Chapter 5 Physiotherapy in intensive care
Historically, physiotherapy in the intensive care unit (ICU) was confined to the treatment of respiratory problems performed routinely on all patients. Evidence-based practice has demonstrated that there is no longer a place for routine physiotherapy treatment in ICU.1 Physiotherapeutic intervention is based on clinical reasoning following the identification of physiotherapy-amenable problems, which are elucidated from a thorough systematic assessment.
There is still some debate about the precise role of the physiotherapist within ICU, which may vary,2 but the main features include:
CARDIOPULMONARY PHYSIOTHERAPY
TREATMENT MODALITIES TO OPTIMISE CARDIOPULMONARY FUNCTION
Patients who are critically ill may present with impaired cardiopulmonary physiology secondary to both the underlying pathology and the therapeutic interventions employed to treat them. In their approach to any individual patient, physiotherapists may use specific treatment techniques targeted at improving ventilation/perfusion (V/Q) disturbances, increasing lung volumes, reducing the work of breathing and removing pulmonary secretions. Physiotherapy treatment modalities may differ depending on the presence of an endotracheal tube, although patient participation with treatment is encouraged and promoted at the earliest point during intubation. Each intervention is rarely used in isolation, but as part of an effective treatment plan. Some physiotherapeutic techniques may have short-lived beneficial effects on pulmonary function, and some have no clear evidence to validate their effectiveness (Table 5.1).
Table 5.1 Treatment modalities to optimise cardiopulmonary function
| Invasively ventilated patients | Non-invasive/self-ventilating patients |
|---|---|
| Manual hyperinflation (MHI) | Active cycle of breathing technique (ACBT) |
| Suction | Manual techniques |
| Manual techniques | Positioning |
| Positioning | Intermittent positive-pressure breathing (IPPB) |
| Mobilization/rehabilitation | Continuous positive airways pressure (CPAP) |
| Non-invasive ventilation (NIV) | |
| Nasopharyngeal/oral suction | |
| Positive expiratory pressure (PEP) mask, flutter valve | |
| Mobilization/rehabilitation |
MANUAL HYPERINFLATION
In this technique a self-inflating circuit is used to deliver a volume of gas 50% greater than tidal volume (VT) via an endotracheal or tracheostomy tube. An augmented VT may recruit atelectatic lung secondary to reduced airflow resistance and enhanced interdependence via the collateral channels of ventilation.3 Bronchial secretions may be mobilised by the increased expiratory flow rate and/or stimulation of a cough.4 However, ventilator hyperinflation, the delivery of an augmented VT via the ventilator, has been shown to be as effective in the removal of secretions and maintenance of static lung compliance as conventional manual hyperinflation (MHI).5 This may also avoid cardiopulmonary instability associated with ventilator disconnection and loss of positive end-expiratory pressure (PEEP). In an emergency situation an Ambu-bag and facemask can be used to perform MHI in the self-ventilating patient. However, an alternative technique such as intermittent postitive-pressure breathing (IPPB) should be considered when an augmented VT is required during a therapeutic intervention (Table 5.2).
Table 5.2 Potential advantages and complications of manual hyperinflation
| Potential advantages |
| Reversal of acute lobar atelectasis3 |
| Alveolar recruitment via channels of collateral ventilation3 |
| Improvement in arterial oxygenation |
| Mobilisation of secretions and contents of aspiration5 |
| Improved static lung compliance5 |
| Effectiveness may be increased when combined with appropriate positioning and manual techniques1 |
| Potential complications |
| Absolute contraindications include undrained pneumothorax and unexplained haemoptysis |
| Cardiovascular and haemodynamic instability6 |
| Loss of PEEP, inducing hypoxia and potential lung damage. This can be minimised by incorporating a PEEP valve into the circuit of a ‘PEEP-dependent’ patient |
| Risk of volutrauma, barotrauma and pneumothorax,7 which can be reduced by including a manometer in the circuit |
| Risk of increased intracranial pressure |
| Increased patient stress and anxiety |
PEEP, positive end-expiratory pressure.
