Chapter 195 Peripheral Nerve Injury
Classification
Much of our knowledge about peripheral nerve injuries and their effects came from World War I, where the number of traumatic nerve divisions was numerous. This afforded an unprecedented opportunity to observe and follow a wide spectrum of injuries and their effects if untreated over time.1 In several cases there was loss of function but maintenance of anatomic continuity, and complete peripheral degeneration ensued. In others there was loss of function but no degeneration, and rapid spontaneous recovery occurred. These observations led Seddon1,2 to develop a classification system of nerve injuries that dealt with peripheral nerve lesions causing loss of function. It could be used as a clinical guide for diagnosis and treatment. He classified nerve injury into three pathologic types based upon the three components of the normal anatomic structure of the nerve. This includes the axon, myelin sheath, and supporting connective tissue structures (endoneurium, perineurium, epineurium).
Seddon’s Classification System1,2
Neurapraxia
Neurapraxia is usually mild and reversible. Motor deficits are greater than sensory deficits. There is selective segmental demyelination, with a focal nerve conduction block across the lesion. The axon and its surrounding connective tissue supporting structures remain intact. No peripheral wallerian degeneration occurs. Complete spontaneous recovery usually occurs within weeks to months of injury. The etiology is least understood but has mostly been attributed to direct mechanical compression or transient ischemia.3,4 Experiments by Bentley and Schlapp5–7 showed that conduction block developed slower and was prolonged after release of direct pressure when compared to the faster onset and rapid recovery after relief of ischemia. Initial electromyography (EMG) shows no motor action potentials.
Axonotmesis
Axonotmesis is more severe than neurapraxia, with complete internal disruption of axons, loss of axon continuity, and demyelination. However, the epineurium, perineurium, and endoneurium remain intact. Complete distal wallerian degeneration occurs. Spontaneous recovery occurs at a regeneration rate of 1 mm per day (1 inch per month). It is usually complete as long as the regenerating fibers grow into their original endoneurial tubes, ensuring the original fiber pattern.3 Full functional recovery is expected but can take weeks, months, or even years. This injury can be caused by direct blunt injury, fractures, dislocations, contusions, or stretch or crush injuries and can result in motor, sensory, or autonomic paralysis in the autonomous distribution of the nerve. In the early phase of injury it is difficult to distinguish axonotmesis from neurotmesis on a clinical basis because the deficits are similar. In both lesions, initial electrical studies show no conduction distal to injury. After 3 weeks, EMG can show fibrillations and denervation potentials distal to the injury site.8–10 If the lesion was a pure axonometric lesion, recovery will be spontaneous and complete.
Sunderland’s Classification System2
Sunderland described five pathologic degrees (grades 1 to 5) of injury based on the effect of the injury on the normal structural anatomy of the nerve trunk. He also addressed axonometric injuries with incomplete recovery and restoration of function that were not included in Seddon’s classification system. In these injuries there is disruption of the myelin sheaths and endoneurial tubes, allowing regenerating axons to escape from their original endoneurial tubes and enter distal foreign tubes. Therefore, continuity is not reestablished with the appropriate end organ originally innervated by it. The result is incomplete recovery with possible formation of neuromas. Sunderland described these lesions based upon disruption of the supportive connective tissue components of the nerve: the endoneurium, perineurium, and epineurium.
Grade 3
Intrafascicular fibrosis causes some axons to enter functionally unrelated tubes, resulting in a new pattern of innervation in the distal end organ. Sunderland refers to this as cross-shunting of axons.11 The consequences are different for an individual fascicle containing fibers from several different branches as opposed to the similar branches. If all of the fascicles are involved there is complete loss of motor, sensory, and sympathetic function in the autonomous distribution of the nerve. For partial lesions the fiber composition of the injured fascicle(s) determines the type and extent of defect. There can be mild to severe motor and sensory losses.
Grade 5
Grade 5 is the most severe grade of injury. There is complete transection, with loss of continuity of the entire nerve trunk, including the epineurium. In the proximal trunk, sprouting axons tangle and can form neuromas. These are usually laceration or severe stretch injuries. There is complete loss of motor, sensory, and sympathetic function in the autonomous distribution of the severed nerve. Wallerian degeneration occurs in the distal stump. Retrograde neuronal degeneration and axonal losses are higher than in grade 4 injury. Regenerating axons do not reach their original fasciculi and endoneurial tubes in the distal stump secondary to large injury gaps. Wasteful regeneration occurs because the axons escape into intervening tissue from the open end of the proximal stump. The distal stump shrinks over time because of denervation. Intervening scar causes obstruction, and cross-shunting occurs. The chance of any functional recovery requires surgical repair with or without nerve grafting. The quality of recovery depends on the degree of axon loss and disorganization of fiber pattern. Complete restoration is not possible.
Partial And Mixed Injuries (Combined)
Sunderland’s classification system2 can be used to describe partial and mixed injuries. In partial lesions, only a portion of the nerve fibers may be injured while the remaining are intact. In mixed (combined) injuries, all parts of the nerve are affected, with some areas more severely affected than others. For example, partial severance or subtotal fourth-degree involvement of the fascicles can coexist with minor degrees of injury to the remaining fibers of the nerve. Fascicles in continuity can contain fibers with neurapraxic, axonometric, or both types of injuries inside the same bundle. There may be complete loss of motor, sensory, and autonomic function in the distribution of the affected nerve(s). The duration, course, and quality of recovery depend on the severity, number, and type of injury to each individual nerve fiber or fascicle.
Preoperative Assessment
Neurologic Examination
Hoffman-Tinel Test
The Hoffman-Tinel test is used to evaluate and monitor the progress of spontaneous regeneration of sensory axons. It is performed several months after injury, when wallerian degeneration has already started. When the nerve is percussed, paresthesias are felt in the distribution of the nerve. This is due to mechanosensitivity of unmyelinated axons early in regeneration. Their disappearance after myelination indicates growing fibers after spontaneous recovery.12
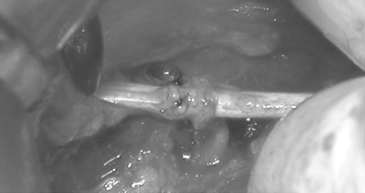
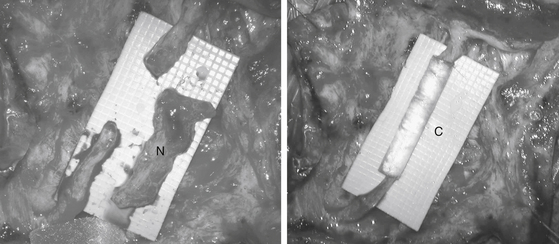
When deciding on when to do exploratory surgery one must determine if the injury is open or closed and whether it is acute or chronic and clean or dirty. Most open traumatic peripheral nerve injuries are caused by acute clean, sharp lacerations. These require immediate exploration and closure with end-to-end epineural sutures if possible (Fig. 195-1).13 Repair of a blunt injury should be delayed to allow the full extent of scar to develop. Most closed traumatic injuries are in continuity. Early after injury, neurapraxic and axonotmetic injuries should be followed for 3 to 4 months with serial clinical and electrodiagnostic examinations to confirm objective signs of spontaneous regeneration and recovery. The EMG findings are normal in neurapraxic lesions. Surgical exploration is indicated if the clinical examination and electrical studies show no improvement. Intraoperative assessment with recording of nerve action potentials (NAPs) is paramount to determine whether resection with or without nerve grafting, internal neurolysis, or only external neurolysis is appropriate.
The critical window for muscle reinnervation is within 2 years after injury for any chance of useful functional recovery. Chronic denervation results in irreversible atrophy.9 Therefore, the evaluation and decision regarding surgery should be done ideally 3 to 4 months after injury.9,12 Clinical and electrodiagnostic studies are similar in complete axonotmetic or neurotmetic injuries. However, axonotmetic injuries undergo spontaneous regeneration whereas neurotmetic injuries in continuity or discontinuity must undergo immediate surgery to reestablish continuity for any chance of useful functional recovery. Surgery is reserved for severe intractable symptoms and neurologic deficits.
Electrodiagnostic Studies
EMG is the clinical study of the electrical activity of muscle.14 It helps to define the etiology of muscle weakness and evaluate the recovery progress during nerve regeneration and muscle healing. It can be performed as early as 10 days after injury but will certainly be accurate by 4 to 6 weeks after injury. It will be normal in neurapraxic injuries and abnormal in axonotmetic or neurotmetic injuries.9,10 Abnormal findings such as fibrillations, fasciculations, and positive sharp waves may be present.8 EMG evidence of reinnervation and regeneration precedes voluntary muscle contraction by several weeks.7,9,14–16 The duration for regeneration depends on the distance from the injury to the end organ.17
In brachial palsy, EMG in combination with nerve conduction studies can help distinguish preganglionic (including avulsion) from postganglionc cervical root injury or plexus injury. Motor axonal injury shows denervation fibrillation potentials beginning approximately 3 weeks after injury.7,9,14 Nerve conduction studies (NCS) assess peripheral motor and sensory nerve function by evaluation of the electronic potential following nerve stimulation.14,18 They can help localize peripheral nerve lesions and differentiate them from disorders of muscle or neuromuscular disease. They also help delineate segmental demyelination from axonal degeneration as in neurapraxic and/or axonometric injuries as early as 1 week after injury. Serial studies are done to monitor progression of disease.
