Peritoneal Procedures
DPL
Root and colleagues introduced DPL in 1964.1 It has withstood the passage of several decades and remains a useful diagnostic adjunct for the management of penetrating torso trauma. Following a blunt mechanism of injury, its greatest utility is as a triage tool in the assessment of hemodynamically unstable, multiply injured patients. The intent is to rapidly discover or exclude the presence of intraperitoneal hemorrhage (IPH). The advent and availability of ultrasound (US) in the emergency department (ED) have rendered this purpose complementary to that of US in the diagnostic armamentarium of emergency clinicians evaluating blunt trauma patients.2
Peritoneal lavage can be used as a therapeutic tool in patients with hypothermia and as a means of removing toxins.3 It has also been used as a diagnostic instrument for suspected intraabdominal infection and nontraumatic sources of hemorrhage.4,5 Although the steps of the procedure are the same regardless of the indication, the primary use of DPL is to determine the need for laparotomy after trauma, and this chapter focuses on that indication.
Indications
Before the advent of computed tomography (CT) and US, DPL was the sole diagnostic option to supplement physical examination for predicting the need for operative intervention (Table 43-1). It was integral to both reduction of unnecessary laparotomies and discovery of unsuspected and life-threatening intraabdominal hemorrhage in patients with significant closed-head injury.6,7
TABLE 43-1
Clinical Indications for Laparotomy after Blunt Trauma
| MANIFESTATION | PITFALL |
| Unstable vital signs with strongly suspected abdominal injury | Alternative sources of shock |
| Unequivocal peritoneal irritation | Unreliable |
| Pneumoperitoneum | Insensitive; may be due to a cardiopulmonary source or invasive procedures (diagnostic peritoneal lavage, laparoscopy) |
| Evidence of diaphragmatic injury | Nonspecific |
| Significant gastrointestinal bleeding | Uncommon, unknown accuracy |
From Marx J, Isenhour J. Abdominal trauma. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 6th ed. St. Louis: Mosby; 2006:509.
This procedure may be undertaken by the emergency physician, but its use is not mandated and often relegated to the trauma service or consulting surgeon. In a number of respected centers in the United States, DPL continues to be a focal diagnostic instrument.2,8 It serves two primary functions.9 First, it can be used to rapidly determine or exclude the presence of IPH (Table 43-2). Thus, a patient with a critical closed-head injury, an unstable motor vehicle crash victim with multiple potential sources of blood loss, or a patient with pelvic fractures and retroperitoneal hemorrhage can be appropriately routed to lifesaving laparotomy.10,11 Furthermore, given its exquisite sensitivity, a negative peritoneal aspiration allows the clinician to proceed to alternative management steps and the patient to forego unnecessary laparotomy. Second, DPL has been used in less exigent circumstances as a means of predicting solid or hollow visceral injury requiring laparotomy.12,13 However, in this venue its sensitivity to the presence of hemorrhage may prompt unnecessary laparotomy in patients with self-limited lacerations of the liver, spleen,14–17 or mesentery.17 CT specifically evaluates all intraperitoneal structures, as well as the retroperitoneum, a region inaccessible to DPL. Because the resolution and the speed with which it can be undertaken have vastly improved, CT has become an invaluable adjunct in the management of blunt trauma and has largely replaced DPL in stable patients. It is most useful in identifying injury to solid organs with accompanying IPH and greatly assists nonoperative management of these injuries. The ability of CT to discern hollow viscus and pancreatic pathology has continued to improve as the modality has evolved.11 With regard to hollow viscus injury, it is when serial clinical evaluations cannot be performed that gut perforation leads to preventable mortality. This is especially true in patients with severe closed-head injury or high spinal cord injury, in whom physical assessment of the abdomen is quite compromised. It is for these express scenarios that some authorities recommend the performance of DPL. The clinician’s concern for hollow viscus injury should be heightened if US or CT demonstrates minimal amounts of free intraperitoneal fluid without evidence of solid organ damage.18
TABLE 43-2
Likelihood of Injury by Entry Site
From Marx JA. Diagnostic peritoneal lavage. In: Ivatury RR, Cayten CG, eds. The Textbook of Penetrating Trauma. Baltimore: Williams & Wilkins; 1996:336.
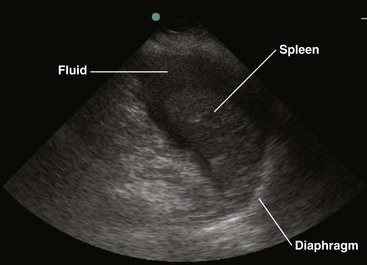
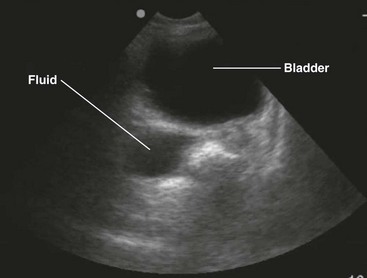
Two paradigms have brought US to the forefront. First, this modality has been adopted as the primary triage instrument, in lieu of DPL, for the detection of IPH on the basis of identifying which pouches and gutters are filled with fluid.19–21 Clinical success in this role has been mixed, with reported sensitivity for IPH of 65% to 95%.22–28 In addition, to be useful in this role, a competent technician and interpreter and the appropriate equipment must be present in real time. It has been demonstrated that emergency clinicians and surgeons can be trained in this technique to a level of competence sufficient for this need.29 In centers that rely on US, DPL should serve as a reliable study when US equipment is unavailable, performance of US is technically difficult, or the results of US are indeterminate, especially when the patient demonstrates hemodynamic compromise.
DPL is a readily available procedure that can be conducted rapidly in the safe confines of the ED. The ability to undertake CT in particular or, to a lesser extent, US in a similar manner requires careful consideration of the clinical circumstances, location of equipment, and capabilities of the personnel available (Fig. 43-1 and Table 43-3).2,11
TABLE 43-3
Diagnostic Studies in Patients with Blunt Abdominal Trauma
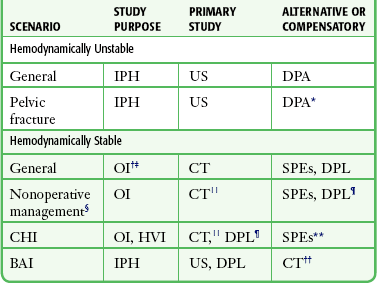
*A positive peritoneal aspirate mandates laparotomy; a positive red blood cell count warrants attention only to the pelvic fracture.
†To discover fluid or blood suggesting injury.
‡US for OI is much less reliable than for IPH.
§Institutional capability should be carefully considered.
||CT is less reliable for HVI than for solid visceral injury.
¶Complementary to CT if HVI is suspected.
**SPEs are unreliable in patients with CHI.
††May be more appropriate if helical CT is the primary study for BAI or can be acquired rapidly.
Adapted from Marx J, Isenhour J. Abdominal trauma. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 6th ed. St. Louis: Mosby; 2006:507.
Penetrating Trauma
The advent of DPL was seminal in the promotion of selective management for penetrating abdominal injury. Here its role is more dominant than for blunt trauma because of the far greater likelihood of occult injury to hollow viscera and the diaphragm after a penetrating mechanism.30,31
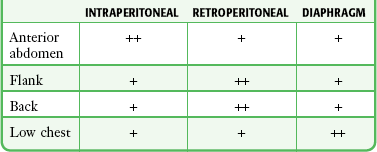
Instruments and missiles may penetrate the abdominal cavity via the anterior abdominal wall, flank, back, or low chest region.32 The intraperitoneal space is vulnerable if penetration occurs as high as the fourth intercostal space anteriorly and the sixth or seventh space laterally and posteriorly because the diaphragm may rise to these levels in the expiratory phase of respiration.33 Coincident thoracic penetration occurs in up to 46% of patients with abdominal injuries.34–36 The likelihood of retroperitoneal injury increases when the entry site is over the flank or back, but the prospect of intraperitoneal pathology remains considerable, with cited incidences of up to 43% for the flank and 14% for the back (Table 43-4).37–39
TABLE 43-4
Likelihood of Injury by Entry Site
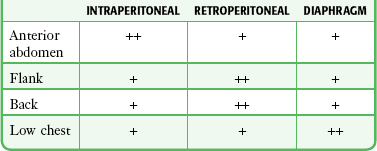
From Marx JA. Diagnostic peritoneal lavage. In: Ivatury RR, Cayten CG, eds. The Textbook of Penetrating Trauma. Baltimore: Williams & Wilkins; 1996:336.
Stab Wounds: Because only one fourth to one third of patients who sustain stab wounds to the anterior aspect of the abdomen require laparotomy, diagnostic algorithms are used to decrease the rate of unnecessary surgery.30,35,40 An optimal approach would not sacrifice sensitivity for morbid intraperitoneal injury. A pathway using a combination of clinical mandates, local wound exploration, and DPL is well established (Fig. 43-2).41 These clinical mandates are reasonably accurate predictors of significant intraperitoneal injury (Table 43-5). Thus, the presence of one or more mandates suggests the need for urgent laparotomy and precludes the undertaking of other diagnostic studies.
TABLE 43-5
Clinical Indications for Laparotomy after Penetrating Trauma
| MANIFESTATION | PREMISE | PITFALL |
| Hemodynamic instability | Major solid visceral or vascular injury | Thorax, mediastinum |
| Peritoneal signs | Intraperitoneal injury | Unreliable, especially immediately after injury |
| Evisceration | Additional bowel, other injury | No injury in one fourth to one third of stab wound cases |
| Diaphragmatic injury | Diaphragmatic herniation | Rare clinical, radiographic findings |
| Gastrointestinal and vaginal hemorrhage | Proximal gut or uterine injury | Uncommon, unknown accuracy |
| Impalement in situ | Vascular impalement | High operative risk, pregnancy |
| Intraperitoneal air | Perforation of a hollow viscus | Insensitive; may be caused by intraperitoneal entry only or be due to a cardiopulmonary source |
Modified from Marx JA. Diagnostic peritoneal lavage. In: Ivatury RR, Cayten CG, eds. The Textbook of Penetrating Trauma. Baltimore: Williams & Wilkins; 1996.
