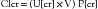Chapter 182 Perioperative Management
General Conditions Affecting Surgical Risk
Age
Infants and young children have a relatively low tolerance for infection, trauma, blood loss, and nutritional and fluid disturbances.1 The management of these disorders in infants and children differs from their treatment in adults. Particular aspects of surgical care deserving special attention include fluid and electrolyte management, nutrition, and temperature maintenance.1,2
Advanced age is an independent risk factor for postoperative morbidity, and the prevalence of coexisting medical problems increases with age.3 As a result, elderly patients who undergo spine procedures have higher rates of postoperative complications, including excessive bleeding, postoperative confusion, and urinary tract infections.4 They tend to recuperate more slowly than do their younger counterparts. A recent study has shown that age is a positive risk factor for postoperative complications in multilevel thoracolumbar spine fusion surgery. Furthermore, it is correlative with higher rates of reoperation.5
It is safe to consider every patient older than 65 years of age to be at high risk for generalized atherosclerosis and for potential limitation of myocardial and renal reserve. Elderly patients often develop cardiac failure if they are fluid overloaded. Close monitoring of vital signs, intake, output, body weight, and serum electrolytes is mandatory. Elderly patients generally require smaller doses of narcotics, sedatives, and anesthetics than do younger patients. Barbiturates, sedatives, and steroids may cause confusion, and narcotics can produce respiratory depression.6
Finally, advanced age is an important independent risk factor for postoperative deep venous thrombosis and pulmonary embolism, which are major causes of morbidity and mortality after surgical procedures.
Obesity
Obesity increases the technical difficulty of surgery and anesthesia. Obese surgical patients have a greater incidence of serious concomitant disease and higher rates of postoperative wound breakdown, thromboembolic complications, and pulmonary disease. Early patient mobilization, aggressive pulmonary toilet, and appropriate prophylaxis against deep venous thrombosis are all necessary adjuncts to good perioperative care. Occasionally, it may be advisable to delay elective surgery until the patient loses weight by appropriate dietary measures. There are increasing data to suggest that minimally invasive techniques, including tubular surgery, diminish the risks incurred by obesity.7
Coagulation Abnormalities
Spine procedures may result in substantial blood loss. Excessive bleeding at the surgical site increases the chances of wound infection and impaired wound healing. Although the prevalence of underlying coagulopathies is no higher in patients undergoing spine surgery, many regularly take nonsteroidal anti-inflammatory drugs (NSAIDs). Because NSAIDs are reversible inhibitors of platelet aggregation, their use can increase postoperative bleeding. NSAIDs should be discontinued at least 1 week before a major spine procedure. NSAIDs have also been shown to inhibit the healing of spine fusions. In patients undergoing spine fusion, NSAIDs should be avoided in the postoperative period as well.8,9
Malnutrition
Increasing evidence suggests that many surgical patients are moderately to severely malnourished.10 The increased metabolic demands of patients undergoing or recovering from spine surgery are often unmet because of insufficient caloric intake. With inadequate caloric intake, the hypercatabolic state induced by trauma or surgery results in significant visceral and skeletal protein depletion. Malnourished patients have increased rates of mortality and morbidity from sepsis, wound complications, impaired healing, and protracted rehabilitation. Although no single test demonstrates malnutrition conclusively, a variety of laboratory studies and physical measurements can help reveal nutritional inadequacies.11 These include preoperative weight loss of more than 10 pounds, a serum albumin level of less than 3.5 g/dL, and a total lymphocyte count less than 1500 to 2000 cells/μL.12
If malnutrition is identified, a vigorous regimen of nutritional supplementation should begin, preferably before surgery, and the patient should be monitored throughout the perioperative period.11 Total or peripheral parenteral nutrition should be considered in patients who either cannot tolerate or cannot meet their caloric needs with enteral nutrition alone.13–16
Smoking
The harmful effects of smoking tobacco on the rate of postoperative pulmonary, cardiac, and thromboembolic complications are well known. In a recent study of 875 patients undergoing orthopaedic reconstructive surgery, the incidence of cardiopulmonary complications in smokers was double that of nonsmokers. In that study, smoking was identified as the single most important risk factor for the development of complications after elective hip or knee arthroplasty. Similarly increased rates of pulmonary complications were identified in a multicenter review of 400 patients undergoing abdominal surgery.17–20
A review of wound infections after dorsal spine operations involving instrumentation implicated smoking as a significant risk factor for development of an infection.21
Wound complications in soft tissue procedures have also been reported to occur much more frequently in smokers. In a study of 425 patients undergoing reconstruction after breast cancer surgery, the risk of skin flap necrosis was nine times as high in heavy smokers as in nonsmokers. Similarly, in a review of patients undergoing face lift, the risk of skin slough was 12.5 times as high in smokers compared with nonsmokers.22,23 In spine surgery, current smoking increases the rate of postoperative wound infection and subsequently increases hospital stay and 30-day mortality rates.24
Stopping smoking before surgery has been shown to decrease the rate of postoperative complications, but only if a significant amount of time elapses between smoking cessation and surgery. A 2-week smoke-free period in a study of 60 patients before colorectal surgery did not decrease the rate of postoperative complications. In contrast, 4 weeks of smoking cessation significantly reduced the rate of wound infections in a randomized, controlled trial of minor dermatologic procedures. In another randomized trial of 120 patients scheduled to undergo hip or knee replacement, enrollment in a smoking cessation program at least 6 weeks before surgery resulted in a substantial decrease in complication rates. The rate of wound complications decreased from 31% to 5%, and of cardiovascular complications from 10% to 0%.25–27
