to induce sustained injury to the arterial endothelial cells that accelerate the development of thrombosis and atherosclerosis. Normal concentrations of total plasma Hcy are in the range of 5–16 µmol/l, although 10 µmol/l is considered to be the desirable upper limit; there is an age-related rise and lower concentrations are found in women. There have been reports of elevated plasma Hcy in Caucasian women with PCOS, and this may be greater in the South Asian population (Rendeva et al 2002). Weight reduction and regular physical exercise are recognized interventions that help to reduce insulin resistance and the metabolic syndrome.
Endometrial cancer
Endometrial adenocarcinoma is the second most common female genital malignancy, but only 4% of cases occur in women under 40 years of age. The risk of developing endometrial cancer has been shown to be adversely influenced by a number of factors including obesity, long-term use of unopposed oestrogens, nulliparity and infertility. The relative risk of endometrial cancer is 1.6 in women with a menarche before the age of 12 years, and 2.4 in women who have their menopause after the age of 52 years (Elwood et al 1977). Women with endometrial carcinoma have had fewer births compared with controls, and it has also been demonstrated that infertility per se gives a relative risk of 2. Hypertension and type 2 diabetes have long been linked to endometrial cancer, with relative risks of 2.1 and 2.8, respectively; these conditions are now known to be associated with PCOS.
A study by Coulam et al (1983) examined the risk of developing endometrial carcinoma in a group of 1270 patients who were diagnosed with chronic anovulation syndrome. The defining characteristics of this group included pathological or macroscopic evidence of the Stein–Leventhal syndrome, or a clinical diagnosis of chronic anovulation. This study identified the excess risk of endometrial cancer to be 3.1 (95% CI 1.1–7.3), and proposed that this might be due to abnormal levels of unopposed oestrogen. However, the true risk of endometrial carcinoma in women with PCOS is difficult to ascertain.
Breast cancer
Obesity, hyperandrogenism and infertility occur frequently in PCOS, and are features known to be associated with the development of breast cancer. However, studies examining the relationship between PCOS and breast carcinoma have not always identified a significantly increased risk. The study by Coulam et al (1983) calculated a relative risk of 1.5 (95% CI 0.75–2.55) for breast cancer in their group of women with chronic anovulation, which was not statistically significant. After stratification by age, however, the relative risk was found to be 3.6 (95% CI 1.2–8.3) in the postmenopausal age group. More recently, Pierpoint et al (1998) reported SMRs calculated for patients with PCOS compared with the normal population. The SMR for all neoplasms was 0.91 (95% CI 0.60–1.32) and the SMR for breast cancer was 1.48 (95% CI 0.79–2.54). In fact, breast cancer was the leading cause of death in this cohort.
Ovarian cancer
A few studies have addressed the possibility of an association between polycystic ovaries and ovarian cancer. The results are conflicting, and generalizability is limited due to problems with the study designs. In the large UK study of Pierpoint et al (1998), the SMR for ovarian cancer was 0.39 (95% CI 0.01–2.17).
Management of Non-Fertility Aspects of Polycystic Ovaries
Psychological support and quality of life
The symptoms typically associated with the condition have also been shown to lead to a significant reduction in health-related quality of life (Jones et al 2004). Health-related quality of life is a multidimensional, dynamic concept that encompasses physical, psychological and social aspects that are associated with a particular disease or its treatment. Therefore, any management of the woman with PCOS needs to consider and understand the negative impact that this condition may have upon these psychosocial parameters. For example, although the management of hirsuitism may be considered as a purely cosmetic issue, excessive facial hair has been shown to be one of the major causes of marked psychological stress in women with PCOS, often caused by embarrassment about the excessive hair growth. Infertility and weight issues have also been found to affect other social and psychological parameters. Infertility can cause tensions within the family, altered self-perception and problems at work. Whilst obesity worsens the symptoms, the metabolic scenario conspires against weight loss; many women experience frustration in attempts to lose weight and suffer from low-esteem and poor body image.
