Chapter 7 Physiologic and Pathophysiologic Responses to Intubation
I Background
Laryngoscopy, endotracheal intubation, and other airway manipulations (e.g., placement of a nasopharyngeal or oropharyngeal supralaryngeal airway) are noxious stimuli that may induce profound changes in cardiovascular physiology, primarily through reflex responses. Although these responses may be of short duration and of little consequence in healthy individuals, serious complications can occur in patients with underlying coronary artery disease,1,2 reactive airways,3,4 or intracranial neuropathology.5,6
II Cardiovascular Responses during Airway Manipulation
A Cardiovascular Reflexes
The cardiovascular responses to noxious airway manipulation are initiated by proprioceptors responding to tissue irritation in the supraglottic region and in the trachea.7 Located in close proximity to the airway mucosa, these proprioceptors consist of mechanoreceptors with small-diameter myelinated fibers, slowly-adapting stretch receptors with large-diameter myelinated fibers, and polymodal endings of nonmyelinated nerve fibers.8 (The superficial location of these proprioceptors and their nerves explains why topical local anesthesia of the airway is such an effective means of blunting cardiovascular responses to airway interventions.) The glossopharyngeal and vagal afferent nerves transmit these impulses to the brainstem, which, in turn, causes widespread autonomic activation through the sympathetic and parasympathetic nervous systems. Bradycardia, often elicited in infants and small children during laryngoscopy or intubation, is the autonomic equivalent of the laryngospasm response. Although seen only rarely in adults, this reflex results from an increase in vagal tone at the sinoatrial node and is virtually a monosynaptic response to a noxious stimulus in the airway.
In adults and adolescents, the more common response to airway manipulation is hypertension (HTN) and tachycardia mediated by the cardioaccelerator nerves and sympathetic chain ganglia. This response includes widespread release of norepinephrine from adrenergic nerve terminals and secretion of epinephrine from the adrenal medulla.9 Some of the hypertensive response to endotracheal intubation also results from activation of the renin-angiotensin system, including release of renin from the renal juxtaglomerular apparatus, which is innervated by β-adrenergic nerve terminals.
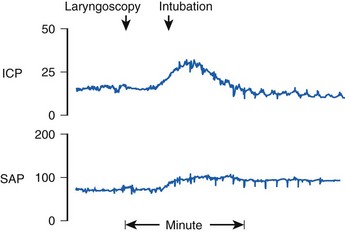
In addition to activation of the autonomic nervous system, laryngoscopy and endotracheal intubation result in stimulation of the central nervous system, as evidenced by increases in electroencephalographic (EEG) activity, cerebral metabolic rate, and cerebral blood flow (CBF).10 In patients with compromised intracranial compliance, the increase in CBF may result in elevated intracranial pressure (ICP), which, in turn, may result in herniation of brain contents and severe neurologic compromise.
B Intubation in the Presence of Cardiovascular Disease
It follows, then, that neuroendocrine responses to airway manipulation resulting in tachycardia and HTN may result in a variety of complications in patients with cardiac disease, myocardial ischemia chief among them. This set of circumstances is responsible for episodes of ischemic electrocardiographic ST-segment depression and increased pulmonary artery diastolic blood pressure (BP) that may be seen when intubation is performed in patients with arteriosclerosis; occasionally, these episodes presage the occurrence of a perioperative myocardial infarction.2 However, short ischemic episodes (<10 minutes) evidenced by electrocardiographic ST-segment depression, such as those that may be experienced only during airway manipulation, have not been shown to correlate with postoperative myocardial infarction. In contrast, ST-segment changes of a single duration lasting longer than 20 minutes (mean SD 20 ± 30 minutes) or cumulative durations of longer than 1 hour (mean SD 1 ± 2 hours) do seem to be an important factor associated with adverse perioperative cardiac outcomes.11,12
C Implications for Patients with Neurovascular Disease
Intracranial aneurysms and arteriovenous malformations (AVMs) often arise with a small “sentinel” hemorrhage that serves as a warning of worse things to come. During subsequent periods of elevated arterial BP, these lesions are likely to rebleed, resulting in sudden and permanent neurologic injury. Many neurosurgeons and interventional neuroradiologists attempt to stabilize cerebral aneurysms and AVMs soon after hospitalization in an effort to minimize the risk of rebleeding. This means that the patient presents for anesthesia at a time when the clot tamponading the aneurysm or AVM is particularly delicate, and a small increase in arterial transmural pressure could cause rerupture. One of the times at which this is most likely to occur is when the arterial BP and the HR are increased in response to endotracheal intubation.5 Therefore, neurosurgical anesthesiologists pay meticulous attention to attenuating these responses during the course of anesthetic induction and endotracheal intubation.
D Intubation in Patients with Neuropathologic Disorders
Reflex responses to endotracheal intubation are also a potential hazard to patients with compromised intracranial compliance resulting from neuropathologic processes such as intracranial mass lesions, brain edema, or acute hydrocephalus. Uncontrolled coughing can result in a marked increase in intrathoracic and intra-abdominal pressure that, in turn, increases cerebrospinal fluid pressure and may result in impairment of cerebral perfusion. In patients with impaired cerebral autoregulation (e.g., brain trauma, cerebrovascular accidents, neoplasms), the normal tendency for CBF to remain constant over the mean BP range of 50 to 150 mm Hg is impaired. When endotracheal intubation causes an increase in arterial BP, there is a marked increase in CBF and cerebral blood volume, which in turn can cause dangerous increases in ICP.6 This effect is magnified by the fact that noxious stimuli, such as airway manipulation, result in increased CBF, which summates with the hypertensive BP response, occasionally causing profound increases in ICP (Fig. 7-1).
E Neuromuscular Blocking Drugs and Cardiovascular Responses
Neuromuscular blocking drugs (NMB) are often administered to optimize conditions for intubation. Accordingly, it is appropriate to consider the cardiovascular and cerebrovascular responses to the administration of these agents. Indeed, the hypertensive-tachycardic response to endotracheal intubation was not identified until NMB agents were introduced into clinical practice, because before that time intubation was performed only with the patient under such deep levels of anesthesia that relatively little cardiovascular response was generated.13
The depressor effects of benzylisoquinolinium relaxants (atracurium and mivacurium) are mediated by histamine release.14 This effect could be viewed as a potential antagonist to the pressor response to laryngoscopy and endotracheal intubation. In the case of patients at risk for intracranial HTN, however, histamine-induced cerebral vasodilation may produce increases in ICP even as the BP falls.15 By contrast, pancuronium, rocuronium, and, to a lesser extent, vecuronium may initiate a hyperdynamic cardiovascular state that can potentiate the cardiovascular responses seen after endotracheal intubation in lightly anesthetized patients. The faster onset of rocuronium (doses of up to 2 mg/kg have a 90% chance of providing perfect intubating conditions) are the reason for its current widespread use as an alternative to succinylcholine for rapid-sequence intubation (RSI) and in operations expected to last longer than 1 hour.16
Succinylcholine, or diacetylcholine, is associated with bradycardia in children, particularly when doses are repeated, but is a cardiovascular stimulant in adults. This phenomenon is often associated with activation of the EEG, and patients with brain tumors may sustain marked increases in ICP after succinylcholine administration if intracranial compliance is compromised and cerebrovascular autoregulation is impaired.17 This has been shown in dogs to be a result of increased CBF related primarily to succinylcholine-induced increases in afferent muscle spindle activity at the time of fasciculation and secondarily to an elevated arterial carbon dioxide tension from fasciculation-induced carbon dioxide production.18 The evidence to substantiate the clinical relevance of these findings is lacking, however. Whereas it has been reported that succinylcholine administered to patients with brain tumors may elevate ICP by a mean of 5 to 12 torr, cerebral perfusion pressure does not change significantly, and a negative effect on neurologic outcome has not been documented.19,20 Additionally, this phenomenon can be prevented by pretreatment with defasciculating doses of nondepolarizing NMB drugs.19 Further, when adequate ventilation is maintained, succinylcholine administered to intubated patients being treated for intracranial HTN of various causes or to those who have suffered severe head injuries caused by blunt trauma had no effect on ICP, cerebral perfusion pressure, or CBF.21,22 As a result, succinylcholine is still considered a first-line agent for RSI in patients with acute head injury but is ideally used after pretreatment with a nondepolarizing agent and in the presence of slight hypocapnia.
F Cardiopulmonary Consequences of Positive-Pressure Ventilation
It should also be noted that both hypoxemia and hypercapnia lead to a stress-induced catecholamine response, which may mask other potential causes of hypotension. This becomes readily apparent only after intubation in critically ill patients. Prophylactic volume expansion and the immediate availability of vasoactive infusions decrease severe hemodynamic collapse in this situation.23
III Prevention of Cardiovascular Responses
A Technical Considerations: Minimizing Stimulation of Airway Proprioceptors
As a general rule, cardiovascular responses to airway maneuvers can be minimized by limiting airway proprioceptor stimulation, starting with manipulation of the larynx itself. For instance, cricoid cartilage pressure with a posterior force of 4.5 kg is widely used to prevent regurgitation of gastric contents or to facilitate laryngeal visualization. In a double-blind study, cricoid pressure resulted in a significantly greater HR and BP response to endotracheal intubation than occurred in patients whose cricoid area was gently palpated.24 This is a little-recognized effect of cricoid pressure that should be considered when estimating the risk-benefit ratio of this procedure in individual patients.
Laryngoscopy itself is a moderately stimulating procedure, and use of a straight blade (Miller blade) with elevation of the vagally innervated posterior aspect of the epiglottis results in significantly higher arterial BP than does use of a curved blade (Macintosh or Corazzelli–London–McCoy [CLM]).25 Newer video and optical laryngoscopes, which do not require alignment of the laryngeal axes for adequate visualization of the vocal cord inlet and subsequent intubation, have the potential to minimize the pressor response to airway manipulation by reducing the amount of force needed to displace oropharyngeal tissues and limiting cervical spine motion compared to traditional laryngoscopy with a Macintosh laryngoscope blade.26 Nonetheless, reports documenting this advantage are few.
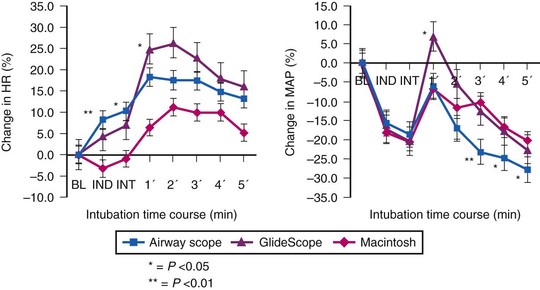
Use of the Pentax-AWS video laryngoscope (Pentax, Tokyo, Japan) has been reported to attenuate the hemodynamic response to endotracheal intubation after fentanyl/propofol induction when compared to either the GlideScope (Verathon, Bothell, WA) or the Macintosh laryngoscope (Fig. 7-2).27 This finding is not universal. An earlier study comparing the Pentax-AWS to Macintosh laryngoscopy reported no significant differences in systolic BP, diastolic BP, or HR after intubation, and a separate study comparing the GlideScope and Macintosh laryngoscopy also failed to find significant differences in hemodynamic values at any point in the study.28 None of these studies included patients with known cardiac disease or chronic HTN, who often have exaggerated pressor responses to stimulation; the newer airway devices may have greater value among this group compared with traditional laryngoscopy.