In addition to the aforementioned general postoperative problems, tobacco smoking has been associated with unique complications after spine surgery. Smoking was identified as a significant risk factor in the development of postoperative airway obstruction after cervical corpectomy. In a review of 133 patients undergoing cervical corpectomy, 6 of 7 patients who developed airway obstruction were smokers. Two of these patients died as a result of this complication. Other risk factors were myelopathy and multilevel surgery. In patients with these risk factors, the authors recommend delayed extubation and careful assessment of the airway for swelling before extubation.28
Smoking has been shown to inhibit the healing of spine fusions in many clinical reviews. This effect has been well documented in patients undergoing spine fusion surgery and in animal models. In a randomly selected retrospective study of 50 smokers and 50 nonsmokers undergoing uninstrumented lumbar dorsolateral fusion, the pseudarthrosis rate was 40% for smokers and 8% for nonsmokers. A corresponding diminution in resting oxygen saturation was identified. The authors theorized that this relative hypoxia was responsible for the failure of the arthrodesis to heal.29
Animal studies, however, have shown that the inhibition of fusion can be attributed directly to the pharmacologic effects of systemically administered nicotine, without hypoxia.30 Inhibition of bone graft vascularization has been shown in animal models. Cytokine expression is decreased, suggesting that the inhibitory effects of nicotine involve more than just local vasoconstriction.31,32
Smoking has been demonstrated to be a risk factor for nonunion after thoracic and cervical fusion surgery as well. In a review of 90 patients undergoing ventral instrumented spine fusion for adolescent scoliosis, 4 of 5 patients who developed a nonunion were smokers.33
A retrospective review of 131 patients who had multilevel cervical discectomies and fusions with autogenous interbody graft without instrumentation found a pseudarthrosis rate of 50% in smokers compared with 24% in nonsmokers.34
In animal models, bone morphogenetic protein was effective in reversing the inhibitory effects of nicotine on spine fusion.35
In some clinical series, the use of instrumentation and electrical stimulation has been reported to help overcome the inhibitory effects of smoking on spine fusion.36,37
Two studies have assessed whether the negative effects on fusion can be reversed by smoking cessation, with conflicting results. Glassman et al.9 reported that whereas smokers had a nonunion rate of 26.5% after lumbar fusion surgery, those who stopped smoking for more than 6 months after surgery had a successful arthrodesis rate of 82.9%. This was not significantly different than the union rate of 85.8% in nonsmokers. However, Deguchi et al.8 found no improvement in arthrodesis rate in their patients who stopped smoking.
Infection
Urinary tract infections are frequent in patients undergoing spine surgery. When associated with bacteremia, these infections are of particular concern because of the possibility of bacterial seeding of hardware. Thus, it is important to identify and treat established urinary tract infections before spinal instrumentation is applied.38
Multiple Trauma
Many spine surgery patients are the victims of severe accidents and have sustained multiple concomitant traumatic injuries. In such cases, urgent repair of spine injuries almost always takes precedence over assessment and treatment of chronic medical problems because acute spine stabilization dramatically reduces mortality, incidence of acute respiratory distress syndrome, length of hospital stay, and need for mechanical ventilation. Nonetheless, it is important to realize that the severity of neurologic injury, number of comorbidities, and use of high-dose steroids increase the risk of complication after thoracolumbar spine fracutrues.39
Rheumatologic Conditions
Patients with rheumatoid arthritis undergo a variety of spine procedures, particularly cervical fusion. These patients experience high complication rates for all surgical procedures. Wound breakdown, infection, loosening of instrumentation, and pseudarthrosis occur more frequently in patients with rheumatoid arthritis and can be attributed to poor tissue integrity, compromised vascular status, and the use of immunosuppressive drugs.40,41
Patients with rheumatoid arthritis should be examined for cervical spine involvement. Dynamic flexion and extension radiographs should be obtained to exclude occult instability before the administration of general endotracheal anesthesia. A variety of other problems, including anemia, pulmonary fibrosis, and pleural effusions, may be present. Chronic steroid use often results in adrenal suppression, requiring administration of perioperative stress-dose steroids. Similarly, patients with systemic lupus erythematosus and other rheumatologic conditions require thorough preoperative evaluation and may prove difficult to manage.42
Nonsurgical Diseases Affecting Surgical Risk
Cardiac Disease
Preoperative Evaluation
The most common symptoms of heart disease are dyspnea, fatigue, chest pain, and palpitation. It is important to inquire about exercise tolerance, paroxysmal nocturnal dyspnea, orthopnea, peripheral edema, irregular heartbeat, and chest pain. One should document significant past illnesses such as congenital heart disease, rheumatic fever, MI, atherosclerotic cerebrovascular and peripheral vascular disease, diabetes mellitus, hypertension, and autoimmune disease. Also, use of cardiac pacemakers, previous cardiac surgery, and past or present use of diuretics, digitalis, coronary vasodilators, antihypertensive drugs, and antiarrhythmic drugs should be noted. A history of angina pectoris, MI, Adams-Stokes attacks, stroke, cerebral ischemic attacks, intermittent claudication, or previous treatment for heart disease or hypertension should alert the surgeon to the possibility of a cardiac abnormality requiring further evaluation.
