Pituitary Surgery
Historical Background
Pituitary surgery was developed and has advanced on the basis of repeated innovations and exchanges between Europe and the New World. The first operation on a pituitary tumor was performed by Horsley in 1889, who published in 19061 the results obtained on a series of 10 patients, first by means of a frontal craniotomy and later through a temporal approach.2 The first surgeon who reported on an operation specifically for a pituitary tumor was a British general surgeon, Paul; in 1893, he performed a temporal decompression in an acromegalic patient without actually reaching the tumor.3,4
The next milestone was the first transsphenoidal approach achieved by the Viennese surgeon Schloffer in Innsbruck, Austria, in 1907.5 The use of a direct route through the nose toward the brain was not absolutely new: Many centuries ago, the Egyptians used to extract cerebral tissue transnasally in the mummification process by means of special hooked instruments, without disfiguring the face. Based on anatomic studies of the Italian physician Giordano, chief surgeon of the Hospital of Venice,6,7 Schloffer performed a lateral rhinotomy, reflecting the nose to the right; removed the turbinates; and opened the maxillary, ethmoid, and sphenoid sinuses before reaching the sella. In the same year, von Eiselsberg,8 in Vienna, performed a similar, if even more extended, procedure. The next evolutionary step, approaching the modern transsphenoidal approach, was realized in 1909 by Kocher, professor of surgery in Berne, Switzerland, who was awarded the Nobel Prize for Medicine and Physiology in 1909 for his contributions concerning the thyroid; he performed a transseptal submucosal approach by means of an external midline incision on the nasal bridge,9,10 but without exenteration of frontal, ethmoidal, and maxillary sinuses. Another remarkable contribution was that of Kanavel,11,12 who proposed an approach through an infranasal skin incision. The first totally endonasal procedure, without complete dislocation of the nose, was achieved in 1910 in five stages with the patient under local anesthesia by Hirsch, a Viennese rhinologist, who was the first to incorporate a nasal speculum.13 He used the technique of his teacher Hajek,14 which was used previously for purulent infections of the sphenoid sinus, first opening the posterior ethmoid sinus, then enlarging the opening into the sphenoid sinus after a submucosal resection of the septum, according to Kocher’s and Kilian’s techniques,9,15 beginning with a hemitransfixion incision in the right nasal cavity. Hirsch moved to the United States in 1938 to escape the Nazis, and worked in Boston with the neurosurgeon Hamlin.16
In 1910, Halstead17,18 was a pioneer of the sublabial approach, which initially was performed as a multistage operation in Chicago. Cushing performed his first transsphenoidal procedure in 1909,19 but his classic sublabial, transseptal, transsphenoidal approach20 was the evolution of his technique and a combination of different methods reported by other authors, such as Halstead, Hirsch, Kanavel, and Kocher (i.e., sublabial incision + submucosal paraseptal approach to the sphenoid sinus + use of the nasal speculum + use of an electric headlamp). Cushing later abandoned this procedure,10,21,22 likely because of better recovery of vision in patients operated transcranially owing to difficulty with hemostasis and completeness of tumor removal in large suprasellar tumors and owing to difficulty in preoperative differential diagnosis. His advocacy of the transcranial option prompted most neurosurgeons to follow his recommendations. Another leading American neurosurgeon, Dandy, stated, “The nasal route is impractical and can never be otherwise.”23 In 1918, at the Johns Hopkins Medical Society, Dandy had presented his experience in about 20 cases operated on through an intracranial intradural approach to the chiasm, according to a frontotemporal route to the pituitary along the sylvian fissure, originally conceived by Heuer in 1914.24,25 The two main transcranial options—subfrontal and frontotemporal—are still used today, together with more recent skull base approaches.
The late 1920s to the 1960s was a relatively dark period for transsphenoidal surgery, because of the absence of antibiotics and replacement therapy for adrenocortical hormones, the lack of adequate illumination, and the opinions of the most authoritative opinion leader, Cushing. The only pupil of Cushing who did not abandon the transsphenoidal method was Dott, neurosurgeon of the Royal Infirmary at Edinburgh.10,26 He had learned the method from Cushing when he had been awarded a 1 year Rockefeller Fellowship at the Peter Bent Brigham Hospital in 1923. It is not clear why Dott did not publish his results, but he kept the procedure alive, improved the technique by adding two light bulbs to the speculum designed by Cushing, and taught the method to the French neurosurgeon Guiot during his visit to the Royal Infirmary in 1956. Guiot at the Hôpital Foch in Paris and Guiot’s trainee Hardy in Montreal deserve credit for the “transsphenoidal renaissance” in the late 1960s and 1970s. Modern transsphenoidal surgery takes advantage of the innovations of intraoperative image intensification and fluoroscopy, introduced by Guiot, and the use of the operating microscope according to Hardy,27 who introduced the concept of microadenoma and selective microsurgical resection.
No new progress was made until the 1990s, when the latest innovation, the endoscope, was introduced. By analogy with the evolution of Picasso’s painting, the “cubist evolution” of transsphenoidal surgery occurred, from devastating transfacial approaches to minimally invasive contemporary procedures,28 and with the endoscope used as a visualizing instrument for pituitary surgery. Used for the first time by Guiot in 196329 as an adjunct to the microscope to expand the field of vision (endoscope-assisted microneurosurgery), then abandoned for many years because it still was technically insufficient, the endoscope has come into regular use as a stand-alone visualizing and operating tool (pure endoscopic transsphenoidal surgery), thanks primarily to the work of Jho in Pittsburgh30,31 and of our group in Naples, Italy32,33; these workers standardized a unilateral endonasal anterior sphenoidotomy approach to the sella, without the use of the operating microscope or of a transsphenoidal retractor. Further advancement and evolution of the technique are expected through intraoperative magnetic resonance imaging (MRI), robotics, and miniaturization, as well as the rapidly emerging biomolecular frontiers, which are expected to change the world of pituitary surgery.
Surgical Anatomy
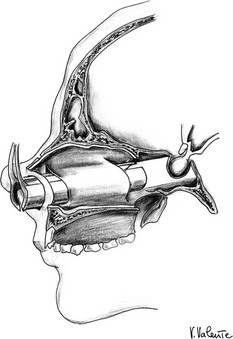
The pituitary gland, or the hypophysis cerebri, is situated within the hypophyseal fossa, a fibro-osseous compartment near the center of the cranial base (Fig. 12-1). This fossa is limited laterally and superiorly by reflections of dura mater, and anteriorly, posteriorly, and inferiorly by the sella turcica, a depression in the body of the sphenoid bone. At the superior edge of the anterior wall of the sella turcica is a bony protrusion called the tuberculum sellae, and its posterior wall is the dorsum sellae.
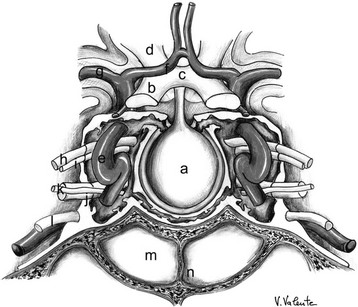
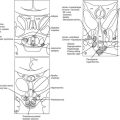
FIGURE 12-1 Frontal view: schematic drawing of the sellar region. a, Pituitary gland. b, Optic nerve. c, Optic chiasm. d, Optic tract. e, Internal carotid artery. f, Anterior cerebral artery. g, Middle cerebral artery. h, Oculomotor nerve. i, Trochlear nerve. j, Ophthalmic branch (V1) of the trigeminal nerve. k, Abducent nerve. l, Maxillary branch (V2) of the trigeminal nerve. m, Sphenoid sinus. n, Sphenoid septum. (Courtesy V. Valente, MD.)
The diaphragma sellae, a fold of dura with a central aperture, forms an incomplete roof above the sella turcica. The diaphragma separates the anterior lobe from the overlying optic chiasm. The central opening of the diaphragma is of variable size and transmits the pituitary stalk and its blood supply. The subarachnoid space of the chiasmatic cistern can extend through the aperture of the diaphragma and into the sella turcica for varying distances above the gland. When there is an incompetent diaphragma sellae (i.e., wide central aperture of the diaphragma), the chiasmatic cistern herniates to fill the sella turcica partially, leading to remodeling and enlargement of the hypophyseal fossa and flattening of the pituitary gland, a condition called empty sella,34 which is found in 5% to 23% of cases at autopsy.35 When this condition is associated with the presence of an adenoma, usually a microadenoma, the surgeon must be careful to avoid entering the subarachnoid space (i.e., the chiasmatic cistern herniated into the sella), to prevent an intraoperative cerebrospinal fluid (CSF) leak, which could increase the difficulty of resecting the lesion.
The folds of the dura mater form the lateral walls of the hypophyseal fossa and the medial wall of the so-called cavernous sinuses, these latter consisting of a series of compartmentalized venous channels separated by fibrous trabeculae and communicating with each other by means of the anterior and posterior intercavernous sinuses.36–40 The oculomotor nerve, the trochlear nerve, and the first two divisions of the trigeminal nerve are embedded in the lateral wall of the cavernous sinus, lying between the endothelial lining and the dura mater, whereas the abducens nerve is contained within the sinus itself. The cavernous sinus also envelops a portion of the internal carotid artery (ICA) and the sympathetic nerve plexus encircling it. The intracavernous segment of the ICA extends forward, adjacent to the superolateral surface of the body of the sphenoid bone, in a groove called the carotid sulcus. The carotid arteries and the bone layers overlying them in the sphenoid sinus form two protuberances, which represent important landmarks in the transsphenoidal approach, particularly at the level of the sellar floor, where they are considered the lateral margins of a correct opening of the sella.
The pituitary gland is overlaid by the visual pathways and the hypothalamus. The relationships between the pituitary gland, diaphragma sellae, sulcus chiasmatis, and optic apparatus are important determinants of the visual deficits produced by an expanding pituitary tumor.41 In some cases, the anterior border of the optic chiasm is closely applied to the sulcus chiasmatis of the sphenoid bone; this leads to a lower position of the optic chiasm, which is much nearer to the diaphragma sellae. This condition, called prefixed chiasm and present in 5% to 10% of cases,42,43 must be considered during transcranial approaches and in the transsphenoidal opening of the upper portion of the sellar floor, as in the extended approaches to the planum sphenoidale, because of the possibility of producing iatrogenic damage to the optic chiasm. In other cases, the optic chiasm is located above the anterior part of the diaphragma sellae, making it extremely vulnerable to the suprasellar extension of a pituitary tumor. This pattern is found in about 12% of cases.42,43 In most cases (75%), the optic chiasm is placed more posteriorly, lying over the posterior aspect of the diaphragma sellae, near the dorsum sellae,42,43 which is a more favorable relationship for the removal of a sellar lesion because in such a circumstance, the suprasellar region is free from the optic chiasm. The remaining pattern is that of an optic chiasm located on and behind the dorsum sellae, called postfixed chiasm (4% to 11% of cases).42,43 In such cases, the intracranial course of the optic nerves is longer, and the medial aspects of the optic nerves are more vulnerable to the suprasellar extension of the pituitary tumor.
