Chapter 9 Pericardial and Myocardial Disease
PERICARDIAL DISEASES
Anatomy
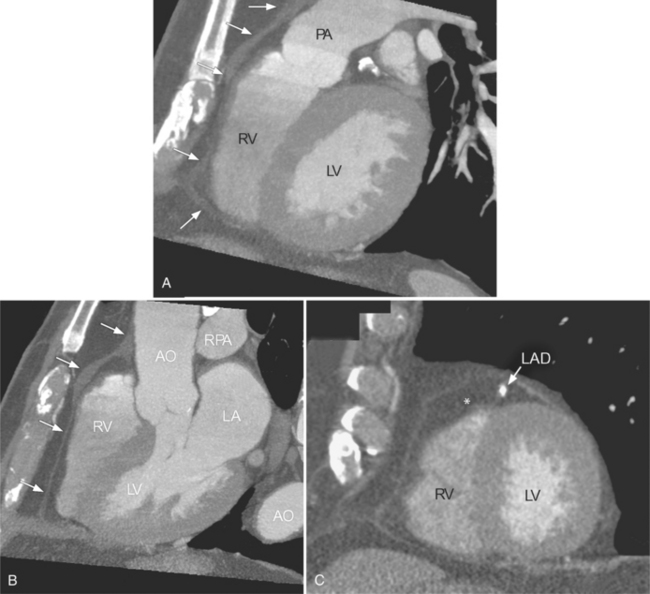
The inner visceral layer of the pericardium, also called serous pericardium or epicardium, is directly attached to the heart and gives a glistening appearance to the heart. The parietal pericardium is attached to the surrounding mediastinum, anteriorly to the superior pericardial sternal ligament, inferiorly to the central tendon of the diaphragm, and posteriorly to the esophagus and descending thoracic aorta. The fat outside the parietal pericardium is called pericardial fat. It is mediastinal fat that is adjacent to the pericardium. The fat inside the visceral pericardium is called epicardial fat, and it is directly attached to the cardiac chambers. The coronary arteries run within the epicardial fat. The visceral pericardium covers both, coronary arteries and epicardial fat. The pericardium does not cover only the heart but also extends about 3 cm into the root of the aorta and pulmonary artery where the visceral and parietal pericardium come together to form the pericardial reflection (Figure 9-1).
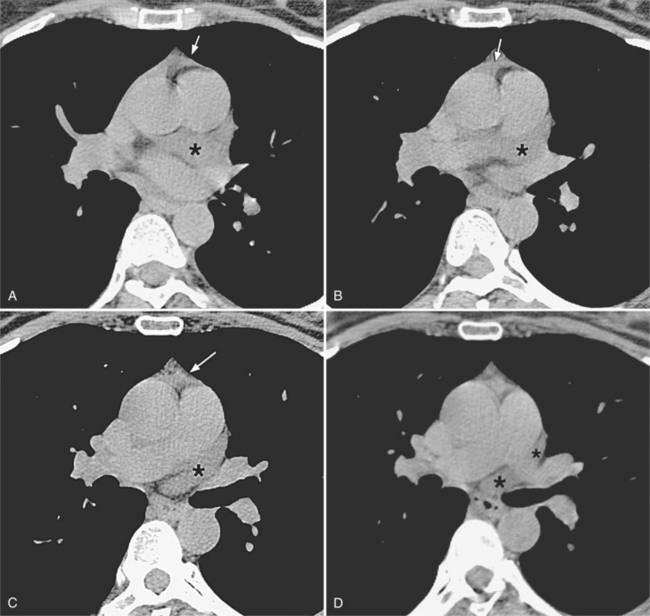
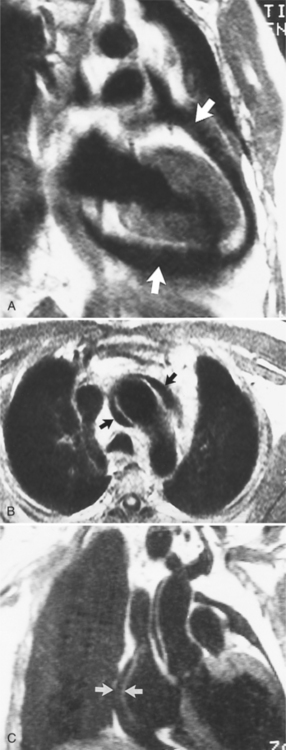
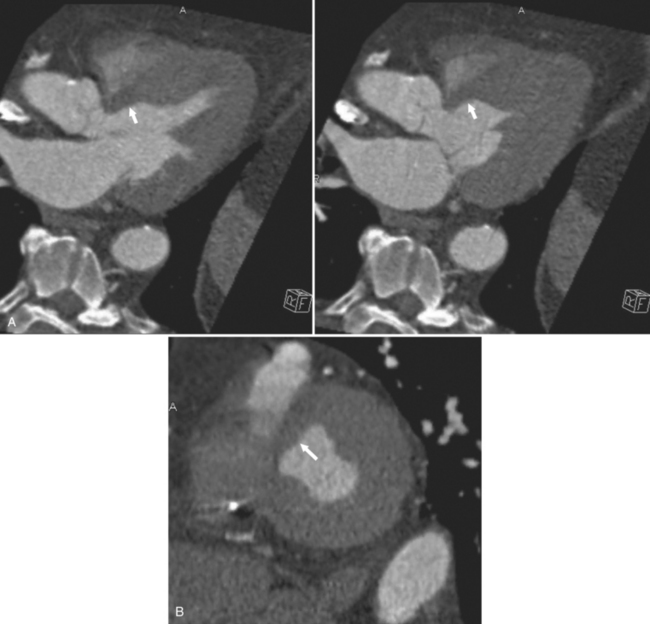
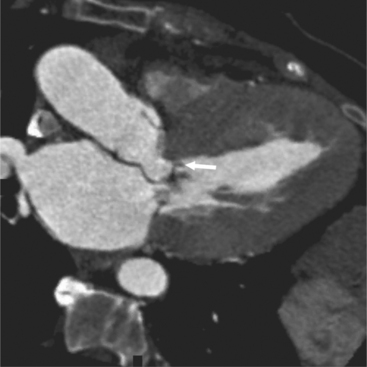
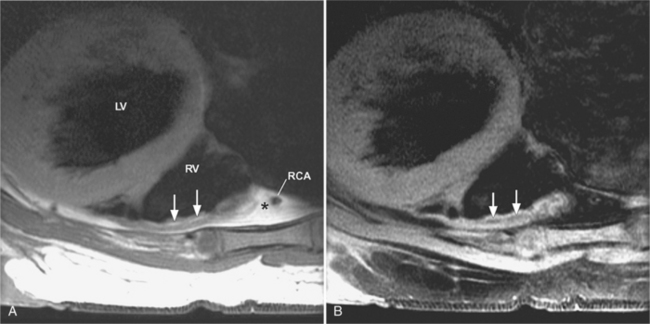
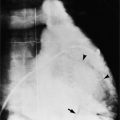
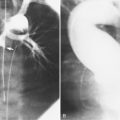
There are a few spaces inside the pericardial cavity in which fluid tends to accumulate and through which surgeons could stick their fingers or pull bypasses if they choose to do so—and sometimes they do. The superior pericardial recess is such a space. It connects the right side and the left side of the pericardial space between the root of the ascending aorta and the right main pulmonary artery (Figure 9-2). Sometimes fluid that has accumulated there may mimic aortic dissection, a thickened aortic wall from arteritis or enlarged lymph nodes. On rare occasions, a surgeon may opt to pull a venous bypass graft through the superior pericardial recess, if the graft is too short to be placed in the typical position anterior to the pulmonary artery (Figure 9-3).