DPL fills three roles in the evaluation of patients with abdominal stab wounds (see Table 43-2): (1) rapid determination of the presence of hemoperitoneum, (2) discovery of intraperitoneal injury requiring surgery in stable patients, and (3) establishment of diaphragmatic violation. As is the case in blunt trauma patients, DPL can be invaluable as a rapid triage tool when the source of hemodynamic instability is not known. Pericardial tamponade, intrathoracic hemorrhage, and IPH may be contributory to hemodynamic instability or wholly causal. Again, as for blunt trauma evaluation, US is the only bedside diagnostic modality for IPH that is competitive in this role, and it carries the added advantage of scanning for intrapericardial and intrathoracic hemorrhage.35 In determining injury after stab wounds, DPL has 90% accuracy.42–44 Serial examinations,45–47 CT, and laparoscopy48–51 are alternative modalities in specific circumstances and centers.52 The diaphragmatic rents created by stab wounds are generally small; thus, at the outset they do not create apparent clinical or radiologic abnormalities.53,54 However, morbidity from delayed herniation of bowel is common and substantive.55 Physical examination is notoriously insensitive and DPL is currently the most sensitive means of discerning this injury in the immediate posttrauma phase.42 There is some evidence that coronal reconstruction of CT images provides greater sensitivity for detecting small diaphragmatic tears, and as CT technology continues to evolve, it may surpass DPL for evaluation of these subtle injuries.56 For these small wounds, magnetic resonance imaging may be diagnostic, but because of safety and accessibility concerns, it should be reserved for the nonacute phase of management. Laparoscopy has demonstrated promise in experienced hands.48,49
Gunshot Wounds: Injury to multiple organs is the rule after gunshot wounds, and mortality is significantly greater than after stab wounds.52 The diagnostic approach is more conservative for gunshot wounds because in some studies the likelihood of intraperitoneal injury requiring operative intervention has exceeded 90% when the projectile has entered the intraperitoneal cavity (Fig. 43-3).57 If clinical mandates are met (see Table 43-5) or if peritoneal violation has occurred, most centers proceed to laparotomy.41 One series, however, cited intraabdominal injury in 70% to 80% of cases, thus supporting the contention that nonoperative management could be applied to a substantial percentage of patients.58 In a separate cohort of 152 patients sustaining solid organ injury from penetrating abdominal trauma (70% gunshot wounds and 30% stab wounds), 27% were successfully managed without laparotomy after selection by a protocol combining clinical examination and CT scanning.59 DPL is reserved for two circumstances: (1) the wound tract is neither obviously superficial nor intraperitoneal, and (2) penetration occurred in the low chest region, where diaphragmatic injury is more likely yet the possibility of intraperitoneal injury also exists.
Procedure
Decompress the stomach and bladder to prevent inadvertent injury. Place the patient in the supine position and administer sedatives and analgesics as appropriate. Perform DPL according to compliance with standards for body fluid precautions. Observe sterile technique throughout the procedure. Before making the skin incisions described later, prepare the site with standard skin antiseptics and drape appropriately. Prophylactic antibiotics are not indicated for routine DPL because local and systemic infections are rare.60
Infiltrate the area for incision and dissection with a local anesthetic such as 1% lidocaine with epinephrine (Fig. 43-4, step 1). Delay the incision for more than 30 seconds after infiltration of local anesthetic to permit local vasospasm, which minimizes bleeding of the wound during the procedure.
Placement of the Catheter
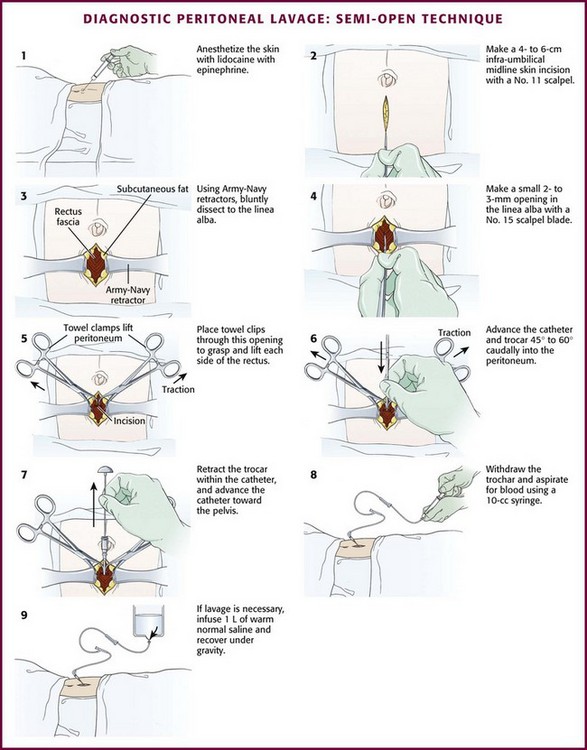
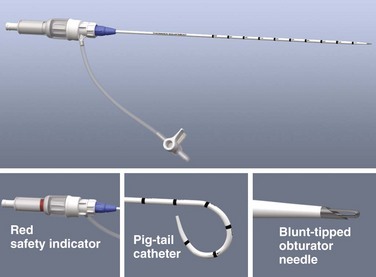
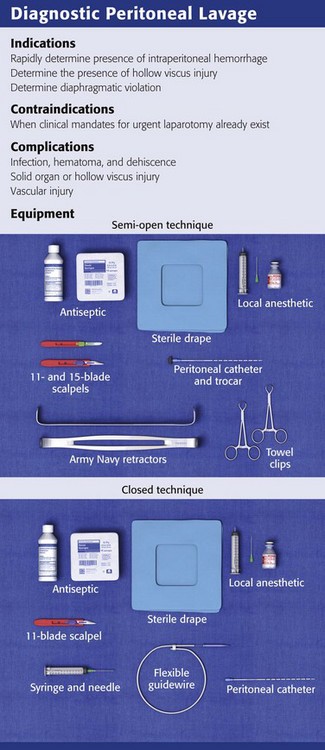
Semi-open Technique: Make a skin incision 4 to 6 cm in length with a No. 11 scalpel blade. Using Army-Navy retractors, proceed with blunt dissection to expose the rectus fascia (Fig. 43-4, steps 2 and 3). With the infraumbilical incision in the midline, continue blunt dissection until the linea alba is seen. Its crossing bands of crural fibers may be apparent.61 Make a small 2- to 3-mm opening in the linea alba with a No. 15 scalpel blade (Fig. 43-4, step 4). You may notice a tough, gritty sensation when cutting the linea alba with the scalpel. Place towel clips through this opening to grasp each side of the rectus fascia (Fig. 43-4, step 5). Ask an assistant to lift the two towel clips and carefully advance the catheter and trocar in a 45- to 60-degree caudad orientation. Proceed through the peritoneum into the peritoneal cavity (see Fig. 43-4, steps 6 and 7).62
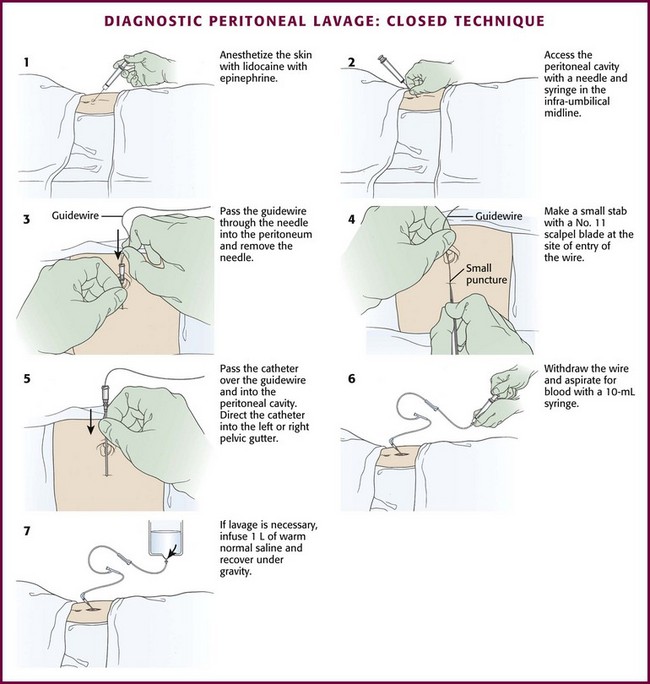
Closed Technique: For the closed technique, introduce the catheter into the peritoneal space in a blind percutaneous fashion.63 Use the simple Seldinger (guidewire) method, in which a small-gauge guide needle is inserted into the peritoneal cavity in the midline just inferior to the umbilicus (Fig. 43-5, step 2). Pass a flexible wire through the needle (Fig. 43-5, step 3), and remove the needle but not the wire. Advance a soft catheter over the wire and into the peritoneal cavity. Make a small stab with a No. 11 scalpel blade at the entry site of the wire to allow easier passage of the catheter through the abdominal wall (Fig. 43-5, step 4). Rotate the catheter while pushing it over the guidewire to facilitate entry into the peritoneal cavity. Place the catheter into the right or left pelvic gutter.
Always control the guidewire to avert intraabdominal migration of the wire. Withdraw the wire and aspirate for blood with a 10-mL syringe. Follow this with peritoneal lavage when necessary. Proponents of the guidewire technique promote its ease and rapidity.64–68 Those who prefer the semi-open method argue that the time until peritoneal aspiration, the more critical interval, is minimally different and that this method may have fewer complications and thus be more accurate than the guidewire technique.69–73 Note that for both the semi-open and closed approaches, the time until aspiration is performed should be no more than 2 to 5 minutes.
Site
The optimum location for DPL is at the infraumbilical ring at the inferior border of the umbilicus (Table 43-6). Here, between the rectus abdominis muscles there is adherence of the peritoneum and relative lack of vascularity and preperitoneal fat.61 Closed DPL should always be conducted here. In the event of second- or third-trimester pregnancy, a suprauterine approach is used. If midline scarring is present, a fully open technique at the lateral border of the rectus abdominis in the left lower quadrant may be necessary. The left side is preferred to avoid later confusion about whether an appendectomy has been performed. It is interesting to note that Moore and associates found no increase in complications or misclassified lavage when the closed technique was used in a small series of patients with previous abdominal surgery.74 In the presence of a pelvic fracture, use a fully open supraumbilical approach. This greatly decreases the likelihood of passing the catheter through a retroperitoneal hematoma that has dissected from the fracture anteriorly and across the abdominal wall.75 In patients with penetrating trauma, do not perform DPL through the stab or missile entry site. This approach can contaminate the intraperitoneal cavity, potentially exacerbate the abdominal wall bleeding, and lead to a false-positive result.
TABLE 43-6
| CLINICAL CIRCUMSTANCE | SITE | METHOD |
| Standard adult | Infraumbilical midline | C or SO |
| Standard pediatric | Infraumbilical midline | C or SO |
| Second- and third-trimester pregnancy | Suprauterine | FO |
| Midline scarring | Left lower quadrant | FO |
| Pelvic fracture | Supraumbilical | FO |
| Penetrating trauma | Infraumbilical midline* | C or SO |
C, closed; DPL, diagnostic peritoneal lavage; FO, fully open; SO, semi-open.
Aspiration and Lavage
Once the catheter has been placed successfully into the peritoneal cavity, attach the right-angle adapter, extension tubing, and a non–Luer-Lok syringe and attempt aspiration (see Fig. 43-4, step 8). If 10 mm of blood is aspirated, the test is positive and the procedure is terminated. With penetrating trauma, acquisition of lesser amounts may be meaningful because of the tendency for the diaphragm and bowel to hemorrhage minimally when injured. However, no rules have been established in this regard.