Cardiac Contraindications
In hypertensive patients the blood pressure should be normalized before surgery. There is a linear correlation between preoperative blood pressure and postoperative myocardial ischemia.34
Postoperative Management
Most postoperative MIs occur on the second or third postoperative day, and hence serial ECGs for 3 days are indicated in patients with known coronary artery disease. Chest pain is often difficult to evaluate in the postoperative period, and an MI may become apparent only because of hypotension or arrhythmia. Serial cardiac isoenzyme studies are especially useful for identifying postoperative infarcts, and should be obtained as well. Postoperative treatment for MI entails vigorous support and monitoring. Arrhythmias and cardiac failure should be treated as they arise.
Pulmonary Disease
Preoperative Evaluation
Laboratory Examination
Yellow, green, or brown sputum suggests active infection. Sputum culture is indicated to identify specific organisms and to determine antibiotic sensitivities. Arterial blood gas measurements help evaluate pulmonary gas exchange. The arterial partial pressure of oxygen (Pao2) is an indicator of oxygen uptake by the blood in its passage through the lungs. Pao2 is affected by the fraction of inspired oxygen, right-to-left shunting, and diffusion capacity across the alveolocapillary membrane. Normally, Pao2 is 70 mm Hg or greater. A Pao2 of 60 mm Hg indicates mild respiratory failure; a Pao2 of 50 mm Hg or less indicates severe pulmonary disease. The oxygen saturation (Sao2) reflects the percentage of hemoglobin actually bound by oxygen. Normally, Sao2 is 93% or more; an Sao2 of 90% indicates mild respiratory failure; an Sao2 of 84% or less indicates severe pulmonary disease. The arterial partial pressure of carbon dioxide (Paco2) reflects the adequacy of ventilation. Normally, Paco2 is 38 to 43 mm Hg. A Paco2 of 44 to 54 mm Hg indicates mild impairment of ventilation; a Paco2 of 55 mm Hg or greater indicates severely impaired ventilation. Arterial blood pH is affected by both metabolic and respiratory factors. Normally, the arterial blood pH is 7.38 to 7.42.
Pulmonary Complications
Pneumothorax is an uncommon complication in elective surgical procedures, but it should be considered in any patient who develops acute respiratory distress or intraoperative deterioration. The principal cause of pneumothorax in hospitalized patients is iatrogenic lung puncture during percutaneous central venous catheter placement. It can also occur in a patient who coughs and thereby ruptures a pulmonary bleb or bulla. Diagnosis is made on the basis of decreased or absent breath sounds on the affected side, with hyperresonance to percussion. When a tension pneumothorax develops there may also be a lateral shift of the trachea away from the affected side. Any patient who develops respiratory distress after insertion of a central venous catheter should be presumed to have a pneumothorax, and a chest tube should be inserted immediately and not delayed for the radiograph. If pneumothorax is suspected but the patient is comfortable, one should first obtain the chest radiograph and insert a chest tube if indicated.
Pulmonary Embolism
Pulmonary embolism accompanied by circulatory and respiratory instability mandates treatment with high-dose IV heparin. Placement of a Greenfield filter or ligation of the inferior vena cava may be required if anticoagulants are contraindicated, if bleeding complications develop in a patient receiving anticoagulants, or if pulmonary embolism recurs in a fully anticoagulated patient.
Renal Disease
Preoperative Evaluation
Laboratory Examination
where U[cr] = urine creatinine concentration in mg/dL, P[cr] = serum creatinine concentration in mg/dL, and V = urine volume in mL/min. Normal creatinine clearance is 125 ± 25 mL/min/1.73 m2. A minimum clearance of 10 mL/min/1.73 m2 is needed to maintain life without dialysis. Serum creatinine level may remain normal until the clearance is reduced by more than half.