Pericardial Effusion
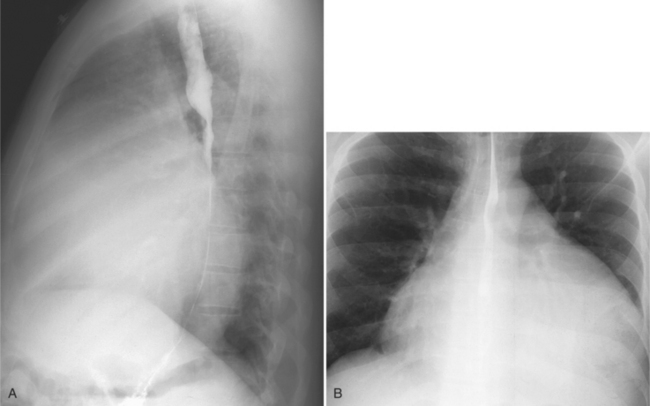
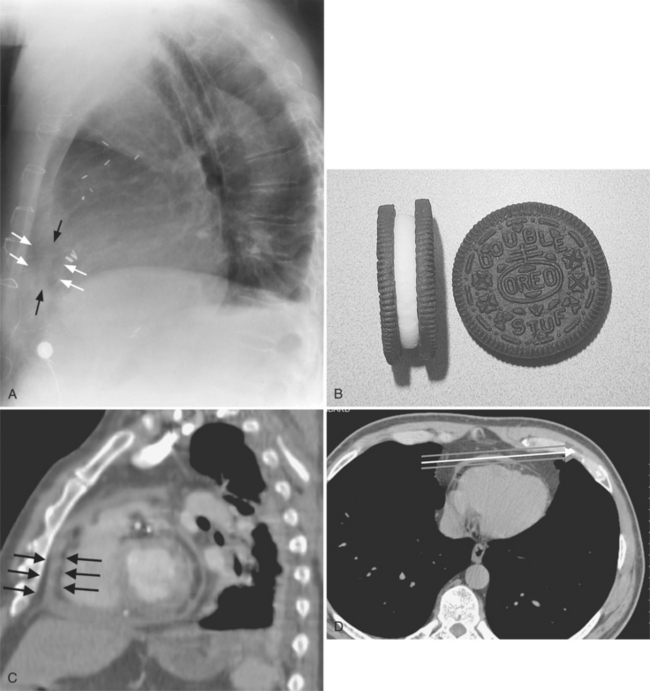
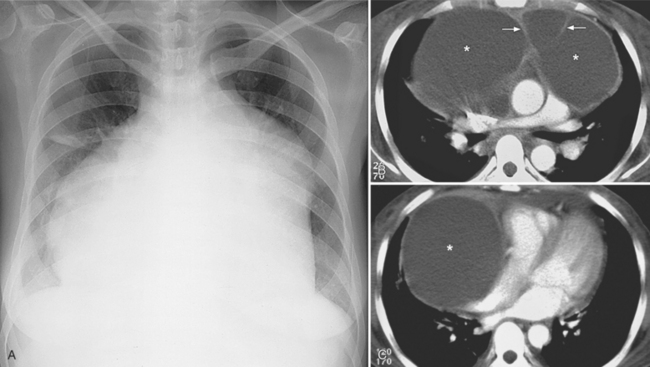
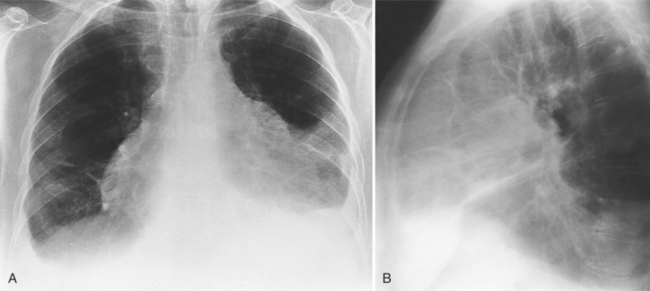
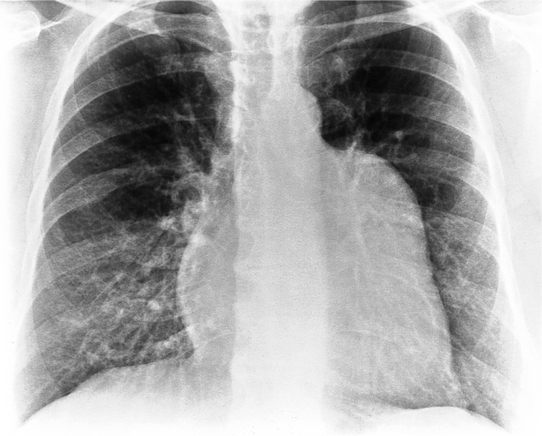
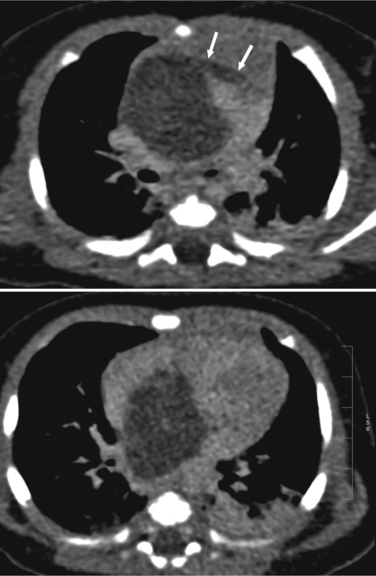
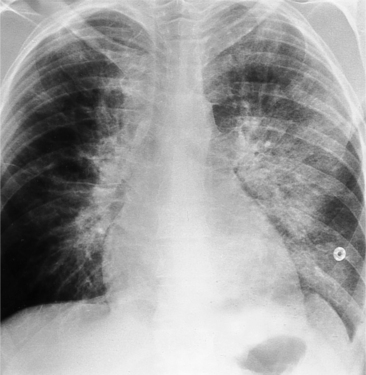
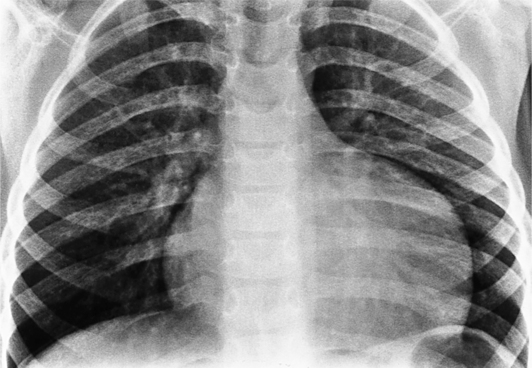
The normal pericardial space in the adult can be distended with 150 to 250 ml of fluid acutely before cardiac tamponade results. Cardiac tamponade is caused by excess fluid in the pericardial space, which compresses the heart and thus causes a low cardiac-output state. In tamponade, the cardiac size on the chest radiograph is slightly to markedly increased. The heart may have a water-bottle appearance in which both sides are rounded and displaced laterally (Figure 9-4). The differential diagnostic considerations for a water-bottle heart are global cardiomegaly, large anterior mediastinal mass, or pericardial effusion. If you are lucky, you may see the Oreo™ cookie sign on the lateral chest x-ray (Figure 9-5). In this sign, a radiolucent stripe behind the sternum (pericardial fat), then a more radiopaque stripe (pericardial effusion), followed by yet another radiolucent stripe (epicardial fat) will be noticed.
Appearance of Effusion on Computed Tomography and Magnetic Resonance Imaging
In MRI on spin echo sequences, pericardial effusions are of low signal intensity, which in part is from low protein content and from the motion of the fluid, which causes phase dispersion (Figure 9-6). Although T2-weighted images demonstrate high signal intensities for fluid in other areas of the body, they are not useful for characterizing pericardial fluid because the motion of the fluid causes signal loss. Gradient echo images depict any fluid in motion with high signal; therefore, pericardial effusion will be bright. Phase contrast images allow for quantification of flow and can be helpful in the characterization of pericardial fluid. The appearance of pericardial hematomas depends heavily on the type of hemoglobin present (e.g., oxyhemoglobin, deoxyhemoglobin, and methemoglobin).
Pericardial Effusion Syndromes
Infection, Collagen Disease, Metabolic Disease, and Tumors
Many infectious and metabolic diseases, tumors, radiation, drug reactions, and collagen disorders, such as systemic lupus erythematosus and scleroderma, typically cause small pericardial effusions. Uremic pericarditis occurs in about 50% of patients with chronic renal failure and is an indication for dialysis. Most effusions do not lead to cardiac tamponade. Common diseases that form pericardial effusions are listed in Box 9-1. Infectious agents that cause pericarditis with resultant effusions are usually coxsackievirus group B and echovirus type 8. Tuberculous pericarditis is uncommon except in patients with acquired immune deficiency syndrome (AIDS).
Although many bacterial, viral, or fungal agents can cause pericarditis, the most common organisms are Staphylococcus, Haemophilus influenzae, and Neisseria meningitidis (Figure 9-7). In addition to a hematogenous source, pericardial infections result from extension from a myocardial abscess related to infective endocarditis, from mediastinal abscess caused by fistula, and from carcinoma of the lung and the esophagus. A loculated pericardial fluid can represent hematoma, abscess, or lymphocele or may be secondary to fibrous adhesions from previous pericarditis. Loculated pericardial effusions can appear similar to pericardial cysts. Neoplastic pericardial effusions are usually related to systemic metastatic disease. The pericardium demonstrates nodular thickening with enhancement of the nodules. Infiltration of the epicardial or pericardial fat, myocardium, or adjacent vascular structures may be seen (Figure 9-8).