If little to no blood is aspirated, lavage the peritoneal cavity with either normal saline or lactated Ringer’s solution (see Fig. 43-4, step 9). Apply a blood pressure cuff or blood infusion pump around the plastic intravenous (IV) bag to speed the influx (i.e., decrease lavage time) if necessary. Large-bore infusion tubing (e.g., urologic irrigation tubing sets, such as the Abbott No. 6543 cystoscopy/irrigation set) also shortens fluid influx time. Infuse 1 L of fluid in adults or 15 mL/kg in children. When possible, roll or shift the patient from side to side after the infusion to increase mixing. Place the IV bag or bottle on the floor (or below abdominal level), and allow the fluid to return by gravity.
Return of 700 mL or more in an adult is generally accepted as adequate for interpretation of the findings. However, as little as 10% to 20% of the infusate may give a representative sample for both gross and microscopic determination. Send 10 mL of fluid from the return to the laboratory for cell count analysis, and send another 10 mL for enzyme analysis (see the section “Interpretation” later in this chapter). Some operators prefer to leave the catheter in place until the returned fluid is analyzed so that lavage may be repeated if the initial results are borderline or an occult bowel perforation is suspected.
Complications
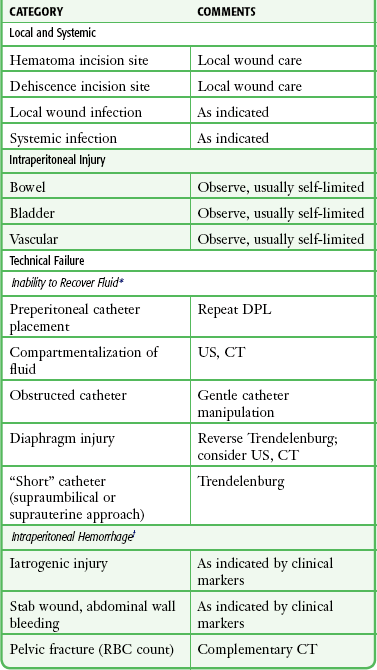
Local wound complications, including infection, hematoma, and dehiscence, occurred in only 0.3% of patients in two large series.42,76 Dehiscence with evisceration is an even rarer condition.77 Systemic infection has been described rarely (Table 43-7).
Technical Failure
Inability to recover peritoneal aspirate or lavage fluid can result in a false-negative interpretation. This can occur in several circumstances. It follows unwitting placement of the catheter into the preperitoneal space, which is less likely to occur with either open technique. Compartmentalization of fluid by adhesions or obstructing omentum can impede the egress of fluid. When a fully open supraumbilical or suprauterine technique is used, the catheter may be too short to access the depths of the intraperitoneal cavity. Finally, the large diaphragmatic tears typical of blunt pathophysiology allow flow of lavage fluid from the intraperitoneal to the thoracic cavity. Saunders and coworkers compared percutaneous DPL and the open technique in a prospective, randomized trial.78 Fluid obtained by the two techniques had similar test performance for intraabdominal pathology. The open technique took, on average, more than 4 minutes longer, but the percutaneous approach had an 11.2% technical failure rate (versus 3.8% with the open approach).
False-positive findings can occur in two ways. First, iatrogenic misadventure may be responsible. Second, in penetrating trauma, particularly stab wounds, bleeding from the abdominal wall injury site into the peritoneal cavity can lead to positive findings when no injury to intraperitoneal structures has occurred.44
Interpretation
Recovery of 10 mL or more of blood via aspiration is considered a positive finding. Aspirates with lesser volume are generally discarded and are not factored into analysis of the lavage fluid. Grossly bloody aspirates are typically indicative of solid visceral or vascular injury, with a positive predictive value of greater than 90%.79,80 Aspiration of blood is responsible for approximately 80% of true-positive DPL findings with blunt trauma and for 50% with stab wounds.43
A positive aspiration in a blunt trauma patient who is hemodynamically stable or has been resuscitated to apparent stability need not mandate urgent surgery. Unnecessary laparotomy will occur if there has been minimal and self-limited damage to the liver, spleen, bowel serosa, or mesentery.81 In this situation, CT and clinical indicators should be used in concert with the findings on DPL.
RBC Count
The recommended red blood cell (RBC) threshold varies according to the mechanism and, in the case of stab wounds, the external site of injury (Table 43-8). The optimum criterion will deliver excellent sensitivity, a high positive predictive value, and a minimal incidence of unnecessary laparotomy. Negative laparotomy incurs a prolongation of hospitalization and increases the cost of care, in addition to creating the potential for procedural complications.82,83 RBC counts greater than 105/mm3 (105/µL) are generally considered positive with a blunt mechanism or after stab wounds in the anterior part of the abdomen, flank, or back. Counts of 20,000 to 100,000/mm3 should be considered indeterminate.43,45,84,85 For stab wounds in the low chest region, where the diaphragm is at increased risk for injury, the RBC criterion should be lowered to 5000/mm3 to maximize sensitivity for isolated injury to this structure.36,43,86,87 With gunshot wounds involving the abdomen or low chest region, the same RBC criterion of 5000/mm3 is applied. This is intended to increase the sensitivity of the test because intraperitoneal entry by a missile carries a 90% or greater likelihood of intraperitoneal injury.36,60,88 An uncomplicated DPL should not result in more than several hundred to several thousand RBCs in the peritoneal lavage fluid.
TABLE 43-8
Diagnostic Peritoneal Lavage RBC Criteria (per mm3)
| POSITIVE | INDETERMINATE | |
| Blunt trauma | 100,000* | 20,000-100,000 |
| Stab wound | ||
| Anterior abdomen | 100,000 | 20,000-100,000 |
| Flank | 100,000 | 20,000-100,000 |
| Back | 100,000 | 20,000-100,000 |
| Low chest | 5000-10,000 | 1000-5000 |
| Gunshot wound | 5000-10,000 | 1000-5000 |
*In a hemodynamically stable patient with a pelvic fracture and a positive or equivocal red blood cell count, computed tomography should be obtained to corroborate or refute intraperitoneal injury.
From Marx J, Isenhour J. Abdominal trauma. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 6th ed. St. Louis: Mosby; 2006:500.
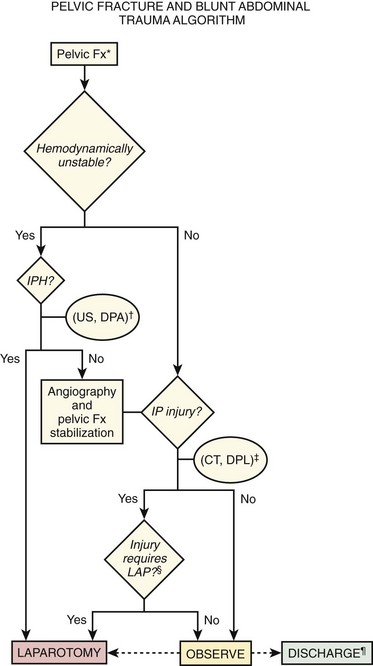
The incidence of false-positive RBC interpretation in the setting of pelvic fracture is considerable. However, aspiration of free blood in patients with critical pelvic fractures predicts active IPH in more than 80% of cases. A positive RBC count should generally prompt corroboration or refutation of intraperitoneal injury by CT. In this fashion, needed pelvic angiography and embolization will not be delayed unnecessarily should active intraperitoneal bleeding not be found (Fig. 43-6).
WBC Count
An inflammatory peritoneal response to a multitude of stimuli can occur, including stool, blood, and enzymes.89 The white blood cell (WBC) count in lavage effluent was formerly touted to predict small bowel injury but has since been proved unreliable.90 It is insensitive in the immediate postinjury period because 3 to 5 hours is necessary before the test becomes positive (Table 43-9).91,92 Moreover, a positive finding is likely to be falsely so.91,93 Therefore, the WBC level in and of itself should not determine the need for laparotomy.
TABLE 43-9

From Marx JA. Diagnostic peritoneal lavage. In: Ivatury RR, Cayten CG, eds. The Textbook of Penetrating Trauma. Baltimore: Williams & Wilkins; 1996:337.
Enzymes
Alkaline phosphatase is contained intramurally in the small bowel, as well as in hepatobiliary secretions released into the proximal part of the intestine. Amylase is contained in the latter only. Perforation of the small bowel allows access of these two markers to the peritoneal cavity, where they can be recovered by peritoneal lavage.94–96 Although levels of the two markers usually rise in tandem, lavage amylase has been shown to be a more accurate marker than lavage alkaline phosphatase (see Table 43-9). In contradistinction to the WBC count, these tests will be positive in the immediate postinjury period. However, they may not be economical if used on a mandatory rather than a selective basis. Neither is helpful in discerning the presence of pancreatic pathology.
Miscellaneous
Routine bile staining, Gram staining, and microscopy to identify vegetable fibers are rarely productive and are of untested accuracy. Deck and Porter reported that finding urine in the lavage fluid, as evidenced by a straw color, and creatinine in the peritoneal fluid should suggest an intraperitoneal bladder or collecting system injury.97
Paracentesis
Therapeutic abdominal paracentesis is one of the oldest medical procedures and dates to approximately 20 bc. Paracentesis was first described in the modern medical literature by Saloman at the beginning of the 20th century, and it became a valued decompressive therapy.98 With the advent of diuretics in the early 1950s, paracentesis fell out of favor as a treatment option. Controlled clinical trials in the late 1980s up to the present have restored its reputation by demonstrating the safety and efficacy of large-volume paracentesis (LVP) in adults and children.99–105 Because this mode is invasive and consumes clinician hours, it is generally reserved for the treatment of patients with chronic ascites who have tense ascites or whose condition is refractory to diuretic therapy.102 However, paracentesis remains an important diagnostic agent for patients with new-onset ascites or to determine the presence of worrisome conditions, notably infection, in those with preexistent ascites.106
Clinical Features
Small amounts of ascites may be asymptomatic. Larger collections typically cause a sense of abdominal fullness, anorexia, early satiety, and perhaps nausea and abdominal pain. Substantial accumulations create symptoms of respiratory distress by virtue of restricting lung capacity.107
The most predictive history and physical examination findings for excluding the diagnosis of ascites are the absence of ankle swelling and increased abdominal girth and an inability to demonstrate bulging flanks, flank dullness, or shifting dullness.108 Positive predictors for the diagnosis are a positive fluid wave, shifting dullness, or peripheral edema.108,109
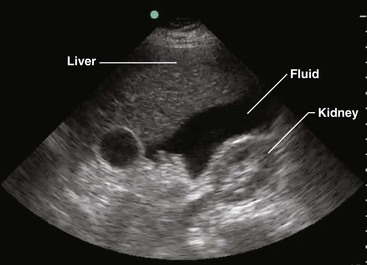
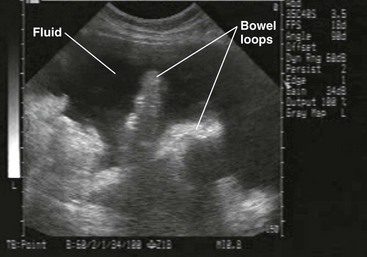
Patients who lack obvious clinical markers may benefit from the performance of US, which can discern the presence of as little as 100 mL of fluid.110 Endoscopically guided US may detect as little as 10 mL. It is more sensitive than CT in this respect and can assist in the identification of malignancy.111 In addition, it is a useful adjunct for determining the location of fluid that may be compartmentalized by preexistent infection or surgical adhesions.
