Perioperative corticosteroids
In the past, the solution to suppression of the HPA axis was to administer corticosteroids in sufficient quantities to match what the adrenal glands would have released when maximally stimulated. Because even 2 weeks of corticosteroid use within the previous 3 months has been found to inhibit the HPA axis, recommendations were developed that such patients should receive exogenous corticosteroids when they were undergoing major operations. For these criteria to have been met, the dose of corticosteroids that the patient should have taken would have had to have been high enough to suppress the HPA axis—20 mg of prednisone or its equivalent (Table 234-1). However, because the dose and duration of the use of corticosteroids varied so much, it was not always clear which patients should receive perioperative corticosteroids. Some patients using topical, ophthalmic, or inhaled corticosteroids were found to have suppressed HPA axes.
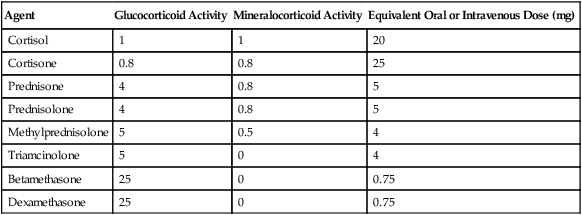
Table 234-1
Glucocorticosteroid Equivalencies
| Agent | Glucocorticoid Activity | Mineralocorticoid Activity | Equivalent Oral or Intravenous Dose (mg) |
| Cortisol | 1 | 1 | 20 |
| Cortisone | 0.8 | 0.8 | 25 |
| Prednisone | 4 | 0.8 | 5 |
| Prednisolone | 4 | 0.8 | 5 |
| Methylprednisolone | 5 | 0.5 | 4 |
| Triamcinolone | 5 | 0 | 4 |
| Betamethasone | 25 | 0 | 0.75 |
| Dexamethasone | 25 | 0 | 0.75 |
Treatment of adrenal suppression
Patients currently taking corticosteroids should take their regular dose of corticosteroids the morning of surgery and should then receive 100 mg of hydrocortisone or its equivalent over the next 24 h (Table 234-2). If dexamethasone is chosen, 10 mg before surgery may be adequate to protect the patient because of its potency and duration. These recommendations are the same if the patient is not currently taking corticosteroids but has taken an HPA axis–suppressive dose for 14 days in the previous 3 months.
Table 234-2
Recommended Perioperative Steroid Coverage for Patients Currently Taking Steroids or Who Have Taken Steroids in the Previous 3 Months
| Current Daily Dose of Steroid (mg) | Type of Operation | Perioperative Hydrocortisone Coverage |
| <10 | Any | No additional coverage needed* |
| ≥10 | Minor | 25 mg at induction |
| ≥10 | Moderate | Usual preoperative dose + 25 mg at induction + 100 mg over the 24 h after surgery |
| ≥10 | Major | Usual preoperative dose + 25 mg at induction + 100 mg/24 h for 48-72 h after surgery |
*Assume normal hypothalamic-pituitary-adrenal axis response.
Treatment of postoperative nausea and vomiting
Postoperative nausea and vomiting (PONV) can occur in up to 33% of patients undergoing anesthesia and surgery (see Chapter 109), depending on the patient’s sex, age, and history (smoking, motion sickness, previous PONV) and type of operation the patient has undergone. As in many other situations, an ounce of prevention is worth a pound of cure. Typically, 5-HT3-receptor blocking agents—because of onset and duration of action—are administered to patients at the end of a surgical procedure, with prochlorperazine and other related compounds used for rescue therapy. In years past, droperidol was administered prophylactically at the beginning of surgical procedures because of its efficacy and duration of action. However, when the U.S. Food and Drug Administration required a boxed warning on the label of droperidol in 2001 regarding the development of torsades de pointes in patients with long QT syndrome who received an intravenously administered bolus of more than 1.25 mg of droperidol, the use of droperidol fell out of favor. Some clinicians and departments of anesthesia continue to use droperidol, albeit in smaller doses (0.625-1.2 mg) for prophylaxis of PONV, but others have changed to the use of corticosteroids. Dexamethasone has been found to have the most efficacy—usually at a dose of 4 to 10 mg administered intravenously while the patient is still in the preoperative holding area prior to the patient’s transport to the operating room.
Adverse effects associated with the use of corticosteroids
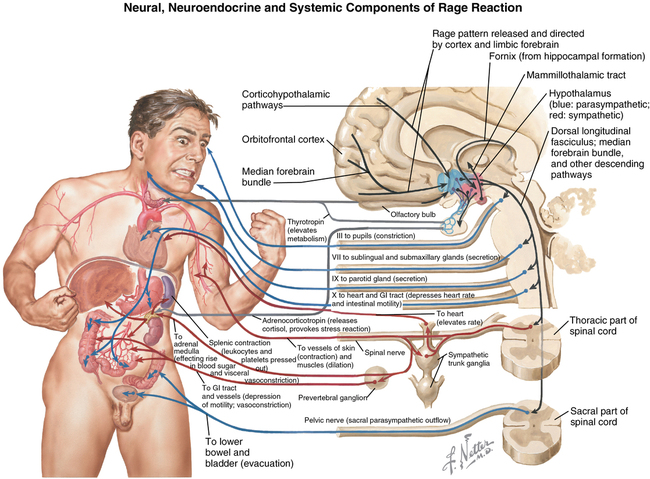
Corticosteroids have multiple actions (Table 234-3) that highlight their role in maintaining homeostasis during a stress of sufficient magnitude to activate the fight-or-flight response (Figure 234-1). The sequelae of corticosteroid use are not so much adverse effects as they are the primary effects associated with the corticosteroids in general. However, for the purpose of this discussion, we consider these effects to be “adverse effects” when one or two doses of corticosteroids are administered for the treatment or prevention of PONV or edema.
Table 234-3
| System | Effect(s) |
| Immune | Up-regulate the expression of antiinflammatory proteins Down-regulate the expression of proinflammatory proteins |
| Metabolic | Stimulate gluconeogenesis Metabolize amino acids from extrahepatic tissue Inhibit glucose uptake in muscle and adipose tissue Stimulate fat breakdown in adipose tissue |
| Fetal development | Stimulate lung maturation |
| Arousal and cognition | Enhance memory, vigilance, and cognition |
| Fluid homeostasis | Normalize extracellular fluid volume: Inhibit dehydration-induced water intake Induce diuresis |