RECRUITMENT MANOEUVRES
Recruitment manoeuvres may be employed to reverse hypoxaemia in patients with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). A recruitment manoeuvre involves a transient increase in transpulmonary pressure in an attempt to reinflate and maintain atelectatic lung units.8 No standard approach exists; however, common options include: the application of incremental levels of continuous positive airways pressure (CPAP) with no tidal excursion; incremental increases in PEEP with additional VT; and the application of intermittent larger ‘sigh’ breaths. In randomised studies, although recruitment manoeuvres may transiently improve oxygenation, there is as yet no proven outcome benefit.9
SUCTION
Suction is used to clear secretions from central airways when a cough reflex is impaired or absent. A suction catheter is passed via an endotracheal or tracheostomy tube or via a nasal/oral airway to the carina, and this may stimulate a cough in a non-paralysed patient (Table 5.3). The catheter is pulled back 1 cm before suction is applied on withdrawal. The suction catheter diameter should not be greater than 50% of the diameter of the airway through which it is inserted as large negative pressure can be generated intrathoracically without air entrainment. The use of suction following effective MHI optimises removal of secretions.10 Instillation of normal saline prior to suctioning remains controversial; however, it may stimulate a cough, maximising secretion mobilisation and clearance.
| Potential advantages |
| Stimulation of a cough when reflex is impaired by mechanical stimulation of the larynx, trachea or large bronchi |
| Removal of secretions from central airways when cough is ineffective or absent |
| Potential complications |
| Tracheal suction is an invasive procedure and should only be undertaken when there is a clear indication |
| Absolute contraindications to suctioning are unexplained haemoptysis, severe coagulopathies, severe bronchospasm, laryngeal stridor, base-of-skull fracture and a compromised cardiovascular system |
| Hypoxaemia can be induced secondary to suctioning. This can be limited by pre- and postoxygenation |
| Cardiac arrhythmias may be more common in the presence of hypoxia |
| Tracheal stimulation may produce increased sympathetic nervous system activity or a vasovagal reflex producing cardiac arrhythmias and hypotension |
MANUAL TECHNIQUES
CHEST SHAKING AND VIBRATIONS
Shaking and vibrations are oscillatory movements of large and small amplitude performed during expiration, and are thought to increase expiratory flow rate, aiding mucociliary clearance.11 These techniques are believed to be more effective when performed at high lung volumes.
CHEST WALL COMPRESSION
Compression of the chest wall can be used to augment an expiratory manoeuvre such as a ‘huff’ (see section on active cycle of breathing technique (ACBT), below) or a cough by providing tactile stimulation, or wound support.
NEUROPHYSIOLOGICAL FACILITATION (NPF) OF RESPIRATION
NPF of respiration is a set of techniques designed for the treatment of the neurologically impaired adult. Manual externally applied stimuli to the thorax, abdomen and mouth can be used to stimulate increased VT, a cough reflex, augmented contraction of the abdominal muscles or an increased conscious level.12,13
POSITIONING
A simple change of position can have a profound effect on cardiopulmonary physiology14,15 (Table 5.4). As such, positioning is commonly utilised to achieve several different goals: drainage of secretions using gravity-assisted positioning (GAP), reduction of the work of breathing/breathlessness or to optimise V/Q matching.
Table 5.4 Potential advantages and complications of mobilisation14
| Potential advantages | |
|---|---|
| Positioning supine to upright | Mobilisation |
| ↑ Lung volumes | ↑ Ventilation |
| ↑ Lung compliance | ↑ V/Q matching |
| ↓ Airway closure | ↑ Recruitment of lung units |
| ↑ PaO2 | ↑ Surfactant production/distribution |
| ↓ Work of breathing | ↑ Mobilisation of secretions |
| ↑ Mobilisation of secretions | ↑ Cardiopulmonary fitness and exercise capacity |
| Potential complications | |
| Cardiovascular/neurological/haematological instability | |
| Increased oxygen/ventilatory requirement | |
(Adapted from Dean E: The effects of positioning and mobilization on oxygen transport. In: Pryor JA, Webber BA (eds) Physiotherapy for Respiratory and Cardiac Problems, 2nd edn. Edinburgh: Churchill Livingstone; 1998: 125.)
GRAVITY-ASSISTED POSITIONING
GAP facilitates the removal of excess bronchial secretions by positioning a specific bronchopulmonary segment perpendicular to gravity (Table 5.5). This technique is not used in isolation but in conjunction with augmented VT, via the ventilator, MHI or ACBT in a spontaneously breathing patient. An individual position exists for each bronchopulmonary segment based on the anatomy of the bronchial tree;16 however, these may need modification in the ICU setting.