The need to define intraoperatively the exact location of a lesion and its relationships with surrounding vascular and nervous structures has led to the development of neuronavigation and intraoperative MRI, which provide continuous anatomic information. The neuronavigator is a computer-based system that offers the surgeon real-time information related to the operating site. The basic function of the navigator is to determine the location of a probe tip within the surgical field and to translate it into CT/MRI coordinates. The patient’s head is related initially to the CT/MRI coordinates; this relationship is established preoperatively by a set of fiducial markers on the patient’s head. During transsphenoidal surgery, this relationship can obviate the need for intraoperative fluoroscopy, avoiding exposure of the operating room staff and the patient to radiation. Its employment is particularly useful in the presence of a conchal or presellar type of sphenoid sinus, in identifying the boundaries of the sella, and in some cases of recurrence in which prior surgery has altered the landmarks needed to reach the sella safely.44–49
Intraoperative MRI, with the use of a magnetic resonance magnet positioned in a specially designed operating room with a movable operating table that allows translation of the patient from the surgical equipment to the MRI imager, offers the opportunity of a second look during the same surgical procedure. In transsphenoidal surgery, intraoperative MRI allows documentation of the extent of surgical resection of the sellar lesion, and removal of the suprasellar portion of the tumor can be evaluated reliably.50–55
Surgery
Therapy for pituitary adenomas is targeted to achieve multiple goals, as follows:
• Normalization of excess hormone secretion
• Preservation or restoration of normal pituitary function
• Preservation or restoration of normal neurologic function, usually with visual acuity or visual field (or both) affected more frequently
• Prevention of tumor recurrence
• Pituitary apoplexy, a relatively rare condition presenting with sudden headache, abrupt visual loss, ophthalmoplegia, altered level of consciousness, and collapse from acute adrenal insufficiency. It is caused by a hemorrhage into the tumor or its acute necrosis, with subsequent swelling and frequent spreading into the subarachnoid space, leading to other signs of meningeal irritation; the related acute and severe clinical syndrome demands glucocorticoid replacement and surgical decompression, usually transsphenoidal, if visual loss is severe and progressive.56–59 If the patient has a mild form of apoplexy and is clinically stable, it is prudent to measure the serum prolactin because some patients with prolactinoma present in this fashion and can be treated successfully with medical therapy.
• Progressive mass effect, producing compression of surrounding neurovascular structures and usually causing visual deficit (due to compression of the optic chiasm) or less frequently cranial nerve palsy (due to compression of cranial nerves inside the cavernous sinus). In cases of prolactin-secreting macroadenomas, dopamine agonist administration can be considered as the first treatment option because of predictable dramatic shrinkage of the lesion, with rapid recovery of neurologic deficits. In such circumstances, frequent visual field and imaging controls are necessary to monitor the clinical evolution.
• Nonfunctioning pituitary tumors
• Cushing’s disease, because of the present inadequacy of pharmacologic agents
• Acromegaly, in combination with medical treatment (preoperative and postoperative, if necessary)
• Failure of or resistance to medical treatment or intolerable side effects of medical therapy
• Recurrence, in combination or in association with the other therapeutic options, medical or radiotherapeutic or both
Indications for surgery have changed over time and with the refinement of surgical techniques and according to the evaluation of results and experiences, the development of knowledge about the biology of pituitary tumors, and the use of effective new pharmacologic agents60–62 and radiation techniques. Large invasive pituitary tumors are difficult to cure regardless of the approach because removal of every fragment of the tumor is often impossible. Extended transsphenoidal approaches sometimes can represent a valid alternative to transcranial options; excellent visual outcomes derive from the transsphenoidal method.58,63 Visual impairment does not indicate the need for a transcranial operation, as Cushing believed at one time.64
After an initial flourishing of transsphenoidal surgery in the early 1900s, transcranial approaches attained success and were popular in the first half of the twentieth century. This fundamental debate lasted for decades, until the introduction of intraoperative fluoroscopy and microscopy effectively put it to rest. With these new imaging techniques, adequate exposure and thorough exploration of the sella turcica became possible without the need for a craniotomy and associated brain retraction. As a result, the transseptal transsphenoidal approach came to be accepted as the procedure of choice for the surgical management of most pituitary lesions.65,66
The success of the transsphenoidal approach is based on solid foundations: It is the least traumatic route to the sella, it lacks visible scars, it provides excellent visualization of the pituitary gland and adjacent pathology, it offers lower morbidity and mortality rates compared with transcranial procedures, and it requires only a brief hospital stay. Indications for transsphenoidal surgery today include more than 95% of the surgical indications in the sellar area and approximately 96% of all pituitary adenomas.67 The well-established indications for this route are as follows:
• Almost all adenomatous lesions68
• Non-neoplastic intrasellar cysts34,69–71
• Craniopharyngiomas, preferably cystic, extra-arachnoidal,72 and infradiaphragmatic,73 with an enlarged sella74–76
Absolute indications were established in the 1970s and still are valid today; they include the following77:
• Elevated surgical risk of the transcranial route
• In long-standing compression of the chiasm, not able to tolerate additional trauma
• In cases of acute endosellar hypertension
• The extended transsphenoidal approaches to the sphenoethmoid planum, for suprasellar craniopharyngiomas, Rathke’s cleft cysts, some tuberculum sellae meningiomas, and anterior cranial base CSF leaks78–88; to the clival area, for chordomas79,89–93; and to the parasellar compartment,37,79,91,94–99 for invasive adenomas and chordomas. The development of extended transsphenoidal approaches has provided transsphenoidal access to several lesions that previously would have been considered accessible by transcranial approaches only. The spectrum of lesions accessible to transsphenoidal surgery is widening. Extended approaches today represent standard procedures in selected centers and in experienced hands and are expected to progress further in the near future with additional technical and instrumental development.
• A sequential transsphenoidal approach, in intrasuprasellar adenomas, as an intentionally two-staged transsphenoidal operation. This operation is designed to encourage the descent of a suprasellar remnant of the adenoma incompletely removed in the first step, to limit the risks for brisk decompression of huge lesions, and to manage lesions with a second surgery.100
Indications for transcranial surgery include the following68,73:
• Tumors with extensive intracranial invasion, into the anterior cranial fossa or lateral or posterior extension, into the middle and posterior cranial fossae101
• Tumors with asymmetric suprasellar development, particularly if major vessel involvement is present
• Tumors with intracranial extension separated from the intrasellar portion by a narrow neck (dumbbell adenoma) and showing an hourglass configuration102
• Suprasellar tumors not completely resectable through the transsphenoidal route103
• Recurrent or residual pituitary tumors in patients who already have had unsuccessful transsphenoidal surgery
• When preoperative MRI assessment, on the basis of long repetition time (TR) signal, suggests a firm consistency of the adenoma, preventing easy debulking with subsequent collapse and descent into the sella, when resected from below.104–106 This may occur after radiotherapy107; increased fibrosis also has been reported after treatment with dopamine agonists108 or somatostatin analogues,109,110 but these reports do not reflect our experience.
• When the sphenoid sinus is not pneumatized and the sella is small or does not make it easy to reach the suprasellar extension of the tumor111
• When coexisting vascular96,112 and tumoral surgical pathology is evident and one-time surgical treatment for both conditions is chosen
Transsphenoidal Approaches
One or another variation of the transsphenoidal approach represents the most physiologic and minimally traumatic corridor of surgical access to the sella, providing direct and superior visualization of the pituitary gland and adjacent pathology.92,113 The transsphenoidal approach represents a midline approach that has been performed since the 1960s with use of the operating microscope as a visualizing tool, through transnasal transseptal, sublabial transseptal, or endonasal procedures (microsurgical transsphenoidal procedures). The transsphenoidal approach also can be performed by using the endoscope as the sole visualizing tool during the entire surgical procedure, realizing a “pure” endoscopic endonasal transsphenoidal approach. The combined use of the microscope and the endoscope during the same approach defines the condition of endoscope-assisted microsurgery.
Microsurgical Transsphenoidal Approaches: Although many different transsphenoidal procedures and variations have been described, three basic microsurgical transsphenoidal approaches to pituitary tumors are used: the transnasal transseptal transsphenoidal approach, the sublabial transseptal transsphenoidal approach, and the endonasal transsphenoidal approach. The patient can be positioned on the operating table supine, as originally proposed by Cushing, with the surgeon behind the patient’s head, or in the semisitting position, as favored by Guiot, with the surgeon standing in front of the patient. The procedure is performed with an operating microscope for visualization, illumination, and magnification of the surgical field. Intermittent fluoroscopy is used for trajectory guidance, or, more recently, neuronavigational systems permit the surgeon to gather information about the current position of anatomic structures or instruments during the procedure iself.44–49 Intraoperative MRI is capable of enhancing safety and providing additional knowledge about the completeness of lesion removal.50,51,53–55 The three main transsphenoidal methods differ slightly one from each other primarily in the initial phase up to the exposure of the sphenoid sinus; they then follow the same surgical sphenoidal and sellar steps.
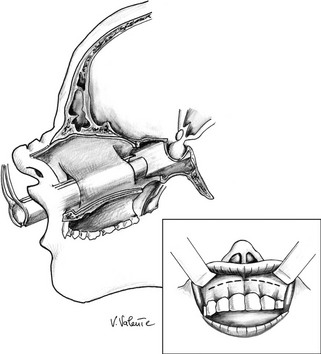
Microsurgical Transnasal Transseptal Transsphenoidal Approach: In a diffused version of the transnasal approach (Fig. 12-2), the operation starts in the right nostril, with retraction of the columella to the patient’s left to expose through the incision in the nostril the anterior edge of the septal cartilage, 2 to 3 cm behind the mucosal-cutaneous junction. The nasal mucosa usually adheres tightly to the most anterior region of the septum: Its dense, fibrous strands are divided through a combination of sharp and blunt dissection. The submucosal dissection is extended posteriorly, elevating the nasal mucosa away from the septal cartilage up to its junction with the bony septum. The cartilaginous septum is dissected from the mucoperichondrium along its right side, then is laterally pushed on the left side, at the junction point, to free the cartilaginous septum from the bony septum. Posterior submucosal tunnels are created along both sides of the bony septum, which is partially removed to facilitate the introduction of a self-retaining transsphenoidal retractor, following the use of a nasal speculum in the dissection of the nasal septum. Care must be taken to avoid mucosal perforation during these maneuvers.
Microsurgical Sublabial Transseptal Transsphenoidal Approach: The upper lip is retracted, and an incision is made along the buccogingival junction, between the two canine fossae (Fig. 12-3). The upper lip and the periosteum are elevated to expose the anterior nasal spine and the inferior border of the pyriform aperture of the nasal cavities. The mucosa of the floor of the nose is elevated first on both sides with a small periosteal elevator, which is introduced along the nasal septum to detach the mucosa from the cartilage. The elevated mucosa is held in place by a nasal speculum, which allows further mucosal elevation from the bony nasal septum. The inferior and posterior portion of the cartilaginous septum is dissected from the bony nasal septum and is deflected laterally. The self-retaining nasal speculum is introduced and opened widely to hold the retracted mucosa out of the field. The sublabial approach permits a more anterior trajectory with respect to the transnasal option; this can be useful in lesions that extend into the suprasellar area or toward the planum sphenoidale.
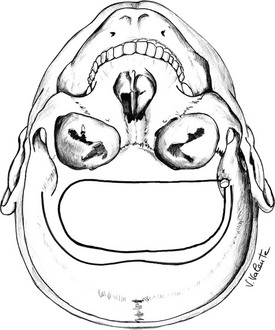
Microsurgical Endonasal Transsphenoidal Approach: A handheld speculum is inserted into the nostril along the middle turbinate, which reliably leads to the sphenoid sinus (Fig. 12-4). In the posterior nasal cavity, an elevator is used to make a vertical mucosal incision at the junction of the keel of the sphenoid bone and the posterior nasal septum. The septum, with its intact mucosa, is pushed off the midline by the medial blade of the handheld speculum. Bilateral mucosal flaps over the keel of the sphenoid bone are elevated and reflected laterally, with identification of the sphenoid ostia. The handheld speculum is replaced by a thin nasal speculum, which is placed up to the face of the sphenoid bone. After lesion removal, the speculum is withdrawn, the nasal septum is returned to the midline, and the ipsilateral outfractured middle turbinate may be moved toward the midline to prevent a maxillary sinus mucocele. Nasal packing is placed for 24 hours in selected cases but is not used routinely.114,115
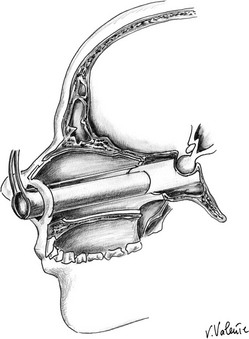
FIGURE 12-4 Sagittal view: schematic drawing of the microsurgical endonasal transsphenoidal approach. (Courtesy V. Valente, MD.)
