Chapter 6 Valvular Heart Disease
Cardiac imaging in suspected valvular disease determines the involvement of the valves, the extent of the stenosis or regurgitation, and the hemodynamic consequence of the pressure or volume overload on the heart. It also evaluates associated conditions, such as aortic dissection or aneurysm and ventricular contractility and enlargement.
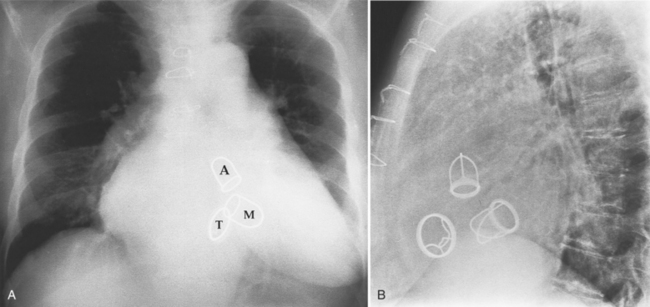
Fig. 6-1 illustrates the anatomic positions of the heart valves.
VALVULAR AORTIC STENOSIS
Chest Film Findings
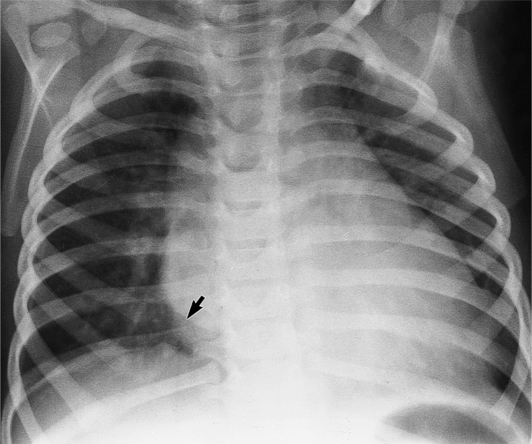
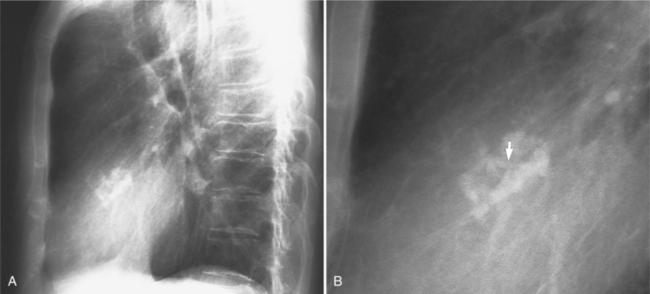
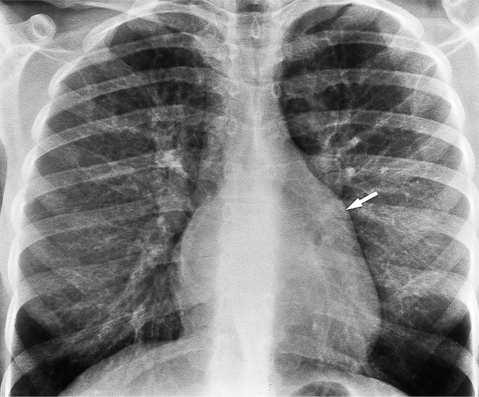
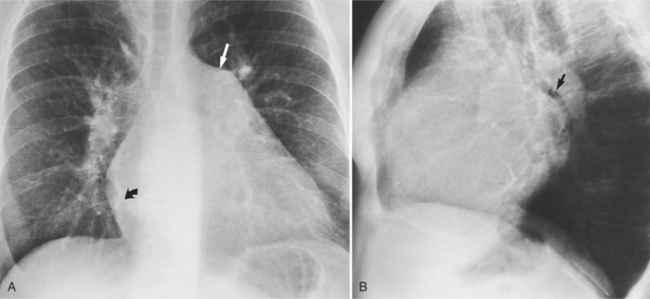
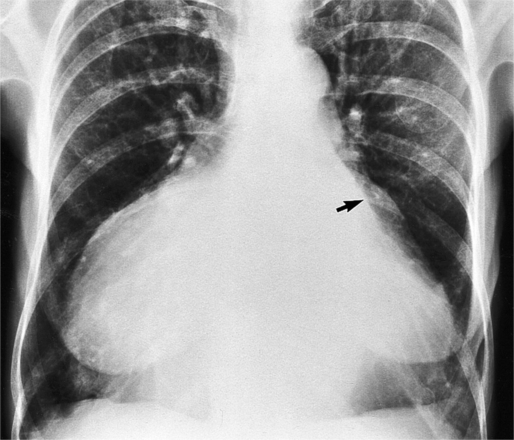
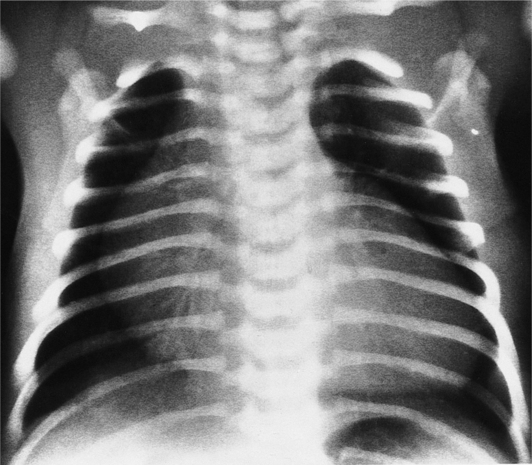
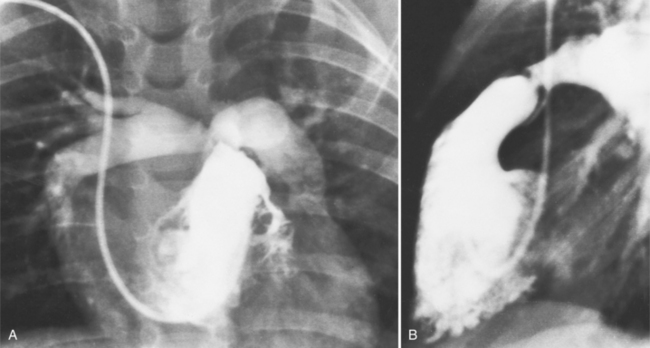
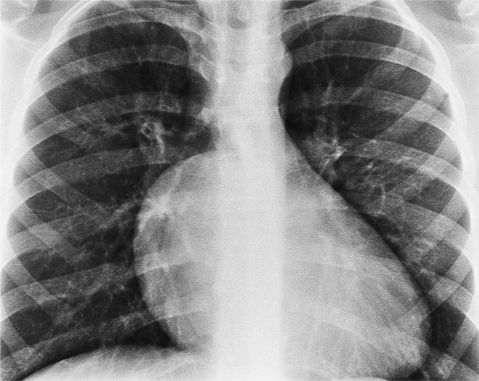
The chest film abnormalities depend on the age of the patient and the severity of the stenosis. In infancy, pulmonary edema with generalized cardiomegaly is the typical appearance of obstruction to blood flow at any point between the pulmonary veins, left heart, and aorta (Fig. 6-2). The adult heart is normal in size, and the lungs are clear unless there is left ventricular failure and dilatation (Fig. 6-3). Calcification in the aortic valve over age 40 years occurs in all types of aortic stenosis and clinically marks the stenosis as severe. Dilatation of the ascending aorta is frequent in aortic stenosis but correlates rather poorly with severity or with the site of the stenosis (one third of patients with subvalvular aortic stenosis have dilated aortas).
Imaging Features
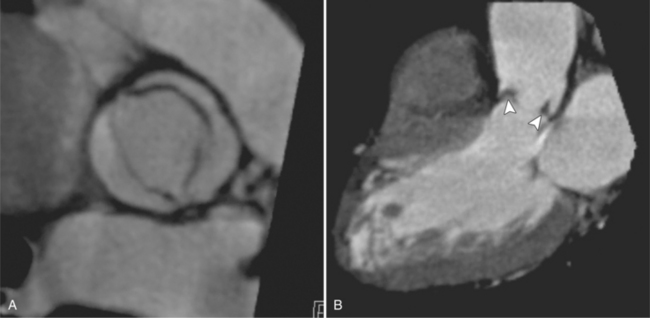
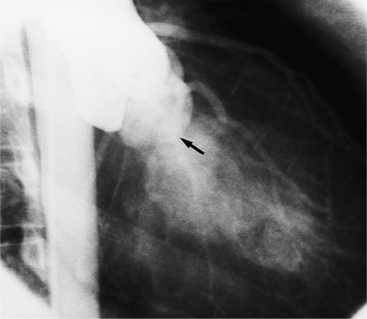
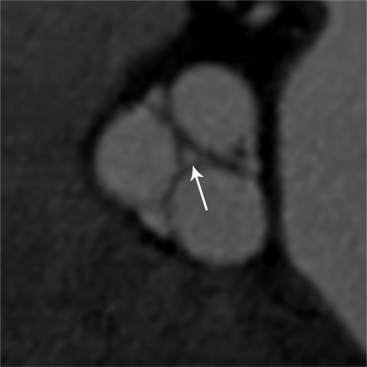