In brachial plexus lesions, sensory nerve evoked potential (SEP) recordings can help differentiate between proximal preganglionic (intraforaminal, intraspinal, intradural) and distal postganglionic (extraforaminal, extraspinal, extradural) lesions. It is well understood that the survival of the sensory nerve fibers depends on the cell bodies within the dorsal root ganglion. The motor nerve fibers depend upon the nerve’s cell bodies within the spinal cord.19
In preganglionic brachial plexus root avulsion from the spinal cord, the cells in the dorsal root ganglion and the peripheral nerve fibers are intact and evoke a sensory nerve action potential (SNAP). SNAPs are absent in postganglionic lesions because of wallerian degeneration of injured nerve fibers. However, these recordings only evaluate the function of the posterior dorsal root and not the ventral root. Therefore, the integrity of an isolated ventral root injury or of combined preganglionic and postganglionic lesions cannot be determined using only this method.20–24 The presence of an intact SNAP in the face of severe EMG changes indicates a preganglionic lesion. If there is absence of the SNAP then the lesion must be postganglionic, but this does not preclude the presence of a combined pre- and postganglionic lesion.
At present, the indirect method of evaluating motor root fibers is via EMG testing of individual muscles or groups of muscles for function via contractile responses. Therefore, the combination of EMG and SNAP recordings is more valuable than any one study for detecting spinal root or cord injury. However, motor evoked potentials (MEPS) are considered the gold standard for evaluating motor function.25 MEPS are produced via transcranial stimulation (electrical or magnetic) of the contralateral motor cortex and recording from the spinal nerve (neurogenic MEP) at its exit from the intervertebral foramen and the EMG from involved muscles (CMAP) or spinal cord (D-wave; direct corticospinal wave).23–27 Transcranial stimulation (TcMEP) produces contralateral motor activity representative of functioning corticospinal tracts. Changes in MEPs are much more sensitive than those in somatosensory evoked potentials (SSEPs) in detecting postoperative motor deficits. This is especially important in isolated motor injury with no change in SSEPs.25,28
The limitation of TcMEPs is that they cannot be continuously monitored. Monitoring requires total intravenous anesthesia for adequate signals, and TcMEPs are technically more difficult to obtain than SSEPs.25 However, continuous monitoring with spontaneous EMG allows detection of possible injury to the spinal nerve, plexus, or roots during cervical or lumbosacral spine procedures and during minimally invasive procedures. Triggered EMG is helpful in localizing functional neural structures in the surgical field as well as evaluating injury to nerve roots after medial wall breach during pedicle screw instrumentation.25 A large prospective study of 1055 patients demonstrated that the combination of EMG, SSEPs, and MEPs increased the chances of detecting neurologic injury during cervical spine procedures.28 The consensus is that the use of multimodality neurophysiologic monitoring in spinal procedures aids in preventing postoperative neurologic deficits.
SSEP studies evaluate the dorsal column and medial lemniscus pathways, which helps assess proximal spinal nerve root injuries,9,29–31 spinal cord tumors, and other spinal disorders.32 SSEP involves electrical stimulation of peripheral nerves (median nerve, common peroneal nerve, posterior tibial nerve, or ulnar nerve). The signals are recorded centrally over the brain in the contralateral parietal cortex or in the spinal cord. A dorsal root (preganglionic) avulsion injury would have an absent SSEP in the presence of a positive postganglionic SNAP because the distal sensory fibers are still connected to the dorsal root ganglion.29,30 In distal postganglionic lesions, stimulation of the root proximal to the level of injury results in a positive SSEP in the absence of a SNAP.29
SSEPs can be invaluable for intraoperative monitoring.25,27,32 Early recognition of changes can signify possible injury and give the surgeon time to make the necessary adjustments to prevent postoperative neurologic deficits. According to Dinner and colleagues,32 significant changes such as loss in signal or decrease in amplitude in monitored SSEPs correlated with a higher possibility of postoperative neurologic deficit (three of seven SSEPs, 43%). If no changes occur, the chances of a deficit are low (1.8%, 4 of 213 cases). Sensory nerve evoked potentials (SNEPs) are useful in the resection of some intraneural tumors by distinguishing and functional from nonfunctional fascicles, thereby rendering safer the separation and resection of tumor from functional fascicles. SNEP is valuable in the surgical management of peripheral neural sheath tumors of major nerves including the brachial plexus.33,34 This is significant, especially in type 1 neurofibromatosis (NF1), which previously was thought to be unsafe for resection.3
Imaging Studies
Plain x-rays can reveal some tumors, cysts, injury to the diaphragm (phrenic nerve), and skeletal fractures where specific locations can imply concomitant nerve injury. However, there is poor delineation of peripheral nerves from surrounding tissue.35
CT can identify soft tissue tumors, cysts, and skeletal fractures or hemorrhage and can help to delineate some peripheral nerve tumors from adjacent blood vessels or tissues, although in most cases visualization is usually poor.36
MRI can delineate the anatomic details of soft tissue, peripheral nerves, and perineural tissue much better than CT. It can give T1 and T2 multiplanar images (axial, coronal, sagittal) of large bulky tumors.37 Coronal and sagittal views show the full linear extent of the tumor with fewer cuts and produce clearer images than CT. CT can require several axial cuts. MRI can be used in patients with nonferromagnetic prosthetics. It is also useful in evaluating and diagnosing traumatic, compressive, and inflammatory lesions of peripheral nerves.35,37 Inadequate images are usually secondary to spatial and resolution limitations and motion artifacts.20,38 Traumatic peripheral nerve injury can result in axonal losses with degeneration and muscle denervation followed by muscle atrophy, if the injury is chronic. MRI of muscle distal to the lesion can show increased T2 and short tau inversion recovery (STIR) signals in the denervated muscle. This correlates well with clinical examination (grade 3 weakness) and EMG findings of fibrillation potentials. These signals can be seen as early as 4 days after nerve injury.39 MRI can in some instances distinguish neurapraxic from axonometric and neurometric injury.39,40 Both STIR and T2-weighted images are normal in neurapraxic lesions.
In axonometric injuries, signs of reinnervation and recovery are reflected by normalization of signals, but the MRI findings can lag.39 Therefore surgical exploration with intraoperative electrical studies are necessary to appropriately assess for axonometric and neurometric injury. This is especially important because neurometric injuries require surgical repair for any chance of useful functional recovery. Increased T2 and STIR signals are also seen in chronically denervated muscles and nerves as in entrapment neuropathies such as carpal tunnel syndrome of the median nerve or in ulnar nerve entrapment at the elbow. In carpal tunnel syndrome these findings correlate well with EMG, nerve conduction studies, clinical examination, and operative findings; however, in the ulnar nerve, where symptom localization with electrodiagnostic studies is difficult, the imaging correlation is less clear.40–43 For reasons unknown, some abnormal MRI signals normalize over months even without signs of recovery.42
In a significant spinal study, MRI with and without contrast (gadolinium) was shown to quantify lumbar peridural fibrosis 6 months after discectomy for herniated lumbar disc.16 The increased scar correlated well with an increased probability of recurrent radicular pain. The imaging in this case looked more at the scar tissue than the nerve itself.
Despite all of the advantages of MRI and CT, in some instances it is still difficult to distinguish vascular structures and surrounding tissues from peripheral nerves. Despite the superior contrast enhancement of tumors with MRI, its utility is variable in peripheral nerve disorders.44 The development of MRN has helped to resolve this by its ability to directly visualize the normal fasicular structure and pattern of the nerve by the interfascicular and intrafascicular suppression of non-neural structures such as fat, muscle, and blood vessels.26,35,44 Therefore, the nerve fascicle signal predominates. Images are obtained using phased-array coils, fast spin echo (FSE) sequences, and fat-suppressed T2-weighted images. Signals are increased (hyperintense) in intraneural disease and correlate with clinical examination, intraoperative electrodiagnostic studies, and pathology.38 T1 spin-echo pulse sequences can show the course of the nerve in relation to adjacent structures.38,44 Longitudinal and cross-sectional fascicular images aid in distinguishing intraneural from perineural masses such as tumors or cysts.44 In one study, preoperative MRN distinguished nonfunctioning and functioning fascicles from tumor, allowing complete resection in 72.5% of patients with NF1.38 After traumatic peripheral nerve injury, MRN can assess nerve continuity and aid in surgical planning.44,45 Cudlip and colleagues17 used the rat sciatic nerve crush model to follow the MRN signal changes immediately after injury and throughout the degenerative and regenerative processes. The signal changes correlated with the functional deficits. Normalization of the signals correlated with functional recovery. Early after severe axonometric or neurometric injury, T2 signals are increased at and distal to the lesion. Electrical studies show no nerve conduction across the lesion.
