Chapter 127 Pharmacology at Surgery
Pharmacologic vitreolysis
Rationale for pharmacologic vitreolysis
Posterior vitreous detachment (PVD) is a progressive physiological process, involving both syneresis (liquefaction) and synchysis (separation). However, spontaneous PVD is very often incomplete and remnants of the vitreous adhere firmly either to areas in the periphery of the retina or to the macular area in some conditions. Tractional forces exerted by vitreous collagen fibers and/or cellular proliferations at the vitreoretinal interface also play an important role in the pathogenesis of tractional maculopathies such as macular holes, vitreomacular traction syndrome or epimacular membranes. In addition, focal abnormal vitreoretinal adhesions may be implicated in certain types of diabetic macular edema and exudative age-related macular degeneration.1,2
Our present therapeutic approach in these tractional maculopathies is to relieve tractional forces surgically by mechanical means inducing a more or less complete PVD using suction exerted by the vitrectomy probe, followed by a removal of remnants of vitreous collagen fibers and cellular proliferations using endgripping forceps. As we have learned that cellular proliferations tend to recur using remnants of collagen at the internal limiting membrane (ILM) as a scaffold for cellular proliferation, most surgeons tend to remove the ILM during the surgical intervention as well, in order to remove all epiretinal tissue. However, it may be hypothesized that direct manipulation in the area of the macula and the removal of the ILM itself may somehow have an impact on function in the macular area or interfere with the morphological integrity of the retinal layers, especially when being removed with the aid of visualizing agents.3 Therefore, although ILM peeling appears safe from a clinical point of view, it may not be the optimum treatment option with regard to the best possible functional results.
Given this background, a pharmacological liquefaction of the vitreous gel and simultaneous enzymatic separation of vitreous fibrils from the inner aspect of the ILM may result in a resolution of focal vitreomacular adhesions, and this represents an alternative approach to treat traction-related retinal and macular diseases. Leaving the ILM in place and cleaving the vitreoretinal interface at the vitreal side of the ILM, may offer a more complete PVD, and be less traumatic as compared with vitrectomy. This pharmacologic induction of a PVD, if achieved early in the course of retinal or macular diseases, may have also a potential as a prophylactic treatment against advanced stages of potentially sight-threatening conditions such as diabetic retinopathy or AMD.4 This concept is referred to as “pharmacologic vitreolysis,” and was initially introduced by Sebag in 1998.5 Additional potential indications include vitreoretinal procedures for retinal detachment in children, in whom firm adherences of the vitreous often complicate surgery, especially in retinopathy of prematurity or ocular trauma with intraocular foreign body in young persons. The hope is to make vitreoretinal surgery procedures more safe and effective using the concept of pharmacologic vitreolysis.
The following substances have been evaluated or are currently under investigation.
Enzymatic vitreolysis – microplasmin, plasmin, and others
Microplasmin
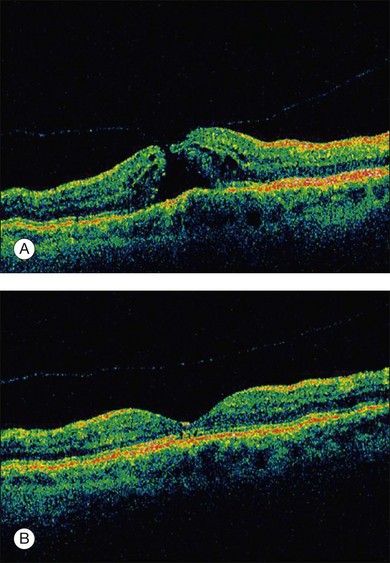
Microplasmin represents a recombinant protein that contains the catalytic domain of human plasmin but is much more stable as compared to the latter; both plasmin and microplasmin are nonspecific serine proteases cleaving a variety of glycoproteins such as fibronectin, laminin, fibrin and thrombospondin.6 These glycoproteins are involved in the adherence of the vitreous cortex to the ILM. Thus cleavage of these enzymes potentially induces a PVD: experimentally, microplasmin has been shown to increase vitreous diffusion coefficients in vitro using the non-invasive technique of dynamic light scattering7 and caused simultaneous vitreolysis and posterior vitreous separation in ex vivo and in vivo animal eye models in an apparent dose- and time-dependent fashion8,9 without morphological alterations in the retina.10 In a human, double-masked, Phase II trial 60 patients were randomized in four treatment cohorts either receiving increasing doses of microplasmin (75, 125, and 175 µg) or an initial injection of 125 µg followed by an injection of 125 µg if no release of adhesion occurred 1 month later or sham injection.11 The study revealed that within 28 days of sham, 75, 125, and 175 µg microplasmin administration, a nonsurgical resolution of vitreomacular adhesion was observed in 8, 25, 44, and 27% of patients; when the 125 µg dose was repeated up to 3 times, adhesion release was observed in 58% of patients 28 days after the final injection.11 Another phase 2, placebo-controlled, double-masked, dose-ranging clinical trial analyzed the effect of a single injection of microplasmin (25, 75, or 125 µg) 7 days prior to scheduled vitrectomy for vitreomacular traction and observed that 125 µg microplasmin was associated with a greater likelihood of induction and progression of PVD than placebo injection.12 Further randomized clinical trials are now ongoing to evaluate the safety and efficacy of microplasmin in association with vitrectomy or even as a monotherapy replacing the need for surgical intervention small macular holes (Fig. 127.1).