Hyperandrogenism and hirsutism
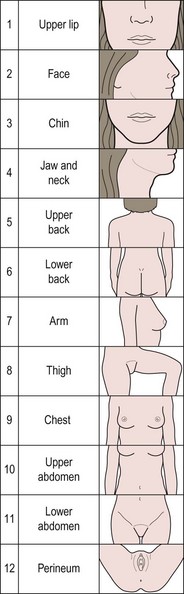
The bioavailability of testosterone is affected by the serum concentration of SHBG. High levels of insulin lower the production of SHBG and therefore increase the free fraction of androgen. Elevated serum androgen concentrations stimulate peripheral androgen receptors, resulting in an increase in 5-α reductase activity, directly increasing the conversion of testosterone to the more potent metabolite, dihydrotestosterone. Symptoms of hyperandrogenism include hirsutism and acne, which are both distressing conditions. Hirsutism is characterized by terminal hair growth in a male pattern of distribution, including chin, upper lip, chest, upper and lower back, upper and lower abdomen, upper arm, thigh and buttocks. A standardized scoring system, such as the modified Ferriman and Gallwey score, should be used to evaluate the degree of hirsutism before and during treatments (Figure 18.7).
Ovulation Induction
Ovulation induction has traditionally involved the use of clomiphene citrate and then gonadotrophin therapy or laparoscopic ovarian surgery in those who are clomiphene resistant. The principles of management of anovulatory infertility are firstly to optimize health before commencing therapy, for example weight loss for those who are overweight, and then induce regular unifollicular ovulation, while minimizing the risks of ovarian hyperstimualtion syndrome (OHSS) and multiple pregnancy (see Chapter 17, Ovulation induction, for more information).
Obesity reduces fertility, and increases the risk of miscarriage, some congenital anomalies and pregnancy-related complications, e.g. pre-eclampsia, gestational diabetes, problems during delivery, and maternal and fetal mortality (Balen et al 2006). Monitoring treatment is also harder in obese women because their ovaries are more difficult to see on ultrasound scans, thus raising the risk of missing multiple ovulation and multiple pregnancy. National guidelines in the UK for managing overweight women with PCOS advise weight loss, preferably to a BMI of less than 30 kg/m2, before commencing drugs for ovarian stimulation (Balen and Anderson 2007).
Obese women (BMI >30 kg/m2) should therefore be encouraged to lose weight. Clark et al (1995) investigated the effects of a weight loss and exercise programme on women with anovulatory infertility, clomiphene resistance and a BMI of more than 30 kg/m2. The emphasis of the study was a realistic exercise schedule combined with positive reinforcement of a suitable eating programme over a 6-month period. Weight loss had a significant effect on endocrine function, ovulation and subsequent pregnancy.
Clomiphene citrate
Antioestrogen therapy with clomiphene citrate or tamoxifen has traditionally been used as first-line therapy for anovulatory PCOS. A recent meta-analysis confirmed that clomiphene is effective in increasing pregnancy rates when compared with placebo as first-line therapy (fixed odds ratio 5.8, 95% CI 1.6–21.5; number needed to treat 5.9, 95% CI 3.6–16.7) (Beck et al 2005). Antioestrogen therapy is usually commenced on day 2 of the cycle and given for 5 days. If the patient has oligomenorrhoea or amenorrhoea, it is necessary to exclude pregnancy and then induce a withdrawal bleed with a short course of a progestogen, such as medroxyprogesterone acetate 5–20 mg/day for 5–10 days. The starting dose of clomiphene citrate is 50 mg/day for 5 days beginning on days 3–5 of the menstrual cycle. The dose of clomiphene citrate should only be increased if there is no response after three cycles, as of those women who will respond to 50 mg/day, only two-thirds will do so in the first cycle. Doses of 150 mg/day or more do not appear to be of benefit. If there is an over-response to 50 mg/day, as in some women with PCOS, the dose can be decreased to 25 mg/day.
Clomiphene citrate may cause an exaggeration in the hypersecretion of LH and have antioestrogenic effects on the endometrium and cervical mucus. All women who are prescribed clomiphene citrate should be carefully monitored with a combination of endocrine and ultrasonographic assessment of follicular growth and ovulation because of the risk of multiple pregnancies, which is approximately 10% (Figures 18.8 and 18.9). Clomiphene therapy should therefore be prescribed and managed by specialists in reproductive medicine.