Differential Diagnosis
Causes of ascites can be categorized in several ways. On a structural basis the causes are divided into diseases of the peritoneum and diseases not involving the peritoneum. The former group includes infection, neoplasm, collagen vascular disease, and idiopathic causes. The latter includes cirrhosis, congestive heart failure, nephrotic syndrome, protein-losing enteropathy, malnutrition, myxedema, pancreatic disease, ovarian disease, chylous effusion, Budd-Chiari syndrome, and hepatic venous occlusive disease. Pathophysiologic categories are listed in Box 43-1. In the United States, parenchymal liver pathology is overwhelmingly the most likely cause. Within this group, alcoholic liver disease is responsible for approximately 80% of cases (Table 43-10).112 Finally, ascites can be classified on the basis of a serum-ascites albumin gradient, that is, the difference between albumin values obtained simultaneously from serum and ascites samples (Box 43-2).113
TABLE 43-10
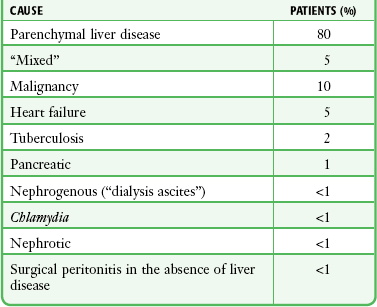
*Based on a series of 1500 paracenteses performed in a predominantly inpatient hepatology/general internal medicine setting (B. A. Runyon, unpublished observations).
From Runyon BA. Ascites and spontaneous bacterial peritonitis. In: Sleisenger MH, Fordtran JS, eds. Gastrointestinal Disease: Pathophysiology/Diagnosis/Management. 5th ed. Philadelphia: Saunders; 1993:1977.
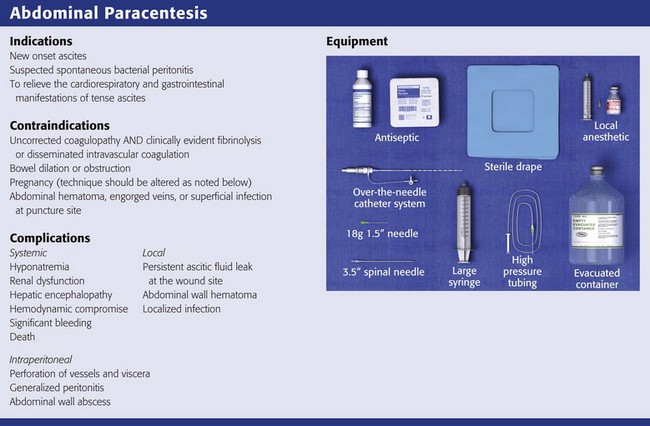
Indications and Contraindications
Therapeutic paracentesis is often undertaken in the ED setting to relieve the cardiorespiratory and gastrointestinal manifestations of tense ascites.114–116 LVP, or removal of more than 5 L, ameliorates the shortness of breath and early satiety that these patients experience. It may also be associated with collateral advantages, such as a reduction in hepatic venous pressure gradients, intravariceal pressure, and variceal wall tension. These parameters are considered important predictors of variceal bleeding, and the improvement after LVP may decrease the risk for bleeding. Diagnostic paracentesis, often relegated to inpatient services, is indicated in any patient whose ascites is of new onset or to disclose the presence of infection in patients with known or suspected ascites, particularly in the context of alcohol-related cirrhotic liver disease.117,118 Diagnostic paracentesis is also useful in the management of patients with acquired immunodeficiency syndrome (AIDS), in whom the etiology of ascites will be non–AIDS related in three quarters of cases.119 There are few relative contraindications to abdominal paracentesis. Certain systemic and anatomic risks should be considered, however.
Systemic
Given the predominance of alcohol-related cirrhotic liver disease as the cause of ascites, as many as two thirds to three quarters of patients who undergo paracentesis will have a coagulopathy. However, the only prospective study that evaluated the complications of paracentesis determined that transfusion-requiring abdominal hematomas occurred in less than 1% of cases despite the fact that 71% of the patients had an abnormal prothrombin time (PT).120 Because transfusion-requiring hematoma is so unlikely, even in this population, prophylactic administration of fresh frozen plasma or platelets is not standard, nor mandated, and imposes considerable cost, in addition to the risk for posttransfusion hepatitis, with little net gain.121 Therefore, for patients undergoing repeated therapeutic paracentesis, in the absence of previous problems or obvious clotting issues, obtaining a platelet count and international normalized ratio (INR) before the procedure is not routine. In an investigation of 628 patients undergoing outpatient LVP, the procedure was safely performed when the PT INR was as high as 8.7 (mean value, 1.7) and the platelet count as low as 19,000/mm3 (mean value, 50/mm3).122 These data countermand older and more conservative recommendations to administer platelets to patients with levels of less than 50,000/mm3 or to give fresh frozen plasma to those with a PT exceeding 20 seconds (1.5 times the therapeutic level).123 These blood products should be reserved for clinically evident fibrinolysis and disseminated intravascular coagulation.
Anatomic
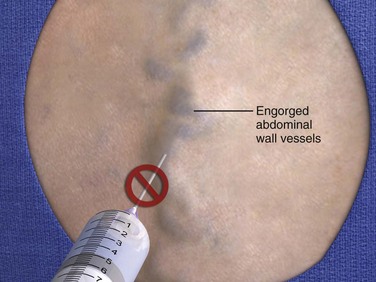
Structural impediments to the safe introduction of a paracentesis needle can include the bladder, bowel, and pregnant uterus. The bladder is normally tucked into the recess of the pelvis. However, bladders that are neuropathologically distended as a result of pharmacologic agents or medical conditions should preferably be emptied by voiding or by catheterization to avoid puncture. The intestines typically float in ascitic fluid and will move safely away from a slowly advancing paracentesis needle.123 Therefore, US guidance may be indicated in cases of suspected adhesions or bowel obstruction. Even if penetrated by an 18- to 22-gauge needle, leakage of intestinal contents will not occur unless intraluminal pressure is 5- to 10-fold greater than normal conditions.124 In second- and third-term pregnancy, an open supraumbilical or US-assisted approach is preferred. The abdomen should be inspected carefully for evidence of abdominal hematoma, engorged veins, or superficial infection, and these sites should be strictly avoided.
Technique
Site of Entry
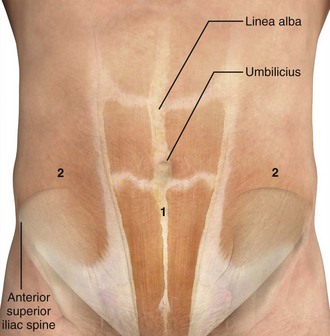
The best site of entrance for repeated paracentesis is determined by the patient’s previous experience, so this question should be asked of the patient. Theoretically, most sites on the abdominal wall can be used, but in absence of previous experience with the individual patient, two sites are preferred. One site is approximately 2 cm below the umbilicus in the midline (Fig. 43-7), where the fasciae of the rectus abdominis muscle join to form the fibrous, thin, avascular linea alba. Large collateral veins may occasionally be present and should be avoided (Fig. 43-8), as should suspected areas of skin infection. If the patient has midline scarring or if previous experience has been positive, the preferred alternative site is in either the right or left lower quadrant, approximately 4 to 5 cm cephalad and medial to the anterior superior iliac spine (see Fig. 43-7). The importance of remaining lateral to the rectus sheath is to avoid the inferior epigastric artery. Patients with a large quantity of ascites can readily undergo the procedure in the supine position with the head of the bed slightly elevated. Those with lesser amounts of fluid may benefit from a lateral decubitus position with introduction of the needle into the midline or dependent lower quadrant (Fig. 43-9). Some clinicians prefer to use the lateral decubitus position routinely because the bowel tends to float upward and away from the path of the needle. Hence, the site of needle entrance is in the midline or on the side closest to the bed. Rarely, patients may need to be placed in a facedown, hands-on-knees position.120 In patients with multiple abdominal scars or suspicion of compartmentalized abdominal fluid for any reason, US guidance is prudent.125
Procedure
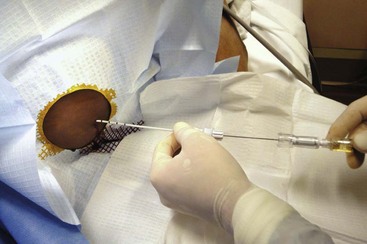
A prepackaged kit, such as the Saf-T-Centesis system, is a convenient way to perform the procedure (Fig 43-10). Following sterile preparation of the skin, inject local anesthetic at the paracentesis site (Fig. 43-11, step 4). Use a standard 3.8-cm (1.5-inch) metal needle in most cases. If necessary, use a longer 8.9-cm (3.5-inch) spinal needle in obese patients. Plastic sheath cannulas tend to kink and run the risk of being sheared off into the peritoneal cavity, but a steel needle can be left in the abdomen during a therapeutic tap for intervals of an hour or longer without injury. The 15-gauge, 3.25-inch Caldwell needle/cannula is an alternative for LVP that has been shown to perform similar to a steel needle in terms of procedural complications and the volume of fluid removed.126 Use a smaller-gauge (20- to 22-gauge) needle for diagnostic taps because such needles lessen the likelihood of leakage of ascitic fluid through the wound site after the procedure. However, for therapeutic LVP, use an 18-gauge needle because it permits expeditious outflow.104,127
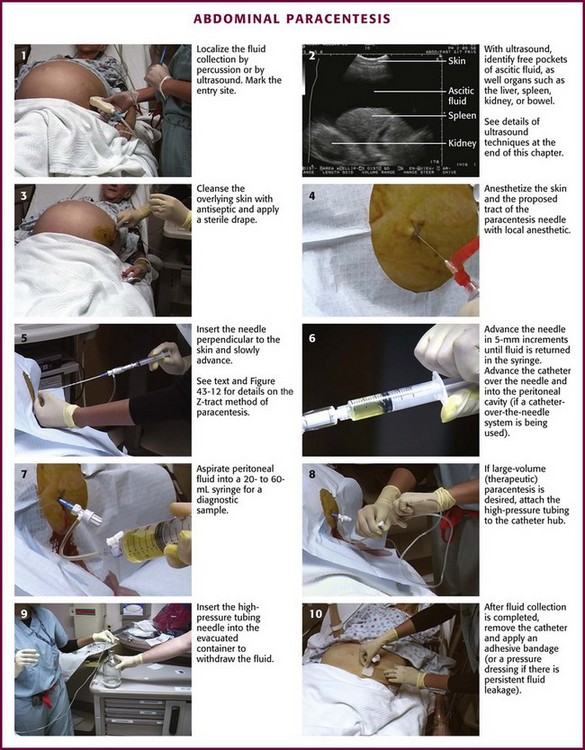
Figure 43-11 Abdominal paracentesis.
