Perioperative sonographic monitoring in cardiovascular surgery
Overview
Despite the well-known advances in cardiovascular surgery over the last 50 years, it remains a high-risk procedure associated with significant morbidity and mortality rates. Currently, patients undergoing cardiovascular operations tend to be older and have more comorbid conditions. This could be attributed to the progress in surgical procedures, as well as to improved preoperative, perioperative, and postoperative care, which includes hemodynamic optimization by implementation of goal-directed therapies and the use of β-blockade in selected patients. Recent guidelines have recommended the use of ultrasound for detection of perioperative complications and for hemodynamic management.1,2 This chapter discusses the use of echocardiography for the evaluation of patients after cardiovascular surgery. Because image quality is usually poor with transthoracic echocardiography, transesophageal echocardiography (TEE) has been used routinely in the intensive care unit (ICU) for the detection of functional and structural cardiovascular abnormalities postoperatively.
Hypovolemia
Hypovolemia is the most frequent hemodynamic alteration after cardiovascular surgery. Even though bleeding is easily assessed by checking the various drains, it is sometimes overlooked because of clotting of drains or accumulation of blood in nonadequately drained cavities (e.g., pleural space). Notably, even when bleeding is detected, volume correction can be insufficient, and it is worth mentioning that cardiopulmonary bypass often provokes capillary leak syndrome secondary to a systemic inflammatory response. Postoperatively, patients may have hypotension or signs of tissue hypoperfusion necessitating close hemodynamic monitoring in the ICU. Whenever possible, fluid responsiveness should be assessed with dynamic indices (Chapter 38). Evaluation of variations in aortic flow with respiration is the most reliable method. Assessment of fluid responsiveness based on respiratory variations in the superior and inferior vena cava is less reliable because of direct compression of the vessel by blood and clots in the mediastinum (superior vena cava) or because of increased pericardial pressure (both vessels). Finally, systolic or diastolic left ventricular (LV) dysfunction (preexisting or related to cardiopulmonary bypass) may result in intolerance of fluid therapy and thus should be carefully evaluated.
Left ventricular dysfunction
LV dysfunction is the second most frequent hemodynamic alteration after cardiovascular surgery and can be attributed to preexisting LV dysfunction or to ischemic events or stunning as a result of cardiopulmonary bypass.3 Preoperative evaluation of ventricular function is fundamental in these patients since it facilitates postoperative identification of a new segmental ventricular wall abnormality, which should prompt discussion of coronary angiography and reperfusion strategies. In addition, demonstration of impaired contractility does not inevitably constitute an indication for the use of inotropic agents, which are indicated only when impaired contractility is associated with an inadequate cardiac output contributing to the impaired tissue perfusion. Indeed, the patient is often hypothermic and metabolic needs are low during and just after surgery. Therefore low cardiac output in isolation should not be treated. Measurements of LV ejection fraction should be used in combination with cardiac output measurements (measured as the aortic flow velocity-time integral) and markers of tissue hypoperfusion (blood lactate levels, oliguria). Finally, right ventricular (RV) dysfunction may also occur, especially after selected procedures such as correction of mitral valve stenosis or pulmonary thromboembolectomy (Chapter 33).4
Pericardial effusion and localized tamponade
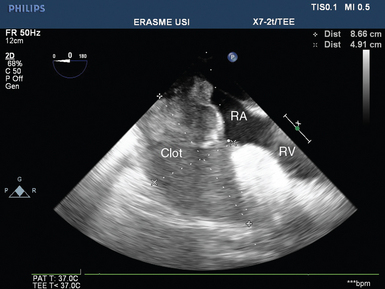
Blood and clots may accumulate in the pericardial space even with the use of drains. Stagnation of blood in the pericardium facilitates the development of clots, which in turn may lead to drain occlusion and global tamponade by enabling continuous accumulation of blood. Localized tamponade may also occur as a result of the development of large clots compressing selected cardiac structures. In most cases these large clots compress the right cardiac cavities and especially the right atrium (Figure 40-1). In the latter clinical scenario, patients typically have severe hypovolemia that responds partially to fluid therapy, and ultrasound detects a very small right atrium and dilatation of the superior and inferior vena cava (increased central venous pressure). Rarely, clots may selectively compress the left atrium, which results in low cardiac output with some evidence of hypovolemia (usually a small left ventricle) and a very small left atrium with occasional pulmonary edema (often predominant on the left side) because of compression of the pulmonary veins (as confirmed by detection of very high color and pulsed wave Doppler velocity [>100 cm/sec] in the ipsilateral pulmonary veins).