With a presellar or a conchal type of sphenoid sinus, the sphenoidotomy calls for some precautions.116 With these two variants of incomplete sphenoid sinus pneumatization, a microdrill is used to open the sellar floor. In these cases, fluoroscopy or neuronavigation is extremely useful, if not essential, for identification of the superior and inferior edges of the sella, even in experienced hands. The method used to open the sellar floor depends on its consistency: If it is intact, opening is achieved by means of a microdrill or bone punches or both; if it is eroded or thinned, opening is achieved by means of a dissector, sometimes realizing an osteoplastic opening useful for sellar repair.117
The dura is incised in a midline position, in a linear or cross fashion, and a fragment of dura can be taken for histologic examination if it appears infiltrated.118 When the dura is incised, the surgeon must keep in mind that the perisellar sinuses,119 and particularly the superior and inferior intercavernous sinuses, are compressed and usually are obliterated by macroadenomas, making the dural incision bloodless. The situation is different with microadenomas, particularly in cases of Cushing’s disease, in which it is not unusual to find the entire sellar dura covered by one or two venous channels that can bleed during tumor resection. Caution is necessary when incising the dura in microadenomas to avoid damaging a possibly ectatic carotid artery, which may be located within the sella, especially in acromegalic patients.
Before removing an adenoma, the surgeon must keep in mind that the pituitary gland is an extra-arachnoid structure, situated below the diaphragma sellae. During the removal of a pituitary adenoma, surgical maneuvers must respect these structures, to avoid postoperative CSF leaks and other major complications. Concerning the removal of a microadenoma, if it is visible on the surface of the gland, a cleavage plane between the microadenoma and the residual anterior pituitary should be found, with the aim of delimiting the lesion. When the microadenoma is not superficial, and no change in the appearance of the overlying anterior pituitary is evident, such as discoloration or attenuated texture, a small incision can be made in the normal pituitary gland on the same side of the microadenoma, and the lesion can be removed with the help of small ring curettes. After curettage of the adenoma, a small cottonoid is inserted inside the tumor cavity and with a forceps is turned in alternate directions to mobilize fragments of the lesion or of the neoplastic capsule. Concerning the removal of macroadenomas, the surgeon first must try to remove the tumor tissue from the interior of the sella and from any lateral extension, to avoid cumbersome obstruction of the surgical field by a down-hanging, inverted diaphragma sella. If a gradual descent of the suprasellar portion of the lesion is not observed, it is useful to ask the anesthetist to perform the Valsalva maneuver, which may cause protrusion into the sellar cavity of a part of the dura and arachnoid covering the suprasellar tumor extension (suprasellar cistern), or to inject air through a lumbar drain preoperatively positioned for the same purpose.120
After lesion removal, closure of the sellar floor is performed, especially when an intraoperative CSF leak has occurred, using a variety of techniques (intradural or extradural closure of the sella, packing of the sella with or without packing of the sphenoid sinus) and different autologous and synthetic materials.121,122 Overpacking of the sella must be avoided to prevent compression of the optic system.
As with removal of lesions that often originate or develop inside the intra-arachnoid compartment, such as craniopharyngiomas or Rathke’s cleft cysts, additional considerations are appropriate. These lesions develop primarily in the suprasellar region, with an intact or only slightly enlarged sellar cavity. In such cases, an extended approach is often necessary to manage the lesion. The anterior sellar wall, the tuberculum sellae, and the posterior portion of the planum sphenoidale are drilled away, according to the circumstances, with the use of a microdrill with a diamond bur. The superior intercavernous sinus is identified, coagulated, and divided in a midline position. When the lesion is exposed, it is removed, with as much respect as possible given to the arachnoid membrane, to avoid intraoperative and postoperative complications, which seem to be more frequent than with conventional transsphenoidal surgery.83
Endoscopic Endonasal Transsphenoidal Approach: Endoscopic endonasal transsphenoidal surgery (Fig. 12-5) is a novel, minimally invasive transsphenoidal approach performed with the endoscope as a stand-alone visualizing and operating instrument, without the need for the transsphenoidal retractor. It has the same indications as the conventional microsurgical technique,33,123 and since the 1990s, it has enjoyed progressive acceptance among surgeons and patients for its minimal invasiveness and for the excellent surgical view it provides.30–33,124–127 This procedure requires specific endoscopic skills and is based on a different concept because the endoscopic view that the surgeon receives on the video monitor is not a transposition of the real image, as it would be if looking through the eyepiece of a microscope, but is the result of a microprocessor’s elaboration.
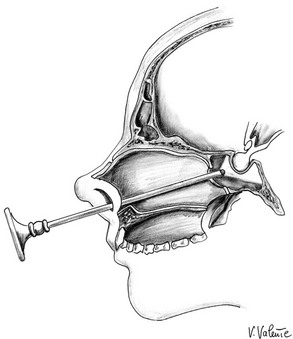
FIGURE 12-5 Sagittal view: schematic drawing of the endoscopic endonasal transsphenoidal approach. (Courtesy V. Valente, MD.)
• After the sphenoid septa have been removed, thanks to the wider view offered by the endoscope, the posterior and lateral walls of the sphenoid sinus are visible, with the sellar floor at the center, the sphenoethmoid planum above it, and the clival indentation below; lateral to the sellar floor, the bony prominences of the ICA and of the optic nerves can be seen and between them the optocarotid recess, molded by pneumatization of the optic strut of the anterior clinoid process. These prominences and depressions, especially in a well-pneumatized sphenoid sinus not invaded by a sellar lesion, define a sort of “fetal face,” where the forehead corresponds to the sphenoid planum, the eyes to the two optocarotid recesses, the eyebrows to the two optic nerve protuberances, the nose to the sella, and the mouth to the clivus, laterally limited by the two paraclival carotid artery protuberances that represent the cheeks. Nevertheless, in most sellar-type sphenoid sinuses, all these landmarks may not be clearly recognizable because of the different degree of sphenoid sinus pneumatization or extension of the lesion, but identification of the sphenoethmoid planum, of the clival indentation, and of the bony protuberances of the ICA can be considered sufficient to determine safely the edges of the sellar floor. Because of the paucity of anatomic landmarks, only in the presence of a presellar or a conchal sphenoid sinus or in some recurrences, a neuronavigation system or C-arm fluoroscopy is needed to avoid lateral misdirection, close to the parasellar and paraclival courses of the ICAs.
• Before the sellar floor is opened, a longer endoscope (4 mm in diameter, 0 degree angled lens, 30 cm in length), fixed to the holder, is positioned inside the upper portion of the nasal cavity to free both of the surgeon’s hands and to allow comfortable introduction of two instruments under the endoscope, without coming into conflict with it.
• Opening of the sellar floor, a dural incision, and lesion removal are performed in accordance with the already well-defined rules of the microsurgical transsphenoidal approach. If enough space is present in the sellar cavity, during or after lesion removal, angled endoscopes (30 degree and 45 degree) can be advanced to verify the presence of possible tumor remnants, which often are imprisoned in the recesses created by the descent of the suprasellar cistern. When the lesion extends toward the medial wall of the cavernous sinus, its removal can be accomplished under direct endoscopic vision, with the use of curved instruments and suction cannulas.
• After removal of the tumor, if evidence or risk for a CSF leak is noted, closure of the sella is performed according to common guidelines. Sellar reconstruction is performed also with the purpose of creating a barrier outward, of reducing the dead space, and of preventing the descent of the chiasm into the sellar cavity122; in certain other cases, no plugging is used.121 Because of the minimal invasiveness of the endoscopic procedure, no autologous bone or cartilage from the nasal septum is usually available, and different synthetic or resorbable materials,128 when necessary,121 must be employed to effect a safe and effective repair of the sella. Lumbar drainage is adopted in cases of intraoperative CSF leakage, only when the closure is not judged absolutely watertight, in extended approaches, or when minimal unexpected postoperative CSF leakage occurs.
• At the end of the procedure, hemostasis is obtained; final irrigation is performed; the endoscope is gradually removed; and the middle turbinate is gently restored in a medial direction, while avoiding contact with the nasal septum to prevent the formation of synechiae. Packing of the nasal cavity is not commonly considered necessary except in cases of diffuse intraoperative bleeding from the nasal mucosa, as can occur in some acromegalic patients or in poorly controlled hypertensive patients, in whom it is applied for a few hours.
• The main advantages of the endoscopic procedure compared with microsurgical procedures are related to the properties of the endoscope itself and to the absence of the nasal speculum.33,125 While avoiding use of the nasal speculum, which creates a “fixed tunnel” and an almost coaxial restriction of the microinstruments, the endoscope discloses its superior properties, permitting a wider vision of the surgical field, with a close-up “look” inside the anatomy. Angled lens endoscopes enable the surgeon to work on tumors located in suprasellar and parasellar regions under direct visual control.
• The whole procedure seems to be less traumatic, and the percentage of many complications is reduced compared with the traditional microsurgical approach.129 Because the real operation starts from the natural ostium of the sphenoid sinus and the submucosal nasal phase is avoided, septal perforations, nasal scars, damage to the nasal spine, and orodental complications due to the incision in the buccogingival junction are prevented.
• In almost all cases, no nasal packing is employed, and postoperative breathing difficulties are reduced.
• The use of an endoscopic approach is particularly advantageous in the case of recurrent or residual tumor already treated by a transsphenoidal operation,130 in which the surgeon usually finds distorted anatomy and may encounter nasal synechiae, septal perforations, mucoceles, and intrasellar scarring. With the endoscopic procedure, thanks to avoidance of the submucosal nasal phase of the microsurgical operation, the real beginning of the operation occurs at the sphenoid sinus, which already is enlarged by the former approach, rendering the procedure faster and more straightforward compared with the microsurgical transsphenoidal method. The wide anatomic view of the surgical field that the endoscope offers in the sphenoid and sellar areas minimizes the chance of a misdirected orientation when the midline anatomic landmarks are not recognizable or are absent, thus reducing the possibility of injury to the intrasellar and parasellar structures.
• The endoscopic endonasal approach can be employed in cases of intentionally two-staged transsphenoidal operations100 because of its excellent ability in reaching the sellar region during the second operation.
Disadvantages of the endoscopic approach include the requirement of a steep learning curve to enhance confidence with the unfamiliar anatomy of the nasal cavities and with specific endoscopic dexterity. Nevertheless, after adequate experience has been attained, the operating time becomes the same as or shorter than that required for transsphenoidal microsurgery, especially in cases of recurrence. The endoscope offers only bidimensional vision on the video monitor. A sense of depth can be gained by the surgeon’s experience, and the endoscope can be made to execute in and out movements, while looking for many useful different anatomic landmarks and referring to many protuberances and depressions in the sphenoid sinus, which represent reflections and shadows that correspond to different structures. Dedicated microsurgical endoscopic instruments with secure grip, straight and not bayonet shaped, which are provided with different and variably angled tips, are necessary to reach the surgical targets, particularly targets that the angled endoscopes are able to show.131,132
Extended Endoscopic Endonasal Approach to the Suprasellar Area: Lesions located in the suprasellar area historically have been approached through different extensive transcranial and/or nasofacial approaches, such as anterior, anterolateral, and/or pterional techniques.133–137 Such surgical routes often cause tissue disruption related to brain retraction and neurovascular manipulation, with sometimes aesthetically disfiguring results, and lead to higher rates of surgical morbidity and mortality.