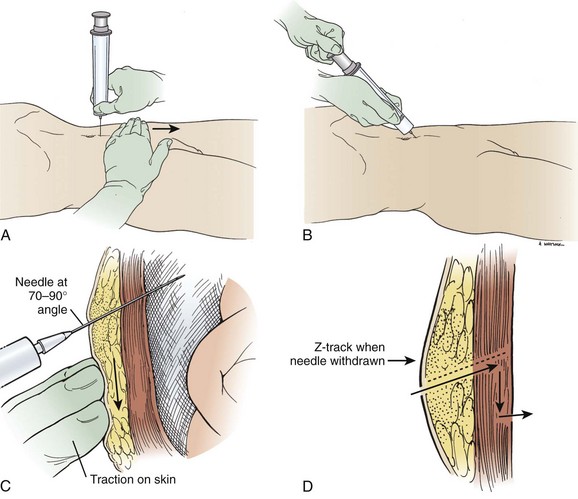
Insert the needle directly perpendicular to the skin at the preferred site (Fig. 43-11, step 5). Alternatively, use the “Z-tract” method. For this method, pull the skin approximately 2 cm caudad to the deep abdominal wall with the non–needle-bearing hand while slowly inserting the paracentesis needle (Fig. 43-12). Release the skin when the needle has penetrated the peritoneum and fluid flows. This technique also holds the draining needle in place without suture or tape. Remove the needle after the procedure, and the skin will slide to its original position and help seal the tract. In any case, insert the needle slowly in 5-mm increments to detect undesired entry of a vessel and to help prevent unnecessary puncture of the small bowel. Avoid continuous suction because it may attract bowel or omentum to the end of the paracentesis needle with resultant occlusion. Once fluid is flowing, stabilize the needle to ensure a steady flow. If flow ceases, gently rotate the needle and advance it inward in 1- to 2-mm increments. When fluid removal is complete, remove the needle and place an adhesive bandage over the puncture site. If there is persistent leakage of fluid, a pressure bandage may be required.
Ultrasound Guidance
US-guided paracentesis may be performed by a radiologist or an experienced emergency clinician ultrasonographer (see the specific US box in this chapter). This technique clearly delineates the pocket of ascitic fluid and allows visualization of loculated collections and avoidance of bowel adherent to the anterior abdominal peritoneum. The ultrasonographer scans the abdomen and marks the skin at the point overlying the optimal puncture site (see Fig. 43-11, step 2). Once the entry site is marked, keep the patient immobile and perform the procedure (as detailed previously) as soon as practical to avoid shifting of the fluid, which may decrease the utility of US guidance.
Volume of Fluid Removed
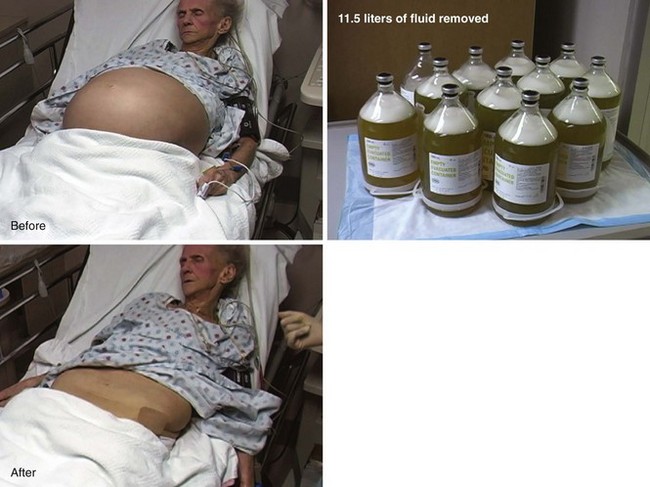
Many patients with chronic ascites are well versed on the procedure and have experienced it many times. Some undergo paracentesis on a regular basis in the outpatient setting. Therefore, the best guide to the volume of fluid to be removed for recurrent ascites is based on the patient’s previous experience, and this question should be asked. Up to 5 or 6 L is routine and well tolerated, and for therapeutic purposes, at least this volume should be removed. Patients seen in the ED are probably less compliant with outpatient regimens and seek care only when in extremis. Hence, their ascites is likely to be much more voluminous than in those treated regularly. In general, the paracentesis volume consists of as much fluid as can be removed without excessive manipulation of the patient. Volumes greater than 5 L are termed LVP. Up to 10 to 12 L may be removed safely in most patients with chronic ascites (Fig. 43-13). For first-time paracentesis and for diagnostic purposes (ruling out bacterial peritonitis, screening for cancer), 200 to 500 mL is usually sufficient, but more can be drained if it flows easily.
Complications
Complications of paracentesis can be divided into systemic, local, and intraperitoneal categories.
Systemic
Because upward of 6 L has been reportedly removed in less than 15 minutes without complication, certain authorities decry this issue as folklore.128 Others believe that rapid total paracentesis is accompanied by marked cardiovascular and humoral changes, some of which are explained by mechanical factors directly or indirectly related to relief of abdominal pressure.129,130 Other changes, including systemic vasodilation and humoral deactivation, are of a nonmechanical nature. Hepatic encephalopathy, hyponatremia, hepatorenal syndrome, and rapid reaccumulation of ascitic fluid have also been ascribed to LVP. In the editor’s clinical experience, PCD of varying degrees is a real syndrome, and it may occur more often than suspected because many patients are discharged after the procedure. The consequences of LVP in ED patients have not been well studied, and the implications are somewhat obscure in this subset of patients. Because many patients require therapeutic paracentesis on a regular basis, ask the patient about previous experience and the usual volume of fluid removed to help guide treatment.
Because fluid and electrolyte shifts tend to be minimal after the removal of large amounts of fluid,131 colloid infusion is considered strictly optional by some for patients with paracentesis of more than 5 L and is universally not recommended for paracentesis of lesser volume.106,132,133 Others support the routine administration of albumin if more than 5 L is removed When colloid is indicated, albumin has been the de facto choice. Being a blood product, albumin has been associated with rare complications, including anaphylaxis. The recommended infusion is 6 to 8 g of IV albumin per liter of ascitic fluid removed, or 50 g.132,134 However, colloid dextran 70 is favored by some authorities because of cost and concern for infection.135–137
Local
Local complications include persistent leakage of ascitic fluid at the wound site, abdominal wall hematoma, and localized infection. Persistent fluid leaks can be corrected with a single suture at the site of puncture.127 An abdominal wall hematoma requiring transfusion is very uncommon, but careful observation in such cases is necessary.
Intraperitoneal
Intraperitoneal complications include perforation of vessels and viscera.138 In experienced hands these complications are uncommon, and in most circumstances they are self-sealing and clinically inconsequential. However, generalized peritonitis and abdominal wall abscess have been reported after paracentesis in rare cases. The most common cause of postparacentesis IPH is bleeding as a result of a coagulopathy rather than large-vessel injury per se.139
Interpretation
Ascitic fluid should undergo gross inspection. Routine laboratory testing includes a differential cell count, albumin assay, and cultures (Box 43-3). The prevalence of occult infection of ascitic fluid in asymptomatic outpatients undergoing therapeutic LVP for resistant ascites is low. As a result, in the absence of concerning symptoms or cloudy fluid, routine laboratory tests and culture of fluid during outpatient or ED paracentesis are not warranted.
Inspection
Ascitic fluid is typically translucent and yellow. A dark greenish brown hue may reflect biliary perforation. Cloudy fluid generally indicates particulate matter, including neutrophils; fluid with WBC counts greater than 5000/µL (i.e., >5000/mm3) are cloudy, and those greater than 50,000/µL are purulent. An opaque, milky appearance may indicate elevated triglyceride levels.140 A blood-tinged appearance requires at least 10,000 RBCs/µL. This may reflect an iatrogenic complication, malignancy, hemorrhagic pancreatitis, or tuberculous peritonitis, although the last diagnosis creates hemorrhagic-appearing fluid in less than 5% of cases.120
Cell Count
Several milliliters of ascitic fluid is sufficient to obtain a differential cell count. Cirrhotic ascites should generally contain less than 250 WBCs/µL (Table 43-11). However, because cells may exit through the peritoneal cavity more slowly than fluid does, the WBC count can rise in the ascitic fluid during the procedure.141 Thus, an upper limit for uncomplicated cirrhotic ascites is reported as 500 cells/µL.142–144 Lymphocytes should predominate, and clinical signs or symptoms of peritoneal infection should be absent.145 In cases in which spontaneous bacterial peritonitis is a clinical consideration, the WBC criterion is 250/µL with greater than 50% polymorphonuclear leukocytes.115,117,145,146
TABLE 43-11
Ascitic Fluid Characteristics in Various Disease States
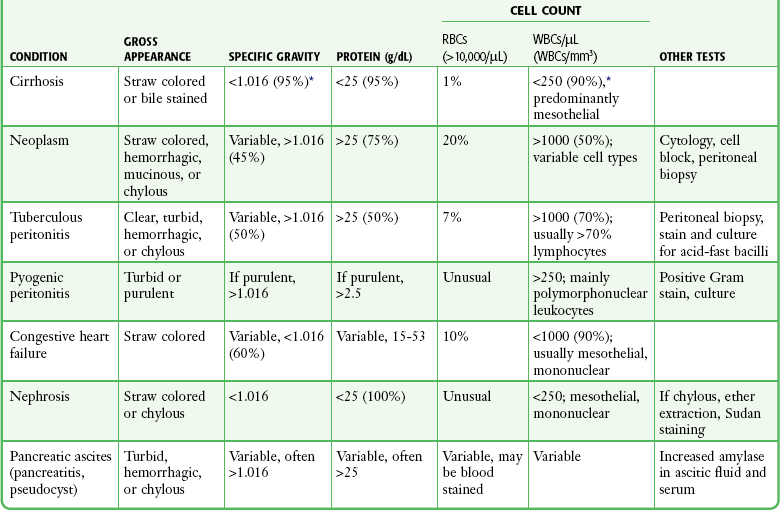
RBC, red blood cell; WBC, white blood cell.
*Because the conditions of examining fluid and selecting patients were not identical in each series, the percentages (in parentheses) should be taken as an indication of the order of magnitude rather than as the precise incidence of any abnormal finding.
From Glickman RM, Isselbacher KJ. Abdominal swelling and ascites. In: Isselbacher KJ, Braunwald E, Wilson JD, et al. eds. Harrison’s Principles of Internal Medicine. 13th ed. New York: McGraw-Hill; 1994:234.