Preoperative Preparation
Anemia is a frequent finding in patients with renal disease and should be evaluated preoperatively. A hemoglobin level of 9 g/dL and a hematocrit of 25% are satisfactory for patients with chronic renal insufficiency. Patients on hemodialysis adapt to hematocrits in the range of 20% and do not need transfusions unless there is significant blood loss. Blood transfusion should be used cautiously to avoid cardiac decompensation.
Postoperative Management
Acute Renal Failure
Measurable fluid losses and insensible losses should be replaced. Fluid intake, fluid output, and body weight should be meticulously assessed. In addition, sodium losses from urine or other measurable sources should be replaced. Hyponatremia developing in the course of ATN is usually indicative of fluid excess rather than of sodium deficit and is best treated by fluid restriction. In addition, hyperkalemia frequently occurs in these patients. A variety of factors contribute to hyperkalemia, including acidosis and potassium release from tissues secondary to excessive catabolism, trauma, or hemolysis. The serum potassium concentration and the ECG must be monitored.
Gastrointestinal Complications
Gastric Distention and Dilation
Paralytic ileus is the cessation of effective gastrointestinal motility after trauma, severe illness, or surgery. Ileus is primarily a gastric phenomenon because the remainder of the gut can usually handle fluids earlier than the stomach. Vomiting and abdominal distention are the main manifestations. Decreased bowel sounds are an unreliable finding. Abdominal radiographs show gas in the stomach, small bowel, and colon. Mechanical bowel obstruction must be excluded in such patients. Any patient with ileus should be given bowel rest (given nothing by mouth) and should have a nasogastric tube inserted and left in place until the ileus resolves.
Prophylaxis
Autologous Blood Donation
Autologous blood transfusion avoids most of the complications associated with transfusion of homologous blood, including transmission of disease, hemolytic transfusion reactions, and other immune phenomena. Despite existing medical problems, most patients are able to donate at least two units of autologous blood before surgery. This could potentially enable over 90% of patients to avoid homologous transfusion during major spine procedures. Autologous blood can normally be stored for up to 40 days (longer storage requires an expensive and complex freezing process). One unit of autologous blood can be processed from a given patient every 3 days up to 3 days before surgery, provided the hematocrit remains at least 34%. Iron supplementation is recommended. Treatment with erythropoietin can increase the patient’s hematocrit and allow more blood to be stored in advance of surgery. It can also be used without predonation to minimize the need for transfusion at the time of surgery.43
Prophylaxis for Thromboembolism
The most important prophylaxis issue in patients undergoing spine surgery is the prevention of deep venous thrombosis and pulmonary embolism. The incidence of deep venous thrombosis in spine surgery is not known. Estimates as high as 60% have been made. Prophylaxis for thromboembolism should therefore be considered in all patients undergoing major spine procedures, especially if patients are not immediately ambulatory. Thigh-length sequential compression devices, thromboembolic stockings, and low-dose heparin serve as prophylaxis for deep venous thrombosis in elective spine surgery. Initial studies suggest that low-molecular-weight heparin and heparinoids may be effective and safe in these patients. The benefits of prophylactic anticoagulation must be balanced against the risk. Cain et al.44 reported a high rate of complications due to therapeutic heparinization after pulmonary embolus in spine surgery patients. In a poll of Scoliosis Research Society members representing more than 13,000 thoracic and lumbar fusions, they identified 9 patients who were treated with heparin anticoagulation. Complications attributable to anticoagulation were reported in two thirds of these patients.
Anticoagulation
Depression of platelet function occurs in some patients after 3 to 4 days of heparin therapy. The risk of hemorrhage is greatest in these patients. This complication can often be anticipated, however, because it is usually preceded by a decrease in the platelet count to below 100,000/mm3.
Heparin should be given in adequate doses. The rate of heparin infusion should be adjusted to maintain a partial thromboplastin time that is 1.5 to 2 times the control value. Enoxaparin, a low-molecular-weight heparin, is an alternative that does not require continuous infusion or such careful monitoring and regulation of laboratory values. The usual dose for prophylaxis is 40 mg given subcutaneously once daily. The dose for treatment of deep venous thrombosis is 1 mg/kg body weight given subcutaneously every 12 hours. Special caution is necessary when treating the elderly, those with renal impairment, or patients weighing less than 45 kg.
Postoperative Complications
Nonspecific Complications
Delayed Wound Healing and Dehiscence
Many systemic factors contribute to wound healing failure by altering collagen metabolism or impairing oxygen delivery to the wound. Local and technical problems may cause impaired blood supply or inadequate resistance to mechanical forces. Infection, corticosteroid or cytotoxic drug use, malnutrition, hypovolemia, hypoxemia, increased blood viscosity, tissue irradiation, and errors in technique may all contribute to impaired wound healing.
