Chapter 103 Pneumatic Retinopexy
Introduction
For more than a half century, the operation most favored for primary retinal detachment had been scleral buckling (SB). Between 75% and 88% of cases attain permanent reattachment with one operation with this procedure.1–3 However, it frequently results in tissue trauma, complications,4 relatively high expense, and use of a hospital or surgicenter operating room. In recent decades, pars plana vitrectomy has been used with increasing frequency for primary retinal detachments, particularly in pseudophakic eyes. This technique also presents its list of potential complications, and also requires an operating room with similar high expense.
Pneumatic retinopexy (PR) was developed in an attempt to minimize these problems. This outpatient procedure for retinal reattachment consists of an intravitreal gas injection with transconjunctival cryopexy or laser photocoagulation, followed by appropriate head positioning.5 No incisions are required. PR is substantially less expensive than scleral buckling or vitrectomy and has become widely accepted as the treatment of choice for selected retinal detachments, with the vast majority of vitreoretinal surgeons employing this procedure.6,7
A multicenter, randomized, controlled clinical trial has demonstrated that the anatomic success rate of PR is comparable to SB, with less morbidity and with significantly better visual results than with SB.8 Cataract surgery was required more often following SB than following PR.9 PR should be considered in patients who do not have detached inferior breaks, extensive retinal breaks, or significant proliferative vitreoretinopathy.
History
Ohm10 performed the first intravitreal air injection for retinal detachment in 1911. In 1938, Rosengren11 reported the use of intravitreal air with drainage of subretinal fluid in a series of 256 retinal detachments. In 1973, Norton12 reported intravitreal sulfur hexafluoride (SF6) injection used with SB or vitrectomy for various surgical problems, such as giant breaks, large posterior breaks, and fishmouthing. Blodi and Folk13 treated detachments due to a macular hole using intravitreal gas, and retinal detachments treated with “repeated insufflations of expansive gas” were described by Dominguez et al.14,15 Hilton and Grizzard5 introduced the term “pneumatic retinopexy” at the 1985 meeting of the American Academy of Ophthalmology, a procedure using only transconjunctival cryotherapy and gas injection without conjunctival incision.
Unlike SB or vitrectomy, PR affords no permanent relief of vitreoretinal traction. The fact that detachments can be cured without permanently relieving traction, demonstrated decades before by Ohm10 and by Rosengren,11 was reconfirmed in 1979 by Lincoff et al.,16 who introduced the use of a balloon to achieve temporary scleral buckling. After cryopexy to the tear, they inserted a deflated balloon directly over the tear through a conjunctival and Tenon’s capsule incision, inflated the balloon, and left it in place for a week. The efficacy of this procedure was demonstrated but it never caught on, and the orbital balloon is not commercially available worldwide. However, the principle of repairing a retinal detachment without permanently relieving vitreoretinal traction, well proven by Lincoff’s technique, combined with the demonstrated safety of intravitreal gas, formed the basis for PR.
In 1989, Tornambe and Hilton8 co-directed a multicenter, randomized, controlled clinical trial comparing PR with SB. Tornambe17 reviewed over 200 studies on PR, including statistical reports on over 1300 cases. The single-operation success rate in these combined series was 80%, increasing to 98% after reoperations18 – results which have been subsequently replicated.19 Chan et al.20 reviewed all published reports on PR cases from 1986 to 2007, totalling 4138 eyes. Single-operation success was 74.4%, and success with one or more operations was 96.1%.
Basic principles
Intraocular gases
Sulfur hexafluoride (SF6) and perfluoropropane (C3F8) are the gases most frequently used with PR. Success also has been reported with sterile room air.21 In 1993, the United States Food and Drug Administration approved certain SF6 and C3F8 products for use in PR.
The value of the intraocular bubble is based on three features: buoyancy, surface tension, and isolation of retinal tears from intraocular currents.22,23 Buoyancy applies upward pressure on the detached retina. The surface tension of the bubble closes the retinal break and prevents the bubble from passing into the subretinal space. With the break closed, the retinal pigment epithelial pump removes the subretinal fluid.
Because of their low solubility in water, SF6 and C3F8 tend to diffuse from the eye very slowly. However, the nitrogen and oxygen that are in solution in the surrounding tissues of the eye are much more soluble and pass relatively quickly into the gas bubble, following the law of partial pressures. The net result is the initial expansion of a bubble of pure SF6 or C3F8 within the vitreous, followed by gradual resorption. Characteristics of gas expansion and resorption for SF6, C3F8, and air are listed in Table 103.1.
SF6 and C3F8 are chemically inert, colorless, odorless, and nontoxic.24 SF6 has been studied extensively in experimental animals and has been found to be nontoxic as judged by electrophysiologic testing and electron microscopy.25 One study of rabbits concluded that “eyes only partially filled with C3F8 showed no permanent damage, with a total recovery of the cortical matrix, gel, and liquid vitreous.”26 Early concerns27 regarding PVR from intravitreal gas injection have not been substantiated. A 0.22 µm Millipore filter is sufficient to render gas sterile.25
Lincoff et al.28 noted that gas bubble contact with the crystalline lens can produce cataract after several days, but this is avoidable by appropriate positioning. Mougharbel et al.29 have demonstrated with Scheimpflug photography that PR does not cause cataract.
Retina–gas interface
Larger areas of tamponade require disproportionate increases in bubble volume. A 0.3 mL gas bubble in humans covers more than 45° of arc of the retina, but it takes approximately a 1.2 mL bubble to cover 80–90°.5 Because surface tension causes the gas to take on a relatively spherical contour, particularly with smaller bubbles, the extent of retina–gas contact is significantly less than that published from studies of model eyes.30 To cover the same arc of the retina, a highly myopic eye requires a larger volume of gas than an emmetropic eye. These factors are considered in deciding how much gas to inject.
Case selection
The multicenter clinical trial excluded cases with the following characteristics:8
1. Breaks larger than 1 clock-hour or multiple breaks extending over more than 1 clock-hour of the retina.
2. Breaks in the inferior 4 clock-hours of the retina.
3. Presence of PVR grade C or D (Retina Society Terminology Committee, 1983).31
4. Physical disability or mental incompetence precluding maintenance of the required positioning.
A review of 1000 consecutive detachments5 revealed that 41% met these limited criteria. Subsequent experience has demonstrated that selected cases that do not strictly meet these criteria can also be successfully treated with PR.32,33 In our hands, about half of all primary retinal detachments are treated with PR.