Over past decades, the evolution of endoscopic surgical techniques and technological developments have boosted the progressive reduction in invasiveness of transcranial approaches,134,138–141 as well as of transsphenoidal surgery, in which endoscopic variance represents the last evolution of the technique. Nevertheless, the transsphenoidal route has been used historically only for sellar lesions, especially for pituitary adenomas. It was Guiot, in the early 1960s, who first renewed and fixed the indications for the transsphenoidal route142; he proposed that such a technique could be used as treatment for craniopharyngiomas and advised practitioners to consider the transsphenoidal approach, preferably for those lesions with a cystic component, with a minimal supradiaphragmatic extension and with an enlarged sella. These suggestions have lasted for over three decades,143,144 so that such a route most often was indicated for sellar or intra-suprasellar intradiaphragmatic lesions.142,144,145 An enormous contribution to the widespread use of the transsphenoidal technique is attributed to Jules Hardy, who introduced technical innovations such as the operating microscope and the concept of microadenoma. He improved the effectiveness of such approaches and, at the same time, conceived their application for the management of different sellar and skull base lesions, such as “a chordoma or a meningioma.”146
In 1987, Weiss147 termed and originally defined the extended transsphenoidal approach, while describing a transsphenoidal approach with removal of additional bone along the tuberculum sellae and the posterior planum sphenoidale between the optic canals. As a matter of fact, the dural opening was completely above the diaphragma sellae.
This approach, which initially was performed via microsurgical technique147,148 through a transnasal or sublabial route, provides midline access and direct visualization of the suprasellar space, thus offering the possibility of treatment for small, midline suprasellar lesions, which traditionally have been approached transcranially, without brain manipulation.
Besides, the possibility of reaching the suprasellar compartment via a different route—through the sellar cavity (i.e., via the transsellar/transdiaphragmatic approach)—has been reported.149,150 Nevertheless, the supradiaphragmatic structures become visible only after the intrasellar portion of the lesion has been removed. Again, it should be kept in mind that the exposure provided by such an approach most often depends on the sellar dimensions; therefore, this approach seems to be more easily amenable for those lesions that already have enlarged the sella and the diaphragma.
To achieve a more comfortable route to the supradiaphragmatic space, other authors138,148,151–160 have described a modified transsphenoidal microsurgical approach, the so-called transsphenoidal transtuberculum approach. As a matter of fact, this technique definitively overcame Guiot’s paradigm, providing enough exposure for the removal of suprasellar/supradiaphragmatic tumors. Indeed, thanks to additional bone removal from the anterior cranial base (tuberculum and posterior planum sphenoidale), such a modified extended transsphenoidal approach makes available excellent direct access to the suprasellar/supradiaphragmatic area, regardless of sellar size (even with a sella that is not enlarged), and avoids the risk for pituitary tissue injury.
More recently, widespread use of the endoscope in transsphenoidal surgery has led to new interest in this surgical technique, affording extension of the transsphenoidal approach.138,161–167 The wider and panoramic view offered by the endoscope has improved the safety of the transsphenoidal approach with the possibility of passing through a less noble structure (nasal cavity) to reach a more noble one (the brain with its neurovascular structures). Because of the enormous contributions of many groups all around the world, the applicability of such a route has been expanded to the removal of different “pure” supradiaphragmatic lesions.128,155,157,165,168–180 Indeed, the extended endoscopic approach has been adopted in many centers as treatment for pathologies such as craniopharyngiomas (intraventricular or extraventricular), tuberculum sellae, planum sphenoidale or olfactory groove meningiomas, Rathke’s cleft cysts, or even pituitary macroadenomas with suprasellar symmetric and/or asymmetric extension—all of which were once considered amenable to open transcranial surgery only (Fig. 12-6). Credit should be given to Kassam and his group in Pittsburgh for having provided the main guidelines for extended endonasal transsphenoidal management of the skull base (i.e., a binostril approach), removal of the middle turbinate on one side (usually the right one), lateralization of the middle turbinate in the other nostril (sometimes even this turbinate can be removed), removal of the posterior portion of the nasal septum, and wider sphenoidotomy, and furthermore for having systematized this strategy according to strict anatomic principles along the coronal and sagittal planes.180,181 In this way, it is possible to use two or three instruments plus the endoscope when working through both nostrils.
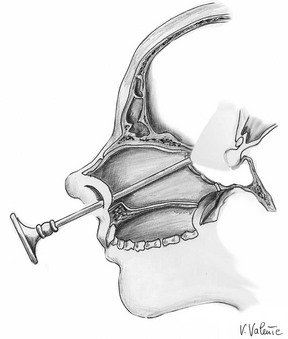
FIGURE 12-6 Sagittal view: schematic drawing of the extended endoscopic endonasal approach to the suprasellar area. (Courtesy V. Valente, MD.)
• Detailed, complete preoperative planning, integrated with three-dimensional (3D) reconstructions, so as to tailor the skull base opening with the 3D volume of the lesion
• An image-guided system (neuronavigator), which is used to intraoperatively identify the limits of the lesion, the midline, and the trajectory, and which offers better precision in defining the boundaries of bone removal and neurovascular relationships
• Dedicated instruments (i.e., high-speed low-profile microdrills, micro-Doppler probes, bipolar forceps with angled tips, low-profile ultrasonic aspirators, or radiofrequency cavitron-like coagulation) to manage properly lesions involving such a delicate environment
Although complete exposure of the posterior wall of the sphenoid sinus is achieved, a series of protuberances and depressions (according to the grade of pneumatization) become visible182; again, it is extremely important to keep in mind perfect knowledge of such anatomic landmarks so as to preserve correct orientation and better refine the bone opening.
From this point forward, the surgeon could proceed in performing a bimanual dissection while a “tuned” coworker holds the endoscope, moving it dynamically, and as requested inserts another surgical instrument.138 This so-called 3 to 4 hands technique183 requires good collaboration between two surgeons that should be tuned as if they were running a rally car race, with one—the “navigator”—holding the endoscope, and another—the “pilot”—handling two surgical instruments inside the surgical field. The pilot and the navigator therefore can pass continuously between the close-up view, as during dissecting maneuvers, and a panoramic view of neurovascular structures. However, it is possible to fix the endoscope to an autostatic holder, which can be settled by a single surgeon.
Because of the conspicuous intraoperative CSF leakage caused by the larger osteodural opening required to approach the suprasellar area, accurate reconstruction of the skull base defect is needed after lesion removal. Reconstruction has to be watertight to prevent postoperative CSF leakage, which could occur even more frequently in cases of a large opening of the arachnoid cistern, or of the third ventricle. First of all, a thin layer of fibrin glue is placed within the intradural space to seal the arachnoid space and create a first barrier against the CSF, thus filling the dead space. Thereafter, the osteodural defect is closed with a heterologous dural substitute combined with an autologous septal or turbinate bone, or even an easy-to-shape, synthetic bone substitute. The reconstruction is performed according to different techniques (i.e., intradural, extradural, and intra-extradural), even though the most effective seems to be the extradural approach.184 In this latter procedure, termed a gasket seal,185 or “grandma’s cap,”184 a single large layer of dural substitute is positioned within the extradural space, covering the dural opening, and a conformed sheath of resorbable solid material then is overlapped with the dural substitute and embedded in the extradural space, dragging the dural substitute in overlay position.
Once closure of the defect has been completed, achieving a watertight barrier, multiple layers of dural substitute are placed over to support the reconstruction; this is reinforced by the mucoperichondrium of the middle turbinate and/or a pedicled flap of septal mucosa (i.e., the Hadad-Carrau flap).186,187 This latter approach, recently introduced into clinical practice, has proved helpful in improving healing of the skull base defect.179 Finally, the sphenoid sinus is filled with cellulose gelatin (Surgicel), surgical glue (Tisseel), and/or Duraseal, to reduce dead spaces and to hold reconstruction material in place.188,189 No lumbar drainage is required at the end of the procedure; nevertheless, we advise our patients to have bed rest for 3 to 5 days, according to the grade of pneumoencephalus (especially in cases of third ventricle craniopharyngioma), while medical therapy with acetazolamide, laxatives, and wide-spectrum antibiotics is administered.
On the other hand, it should be recalled that the extended endoscopic endonasal approach to the suprasellar area is more technically demanding and requires additional skills related to use of the endoscope itself and to the opposite anatomic point of view.190,191 Besides, it is crucial to focus on some parameters related to the lesion and its anatomy, which could affect lesion management via such a route.138 Indeed, a well-pneumatized sphenoid sinus allows better visualization of all important landmarks on its posterior wall, which, again, is needed to maintain surgical orientation while bone is removed.157 On the contrary, a conchal-type sphenoid sinus could hinder the extended endonasal approach, while a small sella, with two close intracavernous carotids, could require a narrower approach.
In terms of lesion-related conditions, it has to be said that the effectiveness of the extended transsphenoidal approach could be reduced in cases of lesions with an eccentric shape and/or a wider lateral extension, with encasement of one or both ICAs and/or of the AcomA complex. Moreover, lesions that displace the optic chiasm posteriorly or that cause this latter structure to be postfixed are easily removed through this route; indeed, internal debulking of the mass from below provides enough space for dissection from the chiasm and from the optic nerves. More limited access to the suprasellar and supradiaphragmatic areas is achieved if the chiasm is prefixed or anteriorly displaced.138,168
It should be recalled that greater risk for postoperative CSF leak,173 as compared with transcranial approaches, remains a challenging matter.179,192,193 Nevertheless, improvements in reconstruction techniques and the use of new, dedicated instruments seem to significantly reduce such risks.184,186,188
Transcranial Approaches
With the selected indications described previously, many different standard transcranial or alternative skull base approaches are used routinely, depending on the direction of the extrasellar growth of the lesion. Most surgeons become familiar with one or two approaches and tend to use them in most cases. We describe just the major variations of transcranial techniques that remain in popular use for the resection of pituitary tumors with extensive suprasellar and parasellar extension: the unilateral subfrontal approach, the pterional approach, and the bilateral subfrontal interhemispheric approach. Depending on the particular compartment where the tumor is located, the size of the opening must be commensurate with the best and safest removal of the tumor—“as small as possible, as large as necessary, but cosmetically optimal.”194 With all variations, the operating microscope and dedicated microinstruments are employed, according to the general principles of central nervous system microneurosurgery, and in more recent times, the condition of endoscope-assisted microsurgery sometimes is realized.
The unilateral subfrontal approach (Fig. 12-7), described for the first time with an extradural version by McArthur in 1912,195 then by Frazier in 1913,196 and by Krause in Berlin in 1914, with a right frontal osteoplastic flap,197 was adopted by Cushing21,64 and is still in current use. This approach is indicated mainly for large suprasellar adenomas with an asymmetric supraparasellar extension and for tumors that have expanded into the upper prepontine cistern. It gives excellent bilateral access to the optic nerves and the chiasm.198
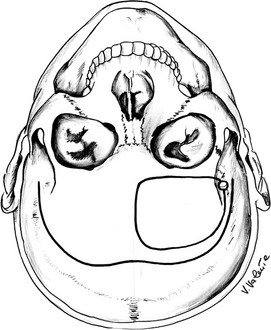
FIGURE 12-7 Schematic drawing of the unilateral subfrontal approach (seen from a surgical point of view). The bottom, curved line shows the skin incision, and the circular line shows the border of the craniotomy. (Courtesy V. Valente, MD.)
The frontolateral craniotomy, initially reported by Dandy in 1918,23,199 and subsequently modified and popularized by Yasargil194 as the pterional approach (Fig. 12-8), is a versatile craniotomy that gives good exposure of the inferolateral portion of the frontal lobe and the anterior temporal lobe. The pterional approach provides a short distance to the suprasellar region and is the craniotomy of choice for adenomas with unilateral extrasellar parasellar extension, when it is necessary to expose the compartment between the optic nerve and the ICA, or between the ICA and the third cranial nerve. It may be useful when cavernous sinus area invasion39 or a significant retrochiasmatic component is present. The opening of the basal cisterns early in the approach is used to minimize brain retraction; mannitol is used when further cerebral relaxation is necessary.
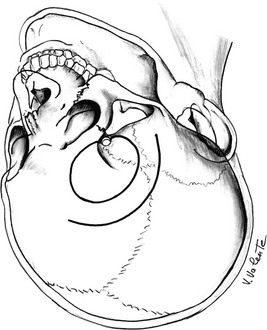
FIGURE 12-8 Schematic drawing of the pterional approach (seen from a surgical point of view). The curved line shows the skin incision, and the circle shows the border of the craniotomy. (Courtesy V. Valente, MD.)