Postoperative Complications Specific to Spine Surgery
Infection
Spine Surgical Wound Infection
Spangfort45 reviewed more than 10,000 laminectomy cases and reported an operative infection rate of approximately 2.9%. More recent series indicate that preoperative antibiotic prophylaxis may lower the incidence of infection.46,47
In 1980, Ramirez and Thisted48 reported an incidence of infection of 0.3% in an analysis of 28,395 patients who underwent lumbar laminectomy for radiculopathy in the United States.
The clinical and radiographic characteristics of interspace infection were described first by Milward,49 in 1936, after the inadvertent introduction of microorganisms into a disc space during lumbar puncture. Gieseking50 reported the first postoperative interspace infection in 1951. Typically, patients with aseptic necrosis or interspace infection are asymptomatic immediately after surgery but begin to experience excruciating spasms in the lower back, with or without radiation into the legs, within 2 weeks. Typically, the white blood cell count and temperature are normal but the erythrocyte sedimentation rate is elevated, often to more than 100 mm/hr. Lumbosacral radiographs may reveal erosion of the cartilaginous plates as the disease progresses. Needle aspirations of the interspace may reveal the offending organisms but are often negative.51 Patients with a clear-cut syndrome should be placed on IV antibiotics.
Discitis
The incidence of postoperative intervertebral disc space infection (discitis) is 0.75%. Disc space infection rates vary from 0.1% to 3.8%.45,52–55 The higher incidence of disc space infection with microsurgery has been attributed to the presence of the microscope over the open wound.56 Postoperative discitis produces persistent, intense back pain with unremarkable associated physical findings 2 weeks to 3 months after discectomy.
Elevated erythrocyte sedimentation rates are typical. Bone scan, CT, and MRI are quite sensitive for detecting discitis and can identify changes associated with discitis earlier than can plain radiographs. CT is effective in the early diagnosis of discitis, with hypodensity of the affected disc space being detected as early as 10 days after surgery. The responsible bacteria are identified in less than 50% of cases, with Staphylococcus species the most common organisms cultured.51 Early diagnosis and immediate treatment are important for preventing chronic infection. Immobilization is often effective for pain relief, and 4 to 6 weeks of IV antibiotic therapy is recommended. Uncomplicated discitis should not require surgery, and most patients undergo spontaneous interbody fusion. Occasionally, lumbar epidural abscesses may develop that produce paresis. Under these circumstances, immediate decompressive laminectomy is indicated.
Postoperative Osteomyelitis
The typical radiologic changes of vertebral osteomyelitis may not be apparent for months. Radionuclide bone scans are quite sensitive and often demonstrate evidence of infection before plain films of the spine show any changes. However, they are not specific; surgical edema and disc changes may yield false-positive results.57 Furthermore, early in the course of disc space infection, the bone scan may be negative in a significant proportion of patients.58 CT scans may show destructive changes of the vertebral bodies before these are evident on plain films. End-plate irregularities on CT scan, however, are not specific for discitis, and normal curettage changes in vertebral end plates may mimic erosions of discitis.59 MRI may show changes of discitis long before any changes are apparent radiologically.60 It is, however, important to note that MRI and CT studies may be negative early in the course of postoperative or posttraumatic discitis,58 and a high index of suspicion is necessary. Any patient with increasing back pain more than 2 weeks after surgery and an erythrocyte sedimentation rate greater than 50 mm/hr should be considered to have discitis until proven otherwise.60 Percutaneous disc biopsy can be helpful in the diagnosis of postoperative discitis,61 but this often produces false-negative results.
In a study comparing MRI, plain radiographs, and radionuclide studies in the evaluation of vertebral osteomyelitis, MRI was judged to be as accurate and sensitive as combined bone and gallium scanning and more sensitive than plain radiography.60 The MRI appearance of pyogenic infection is characteristic, making MRI a rapid, noninvasive method for the detection of vertebral osteomyelitis and its complications, including epidural abscess. On T1-weighted images, infected disc material shows decreased signal intensity from the intervertebral disc space and contiguous vertebral bodies relative to the normal vertebral signal. On T2-weighted images, these tissues show increased signal. MRI provides more anatomic detail than radionuclide scanning and allows differentiation of neoplasm and degenerative disease from osteomyelitis. The disc space is nearly always spared in neoplastic disease, whereas degenerative disease with nucleus desiccation produces decreased disc signal on T2-weighted images. Gallium scans may show positive results earlier than MRI in the course of infection, and this technique is more sensitive to changes arising from treatment and decreasing inflammation.