The act of passing an endotracheal tube (ETT) is far more hemodynamically stimulating than just laryngoscopy. Surprisingly, the use of a lighted intubation stylet fails to prevent hemodynamic stimulation when the ETT is advanced past the vocal cords.29 Insertion of a conventional laryngeal mask airway (LMA) after induction of general anesthesia with thiopental or propofol and fentanyl has been shown to cause less cardiovascular and endocrine response than laryngoscopy or endotracheal intubation.30–33 The LMA has the advantage of avoiding the vagally mediated infraglottic stimulation entailed by the use of a laryngoscope, thus enabling lighter levels of general anesthesia. Furthermore, because muscle relaxation is not required for airway control, spontaneously initiated ventilation is possible, with avoidance of the adverse hemodynamic consequences of PPV. In contrast, endotracheal intubation using the intubating LMA (iLMA) resulted in a hemodynamic and endocrine response similar to that resulting from direct laryngoscopy and intubation after propofol induction.34 Therefore, if endotracheal intubation is necessary, there does not appear to be a hemodynamic advantage to instrumenting the airway with the iLMA.
Whatever the technique employed to manage the airway, it must be emphasized that the hypertensive-tachycardic response to intubation is a manifestation of insufficient anesthesia. Insofar as the pressor response can also be influenced by prolonged intubation time, rapid first-attempt success is also of particular importance.7
B Topical and Regional Anesthesia
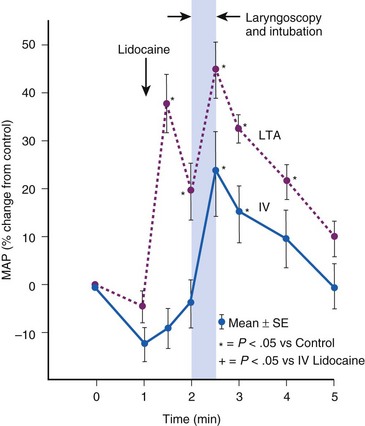
Topical anesthesia applied to the upper airway is effective in blunting hemodynamic responses to endotracheal intubation,35,36 but it has almost invariably proved to be less effective than systemic administration of lidocaine. During general anesthesia, rigid laryngoscopy and instillation of lidocaine solution initiate the same adverse reflexes caused by placement of an ETT (Fig. 7-3).37 Furthermore, a laryngotracheal spray of lidocaine solution may, in itself, produce profound cardiovascular stimulation in adults, and in children it may produce the same sort of bradycardic response associated with endotracheal intubation.38 If topical lidocaine is administered to the upper airway, there should be an intervening period of at least 2 minutes to allow initiation of anesthetic effect before airway instrumentation begins.39
Excellent topical anesthesia of the airway obtained before awake flexible fiberoptic intubation was responsible for reports suggesting that there was less cardiovascular stimulation after this procedure than after intubation with a rigid laryngoscope.40 Later studies performed with patients under general anesthesia demonstrated no difference between the two modes of intubation with regard to hemodynamic impact, probably because the more profound stimulus results from placement of the ETT below the level of the glottis.41–44
Increasing the concentration of lidocaine used, and thus the total dose, also does not appear to mitigate this effect, although it may improve intubating conditions during awake flexible fiberoptic intubation.45,46 Although both 2% and 4% lidocaine administered through an epidural catheter in the working channel of the flexible fiberoptic bronchoscope by a “spray-as-you-go” technique provided similar intubating conditions and hemodynamic profiles, the former resulted in a smaller overall dose, lower plasma levels, and therefore less chance for toxicity reactions.46 Lower concentrations of lidocaine (1%) provided lower plasma levels and similar hemodynamics but appeared to provide less optimal intubating conditions than atomized 2% lidocaine when used for topical anesthesia before airway manipulation.45
In contrast to topical anesthesia of the airway, which appears to provide inconsistent benefit, regional nerve blocks involving the sensory pathways from the airway prevent hemodynamic responses to intubation. The superior laryngeal nerve (SLN) innervates the superior surface of the larynx, and the glossopharyngeal nerve innervates the oropharynx. Depositing local anesthetic on each cornu of the hyoid bone can block the SLN. Blockade of the glossopharyngeal nerve at the tonsillar pillars (sensory distribution above the level of the epiglottis) potentiates this effect by decreasing the stimulus of laryngoscopy.47 The inferior surfaces of the larynx and trachea require topical anesthesia, however, because they are innervated by the recurrent laryngeal nerve and the vagus, which cannot be directly blocked. With the preceding combination, awake patients exhibit little response as the ETT is inserted.
Instillation of lidocaine via an ETT to prevent alterations in cerebrovascular hemodynamics in patients with severe head injury may be of some benefit. A dose of 1.7 mg/kg lidocaine instilled at body temperature given slowly (1 mL/sec) through a fine tube advanced to the end of the ETT but not in contact with the tracheal mucosa was reported to be efficacious in half of the patients treated.48
C Inhalational Anesthetics
For inhalational anesthetics, endotracheal intubation using doses in the range of the minimum alveolar concentration (1 MAC) resulted in marked cardiovascular stimulation during anesthesia with nitrous oxide (N2O) supplemented with either halothane or morphine.49 It should not be surprising that 1 MAC is insufficient, because it is known that approximately 1.5 to 1.6 MAC is needed to block the adrenergic and cardiovascular responses to a simple surgical skin incision (MAC-BAR).50 The dose of anesthetic required to prevent coughing during endotracheal intubation with sevoflurane may exceed MAC by a factor of 2.86 in adults,51 although this factor appears to be close to 1.3 in children.52
Accordingly, it appears that the dose of volatile anesthetic required to block the cardiovascular response to endotracheal intubation must be inordinately high, resulting in profound cardiovascular depression before endotracheal intubation.53 From a cerebrovascular viewpoint, this approach is totally impractical, because high doses of volatile anesthetics cause cerebral vasodilation and marked increases in ICP in patients with compromised intracranial compliance. Furthermore, from a cardiovascular point of view, the arterial hypotension and reduced cerebral perfusion pressure before intubation would be entirely unacceptable for patients with cerebrovascular disease or brain injury.
D Intravenous Agents
Propofol, barbiturates, and benzodiazepines are all associated with profound hypotension at doses that suppress the hemodynamic and ICP responses to intubation.54–56 In the case of etomidate, the effective dose for blocking the cardiovascular response to intubation can be identified by a burst-suppression pattern on the cortical surface EEG, indicating fairly deep cerebral depression.57 Because etomidate supports BP at such deep levels of anesthesia, it is probably the only contemporary agent that, by itself, can achieve suppression of cardiovascular responses without first producing undue arterial hypotension and compromise of coronary and cerebral perfusion.
Opioids are the adjuvants most commonly administered in addition to other IV or inhaled agents to facilitate induction of anesthesia and subsequent airway manipulation. Their use in this capacity relates to their historical use as part of a N2O-narcotic anesthetic often used in patients with marginal cardiac reserve. For example, Bennet and Stanley compared the cardiovascular responses after administration of N2O-morphine 0.4 mg/kg versus N2O-fentanyl 4 µg/kg 10 minutes before intubation. The HR, cardiac output, and systolic and mean BP were reduced compared to baseline and remained unaffected by intubation in the N2O-fentanyl group, but these parameters were all significantly elevated compared with preanesthetic controls in the N2O–morphine group.58 Whereas the assumed potency of fentanyl in this study was 100 times that of morphine, the lack of effect of morphine suggests that, with respect to suppression of pressor responses to laryngotracheal manipulation, fentanyl is more than 100 times as potent.
As reported by Bennett and Stanley58 and later by other investigators,59 fentanyl may not achieve its peak central nervous system effect until 10 minutes after bolus IV injection. Fentanyl appears to provide blunting of hemodynamic responses in a graded manner: 2 µg/kg IV given several minutes before induction only partially prevented HTN and tachycardia during an RSI with thiopental and succinylcholine. In this situation, 6 µg/kg was considerably more effective.60 Chen and coworkers reported almost complete suppression of hemodynamic response to intubation with both 11 and 15 µg/kg of IV fentanyl, whereas higher IV doses (30 to 75 µg/kg) allowed only a very occasional response to intubation.61
In doses that prevent hemodynamic response to intubation, however, fentanyl is not a short-acting agent, and the risk of prolonged postoperative respiratory depression must be weighed against the advantages of perioperative cardiovascular stability. With this risk in mind, it has been observed that pretreatment with 2 µg/kg IV fentanyl given 10 minutes before intubation during an infusion of propofol sufficient to reduce the Bispectral Index Score to 45 prevented a significant increase in HR or BP compared with awake preanesthetic values.10 Similar results were observed when intubation was performed after administration of fentanyl, 2 µg/kg, and propofol bolus doses of 2.0 to 3.5 mg/kg.10
Fentanyl and propofol require 6.4 and 2.9 minutes, respectively, to achieve effect-site equilibrium after IV bolus administration.10 Therefore, the common practice of administering 1 to 2 mL (50 to 100 µg) just before or almost simultaneously with other induction medications would not be expected to have any effect based on inadequate dose and inappropriate timing of administration. Rather, this may provide a more plausible explanation for hypotension during the minutes-long quiescent period between endotracheal intubation and actual surgical incision. It is strongly recommended that laryngoscopy and intubation be timed to coincide with the peak effect of these agents.
Opioids with shorter onset and offset times have some advantages over fentanyl for modulating circulatory responses to intubation. Alfentanil has a smaller steady-state distribution volume and shorter terminal elimination half-life than fentanyl.62 Ausems and colleagues demonstrated that an alfentanil plasma concentration of 600 ng/mL effectively prevented hemodynamic responses to intubation during induction of N2O anesthesia.63 This was achieved by a 30-second infusion of alfentanil at 150 µg/kg. During this induction period, N2O and succinylcholine were also administered. Only 5 of the 35 patients studied sustained an increase in HR or BP greater than 15% above preinduction values.
Remifentanil has been found to be highly effective in preventing hemodynamic responses to intubation, albeit always with the cost of impressive bradycardia or hypotension, or both, before and after airway manipulation.64 Many studies have used vagolytic agents to avoid bradycardia, at the risk of an elevated HR response after intubation. Remifentanil’s half-time for equilibration between blood and effect site is 1.3 minutes,65 and it has a brief half-life of 3 to 5 minutes due to hydrolysis by tissue and blood esterases.66 Typical remifentanil infusion rates used for blunting hemodynamic responses are 0.25 to 1.0 µg/kg/min in association with cautious propofol administration and nondepolarizing neuromuscular blockade.67 For RSI with thiopental and succinylcholine, the optimal dose of remifentanil appears to be 1.0 µg/kg administered over 30 seconds, with laryngoscopy performed 1 minute after induction. A bolus dose of 1.25 µg/kg was associated with unsatisfactory bradycardia, whereas 0.5 µg/kg resulted in excessive cardiovascular stimulation.68 This dosing recommendation is supported by another report that found remifentanil 1 µg/kg given over 30 seconds, followed by thiopental 5 mg/kg and rocuronium 1 mg/kg 100 seconds later, was more effective than lidocaine and esmolol in attenuating the hemodynamic response to RSI.69
IV lidocaine may also blunt hemodynamic and cerebrovascular responses to intubation. When given in a bolus of 1.5 mg/kg IV, it adds approximately 0.3 MAC of anesthetic potency.70 Significant reductions in hemodynamic response to endotracheal intubation have been noted when lidocaine (3 mg/kg) was used as an adjunct to high-dose fentanyl anesthesia,71 as well as during other light anesthetic techniques, such as thiopental-N2O-O2.72 However, smaller doses of lidocaine (1.5 mg/kg) have not been consistently reported to be effective in reducing the hemodynamic response to laryngoscopy and endotracheal intubation.73,74
The general anesthetic properties of lidocaine tend to reduce cerebral metabolic rate for O2 and CBF, thus lowering ICP in patients with compromised intracranial compliance.75 Theoretically, these properties of lidocaine might be exploited to mitigate rises in ICP during airway manipulation in those patients with acute intracranial pathology or compromised intracranial compliance. However, only a single human study has been reported specifically evaluating the ability of IV lidocaine to blunt intubation-related elevations in ICP. Bedford and colleagues compared 1.5 mg/kg IV lidocaine with placebo in 20 patients diagnosed with brain tumor. When administered 2 minutes before intubation, lidocaine failed to prevent a rise in ICP from the preanesthesia baseline, although the increase was more modest than that observed with the placebo (−12.1 mm Hg; 95% confidence interval, −22.8 to −1.4 mm Hg; P = 0.03).76 This dearth of direct benefit was underscored by a systematic review that also failed to identify any evidence that pretreatment with IV lidocaine before RSI consistently reduced ICP or positively affected neurologic outcome.77 This review is now more than a decade old, but because no new direct evidence has been published in the interim, its conclusion remains valid.