At this time, the sequence of cisternal openings depends on the extension of the lesion: The carotid cistern is completely opened, revealing the carotid artery and its branches. The tumor can be found in the optochiasmatic cistern, the interpeduncular cistern (Liliequist’s membrane), and the cistern of the lamina terminalis. In the case of intraventricular extension, the adenoma can be reached through the translamina terminalis corridor (Fig. 12-9).200–203
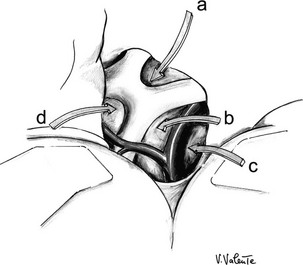
FIGURE 12-9 Schematic drawing of the different surgical corridors available by means of the pterional approach. a, Interoptic corridor. b, Optocarotid corridor. c, Corridor lateral to the internal carotid artery. d, Translamina terminalis corridor. (Courtesy V. Valente, MD.)
When a large pituitary tumor invades the parasellar-cavernous sinus area and the adjacent central skull base regions, this approach can be modified in keeping with Dolenc’s technique.39 A transbasal approach is performed: It is extradural and consists of an osteoplastic frontotemporoparietal craniotomy; unroofing of the orbit; resection of the sphenoid wing and the anterior clinoid process; opening of the optic canal; and exposure of cranial nerves III, IV, and VI.
The bilateral interhemispheric subfrontal approach (Fig. 12-10), initially described by Killiani204 in 1904 for the removal of chiasmatic lesions, and variously used by many neurosurgeons for selected anterior cranial fossa lesions, such as large olfactory groove meningiomas, is not used today as frequently as the other two options described here. It has been revised recently205–207 as treatment for large craniopharyngiomas with retrochiasmatic extension. It offers wide exposure of the anterior cranial base with a good overview of the sellar, suprasellar, and parasellar areas. It affords an excellent midline orientation and may be used as treatment for huge pituitary adenomas with large bilateral suprasellar extension because occasionally, a unilateral approach is insufficient for adequate bilateral management of the lesion. This technique requires patience and time, which can be rewarded fully by the technical advantages provided by excellent exposure of the surgical field.
Radiosurgery
Because the main limitation of gamma knife or LINAC radiosurgery is the distance between the tumor margin and the optic chiasm, appropriate candidates for these treatments208 are patients with a small residual tumor or a tumor confined to the cavernous sinus, in whom the risk for damage to vision is minimized. The delay in reducing excessive hormone secretion to normal is shorter than with conventional radiotherapy.209–211
Complications
Complications of pituitary surgery depend on the surgical route employed to reach the sella. We refer to transsphenoidal (microsurgical and endoscopic) and transcranial complications. Microsurgical transsphenoidal surgery, with its lack of visible scars and lower mortality and morbidity compared with conventional transcranial approaches, is appealing to patients and physicians. Serious complications of transsphenoidal surgery are uncommon and seem to be related to the size of the tumor and the experience of the surgeon. Nevertheless, even if the mortality rate is low (usually <1%),212–215 complications still occur.216 Major morbidity (CSF leak, meningitis, stroke, intracranial hemorrage, and visual loss) occurs in 3.4% of cases, whereas minor complications (sinus disease, nasal septal perforations) are present in approximately 4.6% of procedures.67 Microsurgical transsphenoidal approach complications can be divided into different groups, according to the anatomic structures and the systems that may be involved. The following categories have been identified:
1. Nasofacial complications (approach complications)
2. Sphenoid sinus complications
3. Sella turcica complications
Nasofacial complications, including nasal septal perforations, bleeding from the mucosal branches of the sphenopalatine artery, injury or fracture of the cribriform plate with subsequent CSF leak, anesthesia of the upper lip and of the anterior maxillary teeth, saddle nose, anosmia caused by undue superior nasal septum dissection, diastasis of the maxilla, and fracture of the hard palate due to overspreading of the speculum, have been reported. Sphenoid sinus complications that occur more frequently are sinusitis and mucocele, a rare and usually late-onset disorder caused by obstruction of airflow at the osteomeatal complex. Fracture of the sphenoid body with injury to the optic nerves and the carotid arteries, sometimes due to thin or absent bone, is exceptional but must be kept in mind. Sella turcica complications that may occur include CSF leak due to violation of the arachnoid membrane, subarachnoid hemorrhage, vasospasm, and tension pneumocephalus. A wide range of suprasellar and parasellar complications have been reported: hypothalamic injury, resulting from direct surgical injury or from hemorrage or ischemia provoked by the procedure; visual damage, caused by direct surgical trauma, hemorrhage, or ischemia; vascular injury to one of the vessels of the circle of Willis, which represents one of the main sources of operative mortality66,217; meningitis, related to a CSF leak or to contamination; cavernous sinus injury (ICA; sixth, thirth, and fourth cranial nerve injury), resulting from surgical maneuvers performed to remove the lesion extending into the parasellar area; and brain stem injury, caused by a misdirected approach to the clivus. These complications occur infrequently. The endocrine sequelae are the most frequent complications after a transsphenoidal procedure. Loss of one or more anterior pituitary functional axes occurs in approximately 3% of microadenomas, whereas in macroadenomas, this occurs in about 5% of cases.67 Permanent diabetes insipidus occurs in 3% of cases.67
For the endoscopic transsphenoidal approach, differences in the type of complications are noted compared with complications described with the microsurgical transsphenoidal approach. These differences arise from the different type of approach used and from the absence of a nasal speculum in the endoscopic procedure. The endoscopic approach is endonasal, whereas the microsurgical approach has a phase in which the oral mucosa or nasal septum or both are dissected; this, even if rarely, can be the reason for anesthesia of the upper lip and of the anterior maxillary teeth, nasal septal perforations, saddle nose, and anosmia. The lack of a nasal speculum avoids the development of other rare complications, such as diastasis of the maxilla and fracture of the hard palate, which are due to overspreading of the speculum; fracture of the orbit; and injury or fracture of the cribriform plate and subsequent CSF leak. Bleeding from the mucosal branches of the sphenopalatine artery218 also is possible with the endoscopic approach. Series of endoscopic operations125,127,129 showed an overall decreased incidence of complications compared with historical microsurgical transsphenoidal series.213 In addition to a decrease in functional and esthetic nasofacial complications, a correlated decrease is seen in all the other complications described in the literature. The explanation for the reduced complication rate might be found in the wider “overview inside the anatomy,” facilitated by the endoscope, and in decreased surgical trauma with the endoscopic approach itself.
Transcranial approaches are associated with significantly higher morbidity and mortality compared with the transsphenoidal route; morbidity and mortality have decreased in the microsurgical era. Direct comparison of the complications between the two groups is not possible because the respective inclusion criteria have changed over the years. One aspect that should not be underestimated is that nowadays transcranial surgery usually is employed for giant and invasive pituitary adenomas,73,219,220 or for adenomas invading the parasellar compartment or the central skull base,221 and representing a cohort of subjects with the most difficult surgical management and intricate surgical problems. Despite these considerations, a surprisingly high total tumor resection rate (63% to 96%) has been reported more recently.221 In a similar subset of patients, the recurrence rate has been 15.2%.73
Specific complications, common to any supratentorial craniotomy, are related to traction on the frontal and temporal lobes, dissection of major or perforating vessels, and manipulation of the optic or oculomotor nerves.222 The most feared complication is postoperative hematoma in the sellar and suprasellar region, leading to coma and autonomic deterioration. The most common postoperative complication is diabetes insipidus, immediate, delayed (4 to 5 days), or triphasic; transient (31.8%) or permanent (21.1%); followed by hemiparesis, transient (33.3%), or permanent (9.1%).73 Loss of vision can occur, most commonly as the result of disruption of the blood supply to the chiasm or the optic nerves. Other complications, such as worsening of anterior pituitary function, epilepsy, infection, and CSF leak, also can occur. The strict operative mortality (5%) or mortality from disease-related complications (11.3%) is not negligible.73
References
1. Fahlbusch, R, Buchfelder, M, Nomikos, P. Pituitary Surgery. In: Melmed S, ed. The Pituitary. Malden, MA: Blackwell; 2002:405–417.
2. Horsley, V. Address in surgery on the technic of operation on the central nervous system. Br Med J. 1906;2:411–423.
3. Caton, R, Paul, FT. Notes on a case of acromegaly treated by operation. Br Med J. 1893;2:1421–1423.
4. Jane, JA, Jr., Thapar, K, Laws, ER, Jr. A history of pituitary surgery. Oper Tech Neurosurg. 2002;5:200–209.
5. Schloffer, H. Erfolgreiche Operationen eines Hypophysentumors auf Nasalem Wege. Wien Clin Wochenschr. 1907;20:621–624.
6. Artico, M, Pastore, FS, Fraioli, B, et al. The contribution of Davide Giordano (1864–1954) to pituitary surgery: the transglabellar-nasal approach. Neurosurgery. 1998;42:909–912.
7. Giordano, D. Compendio di Chirurgia Operativa Italiana. Torino: UTET; 1911.
8. von Eiselsberg, A, von Frankl-Hochwart, L. Uber die operative Behandlung der Tumoren der Hypophysisgegend. Neurol Centralblatt. 1907;26:994–1001.
9. Kocher, T. Ein Fall von Hypophysis-Tumor mit Operativer Heilung. Dtsch Zeitschrift Chir. 1909;100:13–37.
10. Lanzino, G, Laws, ER, Jr. Pioneers in the development of transsphenoidal surgery: Theodor Kocher, Oskar Hirsch, and Norman Dott. J Neurosurg. 2001;95:1097–1103.
11. Kanavel, AB. The removal of tumors of the pituitary body by an infranasal route: a proposed operation with a description of the technique. JAMA. 1909;53:1704–1707.
12. Kanavel, AB, Grinker, J. Removal of tumors of the pituitary body with a suggestion as to a two-step route, and a report of a case with a malignant tumor operated upon with primary recovery. Surg Gynecol Obstet. 1910;10:414–418.
13. Hirsch, O. Uber Methoden der Behandlung von Hypophysistumoren auf endonasalem Wege. Arch Laryngol Rhinol. 1911;24:129–177.
14. Hajek, M. Zur Diagnose und intranasalen chirurgischen Behandlung der Eiterungen der Keilbeinhöhle und des hinteren Siebbeinlabyrinthes. Arch Laryngol Rhinol. 1904;16:105–143.
15. Kilian, G. Die submuköse Fensterresektion der Nasenscheidewand. Arch Laryngol Rhinol. 1904;16:362–387.
16. Hamlin, H. Oskar Hirsch. Surg Neurol. 1981;16:391–393.
17. Halstead, AE. The operative treatment of tumors of the hypophysis. Surg Gynecol Obstet. 1910;10:494.
18. Halstead, AE. Remarks on the operative treatment of tumors of the hypophysis: with the report of two cases operated on by an oronasal method. Tran Am Surg Assoc. 1910;28:73–93.
19. Cushing, H. Partial hypophysectomy for acromegaly: with remarks on the functions on the hypophysis. Ann Surg. 1909;30:1002–1017.
20. Cushing, H. Surgical experiences with pituitary disorders. JAMA. 1914;63:1515–1525.
21. Cushing, H. Intracranial Tumors: Notes upon a Series of Two Thousand Verified Cases with Surgical-Mortality Percentages Pertaining Thereto. Springfield, IL: Charles C Thomas; 1932.
22. Rosegay, H. Cushing’s legacy to transsphenoidal surgery. J Neurosurg. 1981;54:448–454.
23. Dandy, WE. The brain. In: Lewis D, ed. Practice of Surgery. Hagerstown, MD: WF Prior; 1934:556–605.
24. Heuer, GJ. Surgical experiences with an intracranial approach to chiasmal lesions. Arch Surg. 1920;1:368–381.
25. Heuer, GJ. The surgical approach and the treatment of tumors and other lesions about the optic chiasm. Surg Gynecol Obstet. 1931;53:489–518.