Albumin
A serum-ascites albumin gradient can be obtained by simultaneously measuring albumin in ascites and serum and calculating the gradient. A serum-ascites albumin gradient greater than 1.1 g/dL indicates portal hypertension with greater than 95% accuracy (see Box 43-2).147–149
Culture and Gram Stain
The most valuable method for determining the presence of infection is culture. The sensitivity of this test is markedly increased by direct inoculation of blood culture bottles at the bedside as opposed to simply delivering the ascitic fluid to the laboratory.150,151 Gram staining of peritoneal fluid is rarely helpful in the evaluation of ascites.118 Approximately 10 bacteria/µL of fluid is required for a positive Gram stain. Thus, the Gram stain is notoriously insensitive in patients with spontaneous bacterial peritonitis, in whom the medium concentration of bacteria is 10−3 organisms/µL of fluid.152 Gram stain can be expected to be helpful only in cases of free gut perforation.
Miscellaneous
Optional tests include measurement of total protein, glucose, lactate dehydrogenase, and amylase. These tests will be beneficial in selected circumstances and need not be obtained on a routine basis. Immunosuppressed patients, including those with AIDS, should undergo microbiologic testing for opportunistic infections, including tuberculosis.119 Cytologic analysis is recommended in patients with suspicious constitutional symptoms and signs.152,153 Triglyceride and bilirubin studies are indicated if the gross appearance of the fluid is suggestive of increased levels.154
Chronic Ambulatory Peritoneal Dialysis
Patients undergoing chronic ambulatory peritoneal dialysis are at an increased risk for peritonitis because of the presence of a chronic indwelling peritoneal catheter. Culture yield is maximized by obtaining a sample of greater than 10 mL of the peritoneal effluent under sterile conditions after a dwell time of at least 2 to 4 hours.155,156 Peritonitis is defined by cloudy fluid with more than 100 WBCs/mm3 and greater than 50% polymorphonuclear cells.156 Although Gram stain is often negative in patients with bacterial peritonitis, it may reveal the presence of yeast and prompt timely initiation of antifungal therapy. Intraperitoneal antibiotics are superior to IV dosing, and removal of the catheter may be required for refractory or recurrent infections. Initial, empirical intraperitoneal therapy usually includes a first-generation cephalosporin along with an aminoglycoside, ceftazidime, cefepime, or carbapenem.156 Vancomycin should be considered in patients with a previous history of methicillin-resistant Staphylococcus aureus colonization or infection, in those who are seriously ill, or in areas with an increased local rate of methicillin resistance. The optimal treatment strategy should be discussed with the consulting nephrologist.
References
1. Root, HD, Hauser, CW, McKinley, CR, et al. Diagnostic peritoneal lavage. Surgery. 1965;57:633–637.
2. Cha, JY, Kashuk, JL, Sarin, EL, et al. Diagnostic peritoneal lavage remains a valuable adjunct to modern imaging techniques. J Trauma. 2009;67:330–334. [discussion 334-336].
3. Weinberg, AD. Hypothermia. Ann Emerg Med. 1993;22:370–377.
4. Roberts, MR, Jackimczyk, K, Marx, J, et al. Diagnosis of ruptured ectopic pregnancy with peritoneal lavage. Ann Emerg Med. 1982;11:556–558.
5. Mozingo, DW, Cioffi, WG, Jr., McManus, WF, et al. Peritoneal lavage in the diagnosis of acute surgical abdomen following thermal injury. J Trauma. 1995;38:5–7.
6. Olsen, WR, Hildreth, DH. Abdominal paracentesis and peritoneal lavage in blunt abdominal trauma. J Trauma. 1971;11:824–829.
7. Parvin, S, Smith, DE, Asher, WM, et al. Effectiveness of peritoneal lavage in blunt abdominal trauma. Ann Surg. 1975;181:255–261.
8. Mele, TS, Stewart, K, Marokus, B, et al. Evaluation of a diagnostic protocol using screening diagnostic peritoneal lavage with selective use of abdominal computed tomography in blunt abdominal trauma. J Trauma. 1999;46:847–852.
9. Brown, CK, Dunn, KA, Wilson, K. Diagnostic evaluation of patients with blunt abdominal trauma: a decision analysis. Acad Emerg Med. 2000;7:385–396.
10. Grieshop, NA, Jacobson, LE, Gomez, GA, et al. Selective use of computed tomography and diagnostic peritoneal lavage in blunt abdominal trauma. J Trauma. 1995;38:727–731.
11. Rozycki, GS, Root, HD. The diagnosis of intraabdominal visceral injury. J Trauma. 2010;68:1019–1023.
12. Gomez, GA, Alvarez, R, Plasencia, G, et al. Diagnostic peritoneal lavage in the management of blunt abdominal trauma: a reassessment. J Trauma. 1987;27:1–5.
13. Fischer, RP, Beverlin, BC, Engrav, LH, et al. Diagnostic peritoneal lavage: fourteen years and 2,586 patients later. Am J Surg. 1978;136:701–704.
14. Olsen, WR, Redman, HC, Hildreth, DH. Quantitative peritoneal lavage in blunt abdominal trauma. Arch Surg. 1972;104:536–543.
15. Bagwell, CE, Ferguson, WW. Blunt abdominal trauma: exploratory laparotomy or peritoneal lavage? Am J Surg. 1980;140:368–373.
16. Thaemert, BC, Cogbill, TH, Lambert, PJ. Nonoperative management of splenic injury: are follow-up computed tomographic scans of any value? J Trauma. 1997;43:748–751.
17. Meredith, JW, Ditesheim, JA, Stonehouse, S, et al. Computed tomography and diagnostic peritoneal lavage. Complementary roles in blunt trauma. Am Surg. 1992;58:44–48.
18. Ekeh, AP, Saxe, J, Walusimbi, M, et al. Diagnosis of blunt intestinal and mesenteric injury in the era of multidetector CT technology—are results better? J Trauma. 2008;65:354–359.
19. Smith, RS, Kern, SJ, Fry, WR, et al. Institutional learning curve of surgeon-performed trauma ultrasound. Arch Surg. 1998;133:530–535. [discussion 535-536].
20. Boulanger, BR, McLellan, BA, Brenneman, FD, et al. Prospective evidence of the superiority of a sonography-based algorithm in the assessment of blunt abdominal injury. J Trauma. 1999;47:632–637.
21. Henderson, SO, Sung, J, Mandavia, D. Serial abdominal ultrasound in the setting of trauma. J Emerg Med. 2000;18:79–81.
22. Rozycki, GS, Ochsner, MG, Jaffin, JH, et al. Prospective evaluation of surgeons’ use of ultrasound in the evaluation of trauma patients. J Trauma. 1993;34:516–526. [discussion 526-527].
23. McKenney, M, Lentz, K, Nunez, D, et al. Can ultrasound replace diagnostic peritoneal lavage in the assessment of blunt trauma? J Trauma. 1994;37:439–441.
24. Chiu, WC, Cushing, BM, Rodriguez, A, et al. Abdominal injuries without hemoperitoneum: a potential limitation of focused abdominal sonography for trauma (FAST). J Trauma. 1997;42:617–623. [discussion 623-625].
25. Shanmuganathan, K, Mirvis, SE, Sherbourne, CD, et al. Hemoperitoneum as the sole indicator of abdominal visceral injuries: a potential limitation of screening abdominal US for trauma. Radiology. 1999;212:423–430.
26. Teitelbaum, DH. Ultrasound is an effective triage tool to evaluate blunt abdominal trauma in the pediatric population. J Trauma. 1999;46:357–359.
27. Patel, JC, Tepas, JJ, 3rd. The efficacy of focused abdominal sonography for trauma (FAST) as a screening tool in the assessment of injured children. J Pediatr Surg. 1999;34:44–47. [discussion 52-54].
28. Rozycki, GS, Ballard, RB, Feliciano, DV, et al. Surgeon-performed ultrasound for the assessment of truncal injuries: lessons learned from 1540 patients. Ann Surg. 1998;228:557–567.
29. Ohta, S, Hagiwara, A, Yukioka, T, et al. Hyperechoic appearance of hepatic parenchyma on ultrasound examination of patients with blunt hepatic injury. J Trauma. 1998;44:135–138.
30. Ross, SE, Dragon, GM, O’Malley, KF, et al. Morbidity of negative coeliotomy in trauma. Injury. 1995;26:393–394.
31. McCarthy, MC, Lowdermilk, GA, Canal, DF, et al. Prediction of injury caused by penetrating wounds to the abdomen, flank, and back. Arch Surg. 1991;126:962–965. [discussion 965-966].
32. Thal, ER. Evaluation of peritoneal lavage and local exploration in lower chest and abdominal stab wounds. J Trauma. 1977;17:642–648.
33. Freeark, RJ. Penetrating wounds of the abdomen. N Engl J Med. 1974;291:185–188.
34. Moore, JB, Moore, EE, Thompson, JS. Abdominal injuries associated with penetrating trauma in the lower chest. Am J Surg. 1980;140:724–730.
35. Tayal, VS, Beatty, MA, Marx, JA, et al. FAST (focused assessment with sonography in trauma) accurate for cardiac and intraperitoneal injury in penetrating anterior chest trauma. J Ultrasound Med. 2004;23:467–472.
36. Jackson, GL, Thal, ER. Management of stab wounds of the back and flank. J Trauma. 1979;19:660–664.
37. Peck, JJ, Berne, TV. Posterior abdominal stab wounds. J Trauma. 1981;21:298–306.
38. Henao, F, Jimenez, H, Tawil, M. Penetrating wounds of the back and flank: analysis of 77 cases. South Med J. 1987;80:21–25.
39. Thompson, JS, Moore, EE. Peritoneal lavage in the evaluation of penetrating abdominal trauma. Surg Gynecol Obstet. 1981;153:861–863.
40. Fang, JF, Chen, RJ, Lin, BC, et al. Small bowel perforation: is urgent surgery necessary? J Trauma. 1999;47:515–520.
41. Henneman, PL, Marx, JA, Moore, EE, et al. Diagnostic peritoneal lavage: accuracy in predicting necessary laparotomy following blunt and penetrating trauma. J Trauma. 1990;30:1345–1355.
42. Feliciano, DV, Bitondo, CG, Steed, G, et al. Five hundred open taps or lavages in patients with abdominal stab wounds. Am J Surg. 1984;148:772–777.
43. Gonzalez, RP, Turk, B, Falimirski, ME, et al. Abdominal stab wounds: diagnostic peritoneal lavage criteria for emergency room discharge. J Trauma. 2001;51:939–943.
44. Zubowski, R, Nallathambi, M, Ivatury, R, et al. Selective conservatism in abdominal stab wounds: the efficacy of serial physical examination. J Trauma. 1988;28:1665–1668.
45. Mariadason, JG, Parsa, MH, Ayuyao, A, et al. Management of stab wounds to the thoracoabdominal region. A clinical approach. Ann Surg. 1988;207:335–340.