E Nonanesthetic Adjuvant Agents
A final means for modifying the cardiovascular responses to endotracheal intubation is prophylactic administration of vasoactive substances that directly affect the cardiovascular system. This approach was introduced in 1960 by DeVault and associates, who found that pretreatment with phentolamine, 5 mg IV, prevented the hypertensive-tachycardic response to endotracheal intubation during a light barbiturate-succinylcholine anesthetic technique.78 Since then, a large number of articles have appeared advocating the use of various vasodilators and adrenergic blocking agents as pretreatment before endotracheal intubation, including diltiazem, verapamil, and nicardipine79–82; hydralazine83; nitroprusside84; nitroglycerin85; labetalol86; esmolol80,87–89; and clonidine.90,91 Virtually all of these agents appear to be somewhat effective when compared to placebo, particularly when used in high doses.
Esmolol is the best studied of the group. In a large, multicenter, placebo-controlled trial, esmolol at doses of 100 or 200 mg suppressed the hemodynamic response to endotracheal intubation, particularly when combined with a moderate-dose opiate.87 However, esmolol doses of 200 mg were associated with a doubling of the incidence of hypotension compared to placebo. In another study, smaller doses of esmolol (1 mg/kg) had no effect on the hemodynamic response to laryngoscopy and intubation compared to placebo.80 Most recently, the combination of lidocaine (1.5 mg/kg) and esmolol at a dose of 1 mg/kg effectively attenuated the pressor response to intubation but was not as effective as 1 µg/kg remifentanil.69 Currently, the optimal use of any of these agents is undefined, although their use as adjuncts to RSI is reasonable taking into account evidence-based dosing recommendations for the situation.
IV Airway Effects of Endotracheal Intubation
B Dead Space
Patients with severe chronic lung disease may find it easier to breathe after intubation or a tracheostomy. The improvement is most likely due to reduced dead space. The normal extrathoracic anatomic dead space, based on cadaver measurements, is between 70 and 75 mL.92 The exact volume (V) of the ETT is easily calculated as that of a cylinder using the formula V = r2l, where r is the radius of the tube and l is the length. For example, an ETT that has an 8-mm inner diameter (ID) of 25 cm and a length of 25 cm has a volume of 12.6 mL. Intubation would therefore result in a reduction in dead space of approximately 60 mL.
Tracheostomy tubes are shorter than oral ETTs and have an even smaller dead space, although the difference as a proportion of tidal volume (Vt) is negligible. In normal individuals, the reduction in dead space with insertion of a tracheostomy tube is negligible compared with the normal Vt, so there is little benefit. In a patient with severe restrictive lung disease such as in end-stage kyphoscoliosis, Vt may be as low as 100 mL and intubation can confer a major benefit. Similarly, patients with emphysema who are changed from mouth breathing to tracheostomy demonstrate a reduction in minute volume required and a decrease in total body O2 consumption, presumably due to a decreased work of breathing.93 Most likely, the decreased volume required more than compensates for the slight increase in resistance.
C Upper Airway Resistance
The apparent resistance of an ETT is influenced by the shape of the tube and by two types of friction: the friction among the gas molecules and the friction between the tube wall and the gas molecules.94,95 Irregular surfaces created by secretions or by ridges from wire reinforcement may create greater friction and greater resistance.96 ETTs and tracheostomies have a higher resistance than the normal upper respiratory tract.97,98
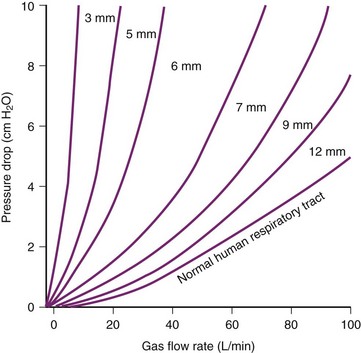
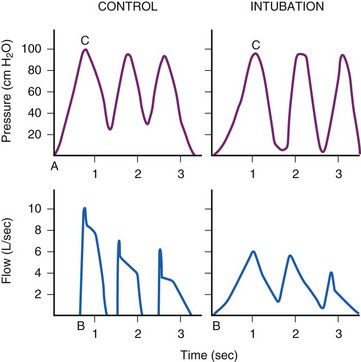
The relationship between pressure difference and flow rate depends on the nature of the flow: laminar, turbulent, or a mixture of the two. In an ETT, turbulent flow predominates. During turbulent flow, the measured resistance is not a constant but varies with the flow rate, becoming markedly higher at high flow rates. Instead of the laminar flow relationship of pressure being directly proportional to flow, the pressure required to move the gas through an ETT with turbulent flow is proportional to the square of the flow. The relationship can therefore be described by a parabolic curve, as in Figure 7-4. The apparent resistance of a tube is proportional to the fourth power of the radius during laminar flow (Poiseuille’s law) but to the fifth power during turbulent flow. Assuming turbulent flow, the relative resistance of a 6-mm ETT versus an 8-mm ETT is 45/35, or 4.2 times as great. However, because flow patterns are not entirely predictable, the exact respiratory pressure-flow relationships may not be predictable without empiric determination. Such determinations are depicted in Figure 7-4. The slope of the pressure-flow graph is the apparent resistance. The parabolic shape of the graphs demonstrates the primarily turbulent nature of the flow through an ETT.
Although the resistance of the ETT may be several-fold greater than the resistance of the normal human upper airway, this is of relatively little consequence at low minute ventilation.99 With a typical peak inspiratory flow of 25 to 30 L/min, approximately 0.5 cm H2O pressure must be generated to overcome the resistance of the upper respiratory tract. This represents about 10% of the total work of breathing. Even a doubling or tripling of that resistance by placement of an ETT does not result in a clinically worrisome increase in the total work of breathing.100
As flow rates increase, however, flow becomes more turbulent and tube resistance may become a problem. For flow rates greater than 15 L/min, flow through any tube smaller than 10 mm ID becomes turbulent.101 At the high flows required by patients in respiratory failure, the resistance of smaller tubes becomes prohibitive.102 At the time of weaning patients with respiratory failure from mechanical ventilation, the importance of tube size often becomes a critical factor, with a common question being whether to change a 7-mm to a larger size. Note in Figure 7-4 that the pressure drop between 7-mm and 9-mm tubes is minimal at low inspiratory flows, but is considerable at flows of 60 L/min and higher.
Theoretically, the patient’s native airway should have less resistance than any size ETT. However, a patient who has been intubated for an extended period may not have a normal upper airway. Indeed, some evidence suggests that work of breathing may actually increase after extubation, perhaps due to high upper airway resistance.103,104 Therefore, if the patient is close to successful weaning, a reasonable approach may be to attempt extubation rather than to change ETTs, recognizing that the need for reintubation is a possibility. Alternatively, pressure support ventilation can be used to compensate for the added work of breathing through the smaller tube until extubation is warranted.105
Tracheostomy tubes have lower resistance than ETTs of comparable diameters because they are shorter. However, there is little, if any, difference in the work of breathing imposed by fresh tracheostomy tubes and ETTs of comparable ID.106 On the other hand, tracheostomy does appear to decrease the work of breathing in patients who have undergone prolonged intubation and mechanical ventilation. This paradox may be explained by a reduction in the ID of an ETT over time, perhaps as a result of inspissated secretions or conformational changes.107 This size reduction may explain the observation that patients being weaned from mechanical ventilation are sometimes more rapidly weaned after a tracheostomy is performed,108 although it may also reflect the increased comfort of clinicians in discontinuing ventilatory support after the airway is secured.
D Lower Airway Resistance
Bronchospasm after induction of anesthesia is a relatively uncommon but well-recognized event and is likely related to a reflex response to endotracheal intubation. Several studies provide some evidence regarding the frequency of bronchospasm. Tiret and associates studied complications at the time of induction of anesthesia and noted that bronchospasm accounted for 5.3% of fatal or near-fatal peri-induction complications.109 The largest population was reported by Olsson, who found 246 cases of bronchospasm to have occurred out of a total of 136,929 anesthesia inductions, for an incidence of 1.7 per 1000.110 However, the exact incidence undoubtedly depends substantially on the patient population.
The incidence of postintubation bronchospasm may be decreasing because of the increasing use of propofol as an induction agent (propofol being more effective than thiopental at preventing this complication). However, ventilation problems combined with hypoxia due to acute bronchospasm still represent important sentinel anesthesia events.111
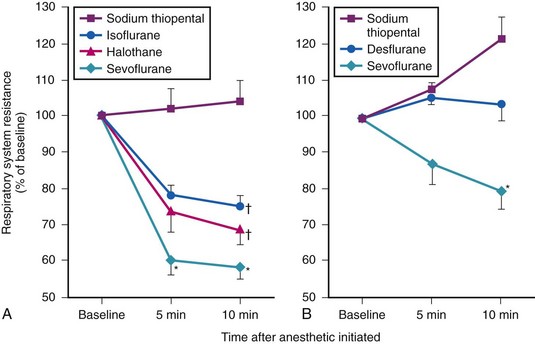
Whereas the incidence of overt clinical bronchospasm is low, a reflex increase in airway resistance may occur much more often. Receptors in the larynx and upper trachea may cause large airway constriction distal to the tube, which in turn may extend to the smaller peripheral airways.112 Support for this hypothesis comes from the work of Gal, who found an increase in lower airway resistance in a series of volunteers whose tracheas were intubated after topical anesthesia (Fig. 7-5).113
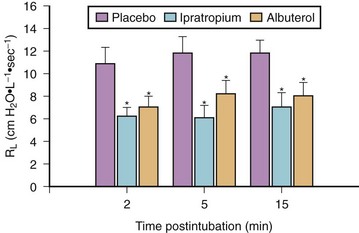
Bronchoconstriction also occurs after endotracheal intubation of normal subjects who have received thiopental/narcotic anesthesia.114 In a series of patients pretreated before anesthesia with either a β-adrenergic agonist (albuterol) or an inhaled anticholinergic agent (ipratropium bromide), measured airway resistance after intubation was markedly lower compared with placebo treatment (Fig. 7-6).
Increases in airway resistance may result from changes in intrinsic smooth muscle tone, airway edema, or intraluminal secretions. These factors are, in turn, controlled by a series of intracellular and extracellular events, including neural and hormonal factors. Rapid changes in airway caliber after airway instrumentation are thought to result largely from parasympathetic nervous system activation of airway smooth muscle.115,116 Cholinergic innervation predominates in the larger central airways, with efferent nerves arising in the vagal nuclei of the brain stem and synapsing with ganglia in the airway walls. Postganglionic parasympathetic nerves release acetylcholine, activating muscarinic receptors on airway smooth muscle that lead to smooth muscle constriction. Such responses can be blocked via muscarinic blockade, using either systemic or inhaled anticholinergic agents.