26. Liu, JK, Das, K, Weiss, MH, et al. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083–1096.
27. Hardy, J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg. 1969;16:185–217.
28. Cappabianca, P, de Divitiis, O, Maiuri, F. Evolution of transsphenoidal surgery. In: de Divitiis E, Cappabianca P, eds. Endoscopic Endonasal Transsphenoidal Surgery. New York: Springer; 2003:1–7.
29. Guiot, G, Rougerie, J, Fourestier, M, et al. Explorations endoscopiques intracraniennes. Presse Med. 1963;71:1225–1228.
30. Carrau, R, Jho, HD, Ko, Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914–918.
31. Jho, HD, Carrau, RL, Ko, Y. Endoscopic pituitary surgery. In: Wilkins H, Rengachary S, eds. Neurosurgical Operative Atlas. Park Ridge, IL: American Association of Neurological Surgeons; 1996:1–12.
32. Cappabianca, P, Alfieri, A, de Divitiis, E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg. 1998;41:66–73.
33. de Divitiis, E, Cappabianca, P, Cavallo, LM. Endoscopic endonasal transsphenoidal approach to the sellar region. In: de Divitiis E, Cappabianca P, eds. Endoscopic Endonasal T Transsphenoidal Surgery. New York: Springer; 2003:91–130.
34. de Divitiis, E, Spaziante, R, Stella, L. Empty sella and benign intrasellar cysts. In: Krayenbühl H, ed. Advances and Technical Standards in Neurosurgery. New York: Springer-Verlag; 1981:3–74.
35. Aron, DC, Findling, JW, Tyrell, JB. Hypothalamus and pituitary. In: Greenspan FS, Strewler GJ, eds. Basic and Clinical Endocrinology. Stanford: Appleton & Lange; 1997:95–156.
36. Alfieri, A, Jho, HD. Endoscopic endonasal cavernous sinus surgery: an anatomical study. Neurosurgery. 2001;48:827–837.
37. Alfieri, A, Jho, HD. Endoscopic endonasal approaches to the cavernous sinus: surgical approaches. Neurosurgery. 2001;49:354–362.
38. Cavallo, LM, Cappabianca, P, Galzio, R, et al. Endoscopic transnasal approach to the cavernous sinus versus transcranial route: anatomic study. Neurosurgery. 2005;56(2 suppl):379–389.
39. Dolenc, VV. Transcranial epidural approach to pituitary tumors extending beyond the sella. Neurosurgery. 1997;41:542–550.
40. Partington, M, Davis, DH, Laws, ER, et al. Pituitary adenomas in childhood and adolescence. J Neurosurg. 1994;80:209–216.
41. Amar, AP, Weiss, MH. Pituitary anatomy and physiology. Neurosurg Clin North Am. 2003;13:11–23.
42. Kirgis, HD, Locke, W. Anatomy and embriology. In: Locke W, Schally AV, eds. The Hypothalamus and Pituitary in Health and Disease. Springfield, IL: Charles C Thomas; 1972:3–65.
43. Schaeffer, JP. Some points in the regional anatomy of the optic pathway, with special reference to tumors of the hypophysis cerebri and resulting ocular changes. Anat Rec. 1924;28:243–279.
44. Elias, WJ, Chadduck, JB, Alden, TD, et al. Frameless stereotaxy for transsphenoidal surgery. Neurosurgery. 1999;45:271–277.
45. Jane, JA, Jr., Thapar, K, Alden, TD, et al. Fluoroscopic frameless stereotaxy for transsphenoidal surgery. Neurosurgery. 2001;48:1302–1308.
46. Kajiwara, K, Nishikazi, T, Ohmoto, Y, et al. Image-guided transsphenoidal surgery for pituitary lesions using Mehrkoordinaten Manipulator (MKM) navigation system. Minim Invasive Neurosurg. 2003;46:78–81.
47. Lasio, G, Ferroli, P, Felisati, G, et al. Image-guided endoscopic transnasal removal of recurrent pituitary adenomas. Neurosurgery. 2002;51:132–137.
48. Ohhashi, G, Kamio, M, Abe, T, et al. Endoscopic transnasal approach to the pituitary lesions using a navigation system (Insta Trak system): technical note. Minim Invasive Neurosurg. 2002;45:120–123.
49. Sandeman, D, Moufid, A. Interactive image-guided pituitary surgery: an experience of 101 procedures. Neurochirurgie. 1998;44:331–338.
50. Bohinski, RJ, Warnick, RE, Gaskill-Shipley, MF, et al. Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery. Neurosurgery. 2001;49:1133–1144.
51. Fahlbusch, R, Ganslandt, O, Buchfelder, M, et al. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg. 2001;95:381–390.
52. Lewin, JS, Metzger, A, Selman, WR. Intraoperative magnetic resonance image guidance in neurosurgery. J Magn Reson Imaging. 2000;12:512–524.
53. Martin, CH, Schwartz, R, Jolesz, F, et al. Transsphenoidal resection of pituitary adenomas in an intraoperative MRI unit. Pituitary. 1999;2:155–162.
54. Nimsky, C, Ganslandt, O, Hofmann, B, et al. Limited benefit of intraoperative low-field magnetic resonance imaging in craniopharyngioma surgery. Neurosurgery. 2003;53:72–81.
55. Steinmeier, R, Fahlbusch, R, Ganslandt, O, et al. Intraoperative magnetic resonance imaging with the magnetom open scanner: Concepts, neurosurgical indications, and procedures: a preliminary report. Neurosurgery. 1998;43:739–748.
56. Bills, D, Meyer, F, Laws, ER, Jr., et al. A retrospective analysis of pituitary apoplexy. Neurosurgery. 1993;33:602–609.
57. Ebersold, MJ, Laws, ER, Jr., Scheithauer, BW, et al. Pituitary apoplexy treated by transsphenoidal surgery: a clinicopathological and immunocytochemical study. J Neurosurg. 1983;58:315–320.
58. Laws, ER, Jr., Trautmann, JC, Hollenhorst, RW, Jr. Trans-sphenoidal decompression of the optic nerve and chiasm: visual results in 62 patients. J Neurosurg. 1977;46:717–722.
59. Laws, ER, Jr. Surgical management of pituitary apoplexy. In: Welch K, Caplan L, Reis D, eds. Primer on Cerebrovascular Diseases. New York: Academic Press; 1997:508–510.
60. Colao, A, Ferone, D, Marzullo, P, et al. Long-term effect of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegaly. J Clin Endocrinol Metab. 2001;86:2779–2786.
61. Colao, A, Di Sarno, A, Cappabianca, P, et al. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med. 2003;349:2023–2033.
62. Di Sarno, A, Landi, ML, Cappabianca, P, et al. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001;86:5256–5261.
63. Cohen, AR, Cooper, PR, Kupersmith, MJ, et al. Visual recovery after transsphenoidal removal of pituiatry adenomas. Neurosurgery. 1985;17:446–452.
64. Henderson, WR. The pituitary adenomata: a follow-up study of the surgical results in 338 cases (Dr Harvey Cushing’s series). Br J Surg. 1939;26:811–921.
65. Laws, ER, Jr. Pituitary surgery. Endocrinol Metab Clin North Am. 1987;16:647–665.
66. Laws, ER, Jr., Thapar, K. Pituitary surgery. Endocrinol Metab Clin North Am. 1999;28:119–131.
67. Thapar, K, Laws, ER, Jr. Pituitary tumors. In: Kaye AW, Laws ER, Jr., eds. Brain Tumors. London: Churchill Livingstone; 2001:804–854.
68. Wilson, CB. Role of surgery in the management of pituitary tumors. Neurosurg Clin North Am. 1990;1:139–159.
69. Baskin, DS, Wilson, CB. Transsphenoidal treatment of non-neoplastic intrasellar cysts: a report of 38 cases. J Neurosurg. 1984;60:8–13.
70. El-Mahdy, W, Powell, M. Transsphenoidal management of 28 symptomatic Rathke’s cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery. 1998;42:7–17.
71. Ross, DA, Norman, D, Wilson, CB. Radiologic characteristics and results of surgical management of Rathke’s cysts in 43 patients. Neurosurgery. 1992;30:173–179.
72. Ciric, IS, Cozzens, JW. Craniopharyngiomas: transsphenoidal method of approach—for the virtuoso only? Clin Neurosurg. 1980;27:169–187.
73. Yasargil, MG. Transcranial surgery for large pituitary adenomas. In: Yasargil MG, ed. Microneurosurgery: Microneurosurgery of CNS Tumors. Stuttgart: Georg Thieme Verlag; 1996:200–204.
74. Abe, T, Lüdecke, DK. Transnasal surgery for infradiaphragmatic craniopharyngiomas in pediatric patients. Neurosurgery. 1999;44:957–966.
75. Laws, ER, Jr. Transsphenoidal microsurgery in the management of craniopharyngioma. J Neurosurg. 1980;52:661–666.
76. Spaziante, R, de Divitiis, E. Drainage techniques for cystic craniopharyngiomas. Neurosurg Quart. 1997;7:183–208.
77. Guiot, G. Transsphenoidal approach in surgical treatment of pituitary adenomas: general principles and indications in non-functioning adenomas. In: Kohler PO, Ross GT, eds. Diagnosis and Treatment of Pituitary Adenomas. Amsterdam: Excerpta Medica; 1973:159–178.
78. Castelnuovo, P, Locatelli, D, Mauri, S, et al. Extended endoscopic approaches to the skull base. Anterior cranial base CSF leaks. In: de Divitiis E, Cappabianca P, eds. Endoscopic Endonasal Transsphenoidal Surgery. New York: Springer; 2003:137–158.
79. de Divitiis, E, Cappabianca, P, Cavallo, LM. Endoscopic transsphenoidal approach: adaptability of the procedure to different sella lesions. Neurosurgery. 2002;51:699–707.
80. Jane, JA, Jr., Thapar, K, Kaptain, GJ, et al. Pituitary surgery: transsphenoidal approach. Neurosurgery. 2002;51:435–444.
81. Jho, HD. The expanding role of endoscopy in skull-base surgery: indications and instruments. Clin Neurosurg. 2001;48:287–305.
82. Jho, HD. Endoscopic endonasal approach to the optic nerve: a technical note. Minim Invasive Neurosurg. 2001;44:190–193.
83. Kaptain, GJ, Vincent, DA, Sheehan, JP, et al. Transsphenoidal approaches for extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2001;49:94–101.
84. Kato, T, Sawamura, J, Abe, H, et al. Transsphenoidal-transtuberculum sellae approach for supradiaphragmatic tumours: technical note. Acta Neurochir. 1998;140:715–719.
85. Kim, J, Choe, I, Bak, K, et al. Transsphenoidal supradiaphragmatic intradural approach: technical note. Minim Invasive Neurosurg. 2000;43:33–37.
86. Kouri, JG, Chen, MY, Watson, JC, et al. Resection of suprasellar tumors by using a modified transsphenoidal approach. J Neurosurg. 2000;92:1028–1035.
87. Mason, RB, Nieman, LK, Doppman, JL, et al. Selective excision of adenomas originating in or extending into the pituitary stalk with preservation of pituitary function. J Neurosurg. 1997;87:343–351.
88. Weiss, WH. The transnasal transsphenoidal approach. In: Apuzzo MLJ, ed. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:476–494.
89. Jho, HD, Carrau, RL, McLaughlin, ML, et al. Endoscopic transsphenoidal resection of a large chordoma in the posterior fossa. Acta Neurochir. 1997;139:343–348.
90. Kelley, TF, Stankiewicz, JA, Chow, JM, et al. Endoscopic closure of postsurgical anterior cranial fossa cerebrospinal fluid leaks. Neurosurgery. 1996;39:743–746.