Table 5.5 Potential advantages and complications of gravity-assisted positioning (GAP)
| Potential advantages |
| Maximises removal of excess bronchial secretions when combined with active cycle of breathing technique |
| Allows accurate treatment of specific bronchopulmonary segments |
| Self-treatment can be included in a home programme on discharge |
| Potential complications |
| Positions need modification when used in the presence of cardiovascular/neurological instability, haemoptysis or gastric reflux |
REDUCTION OF THE WORK OF BREATHING
A reduction in the work of breathing/breathlessness can be achieved by putting a patient in a position that optimises the length–tension relationship of the diaphragm, promotes relaxation of the shoulder girdle and upper chest and facilitates the use of breathing control.17 This approach to positioning is particularly effective when used in conjunction with non-invasive ventilation (NIV). Adequately supported high side-lying is a useful position to promote relaxation of the breathless patient. In addition, it can discourage the overuse of accessory muscles of respiration, which may reduce energy expenditure. Some patients prefer forward lean-sitting with their arms placed in front of them on a high table. In this position the length–tension relationship of the diaphragm is optimised secondary to forward displacement of the abdominal contents.
VENTILATION/PERFUSION
Appropriate positioning of a patient can maximise V/Q.18 In the self-ventilating adult, V/Q matching increases from non-dependent to dependent areas of lung.19 However, in adults receiving positive-pressure ventilation lung mechanics are altered, producing V/Q inequality. In this situation non-dependent areas of lung are preferentially ventilated while dependent regions are optimally perfused; as such, a regular change of position is recommended.
In an extreme form prone positioning has been used to improve refractory hypoxaemia in patients with ALI/ARDS. The mechanisms behind these improvements are complex, but likely centre around a combination of a redistribution of some pulmonary perfusion together with a more homogeneous distribution of ventilation, leading to improved V/Q matching. Although prone positioning improves oxygenation in 70% of patients with ALI/ARDS, its role in improving outcome remains controversial.20
ACTIVE CYCLE OF BREATHING TECHNIQUE
The ACBT is a cycle of breathing exercises used to remove excess bronchial secretions (Table 5.6). The cycle can be adapted for each patient according to existing underlying pathology and presenting clinical signs. It consists of:
Table 5.6 Potential advantages and complications of active cycle of breathing technique (ACBT)
| Potential advantages |
| Mobilises and clears excess bronchial secretions21,22 |
| Improves lung function23 |
| Minimises the work of breathing |
| Individual components of the cycle can be utilised/emphasised to target specific problems |
| Can be used in combination with other manual techniques, gravity-assisted positioning, V/Q matching, positioning to reduce breathlessness and during activities such as walking |
| Self-treatment can be included in a home programme |
| Potential complications |
| Without adequate periods of breathing control, bronchospasm and desaturation can occur |
| Poor technique can lead to ineffective treatment and unnecessary energy expenditure |
MECHANICAL ADJUNCTS
INTERMITTENT POSITIVE-PRESSURE BREATHING
IPPB is a patient-triggered, pressure-cycled mechanical device mainly used in self-ventilating patients to increase ventilation, mobilise bronchial secretions and re-expand lung tissue by augmenting VT24 (Table 5.7). Positive airway pressure is maintained throughout inspiration. Expiration is passive. IPPB requires constant adjustment of pressure and flow rates and careful patient monitoring to maintain effectiveness and cooperation. Effectiveness is increased when used in conjunction with positioning, ACBT and manual techniques24 (Table 5.8).
Table 5.7 Site and action of intermittent positive-pressure breathing (IPPB), continuous positive airways pressure (CPAP) and non-invasive ventilation (NIV)
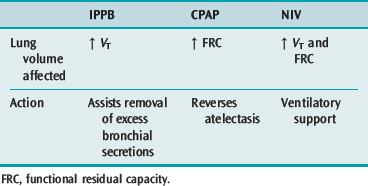
Table 5.8 Potential advantages and complications of intermittent positive-pressure breathing (IPPB), continuous positive airways pressure (CPAP) and non-invasive ventilation (NIV)
| Potential advantages |
| Improves lung volumes |
| Improves gaseous exchange |
| Decreases the work of breathing |
| IPPB and NIV can mobilise excess bronchial secretions by improving VT |
| IPPB and NIV can improve lung and chest wall compliance |
| CPAP reduces left ventricular afterload by reducing the transmural pressure gradient |
| Patients can be mobilised while on CPAP and some modes of NIV. Alteration of ventilator settings might be indicated to maximise patient potential/exercise tolerance during treatment |
| Settings can be adjusted to augment physiotherapy intervention, e.g. increased inspiratory positive airway pressure to assist removal of secretions |
| Potential complications |
| Absolute contraindications include severe bronchospasm, undrained pneumothorax, pneumomediastinum, unexplained haemoptysis and facial fractures. Use with care in pre-existing bullous lung disease |
| Haemodynamic/neurological instability |
| Risk of decreased urine output with CPAP and NIV |
| Risk of carbon dioxide retention with CPAP |
| Risk of aspiration |
IPAP, Inspiratory positive airway pressure.
