Pleural Disease
This chapter discusses the anatomy of the pleura, followed by a presentation of several physiologic principles of fluid formation and absorption by the pleura and a discussion of two types of abnormalities that affect the pleura: liquid in the pleural space (pleural effusion) and air in the pleural space (pneumothorax). A comprehensive treatment of all the disorders that affect the pleura is beyond the scope of this text. Rather, this chapter aims to cover the major categories and give the reader an understanding of how different factors interact in producing pleural disease. The primary malignancy of the pleura, mesothelioma, is discussed in Chapter 21, which deals with neoplastic disease of the thorax.
Anatomy
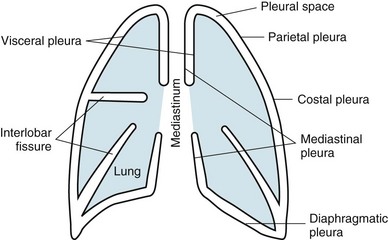
The term pleura refers to the thin lining layer on the outer surface of the lung (visceral pleura), the corresponding lining layer on the inner surface of the chest wall (parietal pleura), and the space between them (pleural space) (Fig. 15-1). Because the visceral and parietal pleural surfaces normally touch each other, the space between them is usually only a potential space. It contains a thin layer of serous fluid coating the apposing surfaces. When air or a larger amount of fluid accumulates in the pleural space, the visceral and parietal pleural surfaces are separated, and the space between the lung and the chest wall becomes more apparent.
The pleura lines not only the surfaces of the lung in direct contact with the chest wall but also the diaphragmatic and mediastinal borders of the lung. These surfaces are called the diaphragmatic and mediastinal pleura, respectively (see Fig. 15-1). Visceral pleura also separates the lobes of the lung from each other; therefore, the major and minor fissures are defined by two apposing visceral pleural surfaces.
Physiology
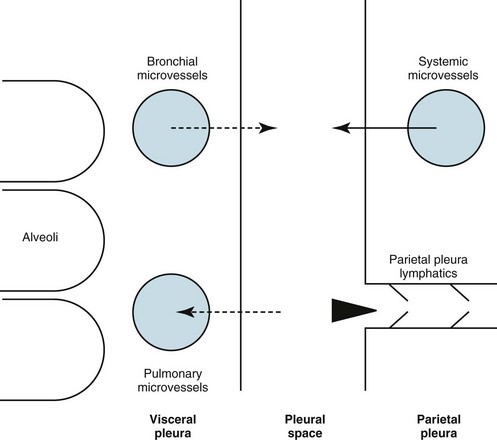
The pleural space normally contains only a small quantity of liquid (≈10 mL), which lubricates the apposing surfaces of the visceral and parietal pleurae. According to the current concept of pleural fluid formation and resorption, formation of fluid is ongoing primarily from the parietal pleural surface, and fluid is resorbed through the stomata into the lymphatic channels of the parietal pleura (Fig. 15-2). The normal rates of formation and resorption of fluid, which must be equal if the quantity of fluid within the pleural space is not changing, are believed to be approximately 15 to 20 mL/day.
where K = filtration coefficient (a function of the permeability of the pleural surface), P = hydrostatic pressure, COP = colloid osmotic pressure, σ = measure of capillary permeability to protein (called the reflection coefficient), and the subscripts c and is refer to the capillary and pericapillary interstitial space, respectively. In this case, the pericapillary interstitial space is essentially the pleural space; therefore, Pis and COPis refer to intrapleural pressure and the colloid osmotic pressure of pleural fluid, respectively. The intrapleural pressure—that is, the hydrostatic pressure within the pleural space—is negative, reflecting the outward elastic recoil of the chest wall and the inward elastic recoil of the lung.
Pleural Effusion
Etiology of Pleural Effusion
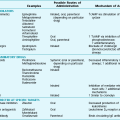
The numerous causes of pleural fluid accumulation are best divided into transudative and exudative categories (Table 15-1). This distinction is generally easy to make and is most important in guiding the physician along the best route for further evaluation. Transudative fluid usually implies that the pathologic process does not primarily involve the pleural surfaces, whereas exudative fluid often suggests that the pleura is affected by the disease process causing the effusion.