Extent of breaks
Breaks spanning more than 1 clock-hour can be treated with PR. Single or multiple tears spanning up to 3 clock-hours pose no particular problem. Detached tears 6 clock-hours apart are difficult to fix with PR, although alternating positioning has been used successfully. Even detachments with giant retinal tears have been cured with PR.34 However, scleral buckling or vitrectomy is usually preferred for detachments with widely separated or giant tears.
Inferior breaks
Most cases involving breaks in the inferior 4 clock-hours of the eye have been difficult to treat with PR, despite various attempts.33 Even for flexible patients, it can be difficult to tilt the head below the horizontal plane for extended periods. Friberg and Eller35 first reported success with inferior breaks in eight of eight patients using dependent positioning. In 2003, Chang et al.36 reported single operation success in 9 of 11 patients with inferior breaks using PR with inverted positioning for only eight hours. Hilton et al.37 treated retinal detachment with inferior breaks by augmenting pneumatic retinopexy with in-office partial vitrectomy, allowing injection of a large enough gas bubble to close the breaks with side positioning. In 2011, Hwang et al.38 reported 10 out of 13 single-operation success with a 10–30 cm downward head tilt.
Lattice degeneration
In several studies, the presence of lattice degeneration did not adversely affect single operation success with PR,17,39 but in other studies, extensive lattice degeneration tended to decrease the chance of success with PR. It does not appear that mild to moderate lattice should be considered a contraindication.
Aphakia and pseudophakia
Retinal detachments in aphakic/pseudophakic eyes have a poorer prognosis than phakic eyes, no matter what surgery is performed.9 In some series, aphakic eyes did poorly with PR,40 especially if the posterior capsule was open,41 but in other reports this was not the case.8,9 Aphakic and pseudophakic eyes are prone to multiple, tiny, far-peripheral breaks and require an especially careful preoperative examination. In pseudophakic eyes, the view of the peripheral retina can be quite limited if much peripheral capsular opacity is present. PR should probably not be performed in these cases unless retinal detachment is rather limited. In our opinion, if the peripheral retina can be adequately examined, aphakia or pseudophakia is not a contraindication to PR.
Posterior vitreous detachment
Phakic eyes with complete posterior vitreous detachment tend to have a higher success rate with PR than those with partial vitreous detachment.42
Cases where pneumatic retinopexy presents a particular advantage
Compared with SB, PR is especially advantageous in the management of the following six situations:
1. Macular breaks and other posterior retinal breaks. Posterior retinal breaks are difficult to treat with SB, so PR is the procedure of choice in many of these cases, especially in phakic eyes. It also has been reported as an effective option in the treatment of optic pits with macular detachment.43,44
2. Redetachment or persistent detachment after scleral buckling. When subretinal fluid accumulates or persists because of a superior break after SB, PR may be much easier to perform than buckle revision.45 This is especially effective if the break is located on or anterior to the buckle.46
3. Isolated tears under the superior rectus. Placing a segmental buckle under a vertically acting muscle runs the risk of iatrogenic diplopia; this is eliminated with PR.
4. Filtering blebs. If a functioning filtering bleb is present, or if a filtering procedure may be necessary in the future, PR should be considered.
5. Impending macular detachment. Because PR can be performed promptly in the office without the delays required to prepare a patient for the operating room, and because the gas bubble can be used proactively to move the fluid away from the macula, a macula at imminent risk of detachment may be best served by PR.
6. Bullous detachment. When retinal detachment is highly bullous, retinal tears can be difficult to localize and treat with SB, a problem which is avoided by two-session PR.
Surgical technique
One-session versus two-session procedure
See Box 103.1 for indications for the one-session and two-session procedures.
Cryopexy versus laser
If a laser indirect ophthalmoscope (LIO) is not available, tears in the far periphery may not be treatable with laser delivered by slit lamp, necessitating cryopexy. The LIO is ideal for PR because it allows treatment of the far periphery and facilitates maneuvering of the gas bubble out of the way.35 Although most tears can be treated with laser through a slit-lamp delivery system by tilting the patient’s head as necessary to move the bubble away from the tear, this is difficult with tears from 11 to 1 o’clock. It is often possible to apply laser treatment through a large gas bubble, but it can be difficult with the smaller bubbles utilized with PR because of optical factors. Application of laser through gas causes concentration of heat due to the insulating effect of gas, and excessively hot burns may result.
Chorioretinal adhesion may be quicker and firmer using laser rather than cryopexy.47,48 In addition, some authors believe that cryopexy may be associated with a higher incidence of proliferative vitreoretinopathy and other complications,49,50 although others could find no such association.51 Laser also has the advantage of less morbidity compared with cryopexy, especially if multiple or large breaks are present.
Transscleral diode laser photocoagulation offers some potential advantages over both cryopexy and transpupillary laser for retinopexy treatment.52 Like transpupillary laser photocoagulation, one can see exactly where the treatment has been and will be applied. As with cryopexy, it allows treatment in detached retina. Treatment can even be applied through a pre-existing buckle.
Amount and type of gas to inject
We believe that it is optimal for the gas bubble to cover the break(s) for 5 days and then disappear as soon as possible. However, successful reattachment has been reported after as little as 6–8 hours of tamponade, as with inverted positioning for inferior tears.36 The longevity of an air bubble is probably sufficient for most cases, but occasionally the chorioretinal adhesion may not be sufficiently mature when the air has resorbed. The use of air also forfeits the advantage of postinjection bubble expansion within the eye, necessitating injection of a larger volume, but it offers the advantages of decreased expense and universal availability.
Sterilization of the ocular surface
Meticulous attention to sterile technique is mandatory. Sterilization of the ocular surface requires topical antiseptics; antibiotics are inadequate. We recommend povidone–iodine solution,53 and we do not recommend other preparations that contain detergent or alcohol.
A sterile lid speculum is utilized. Several drops of povidone–iodine solution are instilled directly onto the conjunctiva and left in contact with the eye while the gas is prepared. The injection site is then dried with a sterile cotton-tipped applicator, and the eye is ready for paracentesis and injection of gas. With these precautions, the occurrence of endophthalmitis after PR is extremely uncommon, with very few reported cases in the literature.54,55
Preparation of the gas
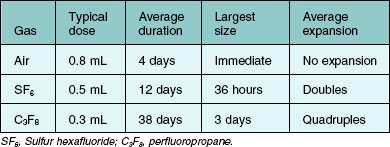
A pressure-reducing system is attached to the gas cylinder to allow drawing of the gas from a low-pressure system. High pressure can blow out the Millipore filter and render it useless in sterilizing the gas.56 A condom catheter can be attached to the cylinder, as shown in Figure 103.1, or a step-down valve system can be used. Alternatively, the gas can be drawn into a large syringe and then transferred to a small syringe.