91. Lalwani, AK, Kaplan, MJ, Gutin, PH. The transsphenoethmoid approach to the sphenoid sinus and clivus. Neurosurgery. 1992;31:1008–1014.
92. Laws, ER, Jr. Transsphenoidal surgery. In: Apuzzo MLJ, ed. Brain Surgery: Complications Avoidance and Management. New York: Churchill Livingstone; 1993:357–362.
93. Maira, G, Pallini, R, Anile, C, et al. Surgical treatment of clival chordomas: the transsphenoidal approach revisited. Neurosurgery. 1996;85:784–792.
94. Fraioli, B, Esposito, V, Santoro, A, et al. Transmaxillosphenoidal approach to tumors invading the medial compartment of the cavernous sinus. J Neurosurg. 1995;82:63–69.
95. Frank, G, Pasquini, E. Extended endoscopic approaches to the skull base: approach to the cavernous sinus. In: de Divitiis E, Cappabianca P, eds. Endoscopic Endonasal Transsphenoidal Surgery. New York: Springer; 2003:159–175.
96. Hermier, M, Turjman, F, Tournut, P, et al. Intracranial aneurysm associated with pituitary adenoma shown by MR angiography: case report. Neuroradiology. 1994;36:115–116.
97. Inoue, T, Rhoton, AL, Jr., Theele, D, et al. Surgical approaches to the cavernous sinus: a microsurgical study. Neurosurgery. 1990;26:903–932.
98. Kitano, M, Taneda, M. Extended transsphenoidal approach with submucosal posterior ethmoidectomy for parasellar tumors: technical note. J Neurosurg. 2001;94:999–1004.
99. Sabit, I, Schaefer, SD, Couldwell, T. Extradural extranasal combined transmaxillary transsphenoidal approach to the cavernous sinus: A minimally invasive microsurgical model. Laryngoscope. 2000;110:286–291.
100. Saito, K, Kuwayama, A, Yamamoto, N, et al. The transsphenoidal removal of non functioning pituitary adenomas with suprasellar extension: the open sella method and intentionally staged operation. Neurosurgery. 1995;36:668–676.
101. Mortini, P, Giovanelli, M. Transcranial approaches to pituitary tumors. Oper Tech Neurosurg. 2002;5:1–13.
102. Van Alpen, HA. Microsurgical fronto-temporal approach to pituitary adenomas with extrasellar extension. Clin Neurol Neurosurg. 1975;78:246–256.
103. Alleyne, CH, Jr., Barrow, DL, Oyesiku, NM. Combined transsphenoidal and pterional craniotomy approach to giant pituitary tumors. Surg Neurol. 2002;57:380–390.
104. Ishii, K, Ikeda, H, Takahashi, S, et al. MR imaging of pituitary adenomas with sphenoid sinus invasion: characteristic MR findings indicating fibrosis. Radiat Med. 1996;14:173–178.
105. Iuchi, T, Saeki, N, Tanaka, M, et al. MRI prediction of fibrous pituitary adenomas. Acta Neurochir. 1998;140:779–786.
106. Snow, RB, Johnson, CE, Morgello, S, et al. Is magnetic resonance imaging useful in guiding the operative approach to large pituitary tumors? Neurosurgery. 1990;26:801–803.
107. Patterson, RH. The role of transcranial surgery in the management of pituitary adenoma. Acta Neurochir. 1996;65(Suppl):16–17.
108. Esiri, M, Bevan, JS, Burke, CW, et al. Effect of bromocriptine treatment on the fibrous tissue content of prolactin-secreting and nonfunctioning macroadenomas of the pituitary gland. J Clin Endocrinol Metab. 1986;63:383–388.
109. Barkan, AL, Lloyd, RV, Chandler, WF, et al. Preoperative treatment of acromegaly with long-acting somatostatin analog SMS 201-995: Shrinkage of invasive pituitary macroadenomas and improved surgical remission rate. J Clin Endocrinol Metab. 1988;67:1040–1048.
110. Ezzat, S, Horvath, E, Harris, AG, et al. Morphological effects of octreotide on growth hormone-producing pituitary adenomas. J Clin Endocrinol Metab. 1994;79:113–118.
111. Rhoton, AL, Jr. Operative techniques and instrumentation for neurosurgery. Neurosurgery. 2003;53:907–934.
112. Revuelta, R, Arriada-Mendicoa, N, Ramirez-Alba, J, et al. Simultaneous treatment of a pituitary adenoma and an internal carotid artery aneurysm through a supraorbital keyhole approach. Minim Invasive Neurosurg. 2002;45:109–111.
113. Elias, WJ, Laws, ER, Jr. Transsphenoidal approach to lesions of the sella. In: Schmidek HH, ed. Schmidek and Sweet Operative Neurosurgical Techniques. Philadelphia: WB Saunders; 2000:373–384.
114. Rhoton, AL, Jr. The supratentorial cranial space: microsurgical anatomy and surgical approaches. Neurosurgery. 2002;51(Suppl 1):335–374.
115. Zada, G, Kelly, DF, Cohan, P, et al. The endonasal transsphenoidal approach for pituitary adenomas and other sellar lesions: an assessment of efficacy, safety and patient impressions. J Neurosurg. 2003;98:350–358.
116. Landolt, AM, Schiller, Z. Surgical technique: transsphenoidal approach. In: Landolt AM, Vanve ML, Reilly PR, eds. Pituitary Adenomas. New York: Churchill Livingstone; 1996:315–331.
117. de Divitiis, E, Spaziante, R. Osteoplastic opening of the sellar floor in transsphenoidal surgery: technical note. Neurosurgery. 1987;20:445–446.
118. Meij, B, Lopes, MB, Ellegala, DB, et al. The long term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg. 2002;96:195–208.
119. de Divitiis, E, Spaziante, R, Iaccarino, V, et al. Phlebography of the cavernous and intercavernous sinuses. Surg Neurol. 1981;15:306–312.
120. Spaziante, R, de Divitiis, E. Forced subarachnoid air in transsphenoidal excision of pituitary tumours (pumping technique). J Neurosurg. 1989;71:864–867.
121. Cappabianca, P, Cavallo, LM, Esposito, F, et al. Sellar repair in endoscopic endonasal transsphenoidal surgery: results of 170 cases. Neurosurgery. 2002;51:1365–1372.
122. Spaziante, R, de Divitiis, E, Cappabianca, P. Repair of the sella after transsphenoidal surgery. In: Schmideck HH, ed. Schmidek and Sweet Operative Neurosurgical Techniques. Philadelphia: WB Saunders; 2000:398–416.
123. Jho, HD. Endoscopic surgery of pituitary adenomas. In: Krisht AF, Tindall GT, eds. Comprehensive Management of Pituitary Disorders. Hagerstown, MD: Lippincott Williams & Wilkins; 1999:389–403.
124. Cappabianca, P, Cavallo, LM, Colao, A, et al. Endoscopic endonasal transsphenoidal approach: Outcome analysis of 100 consecutive procedures. Minim Invasive Neurosurg. 2002;45:1–8.
125. de Divitiis, E, Cappabianca, P. Endoscopic endonasal transsphenoidal surgery. In: Pickard JD, ed. Advances and Technical Standards in Neurosurgery. New York: Springer Verlag; 2002:137–177.
126. Jho, HD, Carrau, RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg. 1997;87:44–51.
127. Jho, HD. Endoscopic transsphenoidal surgery. In: Schmidek HH, ed. Schmidek and Sweet Operative Neurosurgical Techniques. Philadelphia: WB Saunders; 2000:385–397.
128. Cappabianca, P, Cavallo, LM, Mariniello, G, et al. Easy sellar reconstruction in endoscopic transsphenoidal surgery with polyester-silicone dural substitute and fibrin glue: technical note. Neurosurgery. 2001;49:473–476.
129. Cappabianca, P, Cavallo, LM, Colao, A, et al. Surgical complications of the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97:293–298.
130. Cappabianca, P, Alfieri, A, Colao, A, et al. Endoscopic endonasal transsphenoidal surgery in recurrent and residual pituitary adenomas: technical note. Minim Invasive Neurosurg. 2000;43:38–43.
131. Cappabianca, P, Alfieri, A, Thermes, S, et al. Instruments for endoscopic endonasal transsphenoidal surgery. Neurosurgery. 1999;45:392–396.
132. Leonhard, M, Cappabianca, P, de Divitiis, E. The endoscope, endoscopic equipment and instrumentation. In: de Divitiis E, Cappabianca P, eds. Endoscopic Endonasal Transsphenoidal Surgery. New York: Springer; 2003:9–19.
133. Fahlbusch, R, Schott, W. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002;96:235–243.
134. Goel, A, Muzumdar, D, Desai, KI. Tuberculum sellae meningioma: a report on management on the basis of a surgical experience with 70 patients. Neurosurgery. 2002;51:1358–1363.
135. Hakuba, A, Liu, S, Nishimura, S. The orbitozygomatic infratemporal approach: a new surgical technique. Surg Neurol. 1986;26:271–276.
136. Hakuba, A, Tanaka, K, Suzuki, T, et al. A combined orbitozygomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus. J Neurosurg. 1989;71:699–704.
137. Kawase, T, Shiobara, R, Toya, S. Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery. 1991;28:869–875.
138. de Divitiis, E, Cavallo, LM, Cappabianca, P, et al. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: Part 2. Neurosurgery. 2007;60:46–58.
139. Delashaw, JB, Jr., Tedeschi, H, Rhoton, AL. Modified supraorbital craniotomy: technical note. Neurosurgery. 1992;30:954–956.
140. Jallo, GI, Benjamin, V. Tuberculum sellae meningiomas: microsurgical anatomy and surgical technique. Neurosurgery. 2002;51:1432–1439.
141. Reisch, R, Perneczky, A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005;57:242–255.
142. Guiot, G. Transsphenoidal approach in surgical treatment of pituitary adenomas: general principles and indications in non-functioning adenomas. In: Kohler PO, Ross GT, eds. Diagnosis and treatment of pituitary adenomas. Amsterdam: Excerpta Medica; 1973:159–178.
143. Liu, JK, Das, K, Weiss, MH, et al. The history and evolution of transsphenoidal surgery. J Neurosurg. 2001;95:1083–1096.
144. McDonald, TJ, Laws, ER, Jr. Historical aspects of the management of pituitary disorders with emphasis on transsphenoidal surgery. In: Laws ER, Jr., Randall RV, Kern EB, Abboud CF, eds. The Management of Pituitary Adenomas and Related Lesions with Emphasis on Transsphenoidal Microsurgery. New York: Appleton-Century-Crofts; 1982:1–13.
145. Laws, ERJ, Transsphenoidal surgery. Brain surgery: Complications Avoidance and Management. Apuzzo, MLJ, eds. Brain surgery: Complications Avoidance and Management, Vol 1. New York: Churchill Livingstone, 1993:357–362.
146. Hardy, J. Transsphenoidal hypophysectomy. J Neurosurg. 1971;34:582–594.
147. Weiss, MH. The transnasal transsphenoidal approach. In: Apuzzo MLJ, ed. Surgery of the third ventricle. Baltimore: Williams & Wilkins; 1987:476–494.
148. Kato, T, Sawamura, Y, Abe, H, et al. Transsphenoidal-transtuberculum sellae approach for supradiaphragmatic tumours: technical note. Acta Neurochir (Wien). 1998;140:715–719.
149. Mason, RB, Nieman, LK, Doppman, JL, et al. Selective excision of adenomas originating in or extending into the pituitary stalk with preservation of pituitary function. J Neurosurg. 1997;87:343–351.
150. Laws, ER, Kanter, AS, Jane, JA, Jr., et al. Extended transsphenoidal approach. J Neurosurg. 2005;102:825–827.
151. Cook, SW, Smith, Z, Kelly, DF. Endonasal transsphenoidal removal of tuberculum sellae meningiomas: technical note. Neurosurgery. 2004;55:239–244.