Table 15-1
MAJOR CAUSES OF PLEURAL EFFUSION
TRANSUDATE
Increased hydrostatic pressure; “overflow” of liquid from the lung interstitium
Decreased plasma oncotic pressure
Movement of transudative ascitic fluid through the diaphragm
EXUDATE
Diagnostic Approach
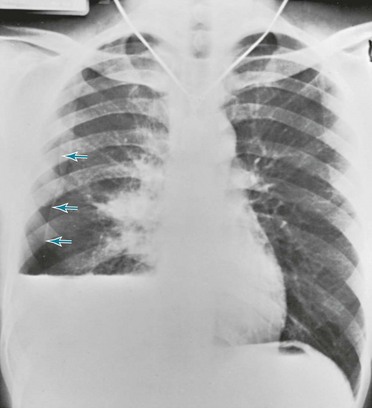
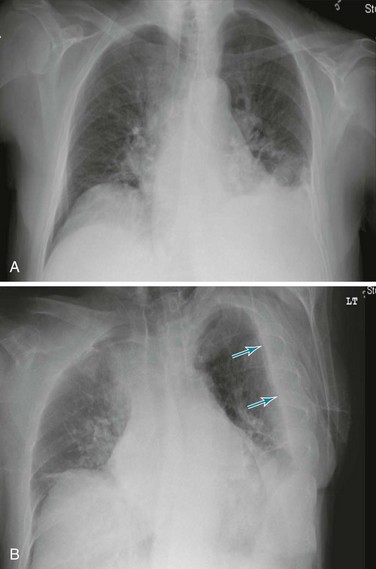
Posteroanterior and lateral chest radiographs are clearly most important in the initial evaluation of the patient with suspected pleural effusion (Fig. 15-3). With a small effusion, blunting of the normally sharp angle between the diaphragm and chest wall (costophrenic angle) is seen. Often this blunting is first apparent on inspection of the posterior costophrenic angle on the lateral radiograph, because this is the most dependent area of the pleural space. With a larger effusion, a homogeneous opacity of liquid density appears and is most obvious at the lung base(s) when the patient is upright. The fluid may track along the lateral chest wall, forming a meniscus. Computed tomography (CT) scanning of the chest is more sensitive than plain film in detecting pleural effusions (Fig. 15-4). Small free-flowing effusions will be seen posteriorly at the bases of the lung, and track up the lung fields posteriorly and laterally as the effusion becomes larger.
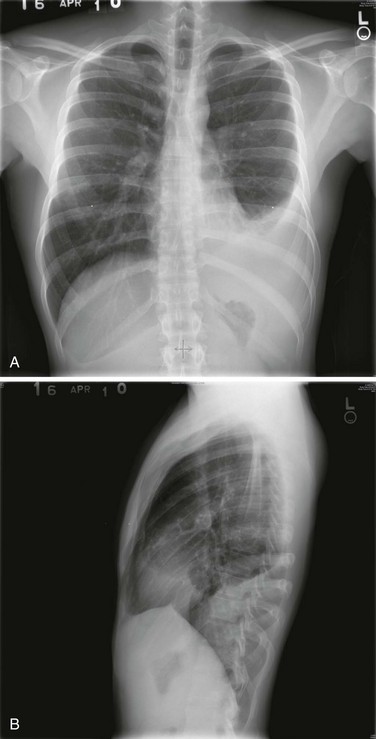
Figure 15-3 Posteroanterior (A) and lateral (B) chest radiographs demonstrating left pleural effusion.
When certain inflammatory effusions persist for a time, fluid may no longer be free-flowing within the pleural space as fibrous bands of tissue (loculations) form within the pleura. In such circumstances, fluid is not necessarily positioned as expected from the effects of gravity, and atypical appearances may be found. To detect whether fluid is free-flowing or whether small costophrenic angle densities represent pleural fluid, a lateral decubitus chest radiograph may be extremely useful. In this view, the patient lies on a side, and free-flowing fluid shifts position to line the most dependent part of the pleural space (Fig. 15-5).
Pneumothorax
Etiology and Pathogenesis
Patients who receive positive pressure to the tracheobronchial tree and alveoli (e.g., with mechanical ventilation) are subject to development of a pneumothorax. In this case, as a result of positive pressure, a preexisting subpleural bleb may rupture, or air may rupture through an alveolar wall into the interstitial space, track through the lung parenchyma to the subpleural surface, and then rupture into the pleural space. Alternatively, and perhaps more commonly, the air following alveolar rupture tracks retrograde to the mediastinum alongside blood vessels and airways and produces a pneumomediastinum (see Chapter 16). A pneumothorax can result when air ruptures through the mediastinal pleura into the pleural space.
Diagnostic Approach
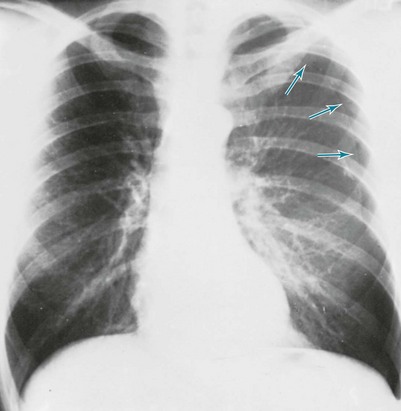
The diagnosis of pneumothorax is established or confirmed by chest radiograph. The characteristic finding is a curved line representing the edge of the lung (the visceral pleura) separated from the chest wall. Between the edge of the lung and the chest wall, the pleural space is lucent, and none of the normal vascular markings of the lung are seen in this region (Figs. 15-6 and 15-7). When the pneumothorax is small, separation of the visceral and parietal pleura appears on upright chest films only at the apex of the lung, where the pleural air generally accumulates first. If the pneumothorax is substantial, the lung loses a significant amount of volume and therefore has a greater density than usual.