Myocardial Infarction (Dressler Syndrome)
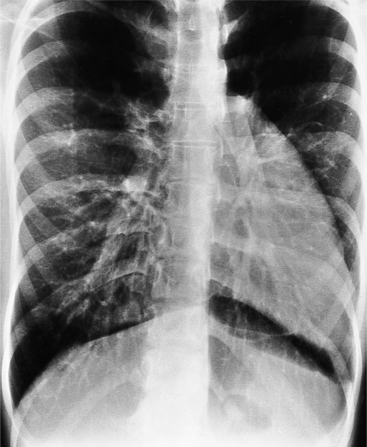
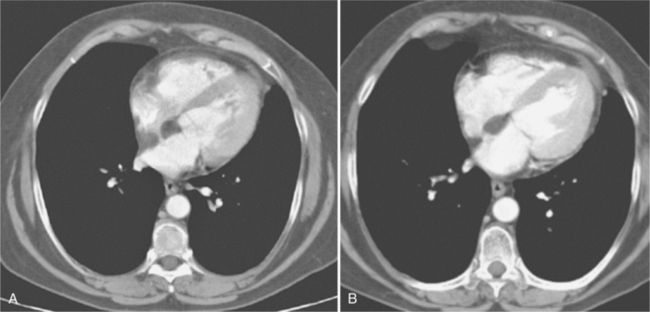
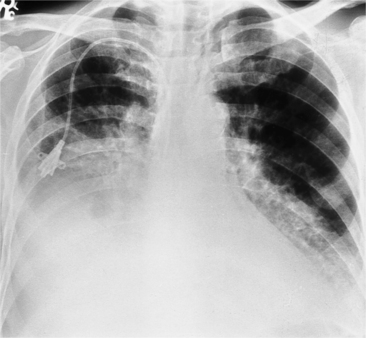
The most common cause of pericardial effusion is myocardial infarction with left ventricular failure. An increase in either right or left heart pressure may also cause a pericardial effusion. About 5% of patients with acute myocardial infarction develop a pericardial effusion. Dressler syndrome is the development of pericardial and pleural effusions 2 to 10 weeks after a myocardial infarction (Figure 9-9). These effusions may be hemorrhagic and can result in cardiac tamponade, particularly if the patients have been given anticoagulant medication.
Constrictive Pericarditis
Constrictive pericarditis is caused by adhesions between the visceral and parietal layers of the pericardium. It occurs after pericarditis from any etiology but is more frequently ascribed to viral or tuberculous pericarditis, uremia with pericardial effusion, and after cardiac surgery. Dense fibrous tissue covers the outer surface of the heart, obliterates the pericardial space, and causes the thickening of the pericardial contour as seen on magnetic resonance and CT. Later calcification may occur. The fibrous adhesions prevent the valve plane from moving toward the cardiac apex in systole and therefore restricts diastolic filling of the heart. Effusive-constrictive pericarditis is a disease in which hemodynamic signs of constriction remain after a pericardial effusion has been aspirated (Table 9-1).
| Modality | Sign |
|---|---|
| Chest x-ray | Eggshell calcification of pericardium |
| Computed tomography | Thickened pericardium |
| Pericardial calcifications | |
| Magnetic resonance | Thickened pericardial contour imaging (>4 mm) in the absence of freeflowing pericardial effusion |
| Septal bounce on cine magnetic resonance images | |
| Pericardial adhesions proven by tagged cine magnetic resonance imaging | |
| Enhancing pericardium |
Calcifications
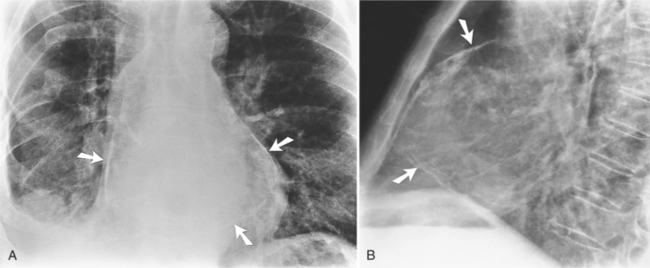
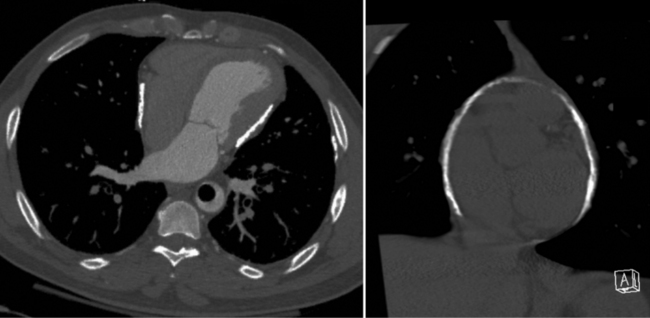
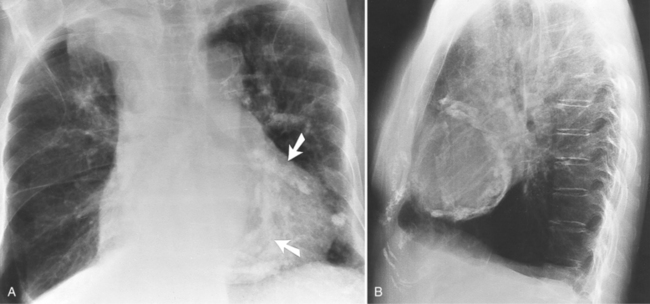
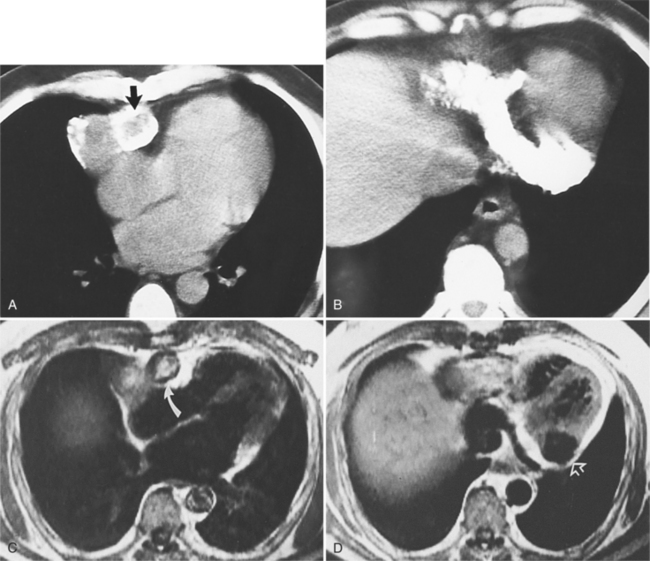
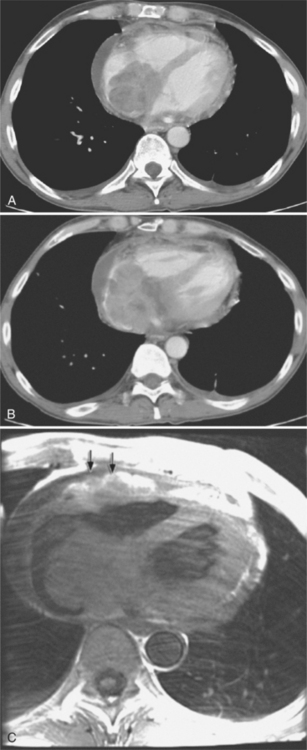
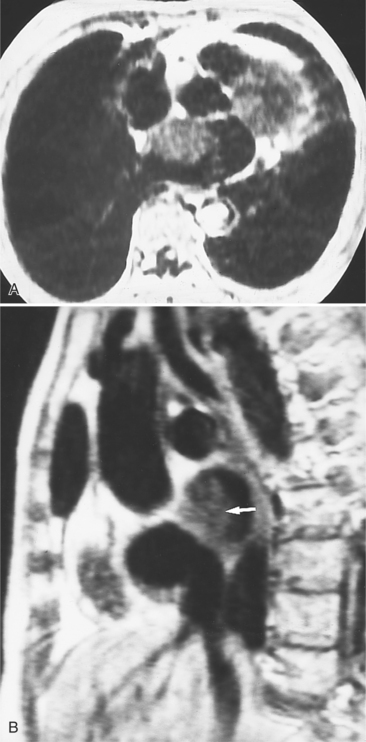
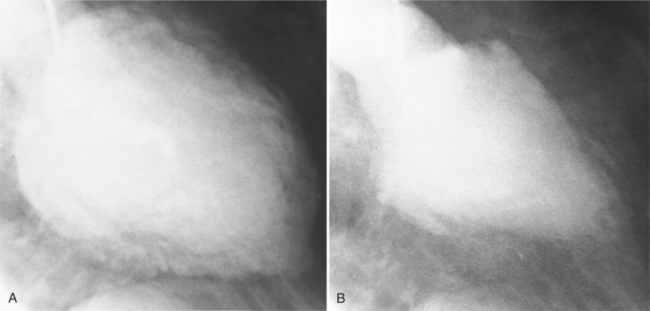
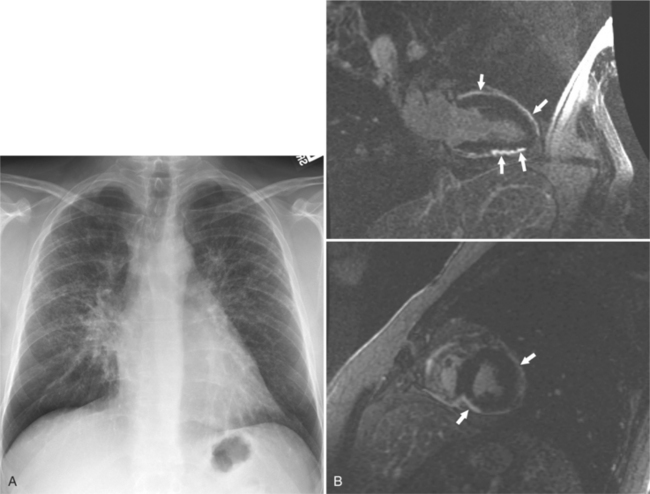
On the chest radiograph or CT, constrictive pericarditis may be suggested by the presence of pericardial calcification. The calcium may be quite thin and linear and appear as “eggshell calcification” around the margins of the heart (Figures 9-10, 9-11). Care must be taken to differentiate this pattern from the calcifications within the myocardium in old infarcts. The etiology of the pericardial calcifications in constriction is speculative, but it is seen mainly after viral and uremic pericarditis. A second type of pericardial calcification is a shaggy, thick, and amorphous deposition, which historically was rather specific for tuberculosis (Figures 9-12, 9-13). The calcium is particularly obvious in regions of the heart in which normal fat is found, namely in the atrioventricular grooves. Calcium in the atrioventricular region may indent the heart focally, producing “extrinsic” tricuspid and mitral stenoses. However, a calcified pericardium does not necessarily imply that constriction exists.