Plasmin
Plasmin was initially used by Verstraeten and coworkers to facilitate a PVD during vitrectomy in rabbit eyes. They found a positive effect but also mentioned a transient reduction of the b-wave amplitude in the electroretinogram as a potential adverse effect.13 This study was followed by other experimental trials confirming the proteolytic activity of plasmin on the vitreoretinal junction. Autologous plasmin was then used in clinical case series investigating the effect of a single injection without vitrectomy as a treatment for refractory diffuse diabetic macular edema,14 tractional diabetic macular edema15 or macular edema associated with branch retinal vein occlusion.16 The reduction of retinal thickness and improvement of visual acuity in these series supported an effectiveness of plasmin and the need for further trials. Autologous plasmin-assisted vitrectomy was performed for stage 3 macular holes, traumatic macular holes, diabetic macular edema and stage 5 retinopathy of prematurity17–22 and all of these trials underlined the potential of plasmin to facilitate vitrectomy and its good safety profile.
Hyaluronidase
Hyaluronidase has a potential to liquefy the vitreous, but does not induce a PVD in animal models.23 Therefore, its value in humans to relieve vitreoretinal traction seems limited. A phase III trial in diabetic humans suffering from vitreous hemorrhage showed a faster resorption of the vitreal hemorrhage as compared to a control group with no treatment-related relevant side-effects noted.24 However, a potential effect on the induction of a complete PVD in these patients was not further evaluated.
Dispase
Dispase represents a neutral protease that preferentially cleaves fibronectin and type IV collagen.25 Although initially shown to induce a PVD without damage to underlying tissue,26 subsequent trials brought up evidence for retinal bleedings, epimacular membrane formation, abnormalities in the electroretinogram responses and ultrastructural retinal damage.27 Thus, this enzyme was not further evaluated for clinical use.
Antiproliferative agents in the management of proliferative vitreoretinopathy
Proliferative vitreoretinopathy (PVR) is a serious and major complication of primary rhegmatogenous retinal detachment, severe diabetic retinopathy, and severe intraocular trauma. The pathophysiology of this disease implies a very complex cascade of events resulting in a subsequent proliferative response within the retina (see Chapter 97, Pathogenesis of PVR). Experimental studies described an intraretinal cellular response to retinal detachment, which is triggered by serum and growth factors present in the vitreous cavity28 and includes all types of non-neural cells such as RPE cells, astrocytes, microglia, macrophages, but predominantly Muller cells29–32 which is then followed by a fibrocellular growth on the subretinal or epiretinal surface.33 Both, subretinal fibrosis and epiretinal proliferation, have an impact on functional results and anatomical failure of retinal detachment surgery.33,34 As these cellular responses to retinal detachment may occur early in the development of the disease and may continue even in the longer term following (successful) surgical intervention, pointing to the need for an early pharmacological intervention and a long-term drug release or pharmacological effect.35
Other contributing factors include a relative hypoxia due to the distance between the neurosensory retina and the choriocapillaris,36 which leads to glial changes and photoreceptor deconstruction. These mechanisms are the target of recent and current approaches for an adjuvant pharmacologic intervention in PVR as these cellular responses can not be controlled by surgery alone.
Antiproliferative and anti-inflammatory agents have been the subject of in vitro investigations including substances such as colchicine, daunomycin and 5-fluorouracil.37,38 However, none of these agents have become part of any standardized procedure to treat or prevent PVR. Only daunomycin and 5-fluorouracil combined with low-molecular heparin were tested in clinical studies, which revealed no clinically relevant impact on PVR.39 Some 20 years ago, steroids applied intravitreally were used to suppress the process of PVR in clinical settings and in experimental studies.40 However, the effect was not lasting enough to avoid PVR.
New concepts include other agents such as alkylphosphocholines (APCs), which are synthetic phospholipid derivatives that have been shown to effectively inhibit cellular proliferation.41 Their mechanism of action involves binding to the membrane-bound G-protein PKC (protein kinase C), which is part of a major intracellular second-messenger system that regulates cell attachment, spreading, migration and proliferation. It was described in the literature that APCs have a potential to inhibit RPE cell spreading and migration as well as proliferation and cell-mediated membrane contraction at nontoxic concentrations in vitro and did not display any toxic effect of APCs to retinal tissue in in-vivo animal models.42 Besides prevention and treatment of PVR, APCs might have the potential for topical application as a single-dose agent to prevent posterior capsule opacification formation following cataract surgery.43
Tissue plasminogen activator in vitreoretinal surgery
This may be related to toxic effects of iron released from subretinal hemoglobin as well as an increased physical barrier for retinal diffusion and fibrotic changes.44–46 To date, there is no consensus on how to treat subretinal hemorrhages associated with neovascular age-related macular degeneration and most surgical treatment strategies seem insufficient to restore or improve vision.47
A displacement of the hemorrhage may be achieved by intravitreally applied expansile gas combined with intravitreal injection of recombinant plasminogen activator (rtPA). However, the subretinal lytic effect of intravitreally applied rtPA was challenged by some authors due to its molecular size and the reduced retinal diffusion.48,49 Therefore, a combination of intravitreal VEGF inhibitors to treat the underlying neovascular process combined with an injection of expansible gas is favored by some authors.50
In our own experience, intravitreal rtPA in combination with expansile gas was in the long-term more effective if combined with subsequent anti-VEGF treatment than intravitreal bevacizumab in combination with expansile gas alone (data submitted). Other groups have suggested triple therapies using rtPA, bevacizumab or ranibizumab and have shown a successful management of the disease with this approach.51,52 If rTPA and pneumatic displacement combination is contraindicated, an anti-VEGF monotherapy may be performed to prevent further visual loss.53 However, after intravitreal injection of any agent combined with expansible gas a vitreous hemorrhage may occur, very often due to a displacement of the subretinal blood into the vitreous cavity. In these cases, vitrectomy is indicated and may lead to good functional results.