The preferred imaging modality for the dynamic assessment of aortic stenosis remains echocardiography. Cardiac-gated multidetector CT (MDCT) angiography is proving to be an excellent modality to detect and quantify aortic valve stenosis. Direct planimetry of the valve opening in systole correlates quite well to echocardiographic grades of aortic stenosis severity. The added advantage of MDCT is that it nicely demonstrates associated aortic root pathologies. Fig. 6-4 shows a case of mild aortic stenosis with the valve in short axis during systole (open) and diastole (closed). Fig. 6-5 shows the same valve in long axis during systole and diastole. Finally, MRI can be useful for aortic stenosis because it can accurately quantify peak aortic valve velocities and pressure gradients. The associated calcium on the valve makes MRI less useful to assess morphology because of the typical signal loss of calcified structures on gradient echo pulse sequences. A narrow orifice to the aortic valve is visible as a jet through the valve. The degree of aortic stenosis can be estimated by comparing the width of the jet with the diameter of the aortic annulus. When the jet is less than 15% of the diameter of the annulus, there is severe aortic stenosis. However, it may also be present when the width of the column of blood through the valve appears to be the same size as the annulus. This latter finding is typical of degenerative aortic stenosis in the elderly and in many cases of rheumatic involvement of the aortic valve. In the former, the valve orifice is irregularly eccentric without commissural fusion, allowing the stream of contrast material adjacent to the three commissures to project over the entire width of the aorta. Calcification may be so extensive that the leaflets are akinetic.