The selected gas passes through a Millipore filter into a 3 mL syringe in sterile fashion. The tube connecting the gas cylinder with the syringe, including the filter, is flushed through with gas to ensure no dilution with room air. The syringe is filled with a few milliliters of gas and the gas is discarded. The syringe is then refilled. Passive filling of the syringe ensures that room air is not drawn in. A disposable 30-gauge, 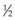 -inch (12 mm) needle is then placed tightly on the syringe, and excess gas is expelled to leave the exact amount intended for injection. The gas should not be stored in the syringe for more than a few minutes before injection because room air infiltrates the syringe and dilutes the gas sample.57
-inch (12 mm) needle is then placed tightly on the syringe, and excess gas is expelled to leave the exact amount intended for injection. The gas should not be stored in the syringe for more than a few minutes before injection because room air infiltrates the syringe and dilutes the gas sample.57
Performing a paracentesis
Although gas can be injected before paracentesis, we generally recommend a paracentesis first. Paracentesis performed after gas injection can result in gas in the anterior chamber. Preinjection paracentesis helps determine how much gas to inject and avoids marked intraocular pressure elevations. Dehiscence of a recent clear corneal cataract incision has been reported following PR when preinjection paracentesis was not performed.58
These recommendations are contrary to the practice of most retina surgeons, 62% of whom perform the paracentesis following injection, with the same percentage preferring C3F8 over SF6.59
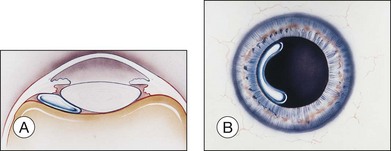
For a paracentesis, we use a 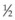 -inch, 30-gauge needle mounted on a 1 mL syringe without a plunger. After sterilization of the ocular surface, the needle is passed obliquely through the limbus into the anterior chamber, with the needle tip kept over the peripheral iris to avoid touching the lens. This maneuver may be facilitated by forcing aqueous toward the angle by applying gentle pressure on the center of the cornea with a cotton-tipped applicator (Fig. 103.2). However, care must be taken to avoid contact between the cornea and the lens. Instead we prefer applying pressure with the cotton-tipped applicator at the equator of the eye, facilitating maximum aqueous removal.
-inch, 30-gauge needle mounted on a 1 mL syringe without a plunger. After sterilization of the ocular surface, the needle is passed obliquely through the limbus into the anterior chamber, with the needle tip kept over the peripheral iris to avoid touching the lens. This maneuver may be facilitated by forcing aqueous toward the angle by applying gentle pressure on the center of the cornea with a cotton-tipped applicator (Fig. 103.2). However, care must be taken to avoid contact between the cornea and the lens. Instead we prefer applying pressure with the cotton-tipped applicator at the equator of the eye, facilitating maximum aqueous removal.
Injection of gas
With the ocular surface still sterile and the patient supine, the head and the eye are turned a total of approximately 45° to one side to place the pars plana injection site uppermost. The gas usually is injected temporally unless the pars plana epithelium is detached or large retinal breaks are present in that area, in which case another site is selected. The injection is made 3–4 mm posterior to the limbus with a 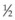 -inch (12 mm), 30-gauge needle. The needle is directed toward the center of the vitreous and inserted to a depth of 7 or 8 mm to ensure penetration of the pars plana epithelium and the anterior hyaloid face. It is then partially withdrawn so that approximately 9 mm of the needle shaft is seen outside the eye, leaving only 3 mm of the needle tip inside the globe. With the injection site uppermost and the needle vertical, the gas is injected moderately briskly. This technique creates one single bubble at the needle tip (Fig. 103.3C) rather than multiple small bubbles, often referred to as “fish eggs” (see below).
-inch (12 mm), 30-gauge needle. The needle is directed toward the center of the vitreous and inserted to a depth of 7 or 8 mm to ensure penetration of the pars plana epithelium and the anterior hyaloid face. It is then partially withdrawn so that approximately 9 mm of the needle shaft is seen outside the eye, leaving only 3 mm of the needle tip inside the globe. With the injection site uppermost and the needle vertical, the gas is injected moderately briskly. This technique creates one single bubble at the needle tip (Fig. 103.3C) rather than multiple small bubbles, often referred to as “fish eggs” (see below).
Assessing intraocular pressure
Despite preinjection paracentesis, the central retinal artery sometimes closes after gas injection. Several studies suggest that the retina can tolerate 60 minutes without blood flow as a result of elevated pressure.26,61–65 If the artery does not reopen within about 10 minutes, intraocular pressure should be lowered by repeat paracentesis.
While waiting for the central retinal artery to reopen, intermittent ocular compression can hasten the return to normal intraocular pressure. A scleral depressor is pressed against the lateral aspect of the eye so that the eye is compressed against the medial orbital wall. This raises the intraocular pressure, augmenting fluid egress and stretching the scleral wall.66 This is not recommended in eyes which have had recent surgery or penetrating trauma.
When checking intraocular pressure in a gas-filled eye, it is important to remember that indentation tonometry (e.g., Schiøtz) can give falsely low pressure readings, necessitating an appropriate correction factor.67 With a 1 mL gas bubble volume, the error of Schiøtz tonometry is approximately 8 mmHg for intraocular pressures in the range of 10–20 mmHg and approximately 15 mmHg for intraocular pressures in the range of 30–40 mmHg. Applanation tonometry is preferred.
Special procedures
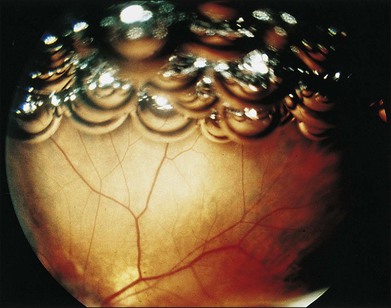
Fish eggs
Multiple small intravitreal gas bubbles, or “fish eggs”18 (Fig. 103.4), can result in gas getting under the retina, particularly in the presence of large retinal breaks. This occurrence is generally preventable by performing the following steps, in order of importance (Fig. 103.5):
1. Make sure that the needle tip is placed shallowly within the vitreous at the time of injection.
2. Make sure that the injection site is uppermost.
3. Inject moderately briskly, not too briskly, nor too slowly.
Gas entrapment at the injection site
After gas injection, the head is turned to the opposite side and mobility of the gas is confirmed ophthalmoscopically. If the gas bubble remains trapped at the injection site, it is probably trapped in the canal of Petit (between the anterior hyaloid, the lens and zonules, and the pars plana epithelium). Typically, the gas is visible peripherally behind the lens, forming a partial ring, variously described as the “bagel,” “donut,” or “sausage” sign (Fig. 103.6).