It has also been suggested to perform a vitrectomy and apply rtPA subretinally followed by fluid gas exchange54 to displace the hemorrhage. This approach led to visual improvement in some cases. The authors also reported that vitrectomy with subretinal injection of rtPA and intravitreal gas tamponade was more effective than vitrectomy with intravitreal injection of rtPA and gas in terms of complete displacement of the submacular blood.55 Although functional improvement in the majority of patients suggests the absence of direct retinal toxicity of subretinally applied rtPA, vitrectomy and subretinal injection of rtPA bares a greater risk for postoperative complications.55
Despite good experiences in clinical use, experimental studies demonstrated dose-dependent negative effects of rtPA as seen by a significant and potentially irreversible reduction of the b-wave amplitude in ERG examination in bovine retinas.56
Visualization of the vitreoretinal interface
The application of vital dyes to visualize transparent and sometimes barely visible target structures such as the internal limiting membrane (ILM), epimacular membranes or vitreous has become very popular among vitreoretinal surgeons. The ILM is the main target structure for “chromodissection.” This is related to the fact that the ILM has been identified as an important scaffold for cellular proliferation. In order to remove all cellular proliferations, vitreous collagen remnants and relieve all relevant tractional forces, the ILM is dissected from the underlying tissue using the retinal surface of the ILM as a cleavage plane. The ILM can be removed without further visualization. A slight whitening of the retinal surface can be used as an indicator where ILM has been successfully peeled. However, this maneuver requires a lot of surgical experience and skills. The introduction of visualizing agents made ILM peeling accessible even to the less experienced surgeon. Several dyes are in clinical use and can be applied to selectively visualize the target structure (Table 127.1).
Table 127.1 Dyes* in clinical use which can be applied to selectively visualize the target structure
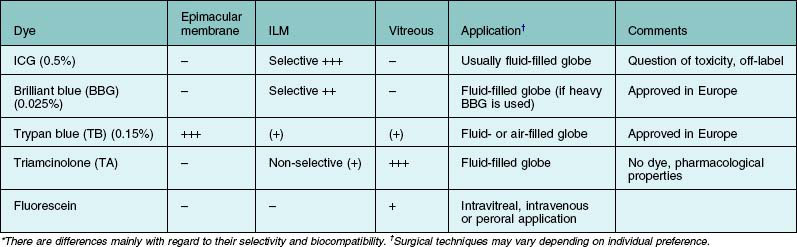
The dyes are either injected into the fluid-filled or air-filled globe and different concentrations are used. Fluid–air exchange can especially be avoided if the dye solution is heavier than water, which can be achieved with specific solvent media such as glucose 5% or deuterium oxide.57
Current dyes for epimacular membranes and the internal limiting membrane
Epimacular membranes can be stained using the anionic disazo dye trypan blue,58 which is commercially available in a concentration of 0.15% for that purpose. The contrast at the level of the internal limiting membrane (ILM) using trypan blue is quite poor. Two other dye classes are currently predominantly used for ILM peeling: the cyanine dye indocyanine green (ICG) and the triarylmethane dye brilliant blue G (BBG). Due to tissue–dye interactions and alterations of its collagen structure, the stained ILM can be peeled off more easily and, postulating that the dye used provides a high biocompatibility, with less damage to underlying retinal structures such as the nerve fibers and Muller cell endfeet. Both dyes selectively stain the ILM,59 although the staining effect seen after ICG use appears more pronounced compared to BBG. In contrast to the good biocompatibility of BBG,60 ICG has revealed toxic effects in some studies which underline the narrow safety margin of this dye.61 Postoperative visual field defects and less favorable functional outcome have been described in the literature.
In addition, ICG appears not as an ideal candidate for ILM staining as its maximum absorption is in the near infrared and not within the spectral sensitivity of the human eye,62 meaning that the majority of light absorption of ICG is useless or of low value during vitreoretinal surgery because it is in the invisible NIR and in the bathochromic region of the visible spectrum. As a consequence, relatively high dye concentrations of 0.5% are required and were used to achieve a sufficient contrast on the vitreoretinal interface in contrast to BBG, where concentrations of 0.025% are sufficient for ILM staining.