In many patients, postoperative discitis and vertebral osteomyelitis resolve spontaneously, and a diagnosis is never made.62 In some patients with postoperative vertebral osteomyelitis, intermittent antibiotic therapy obscures the diagnosis and permits the illness to go undetected for years.63,64 Kern63 reported a patient in whom vertebral osteomyelitis and meningitis became manifest 2.5 years after lumbar spine surgery. The ability of infections to remain dormant and recur after long periods is illustrated by a patient in whom postoperative staphylococcal lumbar vertebral osteomyelitis developed and cleared after 3 years of treatment. Thirty-four years later, after 30 years without symptoms, the patient developed a staphylococcal psoas abscess.64
Epidural Abscess
The importance of early diagnosis of SEA was emphasized by Heusner as early as 1948. Several studies have emphasized the frequently rapid deterioration and substantial permanent morbidity associated with this infection. Despite the recognition of SEA as a potential neurosurgical emergency and the increased sophistication of diagnostic studies, the morbidity and mortality associated with SEA remain significant. Advances in imaging have made the diagnosis of SEA less elusive and the options for therapeutic intervention more rational.65
SEAs are categorized as acute lesions (gross pus in the epidural space), usually with accompanying sepsis, or chronic lesions (granulation tissue in the epidural space) that may persist for months. The clinical presentation of an acute SEA is often stereotypical, but it can be difficult to appreciate in its earliest stages. The classic triad is intense localized back pain, progressive neurologic deficit, and fever. The initial complaint is almost uniformly axial pain. Paresthesias are also very common. Fever or other symptoms of infection are present about 50% of the time. Without treatment, the progression and time course of symptoms beyond this point are astoundingly uniform. Within 3 days, patients generally note radicular symptomatology, followed within 36 hours by weakness. Rapid deterioration to paralysis occurs typically over the next 24 hours. This pattern of symptoms is so uniform that some authors suggest that this establishes the diagnosis until proven otherwise.65
Laboratory findings are often unhelpful. Fever and leukocytosis are useful markers when present but are absent in more than 50% of the cases. Leukocytosis is common in the acute group (average white blood cell count of <16,000/mm3). The erythrocyte sedimentation rate is almost universally elevated but is a nonspecific indicator.
Brooks-Brunn J.A. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111:564-571.
Brown M.D., Seltzer D.G. Perioperative care in lumbar spine surgery. Orthop Clin North Am. 1991;22:353-358.
Forrest J.B., Rehder K., Cahalan M.K., et al. Multicenter study of general anesthesia: III. Predictors of severe perioperative adverse outcomes. Anesthesiology. 1992;76:3-15.
Glassman S.D., Rose S.M., Dimar J.R., et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine (Phila Pa 1976). 1998;23:834-838.
Hilibrand A.S., Fye M.A., Emery S.E., et al. Impact of smoking on the outcome of anterior cervical arthrodesis with interbody or strut-grafting. J Bone Joint Surg [Am]. 2001;83:668-673.
Howell S.J., Hemming A.E., Allman K.G., et al. Predictors of postoperative myocardial ischaemia: the role of intercurrent arterial hypertension and other cardiovascular risk factors. Anaesthesia. 1997;52:107-111.
Lapp M.A., Bridwell K.H., Lenke L.G., et al. Prospective randomization of parenteral hyperalimentation for long fusions with spinal deformity, its effect on complications and recovery from postoperative malnutrition. Spine (Phila Pa 1976). 2001;26:809-817.
Moller A.M., Villebro N., Pedersen T., et al. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359:114-117.
Rubinstein E., Findler G., Amit P., et al. Perioperative prophylactic cephazolin in spinal surgery: a double-blind placebo-controlled trial. J Bone Joint Surg [Br]. 1994;76:99-102.
1. Coran A.G. Perioperative care of the pediatric patient. Surg Annu. 1991;1:31-55.
2. Mammel M., Thompson T.R. Perioperative management of the pediatric patient. Urol Clin North Am. 1983;10:139-148.
3. Miller R.W., Coulthard S.W. Anesthesia for the elderly and debilitated patient. Otolaryngol Clin North Am. 1981;14:715-722.
4. Saleh K.L. The elderly patient in the post anesthesia care unit. Nurs Clin North Am. 1993;28:507-518.
5. Cloyd J.M., Acosta F.L.Jr., Cloyd C., et al. Effects of age on perioperative complications of extensive multilevel thoracolumbar spinal fusion surgery. J Neurosurg Spine. 2010;12:402-408.
6. Brown M.D., Seltzer D.G. Perioperative care in lumbar spine surgery. Orthop Clin North Am. 1991;22:353-358.
7. Tomasino A., Parikh K., Steinberger J., et al. Tubular microsurgery for lumbar discectomies and laminectomies in obese patients: operative results and outcome. Spine (Phila Pa 1976). 2009;34:E664-E672.