Waiting for trapped gas to break free may jeopardize an attached macula imminently threatened by detachment. A trapped bubble can be removed by passing a 27-gauge needle back through the injection site. This needle is mounted on a syringe without the plunger, containing a small amount of sterile saline solution. The injection site is positioned uppermost and the needle passed vertically into the bubble. Sometimes it takes a little manipulation to break the surface tension of the bubble and get it to escape. Most of the gas will escape, bubbling up through the fluid in the syringe. At another site, the gas is reinjected deeper into the vitreous, with 4–5 mm of the needle in the globe. This procedure also may be indicated if trapped gas does not become mobile after face-down positioning, which may constitute the rare and potentially painful occurrence of gas trapped beneath the pars plana epithelium or in the choroid.68,69
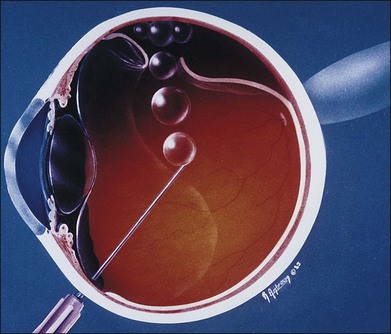
Steamroller
If bullous subretinal fluid extends almost to an attached macula, placement of a bubble against the bullous detachment may push fluid into the macula and cause it to detach.70 This complication, as well as the impending threat of spontaneous macular detachment, usually can be avoided by the “steamroller” technique (Fig. 103.7).60
Possible indications for steamrolling include the following:
1. Prevention of iatrogenic macular detachment
2. Prevention of iatrogenic detachment of an attached retinal break
3. Reduction of a bullous detachment overhanging the optic nerve, preventing visualization of the central retinal artery during the procedure
4. Reduction of subretinal fluid to encourage more rapid resolution of retinal detachment. This might be of use in cases where all retinal breaks cannot be covered at one time by the gas bubble
5. Avoidance of posterior gaping of the tear and subretinal gas in the presence of large retinal breaks. The extreme example of this is a somersault maneuver performed to unroll the inverted flap of a giant retinal tear.
The movement of subretinal fluid into the vitreous has raised theoretical concerns about the production of PVR. However, there were no cases of PVR in the 19 eyes that were managed by the steamroller technique in a multicenter clinical trial.71 Yanyali et al.72 similarly, did not observe a higher rate of PVR with steamrolled cases versus basic PR technique.
Summary of procedure
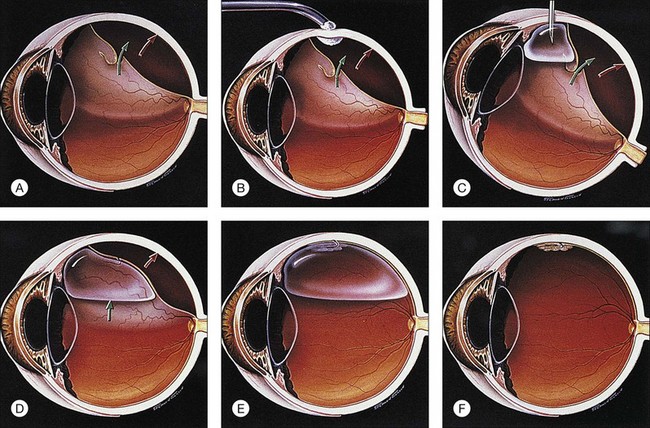
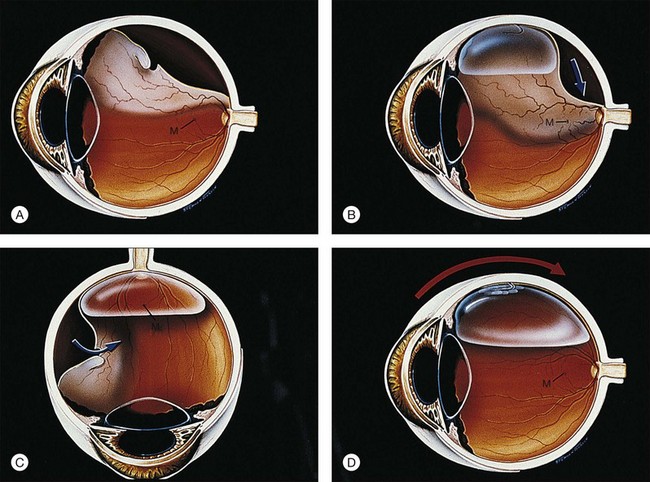
The following constitutes the sequence of a typical pneumatic retinopexy procedure (Fig. 103.3):
1. Anesthetic: topical/subconjunctival or retrobulbar
2. Cryopexy: if one-session procedure, in lieu of laser
3. Sterilization of ocular surface: povidone–iodine solution
4. Paracentesis: limbal, or via pars plana if capsule is open
5. Intravitreal gas injection: 0.4–0.6 mL of SF6
6. Second paracentesis and/or ocular compression: as needed to open artery
7. Special procedures: e.g., steamroller if needed (cryopexy should not be performed before steamroller)
8. Antibiotic and patch: draw arrow
9. Laser: next day or when retina is reattached (in lieu of cryopexy as two-session procedure) with 360° laser if desired.
Postoperative management
The patient is usually examined in the office on the 1st or 2nd postoperative day, at which time the retina is usually reattached (Fig. 103.3E), although an occasional case may take an extra day or two. If the fluid is not resorbing, there may be a new or missed break, traction may be keeping the break open, the bubble may be too small, or the patient may not have maintained proper positioning. The importance of proper positioning should be re-emphasized.
As a general guideline, the patient is examined on about the 1st or 2nd, 5th, and 14th postoperative days and periodically thereafter, looking especially for new retinal breaks. If close follow-up results in early detection and treatment, these breaks do not jeopardize the final outcome.8 At least half of these can be cured with an office procedure without resorting to SB.
The gas bubble will resorb spontaneously (Fig. 103.3F). Until it does, the patient is advised not to fly because the lower atmospheric pressure that exists in flight can cause expansion of the gas bubble with a marked increase in intraocular pressure. We recommend that patients be allowed to return to work in 1–2 weeks.