Gonadotrophins
In order to prevent the risks of overstimulation and multiple pregnancy, the traditional standard step-up regimens (when 75–150 IU is increased by 75 IU every 3–5 days) have been replaced by low-dose step-up regimens. The low-dose step-up regimen employs a starting dose of 25–50 IU, which is only increased after 14 days if there is no response, and then by only half an ampoule every 7 days. Treatment cycles using this approach can be quite long (up to 28–35 days), but the risk of multiple follicular growth is lower than with conventional step-up regimens (Tarlatzis et al 2008) (see Chapter 17, Ovulation induction, for more information).
Insulin-sensitizing agents
It is logical to assume that therapy that achieves a fall in serum insulin concentrations should improve the symptoms of PCOS. The biguanide metformin inhibits the production of hepatic glucose, thereby decreasing insulin secretion, and also enhances insulin sensitivity at the cellular level. Early studies involved a small number of participants and were encourgaing, whereas more recent large studies looking at the use of metformin either alone or combined with clomiphene citrate have failed to demsonstrate significant benefit with respect to weight reduction, improved menstrual cycle function or livebirth rates (Moll et al 2006, Tang et al 2006, Legro et al 2007).
In summary, although the initial studies appeared promising, subsequent large randomized-controlled trials have not demonstrated the anticipated beneficial effects of metformin as a first-line therapy for treatment of the anovulatory patient with PCOS. The study authors concur that the primary aim for overweight women with PCOS is to make lifestyle changes with a combination of diet and exercise in order to lose weight and to improve ovarian function. In conclusion, the position of metformin in the management of PCOS is by no means clear and, on the basis of current evidence, it is not the first-line treatment of choice (Tarlatzis et al 2008).
Surgical ovulation induction
After laparoscopic ovarian surgery, serum concentrations of LH and testosterone fall with restoration of ovarian activity. Whether or not patients respond to laparoscopic ovarian diathermy (LOD) appears to depend on their pretreatment characteristics, with patients with high basal LH concentrations having a better clinical and endocrine response. Ovarian diathermy appears to be as effective as routine gonadotrophin therapy in the treatment of clomiphene-citrate-insensitive PCOS, and the Cochrane Database concludes that whilst there is insufficient evidence to demonstrate a difference between 6–12 months follow-up after LOD and three to six cycles of ovulation induction with gonadotrophins, multiple pregnancy rates are considerably reduced with LOD (Farquhar et al 2002). The largest randomized controlled trial to date is a multicentre study performed in The Netherlands in which 168 patients, resistant to clomiphene citrate, were randomized to either LOD (n = 83) or ovulation induction with recombinant FSH (rFSH, n = 65) (Bayram et al 2004). The initial cumulative pregnancy rate after 6 months was 34% in the LOD arm vs 67% in the rFSH arm. Those who did not ovulate in response to LOD were first given clomiphene citrate and then rFSH, so by 12 months, the cumulative pregnancy rate was similar in each group at 67%. Thus, those treated with LOD took longer to conceive, and 54% required additional medical ovulation induction therapy.
Laparoscopic ovarian diathermy is associated with a low risk of multiple pregnancy and so does not need the same intensity of monitoring as the use of drugs to stimulate ovulation. Indications for LOD are listed in Table 18.4. A strategy for ovulation induction for women with PCOS is outlined in Table 18.5.
Table 18.4 Indications for laparoscopic ovarian diathermy (LOD)
Table 18.5 Strategy for ovulation induction in anovulatory polycystic ovary syndrome
| Slim patient | Overweight patient (body mass index >30 kg/m2) |
|---|---|
IVF, in-vitro fertilization; LOD, laparoscopic ovarian diathermy.
Conclusions
KEY POINTS
Balen AH. Polycystic ovary syndrome and cancer. Human Reproduction Update. 2001;7:522-525.
Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Human Reproduction. 1995;10:2107-2111.