CONTINUOUS POSITIVE AIRWAYS PRESSURE
CPAP maintains a positive airway pressure throughout inspiration and expiration. It is used in both intubated and self-ventilating patients to increase/normalise functional residual capacity (FRC) via recruitment of atelectatic lung. Clinically increased FRC is associated with improved lung compliance, improved oxygenation and a reduced work of breathing.24 Effectiveness is increased when used in conjunction with appropriate positioning. The self-ventilating patient must be able to generate an adequate VT as this volume is not augmented with CPAP (see Table 5.7).
NON-INVASIVE VENTILATION
In recent years there has been an expansion in the role of NIV in the ICU. This includes the prevention of invasive ventilation in patients with chronic obstructive pulmonary disease,25 pulmonary oedema and immunocompromise; early weaning from mechanical ventilation; and potentially the prevention of reintubation in those who suffer extubation failure. In addition, VT may be augmented during physiotherapy treatment to remove secretions, or when mobilising the patient (see Table 5.7). Improved oxygenation may be achieved using NIV when the patient is positioning to optimise V/Q (see Table 5.8).
CRITICAL CARE REHABILITATION
The effects of deconditioning on the cardiovascular (Table 5.9), respiratory (Table 5.10) and neuromusculoskeletal system (Table 5.11) are well documented.26–28 This phenomenon occurs as a result of restricted physical activity, and reduces the ability to perform work. The effects of deconditioning can occur with even relatively short periods of immobility, and are significantly influenced by age, premorbid condition, nature of the illness/injury and pharmacological factors. The consequences of deconditioning are significant in terms of patient outcome, length of hospital stay, duration of rehabilitation and subsequent ability to function independently in the community.29 The psychological impact of deconditioning should not be underestimated. Inability to function at a ‘normal’ level of activity may result in depression and reduced self-efficacy.
Table 5.9 Deconditioning and the cardiovascular system26,28,30
| Cardiovascular system |
| ↓ Stroke volume – ventricular remodelling and reduced preload (see ↓ Plasma volume) |
| ↑ Heart rate (resting and exercising): ↓ vagal tone, ↑ sympathetic catecholamine secretion and ↑ cardiac β-receptor activity |
| ↓ Cardiac output and systemic oxygen delivery |
| ↓ VO2max: magnitude highly correlated to duration, static exercise effective in preventing some decrease. Related to changes centrally (cardiac output) and peripherally (oxygen delivery and utilisation) |
| ↓ Plasma volume: secondary to fluid shift and altered renin–angiotensin–aldosterone activity. Contributes to ↓ orthostatic tolerance |
| Orthostatic intolerance develops more rapidly in the elderly or those with cardiovascular pathology. Often slow to resolve |
| Increased blood viscosity and vascular stasis: predisposition to thromboembolism |
| Altered cardiovascular reflexes: proposed attenuated baroreflex-mediated sympathoexcitation and enhanced cardiopulmonary receptor-mediated sympathoinhibition. Contributes to orthostatic intolerance |
| Altered arterial/venous vascular function |
Table 5.10 Deconditioning and the respiratory system31–33
| Respiratory system |
| Adverse effects on: |
| Functional residual capacity |
| Compliance (lung and chest wall) |
| Resistance |
| Closing volume |
| Respiratory muscle function – impaired strength and endurance, reduced performance of ventilatory pump, ↑ days of mechanical ventilation, complex weaning issues |
| Concept of ventilator-induced diaphragmatic dysfunction proposed (atrophy, fibre remodelling, oxidative stress and structural injury). Time-dependent reduction in force-generating capacity, secondary to disuse and passive shortening |
| Respiratory muscle weakness may be limited by judicious choice of ventilation mode. Role of inspiratory muscle training unclear |