Results
In 1991, a compilation of 26 statistical series from seven countries including 1274 eyes treated with PR, showed a single-operation success rate of 80%, with 98% cured with one or more operations.18 More recently, Chan et al.20 reviewed 81 published PR series from 1986 to 2007, totalling 4138 eyes. Single-operation success was 74.4%, and success with one or more operations was 96.1%. New retinal breaks occurred in 11.7% of eyes, and PVR occurred in 5.2% (Table 103.2).
Table 103.2 Summary of 81 published statistical reports on pneumatic retinopexy, 1986–2007: anatomic results and two postoperative complications

Tornambe17 reviewed the causes of failure and concluded that pseudophakia/aphakia, detachment >50% of the retina, and multiple retinal breaks adversely influence single-operation success.
The multicenter clinical trial9 confirmed the conclusion of Ambler et al.73 that initial failure with PR, subsequently requiring SB, does not adversely affect the final visual outcome compared with SB alone.
Comparison of pneumatic retinopexy with scleral buckle
The multicenter randomized controlled clinical trial compared PR and SB in 198 eyes, establishing two statistically similar groups with similar preoperative visual acuity. The conclusions of the study include the following:8
1. Postoperative morbidity was less and had a shorter duration in the PR group.
2. Postoperative visual acuity was significantly better with PR than with SB for eyes in which the macula was detached for up to 2 weeks (P = 0.05). In this group, 80% of the PR patients regained 20/50 or better vision, compared with only 56% of SB patients.
3. With one operation and with occasional postoperative cryotherapy or laser supplementation, retinal reattachment was 84% in the SB group and 81% in the PR group. This difference was not statistically significant. However, subsequent studies have confirmed a modestly higher single operation success rate with SB.74
4. With reoperations, the final reattachment rate was 98% in the SB group and 99% in the PR group, also not statistically significant. Subsequent studies have confirmed comparable final reattachment rates.74
5. Complications were similar, based on a score system that weighted heavily the need for postoperative laser or cryotherapy.
6. Cataract surgery was required four times more often after SB than after PR.
7. New and missed retinal breaks occurred more frequently after PR but did not usually produce an unfavorable outcome. New and missed retinal breaks after SB had the worst prognosis, requiring more extensive reoperation with decreased visual results.
8. An open or absent posterior capsule was associated with an equally lower single-operation success rate in both groups.
9. Postoperative proliferative vitreoretinopathy occurred in 5% in the SB group and 3% in the PR group (not statistically significant).
Comparison of pneumatic retinopexy with vitrectomy
Unfortunately, similar prospective data for a direct comparison of PR to primary vitrectomy are unavailable. As SB has declined in favor among retinal surgeons, vitrectomy for primary rhegmatogenous retinal detachment has gained tremendous popularity in recent years,75,76 driven by continual advances in vitreoretinal instrumentation, microincisional techniques, endoillumination, and wide-field viewing systems. Similar to PR, vitrectomy avoids some of the morbidity more frequently associated with SB, such as induced myopia. Unlike PR, it tends to induce cataract and demands the time and expense associated with an operating room procedure.
Because vitrectomy tends to induce cataract, it is used most commonly in pseudophakic eyes. Tiny breaks, common in pseudophakic eyes, may be most readily identified by internal search during vitrectomy. In a multicenter clinical trial of SB versus primary vitrectomy for rhegmatogenous retinal detachment,77 vitrectomy yielded significantly better single-operation success rates than SB in pseudophakic eyes, but no difference in final vision. In phakic eyes, vitrectomy yielded poorer visual results than SB with no benefit in single-operation success.
Complications
Fish eggs, gas entrapment, and iatrogenic macular detachment are discussed under “Special procedures”, above. Other intraoperative complications of PR are minimal. A number of postoperative complications have been noted in the literature, but most of these occur with an incidence of less than 2% (Table 103.3). The two major concerns are PVR and new or missed retinal breaks. The management of subretinal gas also warrants discussion.
Table 103.3 Complications* in 565 eyes in 10 series†
| Eyes | ||
|---|---|---|
| n | (%) | |
| Operative | ||
| Incarceration of vitreous | 8 | 1.4 |
| Subconjunctival gas | 6 | 1.1 |
| Postoperative | ||
| New or missed breaks | 75 | 13.3 |
| PVR | 26 | 4.6 |
| Redetachment | 17 | 3.0 |
| Mild macular pucker | 10 | 1.8 |
| Persistent subretinal fluid | 12 | 2.1 |
| Minimal epiretinal membrane | 8 | 1.4 |
| Reopening of original break | 6 | 1.1 |
| Vitreous haze, 3–8 days | 6 | 1.1 |
*Complications of 0.3–1.0% omitted: choroidal detachment, anterior gas entrapment, vitreous hemorrhage, subretinal gas, shift of subretinal fluid, and macular hole. Complications, one case each (0.2%): peripheral subretinal hemorrhage, hyphema, pars plana detachment, cataract, malignant glaucoma, and endophthalmitis. †See references.5,8,33,35,40,41,60,78–80
Proliferative vitreoretinopathy
PVR is the major complication for all types of retinal reattachment surgery. A review of the PR literature revealed an incidence of 4% (Table 103.2). PVR developed in 3% of eyes managed with PR compared with 5% in the SB control group in the multicenter clinical trial.8 Evidence does not support the concern that intravitreal gas might stimulate PVR.
New or missed retinal breaks
The incidence of new and missed retinal breaks in a compilation of 81 series including 4138 eyes was 11.7% (Table 103.2). This is remarkably less than the 14% figure reported in a study of 171 eyes with retinal breaks, treated with laser photocoagulation or cryopexy but without gas injection.81 This, along with other evidence suggests that intravitreal gas may play little role in causing new retinal breaks and underscores the natural history of the disease, which includes the rather frequent development of additional breaks. The most common cause of detachment requiring reoperation following pneumatic retinopexy appears to be development of a new retinal break with new rather than persistent retinal detachment.82
In long-term follow-up, the need for reoperation is evident within the first 3 months in 89% of cases.19 With or without gas, the majority of new breaks appear within the first postoperative month, suggesting that incomplete posterior vitreous detachment may be responsible for some postoperative breaks.8,81 In phakic eyes with complete rather than partial posterior vitreous detachment, as determined by B-scan ultrasonography, Rezende et al.42 reported a higher rate of single-operation retinal reattachment, particularly in eyes treated with PR.