Balen AH, Michelmore K. What is polycystic ovary syndrome? Are national views important? Human Reproduction. 2002;17:2219-2227.
Balen AH, Laven JSE, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Human Reproduction Update. 2003;9:505-514.
Balen AH, Anderson R. Impact of obesity on female reproductive health: British Fertility Society policy and practice guidelines. Human Fertility. 2007;10:195-206.
Balen AH, Dresner M, Scott EM, Drife JO. Should obese women with polycystic ovary syndrome (PCOS) receive treatment for infertility? BMJ. 2006;332:434-435. (Clinical Research Ed.)
Bayram N, van Wely M, Kaaijk EM, Bossuyt PMM, van der Veen F. Using an electrocautery strategy or recombinant FSH to induce ovulation in polycystic ovary syndrome: a randomised controlled trial. BMJ. 2004;328:192-195. (Clinical Research Ed.)
Beck JI, Boothroyd C, Proctor M, Farquhar C, Hughes E 2005 Oral anti-oestrogens and medical adjuncts for subfertility associated with anovulation. Cochrane Database of Systematic Reviews 1:CD002249.
Clark AM, Ledger W, Galletly C, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Human Reproduction. 1995;10:2705-2712.
Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstetrics and Gynecology. 1983;61:403-407.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanisms and implication for pathogenesis. Endocrine Review. 1997;18:774-800.
Elwood JM, Cole P, Rothman KJ, et al. Epidemiology of endometrial cancer. Journal of the National Cancer Institute. 1977;59:1055-1060.
Farquhar C, Vandekerckhove P, Lilford R. Laparoscopic ‘drilling’ by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews. 1, 2002.
Fauser B, Tarlatzis B, Chang J, et al. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reproduction. 2004;19:41-47.
Franks S, Gharani N, McCarthy M. Candidate genes in polycystic ovary syndrome. Human Reproduction Update. 2001;7:405-410.
Jones GL, Benes K, Clark TL, et al. The polycystic ovary syndrome health-related quality of life questionnaire (PCOSQ): a validation. Human Reproduction. 2004;19:317-377.
Kousta E, White DM, Franks S. Modern use of clomifene citrate in induction of ovulation. Human Reproduction Update. 1997;3:359-365.
Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. New England Journal of Medicine. 2007;356:551-566.
Lord J, Wilkin T. Polycystic ovary syndrome and fat distribution: the central issue? Human Fertility. 2002;5:67-71.
Michelmore KF, Balen AH, Dunger DB, Vessey MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clinical Endocrinology. 1999;51:779-786.
Moll E, Bossuyt PMM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ. 2006;332:1485-1488. (Clinical Research Ed.)
Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. Journal of Clinical Epidemiology. 1998;51:581-586.
Rajkowha M, Glass MR, Rutherford AJ, Michelmore K, Balen AH. Polycystic ovary syndrome: a risk factor for cardiovascular disease? British Journal of Obstetrics and Gynaecology. 2000;107:11-18.
Rendeva HS, Lewandowski KC, Drzewoski J, et al. Exercise decreases plasma total homocysteine in overweight young women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2002;87:4496-4501.
Rodin DA, Bano G, Bland JM, Taylor K, Nussey SS. Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Clinical Endocrinology. 1998;49:91-99.
Sampaolo P, Livien C, Montanari L, Paganelli A, Salesi A, Lorini R. Precocious signs of polycystic ovaries in obese girls. Ultrasound Obstetrics and Gynaecology. 1994;4:1-6.
Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined life-style modification and metformin in obese patients with polycystic ovary syndrome (PCOS). A randomised, placebo-controlled, double-blind multi-centre study. Human Reproduction. 2006;21:80-89.
Tarlatzis BC, Fauser BCJM, Chang J, et al. Consensus on infertility treatment related to polycystic ovary syndrome. Human Reproduction. 2008;23:462-477.
Wijeyaratne CN, Balen AH, Barth J, Belchetz PE. Polycystic ovary syndrome in south Asian women: a case control study. Clinical Endocrinology. 2002;57:343-350.
Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clinical Endocrinology. 2000;52:595-600.