Table 5.11 Deconditioning and the neuromusculoskeletal system26,34–37
| Neuromusculoskeletal system |
| Muscle atrophy – protein degradation (loss of contractile protein, increased non-contractile tissue, e.g. collagen) and cytokine activity. Reduction in strength, especially lower-limb antigravity muscles (i.e. those involved with transferring and ambulation). Inactivity amplifies the catabolic response of skeletal muscle to cortisol, therefore there is more marked atrophy following trauma or illness. Particularly significant in patient groups with low relative muscle mass, e.g. the elderly. Nutritional countermeasures should be considered and carefully titrated to meet demands best |
| ↓ Muscle endurance (cf. Table 5.10) – reduced muscle blood flow/red cell volume/capillarisation/oxidative enzymes and biochemical changes. Generally longer to rehabilitate compared to reduction in muscle strength |
| Muscle shortening or changes in peri-/intra-articular connective tissue (including chest wall and thoracic spine) → contractures, ↓ joint range of motion, pain. Positioning and stretching maintain range and delay invasion of non-contractile protein |
| Decreased bone mineral density (particularly trabecular bone) – may be attenuated by standing or resistance exercise. Rate of recovery tends to lag behind that of muscle strength. Increased risk of fracture on remobilisation, especially in elderly |
| Microvascular and biochemical changes in peripheral nerves impair neuromuscular function. Adversely affects maximal voluntary contraction, and balance/proprioceptive activity |
| Critical illness neuropathy and myopathy frequently develop in patients hospitalised in an ICU for > 1 week. Risk factors include sepsis, SIRS and severe MOF. Associated with higher mortality rate, prolonged ventilation and rehabilitation, disability and reduced quality of life |
ICU, intensive care unit; SIRS, systemic inflammatory response syndrome; MOF, multiorgan failure.
An increasing volume of research suggests that the deleterious consequences of immobility may be in part attenuated by selected rehabilitation interventions.38,39 The physiotherapist possessing expertise in rehabilitation and exercise physiology should direct the multiprofessional team in evaluating individual patients, devising a therapeutic strategy and referring to other specialties (e.g. speech and language therapy, occupational therapy) as required. Considerations must include:
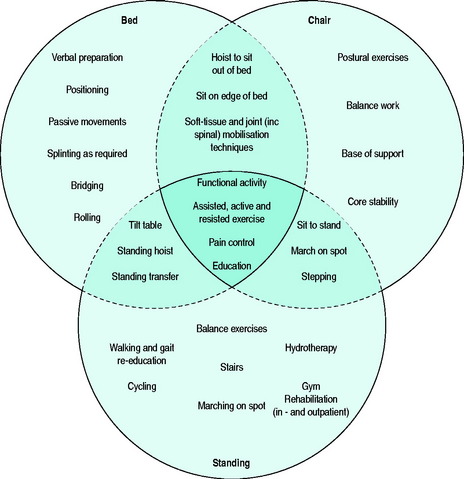
Traditionally, exercise rehabilitation has progressed linearly from activity in bed, then sitting, and finally to standing/walking. The model demonstrated in Figure 5.1 represents a three-stage functional rehabilitation programme. It is supported by evidence that suggests that a multimodal training regime is required to maintain/restore both physiological and psychological performance after a period of immobility and illness.39 The use of interlinking circles is intended to reflect the non-linear pattern of exercise progression more commonly utilised in patients with critical illness, e.g. patients may be able to stand using a tilt-table before they are able to tolerate sitting out of bed. The central shaded area represents the core components that should be addressed at every stage in the patient’s recovery. The areas bordered by the broken lines represent the progression or regression from one stage to the next. During all stages, the patient’s cardiopulmonary response must be closely monitored, and exercise titrated accordingly. Modifications (e.g. temporarily increasing the FiO2 and/or level of ventilatory assistance) during exercise and in the early postexercise period may be necessary. Such modifications are commonly required as increasing physical activity invariably coincides with weaning from ventilatory support – both significant challenges to the physiological reserve.