Endotracheal intubation also may induce bronchospasm by causing coughing. A cough reduces lung volume, which in turn markedly increases bronchoconstriction in response to a stimulus.117 In the patient with known reactive airways, prevention of coughing at the time of endotracheal intubation by use of either a deep level of anesthetic or a muscle relaxant may help to minimize the likelihood of bronchospasm.
E Endotracheal Tube Resistance and Exhalation
In normal patients breathing at moderately elevated minute ventilation, exhalation is usually completed well before the next inhalation begins. By contrast, patients with obstructive airways disease may not complete full exhalation before the start of the next inhalation. In other words, inhalation begins before exhalation to functional residual capacity (FRC), resulting in persistent positive pressure in the alveoli. This phenomenon has been called auto-PEEP or dynamic hyperinflation, and it results in air trapping, elevated intrathoracic pressure, and hemodynamic compromise.118
Auto-PEEP most commonly occurs in patients with obstructive lung disease and high minute ventilation, but it may also rarely occur in patients with relatively normal airways who are ventilated at very high minute ventilation. This has been observed in patients with burns or sepsis, who may require as much as 30 to 40 L of minute ventilation. Under these circumstances, the resistance of the ETT may limit expiratory flow so that full exhalation does not occur. This has been demonstrated experimentally, with the magnitude of the auto-PEEP correlating directly with the resistance of the ETT.119 Among patients under anesthesia, major resistance to exhalation caused by the ETT is of no consequence in routine cases and is only rarely seen in critically ill patients. However, low levels of auto-PEEP due to tube resistance probably occur frequently in patients with high minute ventilation108 and during single-lung ventilation via a double-lumen ETT.
F Functional Residual Capacity
The effect of endotracheal intubation on FRC has been a subject of considerable controversy. Intensivists are well aware of patients recovering from respiratory failure in whom oxygenation improved after extubation. The improvement has been attributed to “physiologic PEEP”—the presumption that a small positive pressure is normally created by the glottis and that this leads to breathing at a higher lung volume. The assumption is that an ETT removes the glottic barrier and may, therefore, lower lung volume. However, the existence of positive intratracheal pressure has never been documented, and in a study of volunteers who underwent awake intubation, no consistent change in FRC could be measured.120–122
By contrast, a different conclusion was reached in a series of patients who were studied just before and after extubation following recovery from respiratory failure. In this situation, both FRC and arterial oxygen tension (PaO2) were found to increase after extubation, supporting the concept that the presence of an ETT decreases FRC.123 Resolution of these disparate results was suggested by a rabbit study in which normal rabbits did not demonstrate a difference in oxygenation or tracheal pressure after intubation, but after respiratory failure was induced, endotracheal intubation worsened oxygenation.124 These results suggest that the rabbits compensated for respiratory failure by using glottic closure to maintain a positive intratracheal pressure and that the effect of an ETT on FRC depends on that underlying respiratory state.
G Cough
Although it is widely recognized that cough efficiency is reduced whenever an ETT is in place, it is a common observation that a disconnected ETT is likely to produce a plug of sputum whenever the patient is stimulated to cough. In awake intubation volunteers, peak airway flow was reduced but was still adequate to enable secretion clearance.113 However, the ETT prevented collapse of the trachea by acting as a stent. Therefore, although secretions could be moved to the central airways, the ETT prevented maximum efficiency of expectoration. Large airway collapse is important for producing maximum force against secretions, and this explains why moving secretions from the trachea out through the ETT often requires the use of a suction catheter.
H Humidification of Gases
Under normal circumstances, the upper airway warms, humidifies, and filters 7000 to 10,000 L of inspired air daily, adding up to 1 L of moisture to the gases. When the upper airway is bypassed by intubation, the gas must be warmed and humidified in the trachea if it is not adequately humidified before inhalation. In an anesthetized patient breathing dry gases, up to 10% of the average metabolic rate may be required to perform these tasks.125 Delivery of cool, dry gases may also have a significant effect on mucociliary transport, a critical defense mechanism of the respiratory tract. Inhalation of unconditioned gas rapidly leads to abnormal mucosal ciliary motion, with subsequent encrustation and inspissation of tracheal secretions.126,127 These changes occur as early as 30 minutes after intubation and, theoretically, may lead to an increase in postoperative complications in patients with limited chest excursion. Accordingly, assurance of adequate gas conditioning should be standard in all but very brief endotracheal intubations.
V Control and Treatment of the Respiratory Responses to Airway Instrumentation
A Preventing Upper Airway Responses
Inhibition of upper airway reflexes can certainly be accomplished by performing endotracheal intubation after the administration of NMB agents. However, both laryngeal and tracheal reflexes are difficult to inhibit by deep levels of general anesthesia alone.128 When circumstances preclude the use of NMB agents, the clinician must give consideration to how best to prevent discomfort, gagging, coughing, and laryngospasm during endotracheal intubation: avoidance of endotracheal intubation, use of regional and topical anesthesia, very deep general anesthesia, or a combination of all modalities.
1 Technical Considerations: Minimizing Airway Stimulation
Although placement of an LMA is likely to be less noxious than direct laryngoscopy and endotracheal intubation, it remains a highly stimulating procedure. For example, Scanlon and colleagues found a 60% incidence of gagging, a 30% incidence of laryngospasm, and a 19% incidence of coughing when the LMA was placed after induction with thiopental 5 mg/kg.129 Induction with propofol 2.5 mg/kg reduced these events by two thirds but did not ablate them. Therefore, instrumentation of the upper airway by any technique will illicit protective reflexes that must be obtunded with local or general anesthesia (or both).
2 Regional and Topical Anesthesia
The surfaces of the mouth and nose are easily anesthetized with topical anesthetic sprays or gels. Lidocaine is equally effective as cocaine and less toxic; it can be combined with a vasoconstrictor to give equivalent intubating conditions.130–132 Administration of an antisialagogue 30 to 60 minutes before application of the topical anesthetic results in better anesthesia as well as better intubating conditions. The lack of secretions probably minimizes dilution of the applied anesthetic and also results in better intubating conditions.
The mouth and pharynx derive their sensory innervation from the trigeminal and glossopharyngeal nerves. The supraglottic larynx derives its sensory innervation from the SLN, a branch of the vagus, and intubation can be facilitated by blocking it bilaterally.133 The nerve block relies on the consistent relationship of the SLN to the lateral horns of the hyoid bone. When combined with topical anesthesia of the nose or mouth and adequate anesthesia of the infraglottic larynx, the nerve block provides excellent intubating conditions, and most patients are able to accept an ETT without cough, gag, or laryngospasm. Equal success in blunting upper airway reflexes can be achieved by careful spraying of the larynx with topical anesthesia. A nasal trumpet helps ensure that solution reaches the larynx. Topical anesthesia spares the patient the need for two injections.
The efficacy of topical and nerve block anesthesia at suppressing airway reflexes during intubation is evident. Several studies have documented that topical anesthesia applied preoperatively (for brief cases) or intraoperatively can suppress cough and laryngospasm at the time of extubation.134 A randomized study of patients undergoing tonsillectomy found that the incidence of stridor or laryngospasm at the time of extubation could be reduced from 12% to 3% by application of topical lidocaine at the time of intubation.135 The LITA endotracheal tube (Laryngotracheal Instillation of Topical Anesthetic, Sheridan Corporation, Argyle, NY) contains a small channel that can be used to spray the upper airway while an ETT is in place. When this method was used to spray the ETT before extubation, coughs were reduced by more than 60%, and the severity of the coughing was decreased.136
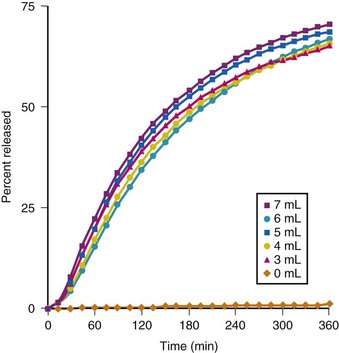
The use of liquid, in the form of a lidocaine-bicarbonate mixture, rather than air to inflate the cuff of the ETT after intubation has also been reported to be effective in diminishing emergence phenomena.137,138 Inflating the ETT cuff with 40 mg of lidocaine (2 mL of 2% solution) and then adding 3 to 7 mL of 8.4% sodium bicarbonate (NaHCO3) until no cuff leak was present resulted in significant reductions in coughing, restlessness, and BP during emergence. In addition, sore throat complaints assessed at 15 minutes and at 1, 2, 3, and 24 hours postoperatively; postoperative dysphonia; and hoarseness after extubation were all reduced when compared to cuff inflation with air. When this technique was used, more than 50% of the original 40 mg of lidocaine still remained in solution at 2 hours after inflation of the cuff, and only about 75% was released even after 6 hours of surgery (Fig. 7-7).137 Because standard 8.4% NaHCO3 is a basic solution with a calculated pH of 7.8 (range 7 to 8.5), the addition of more than 2 mL of bicarbonate to the 2 mL of 2% lidocaine (calculated pH 6, range 5 to 7) already injected into the ETT cuff results in a solution with a pH 7.95 to 8.09; this leads to concern about tracheal mucosal damage from flash burn injury in the event of a cuff rupture. However, a direct comparison between solutions of 2 mL of 2% lidocaine with 8.4% versus 1.4% bicarbonate reported similar efficacy in reducing postoperative sore throat complaints and the occurrence of various emergence phenomena.138 Therefore, in actual clinical practice, a favorable risk-benefit balance can be achieved by using the following combination in a 10-mL syringe: 5 mL 1% lidocaine, 1 mL 8.4% NaHCO3 solution, and 4 to 5 mL of sterile diluent (J.P. Estebe, personal communication, 2010).
3 Intravenous Agents
Given a high enough dose, virtually all agents used as IV anesthetics will suppress the cough response to intubation. However, different agents appear to vary in their ability to inhibit upper airway reflexes when judged on the basis of equal potency in depressing consciousness and in depressing the cardiovascular system. Propofol/narcotic anesthesia may be adequate for intubating the trachea in some patients even without the use of muscle relaxants.139 On the other hand, ketamine clinically appears to enhance laryngeal reflexes at doses that provide adequate anesthesia for surgery.
IV lidocaine is frequently used to prevent cough and laryngospasm at the time of intubation or extubation. Although the studies are not uniform in documenting efficacy, the preponderance of evidence supports the use of lidocaine.140,141 Studies that did not document efficacy are sometimes flawed by the lack of documentation that adequate serum levels were reached. The maximal efficacy of IV lidocaine occurs 1 to 3 minutes after injection and requires a dose of 1.5 mg/kg or more. This corresponded to a plasma level in excess of 4 µg/mL.
The ability of IV lidocaine to suppress cough appears to be related to factors beyond induction of general anesthesia, because cough suppression occurs at levels routinely seen in awake patients being treated with the drug. A comparison of the antitussive effects of lidocaine compared with meperidine and thiopental demonstrated that severe respiratory depression occurs with the latter drugs in achieving the same antitussive efficacy that can be achieved with lidocaine with virtually no respiratory depression.142
Whether IV lidocaine suppresses laryngospasm remains controversial. A study in which tonsillectomy patients were given 2 mg/kg of IV lidocaine and then extubated 1 minute later found suppression of laryngospasm.143 Another study of tonsillectomy patients given 1.5 mg/kg of lidocaine found no clear effect.144 A major difference in the latter study was the authors’ design of not extubating the patient until swallowing had begun. This may have resulted in a significant difference in the anesthetic depth at which the children were extubated.