8. Deguchi M., Rapoff A.J., Zdeblick T.A. Posterolateral fusion for isthmic spondylolisthesis in adults: analysis of fusion rate and clinical results. J Spinal Disord. 1998;11:459-464.
9. Glassman S.D., Rose S.M., Dimar J.R., et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine (Phila Pa 1976). 1998;23:834-838.
10. Buzby G.P. Perioperative nutritional support. JPEN J Parenter Enteral Nutr. 1990;14(Suppl 5):1975-1995.
11. Ellis L.M., Copeland E.D., Souba W.W. Perioperative nutritional support. Surg Clin North Am. 1991;71:493-507.
12. Klein J.D., Hey L.A., Yu C.S., et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine (Phila Pa 1976). 1996;21:2676-2682.
13. Hu S.S., Fontaine F., Kelly B., et al. Nutritional depletion in staged spinal reconstructive surgery: the effect of total parenteral nutrition. Spine (Phila Pa 1976). 1998;23:1401-1405.
14. Jevsevar D.S., Karlin L.I. The relationship between preoperative nutritional status and complications after an operation for scoliosis in patients who have cerebral palsy. J Bone Joint Surg [Am]. 1993;75:880-884.
15. Lapp M.A., Bridwell K.H., Lenke L.G., et al. Prospective randomization of parenteral hyperalimentation for long fusions with spinal deformity, its effect on complications and recovery from postoperative malnutrition. Spine (Phila Pa 1976). 2001;26:809-817.
16. Mandelbaum B.R., Tolo V.T., McAfee P.C., et al. Nutritional deficiencies after staged anterior and posterior spinal reconstructive surgery. Clin Orthop Relat Res. 1988;234:5-11.
17. Brooks-Brunn J.A. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111:564-571.
18. Forrest J.B., Rehder K., Cahalan M.K., et al. Multicenter study of general anesthesia: III. Predictors of severe perioperative adverse outcomes. Anesthesiology. 1992;76:3-15.
19. Moller A.M., Pedersen T., Villebro N., et al. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg [Br]. 2003;85:178-181.
20. Weis J.C., Betz R.R., Clements D.H.III, et al. Prevalence of perioperative complications after anterior spinal fusion for patients with idiopathic scoliosis. J Spinal Disord. 1997;10:371-375.
21. Thalgott J.S., Cotler H.B., Sasso R.C., et al. Postoperative infections in spinal implants: classification and analysis—a multicenter study. Spine (Phila Pa 1976). 1991;16:981-984.
22. Rees T.D., Liverett D.M., Guy C.L. The effect of cigarette smoking on skin-flap survival in the face lift patient. Plast Reconstr Surg. 1984;73:911-915.
23. Sorensen L.T., Horby J., Friis E., et al. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol. 2003;29:815-820.
24. Veeravagu A., Patil C.G., Lad S.P., et al. Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976). 2009;34:1869-1872.
25. Moller A.M., Villebro N., Pedersen T., et al. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet. 2002;359:114-117.
26. Sorensen L.T., Jorgensen T. Short-term pre-operative smoking cessation intervention does not affect postoperative complications in colorectal surgery: a randomized clinical trial. Int J Colorectal Dis. 2003;5:347-352.
27. Sorensen L.T., Karlsmark T., Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238:1-5.
28. Emery S.E., Smith M.D., Bohlman H.H. Upper-airway obstruction after multilevel cervical corpectomy for myelopathy. J Bone Joint Surg [Am]. 1991;73:544-551.
29. Brown C.W., Orme T.J., Richardson H.D. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine (Phila Pa 1976). 1986;11:942-943.
30. Silcox D.H.III, Daftari T., Boden S.D., et al. The effect of nicotine on spinal fusion. Spine (Phila Pa 1976). 1995;20:1549-1553.
31. Daftari T.K., Whitesides T.E.Jr., Heller J.G., et al. Nicotine on the revascularization of bone graft: an experimental study in rabbits. Spine (Phila Pa 1976). 1994;19:904-911.
32. Theiss S.M., Boden S.D., Hair G., et al. The effect of nicotine on gene expression during spine fusion. Spine (Phila Pa 1976). 2000;25:2588-2594.
33. Sweet F.A., Lenke L.G., Bridwell K.H., et al. Prospective radiographic and clinical outcomes and complications of single solid rod instrumented anterior spinal fusion in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2001;26:1956-1965.
34. Howell S.J., Hemming A.E., Allman K.G., et al. Predictors of postoperative myocardial ischaemia: the role of intercurrent arterial hypertension and other cardiovascular risk factors. Anaesthesia. 1997;52:107-111.
35. Silcox D.H.III, Boden S.D., Schimandle J.H., et al. Reversing the inhibitory effect of nicotine on spinal fusion using an osteoinductive protein extract. Spine (Phila Pa 1976). 1998;23:291-296.