Comparison with Restrictive Cardiomyopathy
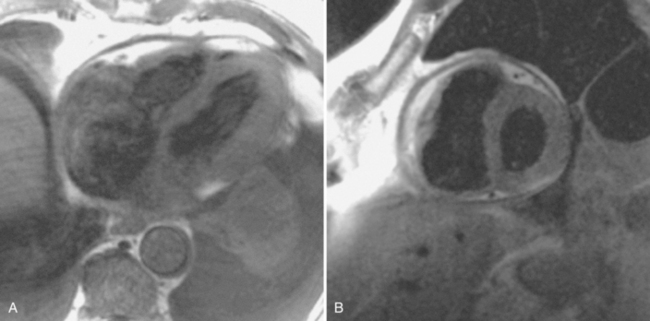
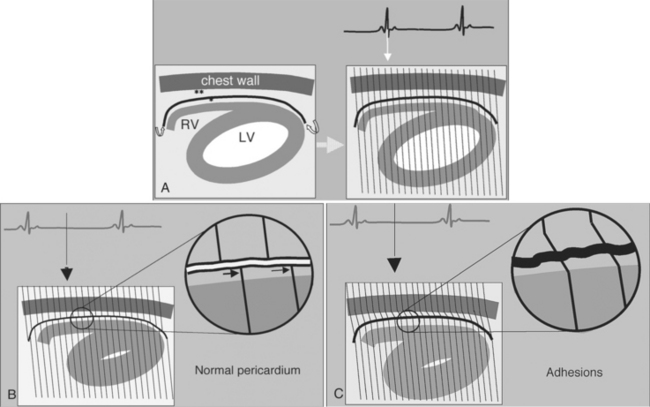
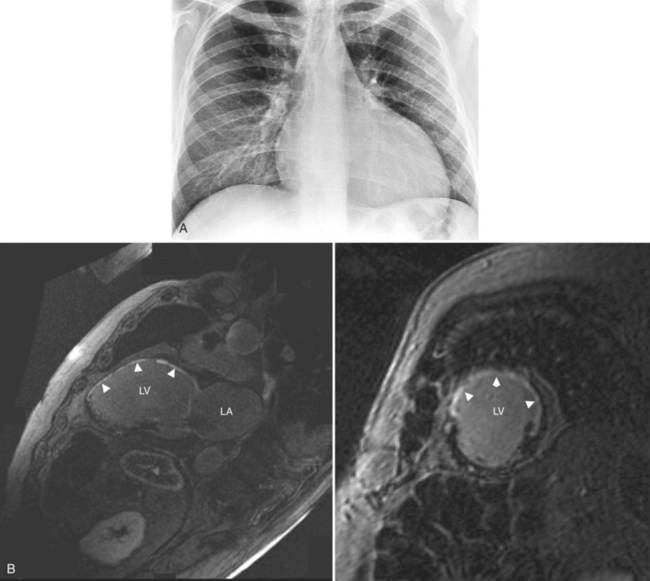
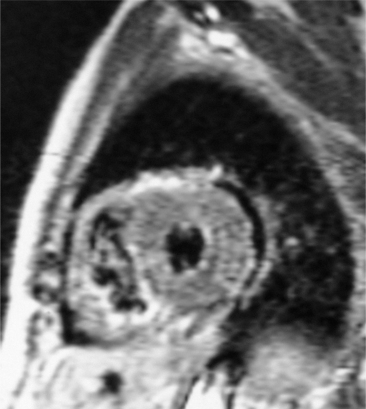
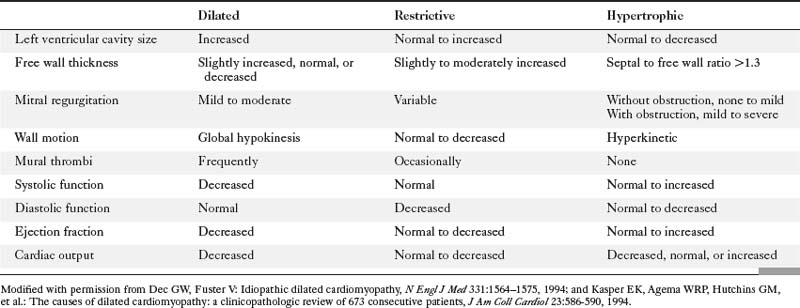
Constrictive pericarditis may be impossible to distinguish from restrictive cardiomyopathy based on hemodynamic tracings alone (see Table 9-1). Although MRI and echocardiography may show an abnormally thick pericardium, CT is the best imaging procedure to reveal calcified pericardium (see Figure 9-11). The pericardium in a restrictive cardiomyopathy does not calcify. Although the presence of pericardial calcium is strong evidence that a constrictive and not a restrictive physiology is present, the absence of calcification does not rule out constriction. Patients with constrictive pericarditis typically have a pericardial thickness greater than 4 mm (Figure 9-14). However, a thickened pericardial contour alone does not necessarily mean that constriction is present. The most reliable sign indicating constriction is the presence of pericardial adhesions. MRI can be used to determine if adhesions are present. Cine gradient echo movies with tag lines placed orthogonal to the pericardium would have to be prescribed. These black tag lines are placed early in systole and then move with the tissue during the cardiac cycle. Along normal pericardium the tag lines are expected to break because the two layers of pericardium can freely move with respect to each other. In constriction, adhesions limit the motion between the pericardial layers, and the tag lines do not break across the pericardium but have a stretched appearance (Figures 9-15, 9-16). Cine images may also show a paradoxical motion of the ventricular septum.
Because of the restriction to right ventricular filling, the right atrium, venae cavae, and hepatic veins are dilated. A pitfall in examining calcific pericarditis with MRI is that the calcified pericardium has a signal void (see Figure 9-13).
Congenital Absence of the Pericardium
Location of Defects
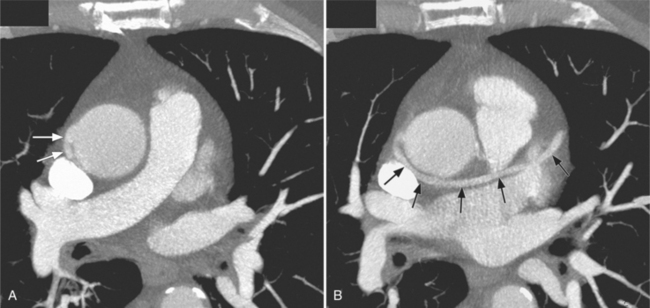
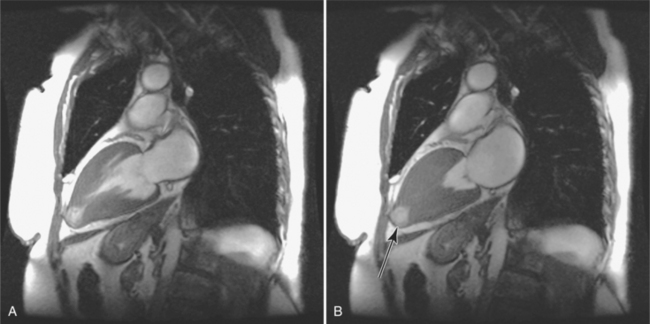
Congenital absence of the pericardium may involve all or part of the parietal pericardium. Most defects are partial and involve a defect over the left atrial appendage and adjacent pulmonary artery (Figure 9-17). Defects in the diaphragmatic part of the pericardium and partial defects over the right atrium and superior vena cava are much less common, and total absence is extremely rare (Figure 9-18). About 20% of patients with pericardial defects have associated heart and mediastinal abnormalities, including atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, bronchogenic cysts, and pulmonary sequestration. Patients with partial pericardial defects are at risk for having part of the heart herniate through the defect, which could cause local strangulation of that part of the heart. In partial absence over the left side, the left atrial appendage may be strangulated.