B Preventing Bronchoconstriction
Bronchospasm after intubation may be cholinergically mediated. Afferent parasympathetic fibers travel to bronchial smooth muscle and then produce bronchoconstriction by stimulating the M3 cholinergic receptors on bronchial smooth muscle. In addition, stimulation of M2 cholinergic receptors on airway smooth muscle potentiates bronchospasm by inhibiting β-adrenergic–mediated smooth muscle relaxation.145
1 Technical Considerations: Minimizing Airway Stimulation
Avoidance of endotracheal intubation is the most logical first step in terms of limiting airway irritation and bronchoconstriction. If general anesthesia is required, the LMA may be preferable to the ETT in terms of provocation of bronchospasm, but, as alluded to earlier, the LMA will not prevent coughing in the absence of NMB.129 However, the LMA does appear to result in reduced lower airway resistance when compared with endotracheal intubation after induction of general anesthesia. This difference is assumed to result from induction of reversible bronchospasm by the ETT.146,147 In addition, use of the LMA results in fewer pulmonary complications and improved pulmonary function when compared with endotracheal intubation in infants born prematurely with bronchopulmonary dysplasia and in adults without lung disease.148,149
2 Topical Anesthesia
The studies of Gal and Surratt demonstrated a doubling of lower airway resistance after endotracheal intubation of awake volunteers under topical anesthesia.120 The bronchoconstrictive response must indeed be a powerful one if local anesthesia sufficient to permit volunteers to be intubated was not sufficient to prevent the reflex bronchoconstriction. A study of awake fiberoptic intubation in asthmatics demonstrated a marked decrease in forced expiratory volume in 1 minute (FEV1) after intubation. This decrease was somewhat mitigated by topical lidocaine although lidocaine was not as effective as albuterol in preventing the bronchoconstriction.150 However, a lidocaine aerosol given to dogs before a challenge with inhaled citric acid did not attenuate the response to this irritant.151 Because the aerosol itself produces a slight increase in lung resistance, the efficacy of the inhaled aerosol lidocaine may in part have been due to IV absorption of the drug. Given these considerations and the time required to administer the aerosol compared to the immediacy and efficacy of IV drugs or other inhaled drugs, aerosolized lidocaine is probably a poor choice for the attenuation of bronchoconstriction associated with endotracheal intubation.
3 Intravenous Agents
A variety of drugs have been studied for their bronchodilating properties. Although IV β-agonists clearly produce bronchodilation, there is no benefit to parenteral administration of these drugs rather than inhalational administration. Among anesthetic induction agents, considerable experimental evidence suggests that ketamine has both direct and indirect relaxant effects on airway smooth muscle through non–β-receptor mechanisms.152–157 However, the clinical data supporting the use of ketamine for prevention or treatment of bronchospasm is largely anecdotal,158 or in more rigorous trials unimpressive.159–161 This may relate to reluctance to routinely use ketamine at high doses because of its side effects, including dysphoria and sympathetic stimulation, rather than a lack of benefit of the drug.
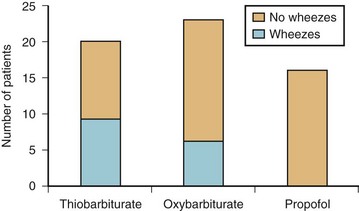
Propofol, midazolam, and etomidate all relax airway smooth muscle in vitro, although generally at higher site concentrations than would be used clinically.162–166 In contrast, barbiturates may have direct bronchoconstricting effects.167 Propofol may also have indirect effects on airway constriction, perhaps though inhibition of vagal tone.157,168 Clinically, propofol has been shown to be superior to the barbiturates and to etomidate in reducing wheezing and airway resistance in both asthmatic and nonasthmatic subjects.169–171 When asthmatics were induced with either thiopental, methohexital, or propofol at equipotent doses, none of the patients given propofol wheezed after endotracheal intubation, whereas both of the barbiturates resulted in a significant incidence of wheezing (Fig. 7-8).169
In animals, IV lidocaine has been reported to attenuate bronchoconstriction induced by a variety of experimental means.172,173 In humans with bronchial hyperreactivity, IV lidocaine reduced the bronchoconstrictor response to histamine challenge and had an additive effect with albuterol in reducing this response.174 However, a double-blind, placebo-controlled trial of IV lidocaine (1.5 mg/kg) or inhaled albuterol in asthmatics found that albuterol but not lidocaine prevented postintubation bronchoconstriction.175 Although lidocaine may inhibit reflex-induced bronchospasm, it may also cause contraction of bronchial smooth muscle in the absence of reflex mechanisms. In a study of 15 asthmatics, IV lidocaine reduced airway diameter at total lung capacity assessed by computed tomography and resulted in significant decreases in FEV1.176 These untoward effects were underscored by a case report in which IV lidocaine 1.5 mg/kg administered to facilitate intubation was temporally associated with transient bronchospasm in a 17-month-old child with mild intermittent asthma.177 A published best-evidence review also failed to find good evidence for use of IV lidocaine during intubation in patients with status asthmaticus.178 Therefore, the evidence in support of IV lidocaine to prevent postintubation bronchospasm when used without the concomitant administration of an inhaled β-agonist is scant, and there is a potential risk of worsening airway resistance with its use. Therefore, the use of IV lidocaine for this indication cannot be endorsed.
4 Inhaled Agents
All of the volatile anesthetics have direct and perhaps indirect relaxant effects on airway smooth muscle in experimental models.94,179–182 Although these agents have differences in potency in vitro, the clinical importance of these differences is unclear.180,182,183 In adult patients, sevoflurane is more effective than isoflurane, desflurane, or halothane in reducing airway resistance after endotracheal intubation,184–186 but does not prevent an increase in airway resistance after intubation of asthmatic children.187 However, given the available data, sevoflurane is probably the volatile agent of choice, and desflurane is a poor choice for use in high-risk patients (Fig. 7-9).
Pretreatment of patients with inhaled β2-adrenergic agonists or an inhaled anticholinergic markedly reduced lung resistance following endotracheal intubation and should be used routinely in patients known to have bronchospasm.114,188
5 Choice of Neuromuscular Blocking Drug
The choice of muscle relaxants can influence bronchial tone after endotracheal intubation. Rapacuronium was withdrawn from the market after a number of reports of severe bronchospasm, most likely due to antagonism at the M2 receptor.189 Mivacurium releases significant amounts of histamine and leads to mast cell degranulation; it should be used extremely cautiously, if at all, in patients with a history of atopy or asthma.190 Studies in France and Norway have suggested a high incidence of anaphylaxis with rocuronium, although this finding does not appear to be supported in literature from other countries.
VII Clinical Pearls
• Laryngoscopy can induce bradycardia, by increasing vagal tone at the sinoatrial node, or HTN and tachycardia mediated by the cardioaccelerator nerves and sympathetic chain ganglia. The former is most common in infants and children, whereas the latter is typical for adolescents and adults.
• Laryngoscopy and intubation result in stimulation of the CNS and may increase cerebral blood flow (CBF), which may result in elevation of intracranial pressure (ICP) and brain herniation.
• Ischemic electrocardiographic changes lasting less than 10 minutes during airway manipulation have not been shown to correlate with postoperative myocardial infarction.
• Succinylcholine is associated with bradycardia in children, particularly when doses are repeated, but it is a cardiovascular stimulant in adults.
• Succinylcholine may directly elevate CBF and ICP, an effect that can be blunted by pretreatment with a nondepolarizing agent and strict maintenance of normocapnia.
• The application of cricoid pressure can result in greater HR and BP response to endotracheal intubation than when it is not used, and it should be considered when estimating the risk-benefit ratio of this procedure in individual patients.
• Fentanyl provides a graded response in blunting hemodynamic responses to intubation, with 2 µg/kg IV fentanyl given several minutes before induction only partially preventing hypertension and tachycardia during a rapid-sequence intubation (RSI).
• Fentanyl and propofol require 6.4 and 2.9 minutes, respectively, to achieve effect-site equilibrium after IV bolus administration. Therefore, the commonly observed practice of administering 1 to 2 mL of fentanyl (50 to 100 µg) just before or almost simultaneously with administration of other induction medications would not be expected to have any effect based on inadequate dose and inappropriate timing of administration.
• When given in a bolus of 1.5 mg/kg IV, lidocaine adds approximately 0.3 MAC of anesthetic potency, but it is not reliable at blunting the cardiovascular or airway response to laryngoscopy or intubation. Additionally, pretreatment with IV lidocaine before RSI does not consistently reduce ICP or positively affect neurologic outcome.
• For surgeries lasting longer than 2 hours, cough and throat complaints may be decreased by inflating the cuff of the ETT with a buffered solution containing 40 mg of lidocaine. This can be accomplished by using a 10-mL syringe containing 5 mL 1% lidocaine, 1 mL 8.4% NaHCO3 solution, and 4 to 5 mL of sterile diluent and inflating the cuff until no leak is present.
• Propofol, midazolam, and etomidate are preferred to barbiturates for anesthetic induction in patients with known reactive airways and in those in whom acute bronchoconstriction is to be avoided.
All references can be found online at expertconsult.com.
12 Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: The role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37:1839–1845.
18 Lanier W, Milde J, Michenfelder J. Cerebral stimulation following succinylcholine in dogs. Anesthesiology. 1986;64:551–559.
24 Saghaei M, Masoodifar M. The pressor response and airway effects of cricoid pressure during induction of general anesthesia. Anesth Analg. 2001;93:787–790.
32 Wood ML, Forrest ET. The haemodynamic response to the insertion of the laryngeal mask airway: A comparison with laryngoscopy and tracheal intubation. Acta Anaesthesiol Scand. 1994;38:510–513.
61 Chen CT, Toung TJK, Donham RT, et al. Fentanyl dosage for suppression of circulatory response to laryngoscopy and endotracheal intubation. Anesthesiol Rev. 1986;13:37–42.
69 Min JH, Chai HS, Kim YH, et al. Attenuation of hemodynamic responses to laryngoscopy and tracheal intubation during rapid sequence induction: Remifentanil vs. lidocaine with esmolol. Minerva Anestesiol. 2010;76:188–192.
73 Miller CD, Warren SJ. Intraveous lignocaine fails to attenuate the cardiovascular response to laryngoscopy and tracheal intubation. Br J Anaesth. 1990;65:216–219.
77 Robinson N, Clancy M. In patients with head injury undergoing rapid sequence intubation, does pretreatment with intravenous lignocaine/lidocaine lead to an improved neurological outcome? A review of the literature. Emerg Med J. 2001;18:453–457.
87 Miller DR, Martineau RJ, Wynands JE, et al. Bolus administration of esmolol for controlling the haemodynamic response to tracheal intubation: The Canadian Multicentre Trial. Can J Anaesth. 1991;38:849–858.
138 Estebe JP, Gentili M, Le Corre P, et al. Alkalinization of intracuff lidocaine: Efficacy and safety. Anesth Analg. 2005;101:1536–1541.
1 Loeb HS, Saudye A, Croke RP, et al. Effects of pharmacologically-induced hypertension on myocardial ischemia and coronary hemodynamics in patients with fixed coronary obstruction. Circulation. 1978;57:41–46.
2 Slogoff S, Keats A. Does perioperative myocardial ischemia lead to postoperative myocardial infarction? Anesthesiology. 1981;55:212–217.