After surgical repair of a peripheral nerve, the course of healing can be followed by the signal changes.26,44 After several weeks or months, signs of regeneration can be confirmed by clinical examination, decreased fibrillations on EMG, and distal normalization of the T2 signals. However, for reasons unknown, these signals can remain high in interpositional grafts.26 Overall, these findings show that MRN correlates well with the clinical and intraoperative electrodiagnostic findings during the course of recovery.26,38
MRI, CT, and myelography can be very sensitive but not specific in detecting multilevel degenerative disc disease of the cervical and lumbar spine.43 There are many false positives in asymptomatic patients. In these cases, MRN could be considered. It may be a useful adjunct because it has already been shown to help evaluate and diagnose traumatic and compressive lesions of peripheral nerves, nerve roots, and the brachial plexus.35,45 The MRN appearance can differ in the symptomatic root, allowing identification of the pathologic spinal level.
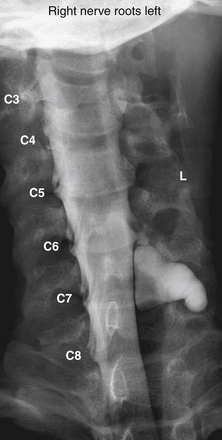
The assessment of acute brachial paralysis secondary to cervical root avulsion can be complex and difficult. It requires a thorough clinical evaluation, imaging, and electrodiagnostic studies. Traumatic meningoceles (pseudomeningoceles) on myelogram studies were considered to be pathognomonic for intradural (intraspinal) spinal root avulsions (Fig. 195-2).20,22 However, surgical exploration in some cases revealed intradural root avulsion even with a normal preoperative myelogram. Currently, CT myelogram done in 1- to 3-mm axial slices is considered accurate for assessment of avulsion of spinal roots, especially in the brachial plexus.20 A significant study has shown that 85% of roots evaluated by preoperative CT myelogram correlate to intraoperative findings of intradural avulsed cervical spinal roots compared to 52% evaluated by MRI.20,42 Avulsed spinal roots are mistakenly diagnosed more commonly at C5 or C6 in both imaging studies. MRI is less reliable at visualizing the intradural (intraspinal) dorsal and ventral roots.20 Intradural fibrosis or traumatic meningoceles (pseudomeningocele) may be contributing factors for poor visualization. For inconclusive or complex injuries of the brachial plexus, MRN should be considered in conjunction with conventional MRI and electrophysiologic studies to assist with localizing nerve root and brachial plexus lesions.45
Operative Management
The goal of any nerve repair is to reestablish continuity and provide an optimal environment and conditions for adequate axonal regeneration that will ultimately produce peripheral innervation patterns similar to the original ones and leading to useful functional recovery. In the latter half of the 20th century, many innovative techniques and instrumentation were developed to assist in achieving this goal. Sunderland11 suggested using the operating microscope in nerve repairs, but it did not come into favor clinically until the 1960s.13 Because most injuries are associated with some form of external fibrosis, the initial step in surgical decompression involves an external neurolysis. The procedure required is dictated by the type and severity of injury. Some of these are described later in further detail.
External Neurolysis
External neurolysis is indicated when electrical studies fail to improve or when they worsen after 3 to 4 months from the time of injury. This could be secondary to nerve compression, constriction, or tethering by scar tissue.1,14,46 Fibrosis can slow down and/or prevent spontaneous regeneration, resulting in poor, if any, recovery. It can also cause significant neuritic pain. Preoperative electrical studies are done to localize the area(s) of slowing or conduction block. Under general anesthesia, fibrotic tissue is resected until healthy planes are established around the nerve lesion and immediate injury site. Electrical studies are repeated intraoperatively to assess for any improvement. A nonconducting lesion requires resection and end-to-end repair or interpositional nerve grafting.
Internal Neurolysis
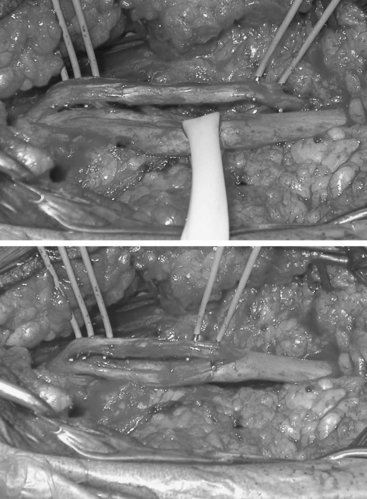
Internal neurolysis is usually done for patients with severe neuritic pain and incomplete losses distal to the injury.14 Electrical studies show no improvement or worsen after 3 to 4 months from the time of initial injury.14 The lesion must be in continuity and conduct a compound NAP preoperatively and intraoperatively. These are usually partial axonotmetic injuries with or without regeneration.8 After external neurolysis, recordings are repeated to identify the reduced conductive areas. These sites undergo an interfascicular dissection, with resection of fibrotic tissue surrounding individual fascicles.8 Each fascicle is tested individually for function and resected if nonconducting (Fig. 195-3). For partial lesions, a split (partial) repair is done in which the nonconducting portion is resected and repaired end-to-end or with an interpositional nerve graft if the defect is too large.14,46,47 Decompression by manipulation of the fascicles can induce fibrosis, which can cause injury to the regenerating axons as well as the normal fascicles, thereby further delaying or worsening recovery. The surgeon must weigh the risk of possible irreversible muscle loss from chronic denervation against that of surgery.
Oberle and colleagues47 demonstrated in a small subset of cases that some nonconducting lesions in continuity do not need to be resected. These lesions have a normal appearance and had no change in caliber. It was assumed that their inability to conduct was secondary to compression by intraneural fibrosis, which slowed axonal regeneration. Therefore, at the time of the surgery there would not be enough matured axonal fibers to produce a recordable NAP. If the lesion were axonometric, then decompression by internal neurolysis would be appropriate. Based on this assumption, Oberle’s group performed an epineurotomy and internal neurolysis on six patients who had complete loss of nerve function and no recordable intraoperative NAP across the lesion but who had normal nerve caliber and appearance. Three patients had good results and the remaining three had useful function. Oberle concluded that the presence or absence of NAPs cannot be used to distinguish this group from those who have irreversible intrafascicular damage.47 Therefore, for these rare cases, the surgeon must rely on his or her expertise and clinical judgment in determining if internal neurolysis is appropriate.
Graft Repair
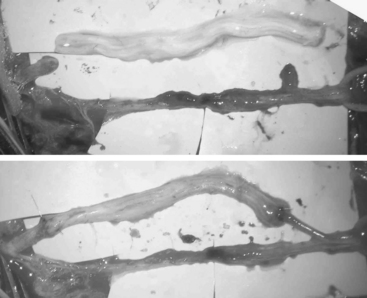
Nerve grafts with donor autografts are ideal because autografts are not rejected.48 They are usually placed when a large segment defect is left after resection of a nerve lesion in continuity, after significant scar is removed within a nerve, or when a primary end-to-end repair is not possible owing to retraction of the ends.49 These gaps are usually too large (greater than 5 cm) for direct end-to-end repair.46 The graft should be long enough so that the repair can be performed without tension (Fig. 195-4). Tension can cause fibrosis, which can cause regenerative failure along the suture site.13,14,46,50 Sensory nerve grafts are preferred over mixed motor-sensory nerves because there is less postoperative deficit. The sural nerve is the preferred graft because it is easily accessible and leaves the patient with few side effects. Other options are the medial antebrachial cutaneous nerve, superficial sensory radial nerve, dorsal cutaneous branch of the ulnar nerve, saphenous nerve, or ulnar nerve.