Since PR failures are sometimes caused by new retinal breaks in untreated areas of the retina, Tornambe has recommended that 360° laser retinopexy be applied between the insertion of the vitreous base and the ora serrata.17 He reported a significant improvement in single-operation success with this approach, but only about 1 in 12 PR surgeons employs this technique.59
Subretinal gas
Subretinal gas is usually a preventable complication, as described above (see under “Fish eggs”). However, if gas does get under the retina, the bubble can generally be teased back through the break if the break is larger than the gas bubble or if the diagnosis is made promptly before gas expansion. Under indirect ophthalmoscopic visualization, scleral depression is used to maneuver the bubble to the break and force it through.
If this fails but the amount of subretinal gas is small relative to the intravitreal gas, it may not be necessary to remove the subretinal gas. In this instance, strict prolonged positioning may still resolve the detachment, since the intravitreal gas will outlast the subretinal gas. Larger amounts of subretinal gas can be removed with a needle passed through the sclera and into the subretinal space, similar to the technique described above in the section “Gas entrapment at the injection site”. Rarely, vitrectomy may be necessary to remove the gas.
Acceptance of Pneumatic Retinopexy
The popularity of PR increased markedly in the 1990s, following the multicenter clinical trial.7 In a 2002 survey of the American Society of Retinal Specialists (then called the “Vitreous Society”), 72% of respondents selected PR for a phakic macula-on detachment with a 12 : 00 tear, and PR was also the most commonly selected option if the eye was pseudophakic (45% for PR versus 13% for primary vitrectomy).59 However, acceptance of PR in Europe has lagged far behind that in the USA.83,84
Recent gains in popularity of vitrectomy have been mostly at the expense of scleral buckling. A 2010 analysis of Medicare claims showed that vitrectomies for primary retinal detachment increased 72% from 1997 to 2007, while SB decreased 69% and PR remained relatively unchanged.75 Microincisional vitrectomy may present a new alternative to PR in selected cases.
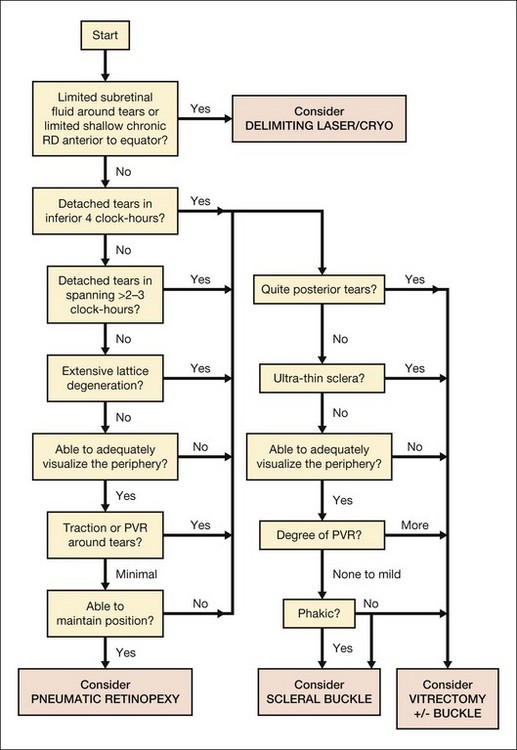
Algorithm for choosing Pneumatic Retinopexy versus other procedures for retinal detachment
In Retinal Detachment: Principles and Practice, Brinton and Wilkinson1 presented an algorithm (Fig. 103.8) intended to organize the decision of type of surgery to use for a given retinal detachment case. It does not reflect all factors which may influence a decision, and the individual circumstances of each specific patient, as well as the experience of the surgeon and the availability of equipment, should also be taken into account. This algorithm provides only guidelines reflecting the opinion of the authors and does not establish the standard of care for a given case.
1 Brinton DA, Wilkinson CP. Retinal detachment: principles and practice, 3rd ed. New York: Oxford University Press/American Academy of Ophthalmology; 2009.
2 Lincoff H, Kreissig I, Goldbaum M. Reasons for failure in non-drainage operations. Mod Probl Ophthalmol. 1974;12:40–48.
3 Rachal WF, Burton TC. Changing concepts of failures after retinal detachment surgery. Arch Ophthalmol. 1979;97:480–483.
4 Wilkinson CP. Surgery for retinal detachment. In: Iliff NT, ed. Complications in ophthalmic surgery. New York: Churchill Livingstone, 1983.
5 Hilton GF, Grizzard WS. Pneumatic retinopexy. A two-step outpatient operation without conjunctival incision. Ophthalmology. 1986;93:626–641.
6 Ai E, Gardner TW. Current patterns of intraocular gas use in North America. Arch Ophthalmol. 1993;111:331–332.
7 Benson WE, Chan P, Sharma S, et al. Current popularity of pneumatic retinopexy. Retina. 1999;19:238–241.
8 Tornambe PE, Hilton GF, the Retinal Detachment Study Group. Pneumatic retinopexy: a multicenter, randomized, controlled clinical trial comparing pneumatic retinopexy with scleral buckling. Ophthalmology. 1989;96:772–783.
9 Tornambe PE, Hilton GF, Brinton DA, et al. Pneumatic retinopexy: a two-year follow-up study of the multicenter clinical trial comparing pneumatic retinopexy with scleral buckling. Ophthalmology. 1991;98:1115–1123.
10 Ohm J. Uber die Behandlung der Netzhautablosung durch operative Entleerung der subretinalen Flussigkeit und Einspritzung von Luft in den Glaskoper. Graefes Arch Clin Exp Ophthalmol. 1911;79:442–450.
11 Rosengren B. Results of treatment of detachment of the retina with diathermy and injection of air into the vitreous. Acta Ophthalmol. 1938;16:573–579.
12 Norton EW. Intraocular gas in the management of selected retinal detachments. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:85–98.
13 Blodi CF, Folk JC. Treatment of macular hole retinal detachments with intravitreal gas. Am J Ophthalmol. 1984;98:811.
14 Dominguez DA, Boyd BF, Gordon S. Repeated insufflation of expansive gas. Highlights Ophthalmol Lett. 1986;14:1–14.
15 Dominguez DA, Fonseca A, Gomez-Montana J. Gas tamponade for ambulatory treatment of retinal detachment. In: Proceedings of the 25th International Congress of Ophthalmology, Rome, May 4–10, 1986. Amsterdam: Kugler and Ghedini; 1987.