Radiologic signs
Most of these defects can be identified on the plain chest film (see Figures 9-17, 9-18). Defects on the left side of the mediastinum rotate the heart in that direction, producing levocardia. The radiologic signs of absent pericardium include:
Pericardial Masses
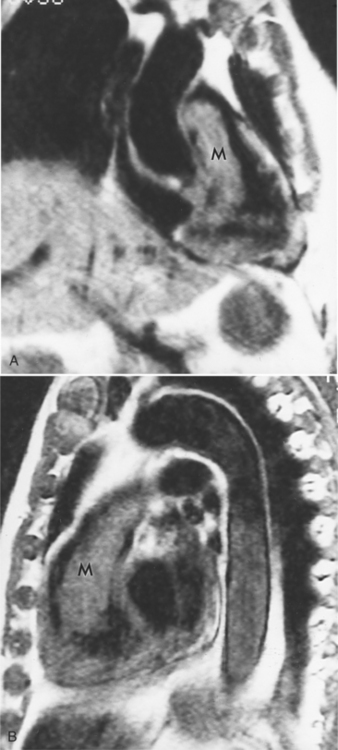
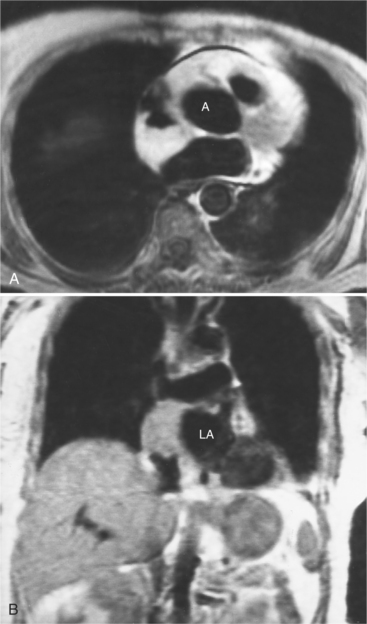
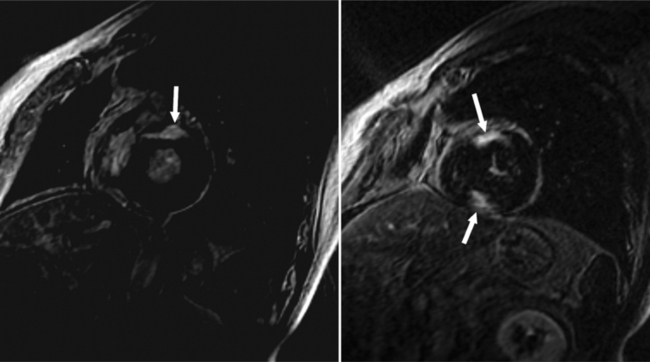
Focal masses in the pericardium may originate in the heart, in the pericardium, or in adjacent structures. Pericardial metastases are common (Figure 9-19). They are found in half of patients dying from breast or lung carcinoma. Primary pericardial masses are usually cysts or lipomas. Cysts in pericardial teratomas contain all three germ layers, whereas intrapericardial bronchogenic cysts contain two germ layers. Some 70% of cysts occur in the right cardiophrenic angle; the rest occur in the left cardiophrenic angle and in the anterior mediastinum (Figure 9-20).
Not all pericardial masses are tumors. Bronchogenic cysts develop in the neonate and are quite rare. Herniation of abdominal contents into the pericardium can occur through a partial absence of the diaphragm. Purulent pericarditis can progress to a pericardial abscess (see Figure 9-7).
MYOCARDIAL DISEASES
Cardiac Masses
Normal Variants
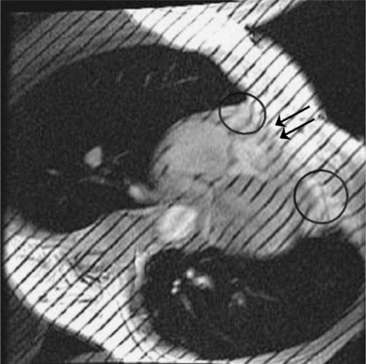
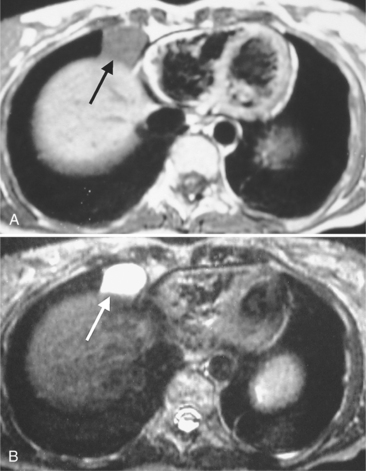
Not all cardiac masses are tumors. Extracardial masses may indent the heart, particularly the low-pressure right and left atria, and produce a concavity that may be mistaken for a primary cardiac tumor. An example is an aneurysm in the aortic root that deforms the right atrium and compresses the superior vena cava and adjacent pulmonary artery. The eustachian valve of the inferior vena cava may be large and mobile and misinterpreted as a vegetation; however, its flaplike appearance adjacent to the inferior vena cava should distinguish it from a pathologic right atrial mass. Fat protrusion into the inferior vena cava may mimic a lipomatous intravascular mass; however, its typical appearance and location allow for its recognition as a normal variant (Figure 9-21). If in doubt, coronal or sagittal reconstructions can be helpful.
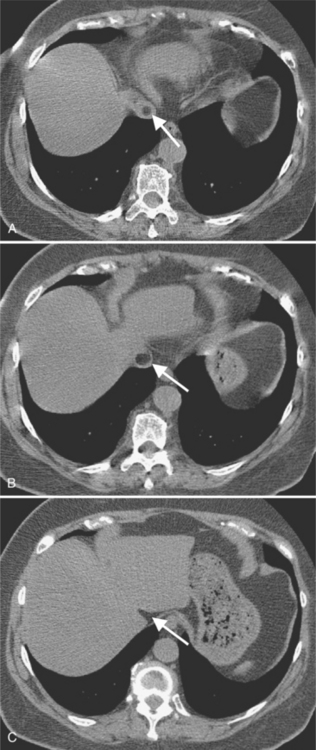
FIGURE 9-21 A through C, Fat protruding into inferior vena cava, simulating intraluminal mass (arrows).
A tumor in the mediastinum can extrinsically displace the superior vena cava and the pulmonary artery. Rarely, the mitral and tricuspid valves may have a large amount of redundant tissue, which appears as a mobile mass on the valve. Atrioventricular canal defects also have an unusual amount of valve tissue, which is rarely large enough to prolapse into the right ventricular outflow region and cause pulmonary stenosis. Large trabeculations in the right ventricle usually are not confused with a tumor, but occasionally a large moderator band has the appearance of a mass if it extends to the apex. The moderator band can be distinguished from a thrombus because the band thickens as it contracts during systole and is on a moving wall. Linear filling defects in the left ventricle unrelated to the mitral valve may be seen occasionally and are false tendons that traverse the lower third of the left ventricular cavity.
Metastatic Tumors
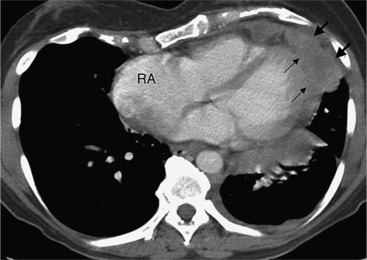
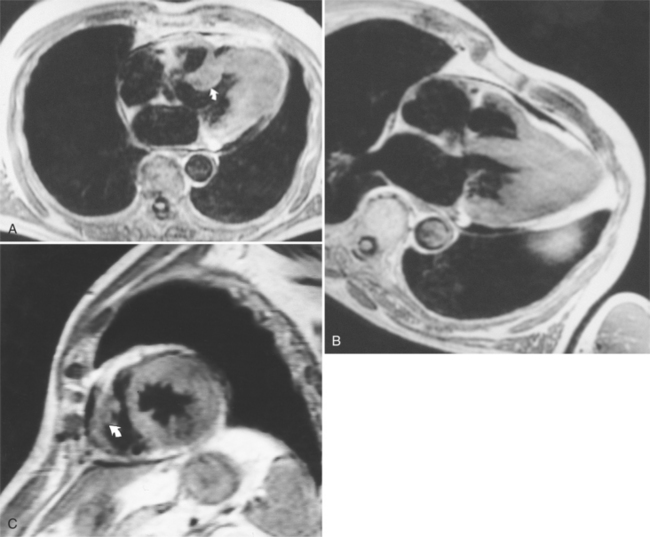
Metastatic tumors to the heart and pericardium are 20 to 40 times more frequent than primary heart tumors (see Figure 9-19). Melanoma, leukemia, and malignant lymphoma are the tumors that more frequently metastasize to the heart. Because of their adjacent location, lung and breast tumors frequently go to the pericardium during the terminal stage of the disease. However, almost any malignant tumor, except those arising from the central nervous system, may metastasize to the heart and pericardium. Tumors that metastasize to the lungs via the bloodstream may extend into the heart along a pulmonary vein. Seeding of the endocardium (Figure 9-22) can be difficult to distinguish from an embolus because many patients with cancer have a hypercoagulable state. A nodular deformity of a heart chamber is strong evidence that the mass is a tumor.