The proximal and distal ends of the nerve defect are trimmed until healthy tissue and normal fascicle are reached. Then both ends are anatomically oriented to the donor graft so that their fascicular patterns are aligned as close as possible in order to optimize good coaptation and chance of functional recovery. The fascicular pattern is a very important factor in determining if epineural or perineural repair will give the best coaptation. A monofascicular or oligofascicular pattern with a few large fascicles can have good fascicular coaptation with superficial epineural sutures. Millesi refers to this as “trunk-to-trunk coaptation.”46 However, oligofascicular patterns with several fascicles with different functions can require interfasicular dissection and separation for adequate coaptation of individual fascicles using perineural sutures. Nerve segment defects with polyfascicular patterns with fascicles of different sizes arranged into groups have a better chance for coaptation between donor nerve graft and each fascicle group if interfascicular sutures are used. Most commonly, sectoral nerve grafting to corresponding sectors is recommended to minimize trauma to individual fascicles or groups.46
End-to-End Repair
End-to-end repair is the preferred treatment for clean transections and acute sharp lacerations.38,46 It is also done when a small gap is left after the resection of an injured nerve segment. The proximal and distal stump ends must be close enough to be approximated without tension.2,14,38,43,44,46 Sometimes additional length can be provided by mobilization, joint flexion, or bone removal. Coaptation is achieved by joining the proximal and distal ends with superficial epineural sutures (nonabsorbable monofilament). Irregular lesions may be complex and technically challenging. More extensive dissection and resection of nerve can be required before reaching normal healthy fascicles, which can result in larger defects. In these cases interfascicular dissection between fascicles and fascicle groups with resection of superficial epineurium should be considered.46 This repair requires a more exact alignment of fascicles to ensure good coaptation. Therefore, end-to-end repair with superficial epineural sutures is not sufficient. Perineural sutures may be more appropriate, depending on fascicular pattern. These complex repairs are fortunately less common.
Conduit Repair
Autologous nerve grafting is considered the gold standard for repairing large nerve defects after lesion resection or when direct end-to-end repair is not possible in peripheral nerve injuries. The introduction of an autologous nerve segment provides a physical and biologic scaffolding over which axonal outgrowth can occur. In this way, growing axons are directed and stabilized as they extend to their appropriate targets. Complications arise, however, because of the limited supply of donor nerves and the risks associated with the harvesting surgery. Donor nerves must approximately match the dimensions of the peripheral deficit and be surgically harvested from a secondary site. Donor sites are vulnerable to infections, the formation of painful neuromas, and loss of function associated with the harvested nerve.46
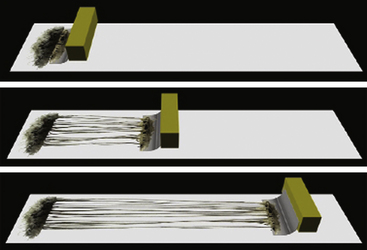
In recent years there has been considerable interest in developing alternative strategies to repair damaged peripheral nerves through transplanted materials of biological or synthetic origin if donor nerves are scarce. Arteries, veins, and musculature have been somewhat discredited as conduits because they have been associated with tissue reaction, early fibrosis, and scar infiltration. Currently in clinical use are polyglycolic acid (PGA) or collagen tubes often filled with a matrix gel and trophic factors. These conduits provide a physical guide for axons sprouting from the proximal nerve stump to reach the disconnected nerve segment. Thereafter chemical and physical cues from the disconnected nerve form a labeled pathway, which then directs the continued growth of regenerating axons to ultimately reinnervate the target tissue. However, synthetic conduits have only been clinically successful for the repair of relatively short nerve lesions and are typically used for gaps less than 2 to 3 cm (Fig. 195-5).
Research is ongoing into designing and constructing better conduits as well as novel tissue-engineered live biomaterials51 (Fig. 195-6). Multiple factors are considered. Of importance are the type of biomaterial and fabrication technique, the physical properties of the tube, and addition of factors that enhance and support axon regeneration.52 The problems and solutions are complex but the future looks promising.
Nerve Transfer, Neurotization, and Reimplantation
Nerve transfer, neurotization, and reimplantation are done to restore lost function to the extremity after severe injury, such as avulsion of spinal nerve roots, where there is no viable proximal nerve for repair. In some cases the proximal stump is present but severely fibrotic.52–56 The chosen donor nerve is normal and is taken from muscles with good collateral innervation or those that have less functional value.1,53–57 The proximal stump is coapted to the distal stump of the damaged nerve trunk whose proximal root has been avulsed or severely injured.9,53,56,57 If the procedure is successful, the regenerating axons will grow into the distal nerve stump or motor endplate of muscles, resulting in reinnervation and restoration of muscle function. Examples are the transfer of the distal spinal accessory nerve1,57,58 or the upper intercostal nerves to the musculocutaneous nerve supplying the biceps and brachialis muscles after avulsion of C5 and C6 spinal nerve roots,9,57,59 coaptation of C5 or C6 to the musculocutaneous nerve via interpostional sural nerve grafts to restore elbow flexion,11 or coaptation of C3 and C4 anterior primary rami to the upper trunk of the brachial plexus to restore function to the biceps and shoulder girdle muscles after avulsion of C5 and C6 spinal nerve roots.11 The direct repair of avulsed spinal motor roots by reimplantation of roots into the spinal cord has exhibited limited functional recovery of proximal muscles. However, these findings are very encouraging.60
Mechanisms and Treatment of Specific Nerve Injuries
There is a wide variety of mechanisms leading to peripheral nerve injury. Nerve injuries are generally classified according to their mechanism of injury, because the mechanism determines the timing and nature of treatment. Examples of these mechanisms include sharp lacerating injury, blunt lacerating injury, stretch and compression injury, gunshot wounds, and nerve root avulsion. In general, within the noncombat population, stretch injuries are the most common.61 Iatrogenic injuries can occur as complications of medical interventions. The most common of these include injection injuries, obstetric brachial plexus injuries, nerve transections, and intraoperative positioning nerve injuries.61,62
Sharp Lacerating Injury
Description
Sharp lacerating nerve injuries result from a focal (partial or total) transection of a peripheral nerve with a sharp object such as glass or a knife blade. This mechanism of injury is responsible for about 30% of serious nerve injuries.61 In contrast to blunt injuries, minimal energy is transmitted to the stumps proximally and distally to the nerve lesion. Thus, there is little to no damage along the stumps (other than the transection itself), enabling acute repair with a reasonable outcome.
Treatment
Ideally, once the patient has been stabilized, these lesions should be repaired immediately with direct, tension-free suture end-to-end coaptation.63 Minimizing the delay decreases the retraction of the nerve stumps, avoiding the need for nerve grafts or conduits.64,65 At the time of surgery, the nerve ends should undergo external neurolysis if needed, and healthy nerve stumps should be approximated.
Blunt Lacerating Injury
Treatment
Blunt lacerating nerve injuries should be repaired in a delayed fashion. Acute wound exploration might be needed, however, for vascular or other tissue repair. If the wound is to be surgically explored, the cut ends of nerve may be sutured to adjacent tissues to prevent additional retraction during the waiting period. Surgical repair of the nerve injuries should be postponed 2 to 4 weeks at minimum to allow the severely injured nerve tissue to scar and provide a clear demarcation of viable tissue that should be repaired.63,65 At the time of definitive repair, external neurolysis is performed to resect the scarred stump back to normal fascicular structure, and a graft repair is typically performed. Occasionally, direct end-to-end tension-free coaptation might be performed if the tissues have not undergone significant retraction and the stumps are in close proximity.
Stretch and Compression Injury
Description
Nerve stretch and compression injuries can disrupt the vasa nervorum, leading to patchy nerve ischemia, or can tear the intraneural connective tissue, leading to hemorrhage and/or necrosis.66 Low-grade injury can cause a temporary conduction block lasting hours to days. More-robust injury can demyelinate the axons, causing a conduction block lasting the several weeks necessary for the myelin to regrow. Greater degrees of stretch or compression can cause an axonotmetic injury, in which the axons are destroyed. In that circumstance, recovery requires the axons to regrow, a process that can take months to years, depending upon the length needed to regrow.65 Stretch injuries to a variety of nerves can occur with joint dislocations, and brachial plexus injuries can occur following motorcycle accidents where the head and shoulder undergo violent distraction. Examples of compression injuries include ulnar nerve compression of the elbow following inadequate positioning during surgery and radial nerve compression seen in Saturday night palsy.66
Treatment
Patients who sustain nerve stretch or compression injury in the absence of a persistent mass lesion undergo observation and physical therapy for 3 months. If a mass lesion (such as a compressive hematoma) is responsible for the neurologic deficit, then decompression of the neurologic elements is performed emergently prior to the waiting period. Many patients recover spontaneously during this time period. Those who do not recover undergo nerve exploration. At surgery, the affected nerve segment is subjected to an external neurolysis, and intraoperative NAP recordings are performed. If a recovering NAP is present, then no further repair is necessary. If a recovering NAP is absent, then the nonconducting neuroma is excised and a graft repair is performed.65,67
Outcome
Patients who spontaneously improve by 3 months generally have excellent outcomes. Patients who do not recover by 3 months but who show a recovering NAP at the time of surgery have a roughly 90% chance of good outcome. Patients who require a graft repair have a highly variable outcome depending on the proximal level of the injury, delay in repair, and length of graft repair needed.66 Generally, short-segment graft repairs (less than 4 cm) have a roughly 50% chance of good recovery.