16 Lincoff H, Kreissig I, Hahn YS. A temporary balloon buckle for the treatment of small retinal detachments. Ophthalmology. 1979;86:586–592.
17 Tornambe P. Pneumatic retinopexy: the evolution of case selection and surgical technique – a 12-year study of 302 eyes. Trans Am Ophthalmol Soc. 1997;95:551–578.
18 Hilton GF, Tornambe PE, and the Retinal Detachment Study Group. Pneumatic retinopexy: an analysis of intraoperative and postoperative complications. Retina. 1991;11:285–294.
19 Eter N, Böker T, Spitznas M. Long-term results of pneumatic retinopexy. Graefe’s Arch Clin Exp Ophthalmol. 2000;238:677–681.
20 Chan CK, Lin SG, Nuthi AS, et al. Pneumatic retinopexy for the repair of retinal detachments: A comprehensive review (1986–2007). Surv Ophthalmol. 2008;53:443–478.
21 Sebag J, Tang M. Pneumatic retinopexy using only air. Retina. 1993;13:8–12.
22 DeJuan E, Jr., McCuen B, Tiedeman J. Intraocular tamponade and surface tension. Surv Ophthalmol. 1985;30:47–51.
23 Machemer R. The importance of fluid absorption, traction, intraocular currents, and chorioretinal scars in the therapy of rhegmatogenous retinal detachments. Am J Ophthalmol. 1984;98:681–693.
24 Braker W, Mossman AL. Matheson gas book. Secaucus, NJ: Matheson Gas Products; 1980.
25 Fineberg B, Machemer R, Sullivan P, et al. Sulfur hexafluoride in the owl monkey vitreous cavity. Am J Ophthalmol. 1975;79:67–76.
26 Panessa-Warren B, Maisel JM, Warren J. Alterations in rabbit vitreal fine structure following C3F8 injection. Graefes Arch Clin Exp Ophthalmol. 1990;228:541–551.
27 Lincoff H, discussion of Chen JC, Robertson JE, et al. Results and complications of pneumatic retinopexy. Ophthalmology. 1988;95:601–608.
28 Lincoff H, Coleman J, Kreissig I, et al. The perfluorocarbon gases in the treatment of retinal detachment. Ophthalmology. 1983;90:546–551.
29 Mougharbel M, Koch FHJ, Boker T, et al. No cataract two years after pneumatic retinopexy. Ophthalmology. 1994;101:1191–1194.
30 Parver LM, Lincoff H. Geometry of intraocular gas used in retinal surgery. Mod Probl Ophthalmol. 1977;18:338–343.
31 Retina Society Terminology Committee. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983;90:121–125.
32 McAllister IL, Zegarra H, Meyers SM, et al. Treatment of retinal detachments with multiple breaks by pneumatic retinopexy. Arch Ophthalmol. 1987;105:913–916.
33 Tornambe PE, Hilton GF, Kelley NF, et al. Expanded indications for pneumatic retinopexy. Ophthalmology. 1988;95:597–600.
34 Irvine AR, Lahey JM. Pneumatic retinopexy for giant retinal tears. Ophthalmology. 1994;101:524–528.
35 Friberg TR, Eller AW. Pneumatic repair of primary and secondary detachments using a binocular indirect ophthalmoscope laser delivery system. Ophthalmology. 1988;95:187–193.
36 Chang TS, Pelzek CD, Nguyen RL, et al. Inverted pneumatic retinopexy: A method of treating retinal detachments associated with inferior retinal breaks. Ophthalmology. 2003;110:589–594.
37 Hilton GH, Josephberg RG, Halperin LS, et al. Office-based sutureless transconjunctival pars plana vitrectomy. Retina. 2002;22:725–732.
38 Hwang JF, Chen SN, Lin CJ. Treatment of inferior rhegmatogenous retinal detachment by pneumatic retinopexy technique. Retina. 2011;31:257–261.
39 Kulkarni KM, Roth DB, Prenner JL. Current visual and anatomic outcomes of pneumatic retinopexy. Retina. 2007;27:1065–1070.
40 Chen JC, Robertson JE, Coonan P, et al. Results and complications of pneumatic retinopexy. Ophthalmology. 1988;95:601–608.
41 McAllister IL, Meyers SM, Zegarra H, et al. Comparison of pneumatic retinopexy with alternative surgical techniques. Ophthalmology. 1988;95:877–883.
42 Rezende FA, Kapusta MA, Burnier MN, Jr., et al. Preoperative B-scan ultrasonography of the vitreoretinal interface in phakic patients undergoing rhegmatogenous retinal detachment repair and its prognostic significance. Graefes Arch Clin Exp Ophthalmol. 2007;245:1295–1301.
43 Hendrikse F. Pneumatic retinopexy to treat optic nerve pits and retinal detachments. Vitreoretinal. Surg Technol. 1991;3:1–2.
44 McDonald HR, Schatz H, Johnson RN. Treatment of bullous rhegmatogenous retinal detachment associated with optic pits. Int Ophthalmol Clin. 1992;32:35–42.
45 Friberg TR, Eller AW. Laser pneumatic retinopexy for repair of recurrent retinal detachment after failed scleral buckle – ten years experience. Ophthalmic Surg Lasers. 2001;32:13–18.
46 Sharma T, Badrinath SS, Mukesh BN, et al. A multivariate analysis of anatomic success of recurrent retinal detachment treated with pneumatic retinopexy. Ophthalmology. 1997;104:2014–2017.
47 Friberg TR. Laser photocoagulation to produce chorioretinal adhesion. Paper presented at the Second International Conference on Pneumatic Retinopexy, Tampa, FL, October 6, 1989.
48 Folk JC, Sneed SR, Folberg R, et al. Early retinal adhesion from laser photocoagulation. Ophthalmology. 1989;96:1523–1525.
49 Campochiaro PA, Kaden IH, Vidaurri-Leal J, et al. Cryotherapy enhances intravitreal dispersion of viable retinal pigment epithelial cells. Arch Ophthalmol. 1985;103:434–436.
50 Cowley M, Conway BP, Campochiaro PA, et al. Clinical risk factors for proliferative vitreoretinopathy. Arch Ophthalmol. 1989;107:1147–1151.
51 Grizzard WS, Hilton GF, Hammer ME, et al. A multivariant analysis of anatomic success of retinal detachments treated with scleral buckling. Graefes Arch Clin Exp Ophthal. 1994;232:1–7.