Dynamic obstruction of the left ventricular outflow tract
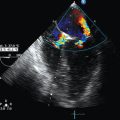
Dynamic LV outflow tract (LVOT) obstruction could be attributed to a Venturi effect in the context of excessive adrenergic (endogenous or exogenous) stimulation of a usually well-contractile hypovolemic left ventricle.5 LVOT obstruction is commonly associated with LV concentric or localized septal hypertrophy, a mitral-aortic angle smaller than 120 degrees, and excess tissue in the mitral valve.5,6 Sometimes it may also occur in the context of mitral valvuloplasty with a flail or redundant subvalvular appendix. As a result of the Venturi effect, the anterior leaflet of the mitral valve is aspirated into the LVOT during systole (systolic anterior motion of the mitral valve leaflet), which causes partial obstruction (Figure 40-2A) and generates a high pressure gradient between the left ventricle and the aorta; this in turn results in a marked decrease in stroke volume. Mitral valve regurgitation may occur as a result of absent mitral valve leaflet coaptation during systole, with an eccentric regurgitant jet being observed in the direction of the left pulmonary veins (Figure 40-2B).
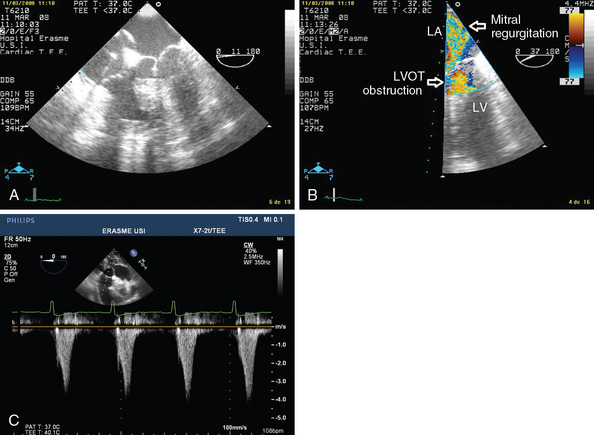
Figure 40-2 Dynamic left ventricular outflow tract (LVOT) obstruction. A, Two-dimensional image showing aspiration of the mitral valve leaflet into the LVOT during systole. B, Color Doppler view showing high velocity in the LVOT and a mitral valve regurgitation jet (LA, left atrium; LV, left ventricle). C, Pulsed wave Doppler showing the typical dagger-shaped pattern.
Identification of dynamic LVOT obstruction should be performed in several steps with TEE. Using different views, color Doppler techniques allow identification of the turbulent flow associated with LVOT obstruction. The midesophageal longitudinal and transverse views are used to evaluate motion of the anterior mitral valve leaflet, which moves anteriorly and partially obstructs the LVOT during systole (a good electrocardiographic signal is mandatory) (Figure 40-2A). This view is also useful for detecting premature closure of the aortic valve, but admittedly this sign is not specific because it is only a marker of low stroke volume. Via a deep transgastric view, pulsed wave Doppler should be applied from the midseptal area of the left ventricle to the LVOT (up to the aortic valve) in an effort to identify the location of the obstruction. Pulsed wave Doppler usually shows a brisk increase in velocity at the LVOT entrance. Continuous wave Doppler should also be used, in the absence of significant aortic valve disease, to better identify the typical dagger-shaped pattern (Figure 40-2C). Mitral valve regurgitation occurs frequently, and this pattern should not be confounded with mitral valve disease.