3 Nadel J, Widdicombe J. Reflex effects of upper airway irritation on total lung resistance and blood pressure. J Appl Physiol. 1962;17:861–865.
4 Dohi S, Gold M. Pulmonary mechanics during general anesthesia. Br J Anaesth. 1979;51:205–213.
5 Fox EJ, Sklar GS, Hill CH, et al. Complications related to the pressor response to endotracheal intubation. Anesthesiology. 1977;47:524–525.
6 Shapiro HM, Wyte SR, Harris AB, et al. Acute intraoperative intracranial hypertension in neurosurgical patients: Mechanical and pharmacologic factors. Anesthesiology. 1972;37:399–405.
7 Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987;59:295–299.
8 Sant’Ambrogio G. Nervous receptors of the tracheobronchial tree. Annu Rev Physiol. 1987;49:611–627.
9 Hassan HG, el-Sharkawy TY, Renck H, et al. Hemodynamic and catecholamine responses to laryngoscopy with vs. without endotracheal intubation. Acta Anaesthesiol Scand. 1991;35:442–447.
10 Mi WD, Sakai T, Takahashi S, et al. Haemodynamic and electroencephalograph responses to intubation during induction with propofol or propofol/fentanyl. Can J Anaesth. 1998;45:19–22.
11 Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet. 1993;341:715–719.
12 Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: The role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37:1839–1845.
13 King B. Reflex circulatory responses to direct laryngoscopy and tracheal intubation performed during general anesthesia. Anesthesiology. 1951;12:556–566.
14 Ali HH, Lien CA, Witkowski T, et al. Efficacy and safety of divided dose administration of mivacurium for a 90-second tracheal intubation. J Clin Anesth. 1996;8:276–281.
15 Tarkkanen L, Laitinen L, Johansson G. Effects of d-tubocurarine on intracranial pressure and thalamic electrical impedance. Anesthesiology. 1974;40:247–251.
16 Heier T, Caldwell JE. Rapid tracheal intubation with large-dose rocuronium: A probability-based approach. Anesth Analg. 2000;90:175–179.
17 Mori K, Iwabuchi K, Fujita M. The effects of depolarizing muscle relaxants on the electroencephalogram and the circulation during halothane anaesthesia in man. Br J Anaesth. 1973;45:604–610.
18 Lanier W, Milde J, Michenfelder J. Cerebral stimulation following succinylcholine in dogs. Anesthesiology. 1986;64:551–559.
19 Minton MD, Grosslight K, Stirt JA, et al. Increases in intracranial pressure from succinylcholine: Prevention by prior nondepolarizing blockade. Anesthesiology. 1986;65:165–169.
20 Stirt JA, Grosslight KR, Bedford RF, et al. “Defasciculation” with metocurine prevents succinylcholine-induced increases in intracranial pressure. Anesthesiology. 1987;67:50–53.
21 Brown MM, Parr MJ, Manara AR. The effect of suxamethonium on intracranial pressure and cerebral perfusion pressure in patients with severe head injuries following blunt trauma. Eur J Anaesthesiol. 1996;13:474–477.
22 Clancy M, Halford S, Walls R, et al. In patients with head injuries who undergo rapid sequence intubation using succinylcholine, does pretreatment with a competitive neuromuscular blocking agent improve outcome? A literature review. Emerg Med J. 2001;18:373–375.
23 Jaber S, Jung B, Corne P, et al. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: A prospective, multiple-center study. Intensive Care Med. 2010;36:248–255.
24 Saghaei M, Masoodifar M. The pressor response and airway effects of cricoid pressure during induction of general anesthesia. Anesth Analg. 2001;93:787–790.
25 Nishiyama T, Higashizawa T, Bito H, et al. [Which laryngoscope is the most stressful in laryngoscopy; Macintosh, Miller, or McCoy?]. Masui. 1997;46:1519–1524.
26 Cooper RM, Pacey JA, Bishop MJ, et al. Early clinical experience with a new videolaryngoscope (GlideScope) in 728 patients. Can J Anaesth. 2005;52:191–198.
27 Tsai PB, Chen B. Hemodynamic responses to endotracheal intubation comparing the Airway Scope, Glidescope, and Macintosh laryngoscopes. Internet J Anesthesiol. 24(2), 2010.
28 Nishikawa K, Matsuoka H, Saito S. Tracheal intubation with the PENTAX-AWS (airway scope) reduces changes of hemodynamic responses and bispectral index scores compared with the Macintosh laryngoscope. J Neurosurg Anesthesiol. 2009;21:292–296.
29 Takahashi S, Mizutani T, Miyabe M, et al. Hemodynamic responses to tracheal intubation with laryngoscope versus lightwand intubating device (Trachlight) in adults with normal airway. Anesth Analg. 2002;95:480–484.
30 Braude N, Clements EA, Hodges UM, et al. The pressor response and laryngeal mask insertion: A comparison with tracheal intubation. Anaesthesia. 1989;44:551–554.
31 Wilson IG, Fell D, Robinson SL, Smith G. Cardiovascular responses to insertion of the laryngeal mask. Anaesthesia. 1992;47:300–302.
32 Wood ML, Forrest ET. The haemodynamic response to the insertion of the laryngeal mask airway: A comparison with laryngoscopy and tracheal intubation. Acta Anaesthesiol Scand. 1994;38:510–513.
33 Oczenski W, Krenn H, Dahaba AA, et al. Hemodynamic and catecholamine stress responses to insertion of the Combitube, laryngeal mask airway or tracheal intubation. Anesth Analg. 1999;88:1389–1394.
34 Choyce A, Avidan MS, Harvey A, et al. The cardiovascular response to insertion of the intubating laryngeal mask airway. Anaesthesia. 2002;57:330–333.
35 Stoelting RK. Circulatory changes during direct laryngoscopy and tracheal intubation: Influence of duration of laryngoscopy with or without prior lidocaine. Anesthesiology. 1977;47:381–384.
36 Stoelting RK. Circulatory response to laryngoscopy and tracheal intubation with or without prior oropharyngeal viscous lidocaine. Anesth Analg. 1977;56:618–621.
37 Youngberg J, Graybar G, Hutchings D. Comparison of intravenous and topical lidocaine in attenuating the cardiovascular responses to endotracheal intubation. South Med J. 1983;76:1122–1124.
38 Mirakhur R. Bradycardia with laryngeal spraying in children. Acta Anaesth Scand. 1982;26:130–132.
39 Takita K, Morimoto Y, Kemmotsu O. Tracheal lidocaine attenuates the cardiovascular response to endotracheal intubation. Can J Anaesth. 2001;48:732–736.
40 Hawkyard SJ, Morrison A, Doyle LA, et al. Attenuating the hypertensive response to laryngoscopy and endotracheal intubation using awake fibreoptic intubation. Acta Anaesthesiol Scand. 1992;36:1–4.
41 Finfer SR, MacKenzie SI, Saddler JM, Watkins TG. Cardiovascular responses to tracheal intubation: A comparison of direct laryngoscopy and fiberoptic intubation. Anaesth Intens Care. 1989;17:44–48.
42 Schaefer H, Marsch S. Comparison of orthodox with fiberoptic orotracheal intubation under total IV anaesthesia. Br J Anaesth. 1991;66:608–610.
43 Smith J. Heart rate and arterial pressure changes during fiberoptic tracheal intubation under general anesthesia. Anaesthesia. 1988;43:629–632.
44 Smith J, Mackenzie A, Scott-Knight V. Comparison of two methods of fiber-scope-guided tracheal intubation. Br J Anaesth. 1991;66:546–550.
45 Woodruff C, Wieczorek PM, Schricker T, et al. Atomised lidocaine for airway topical anaesthesia in the morbidly obese: 1% compared with 2%. Anaesthesia. 2010;65:12–17.
46 Xue FS, Liu HP, He N, et al. Spray-as-you-go airway topical anesthesia in patients with a difficult airway: A randomized, double-blind comparison of 2% and 4% lidocaine. Anesth Analg. 2009;108:536–543.
47 Rovenstine E, Papper E. Glossopharyngeal nerve block. Am J Surg. 1948;75:713–715.
48 Bilotta F, Branca G, Lam A, et al. Endotracheal lidocaine in preventing endotracheal suctioning-induced changes in cerebral hemodynamics in patients with severe head trauma. Neurocrit Care. 2008;8:241–246.
49 Bedford R, Marshall W. Cardiovascular response to endotracheal intubation during four anesthetic techniques. Acta Anaesthesiol Scand. 1984;28:563–566.
50 Roizen MF, Horrigan R, Frazer B. Anesthetic doses that block adrenergic (stress) and cardiovascular responses to incision—MAC-BAR. Anesthesiology. 1981;54:390–398.
51 Kimura T, Watanabe S, Asakura N, et al. Determination of end-tidal sevoflurane concentration for tracheal intubation and minimum alveolar anesthetic concentration in adults. Anesth Analg. 1994;79:378–381.
52 Nishina K, Mikawa K, Shiga M, et al. Oral clonidine premedication reduces minimum alveolar concentration of sevoflurane for tracheal intubation in children. Anesthesiology. 1997;87:1324–1327.
53 Zbinden AM, Petersen-Felix S, Thomson DA. Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen anesthesia: II. Hemodynamic responses. Anesthesiology. 1994;80:261–267.
54 Moss E, Powell D, Gibson RM, et al. Effects of tracheal intubation on intracranial pressure following induction of anaesthesia with thiopentone or althesin in patients undergoing neurosurgery. Br J Anaesth. 1978;50:353–360.
55 Ravussin P, Guinard JP, Ralley F, et al. Effect of propofol on cerebrospinal fluid pressure and cerebral perfusion pressure in patients undergoing craniotomy. Anaesthesia. 1988;43:S37–S41.
56 Giffin JP, Cottrell JE, Shwiry B, et al. Intracranial pressure, mean arterial pressure, and heart rate following midazolam or thiopental in humans with brain tumors. Anesthesiology. 1984;60:491–494.
57 Modica PA, Tempelhoff R. Intracranial pressure during induction of anaesthesia and tracheal intubation with etomidate-induced EEG burst suppression. Can J Anaesth. 1992;39:236–241.
58 Bennett GM, Stanley TH. Human cardiovascular responses to endotracheal intubation during morphine—N2O and fentanyl—N2O anesthesia. Anesthesiology. 1980;52:520–522.
59 Billard V, Moulla F, Bourgain JL, et al. Hemodynamic response to induction and intubation: Propofol/fentanyl interaction. Anesthesiology. 1994;81:1384–1393.
60 Kautto H. Attenuation of the circulatory response to laryngoscopy and intubation by fentanyl. Acta Anaesth Scand. 1982;26:217–221.
61 Chen CT, Toung TJK, Donham RT, et al. Fentanyl dosage for suppression of circulatory response to laryngoscopy and endotracheal intubation. Anesthesiol Rev. 1986;13:37–42.
62 Scott JC, Ponganis KV, Stanski DR. EEG quantitation of narcotic effect: The comparative pharmacodynamics of fentanyl and alfentanil. Anesthesiology. 1985;62:234–241.
63 Ausems ME, Hug CC, Jr., Stanski DR, et al. Plasma concentrations of alfentanil required to supplement nitrous oxide anesthesia for general surgery. Anesthesiology. 1986;65:362–373.
64 Thompson JP, Hall AP, Russell J, et al. Effect of remifentanil on the haemodynamic response to orotracheal intubation. Br J Anaesth. 1998;80:467–469.