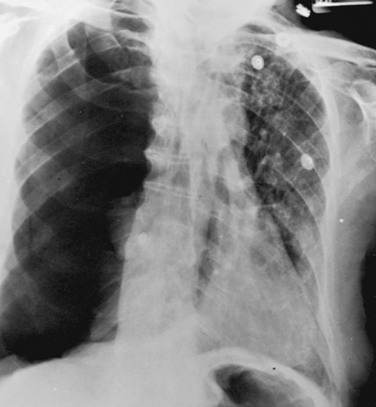
When both fluid and air are present in the pleural space (hydropneumothorax), the fluid no longer appears as a meniscus tracking up along the lateral chest wall. Rather, the fluid falls to the most dependent part of the pleural space and appears as a liquid density with a perfectly horizontal upper border (see Fig. 15-7). Finally, when gas in the pleural space is under tension, evidence is often seen of structures (e.g., trachea and mediastinum) being “pushed” away from the side of the pneumothorax (Fig. 15-8).
Light, RW. Pleural diseases, ed 5. Philadelphia: Lippincott Williams & Wilkins; 2007.
Loddenkemper, R, Antony, VB. Pleural diseases. Eur Respir Mon. 2002;7(monograph 22):1–326.
Natanzon, A, Kronzon, I. Pericardial and pleural effusions in congestive heart failure—anatomical, pathophysiologic, and clinical considerations. Am J Med Sci. 2009;338:211–216.
Pistolesi, M, Miniati, M, Giuntini, C. Pleural liquid and solute exchange. Am Rev Respir Dis. 1989;140:825–847.
Qureshi, NR, Gleeson, FV. Imaging of pleural disease. Clin Chest Med. 2006;27:193–213.
Sahn, SA. Pleural disease. editor. Clin Chest Med. 2006;27:157–394.
Wiener-Kronish, JP, Broaddus, VC. Interrelationship of pleural and pulmonary interstitial liquid. Annu Rev Physiol. 1993;55:209–226.
Zocchi, L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 2002;20:1545–1558.
Colice, GL. Medical and surgical treatment of parapneumonic effusions. An evidence-based guideline. Chest. 2000;18:1158–1171.
Heffner, JE, Klein, SJ, Hampson, C. Interventional management of pleural infections. Chest. 2009;136:1148–1159.
Heffner, JE. Discriminating between transudates and exudates. Clin Chest Med. 2006;27:241–252.
Heffner, JE. Management of the patient with a malignant pleural effusion. Semin Respir Crit Care Med. 2010;31:723–733.
Light, RW. Pleural effusion. N Engl J Med. 2002;346:1971–1977.
Light, RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3:75–80.
Rahman, NM, Maskell, NA, West, A, et al. Intrapleural use of tissue plasminogen activator and DNAase in pleural infection. N Engl J Med. 2011;365:518–526.
Rodriguez-Panadero, F, Janssen, JP, Astoul, P. Thoracoscopy: general overview and place in the diagnosis and management of pleural effusion. Eur Respir J. 2006;28:409–422.
Sahn, SA. The value of pleural fluid analysis. Am J Med Sci. 2008;335:7–15.
Thomsen, TW, DeLaPena, J, Setnik, GS. Videos in clinical medicine: thoracentesis. N Engl J Med. 2006;355:e16.
Haynes, D, Baumann, MH. Management of pneumothorax. Semin Respir Crit Care Med. 2010;31:769–780.
Baumann, MH. Management of spontaneous pneumothorax. Clin Chest Med. 2006;27:369–381.
Leigh-Smith, S, Harris, T. Tension pneumothorax—time for a re-think? Emerg Med J. 2005;22:8–16.
O’Connor, AR, Morgan, WE. Radiological review of pneumothorax. BMJ. 2005;330:1493–1497.
Tschopp, JM, Rami-Porta, R, Noppen, M, Astoul, P. Management of spontaneous pneumothorax: state of the art. Eur Respir J. 2006;28:637–650.
Wakai, A, O’Sullivan, RG, McCabe, G. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. (1):2007. CD004479
Weissberg, D, Refaely, Y. Pneumothorax. Experience with 1,199 patients. Chest. 2000;117:1279–1285.

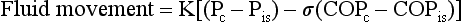
 × upper limit of normal serum LDH
× upper limit of normal serum LDH