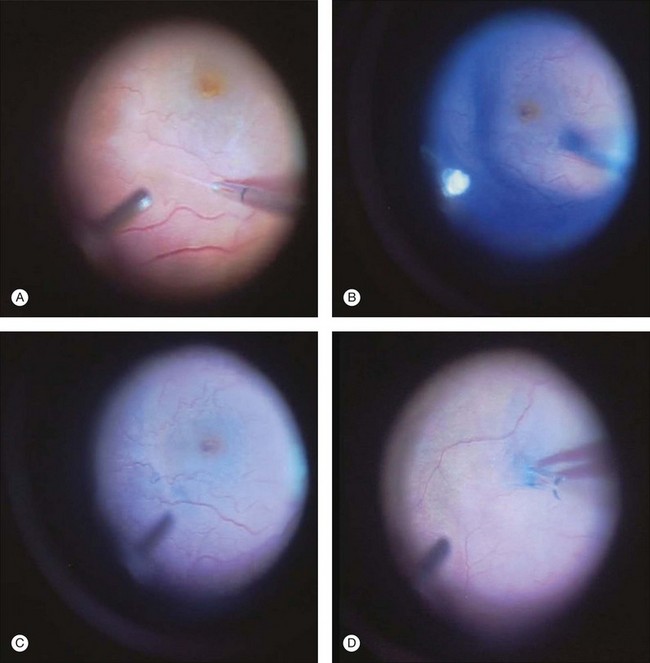
The availability of different dyes with selective staining properties allows for variable operative techniques and sequential “chromodissection” of the tissue. In theory, one could stain the vitreous using triamcinolone and stain epimacular membranes using trypan blue, followed by ILM staining using brilliant blue. In epimacular membrane surgery, where the vitreous is detached already in most cases, a reasonable approach is to peel the epimacular membrane without adjuncts first, and then visualize the ILM and peel areas where ILM can be detected (Fig. 127.2). In macular hole surgery, the ILM can be stained without prior removal of epimacular tissue.
Perspectives
In general, any dye should be used with care as long as the tissue–dye interactions are not completely understood. There is an ongoing effort to identify better dyes with appropriate staining properties and a high biocompatibility. It seems that an ideal candidate dye would be a dye incorporating the excellent contrast provided by ICG and the high biocompatibility of brilliant blue (i.e. strongly absorbing at visible wavelengths, conveniently tissue binding, non toxic, and physiologically degradable at a practical time scale). This may be reached by synthesizing new cyanine dyes and thereby improving the absorption qualities and the affinity of the dye molecule to the target structure.63
These new molecules provide improved absorption and fluorescent qualities, equal staining properties but potentially a better safety profile compared to ICG as it is adapted to the spectral sensitivity of the human eye and to the standard illumination used during surgery. In addition, the contrast is thereby enhanced as it implies both the blue absorption color and an even stronger purple fluorescence color. Additionally, several other absorbent dyes have been the subject of experimental in vivo and ex vivo experiments including among others methyl violet, crystal violet, eosin Y, sudan black B, methylene blue, toluidine blue, light green, indigo carmine, fast green, congo red, evans blue, and bromophenol blue.64,65
VEGF inhibitors in vitreoretinal surgery
Proliferative diabetic retinopathy and macular edema
The development of diabetic retinopathy is a multifactorial process. Much of the retinal damage that characterizes the disease is understood to result from retinal vascular leakage and nonperfusion,66 mediated by numerous growth factors including vascular endothelial growth factor (VEGF).67 High concentrations of VEGF have been identified in the vitreous of patients with proliferative diabetic retinopathy68 indicating that VEGF plays a major part in mediating active intraocular neovascularization in patients with ischemic retinal diseases. The upregulation of VEGF is not only associated with a breakdown of the blood–retina barrier resulting in retinal edema, but also with a stimulation of endothelial cell growth and neovascularization.69 Therefore, the inhibition of VEGF has become a widely used treatment option in these conditions. Available VEGF inhibitors include bevacizumab, ranibizumab and pegaptanib. For intraocular application, the drug is administered via the pars plana using a sterile 27- or 30-gauge needle.
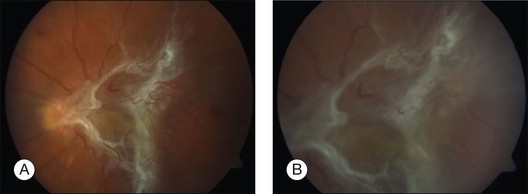
The intravitreal injection of VEGF inhibitors is approved to treat center involving macular edema associated with retinal ischemic disease such as diabetic macular edema or retinal vein occlusion. Nevertheless, another (at present “off label”) indication for the intravitreal application of VEGF inhibitors might be the treatment of proliferative diabetic retinopathy,70 resulting in a marked regression of neovascularization and possibly a resolution of vitreous hemorrhage within a short period of time in some cases. This treatment effect may be of relevance for patients awaiting vitrectomy for dense vitreous hemorrhage or tractional retinal detachment associated with active proliferative vascular diseases (Fig. 127.3). The injection of VEGF inhibitors prior to vitrectomy does help to minimize the risk of perioperative bleeding from active neovascularization and reduces the incidence of postoperative intraocular hemorrhages.71 It has been shown that the vascular component of proliferation is markedly reduced by day 10 post-injection, whereas the contractile components are not yet abundant.72 Therefore, patients having been treated with intravitreal injections of VEGF inhibitors before surgery should be followed on a daily basis, as an exacerbation of the tractional forces may occur due to fibrotic changes of the neovascularization and require immediate intervention. In clinical practice, vitrectomy should be scheduled 2–7 days after intravitreal injection since in most instances the marked reduction of neovascularization is usually seen within 48 hours. This holds true for retinal neovascularizations as well as iris neovascularizations.
In addition some case reports have demonstrated that injection of bevacizumab at the end of vitreous surgery can reduce the recurrence of hemorrhages after vitrectomy.73
Retinopathy of prematurity
Retinopathy of prematurity is a disease of the immature retina affecting children before 31 weeks’ gestational age. It represents one of the three leading causes of legal blindness in childhood in the developed countries. The pathophysiology of the disease implies tissue hypoxia resulting in an up regulation of VEGF and the development of abnormal retinal fibrovascular tissue leading to tractional retinal detachment and vision loss in progressive stages. The well-established treatment option for this condition is peripheral retinal ablation with conventional (confluent) laser therapy. However, laser photocoagulation is destructive and may cause complications later on. In addition, it does not prevent all vision loss, especially in cases of retinopathy of prematurity affecting zone I of the eye.74 Since the introduction of VEGF inhibitors for the treatment of neovascular diseases such as age-related macular degeneration, diabetic retinopathy or retinal vein occlusion, case series described a positive effect of single injections of the VEGF inhibitor bevacizumab in retinopathy of prematurity75 indicating that this agent may be a useful alternative to laser ablation, especially as it is not destructive to retinal tissue. As a rule this agent is applied only once in this disease.