46. Demetriades, D, Rabinowitz, B, Sofianos, C, et al. The management of penetrating injuries of the back. A prospective study of 230 patients. Ann Surg. 1988;207:72–74.
47. Ivatury, RR, Simon, RJ, Stahl, WM. A critical evaluation of laparoscopy in penetrating abdominal trauma. J Trauma. 1993;34:822–827. [discussion 827-828].
48. Salvino, CK, Esposito, TJ, Marshall, WJ, et al. The role of diagnostic laparoscopy in the management of trauma patients: a preliminary assessment. J Trauma. 1993;34:506–513. [discussion 513-515].
49. Zantut, LF, Ivatury, RR, Smith, RS, et al. Diagnostic and therapeutic laparoscopy for penetrating abdominal trauma: a multicenter experience. J Trauma. 1997;42:825–829. [discussion 829-831].
50. Marks, JM, Youngelman, DF, Berk, T. Cost analysis of diagnostic laparoscopy vs laparotomy in the evaluation of penetrating abdominal trauma. Surg Endosc. 1997;11:272–276.
51. Boyle, EM, Jr., Maier, RV, Salazar, JD, et al. Diagnosis of injuries after stab wounds to the back and flank. J Trauma. 1997;42:260–265.
52. Feliciano, DV, Cruse, PA, Mattox, KL, et al. Delayed diagnosis of injuries to the diaphragm after penetrating wounds. J Trauma. 1988;28:1135–1144.
53. Kanowitz, A, Marx, JA. Delayed traumatic diaphragmatic hernia simulating acute tension pneumothorax. J Emerg Med. 1989;7:619–622.
54. Madden, MR, Paull, DE, Finkelstein, JL, et al. Occult diaphragmatic injury from stab wounds to the lower chest and abdomen. J Trauma. 1989;29:292–298.
55. Feliciano, DV, Burch, JM, Spjut-Patrinely, V, et al. Abdominal gunshot wounds. An urban trauma center’s experience with 300 consecutive patients. Ann Surg. 1988;208:362–370.
56. Bhullar, IS, Block, EFJ. CT with coronal reconstruction identifies previously missed smaller diaphragmatic injuries after blunt trauma. Am Surg. 2011;77:55–58.
57. Lowe, RJ, Saletta, JD, Read, DR, et al. Should laparotomy be mandatory or selective in gunshot wounds of the abdomen? J Trauma. 1977;17:903–907.
58. Moore, EE, Moore, JB, Van Duzer-Moore, S, et al. Mandatory laparotomy for gunshot wounds penetrating the abdomen. Am J Surg. 1980;140:847–851.
59. Demetriades, D, Velmahos, G, Cornwell, E, 3rd., et al. Selective nonoperative management of gunshot wounds of the anterior abdomen. Arch Surg. 1997;132:178–183.
60. Gruenberg, JC, Brown, RS, Talbert, JG, et al. The diagnostic usefulness of peritoneal lavage in penetrating trauma: a prospective evaluation and comparison with blunt trauma. Am Surg. 1982;48:402–407.
61. Markovchick, VJ, Elerding, SC, Moore, EE, et al. Diagnostic peritoneal lavage. JACEP. 1979;8:326–328.
62. Moore, JB, Moore, EE, Markovchick, VJ, et al. Diagnostic peritoneal lavage for abdominal trauma: superiority of the open technique at the infraumbilical ring. J Trauma. 1981;21:570–572.
63. Myers, RA, Agarwal, NN, Cowley, RA. A safe, semi-open procedure for diagnostic peritoneal lavage. Surg Gynecol Obstet. 1981;153:739–740.
64. Lazarus, HM, Nelson, JA. A technique for peritoneal lavage without risk or complication. Surg Gynecol Obstet. 1979;149:889–892.
65. Troop, B, Fabian, T, Alsup, B, et al. Randomized, prospective comparison of open and closed peritoneal lavage for abdominal trauma. Ann Emerg Med. 1991;20:1290–1292.
66. Cué, JI, Miller, FB, Cryer, HM, 3rd., et al. A prospective, randomized comparison between open and closed peritoneal lavage techniques. J Trauma. 1990;30:880–883.
67. Sherman, JC, Delaurier, GA, Hawkins, ML, et al. Percutaneous peritoneal lavage in blunt trauma patients: a safe and accurate diagnostic method. J Trauma. 1989;29:801–804. [discussion 804-805].
68. Howdieshell, TR, Osler, TM, Demarest, GB. Open versus closed peritoneal lavage with particular attention to time, accuracy, and cost. Am J Emerg Med. 1989;7:367–371.
69. Adkinson, C, Roller, B, Clinton, J, et al. A comparison of open peritoneal lavage with modified closed peritoneal lavage in blunt abdominal trauma. Am J Emerg Med. 1989;7:352–356.
70. Hernandez, EH, Stein, JM. Comparison of the Lazarus-Nelson peritoneal lavage catheter with the standard peritoneal dialysis catheter in abdominal trauma. J Trauma. 1982;22:153–154.
71. Pachter, HL, Hofstetter, SR. Open and percutaneous paracentesis and lavage for abdominal trauma: a randomized prospective study. Arch Surg. 1981;116:318–319.
72. Lopez-Viego, MA, Mickel, TJ, Weigelt, JA. Open versus closed diagnostic peritoneal lavage in the evaluation of abdominal trauma. Am J Surg. 1990;160:594–596. [discussion 596-597].
73. Marx, JA. Methods of diagnostic peritoneal lavage—better to be safe. Am J Emerg Med. 1989;7:452–453.
74. Moore, GP, Alden, AW, Rodman, GH. Is closed diagnostic peritoneal lavage contraindicated in patients with previous abdominal surgery? Acad Emerg Med. 1997;4:287–290.
75. Evers, BM, Cryer, HM, Miller, FB. Pelvic fracture hemorrhage. Priorities in management. Arch Surg. 1989;124:422–424.
76. Engrav, LH, Benjamin, CI, Strate, RG, et al. Diagnostic peritoneal lavage in blunt abdominal trauma. J Trauma. 1975;15:854–859.
77. Frame, SB, Hendrikson, MF, Boozer, AG, et al. Dehiscence with evisceration: a rare complication of diagnostic peritoneal lavage. J Emerg Med. 1989;7:599–602.
78. Saunders, CJ, Battistella, FD, Whetzel, TP, et al. Percutaneous diagnostic peritoneal lavage using a Veress needle versus an open technique: a prospective randomized trial. J Trauma. 1998;44:883–888.
79. Thal, ER, Shires, GT. Peritoneal lavage in blunt abdominal trauma. Am J Surg. 1973;125:64–69.
80. Sartorelli, KH, Frumiento, C, Rogers, FB, et al. Nonoperative management of hepatic, splenic, and renal injuries in adults with multiple injuries. J Trauma. 2000;49:56–61. [discussion 61-62].
81. Petersen, SR, Sheldon, GF. Morbidity of a negative finding at laparotomy in abdominal trauma. Surg Gynecol Obstet. 1979;148:23–26.
82. Shah, R, Max, MH, Flint, LM, Jr. Negative laparotomy: mortality and morbidity among 100 patients. Am Surg. 1978;44:150–154.
83. Zappa, MJ, Harwood-Nuss, AL, Wears, RL, et al. Objective determination of the optimal red blood cell count in diagnostic peritoneal lavage done for abdominal stab wounds. J Emerg Med. 1992;10:553–558.
84. DeMaria, EJ. Management of patients with indeterminate diagnostic peritoneal lavage results following blunt trauma. J Trauma. 1991;31:1627–1631.
85. Freeman, T, Fischer, RP. The inadequacy of peritoneal lavage in diagnosing acute diaphragmatic rupture. J Trauma. 1976;16:538–542.
86. Nagy, KK, Krosner, SM, Joseph, KT, et al. A method of determining peritoneal penetration in gunshot wounds to the abdomen. J Trauma. 1997;43:242–245. [discussion 245-246].
87. Hornyak, SW, Shaftan, GW. Value of “inconclusive lavage” in abdominal trauma management. J Trauma. 1979;19:329–333.
88. Thal, ER, May, RA, Beesinger, D. Peritoneal lavage. Its unreliability in gunshot wounds of the lower chest and abdomen. Arch Surg. 1980;115:430–433.
89. Root, HD, Keizer, PJ, Perry, JF, Jr. The clinical and experimental aspects of peritoneal response to injury. Arch Surg. 1967;95:531–537.
90. Mueller, GL, Burney, RE, Mackenzie, JR. Sequential peritoneal lavage and early diagnosis of colon perforation. Ann Emerg Med. 1981;10:131–134.
91. Jacobs, DG, Angus, L, Rodriguez, A, et al. Peritoneal lavage white count: a reassessment. J Trauma. 1990;30:607–612.
92. D’Amelio, LF, Rhodes, M. A reassessment of the peritoneal lavage leukocyte count in blunt abdominal trauma. J Trauma. 1990;30:1291–1293.
93. Soyka, JM, Martin, M, Sloan, EP, et al. Diagnostic peritoneal lavage: is an isolated WBC count greater than or equal to 500/mm3 predictive of intra-abdominal injury requiring celiotomy in blunt trauma patients? J Trauma. 1990;30:874–879.
94. Marx, JA, Moore, EE, Bar-Or, D. Peritoneal lavage in penetrating injuries of the small bowel and colon: value of enzyme determinations. Ann Emerg Med. 1983;12:68–70.
95. Marx, JA, Bar-Or, D, Moore, EE, et al. Utility of lavage alkaline phosphatase in detection of isolated small intestinal injury. Ann Emerg Med. 1985;14:10–14.
96. Jaffin, JH, Ochsner, MG, Cole, FJ, et al. Alkaline phosphatase levels in diagnostic peritoneal lavage fluid as a predictor of hollow visceral injury. J Trauma. 1993;34:829–833.
97. Deck, AJ, Porter, JR. Diagnostic peritoneal lavage as sole indicator of intraperitoneal bladder rupture: case report. J Trauma. 2000;49:946–947.
98. Saloman, H. Die diagnostische Punktion des Bauches. Berl Klin Wochenschr. 1906;43:45–47.
99. Ginés, P, Arroyo, V, Quintero, E, et al. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234–241.
100. Kao, HW, Rakov, NE, Savage, E, et al. The effect of large volume paracentesis on plasma volume—a cause of hypovolemia? Hepatology. 1985;5:403–407.
101. Pinto, PC, Amerian, J, Reynolds, TB. Large-volume paracentesis in nonedematous patients with tense ascites: its effect on intravascular volume. Hepatology. 1988;8:207–210.
102. Runyon, BA, Antillon, MR, Montano, AA. Effect of diuresis versus therapeutic paracentesis on ascitic fluid opsonic activity and serum complement. Gastroenterology. 1989;97:158–162.