Primary Tumors in Children (Rhabdomyoma, Fibroma)
Primary tumors of the heart are rare. In children, rhabdomyomas constitute 40% of all cardiac tumors; fibromas, myxomas (Figures 9-23, 9-24), and teratomas occur less frequently. Patients with tuberous sclerosis have a propensity to develop rhabdomyomas. These tumors are frequently multiple and most occur in the ventricular septum. Metastatic Wilms tumor is frequently identified as a filling defect in the inferior vena cava extending into the heart. Table 9-2 lists tumors and cysts of the heart and pericardium.
| Type | Percent |
|---|---|
| BENIGN | |
| Myxoma | 24 |
| Lipoma | 8 |
| Papillary fibroelastoma | 8 |
| Rhabdomyoma | 7 |
| Fibroma | 3 |
| Hemangioma | 3 |
| Teratoma | 3 |
| Mesothelioma of the atrioventricular node | 2 |
| Granular cell tumor | 1 |
| Neurofibroma | 1 |
| Lymphangioma | <1 |
| Subtotal | 60 |
| Pericardial cyst | 15 |
| Bronchogenic cyst | 1 |
| Subtotal | 16 |
| MALIGNANT | |
| Angiosarcoma | 7 |
| Rhabdomyosarcoma | 5 |
| Mesothelioma | 4 |
| Fibrosarcoma | 3 |
| Malignant lymphoma | 1 |
| Extraskeletal osteosarcoma | 1 |
| Neurogenic sarcoma | 1 |
| Malignant teratoma | 1 |
| Thymoma | 1 |
| Leiomyosarcoma | <1 |
| Liposarcoma | <1 |
| Synovial sarcoma | <1 |
| Subtotal | 16 |
Modified with permission from McAllister HA, Fenoglia JJ: Tumors of the cardiovascular system. In Hartmann WH, Cowan WR, eds.: Atlas of tumor pathology (Fasc. 15, 2nd series), Washington, DC, 1978, Armed Forces Institute of Pathology.
Primary Tumors in Adults (Myxoma and Papillary Fibroelastoma)
In adults, myxomas constitute 25% of benign cardiac tumors. Angiosarcomas, rhabdomyosarcomas, mesotheliomas, and fibrosarcomas are the most common primary malignant cardiac tumors. Although cardiac neoplasms do not metastasize or invade locally, many of these tumors act in a malignant manner by causing arrhythmias, obstruction, and embolism (Figure 9-25).
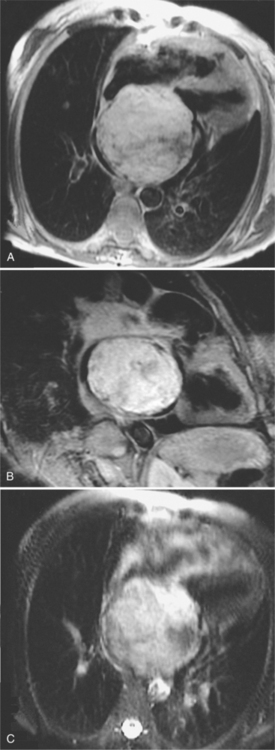
Myxoma is the most common primary benign heart tumor (see Figures 9-23, 9-24). Most myxomas arise in the atria with the left atrium involved four times as often as the right atrium. Most atrial myxomas arise from the interatrial septum. A “dumbbell” myxoma is one that has grown through the fossa ovalis and extends into both the right and left atrium. Some patients may have a syndrome of multiple myxomas that appear throughout life and require multiple surgical resections.
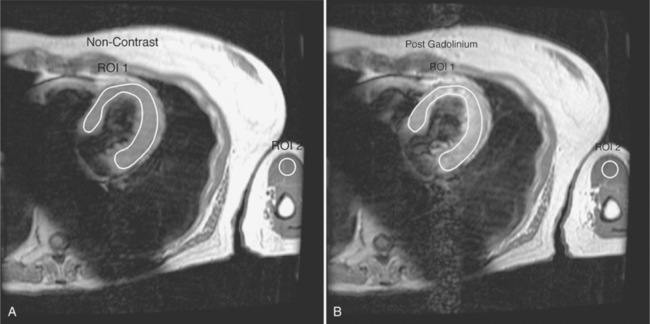
Most myxomas are polypoid and are quite changeable during the cardiac cycle. Many have fronds that may move erratically as the tumor prolapses through a valve. Tumor emboli from left atrial myxoma may obstruct the coronary arteries and cause a myocardial infarction. The appearance of myxomas in the ventricles is similar. These ventricular myxomas can be distinguished from vegetations in that they do not arise from the valve leaflets. On spin echo sequences myxomas may have the same signal intensity as adjacent myocardium on T1-weighted images but are usually bright on T2-weighted images and enhance with gadolinium (see Figure 9-24). They are easily distinguished from lipomas, which are bright on T1-weighted images and demonstrate a signal drop-out on fat-suppressed images (Figure 9-26).
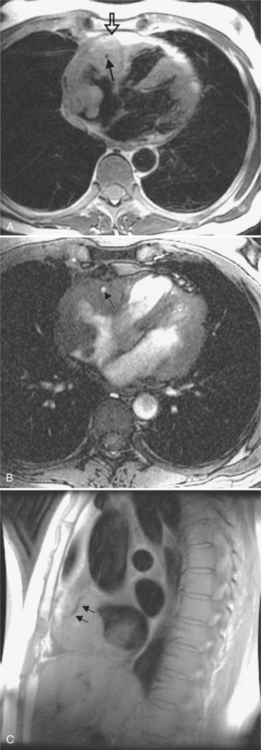
Lipomas are benign tumors that have a spherical shape and a sharp demarcation from adjacent ventricular myocardium. Lipomatous hypertrophy of the interatrial septum, on the other hand, is a common phenomenon of the interatrial septum (Figure 9-27). Best studied by MRI, it consists of large masses of fatty tissue in the interatrial septum with occasional extension into the left and right atria (Figure 9-28). Because of sparing of the fossa ovalis, the mass frequently demonstrates the typical dumbbell configuration. These masses are benign and not associated with obesity, but they can produce supraventricular arrhythmias. The MRI examination may be used to confirm the diagnosis with fat suppression sequences, and occasionally to map the extent of the fat and to document any potential caval obstruction.
Malignant Cardiac Tumors
Malignant cardiac tumors may have a similar appearance regardless of their histologic diagnoses (Figures 9-22, 9-25, 9-29). Angiosarcoma typically involves the right side of the heart and is an irregular infiltrating mass that diffusely involves many structures (see Figure 9-29). The venae cavae may be partially obstructed, the right atrial cavity narrowed, the right ventricle distorted, and the pulmonary outflow region narrowed. Coronary angiography frequently demonstrates patent arteries but extrinsic stenosis and obstruction are well known.
Imaging Abnormalities
Box 9-2 lists the variety of imaging abnormalities that may be seen with cardiac tumors.
Thrombus
Whether a cardiac mass is a tumor or a thrombus can frequently be decided by the clinical situation. Thrombi are common in the left ventricle after a myocardial infarction or in the presence of a known ventricular aneurysm. In patients with atrial fibrillation, particularly when they have rheumatic heart disease, left atrial thrombi are common, whereas myxoma is quite rare. Conversely, peripheral embolization in a patient with no overt sign of heart disease is commonly the first sign of a left atrial myxoma. Thrombi can develop in any part of the heart, but the etiology of the thrombus may be unique to a particular chamber (Box 9-3).
Cardiac Lesions in Acquired Immune Deficiency Syndrome
Myocarditis is a common autopsy finding in patients who have died from AIDS, with nearly half having disease in the heart or pericardium. Even though these patients have multiple infections, an agent is rarely identified in the heart. The histopathologic picture is a focal lymphocytic myocarditis. Many infectious agents are occasionally cultured, such as Mycobacterium aviumintracellulare, Toxoplasma (Figure 9-30), Cryptococcus, cytomegalovirus, and human immunodeficiency virus (HIV). Kaposi’s sarcoma, which is histologically difficult to distinguish from angiosarcoma, and lymphoma are the major pericardial and heart tumors (Figure 9-31). The pericardium frequently has fibrinous pericarditis from uremia or infection and may have a moderate effusion. Vegetations of both infective and nonbacterial endocarditis have been found on all four heart valves. Congestive heart failure with pulmonary edema from left ventricular failure may have a similar clinical presentation as a dilated cardiomyopathy. The right ventricle may be dilated from pulmonary artery hypertension secondary to pulmonary infection or emboli.
Cardiomyopathies
Cardiomyopathies are heart muscle diseases in which congenital, pericardial, valvular, and coronary causes have been excluded by appropriate clinical, hemodynamic, or imaging methods. Primary cardiomyopathies have an unknown etiology, whereas secondary cardiomyopathies have an etiologic diagnosis and therefore are potentially reversible with appropriate therapy. Alcoholic, Adriamycin (doxorubicin), and ischemic cardiomyopathies are examples of known causes and produce similar clinical signs, such as ventricular failure, but may require different therapy.