65 Glass PS, Hardman D, Kamiyama Y, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: Remifentanil (GI87084B). Anesth Analg. 1993;77:1031–1040.
66 Kapila A, Glass PS, Jacobs JR, et al. Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology. 1995;83:968–975.
67 Maguire AM, Kumar N, Parker JL, et al. Comparison of effects of remifentanil and alfentanil on cardiovascular response to tracheal intubation in hypertensive patients. Br J Anaesth. 2001;86:90–93.
68 O’Hare R, McAtamney D, Mirakhur RK, et al. Bolus dose remifentanil for control of haemodynamic response to tracheal intubation during rapid sequence induction of anaesthesia. Br J Anaesth. 1999;82:283–285.
69 Min JH, Chai HS, Kim YH, et al. Attenuation of hemodynamic responses to laryngoscopy and tracheal intubation during rapid sequence induction: Remifentanil vs. lidocaine with esmolol. Minerva Anestesiol. 2010;76:188–192.
70 Himes RJ, DiFazio C, Burney R. Effects of lidocaine on the anesthetic requirements for nitrous oxide and halothane. Anesthesiology. 1977;47:437–440.
71 Kasten GW, Owens E. Evaluation of lidocaine as an adjunct to fentanyl anesthesia for coronary artery bypass graft surgery. Anesth Analg. 1986;65:511–515.
72 Abou-Madi M, Keszler H, Yacoub J. Cardiovascular reactions to laryngoscopy and tracheal intubation following small and large intravenous doses of lidocaine. Canad Anaesth Soc J. 1977;24:12–19.
73 Miller CD, Warren SJ. Intraveous lignocaine fails to attenuate the cardiovascular response to laryngoscopy and tracheal intubation. Br J Anaesth. 1990;65:216–219.
74 Splinter WM. Intravenous lidocaine does not attenuate the haemodynamic response of children to laryngoscopy and tracheal intubation. Can J Anaesth. 1990;37:440–443.
75 Bedford R. Intracranial pressure response to endotracheal intubation: Efficacy of intravenous lidocaine pretreatment for patients with brain tumors. Shulman K, Marmarou A, Miller JD, et al. Intracranial pressure, ed 4, New York: Springer-Verlag, 1980.
76 Bedford RF, Persing JA, Pobereskin L, et al. Lidocaine or thiopental for rapid control of intracranial hypertension? Anesth Analg. 1980;59:435–437.
77 Robinson N, Clancy M. In patients with head injury undergoing rapid sequence intubation, does pretreatment with intravenous lignocaine/lidocaine lead to an improved neurological outcome? A review of the literature. Emerg Med J. 2001;18:453–457.
78 De Vault M, Greifenstein F, Harris LJ. Circulatory responses to endotracheal intubation in light general anesthesia: The effect of atropine and phentolamine. Anesthesiology. 1960;21:360–362.
79 Mikawa K, Nishina K, Maekawa N, et al. Comparison of nicardipine, diltiazem and verapamil for controlling the cardiovascular responses to tracheal intubation. Br J Anaesth. 1996;76:221–226.
80 Atlee JL, Dhamee MS, Olund TL, et al. The use of esmolol, nicardipine, or their combination to blunt hemodynamic changes after laryngoscopy and tracheal intubation. Anesth Analg. 2000;90:280–285.
81 Fujii Y, Kihara S, Takahashi S, et al. Calcium channel blockers attenuate cardiovascular responses to tracheal extubation in hypertensive patients. Can J Anaesth. 1998;45:655–659.
82 Fujii Y, Saitoh Y, Takahashi S, et al. Diltiazem-lidocaine combination for the attenuation of cardiovascular responses to tracheal intubation in hypertensive patients. Can J Anaesth. 1998;45:933–937.
83 Davies MJ, Cronin K, Cowie R. The prevention of hypertension at intubation: A controlled study of intravenous hydralazine on patients undergoing intracranial surgery. Anaesthesia. 1981;36:147–153.
84 Stoelting R. Attenuation of blood pressure response to laryngoscopy and tracheal intubation with sodium nitroprusside. Anesth Analg. 1979;58:116–119.
85 Gallagher JD, Moore RA, Jose AB, et al. Prophylactic nitroglycerin infusions during coronary artery bypass surgery. Anesthesiology. 1986;64:785–789.
86 Van Aken H, Puchstein C, Hidding J. The prevention of hypertension at intubation. Anaesthesia. 1982;37:82–83.
87 Miller DR, Martineau RJ, Wynands JE, et al. Bolus administration of esmolol for controlling the haemodynamic response to tracheal intubation: The Canadian Multicentre Trial. Can J Anaesth. 1991;38:849–858.
88 Sharma S, Mitra S, Grover VK, et al. Esmolol blunts the haemodynamic responses to tracheal intubation in treated hypertensive patients. Can J Anaesth. 1996;43:778–782.
89 Gold MI. Heart rate and blood pressure effects of esmolol after ketamine induction and intubation. Anesthesiology. 1986;64:718–723.
90 Mikawa K, Nishina K, Maekawa N, et al. Attenuation of the catecholamine response to tracheal intubation with oral clonidine in children. Can J Anaesth. 1995;42:869–874.
91 Ghignone M, Quintin L, Duke PC, et al. Effects of clonidine on narcotic requirements and hemodynamic response during induction of fentanyl anesthesia and endotracheal intubation. Anesthesiology. 1986;64:36–42.
92 Nunn J, Campbell E, Peckett B. Anatomical subdivisions of the volume of respiratory dead space and effect of position of the jaw. J Appl Physiol. 1959;14:174–176.
93 Cullen J. An evaluation of tracheostomy in pulmonary emphysema. Ann Intern Med. 1963;58:953–960.
94 Hirshman C, Bergman M. Factors influencing intrapulmonary airway calibre during anaesthesia. Br J Anaesth. 1990;65:30–42.
95 Habib M. Physiologic implications of artificial airways. Artificial airways in patients receiving mechanical ventilation (consensus conference). Chest. 1989;96:180–184.
96 Kil HK, Bishop MJ. Head position and oral vs nasal route as factors determining endotracheal tube resistance. Chest. 1994;105:1794–1797.
97 Cavo J, Ogura JH, Sessions DG, et al. Flow resistance in tracheotomy tubes. Ann Otol Rhinol Laryngol. 1973;82:827–830.
98 Yung M, Snowdon S. Respiratory resistance of tracheostomy tubes. Arch Otolaryngol. 1984;110:591–595.
99 Colgan F, Lian J, Borrow R. Non-invasive assessment by capacitance respirometry of respiration before and after extubation. Anesth Analg. 1975;54:807–813.
100 Bolder PM, Healy TE, Bolder AR, et al. The extra work of breathing through adult endotracheal tubes. Anesth Analg. 1986;65:853–859.
101 Hill D. Properties of liquids, gases and vapours. In: Hill D, ed. Physics Applied to Anaesthesia. ed 4. London: Butterworth-Heineman; 1980:174.
102 Shapiro M, Wilson RK, Casar G, et al. Work of breathing through different sized endotracheal tubes. Crit Care Med. 1986;14:1028–1031.
103 Nathan SD, Ishaaya AM, Koerner SK, et al. Prediction of minimal pressure support during weaning from mechanical ventilation. Chest. 1993;103:1215–1219.
104 Ishaaya AM, Nathan SD, Belman MJ. Work of breathing after extubation. Chest. 1995;107:204–209.
105 Brochard L, Rua F, Lorino H, et al. Inspiratory pressure support compensates for the additional work of breathing caused by the endotracheal tube. Anesthesiology. 1991;75:739–745.
106 Vines D, Peters J, Merritt J, et al. A comparison of total patient work of breathing (TPWOB) between 8.0 endotracheal tubes (ETT) and tracheostomy tubes (TT) during spontaneous breathing in a lung model. Am J Respir Crit Care Med. 2003;167:A460.
107 Diehl JL, El Atrous S, Touchard D, et al. Changes in the work of breathing induced by tracheotomy in ventilator-dependent patients. Am J Respir Crit Care Med. 1999;159:383–388.
108 Davis K, Jr., Campbell RS, Johannigman JA, et al. Changes in respiratory mechanics after tracheostomy. Arch Surg. 1999;134:59–62.
109 Tiret L, Desmonts JM, Hatton F, et al. Complications associated with anaesthesia: A prospective survey in France. Can Anaesth Soc J. 1986;33:336–344.
110 Olsson G. Bronchospasm during anaesthesia: A computer-aided incidence study of 136,929 patients. Acta Anaesthesiol Scand. 1987;31:244–252.
111 Cook TM, Scott S, Mihai R. Litigation related to airway and respiratory complications of anaesthesia: An analysis of claims against the NHS in England 1995–2007. Anaesthesia. 2010;65:556–563.
112 Nadel J. Mechanisms controlling airway size. Arch Environ Health. 1963;7:179–182.
113 Gal T. Pulmonary mechanics in normal subjects following endotracheal intubation. Anesthesiology. 1980;52:27–35.
114 Kil HK, Rooke GA, Ryan-Dykes MA, et al. Effect of prophylactic bronchodilator treatment on lung resistance after tracheal intubation. Anesthesiology. 1994;81:43–48.
115 Hirshman C. Airway reactivity in humans. Anesthesiology. 1983;58:170–177.
116 Boushey H. Bronchial hyperreactivity. Am Rev Respir Dis. 1980;121:389–413.
117 Ding D, Martin J, Macklem P. Effects of lung volume on maximal methacholine-induced bronchoconstrictions in normal humans. J Appl Physiol. 1987;62:1324–1330.
118 Marini J, Pepe P. Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction. Am Rev Respir Dis. 1982;126:166–170.
119 Scott L, Benson M, Bishop M. Relationship of endotracheal tube size to auto-PEEP at high minute ventilation. Respir Care. 1986;31:1080–1082.
120 Gal T, Suratt P. Resistance to breathing in healthy subjects after endotracheal intubation under topical anesthesia. Anesthesiology. 1980;59:270–274.
121 Gal T, Arora N. Respiratory mechanics in supine subjects during progressive partial curarization. J Appl Physiol. 1982;52:57–63.
122 Rodenstein D, Stanescue D, Francis C. Demonstration of failure of body plethysmography in airway obstruction. J Appl Physiol. 1982;52:949–954.
123 Quan S, Falltrick R, Schlobohm R. Extubation from ambient or expiratory positive airway pressure in adults. Anesthesiology. 1984;55:53–56.
124 Smith RA, Venus B, Johnson MT, et al. Influence of glottic mechanism on pulmonary function after acute lung injury. Chest. 1990;98:206–208.
125 Bickler P, Sessler D. Efficiency of airway heat and moisture exchangers in anesthetized humans. Anesth Analg. 1990;71:415–418.
126 Casthely P, Chalon J. Tracheobronchial cytologic changes during prolonged cannulation. Anesth Analg. 1980;59:759–763.
127 Klainer A, Turndorf H. Surface alterations due to endotracheal intubation. Am J Med. 1975;58:674–683.
128 Nishino T, Kochi T, Ishii M. Differences in respiratory reflex responses from the larynx, trachea, and bronchi in anesthetized female subjects. Anesthesiology. 1996;84:70–74.
129 Scanlon P, Carey M, Power M, et al. Patient response to laryngeal mask insertion after induction of anaesthesia with propofol or thiopentone. Can J Anaesth. 1993;40:816–818.
130 Sessler CN, Vitaliti JC, Cooper KR, et al. Comparison of 4% lidocaine/0.5% phenylephrine with 5% cocaine: Which dilates the nasal passage better? Anesthesiology. 1986;64:274–277.