152. Couldwell, WT, Weiss, MH, Rabb, C, et al. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55:539–550.
153. Kim, J, Choe, I, Bak, K, et al. Transsphenoidal supradiaphragmatic intradural approach: technical note. Minim Invasive Neurosurg. 2000;43:33–37.
154. Kouri, JG, Chen, MY, Watson, JC, et al. Resection of suprasellar tumors by using a modified transsphenoidal approach. Report of four cases. J Neurosurg. 2000;92:1028–1035.
155. Dusick, JR, Esposito, F, Kelly, DF, et al. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102:832–841.
156. Kaptain, GJ, Vincent, DA, Sheehan, JP, et al. Transsphenoidal approaches for the extracapsular resection of midline suprasellar and anterior cranial base lesions. Neurosurgery. 2001;49:94–101.
157. Cappabianca, P, Frank, G, Pasquini, E, et al. Extended endoscopic endonasal transsphenoidal approaches to the suprasellar region, planum sphenoidale and clivus. In: de Divitiis E, Cappabianca P, eds. Endoscopic endonasal transsphenoidal surgery. Wien – New York: Springer; 2003:176–187.
158. Laufer, I, Anand, VK, Schwartz, TH. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106:400–406.
159. Kitano, M, Taneda, M. Extended transsphenoidal surgery for suprasellar craniopharyngiomas: infrachiasmatic radical resection combined with or without a suprachiasmatic trans-lamina terminalis approach. Surg Neurol. 2009;71:290–298.
160. Kitano, M, Taneda, M, Nakao, Y. Postoperative improvement in visual function in patients with tuberculum sellae meningiomas: results of the extended transsphenoidal and transcranial approaches. J Neurosurg. 2007;107:337–346.
161. Cappabianca, P, Alfieri, A, de Divitiis, E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg. 1998;41:66–73.
162. Cappabianca, P, de Divitiis, E. Endoscopic pituitary surgery. Anatomy and surgery of the transsphenoidal approach to the sellar region. Tuttlingen: Endo-Press; 2001.
163. Cappabianca, P, de Divitiis, E. Endoscopy and transsphenoidal surgery. Neurosurgery. 2004;54:1043–1048.
164. Cappabianca, P, de Divitiis, E. Back to the Egyptians: neurosurgery via the nose. A five-thousand year history and the recent contribution of the endoscope. Neurosurg Rev. 2007;30:1–7.
165. Cavallo, LM, Messina, A, Cappabianca, P, et al. Endoscopic endonasal surgery of the midline skull base: anatomical study and clinical considerations. Neurosurg Focus. 2005;19:E2.
166. Maroon, JC. Skull base surgery: past, present, and future trends. Neurosurg Focus. 2005;19:E1.
167. Prevedello, DM, Doglietto, F, Jane, JA, Jr., et al. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg. 2007;107:206–213.
168. Cavallo, LM, de Divitiis, O, Aydin, S, et al. Extended endoscopic endonasal transsphenoidal approach to the suprasellar area: anatomic considerations—part 1. Neurosurgery. 2007;61:ONS-24–ONS-34.
169. Frank, G, Pasquini, E, Mazzatenta, D. Extended transsphenoidal approach. J Neurosurg. 2001;95:917–918.
170. Kassam, A, Snyderman, CH, Mintz, A, et al. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19:E4.
171. Kassam, A, Snyderman, CH, Mintz, A, et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19:E3.
172. de Divitiis, E, Cappabianca, P, Cavallo, LM. Endoscopic transsphenoidal approach: adaptability of the procedure to different sellar lesions. Neurosurgery. 2002;51:699–705.
173. Cappabianca, P, Cavallo, LM, Esposito, F, et al. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. In: Pickard JD, Akalan N, Di Rocco C, Dolenc VV, Lobo Antunes J, Mooij JJA, Schramm J, Sindou M, eds. Advances and Technical Standards in Neurosurgery. Wien New York: Springer; 2008:152–199.
174. Frank, G, Pasquini, E, Doglietto, F, et al. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery. 2006;59(suppl 1):ONS75–ONS83.
175. de Divitiis, E, Cappabianca, P, Cavallo, LM, et al. Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery. 2007;61:219–227.
176. Kassam, AB, Gardner, PA, Snyderman, CH, et al. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108:715–728.
177. de Divitiis, E, Cappabianca, P, Esposito, F, et al. Tuberculum sellae meningiomas: High route or low route? A series of 51 consecutive cases. Neurosurgery. 2008;62:556–563.
178. Gardner, PA, Prevedello, DM, Kassam, AB, et al. The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg. 2008;108:1043–1047.
179. Gardner, PA, Kassam, AB, Snyderman, CH, et al. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg. 2008;109:6–16.
180. Kassam, A, Thomas, AJ, Snyderman, C, et al. Fully endoscopic expanded endonasal approach treating skull base lesions in pediatric patients. J Neurosurg. 2007;106:75–86.
181. Kassam, A, Snyderman, C, Carrau, R. An evolving paradigm to the ventral skull base. Skull Base. 14(suppl 1), February, 2004.
182. Cappabianca, P, Cavallo, LM, de Divitiis, E. Endoscopic endonasal transsphenoidal surgery. Neurosurgery. 2004;55:933–940.
183. Castelnuovo, P, Pistochini, A, Locatelli, D. Different surgical approaches to the sellar region: focusing on the “two nostrils four hands technique”. Rhinology. 2006;44:2–7.
184. Cavallo, LM, Messina, A, Esposito, F, et al. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg. 2007;107:713–720.
185. Leng, LZ, Brown, S, Anand, VK, et al. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008;62(5(suppl 2)):ONSE342–343.
186. Hadad, G, Bassagasteguy, L, Carrau, RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–1886.
187. Pinheiro-Neto, CD, Prevedello, DM, Carrau, RL, et al. Improving the design of the pedicled nasoseptal flap for skull base reconstruction: a radioanatomic study. Laryngoscope. 2007;117:1560–1569.
188. Kassam, A, Carrau, RL, Snyderman, CH, et al. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005;19:E8.
189. Cappabianca, P, Cavallo, LM, de Divitiis, E. Endoscopic pituitary and skull base surgery. Anatomy and surgery of the endoscopic endonasal approach. Tuttlingen: Endo-Press; 2008.
190. Snyderman, C, Kassam, A, Carrau, R, et al. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117:699–705.
191. Prevedello, DM, Kassam, AB, Snyderman, C, et al. Endoscopic cranial base surgery: ready for prime time? Clin Neurosurg. 2007;54:48–57.
192. Cappabianca, P, Cavallo, LM, Esposito, F, et al. Craniopharyngiomas. J Neurosurg. 2008;109:1–3.
193. Rutka, JT. Endonasal resection of craniopharyngiomas. J Neurosurg. 2008;109:1.
194. Yasargil, MG, General operative techniques. Microneurosurgery: Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms. Yasargil, MG, eds. Microneurosurgery: Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms, vol I. New York: Georg Thieme Verlag, 1984:215–233.
195. McArthur, LL. An aseptic surgical access to the pituitary body and its neighbourhood. JAMA. 1912;58:2009–2011.
196. Frazier, CH. Lesions of the hypophysis from the viewpoint of the surgeon. Surg Gynecol Obstet. 1913;17:724–736.
197. Krause, F. Freilegung der Hypophyse. In: Krause F, ed. Chirurgie der Gehirkrankheiten. Stuttgart: Ferdinand Enke; 1914:465–470.
198. Powell, MP, Pollock, JR. Transcranial surgery. In: Powell MP, Lightman SL, Laws ER, Jr., eds. Management of Pituitary Tumors. Totowa, NJ: Humana Press; 2003:147–159.
199. Dandy, WE. A new hypophysis operation. Bull Johns Hopkins Hosp. 1918;29:154–155.
200. Bhagwati, SN, Deopujari, CE, Parulekar, GD. Lamina terminalis approach for retrochiasmal craniopharyngiomas. Childs Nerv Syst. 1990;6:425–429.
201. de Divitiis, O, Angileri, F, d’Avella, D, et al. Microsurgical anatomic features of the lamina terminalis. Neurosurgery. 2002;50:563–570.
202. King, TT. Removal of intraventricular craniopharyngiomas through the lamina terminalis. Acta Neurochir. 1979;45:277–286.
203. Maira, G, Anile, C, Colosimo, C, et al. Craniopharyngiomas of the third ventricle: trans-lamina terminalis approach. Neurosurgery. 2000;47:563–570.
204. Killiani, OGT. Some remarks on tumors of the chiasm, with a proposal how to reach the same by operation. Ann Surg. 1904;40:35–43.
205. Fahlbusch, R, Honegger, J, Paulus, W, et al. Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg. 1999;90:237–250.
206. Samii, M, Tatagiba, M. Craniopharyngioma. In: Kaye AW, Laws ER, Jr., eds. Brain Tumors. London: Churchill Livingstone; 1995:873–894.
207. Suzuki, J. The bifrontal anterior interhemispheric approach. In: Apuzzo MLJ, ed. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1998:489–515.
208. Ganz, JC. Gamma knife treatment of pituitary adenomas. In: Landolt A, Vance M, Reilly P, eds. Pituitary Adenomas. Edinburgh: Churchill Livingstone; 1996:461–474.
209. Degerblad, M, Rahn, T, Bergstrand, G, et al. Long-term results of stereotactic radiosurgery to the pituitary gland in Cushing’s disease. Acta Endocrinol. 1986;112:310–314.
210. Landolt, A, Haller, D, Lomax, N, et al. Stereotactic radiosurgery for recurrent surgically treated acromegaly: comparison with fractionated radiotherapy. J Neurosurg. 1998;88:1002–1008.
211. Vance, ML, Laws, ER, Jr. Gamma Knife radiosurgery for secretory pituitary adenomas. In: Powell SL, Lightman SL, Laws ER, Jr., eds. Management of Pituitary Tumors. Totowa, NJ: Humana Press; 2003:221–229.
212. Black, PMcL, Zervas, NT, Candia, GL. Incidence and management of complications of transsphenoidal operation for pituitary adenomas. Neurosurgery. 1987;20:920–924.
213. Ciric, I, Ragin, A, Baumgartner, C, et al. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40:225–237.
214. Laws, ER, Jr., Kern, EB. Complications of trans-sphenoidal surgery. Clin Neurosurg. 1976;23:401–416.
215. Zervas, NT. Surgical results in pituitary adenomas: Results of an international survey. In: Black PMcL, Zervas NT, Ridgway EC, Jr., Martin JB, eds. Secretory Tumors of the Pituitary Gland. New York: Raven Press; 1984:377–385.
216. Laws, ER, Jr., Kern, EB. Complications of trans-sphenoidal surgery. In: Laws ER, Jr., Randall RV, Kern EB, eds. Management of Adenomas and Related Lesions. New York: Appleton-Century-Crofts; 1982:329–346.
217. Laws, ER, Jr. Vascular complications of transsphenoidal surgery. Pituitary. 1999;2:163–170.
218. Cockroft, KM, Carew, JF, Trost, D, et al. Delayed epistaxis resulting from external carotid artery injury requiring embolization: a rare complication of transsphenoidal surgery: Case report. Neurosurgery. 2000;47:236–239.
219. Guiot, G, Derome, P. Surgical problems of pituitary adenomas. In: Krayenbühl H, ed. Advances and Technical Standards in Neurosurgery. New York: Springer Verlag; 1976:3–33.
220. Mohr, G, Hardy, J, Comtois, R, et al. Surgical management of giant adenomas. Can J Neurol Sci. 1990;17:62–66.
221. Dolenc, VV. Pituitary tumors extending beyond the sella. In: Microsurgical Anatomy and Surgery of the Central Skull Base. New York: Springer; 2003:236–252.
222. Fahlbusch, R, Buchfelder, M. Surgical complications. In: Landolt AM, Vance ML, Reilly PR, eds. Pituitary Adenomas. New York: Churchill Livingstone; 1996:395–408.