Dilated Cardiomyopathy
Because globally decreased wall motion is not unique to this abnormality, cardiac imaging not only measures the left ventricular ejection fraction but also helps exclude other types of heart disease. The chest film generally shows cardiomegaly and pulmonary venous hypertension with little or no pulmonary edema (Figure 9-32). The paradox of a huge heart and clear lungs is a diagnostic clue that a dilated cardiomyopathy may be present. Global reduction in left ventricular wall motion may also be seen in acute viral myocarditis; in eosinophilic myocarditis; and as atypical presentation of other types of cardiomyopathies such as those with infective etiologies (Chagas disease), granulomatous heart disease (sarcoid), and infiltrative heart disease (amyloid) (Figure 9-33). Peripartum cardiomyopathy (Figure 9-34) also has global hypokinesis of both ventricles and occurs during the last trimester of pregnancy or during the first several months postpartum.
Known causes of dilated cardiomyopathy are listed in Box 9-4.
Box 9-4 Known causes of dilated cardiomyopathy
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy is characterized by disproportionate hypertrophy of the left ventricle and occasionally of the right ventricle. The disease is distinctive and needs to be separated from secondary and acquired hypertensive heart disease (Box 9-5). Initially this disease was called idiopathic hypertrophic subaortic stenosis or hypertrophic obstructive cardiomyopathy. Subsequently it became clear that subaortic obstruction was only one feature of cardiac hypertrophy. Now hypertrophic cardiomyopathy is typically subdivided into patients with left ventricular outflow obstruction and those without. Mild, nonobstructive hypertrophy of the left ventricle is common in many conditions that cause an increased afterload of the left ventricle, such as hypertension, aortic stenosis, and coarctation. The hypertrophy in these diseases usually is concentric and uniform.
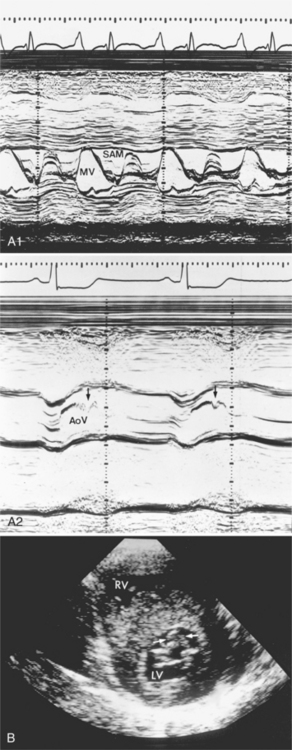
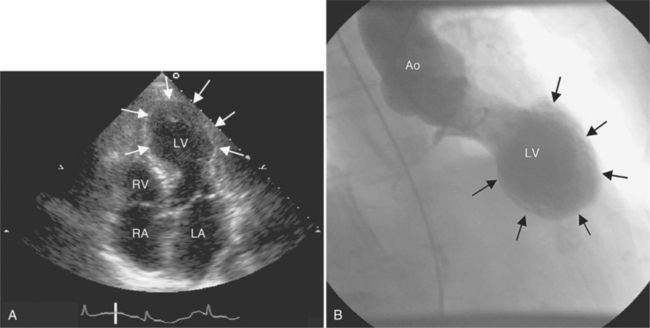
The hallmark of obstructive hypertrophic cardiomyopathy is a dynamic subvalvular aortic stenosis. In diastole the left ventricular outflow tract is open or slightly stenotic because of upper septal hypertrophy. But in systole an increasing stenosis develops as the anterior leaflet of the mitral valve moves into the outflow region. This abnormal motion of the mitral valve increases throughout systole and frequently causes the valve to touch the ventricular septum, creating an occlusion of the outflow from the left ventricle. The systolic anterior motion (SAM) of the mitral valve is variable (Figure 9-35). Physiologic maneuvers that decrease the size of the left ventricle or increase its contractility worsen the aortic stenosis, whereas those maneuvers that increase its size or decrease its contractility lessen or abolish the pressure gradient.
In the same way, SAM is not entirely specific for hypertrophic cardiomyopathy. In transposition of the great arteries the anterior leaflet of the mitral valve may move abnormally in one third of newborns with this malformation. In this instance the SAM is a dynamic subpulmonary stenosis because the pulmonary artery is connected to the left ventricle (Box 9-6).
In hypertrophic cardiomyopathy, the heart size is usually normal on chest films but it can have severe biventricular enlargement from hypertrophy (Figure 9-36). Left atrial enlargement and pulmonary edema occur when the left ventricle is too stiff to allow normal diastolic filling. Tomographic imaging is required to assess the location and amount of hypertrophy (Figure 9-37). Echocardiography and occasionally angiography show the abnormal mitral valve motion (see Figure 9-35).
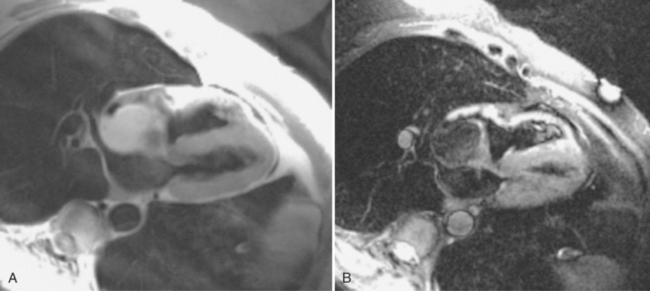
Several types of contraction abnormality are frequently seen in the left ventricle. Lack of base-to-apex shortening, which appears as apical akinesis, is a sign of the muscle-bound heart and not an area of scar from coronary disease. Cavity obliteration usually occurs at the apex at end systole (Figure 9-38), although it may occur only in the middle segment of the ventricle or rarely in the outflow region. The left ventricle may have an unusual shape because of the segmental hypertrophy (Figure 9-39). The papillary muscles may touch at end systole, producing obliteration of the cavity at the midventricular level (Figure 9-40).
Mitral regurgitation in obstructive hypertrophic cardiomyopathy is directly related to the degree of SAM of the anterior leaflet (Figure 9-41). The mitral regurgitation is usually mild and alleviated with medical therapy. However, a small number of patients have such severe mitral regurgitation that septal myotomy or mitral valve replacement is necessary.
Cardiac MRI is now being used to assess the risk for sudden cardiac death in patients with a family history of obstructive hypertrophic cardiomyopathy. Tagged cine sequences can be used to evaluate regional myocardial strain and function. Delayed enhanced images show areas of scarring within the thickened myocardium, the volume of which shows correlation with the risk scores for sudden cardiac death (SCD). The areas of hyperenhancement are not confined to coronary artery territories. They are patchy in nature and are found in the center of thickened myocardium and at the junction of right and left ventricular myocardium.
Restrictive Cardiomyopathy
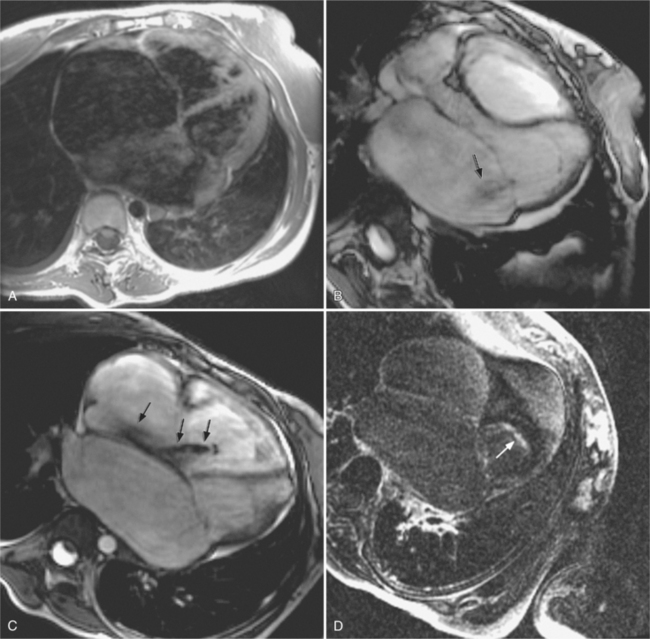
Restrictive cardiomyopathies are associated with extracellular infiltration of protein, granulomas, and cells, radiation fibrosis, carcinoid, and endomyocardial fibrosis. Other causes of secondary restrictive cardiomyopathy include sarcoidosis, amyloidosis, hemochromatosis, scleroderma, and others. Endomyocardial fibrosis affects children and young adults living near the equator. Both right and left ventricle may be involved. Imaging findings include large pericardial effusions, enlarged atria, mitral and tricuspid regurgitation with distortion of the valve leaflets, and apical obliteration (Figure 9-42). Patients with hemochromatosis and the glycogen storage diseases—inborn errors of metabolism—develop restrictive cardiomyopathies because of an intracellular accumulation of excessive iron or lipids (Box 9-7).
Cardiac sarcoidosis is difficult to diagnose clinically. In patients with histologically proven extracardiac sarcoidosis, the diagnosis of cardiac sarcoid is traditionally suggested, if a complete right bundle branch block is present and either an atrioventricular block or other specific ECG findings are present; or abnormal left ventricular dilatation, wall motion, or wall thickness are noted on echocardiography; or myocardial perfusion defects by thallium scintigraphy are present; or if a biopsy showed myocardial infiltration or fibrosis. Cardiac MRI has the potential to noninvasively identify cardiac granulomas or fibrosis using delayed enhanced imaging. Delayed hyperenhancement usually spares the subendocardium. It typically consists of patchy mesocardial or subepicardial hyperenhancement that is not confined to coronary territories (Figure 9-43). The chamber sizes are usually normal.