A recent prospective, controlled, randomized, multicenter trial compared the effect of a 0.625 mg intravitreal bevacizumab monotherapy for zone I or zone II posterior stage 3+ (i.e., stage 3 with plus disease) to conventional laser therapy.76 The recurrence of retinopathy of prematurity in one or both eyes requiring retreatment before 54 weeks’ postmenstrual age was the primary ocular outcome. The study revealed that intravitreal bevacizumab monotherapy, as compared with conventional laser therapy, in infants with stage 3+ retinopathy of prematurity is associated with a significant benefit for zone I but not zone II disease. While development of peripheral retinal vessels continued after treatment with intravitreal bevacizumab, conventional laser therapy led to permanent destruction of the peripheral retina. However, as compared with conventional laser therapy in treating patients with zone I retinopathy of prematurity, intravitreal bevacizumab represents a true breakthrough in disease management of ROP.
Therefore, based on the current literature, the use of VEGF inhibitors in retinopathy of prematurity may provide an advantage compared to laser treatment for acute zone I or zone II posterior stage 3+ disease. A monotherapy using VEGF inhibitors appears advantageous when media opacities such as a tunica vasculosa or hemorrhages in the anterior or posterior segment preclude diode laser photocoagulation.77 In contrast, the treatment with VEGF inhibitors should be avoided in stage 4 or 5 disease, as fibrotic changes may be accelerated which then lead to tractional retinal detachment. Whether a combination of anti-VEGF treatment in combination with vitrectomy in stage 4 or 5 disease in ROP has advantages has not yet been examined. Also, it is not known whether the combination of bevacizumab and laser treatment has advantages.
As intravitreal bevacizumab reaches the systemic circulation78 of the infant there are concerns of negative systemic effects, which have not been demonstrated up to now. However, vigilance is mandatory and a longer follow-up of patients will help us to outline the best concentration of the agent and the indications (and relative contraindications) for the use of VEGF inhibitors for retinopathy of prematurity.
Neovascular glaucoma
Another indication for the use of VEGF inhibitors is neovascular glaucoma. Neovascular glaucoma is still a sight-threatening complication of retinal ischemia as seen in a variety of diseases such as diabetic retinopathy, or retinal vascular occlusion. Vitrectomy for conditions associated with neovascular glaucoma has been considered as resulting in very bad results. Therefore neovascular glaucoma had to be controlled before vitrectomy could be considered. The first-line treatment is laser photocoagulation of ischemic areas of the retina in order to prevent a progression of the neovascularization and to finally stop neovascularization at the iris. In cases where the retina cannot be visualized due to media opacities such as corneal edema or vitreous hemorrhage, transscleral retinal cryotherapy can be performed instead.79 Vitrectomy and endolaser photocoagulation is indicated in the presence of retinal detachment, progressive disease or in the presence of vitreous hemorrhage. While filtration surgery often fails in neovascular glaucoma, laser cyclophotocoagulation or cyclocryotherapy are effective means to lower intraocular pressure with fewer complications seen after laser cyclophotocoagulation. Vitrectomy with peripheral retinectomy had also been suggested as a treatment for neovascular glaucoma, but retinal complications were frequent.80 However, despite several treatment modalities being available functional outcome is often unfavorable. Therefore the addition of pharmacotherapy to vitreoretinal surgery in such cases is almost mandatory today. Again anti-VEGF substances are used.
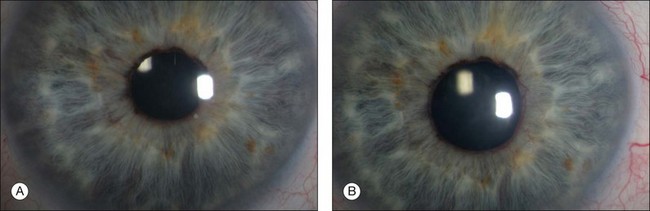
Several studies81 have shown that the monoclonal antibody bevacizumab can be used intravitreally as well as intracamerally to reduce not only retinal, but also iris and anterior chamber angle neovascularization (Fig. 127.4). Often, the regression of iris and chamber angle neovascularization is followed by a fast and effective decrease of intraocular pressure. However, recurrence of increased IOP is frequent.82 Therefore, the intracameral application of VEGF inhibitors may extend our therapeutic options in the management of neovascular glaucoma, especially as other options such as photocoagulation of ischemic retinal areas alone may not be enough to prevent the progression of neovascular glaucoma, and reduce the need for IOP-lowering drugs. The early treatment effect seen after intracameral application of VEGF inhibitors may also help to bridge the time until the treatment effect of other strategies, such as laser photocoagulation, is noted. Almost as a rule iris neovascularization is gone within 24 hours after intraocular injection of bevacizumab and necessary vitreoretinal surgery can be performed 1 day after injection if needed.