52 Haller JA, Walsh J, Smiddy W, et al. Multicenter trial of transscleral diode laser retinopexy in retinal detachment surgery. Trans Am Ophthalmol Soc. 1997;95:221–230.
53 Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769–1775.
54 Eckardt C. Staphylococcus epidermidis endophthalmitis after pneumatic retinopexy. Am J Ophthalmol. 1987;103:720–721.
55 Sharma T. Post-Pneumatic Retinopexy Endophthalmitis: Management of Infection and Persistent Retinal Detachment. Ophthalmic Surg Lasers Imaging. 2010;Mar 9:1–2.
56 Packo KH. Gas injection techniques. Paper presented at the Second International Conference on Pneumatic Retinopexy, Tampa, FL, October 6, 1989.
57 Humayun MS, Yeo JH, Koski WS, et al. The rate of sulfur hexafluoride escape from a plastic syringe. Arch Ophthalmol. 1989;107:853–854.
58 Jun AS, Pieramici DJ, Bridges WZ. Clear corneal cataract wound dehiscence during pneumatic retinopexy. Arch Ophthalmol. 2000;118:847–848.
59 Pollack JS, Packo KH. American Society of Retina Specialists Preferences and Trends Survey. http://www.asrs.org, 2002.
60 Hilton GF, Kelly NE, Salzano TC, et al. Pneumatic retinopexy: a collaborative report of the first 100 cases. Ophthalmology. 1987;94:307–314.
61 Bock J, Bornschein H, Hommer K. Die Wiederbelebungszeit der menschlichen Netzhaut: eine elektroretinographische Studie. Graefes Arch Clin Exp Ophthalmol. 1963;165:437–451.
62 Foulds WS, Johnson NF. Rabbit electroretinogram during recovery from induced ischaemia. Trans. Ophthalmol Soc UK. 1974;94:383–393.
63 Fujino T, Hamasaki DI. Effect of intraocular pressure on the electroretinogram. Arch Ophthalmol. 1967;78:757–765.
64 Popp C. Die Retinafunktion nach intraocularer Ischamie. Graefes Arch Clin Exp Ophthalmol. 1955;156:395–403.
65 Vassileva PL, Dabov SB. Changes in the glycogen content and the electroretinogram in retinal ischaemia experimentally induced in rabbits. In: Cant JS, ed. Vision and circulation: Proceedings of the Third William Mackenzie Memorial Symposium. London: Kimpton, 1976.
66 Shaffer RN. Paper presented in discussion at the American Ophthalmological Society, Hot Springs, VA, March 19–22, 1985.
67 Moses RA. Schiøtz tonometry with an air bubble in the eye. Am J Ophthalmol. 1966;62:281–282.
68 Baker SR, Hainsworth DP. Suprachoroidal gas as a complication of pneumatic retinopexy. Retina. 2000;20:224–225.
69 Jabaly-Habib HY, et al. Prolonged pain following unintentional injection of gas into the suprachoroidal space during pneumatic retinopexy. Retina. 2003;23:722–723.
70 Yeo JH, Vidaurri-Leal J, Glaser BM. Extension of retinal detachments as a complication of pneumatic retinopexy. Arch Ophthalmol. 1986;104:1161–1163.
71 Tornambe PE, Hilton GF. The steamroller maneuver and proliferative vitreoretinopathy (Correspondence). Arch Ophthalmol. 1992;110:15.
72 Yanyali A, Horozoglu F, Bayrak YI, et al. Steamroller versus basic technique in pneumatic retinopexy for primary rhegmatogenous retinal detachment. Retina. 2007;27:74–82.
73 Ambler JS, Meyers SM, Zegarra H, et al. Reoperations and visual results after failed pneumatic retinopexy. Ophthalmology. 1990;97:786–790.
74 Saw SM, Gazzard G, Wagle AM, et al. An evidence-based analysis of surgical interventions for uncomplicated rhegmatogenous retinal detachment. Acta Ophthalmol Scand. 2006;84:606–612.
75 Ramulu PY, Do DV, Corcoran KJ, et al. Use of retinal procedures in medicare beneficiaries from 1997 to 2007. Arch Ophthalmol. 2010;128:1335–1340.
76 Minihan M, Tanner V, Williamson TH. Primary rhegmatogenous retinal detachment: 20 years of change. Br J Ophthalmol. 2001;85:546–548.
77 Heimann H, Bartz-Schmidt KU, Bornfeld N, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114:2142–2154.
78 Algvere P, Hallnas K, Palmquist BM. Success and complications of pneumatic retinopexy. Am J Ophthalmol. 1988;106:400–404.
79 Lowe MA, McDonald HR, Campo RV, et al. Pneumatic retinopexy: surgical results. Arch Ophthalmol. 1988;106:1672–1676.
80 van Effenterre G, Abi-Rached J, Vachet JM, et al. Utilisation exclusive d’une injection de gaz dans le traitement de certain decollements de retine. Ophtalmologie. 1987;2:209–212.
81 Smiddy WE, Flynn HW, Nicholson DH, et al. Results and complications in treated retinal breaks. Am J Ophthalmol. 1991;112:623–631.
82 Mudvari SS, Ravage ZB, Rezaei KA. Retinal detachment after primary pneumatic retinopexy. Retina. 2009;29:1474–1478.
83 Laqua H, Honnicke K. Is scleral buckling still current? Ophthalmology. 2001;98:881–885.
84 Assi AC, Charteris DG, Gregor ZJ. Practice patterns of pneumatic retinopexy in the United Kingdom. Br J Ophthalmol. 2001;85:244.

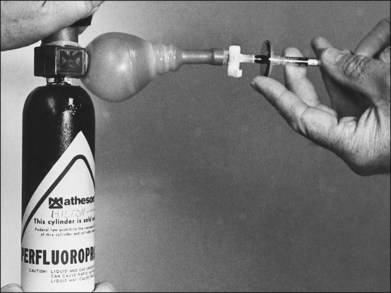
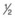 -inch, 30-gauge disposable needle on a 1 mL syringe without a plunger. The lumen is open toward the cornea and positioned over the peripheral iris. Gentle pressure on the cornea with a cotton-tipped applicator forces aqueous into the periphery of the anterior chamber near the needle tip, thereby facilitating more complete drainage.
-inch, 30-gauge disposable needle on a 1 mL syringe without a plunger. The lumen is open toward the cornea and positioned over the peripheral iris. Gentle pressure on the cornea with a cotton-tipped applicator forces aqueous into the periphery of the anterior chamber near the needle tip, thereby facilitating more complete drainage.