Gunshot Wounds
Diagnosis
Identification of bullet caliber, velocity, and distance can be used to estimate the degree of injury. Small-caliber, low-velocity civilian gunshot wounds often lead to a partial nerve injury, with only 21% having complete loss of function.68 High-velocity hunting or military gunshot injuries typically lead to more devastating loss of tissue and neurologic function.
Treatment
In general, gunshot nerve injuries are managed in a similar fashion as stretch and compression injuries. Unless immediate surgery is warranted by a progressive neurologic deficit (suggesting an expanding mass lesion), the patient is observed and electrodiagnostic studies are performed 3 weeks after the incident. Lack of recovery by 3 months should prompt surgical exploration. If the nerve has been completely transected, then external neurolysis followed by primary repair or graft is performed. Intraoperative NAP recordings are used to evaluate lesions in continuity, and those that show no conduction are treated by resection and grafting.68
Nerve Root Avulsion
Diagnosis
History is important because the trajectory of the force might provide a clue for the presence of nerve root avulsion and its localization. In fact, root avulsion is present in 70% of severe brachial plexus traction injuries.69 Physical examination findings that support the diagnosis of nerve root avulsion include Horner syndrome,70 paraspinal or rhomboid muscle denervation, winged scapula, and diaphragm paralysis,.71,72 Electrodiagnostic studies are typically performed 3 weeks after injury and can show absent motor responses in the injured distribution, with preservation of the sensory component. This pattern occurs because avulsion occurs in a preganglionic location, damaging the motor axons but sparing the sensory fibers whose cell bodies reside in the dorsal root ganglion. CT myelography often shows one or more pseudomeningoceles and absent rootlets at the avulsed level, and it is considered to be the imaging study of choice for these lesions.20
Treatment
Brachial plexus avulsion injuries initially are treated in a similar fashion as stretch and compression injuries. Repair is undertaken following a 3- to 4-month waiting period because these injuries are often of mixed pathology and there may be some stretched components that require a primary repair. In contrast to the other forms of injury, root avulsion injuries are not generally repairable with nerve grafts, because the spinal cord anterior horn cells are completely disconnected from the peripheral nervous system. Thus nerve transfers from other, uninjured nerves are needed to create a repair.73,74 Examples include accessory to suprascapular nerve to yield supraspinatus recovery, triceps branch to axillary nerve to enable deltoid recovery, and ulnar to biceps branch of musculocutaneous nerve to enable biceps recovery.75–77
Outcome
In general, the prognosis for patients with nerve root avulsions is worse than that seen for other kinds of nerve injuries, because spontaneous recovery is essentially impossible in the avulsed distributions. Functional recovery can be achieved by nerve transfers in the majority of cases. For instance, 64% to 88% of patients with intercostal-musculocutaneous nerve transfer show significant improvement, achieving elbow flexion.59,73,78,79
Injection Injury
Description
Injection injury is common among iatrogenic causes of nerve damage.63,64 The mechanism of injury can be elicited by direct trauma induced with the needle or by the compression from an expanding lesion such a pseudoaneurysm or hematoma formed secondary to vascular injury. It can also result from chemical irritation and disruption of the nerve tissue.
Diagnosis
The most commonly affected nerves include the sciatic and the radial nerves, because the gluteal region and shoulder constitute two common intramuscular injection sites. Other nerves that have been found to be affected include the median, ulnar, and femoral nerves, which can be injured by needles while attempting to gain intravascular access. The injury can lead to acute sharp pain that radiates in the distribution of the affected nerve, or it can develop as deep burning pain and paresthesias in a progressive fashion, usually as the result of a chemical or compressive process. At surgery, nerve pathology might have minimal manifestations or can manifest with hematomas, adhesions, or a change in nerve caliber.63
Treatment
Initially patients should be observed for a period of 3 months to allow spontaneous recovery. During this time the patient should be encouraged to use the limb as much as possible and to maintain normal range of motion. Surgery is reserved for patients who do not show signs of significant recovery,63 as for other nerve injuries. Surgery consists of external neurolysis followed by NAP studies to assess the need for graft repair, required in the setting of a nonconducting neuroma-in-continuity.
Obstetric Brachial Plexus Palsy
Description
Obstetric brachial plexus palsies are neonatal injuries of the brachial plexus that can occur owing to traction or other forces applied to nerves during labor. Whereas the mechanism is similar to stretch and compression injuries of adults, these lesions have a more benign course, perhaps due to the regenerative capacity of the of neonate’s nerves or due to the milder forces than the ones that can be encountered during a motor vehicle trauma.80
Diagnosis
Some of the most classic presentations include Erb’s palsy (C5–6), Erb’s plus palsy (C5, C6, and C7), and Klumpke’s palsy (C8–T1). The combination of these is known as Erḃ-Klumpke’s palsy (C5–T1) and manifests as a flail arm. Often these nerve injuries are complicated by extremity deformities of shoulder, elbow, forearm, and hand.81
Treatment
An initial period of observation for up to 3 months is suggested in order to distinguish the population that will recover without intervention. Some authors have advocated for the lack of biceps function by 3 months as a cutoff for intervention.82,83 Other authors have a more conservative approach and observe for 9 months to 1 year.63 Failure to improve warrants surgical intervention. Some of the surgical treatments include neurolysis, graft repair, and nerve transfers,63 as for other nerve injuries. Nerve repair strategies may be supplemented by orthopedic reconstructions as well to optimize upper extremity function.80,81
Outcome
The vast majority of lesions that improve during the first 2 months of life recover completely. The prognosis worsens if the patient fails to show signs of improvement by 3 months and more so by 6 months of age.63,80,81 Other signs of poor outcome include Horner syndrome and nerve root avulsion injury.
Sensory Neuroma
Description
A focal nerve injury often leads to a poorly organized regrowth of axons within a fibrous scar at the site of the lesion.84,85 Sensory neuromas can be found at end lesions in the case of total disruption of the nerve, or they can be found as neuromas in continuity. These lesions are mechanosensitive and often painful.86,87 If the neuroma is not treated, the pain can become central, decreasing the possibility of adequate pain control.85
Diagnosis
Sensory neuromas usually occur in the context of a traumatic lesion in which a nerve is involved. A palpable nodule with a prominent Tinel sign in the setting of previous trauma is typically diagnostic of a sensory neuroma. These lesions can also elicit pain by the movement of the surrounding tissues (skin or muscle during normal activities). The diagnosis can be confirmed by injecting a local anesthetic, which alleviates the pain temporarily, but this technique is not perfect and can be confounded by injection errors and placebo effects.
Treatment
The treatment paradigm should begin with noninvasive treatments such as neuropathic pain medications. Nerve blocks and steroid injection may be used subsequently to provide pain relief and to confirm the diagnosis. Usually these therapies only offer a temporary improvement. Alternatively, patients might be treated with radiofrequency or ethanol ablation. If these measures fail, surgical intervention is warranted. At surgery, the neuroma is excised and the stump is transferred into an alternative site such as bone or muscle to prevent scar regeneration.88
Outcome
Pain relief following sensory neuroma surgery is very variable and is highly dependent on the chronicity of the lesion, with longer duration of symptoms associated with worse pain control. In general, the majority of patients achieve symptomatic improvement with treatment, and it is important to proceed from the least-invasive to the most-invasive treatments to minimize unnecessary interventions. Prognosis is influenced by multiple factors, including the presence of central nervous system changes due to chronic pain, as well as psychosocial factors such as worker’s compensation, employment, and other factors that are key in the outcome of patients with chronic pain.85
Radiation Injury
Description
Relatively uncommon, this injury usually manifests as brachial plexopathy following radiation to the thoracic outlet, axilla, neck, or chest, and is found in 1.2% of women who receive radiation therapy for breast cancer with contemporaneous fractionated radiation protocols.89 The effect of radiation on long-term neuropathies has been proved to be dose dependent,90 and the incidence can be further increased by exposure to chemotherapy.89,91
Diagnosis
Symptom onset following exposure can be delayed for a variable period that can be as long as 30 years.90–92 Sensory symptoms such as numbness, paresthesias, and pain are more common, although motor symptoms can be seen. Electrodiagnostic studies can be useful for distinguishing radiation-induced plexopathy from neoplastic disease, because in plexopathy the former is usually associated with myokymia (spontaneous discharges followed by muscle quivering.92,93 MRI is useful for distinguishing this entity from a neoplastic lesion. Radiation-induced plexopathy can lead to enlargement of the plexus with contrast enhancement, but neoplastic lesions tend to appear with a focal mass lesion.