36. Bose B. Anterior cervical instrumentation enhances fusion rates in multilevel reconstruction in smokers. J Spinal Disord. 2001;14:3-9.
37. Mooney V., McDermott K.L., Song J. Effects of smoking and maturation on long-term maintenance of lumbar spinal fusion success. J Spinal Disord. 1999;12:380-385.
38. David T.S., Vrahas M.S. Perioperative lower urinary tract infections and deep sepsis in patients undergoing total joint arthroplasty. J Am Acad Orthop Surg. 2000;8:66-74.
39. Dimar J.R., Fisher C., Vaccaro A.R., et al. Predictors of complications after spinal stabilization of thoracolumbar spine injuries. J Trauma. 2010;69:1497-1500.
40. Sawin P.D., Dickman C.A., Crawford N.R., et al. The effects of dexamethasone on bone fusion in an experimental model of posterolateral lumbar spinal arthrodesis. J Neurosurg. 2001;94(Suppl 1):76-81.
41. Skues M.A., Welchew E.A. Anaesthesia and rheumatoid arthritis. Anaesthesia. 1993;48:989-997.
42. Khanam T. Anaesthetic risks in rheumatoid arthritis. Br J Hosp Med. 1994;52:320-325.
43. Lee J.H., Lee S.H., Oh J.H. Minimal effective dosage of recombinant human erythropoietin in spinal surgery. Clin Orthop Relat Res. 2003;412:71-76.
44. Cain J.E.Jr., Major M.R., Lauerman W.C., et al. The morbidity of heparin therapy after development of pulmonary embolus in patients undergoing thoracolumbar or lumbar spinal fusion. Spine (Phila Pa 1976). 1995;20:1600-1603.
45. Spangfort E. The lumbar disc herniation: a computer-aided analysis of 2,504 operations. Acta Orthop Scand Suppl. 1972;142:1-95.
46. Rubinstein E., Findler G., Amit P., et al. Perioperative prophylactic cephazolin in spinal surgery: a double-blind placebo-controlled trial. J Bone Joint Surg [Br]. 1994;76:99-102.
47. Schnoring M., Brock M. Prophylactic antibiotics in lumbar disc surgery: analysis of 1,030 procedures. Zentralbl Neurochir. 2003;64:24-29.
48. Ramirez L., Thisted R. Complication and demographic characteristics of patients undergoing lumbar discectomy in community hospitals. Neurosurgery. 1989;25:226-231.
49. Milward F. Changes in the intervertebral discs following lumbar puncture. Lancet. 1936;2:183-185.
50. Gieseking H. Lokalisierte spondylitis nach operiertem Bandscheibenvorfall. Zentralbl Chir. 1951;76:1470-1477.
51. Pilgaard S., Aarhus N. Discitis following removal of lumbar intervertebral disc. J Bone Joint Surg [Am]. 1969;51:713-716.
52. Dauch W. Infection of the intervertebral space following conventional and microsurgical operation on the herniated lumbar intervertebral disc. Acta Neurochir (Wien). 1986;82:43-49.
53. Hudgins W. The role of microdiscectomy. Orthop Clin North Am. 1983;14:589-603.
54. Mayfield F. Complications of laminectomy. Clin Neurosurg. 1976;23:435-439.
55. Roberts M. Complications of lumbar disc surgery. Spinal Surg. 1988;2:13-19.
56. McCulloch J. Principles of microsurgery for lumbar disc disease. Philadelphia: Lippincott-Raven; 1989.
57. Fraser R., Ostio, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg [Br]. 1987;69:26-35.
58. Guyer R.D., Collier R.R., Ohnmeiss D.D., et al. Extraosseous spinal lesions mimicking disc disease. Spine (Phila Pa 1976). 1988;13:328-331.
59. Kopecky K., Gilmor R.L., Scott J.A., et al. Pitfalls of computed tomography in diagnosis of discitis. Neuroradiology. 1985;27:57-66.
60. Bircher M.D., Tasker T., Crawshaw C., Mulholland R.C. Discitis following lumbar surgery. Spine (Phila Pa 1976). 1988;13:98-102.
61. Fouquet B., Goupille P., Jattiot F. Discitis after lumbar disc surgery. Spine (Phila Pa 1976). 1992;17:356-358.
62. Scherbel A., Gardner W. Infections involving the intervertebral discs: diagnosis and management. JAMA. 1960;174:370-374.
63. Kern C. Delayed death following disc surgery. Texas State Med J. 1954;50:158-160.
64. Mansour A., Nabos J., Taddonio R. Psoas abscess: thirty-four years after pyogenic osteomyelitis of the spine. Orthopedics. 1979;2:262-264.
65. Rigamonti D., Liem L., Sampath P., et al. Spinal epidural abscess. Surg Neurol. 1999;52:189-196.