It has been suggested that, in order to improve long-term outcomes for survivors of ICU (e.g. late mortality, ongoing morbidity, neurocognitive defects, functional disability, quality of life, economic burden), critical illness and its management should be viewed on a continuum and not merely the time spent in a critical care facility.29 Consequently, rehabilitation must also reflect this change in focus, continuing into the community, outpatient or follow-up clinic setting.40
In recent years the concept of ‘prehabilitation’ has been introduced. Some have argued the case for exercise training prior to planned ICU admissions in order to ameliorate functional capacity. It is proposed that this would enable an individual to withstand better the stressor of inactivity and decrease the duration of dependency post critical care discharge.26
PATIENT PROBLEMS AFTER ICU DISCHARGE
A prolonged stay in ICU can be debilitating mentally and physically and can affect recovery after discharge (Table 5.12). In order to optimise a fast and effective recovery, a patient’s care plan should be multidisciplinary in origin with ongoing appropriate rehabilitation following discharge from the ICU and indeed hospital.
Table 5.12 Psychological, cardiopulmonary and functional problems often encountered after discharge from the intensive care unit
| Psychological | Cardiopulmonary | Functional |
|---|---|---|
| Depression | Compromised cardiopulmonary system | Back pain |
| Fear | Difficulty clearing retained secretions (trache tube, mini-trach in situ) | Shoulder pain |
| Anxiety | Muscle atrophy/decreased strength | |
| Confusion | Decreased lung volumes | Inability to carry out activities of daily living independently |
| Disorientation | Oxygen dependence | |
| Flashbacks | Limited mobility | |
| Lack of motivation | Poor exercise tolerance | |
| Functional dependence | Poor gait pattern |
PHYSIOTHERAPY AND CRITICAL CARE OUTREACH TEAMS
The development of specialist physiotherapist posts within critical care outreach teams (CCOTs) constitutes a prime example of role expansion. Following the publication of Comprehensive Critical Care42 CCOT services were developed to meet the actual or potential needs of patients through critical care provision ‘without walls’:
The introduction of CCOT has been associated with a varied approach to team configuration; however it has been suggested that those following a multiprofessional model are most likely to affect clinical and organisational improvements.43 Consequently, many teams have elected to employ a designated specialist physiotherapist who can bring physiotherapeutic expertise to the service whilst also developing generic outreach practitioner skills (e.g. advanced tracheostomy management, cannulation, venopuncture, prescription via patient group directions, arterial blood gas sampling, drug administration, advanced life support, management of central/peripheral lines, 12-lead electrocardiogram interpretation, ordering/interpreting blood results and chest X-rays).
1 Stiller K. Physiotherapy in intensive care. Towards an evidence-based practice. Chest. 2000;118:1801-1813.
2 Norrenberg M, Vincent JL. Intensive care medicine. A profile of European intensive care unit physiotherapists. Eur Soc Intens Care Med. 2000;7:988-994.
3 Denehy L. The use of manual hyperinflation in airway clearance. Eur Respir J. 1999;14:958-965.
4 Hodgson C, Denehy L, Ntoumenopoulos G, et al. An investigation of the early effects of manual lung hyperinflation in critically ill patients. Anaesth Intens Care. 2000;28:255-261.
5 Berney S, Denehy L. A comparison of the effects of manual and ventilator hyperinflation on static lung compliance and sputum production in intubated and ventilated intensive care patients. Physiother Res Int. 2001;7:100-108.
6 Singer M, Vermaat J, Hall G, et al. Haemodynamic effects of manual hyperinflation in critically ill mechanically ventilated patients. Chest. 1994;106:1182-1187.
7 Clarke RCN, Kelly BE, Convery PN, et al. Ventilatory characteristics in mechanically ventilated patients during manual hyperventilation for chest physiotherapy. Anaesthesia. 1999;54:936-940.
8 Lapinsky SE, Sangeeta M. Bench-to-bedside review: recruitment and recruiting maneuvers. Crit Care. 2005;9:60-65.
9 Brower RG, Lanken PN, MacIntyre NR, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome NIH/NHLBI ARDSNET. N Engl J Med. 2004;351:327-336.
10 Choi JS, Jones AY. Effects of manual hyperinflation and suctioning on respiratory mechanics in mechanically ventilated patients with ventilator acquired pneumonia. Aust J Physiother. 2005;51:25-30.
11 McCarren B, Alison JA, Herbert RD. Vibration and its effect on the respiratory system. Aust J Physiother. 2006;52:39-43.
12 Bethune D. Neurophysiological facilitation of respiration in the unconscious adult patient. Physiother Canada. 1975;27:241-245.