103. Ginès, P, Titó, L, Arroyo, V, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–1502.
104. Schlottmann, K, Gelbmann, C, Grüne, S, et al. [A new paracentesis needle for ascites and pleural effusion compared with the venous indwelling catheter. A prospective, randomized study. Med Klin (Munich). 2001;96:321–324.
105. Moreau, R, Asselah, T, Condat, B, et al. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomised pilot study. Gut. 2002;50:90–94.
106. Yu, AS, Hu, KQ. Management of ascites. Clin Liver Dis. 2001;5:541–568. [viii].
107. Angueira, CE, Kadakia, SC. Effects of large-volume paracentesis on pulmonary function in patients with tense cirrhotic ascites. Hepatology. 1994;20:825–828.
108. Guarino, JR. Auscultatory percussion to detect ascites. N Engl J Med. 1986;315:1555–1556.
109. Cattau, EL, Jr., Benjamin, SB, Knuff, TE, et al. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA. 1982;247:1164–1166.
110. McGahan, JP, Anderson, MW, Walter, JP. Portable real-time sonographic and needle guidance systems for aspiration and drainage. AJR Am J Roentgenol. 1986;147:1241–1246.
111. Nguyen, PT, Chang, KJ. EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc. 2001;54:336–339.
112. Rocco, VK, Ware, AJ. Cirrhotic ascites. Pathophysiology, diagnosis, and management. Ann Intern Med. 1986;105:573–585.
113. Paré, P, Talbot, J, Hoefs, JC. Serum-ascites albumin concentration gradient: a physiologic approach to the differential diagnosis of ascites. Gastroenterology. 1983;85:240–244.
114. Arroyo, V, Ginès, P, Planas, R. Treatment of ascites in cirrhosis. Diuretics, peritoneovenous shunt, and large-volume paracentesis. Gastroenterol Clin North Am. 1992;21:237–256.
115. Luca, A, Feu, F, García-Pagán, JC, et al. Favorable effects of total paracentesis on splanchnic hemodynamics in cirrhotic patients with tense ascites. Hepatology. 1994;20:30–33.
116. Chao, Y, Wang, SS, Lee, SD, et al. Effect of large-volume paracentesis on pulmonary function in patients with cirrhosis and tense ascites. J Hepatol. 1994;20:101–105.
117. Ljubicic, N, Spajic, D, Vrkljan, MM, et al. The value of ascitic fluid polymorphonuclear cell count determination during therapy of spontaneous bacterial peritonitis in patients with liver cirrhosis. Hepatogastroenterology. 2000;47:1360–1363.
118. Chinnock, B, Afarian, H, Minnigan, H, et al. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52:268–273.
119. Cappell, MS, Shetty, V. A multicenter, case-controlled study of the clinical presentation and etiology of ascites and of the safety and clinical efficacy of diagnostic abdominal paracentesis in HIV seropositive patients. Am J Gastroenterol. 1994;89:2172–2177.
120. Runyon, BA. Paracentesis of ascitic fluid. A safe procedure. Arch Intern Med. 1986;146:2259–2261.
121. McVay, PA, Toy, PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31:164–171.
122. Grabau, CM, Crago, SF, Hoff, LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40:484–488.
123. Mallory, A, Schaefer, JW. Complications of diagnostic paracentesis in patients with liver disease. JAMA. 1978;239:628–630.
124. Rao, RN, Ravikumar, TS. Diagnostic peritoneal tap. Int Surg. 1977;62:14–16.
125. Ross, GJ, Kessler, HB, Clair, MR, et al. Sonographically guided paracentesis for palliation of symptomatic malignant ascites. AJR Am J Roentgenol. 1989;153:1309–1311.
126. Shaheen, NJ, Grimm, IS. Comparison of the Caldwell needle/cannula with Angiocath needle in large volume paracentesis. Am J Gastroenterol. 1996;91:1731–1733.
127. Wilcox, CM, Woods, BL, Mixon, HT. Prospective evaluation of a peritoneal dialysis catheter system for large volume paracentesis. Am J Gastroenterol. 1992;87:1443–1446.
128. Reynolds, TB. Therapeutic paracentesis. Have we come full circle? Gastroenterology. 1987;93:386–388.
129. Pozzi, M, Osculati, G, Boari, G, et al. Time course of circulatory and humoral effects of rapid total paracentesis in cirrhotic patients with tense, refractory ascites. Gastroenterology. 1994;106:709–719.
130. Cabrera, J, Falcón, L, Gorriz, E, et al. Abdominal decompression plays a major role in early postparacentesis haemodynamic changes in cirrhotic patients with tense ascites. Gut. 2001;48:384–389.
131. Vila, MC, Coll, S, Solà, R, et al. Total paracentesis in cirrhotic patients with tense ascites and dilutional hyponatremia. Am J Gastroenterol. 1999;94:2219–2223.
132. Runyon, BA. Management of adult patients with ascites caused by cirrhosis. Hepatology. 1998;27:264–272.
133. Runyon, BA. Patient selection is important in studying the impact of large-volume paracentesis on intravascular volume. Am J Gastroenterol. 1997;92:371–373.
134. Ginès, A, Fernández-Esparrach, G, Monescillo, A, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010.
135. Terg, R, Berreta, J, Abecasis, R, et al. Dextran administration avoids hemodynamic changes following paracentesis in cirrhotic patients. A safe and inexpensive option. Dig Dis Sci. 1992;37:79–83.
136. Planas, R, Ginès, P, Arroyo, V, et al. Dextran-70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Results of a randomized study. Gastroenterology. 1990;99:1736–1744.
137. Solà, R, Vila, MC, Andreu, M, et al. Total paracentesis with dextran 40 vs diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282–288.
138. Qureshi, WA, Harshfield, D, Shah, H, et al. An unusual complication of paracentesis. Am J Gastroenterol. 1992;87:1209–1211.
139. Martinet, O, Reis, ED, Mosimann, F. Delayed hemoperitoneum following large-volume paracentesis in a patient with cirrhosis and ascites. Dig Dis Sci. 2000;45:357–358.
140. Arroyo, V. Diuretic-resistant ascites in cirrhosis. Mechanism and treatment. Acta Gastroenterol Belg. 1990;53:249–255.
141. Antillon, MR, Runyon, BA. Effect of marked peripheral leukocytosis on the leukocyte count in ascites. Arch Intern Med. 1991;151:509–510.
142. Bar-Meir, S, Lerner, E, Conn, HO. Analysis of ascitic fluid in cirrhosis. Dig Dis Sci. 1979;24:136–144.
143. Hoefs, JC. Increase in ascites white blood cell and protein concentrations during diuresis in patients with chronic liver disease. Hepatology. 1981;1:249–254.
144. Runyon, BA, Hoefs, JC, Morgan, TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology. 1988;8:1104–1109.
145. Brown, MW, Burk, RF. Development of intractable ascites following upper abdominal surgery in patients with cirrhosis. Am J Med. 1986;80:879–883.
146. Jeffries, MA, Stern, MA, Gunaratnam, NT, et al. Unsuspected infection is infrequent in asymptomatic outpatients with refractory ascites undergoing therapeutic paracentesis. Am J Gastroenterol. 1999;94:2972–2976.
147. Hoefs, JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260–273.
148. Runyon, BA, Montano, AA, Akriviadis, EA, et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215–220.
149. Dittrich, S, Yordi, LM, de Mattos, AA. The value of serum-ascites albumin gradient for the determination of portal hypertension in the diagnosis of ascites. Hepatogastroenterology. 2001;48:166–168.
150. Bobadilla, M, Sifuentes, J, Garcia-Tsao, G. Improved method for bacteriological diagnosis of spontaneous bacterial peritonitis. J Clin Microbiol. 1989;27:2145–2147.
151. Runyon, BA, Canawati, HN, Akriviadis, EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351–1355.
152. Johnson, WD. The cytological diagnosis of cancer in serous effusions. Acta Cytol. 1966;10:161–172.
153. Aslam, N, Marino, CR. Malignant ascites: new concepts in pathophysiology, diagnosis, and management. Arch Intern Med. 2001;161:2733–2737.
154. Runyon, BA, Akriviadis, EA, Keyser, AJ. The opacity of portal hypertension–related ascites correlates with the fluid’s triglyceride concentration. Am J Clin Pathol. 1991;96:142–143.
155. Johnson, DW, Gray, N, Snelling, P. A peritoneal dialysis patient with fatal culture-negative peritonitis. Nephrology (Carlton). 2003;8:49–55.
156. Li, PK-T, Szeto, CC, Piraino, B, et al. Peritoneal dialysis–related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393–423.

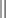 The duration of observation should be 6 to 24 hours, depending on whether diagnostic tests have been performed, the results of the tests, and clinical circumstances, including the absence of factors rendering the examination unreliable. CT, computed tomography; D/C, discharge; DPA, diagnostic peritoneal aspiration; DPL, diagnostic peritoneal lavage; IP, intraperitoneal; IPH, intraperitoneal hemorrhage; LAP, laparotomy; SPE, serial physical examination; US, ultrasound.
The duration of observation should be 6 to 24 hours, depending on whether diagnostic tests have been performed, the results of the tests, and clinical circumstances, including the absence of factors rendering the examination unreliable. CT, computed tomography; D/C, discharge; DPA, diagnostic peritoneal aspiration; DPL, diagnostic peritoneal lavage; IP, intraperitoneal; IPH, intraperitoneal hemorrhage; LAP, laparotomy; SPE, serial physical examination; US, ultrasound. 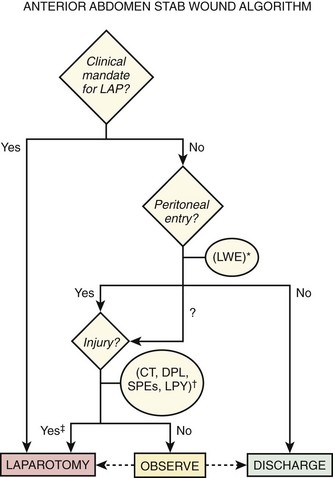
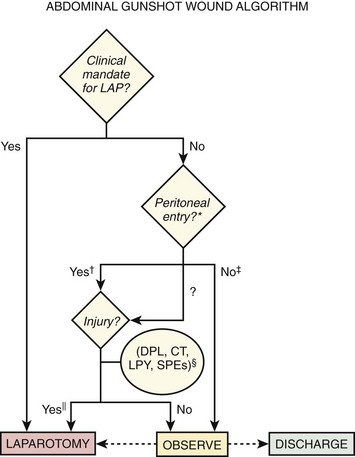
 Expectant management of injuries caused by gunshot wounds is rarely attempted. CT, computed tomography; D/C, discharge; DPL, diagnostic peritoneal lavage; LAP, laparotomy; LPY, laparoscopy; SPE, serial physical examination.
Expectant management of injuries caused by gunshot wounds is rarely attempted. CT, computed tomography; D/C, discharge; DPL, diagnostic peritoneal lavage; LAP, laparotomy; LPY, laparoscopy; SPE, serial physical examination.