Chapter 405 Pneumothorax
Pneumothorax is the accumulation of extrapulmonary air within the chest, most commonly from leakage of air from within the lung. Air leaks can be primary or secondary and can be spontaneous, traumatic, iatrogenic, or catamenial (Table 405-1). Pneumothorax in the neonatal period is also discussed in Chapter 95.12.
Table 405-1 CAUSES OF PNEUMOTHORAX IN CHILDREN
SPONTANEOUS
TRAUMATIC
* Associated with renal agenesis, diaphragmatic hernia, amniotic fluid leaks.
From Kuhn JP, Slovis TL, Haller JO: Caffey’s pediatric diagnostic imaging, vol 1, ed 10, Philadelphia, 2004, Mosby.
Etiology and Epidemiology
A primary spontaneous pneumothorax occurs without trauma or underlying lung disease. Spontaneous pneumothorax with or without exertion occurs occasionally in teenagers and young adults, most frequently in males who are tall, thin, and thought to have subpleural blebs. Familial cases of spontaneous pneumothorax occur and have been associated with mutations in the folliculin gene. Patients with collagen synthesis defects, such as Ehlers-Danlos disease (Chapter 651) and Marfan syndrome (Chapter 693) are unusually prone to the development of pneumothorax.
A pneumothorax arising as a complication of an underlying lung disorder but without trauma is a secondary spontaneous pneumothorax. Pneumothorax can occur in pneumonia, usually with empyema; it can also be secondary to pulmonary abscess, gangrene, infarct, rupture of a cyst or an emphysematous bleb (in asthma), or foreign bodies in the lung. In infants with staphylococcal pneumonia, the incidence of pneumothorax is relatively high. It is found in ≈5% of hospitalized asthmatic children and usually resolves without treatment. Pneumothorax is a serious complication in cystic fibrosis (CF; Chapter 395). Pneumothorax also occurs in patients with lymphoma or other malignancies, and in graft versus host disease with bronchiolitis obliterans.
Diagnosis and Differential Diagnosis
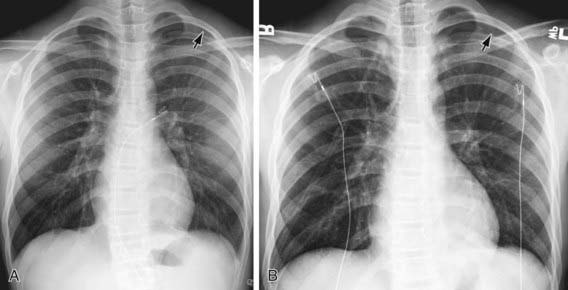
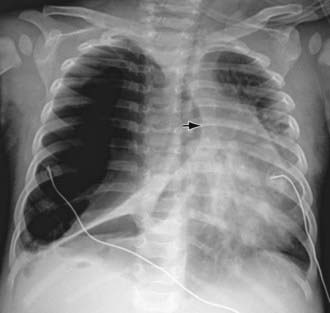
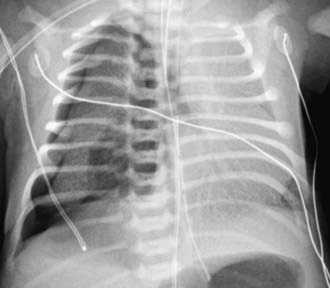
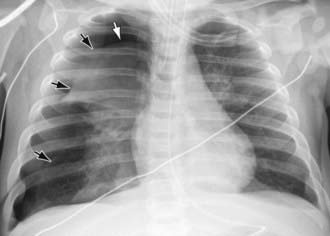
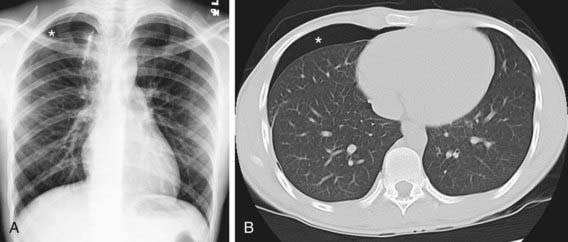
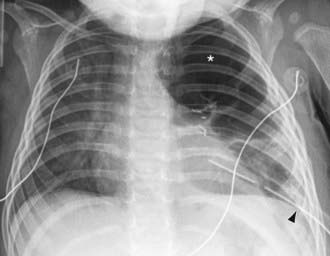
The diagnosis of pneumothorax is usually established by radiographic examination (Figs. 405-1 to 405-6). The amount of air outside the lung varies with time. A radiograph that is taken early shows less lung collapse than one taken later if the leak continues. Expiratory views accentuate the contrast between lung markings and the clear area of the pneumothorax (see Fig. 405-1). When the possibility of diaphragmatic hernia is being considered, a small amount of barium may be necessary to demonstrate that it is not free air but is a portion of the gastrointestinal tract that is in the thoracic cavity. Ultrasound can also be used to establish the diagnosis.
It may be difficult to determine whether a pneumothorax is under tension. Evidence of tension includes shift of mediastinal structures away from the side of the air leak. A shift may be absent in situations in which the other hemithorax resists the shift, such as in the case of bilateral pneumothorax. When the lungs are both stiff, such as in CF or respiratory distress syndrome, the unaffected lung may not collapse easily and shift may not occur (see Fig. 405-3). On occasion, the diagnosis of tension pneumothorax is made only on the basis of evidence of circulatory compromise or on hearing a “hiss” of rapid exit of air under tension with the insertion of the thoracostomy tube.
Agarwal R, Aggarwal AN, Gupta D, et al. Efficacy and safety of iodopovidone in chemical pleurodesis: a meta-analysis of observational studies. Respir Med. 2006;100:2043-2047.
Amin R, Noone PG, Ratien F: Chemical pleurodesis versus surgical intervention for persistent and recurrent pneumothoraces in cystic fibrosis, Cochrane Database Syst Rev (2):CD007481, 2009.
Bialas RC, Weiner TM, Phillips JD. Video-assisted thoracic surgery for primary spontaneous pneumothorax in children: is there an optimal technique? J Pediatr Surg. 2008;43:2151-2155.
Chen JS, Hsu HH, Tsai KT, et al. Salvage for unsuccessful aspiration of primary pneumothorax: thoracoscopic surgery or chest tube drainage? Ann Thorac Surg. 2008;85:1908-1913.
Miller MP, Sagy M. Pressure characteristics of mechanical ventilation and incidence of pneumothorax before and after the implementation of protective lung strategies in the management of pediatric patients with severe ARDS. Chest. 2008;134:969-973.
Posner K, Needleman J. Pneumothorax. Pediatr Rev. 2008;29:69-70.
Ren HZ, Zhu CC, Yang C, et al. Mutation analysis of the FLCN gene in Chinese patients with sporadic and familial isolated primary spontaneous pneumothorax. Clin Genet. 2008;74:178-183.
Treasure T. Minimal access surgery for pneumothorax. Lancet. 2007;370:294-295.
Zehtabchi S, Rios CL. Management of emergency department patients with primary spontaneous pneumothorax: needle aspiration or tube thoracostomy? Ann Emerg Med. 2008;51:91-100.