Endophthalmitis
Endophthalmitis is a serious intraocular inflammatory condition sometimes requiring immediate vitreoretinal intervention (see Chapter 122, Vitrectomy for infectious endophthalmitis). It can be the result of exogenous or endogenous spread of infecting organisms into the eye. The prognosis for visual function is often poor independently from the etiology of the disease. Exogenous endophthalmitis is mostly seen after ophthalmic surgical procedures. As cataract surgery is the most frequently performed surgery in ophthalmology, 90% of exogenous endophthalmitis cases are related to this operation.83 The incidence is reported to range between 0.08% and 0.7%.84,85
Although the incidence of endophthalmitis has decreased over the years due to continuous improvement of preoperative disinfection86 and perioperative prophylaxis, endophthalmitis is still a very severe disease with indistinct prognosis and requires immediate intervention to maintain at least an option for visual recovery. Early diagnosis and treatment with antimicrobial therapy are crucial. Therapy is usually initiated empirically while microbiologic testing of intraocular samples, obtained for example during vitrectomy in severe cases, is being performed. After culturing and identification of the causing organism the spectrum of the antimicrobial therapy may be altered according to the sensitivity of the pathogen. However, unless there is no other unequivocal result from culturing, endophthalmitis therapy should cover both Gram-positive organisms, which play a predominant role in exogenous endophthalmitis, and Gram-negative organisms, as they are associated with higher virulence and poorer outcome. According to the literature, current antibiotic standard protocols for intravitreal application include the peptide antibiotic vancomycin (1.0 mg/0.1 mL) for Gram-positive coverage87,88 in combination with the β-lactam antibiotic ceftazidime (2.25 mg/0.1 mL) for Gram-negative coverage. In patients hypersensitive to β-lactam drugs, amikacin (400 µg/0.1 mL), an aminoglycoside antibiotic, might be considered instead of ceftazidime.87
Other antibiotics such as fluoroquinolone, especially the recently developed third- and fourth-generation such as levofloxacin and moxifloxacin, have been discussed as a potential alternative treatment for Gram-positive pathogens due to their enhanced activity.87
1 Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003;110:62–64.
2 Gandorfer A, Messmer EM, Ulbig MW, et al. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000;20:126–133.
3 Haritoglou C, Gass CA, Schaumberger M, et al. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol. 2001;132:363–369.
4 Gandorfer A. Objective of pharmacologic vitreolysis. Dev Ophthalmol. 2009;44:1–6.
5 Sebag J. Pharmacologic vitreolysis. Retina. 1998;18:1–3.
6 Bishop PN. Vitreous as a substrate for vitreolysis. Dev Ophthalmol. 2009;44:7–19.
7 Sebag J, Ansari RR, Suh KI. Pharmacologic vitreolysis with microplasmin increases vitreous diffusion coefficients. Graefes Arch Clin Exp Ophthalmol. 2007;245:576–580.
8 de Smet MD, Valmaggia C, Zarranz-Ventura J, et al. Microplasmin: ex vivo characterization of its activity in porcine vitreous. Invest Ophthalmol Vis Sci. 2009;50:814–819.
9 Chen W, Huang X, Ma XW, et al. Enzymatic vitreolysis with recombinant microplasminogen and tissue plasminogen activator. Eye. 2008;22:300–307.
10 Gandorfer A, Rohleder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004;45:641–647.
11 Stalmans P, Delaey C, de Smet MD, et al. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: results of a prospective, randomized, sham-controlled phase II trial (the MIVI-IIT trial). Retina. 2010;30:1122–1127.
12 Benz MS, Packo KH, Gonzalez V, et al. A placebo-controlled trial of microplasmin intravitreous injection to facilitate posterior vitreous detachment before vitrectomy. Ophthalmology. 2010;117:791–797.
13 Verstraeten TC, Chapman C, Hartzer M, et al. Pharmacologic induction of posterior vitreous detachment in the rabbit. Arch Ophthalmol. 1993;111:849–854.
14 Diaz-Llopis M, Udaondo P, Arevalo F, et al. Intravitreal plasmin without associated vitrectomy as a treatment for refractory diabetic macular edema. J Ocul Pharmacol Ther. 2009;25:379–384.
15 Elbendary AM, Elwan MM, Azzam HA, et al. Predictability of vitreous detachment following intravitreal plasmin injection in diabetic macular edema associated with vitreomacular traction. Curr Eye Res. 2011;36:534–539.
16 Udaondo P, Díaz-Llopis M, García-Delpech S, et al. Intravitreal plasmin without vitrectomy for macular edema secondary to branch retinal vein occlusion. Arch Ophthalmol. 2011;129:283–287.
17 Wu WC, Drenser KA, Lai M, et al. Plasmin enzyme-assisted vitrectomy for primary and reoperated eyes with stage 5 retinopathy of prematurity. Retina. 2008;28:S75–S80.
18 Wu WC, Drenser KA, Trese MT, et al. Pediatric traumatic macular hole: results of autologous plasmin enzyme-assisted vitrectomy. Am J Ophthalmol. 2007;144:668–672.
19 Sakuma T, Tanaka M, Inoue M, et al. Efficacy of autologous plasmin for idiopathic macular hole surgery. Eur J Ophthalmol. 2005;15:787–794.
20 Margherio AR, Margherio RR, Hartzer M, et al. Plasmin enzyme-assisted vitrectomy in traumatic pediatric macular holes. Ophthalmology. 1998;105:1617–1620.
21 Hirata A, Takano A, Inomata Y, et al. Plasmin-assisted vitrectomy for management of proliferative membrane in proliferative diabetic retinopathy: a pilot study. Retina. 2007;27:1074–1078.