Treatment
Physical therapy is useful for preventing frozen shoulder and atrophy. The pain can be treated medically but is often poorly controlled by this means. Thus, these patients are best managed by a multidisciplinary, comprehensive pain management program and might ultimately benefit from invasive therapies such as neurolysis, nerve transfers, dorsal root entry zone lesions, or neurostimulation.94–96
Confounding Disorders
Neuralgic Amyotrophy
Description
Neuralgic amyotrophy, otherwise known as Parsonage-Turner syndrome, brachial plexopathy, or shoulder-gridle neuritis, is a poorly understood entity that consists of an autoimmune inflammatory process of the brachial plexus.97,98 The reported annual incidence is 1.6 cases per 100,000.99 Some of the predisposing factors include surgery, pregnancy, trauma, vaccinations, and other processes involving systemic immune responses.98,100–103
Diagnosis
Neuralgic amyotrophy can be distinguished from stretch and compression nerve injuries (such as intraoperative positioning injuries) because the neuralgic amyotrophy tends to develop weakness over time whereas stretch and compression injuries manifest with immediate onset of neurologic deficit. In addition, the EMG findings of compression and stretch injuries are usually circumscribed in a region of the plexus, whereas in neuralgic amyotrophy the changes can be extensive and bilateral, despite the lack of symptoms on all the distributions.66
Lumbosacral Plexopathy
Neuropathy of the lumbosacral plexus has a wide range of etiologies. Some of the entities include cancer, trauma (especially in the case of vertical fracture of pelvic ring), radiation-induced plexopathy, and obstetric causes. Some of the most frequent obstetric causes include compression injury at the pelvic brim that often leads to a foot-drop after delivery, piriformis syndrome, and iatrogenic causes (due to prolonged lithotomy position or direct trauma with the retractors) among others.104–108
Idiopathic lumbosacral plexopathy is a distinct entity, and it is considered a diagnosis of exclusion. This is the lumbosacral variant of neuralgic amyotrophy, and it has a similar clinical course. The clinical presentation of idiopathic lumbosacral plexopathy is characterized by acute onset of pain followed by weakness (with variable losses of stretch reflexes), with subsequent atrophy of the involved muscles, and sensory loss.109 In contrast to many other causes of lumbar plexopathy, idiopathic plexopathy is not evident on imaging.104 The heterogeneous list of etiologies for lumbosacral plexopathy leads to a very variable clinical presentation, and subsequently it requires different diagnostic and therapeutic approaches depending on the etiology. In the case of the idiopathic variant, patients usually recover, although this recovery might be partial.109,110
Complex Regional Pain Syndrome
Complex regional pain syndrome (CRPS) is a neuropathic pain syndrome that can occur in the setting of nerve injury (type 2) or can mimic nerve injury in the absence of discernible damage to the peripheral nerves (type 1). The diagnosis of these entities is based on history and physical examination.111 Both types are characterized by intense pain beyond that expected from the initial mechanism; swelling, color, temperature, or trophic changes; and allodynia, hyperpathia, or mechanical hyperalgesia on examination. All of these symptoms and signs can be found in straightforward nerve injuries as well. The key to distinguishing CRPS from a simple nerve injury is that the findings exceed the confines of the injured nerve distribution in CRPS (hence “regional” pain syndrome), whereas findings in a simple nerve injury occur only in the territory of the injured nerve.
A comprehensive, multidisciplinary pain management strategy is most effective in the treatment of CRPS. Many of these patients have psychological overlay, complicating treatment efforts. Pharmacologic management, psychological support, complementary and alternative therapies, sympathetic blocks, and neurostimulation are all potentially helpful for these patients.111
Pain Management Following Nerve Injury
Neuropathic pain following nerve injury is a common manifestation that often can be incapacitating. Pain can improve with the specific management for the nerve injury (as described earlier); nevertheless, analgesic treatment is required in some cases. In general, the first line of treatment for neuropathic pain is pharmacologic. Antidepressants and anticonvulsant medications are the two groups that show the highest efficacy for pain relief.112–115 Common anticonvulsants include pregabalin, oxcarbazepine, and gabapentin.112–114116 The combination of an anticonvulsant with an antidepressant can offer higher efficacy of pain relief.112 Other medications such as opiates and nonsteroidal antiinflammatory drugs have been employed but do not seem to be as effective and are suboptimal for long-term use.115,117
If pain control is not achieved through medical management, then surgical interventions might provide pain relief. Spinal cord stimulation may be effective for treating pain in fairly extensive regions of the body, such as an upper or lower extremity. Spinal nerve root stimulation, which targets pain relief to one or more spinal dermatomes, may be more appropriate for more limited distributions of pain, where more extensive coverage is not needed. Peripheral nerve stimulation targets pain relief to the distribution of a single peripheral nerve, and it may be most appropriate for localized nerve distribution pain.118
Burchiel K.J., Johans T.J., Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78(5):714-719.
Carvalho G.A., Nikkhah G., Matthies C., et al. Diagnosis of root avulsions in traumatic brachial plexus injuries: value of computerized tomography myelography and magnetic resonance imaging. J Neurosurg. 1997;86(1):69-76.
De Ruiter G.C., Malessy M.J., Yaszemski M.J., et al. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26(2):E5.
Du R., Auguste K.I., Chin C.T., et al. Magnetic resonance neurography for the evaluation of peripheral nerve, brachial plexus, and nerve root disorders. J Neurosurg. 2010;112(2):362-371.
Huang J.H., Cullen D.K., Browne K.D., et al. Long-term survival and integration of transplanted engineered nervous tissue constructs promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15(7):1677-1685.
Gonzalez A.A., Jeyanandarajan D., Hansen C., et al. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27(4):E6.
Grant G.A., Goodkin R., Kliot M. Evaluation and surgical management of peripheral nerve problems. Neurosurgery. 1999;44(4):825-839. discussion 839-840
Hsu E.S. Practical management of complex regional pain syndrome. Am J Ther. 2009;16(2):147-154.
Kelleher M.O., Tan G., Sarjeant R., et al. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: a prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8(3):215-221.
Kline D.G., Hudson A.R. Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. Philadelphia: Saunders; 1995.
Kline D.G. Surgical repair of peripheral nerve injury. Muscle Nerve. 1990;13(9):843-852.
Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16(5):E5.
Millesi H. Reappraisal of nerve repair. Surg Clin North Am. 1981;61(2):321-340.
Oberle J.W., Antoniadis G., Rath S.A., et al. Value of nerve action potentials in the surgical management of traumatic nerve lesions. Neurosurgery. 1997;41(6):1337-1342. discussion 1342-1344
Spinner R.J., Kline D.G. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000;23(5):680-695.
Stuart R.M., Winfree C.J. Neurostimulation techniques for painful peripheral nerve disorders. Neurosurg Clin North Am. 2009;20(1):111-120. vii-viii
Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74(4):491-516.
Tiel R.L., Happel L.T.Jr., Kline D.G. Nerve action potential recording method and equipment. Neurosurgery. 1996;39(1):103-108. discussion 108-109
Turner J.W., Parsonage M.J. Neuralgic amyotrophy (paralytic brachial neuritis); with special reference to prognosis. Lancet. 1957;273(6988):209-212.
Winfree C.J., Kline D.G. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63(1):5-18. discussion 18
Yee T. Recurrent idiopathic lumbosacral plexopathy. Muscle Nerve. 2000;23(9):1439-1442.
1. Seddon H. Three types of nerve injury. Brain. 1943;66:237-288.
2. Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74(4):491-516.
3. Castaldo J.E., Ochoa J.L. Mechanical injury of peripheral nerves. Fine structure and dysfunction. Clin Plast Surg. 1984;11(1):9-16.
4. Roberts J.T. The effect of occlusive arterial diseases of the extremities on the blood supply of nerves: experimental and clinical studies on the role of the vasa nervorum. Am Heart J. 1948;35(3):369-392.
5. Bentley F.H., Schlapp W. Experiments on the blood supply of nerves. J Physiol. 1943;102(1):62-71.
6. Bentley F.H., Schlapp W. The effects of pressure on conduction in peripheral nerve. J Physiol. 1943;102(1):72-82.
7. Kline D.G., Hackett E.R., May P.R. Evaluation of nerve injuries by evoked potentials and electromyography. J Neurosurg. 1969;31(2):128-136.
8. Brown B.A. Internal neurolysis in traumatic peripheral nerve lesions in continuity. Surg Clin North Am. 1972;52(5):1167-1175.
9. Grant G.A., Goodkin R., Kliot M. Evaluation and surgical management of peripheral nerve problems. Neurosurgery. 1999;44(4):825-839. discussion 839-840
10. Howard F.M.Jr. Electromyography and conduction studies in peripheral nerve injuries. Surg Clin North Am. 1972;52(5):1343-1352.
11. Sunderland S. Funicular suture and funicular exclusion in the repair of severed nerves. Br J Surg. 1953;40(164):580-587.