13 Chang AT, Boots RJ, Brown MG, et al. Ventilatory changes following head up tilt and standing in healthy subjects. Eur J Appl Physiol. 2005;95:409-417.
14 Dean E. The effects of positioning and mobilization on oxygen transport. In: Pryor JA, Webber BA, editors. Physiotherapy for Respiratory and Cardiac Problems. 2nd edn. Edinburgh: Churchill Livingstone; 1998:125.
15 Jones AY, Dean E. Body position change and its effect on haemodynamic and metabolic status. Heart Lung. 2004;33:281-290.
16 Thoracic Society. The nomenclature of bronchopulmonary anatomy. Thorax. 1950;5:222-228.
17 Dean E. Effects of position on pulmonary function. Phys Ther. 1985;65:613-618.
18 Fink JB. Positioning versus postural drainage. Respir Care. 2002;47:769-777.
19 West JB. Respiratory Physiology, 5th edn., Baltimore: Williams & Wilkins; 1995:51-69.
20 Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568-573.
21 Webber BA, Pryor JA. Physiotherapy techniques. In: Pryor JA, Webber BA, editors. Physiotherapy for Respiratory and Cardiac Problems. 2nd edn. Edinburgh: Churchill Livingstone; 1998:137-209.
22 Pryor JA, Webber BA, Hodson ME, et al. Evaluation of the forced expiration technique as an adjunct to postural drainage in treatment of cystic fibrosis. Br Med J. 1979;2:417-418.
23 Webber BA, Hofmeyr JL, Morgan MDL, et al. Effects of postural drainage, incorporating forced expiratory technique on pulmonary function in cystic fibrosis. Br J Dis Chest. 1986;80:353-359.
24 Denehy L, Berney S. The use of positive pressure devices by physiotherapists. Eur Respir J. 2001;17:821-829.
25 British Thoracic Society guidelines. Noninvasive ventilation in acute respiratory failure. Thorax. 2002;57:192.
26 Topp R, Ditmyer M, King K, et al. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues. 2002;13:263-276.
27 Convertino VA, Bloomfield SA, Greenleaf JE. An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187-190.
28 Convertino VA. Cardiovascular consequences of bed rest: effect on maximal oxygen uptake. Med Sci Sports Exerc. 1997;29:191-196.
29 Angus DC, Carlet J. Surviving intensive care: a report from the 2002 Brussels roundtable. Intens Care Med. 2003;29:368-377.
30 Mueller PJ, Cunningham JT, Patel KP, et al. Proposed role of the paraventricular nucleus in cardiovascular deconditioning. Acta Physiol Scand. 2003;177:27-35.
31 Jubran A. Critical illness and mechanical ventilation: effects on the diaphragm. Respir Care. 2006;51:1054-1061.
32 Chang AT, Boots RJ, Brown MG, et al. Reduced inspiratory muscle endurance following successful weaning from prolonged mechanical ventilation. Chest. 2005;128:553-559.
33 Gayan-Ramirez G, Decramer M. Effects of mechanical ventilation on diaphragm function and biology. Eur Respir J. 2002;20:1579-1586.
34 Bloomfield S. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;2:197-206.
35 Kawakami Y, Akima H, Kubo K, et al. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol. 2001;84:7-12.
36 Latronico N, Peli E, Botteri M. Critical illness myopathy and neuropathy. Curr Opin Crit Care. 2005;11:126-132.
37 Paddon-Jones D. Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J Nutr. 2006;136:2123-2126.
38 Vernikos J, Ludwig DA, Ertl AC, et al. Effect of standing or walking on physiological changes induced by head down bed rest: implications for spaceflight. Aviat Space Environ Med. 1996;67:1069-1079.
39 Greenleaf JE. Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207-215.
40 Elliott D, McKinley S, Alison JA, et al. Study protocol: home-based physical rehabilitation for survivors of a critical illness. Crit Care. 2006;10:R90.
41 Douglas E. The Nottingham Critical Care Rehabilitation Model, University of Nottingham Division of Physiotherapy. Personal communication, 2006.
42 Department of Health. Comprehensive Critical Care: A Review of Adult Critical Care Services. London: Department of Health, 2002.
43 Wood D. Designing an outreach service. In: Cutler L, Robson W, editors. Critical Care Outreach. Chichester: John Wiley; 2006:13-30.