22 Rizzo S, Pellegrini G, Benocci F, et al. Autologous plasmin for pharmacologic vitreolysis prepared 1 hour before surgery. Retina. 2006;26:792–796.
23 Hikichi T, Kado M, Yoshida A. Intravitreal injection of hyaluronidase cannot induce posterior vitreous detachment in the rabbit. Retina. 2000;20:195–198.
24 Kuppermann BD, Thomas EL, de Smet MD, et al. Safety results of two phase III trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Vitrase for Vitreous Hemorrhage Study Groups. Am J Ophthalmol. 2005;140:585–597.
25 Stenn KS, Link R, Moelmann G. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93:287–290.
26 Tezel TH, Del Priore LV, Kaplan HJ. Posterior vitreous detachment with dispase. Retina. 1998;18:7–15.
27 Jorge R, Oyamabuchi EK, Cardillo JA, et al. Intravitreal injection of dispase causes retinal hemorrhages in rabbit and human eyes. Curr Eye Res. 2003;26:107–112.
28 Hollborn M, Tenckhoff S, Jahn K, et al. Changes in retinal gene expression in proliferative vitreoretinopathy: glial cell expression of HB-EGF. Mol Vis. 2005;11:397–413.
29 Coblentz FE, Radeke MJ, Lewis GP, et al. Evidence that ganglion cells react to retinal detachment. Exp Eye Res. 2003;76:333–342.
30 Fisher SK, Lewis GP. Muller cell and neuronal remodelling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vis Res. 2003;43:887–897.
31 Francke M, Faude F, Pannicke T, et al. Glial cell-mediated spread of retinal degeneration during detachment: a hypothesis based upon studies in rabbits. Vis Res. 2005;45:2256–2267.
32 Fisher SK, Erickson PA, Lewis GP, et al. Intraretinal proliferation induced by retinal detachment. Invest Ophthalmol Vis Sci. 1991;32:1739–1748.
33 Anderson DH, Guerin CJ, Erickson PA, et al. Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci. 1986;27:168–183.
34 Charteris DG, Downie J, Aylward GW, et al. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:93–100.
35 Lewis GP, Sethi CS, Linberg KA, et al. Experimental retinal reattachment: a new perspective. Mol Neurobiol. 2003;28:159–175.
36 Shen WG, Peng WX, Shao Y, et al. Localization and activity of calmodulin is involved in cell-cell adhesion of tumor cells and endothelial cells in response to hypoxic stress. Cell Biol Toxicol. 2007;23:323–335.
37 Kirchhof B. Strategies to influence PVR development. Graefes Arch Clin Exp Ophthalmol. 2004;242:699–703.
38 Cai J, Wei R, Ma X, et al. Cytotoxic effects of antiproliferative agents on human retinal glial cells in vitro. Int Ophthalmol. 2001;24:225–231.
39 Charteris DG, Aylward GW, Wong D, et al. A randomised controlled trial of combined 5-fluouracil and low-molecular weight heparin in management of established proliferative vitreoretinopathy. PVR Study Group. Ophthalmology. 2004;111:2240–2245.
40 Chandler DB, Hida T, Rozakis G, et al. The lack of an effect of intraocular steroids on irradiated fibroblasts in experimental proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 1992;230:188–191.
41 Eibl KH, Lewis GP, Betts K, et al. The effect of alkylphosphocholines on intraretinal proliferation initiated by experimental retinal detachment. Invest Ophthalmol Vis Sci. 2007;48:1305–1311.
42 Schuettauf F, Eibl KH, Thaler S, et al. Toxicity study of erucylphosphocholine in a rat model. Curr Eye Res. 2005;30:813–820.
43 Eibl KH, Liegl R, Kernt M, et al. Alkylphosphocholines as a potential pharmacologic prophylaxis for posterior capsule opacification. J Cataract Refract Surg. 2009;35:900–905.
44 Avery RL, Fekrat S, Hawkins BS, et al. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16:183–189.
45 Scupola A, Coscas G, Soubrane, et al. Natural history of macular subretinal hemorrhage in age-related macular degeneration. Ophthalmologica. 1999;213:97–102.
46 Toth CA, Morse LS, Hjelmeland LM, et al. Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol. 1991;109:723–729.
47 Bressler NM, Bressler SB, Childs AL, et al. Surgery for hemorrhagic choroidal neovascular lesions of age-related macular degeneration: ophthalmic findings: SST report no. 13. Ophthalmology. 2004;111:1993–2006.
48 Ohji M, Saito Y, Hayashi A, et al. Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Arch Ophthalmol. 1998;116:1326–1332.
49 Kamei M, Misono K, Lewis H. A study of the ability of tissue plasminogen activator to diffuse into the subretinal space after intravitreal injection in rabbits. Am J Ophthalmol. 1999;128:739–746.
50 Hohn F, Mirshahi A, Hattenbach LO. Combined intravitreal injection of bevacizumab and SF(6) gas for treatment of submacular hemorrhage secondary to age-related macular degeneration. Ophthalmologe. 2010;107:328–332.
51 Meyer CH, Scholl HP, Eter N, et al. Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol. 2008;86:490–494.
52 Guthoff R, Guthoff T, Meigen T, et al. Intravitreous injection of bevacizumab, tissue plasminogen activator, and gas in the treatment of submacular hemorrhage in age-related macular degeneration. Retina. 2011;31:36–40.