12. Henderson W.R. Clinical assessment of peripheral nerve injuries: Tinel’s test. Lancet. 1948;2(6534):801-805.
13. Sunderland S. Nerve injuries and their repair: a critical appraisal. Edinburgh: Churchill Livingstone; 1991.
14. Kline D.G. Surgical repair of peripheral nerve injury. Muscle Nerve. 1990;13(9):843-852.
15. Raimbault J. Electrical assessment of muscle denervation. Clin Plast Surg. 1984;11(1):53-58.
16. Ross J.S., Robertson J.T., Frederickson R.C., et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38(4):855-861. discussion 861-863
17. Cudlip S.A., Howe F.A., Griffiths J.R., et al. Magnetic resonance neurography of peripheral nerve following experimental crush injury, and correlation with functional deficit. J Neurosurg. 2002;96(4):755-759.
18. Publicover N., Terzis J.K. Experimental electrophysiologic recordings. Interpretation of the compound action potential. Clin Plast Surg. 1984;11(1):39-45.
19. Ochs S. Waller’s concept of the trophic dependence of the nerve fiber on the cell body in the light of early neuron theory. Clio Med. 1975;10(4):253-265.
20. Carvalho G.A., Nikkhah G., Matthies C., et al. Diagnosis of root avulsions in traumatic brachial plexus injuries: value of computerized tomography myelography and magnetic resonance imaging. J Neurosurg. 1997;86(1):69-76.
21. Cracco R.Q. Evaluation of conduction in central motor pathways: techniques, pathophysiology, and clinical interpretation. Neurosurgery. 1987;20(1):199-203.
22. Kewalramani L.S., Taylor R.G. Brachial plexus root avulsion: role of myelography—review of diagnostic procedures. J Trauma. 1975;15(7):603-608.
23. Lee W.Y., Hou W.Y., Yang L.H., et al. Intraoperative monitoring of motor function by magnetic motor evoked potentials. Neurosurgery. 1995;36(3):493-500.
24. Morota N., Deletis V., Constantini S., et al. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery. 1997;41(6):1327-1336.
25. Gonzalez A.A., Jeyanandarajan D., Hansen C., et al. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27(4):E6.
26. Dailey A.T., Tsuruda J.S., Filler A.G., et al. Magnetic resonance neurography of peripheral nerve degeneration and regeneration. Lancet. 1997;350(9086):1221-1222.
27. Fehlings M.G., Houldon D., Vajkoczy P. Introduction. Intraoperative neuromonitoring: an essential component of the neurosurgical and spinal armamentarium. Neurosurg Focus. 2009;27(4):E1.
28. Kelleher M.O., Tan G., Sarjeant R., et al. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: a prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8(3):215-221.
29. Jones S.J. Diagnostic value of peripheral and spinal somatosensory evoked potentials in traction lesions of the brachial plexus. Clin Plast Surg. 1984;11(1):167-172.
30. Kline D.G., Hackett E.R., Happel L.H. Surgery for lesions of the brachial plexus. Arch Neurol. 1986;43(2):170-181.
31. Synek V.M., Cowan J.C. Somatosensory evoked potentials in patients with supraclavicular brachial plexus injuries. Neurology. 1982;32(12):1347-1352.
32. Dinner D.S., Luders H., Lesser R.P., et al. Intraoperative spinal somatosensory evoked potential monitoring. J Neurosurg. 1986;65(6):807-814.
33. Donner T.R., Voorhies R.M., Kline D.G. Neural sheath tumors of major nerves. J Neurosurg. 1994;81(3):362-373.
34. Kline D.G., Judice D.J. Operative management of selected brachial plexus lesions. J Neurosurg. 1983;58(5):631-649.
35. Filler A.G., Howe F.A., Hayes C.E., et al. Magnetic resonance neurography. Lancet. 1993;341(8846):659-661.
36. Powers S.K., Norman D., Edwards M.S. Computerized tomography of peripheral nerve lesions. J Neurosurg. 1983;59(1):131-136.
37. Sundaram M., McGuire M.H., Herbold D.R. Magnetic resonance imaging of soft tissue masses: an evaluation of fifty-three histologically proven tumors. Magn Reson Imaging. 1988;6(3):237-248.
38. Kuntz C.T., Blake L., Britz G., et al. Magnetic resonance neurography of peripheral nerve lesions in the lower extremity. Neurosurgery. 1996;39(4):750-756. discussion 756-757
39. West G.A., Haynor D.R., Goodkin R., et al. Magnetic resonance imaging signal changes in denervated muscles after peripheral nerve injury. Neurosurgery. 1994;35(6):1077-1085. discussion 1085-1086
40. Britz G.W., Haynor D.R., Kuntz C., et al. Carpal tunnel syndrome: correlation of magnetic resonance imaging, clinical, electrodiagnostic, and intraoperative findings. Neurosurgery. 1995;37(6):1097-1103.
41. Britz G.W., Haynor D.R., Kuntz C., et al. Ulnar nerve entrapment at the elbow: correlation of magnetic resonance imaging, clinical, electrodiagnostic, and intraoperative findings. Neurosurgery. 1996;38(3):458-465. discussion 465
42. Grant G.A., Britz G.W., Goodkin R., et al. The utility of magnetic resonance imaging in evaluating peripheral nerve disorders. Muscle Nerve. 2002;25(3):314-331.
43. Dailey A.T., Tsuruda J.S., Goodkin R., et al. Magnetic resonance neurography for cervical radiculopathy: a preliminary report. Neurosurgery. 1996;38(3):488-492. discussion 492
44. Filler A.G., Kliot M., Howe F.A., et al. Application of magnetic resonance neurography in the evaluation of patients with peripheral nerve pathology. J Neurosurg. 1996;85(2):299-309.
45. Du R., Auguste K.I., Chin C.T., et al. Magnetic resonance neurography for the evaluation of peripheral nerve, brachial plexus, and nerve root disorders. J Neurosurg. 2010;112(2):362-371.
46. Millesi H. Reappraisal of nerve repair. Surg Clin North Am. 1981;61(2):321-340.
47. Oberle J.W., Antoniadis G., Rath S.A., et al. Value of nerve action potentials in the surgical management of traumatic nerve lesions. Neurosurgery. 1997;41(6):1337-1342. discussion 1342-1344
48. Marmor L. Nerve grafting in peripheral nerve. Surg Clin North Am. 1972;52(5):1177-1187.
49. Carlstedt T., Noren G. Repair of ruptured spinal nerve roots in a brachial plexus lesion. Case report. J Neurosurg. 1995;82(4):661-663.
50. Samii M., Carvalho G.A., Nikkhah G., et al. Surgical reconstruction of the musculocutaneous nerve in traumatic brachial plexus injuries. J Neurosurg. 1997;87(6):881-886.
51. Huang J.H., Cullen D.K., Browne K.D., et al. Long-term survival and integration of transplanted engineered nervous tissue constructs promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15(7):1677-1685.
52. De Ruiter G.C., Malessy M.J., Yaszemski M.J., et al. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26(2):E5.
53. Friedman A.H., Nunley J.A.2nd, Goldner R.D., et al. Nerve transposition for the restoration of elbow flexion following brachial plexus avulsion injuries. J Neurosurg. 1990;72(1):59-64.
54. Dolenc V.V. Intercostal neurotization of the peripheral nerves in avulsion plexus injuries. Clin Plast Surg. 1984;11(1):143-147.
55. Brunelli G., Monini L. Neurotization of avulsed roots of brachial plexus by means of anterior nerves of cervical plexus. Clin Plast Surg. 1984;11(1):149-152.
56. Yamada S., Peterson G.W., Soloniuk D.S., et al. Coaptation of the anterior rami of C-3 and C-4 to the upper trunk of the brachial plexus for cervical nerve root avulsion. J Neurosurg. 1991;74(2):171-177.
57. Narakas A.O. Thoughts on neurotization or nerve transfers in irreparable nerve lesions. Clin Plast Surg. 1984;11(1):153-159.
58. Samardzic M., Grujicic D., Antunovic V., et al. Reinnervation of avulsed brachial plexus using the spinal accessory nerve. Surg Neurol. 1990;33:7-11.
59. Malessy M.J., Thomeer R.T. Evaluation of intercostal to musculocutaneous nerve transfer in reconstructive brachial plexus surgery. J Neurosurg. 1998;88(2):266-271.
60. Carlstedt T., Grane P., Hallin R.G., et al. Return of function after spinal cord implantation of avulsed spinal nerve roots. Lancet. 1995;346(8986):1323-1325.
61. Campbell W.W. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119(9):1951-1965.
62. Robinson L.R. Traumatic injury to peripheral nerves. Suppl Clin Neurophysiol. 2004;57:173-186.
63. Spinner R.J., Kline D.G. Surgery for peripheral nerve and brachial plexus injuries or other nerve lesions. Muscle Nerve. 2000;23(5):680-695.
64. Kline D.G., Hudson A.R. Nerve Injuries: Operative Results for Major Nerve Injuries, Entrapments, and Tumors. Philadelphia: Saunders; 1995.