Arrhythmogenic Right Ventricular Dysplasia
Arrhythmogenic right ventricular dysplasia (ARVD) is a rare cardiomyopathy associated with arrhythmias and sudden death in otherwise healthy young individuals. ARVD is characterized by fibrofatty infiltration of the right ventricular myocardium (Figure 9-44). Focal or global right ventricular wall motion abnormalities and focal aneurysms may be present. End-stage patients will have severe right ventricular dilatation and sometimes left ventricular involvement with relative sparing of the septum.
Currently, the diagnosis is made using a set of major and minor criteria. ARVD is diagnosed if two major criteria, one major and two minor criteria, or four minor criteria are present. Minor criteria include a family history of suspected ARVD or premature sudden cardiac death, electrocardiographic abnormalities in the right precordial leads (V1 to V3), and mild global or segmental right ventricular wall motion abnormalities. Major criteria include family disease confirmed at necropsy or surgery, epsilon waves on ECG, severe segmental or global right ventricular dilatation, right ventricular aneurysms, and fibrofatty replacement of right ventricular myocardium.
Distinguishing Features of the Cardiomyopathies
The diagnostic evaluation of the cardiomyopathies frequently requires cardiac imaging, hemodynamic pressure measurements, and occasionally an endomyocardial biopsy. Table 9-3 lists imaging features that distinguish the different categories of cardiomyopathy.
Myocarditis
A variety of etiologies can lead to myocarditis. Perhaps the most important is the infectious group, with viral myocarditis being the leading cause in the Western world. However, other etiologies, such as systemic diseases, toxins, and drugs, have also been identified.
Endomyocardial biopsy was thought to be the gold standard; however, because the disease may have a patchy distribution, false-negative samples are not uncommon. Cardiac MRI plays an increasing role in the detection of myocarditis. Friedrich and others have introduced a method that investigates the global relative signal enhancement of the left ventricular myocardium related to skeletal muscle. If the enhancement ratio between myocardium and skeletal muscle is above 3.4 then the diagnosis of myocarditis can be made (Figure 9-45). Myocarditis typically displays subepicardial or linear septal midmyocardial delayed enhancement, which differs from the typically subendocardial or transmural enhancement that we see in infarcts.
Takotsubo Syndrome (Apical Ballooning Syndrome)
Apical ballooning syndrome is a recently described heart syndrome with transient left ventricular extensive akinesia of the apical and mid portions of the left ventricle. The resulting shape is described as “apical ballooning” or as “takotsubo-shaped” because of the resemblance of the systolic left ventricular image to the Japanese octopus trap called tako tsubo.
Despite the dramatic initial presentation, the left ventricle function typically recovers completely. Imaging findings include the demonstration of the apical dilatation and akinesis on left ventriculogram during coronary angiography (Figure 9-46). MRI can be used to depict the findings at baseline and to demonstrate resolution of the akinesis at follow-up examinations. If apical ballooning syndrome is suspected clinically, coronary CTA may be an acceptable alternative to rule out coronary artery stenosis and to depict the apical left ventricular dilatation.
Abbara S, Migrino RQ, Sosnovik DE, et al. The value of fat suppression in the MRI evaluation of suspected arrhythmogenic right ventricular dysplasia. Am J Roentgenol. 2004;182:587-591.
Auffermann W, Wichter T, Breithardt G, et al. Arrhythmogenic right ventricular disease: MR imaging vs angiography. Am J Roentgenol. 1993;161:549-555.
Baumhäkel M, Janzen I, Kindermann M, et al. Images in cardiovascular medicine. Cardiac imaging in isolated noncompaction of ventricular myocardium. Circulation. 2002;106:e16.
Black CM, Hedges LK, Javitt MC. The superior pericardial sinus: normal appearance on gradient-echo MR images. Am J Roentgenol. 1993;160:749-751.
Brady TJ, Grist TM, Westra SJ, et al. Pocket radiologist: cardiac. Top 100 diagnoses. Salt Lake City: Amirsys, 2002.
Braunwald E, Zipes DP, Libby P. Heart disease: a textbook of cardiovascular medicine, ed 6. Philadelphia: WB Saunders, 2001.
Castillo E, Lima JA, Bluemke DA. Regional myocardial function: advances in MR imaging and analysis. Radiographics. 2003;23:S127-S140.
Chiles C, Woodard PK, Gutierrez FR, et al. Metastatic involvement of the heart and pericardium: CT and MR imaging. Radiographics. 2001;21:439-449.
Choe YH, Im J, Park JH, et al. The anatomy of the pericardial space: a study in cadavers and patients. Am J Roentgenol. 1987;149:693.
Corrado D, Basso C, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy: current diagnostic and management strategies. Cardiol Rev. 2001;9:259-265.
Davies MJ. The cardiomyopathies: an overview. Heart. 2000;83:469-474.
Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart. 2003;89:1027-1031.
Friedrich MG, Strohm O, Schulz-Menger J, et al. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802-1809.
Gaerte SC, Meyer CA, Winer-Muram HT, et al. Fat-containing lesions of the chest. Radiographics. 2002;22:S61-S78.
Gilkeson RC, Markowitz AH, Ciancibello L. Multisection CT evaluation of the reoperative cardiac surgery patient. Radiographics. 2003;23:S3-S17.
Grebenc ML, Rosado de Christenson ML, Burke AP, et al. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073-1103.
Grebenc ML, Rosado de Christenson ML, Green CE, et al. Cardiac myxoma: imaging features in 83 patients. Radiographics. 2002;22:673-689.
Hamamichi Y, Ichida F, Hashimoto I, et al. Isolated noncompaction of the ventricular myocardium: ultrafast computed tomography and magnetic resonance imaging. Int J Cardiovasc Imaging. 2001;17:305-314.
Higgins CB, De Roos A, Streubert HJ. Cardiovascular MRI and MRA. Philadelphia: Lippincott Williams & Wilkins, 2002.
Hoffmann U, Globits S, Schima W, et al. Usefulness of magnetic resonance imaging of cardiac and paracardiac masses. Am J Cardiol. 2003;92:890-895.
Kasper EK, Agema WR P, Hutchins GM, et al. The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. J Am Coll Cardiol. 1994;23:586-590.
Kaul S, Fishbein MC, Siegal RJ. Cardiac manifestations of acquired immune deficiency syndrome: a 1991 update. Am Heart J. 1991;122:535-544.
Kayser HW, van der Wall EE, Sivananthan MU, et al. Diagnosis of arrhythmogenic right ventricular dysplasia: a review. Radiographics. 2002;22:639-650.
Kayser HW, de Roos A, Schalij MJ, et al. Usefulness of magnetic resonance imaging in diagnosis of arrhythmogenic right ventricular dysplasia and agreement with electrocardiographic criteria. Am J Cardiol. 2003;91:365-367.
Keren A, Popp RL. Assignment of patients into the classification of cardiomyopathies. Circulation. 1992;86:1622-1633.
Kodoma F, Flutz PJ, Wandtke JC. Comparing thin-section and thick-section CT of pericardial sinuses and recesses. Am J Roentgenol. 2003;181:1101-1108.
Laissy JP, Messin B, Varenne O, et al. MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest. 2002;122:1638-1648.
Manning WJ, Pennell DJ. Cardiovascular magnetic resonance. Edinburgh: Churchill Livingstone, 2002.
Marcus F, Towbin JA, Zareba W, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C): a multidisciplinary study: design and protocol. Circulation. 2003;107:2975-2978.
Masui T, Finck S, Higgins CB. Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology. 1992;182:369-373.
Miller SW, Holmvang G. Differentiation of slow flow from thrombus in thoracic magnetic resonance imaging, emphasizing phase images. J Thorac Imaging. 1993;8:98-107.
Park JH, Kim YM, Chung JW, et al. MR imaging of hypertrophic cardiomyopathy. Radiology. 1992;185:441-446.
Protopapas Z, Westcott J. Left pulmonic recess of the pericardium: findings at CT and MR imaging. Radiology. 1995;196:85-88.
Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610-1617.
Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841-842.
Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest. 1993;103:253-258.
Shirani J, Roberts WC. Clinical, electrocardiographic and morphologic features of massive fatty deposits (‘lipomatous hypertrophy’) in the atrial septum. J Am Coll Cardiol. 1993;22:225-238.
Topol EJ, Robert M, Califf RM, et al. Textbook of cardiovascular medicine. Philadelphia: Lippincott Williams & Wilkins, 2002.
Truong MT, Erasmus JJ, Gladish GW, et al. Anatomy of pericardial recesses on multidetector CT: implications for oncologic imaging. Am J Roentgenol. 2003;181:1109-1113.
Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11-18.
Wang ZJ, Reddy GP, Gotway MB, et al. CT and MR imaging of pericardial disease. Radiographics. 2003;23:S167-S180.