53 Sacu S, Stifter E, Vecsei-Marlovits PV, et al. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye. 2009;23:1404–1410.
54 Treumer F, Klatt C, Roider J, et al. Subretinal coapplication of recombinant tissue plasminogen activator and bevacizumab for neovascular age-related macular degeneration with submacular haemorrhage. Br J Ophthalmol. 2010;94:48–53.
55 Hillenkamp J, Surguch V, Framme C, et al. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2010;248:5–11.
56 Luke M, Januschowski K, Warga M, et al. The retinal tolerance to bevacizumab in co-application with a recombinant tissue plasminogen activator. Br J Ophthalmol. 2007;91:1077–1082.
57 Haritoglou C, Schumann RG, Kampik A, et al. Heavy brilliant blue G for internal limiting membrane staining. Retina. 2011;31:405–407.
58 Feron EJ, Veckeneer M, Parys-Van Ginderdeuren R, et al. Trypan blue staining of epiretinal membranes in proliferative vitreoretinopathy. Arch Ophthalmol. 2002;120:141–144.
59 Henrich PB, Priglinger SG, Haritoglou C, et al. Quantification of Contrast Recognizability During Brilliant Blue G (BBG) and Indocyanine Green (ICG) Assisted Chromovitrectomy. Invest Ophthalmol Vis Sci. 2011;2011(52):4345–4349.
60 Lüke M, Januschowski K, Beutel J, et al. Electrophysiological effects of Brilliant Blue G in the model of the isolated perfused vertebrate retina. Graefes Arch Clin Exp Ophthalmol. 2008;246:817–822.
61 Haritoglou C, Gandorfer A, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: a clinicopathologic correlation. Am J Ophthalmol. 2002;134:836–841.
62 Langhals H, Haritoglou C. Chemical and spectroscopic aspects of the application of dyes in vitreoretinal surgery. Ophthalmologe. 2009;106:16–20.
63 Langhals H, Varja A, Laubichler P, et al. Cyanine dyes as optical contrast agents for ophthalmological surgery. J Med Chem. 2011;54:3903–3925.
64 Jackson TL, Griffin L, Vote B, et al. An experimental method for testing novel retinal vital stains. Exp Eye Res. 2005;81:446–454.
65 Rodrigues EB, Penha FM, de Paula Fiod Costa E, et al. Ability of new vital dyes to stain intraocular membranes and tissues in ocular surgery. Am J Ophthalmol. 2010;149:265–277.
66 Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452.
67 Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–450.
68 Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487.
69 Frank RN, Amin RH, Eliott D, et al. Basic fibroblast growth factor and vascular endothelial growth factor are present in epiretinal and choroidal neovascular membranes. Am J Ophthalmol. 1996;122:393–403.
70 Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–278.
71 Abdelhakim MA, Macky TA, Mansour KA, et al. Bevacizumab (Avastin) as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Ophthalmic Res. 2011;45:23–30.
72 El-Sabagh HA, Abdelghaffar W, Labib AM, et al. Preoperative intravitreal bevacizumab use as an adjuvant to diabetic vitrectomy: histopathologic findings and clinical implications. Ophthalmology. 2011;118:636–641.
73 Cheema RA, Mushtaq J, Al-Khars W, et al. Role of intravitreal bevacizumab (Avastin) injected at the end of diabetic vitrectomy in preventing postoperative recurrent vitreous hemorrhage. Retina. 2010;30:1646–1650.
74 Reynolds JD. Bevacizumab for retinopathy of prematurity. N Engl J Med. 2011;364:677–678.
75 Lalwani G, Berrocal A, Murray T, et al. Off-Label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina. 2008;28:13–18.
76 Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–615.
77 Oberacher-Velten IM, Helbig H. VEGF antibodies as therapy for retinopathy of prematurity. Klin Monbl Augenheilkd. 2010;227:694–700.
78 Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology. 2007;114:855–859.
79 Caprioli J, Strang SL, Spaeth GL, et al. Cyclocryotherapy in the treatment of advanced glaucoma. Ophthalmology. 1985;92:947–954.
80 Schlote T, Bartz-Schmidt KU. Treatment of rubeotic secondary glaucoma. In: Joussen AM, Gardner T, Kirchhof B, et al. Retinal vascular disease. Heidelberg: Springer; 2007:274–282.
81 Grisanti S, Biester S, Peters S, et al. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006;142:158–160.
82 Wolf A, von Jagow B, Ulbig M, et al. Intracameral injection of bevacizumab for the treatment of neovascular glaucoma. Ophthalmologica. 2011;226:51–56.
83 Essex RW, Yi Q, Charles PG, et al. Post-traumatic endophthalmitis. Ophthalmology. 2004;111:2015–2022.
84 Javitt JC, Street DA, Tielsch JM, et al. National outcomes of cataract extraction. Retinal detachment and endophthalmitis after outpatient cataract surgery. Cataract Patient Outcomes Research Team. Ophthalmology. 1994;101:100–105.
85 Mamalis N, Nagpal M, Nagpal K, et al. Endophthalmitis following cataract surgery. Ophthalmol Clin North Am. 2001;14:661–674.
86 Ta CN, Singh K, Egbert PR, et al. Prospective comparative evaluation of povidone-iodine (10% for 5 min versus 5% for 1 minute) as prophylaxis for ophthalmic surgery. J Cataract Refract Surg. 2008;34:171–172.
87 Kernt M, Kampik A. Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives. Clin Ophthalmol. 2010;4:121–135.
88 Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol. 1995;113:1479–1496.