65. Winfree C.J. Peripheral nerve injury evaluation and management. Curr Surg. 2005;62(5):469-476.
66. Winfree C.J., Kline D.G. Intraoperative positioning nerve injuries. Surg Neurol. 2005;63(1):5-18. discussion 18
67. Tiel R.L., Happel L.T.Jr, Kline D.G. Nerve action potential recording method and equipment. Neurosurgery. 1996;39(1):103-108. discussion 108-109
68. Kline D.G. Civilian gunshot wounds to the brachial plexus. J Neurosurg. 1989;70(2):166-174.
69. Narakas A.O. Lesions found when operating traction injuries of the brachial plexus. Clin Neurol Neurosurg. 1993;95(Suppl):S56-S64.
70. Dejerine-Klumpke A. Paralysie radiculaire totale du plexus brachial avec phénomène oculo-pupillaires autopsiée trente-six jours après l’accident. Rev Neurol. 1908;16:637-645.
71. Dubuisson A., Kline D.G. Indications for peripheral nerve and brachial plexus surgery. Neurol Clin. 1992;10(4):935-951.
72. McGillicuddy J.E. Clinical decision making in brachial plexus injuries. Neurosurg Clin North Am. 1991;2(1):137-150.
73. Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16(5):E5.
74. Kim D.H., Cho Y.J., Tiel R.L., et al. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana Staate University Health Sciences Center. J Neurosurg. 2003;98(5):1005-1016.
75. Ruchelsman D.E., Ramos L.E., Alfonso I., et al. Outcome following spinal accessory to suprascapular (spinoscapular) nerve transfer in infants with brachial plexus birth injuries. Hand (N Y), epub ahead of print. 2009.
76. Rohde R.S., Wolfe S.W. Nerve transfers for adult traumatic brachial plexus palsy (brachial plexus nerve transfer). HSS J. 2007;3(1):77-82.
77. Wahegaonkar A.L., Doi K., Hattori Y., et al. Technique of intercostal nerve harvest and transfer for various neurotization procedures in brachial plexus injuries. Tech Hand Up Extrem Surg. 2007;11(3):184-194.
78. El-Gammal T.A., Fathi N.A. Outcomes of surgical treatment of brachial plexus injuries using nerve grafting and nerve transfers. J Reconstr Microsurg. 2002;18(1):7-15.
79. Kawai H., Kawabata H., Masada K., et al. Nerve repairs for traumatic brachial plexus palsy with root avulsion. Clin Orthop Relat Res, 1988;237:75-86
80. Michelow B.J., Clarke H.M., Curtis C.G., et al. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93(4):675-680. discussion 681
81. Waters P.M. Obstetric brachial plexus injuries: evaluation and management. J Am Acad Orthop Surg. 1997;5(4):205-214.
82. Gilbert A., Brockman R., Carlioz H.. Surgical treatment of brachial plexus birth palsy. Clin Orthop Relat Res, 1991;264:39-47
83. Gilbert A., Razaboni R., Amar-Khodja S. Indications and results of brachial plexus surgery in obstetrical palsy. Orthop Clin North Am. 1988;19(1):91-105.
84. Ernberg L.A., Adler R.S., Lane J. Ultrasound in the detection and treatment of a painful stump neuroma. Skeletal Radiol. 2003;32(5):306-309.
85. Stokvis A., Coert J.H., van Neck J.W. Insufficient pain relief after surgical neuroma treatment: prognostic factors and central sensitisation. J Plast Reconstr Aesthet Surg. 2010;63(9):1538-1543.
86. Vernadakis A.J., Koch H., Mackinnon S.E. Management of neuromas. Clin Plast Surg. 2003;30(2):247-268. vii
87. Koch H., Haas F., Hubmer M., et al. Treatment of painful neuroma by resection and nerve stump transplantation into a vein. Ann Plast Surg. 2003;51(1):45-50.
88. Burchiel K.J., Johans T.J., Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78(5):714-719.
89. Pierce S.M., Recht A., Lingos T.I., et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23(5):915-923.
90. Svensson J., Johannson G. Long-term efficacy and safety of somatropin for adult growth hormone deficiency. Treat Endocrinol. 2003;2(2):109-120.
91. Olsen N.K., Pfeiffer P., Johannsen L., et al. Radiation-induced brachial plexopathy: neurological follow-up in 161 recurrence-free breast cancer patients. Int J Radiat Oncol Biol Phys. 1993;26(1):43-49.
92. Schierle C., Winograd J.M. Radiation-induced brachial plexopathy: review. Complication without a cure. J Reconstr Microsurg. 2004;20(2):149-152.
93. Harper C.M.Jr, Thomas J.E., Cascino T.L., et al. Distinction between neoplastic and radiation-induced brachial plexopathy, with emphasis on the role of EMG. Neurology. 1989;39(4):502-506.
94. Gosk J., Rutowski R., Urban M., et al. Brachial plexus injuries after radiotherapy—analysis of 6 cases. Folia Neuropathol. 2007;45(1):31-35.
95. Teixeira M.J., Fonoff E.T., Montenegro M.C. Dorsal root entry zone lesions for treatment of pain-related to radiation-induced plexopathy. Spine (Phila Pa 1976). 2007;32(10):E316-E319.
96. Wong M., Tang A.L., Umapathi T. Partial ulnar nerve transfer to the nerve to the biceps for the treatment of brachial plexopathy in metastatic breast carcinoma: case report. J Hand Surg Am. 2009;34(1):79-82.
97. Turner J.W., Parsonage M.J. Neuralgic amyotrophy (paralytic brachial neuritis); with special reference to prognosis. Lancet. 1957;273(6988):209-212.
98. Parsonage M.J., Turner J.W. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
99. James J.L., Miles D.W. Neuralgic amyotrophy: a clinical and electromyographic study. Br Med J. 1966;2(5521):1042-1043.
100. Griffin A.C., Wood W.G. Brachial plexitis: a rare and often misdiagnosed postoperative complication. Aesthetic Plast Surg. 1996;20(3):263-265.
101. Malamut R.I., Marques W., England J.D., et al. Postsurgical idiopathic brachial neuritis. Muscle Nerve. 1994;17(3):320-324.
102. Weintraub M.I., Chia D.T. Paralytic brachial neuritis after swine flu vaccination. Arch Neurol. 1977;34(8):518.
103. Redmond J.M., Cros D., Martin J.B., et al. Relapsing bilateral brachial plexopathy during pregnancy. Report of a case. Arch Neurol. 1989;46(4):462-464.
104. Planner A.C., Donaghy M., Moore N.R. Causes of lumbosacral plexopathy. Clin Radiol. 2006;61(12):987-995.
105. Ozaki S., Hamabe T., Muro T. Piriformis syndrome resulting from an anomalous relationship between the sciatic nerve and piriformis muscle. Orthopedics. 1999;22(8):771-772.
106. Solheim L.F., Siewers P., Paus B. The piriformis muscle syndrome. Sciatic nerve entrapment treated with section of the piriformis muscle. Acta Orthop Scand. 1981;52(1):73-75.
107. Kerimoglu U., Canyigit M. Paradoxic hypertrophy of the sciatic nerve in adult patients after above-knee amputation. Acta Radiol. 2007;48(9):1028-1031.
108. Hill S.C., Baker A.R., Barton N.W., et al. Sciatic nerve: paradoxic hypertrophy after amputation in young patients. Radiology. 1997;205(2):559-562.
109. Yee T. Recurrent idiopathic lumbosacral plexopathy. Muscle Nerve. 2000;23(9):1439-1442.
110. Evans B.A., Stevens J.C., Dyck P.J. Lumbosacral plexus neuropathy. Neurology. 1981;31(10):1327-1330.
111. Hsu E.S. Practical management of complex regional pain syndrome. Am J Ther. 2009;16(2):147-154.
112. Gilron I., Bailey J.M., Tu D., et al. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374(9697):1252-1261.
113. Stacey B.R., Dworkin R.H., Murphy K., et al. Pregabalin in the treatment of refractory neuropathic pain: results of a 15-month open-label trial. Pain Med. 2008;9(8):1202-1208.
114. Silver M., Blum D., Grainger J., et al. Double-blind, placebo-controlled trial of lamotrigine in combination with other medications for neuropathic pain. J Pain Symptom Manage. 2007;34(4):446-454.
115. Sindrup S.H., Otto M., Finnerup N.B., et al. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005;96(6):399-409.
116. Stacey B.R., Barrett J.A., Whalen E., et al. Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain. 2008;9(11):1006-1017.
117. Moise G., Capodice J.L., Winfree C.J. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother. 2007;7(5):507-520.
118. Stuart R.M., Winfree C.J. Neurostimulation techniques for painful peripheral nerve disorders. Neurosurg Clin North Am. 2009;20(1):111-120. vii-viii