131 Goodell J, Gilroy G, Huntress J. Reducing cocaine solution use by promoting the use of a lidocaine-phenylephrine solution. Am J Hosp Pharm. 1988;45:2510–2513.
132 Gross J, Hartigan M, Schaffer D. A suitable substitute for 4% cocaine before blind nasotracheal intubation: 3% lidocaine–0.25% phenylephrine nasal spray. Anesth Analg. 1984;63:915–918.
133 Gotta A, Sullivan C. Anaesthesia of the upper airway using topical anaesthetic and superior laryngeal nerve block. Br J Anaesth. 1981;53:1055–1057.
134 Viguera M, Diakum TA, Shelsky R, et al. [Efficacy of topical administration of lidocaine through a Malinckrodt Hi-Lo Jet tube in lessening cough during recovery from general anesthesia]. Rev Esp Anestesiol Reanim. 1992;39:316–318.
135 Staffel JG, Weissler MC, Tyler EP, et al. The prevention of postoperative stridor and laryngospasm with topical lidocaine. Arch Otolaryngol Head Neck Surg. 1991;117:1123–1128.
136 Gonzalez RM, Bjerke RJ, Drobycki T, et al. Prevention of endotracheal tube-induced coughing during emergence from general anesthesia. Anesth Analg. 1994;79:792–795.
137 Estebe JP, Dollo G, Le Corre P, et al. Alkalinization of intracuff lidocaine improves endotracheal tube-induced emergence phenomena. Anesth Analg. 2002;94:227–230.
138 Estebe JP, Gentili M, Le Corre P, et al. Alkalinization of intracuff lidocaine: Efficacy and safety. Anesth Analg. 2005;101:1536–1541.
139 Scheller M, Zornow M, Saidman L. Tracheal intubation without use of muscle relaxants: A technique using propofol and varying doses of alfentanil. Anesth Analg. 1992;75:788–793.
140 Yukioka H, Hayashi M, Terai T, Fujimori M. Intravenous lidocaine as a suppressant of coughing during tracheal intubation in elderly patients. Anesth Analg. 1993;77:309–312.
141 Steinhaus J, Howland D. Intravenously administered lidocaine as a supplement to nitrous oxide thiobarbiturate anesthesia. Anesth Analg. 1958;37:40–46.
142 Steinhaus J, Gaskin L. A study of intravenous lidocaine as a suppressant of cough reflex. Anesthesiology. 1963;24:285–290.
143 Baraka A. Intravenous lidocaine controls extubation laryngospasm in children. Anesth Analg. 1978;57:506–507.
144 Leicht P, Wisborg T, Chraemmer-Jorgensen B. Does intravenous lidocaine prevent laryngospasm after extubation in children? Anesth Analg. 1985;64:1193–1196.
145 Hirshman CA, Lande B, Croxton TL. Role of M2 muscarinic receptors in airway smooth muscle contraction. Life Sci. 1999;64:443–448.
146 Berry A, Brimacombe J, Keller C, et al. Pulmonary airway resistance with the endotracheal tube versus laryngeal mask airway in paralyzed anesthetized adult patients. Anesthesiology. 1999;90:395–397.
147 Kim ES, Bishop MJ. Endotracheal intubation, but not laryngeal mask airway insertion, produces reversible bronchoconstriction. Anesthesiology. 1999;90:391–394.
148 Ferrari LR, Goudsouzian NG. The use of the laryngeal mask airway in children with bronchopulmonary dysplasia. Anesth Analg. 1995;81:310–313.
149 Natalini G, Franceschetti ME, Pletti C, et al. Impact of laryngeal mask airway and tracheal tube on pulmonary function during the early postoperative period. Acta Anaesthesiol Scand. 2002;46:525–528.
150 Groeben H, Schlicht M, Stieglitz S, et al. Both local anesthetics and salbutamol pretreatment affect reflex bronchoconstriction in volunteers with asthma undergoing awake fiberoptic intubation. Anesthesiology. 2002;97:1445–1450.
151 Downes H, Hirshman CA. Lidocaine aerosols do not prevent allergic bronchoconstriction. Anesth Analg. 1981;60:28–32.
152 Cabanas A, Souhrada JF, Aldrete JA. Effects of ketamine and halothane on normal and asthmatic smooth muscle of the airway in guinea pigs. Can Anaesth Soc J. 1980;27:47–51.
153 Hirshman CA, Downes H, Farbood A, et al. Ketamine block of bronchospasm in experimental canine asthma. Br J Anaesth. 1979;51:713–718.
154 Hill GE, Anderson JL, Whitten CW. Ketamine inhibits agonist-induced cAMP accumulation increase in human airway smooth muscle cells. Can J Anaesth. 1999;46:1172–1177.
155 Pabelick CM, Jones KA, Street K, et al. Calcium concentration-dependent mechanisms through which ketamine relaxes canine airway smooth muscle. Anesthesiology. 1997;86:1104–1111.
156 Sato T, Matsuki A, Zsigmond EK, et al. Ketamine relaxes airway smooth muscle contracted by endothelin. Anesth Analg. 1997;84:900–906.
157 Brown RH, Wagner EM. Mechanisms of bronchoprotection by anesthetic induction agents: Propofol versus ketamine. Anesthesiology. 1999;90:822–828.
158 Sarma VJ. Use of ketamine in acute severe asthma. Acta Anaesthesiol Scand. 1992;36:106–107.
159 Lau TT, Zed PJ. Does ketamine have a role in managing severe exacerbation of asthma in adults? Pharmacotherapy. 2001;21:1100–1106.
160 Howton JC, Rose J, Duffy S, et al. Randomized, double-blind, placebo-controlled trial of intravenous ketamine in acute asthma. Ann Emerg Med. 1996;27:170–175.
161 Hemmingsen C, Nielsen PK, Odorico J. Ketamine in the treatment of bronchospasm during mechanical ventilation. Am J Emerg Med. 1994;12:417–420.
162 Pedersen CM, Thirstrup S, Nielsen-Kudsk JE. Smooth muscle relaxant effects of propofol and ketamine in isolated guinea-pig trachea. Eur J Pharmacol. 1993;238:75–80.
163 Cheng EY, Mazzeo AJ, Bosnjak ZJ, et al. Direct relaxant effects of intravenous anesthetics on airway smooth muscle. Anesth Analg. 1996;83:162–168.
164 Hashiba E, Sato T, Hirota K, et al. The relaxant effect of propofol on guinea pig tracheal muscle is independent of airway epithelial function and beta-adrenoceptor activity. Anesth Analg. 1999;89:191–196.
165 Ouedraogo N, Marthan R, Roux E. The effects of propofol and etomidate on airway contractility in chronically hypoxic rats. Anesth Analg. 2003;96:1035–1041.
166 Mehr EH, Lindeman KS. Effects of halothane, propofol, and thiopental on peripheral airway reactivity. Anesthesiology. 1993;79:290–298.
167 Ouedraogo N, Roux E, Forestier F, et al. Effects of intravenous anesthetics on normal and passively sensitized human isolated airway smooth muscle. Anesthesiology. 1998;88:317–326.
168 Hirota K, Sato T, Hashimoto Y, et al. Relaxant effect of propofol on the airway in dogs. Br J Anaesth. 1999;83:292–295.
169 Pizov R, Brown RH, Weiss YS, et al. Wheezing during induction of general anesthesia in patients with and without asthma: A randomized, blinded trial. Anesthesiology. 1995;82:1111–1116.
170 Wu RS, Wu KC, Sum DC, et al. Comparative effects of thiopentone and propofol on respiratory resistance after tracheal intubation. Br J Anaesth. 1996;77:735–738.
171 Eames WO, Rooke GA, Wu RS, et al. Comparison of the effects of etomidate, propofol, and thiopental on respiratory resistance after tracheal intubation. Anesthesiology. 1996;84:1307–1311.
172 Downes H, Gerber N, Hirshman C. I.V. lignocaine in reflex and allergic bronchoconstriction. Br J Anaesth. 1980;52:873–878.
173 Hirota K, Hashimoto Y, Sato T, et al. Bronchoconstrictive and relaxant effects of lidocaine on the airway in dogs. Crit Care Med. 2001;29:1040–1044.
174 Groeben H, Silvanus MT, Beste M, et al. Combined intravenous lidocaine and inhaled salbutamol protect against bronchial hyperreactivity more effectively than lidocaine or salbutamol alone. Anesthesiology. 1998;89:862–868.
175 Maslow AD, Regan MM, Israel E, et al. Inhaled albuterol, but not intravenous lidocaine, protects against intubation-induced bronchoconstriction in asthma. Anesthesiology. 2000;93:1198–1204.
176 Chang HY, Togias A, Brown RH. The effects of systemic lidocaine on airway tone and pulmonary function in asthmatic subjects. Anesth Analg. 2007;104:1109–1115.
177 Burches BR, Jr., Warner DO. Bronchospasm after intravenous lidocaine. Anesth Analg. 2008;107:1260–1262.
178 Butler J, Jackson R. Best evidence topic report: Lignocaine as a pretreatment to rapid sequence intubation in patients with status asthmaticus. Emerg Med J. 2005;22:732.
179 Brichant JF, Gunst SJ, Warner DO, et al. Halothane, enflurane, and isoflurane depress the peripheral vagal motor pathway in isolated canine tracheal smooth muscle. Anesthesiology. 1991;74:325–332.
180 Mazzeo AJ, Cheng EY, Bosnjak ZJ, et al. Differential effects of desflurane and halothane on peripheral airway smooth muscle. Br J Anaesth. 1996;76:841–846.
181 Mitsuhata H, Saitoh J, Shimizu R, et al. Sevoflurane and isoflurane protect against bronchospasm in dogs. Anesthesiology. 1994;81:1230–1234.
182 Wiklund CU, Lim S, Lindsten U, et al. Relaxation by sevoflurane, desflurane and halothane in the isolated guinea-pig trachea via inhibition of cholinergic neurotransmission. Br J Anaesth. 1999;83:422–429.
183 Brown RH, Zerhouni EA, Hirshman CA. Comparison of low concentrations of halothane and isoflurane as bronchodilators. Anesthesiology. 1993;78:1097–1101.
184 Dikmen Y, Eminoglu E, Salihoglu Z, et al. Pulmonary mechanics during isoflurane, sevoflurane and desflurane anaesthesia. Anaesthesia. 2003;58:745–748.
185 Goff MJ, Arain SR, Ficke DJ, et al. Absence of bronchodilation during desflurane anesthesia: A comparison to sevoflurane and thiopental. Anesthesiology. 2000;93:404–408.
186 Rooke GA, Choi JH, Bishop MJ. The effect of isoflurane, halothane, sevoflurane, and thiopental/nitrous oxide on respiratory system resistance after tracheal intubation. Anesthesiology. 1997;86:1294–1299.
187 Habre W, Scalfaro P, Sims C, et al. Respiratory mechanics during sevoflurane anesthesia in children with and without asthma. Anesth Analg. 1999;89:1177–1181.
188 Scalfaro P, Sly PD, Sims C, et al. Salbutamol prevents the increase of respiratory resistance caused by tracheal intubation during sevoflurane anesthesia in asthmatic children. Anesth Analg. 2001;93:898–902.
189 Jooste E, Klafter F, Hirshman CA, et al. A mechanism for rapacuronium-induced bronchospasm: M2 muscarinic receptor antagonism. Anesthesiology. 2003;98:906–911.
190 Bishop MJ, O’Donnell JT, Salemi JR. Mivacurium and bronchospasm. Anesth Analg. 2003;97:484–485.