Once LVOT obstruction is identified, use of adrenergic (especially inotropic) agents should be discontinued. Fluids should be administered cautiously since these patients often have LV diastolic dysfunction. The fluid challenge technique should be applied in small aliquots (100 to 200 mL), and fluid administration should be stopped when stroke volume fails to increase or when fluids are not tolerated because of increments in filling pressure or an exacerbation of mitral valve regurgitation. β-Blockers, often proposed in the cardiology suite, are difficult to use in shocked patients. The cause of the LVOT obstruction should be identified promptly to guide therapy. For example, if the LVOT obstruction is attributed to excess mitral valve tissue, surgical reintervention should be considered when medical therapy fails. Valvular dysfunction (mostly regurgitation) may occur after valve repair or replacement. It is beyond the scope of this chapter to analyze this subject in detail; however, it is recommended that an expert echocardiographer evaluate valvular structure and function before the end of the surgical procedure.1,2,7 Occasionally, valvular dysfunction may develop as a late complication.
Aortic dissection
Aortic cannulation and irruption of the cardiopulmonary bypass directly into the ascending aorta occasionally leads to iatrogenic aortic type A dissection (Chapter 8). After abdominal aortic surgery, thoracic aortic dissection may also occur as a result of the acute increase in blood pressure from aortic cross-clamping. These complications are rare and occur in less than 0.01% of all cardiac surgeries. Nevertheless, the perioperative echocardiographic examination should always include visualization of the aorta.8
Evaluation of intraaortic balloon pump position and the pleural space
Despite the fact that the pleural space is usually opened during cardiac surgery and drained via mediastinal drains, accumulation of fluid and pneumothorax may occur postoperatively. Evaluation of a patient with hemodynamic instability or respiratory failure after cardiac surgery should include assessment of the pleura by means of ultrasound (Chapter 20).
Pearls and highlights
• TEE is the preferred imaging modality for hemodynamic monitoring, as well as for structural and functional cardiac examination, after cardiovascular surgery.
• Preoperative structural and functional evaluation of the heart is fundamental in patients undergoing cardiovascular surgery.
• Hypovolemia, LV and RV dysfunction, and cardiac tamponade are the most common findings in the early postoperative period.
• Localized cardiac tamponade may occur after cardiac surgery, with selective compression of the right atrium being the most frequent finding.
• Dynamic LVOT obstruction typically occurs in hypovolemic patients with a hypertrophic left ventricle when treated with adrenergic agents. Typical echocardiographic features include turbulent flow in the LVOT, anterior septal movement of the mitral valve in systole, and a dagger-shaped pattern on pulsed or continuous wave Doppler.
• The perioperative echocardiographic examination should include evaluation of the aorta and pleural space.
• When an IABP is used, correct position of the balloon should be assessed by echocardiography.
• Evaluation of cardiac filling pressure and function should not be based on atrial size (markedly enlarged) following cardiac transplantation.
References
1. Flachskampf, FA, Badano, L, Daniel, WG, et al, Recommendations for transoesophageal echocardiography: update 2010. Eur J Echocardiog. 2010; 11:557–576.
2. Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010; 112:1084–1096.
3. De Hert, SG, Rodrigus, IE, Haenen, LR, et al. Recovery of systolic and diastolic left ventricular function early after cardiopulmonary bypass. Anesthesiology. 1996; 85:1063–1075.
4. Diller, GP, Wasan, BS, Kyriacou, A, et al. Effect of coronary artery bypass surgery on myocardial function as assessed by tissue Doppler echocardiography. Eur J Cardiothorac Surg. 2008; 34:995–999.
5. Mingo, S, Benedicto, A, Jimenez, MC, et al. Dynamic left ventricular outflow tract obstruction secondary to catecholamine excess in a normal ventricle. Int J Cardiol. 2006; 112:393–396.
6. Mihaileanu, S, Marino, JP, Chauvaud, S, et al. Left ventricular outflow obstruction after mitral valve repair (Carpentier’s technique). Proposed mechanisms of disease. Circulation. 1988; 78:I78–I84.
7. Quigley, RL. The role of echocardiography in mitral valve dysfunction after repair. Minerva Cardioangiol. 2007; 55:239–246.
8. Leontyev, S, Borger, MA, Legare, JF, et al, Iatrogenic type A aortic dissection during cardiac procedures: early and late outcome in 48 patients. Eur J Cardiothorac Sur. 2012; 41:641–646.