Box 6-1 lists the causes of aortic stenosis.
Congenital Valvular Aortic Stenosis
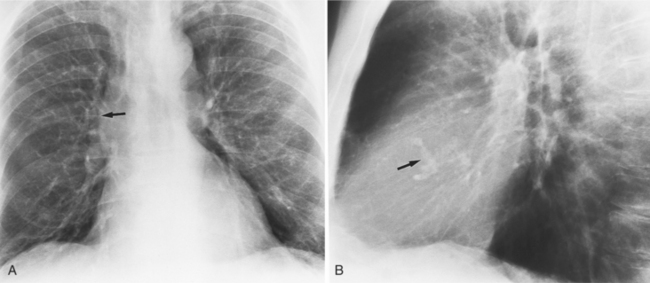
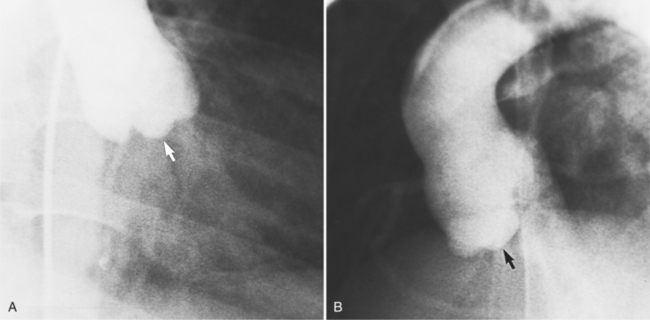
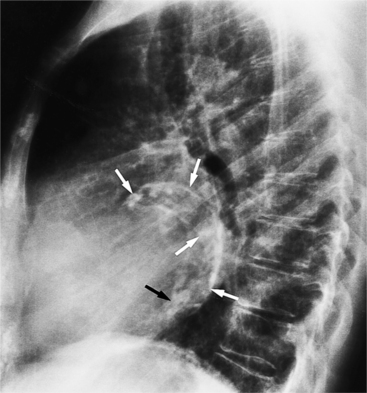
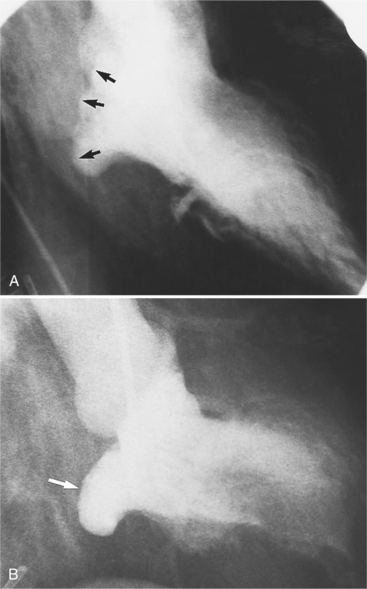
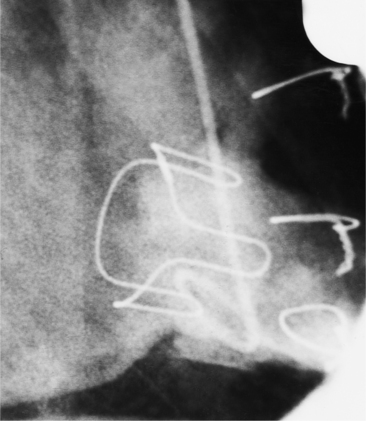
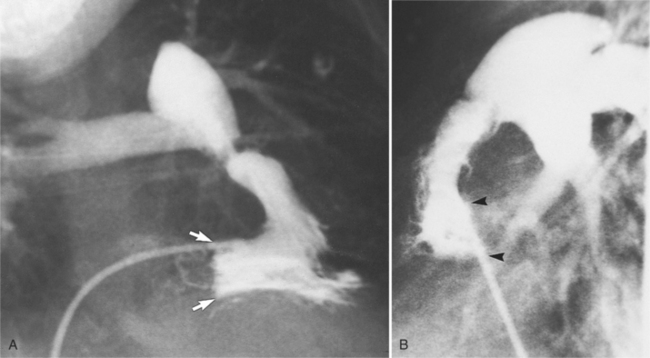
Distinctive calcifications on the lateral chest film characterize the congenital bicuspid aortic valve. Calcium is visible in the raphe of the valve and in the line of insertion of the shallow conjoint leaflet and the convex nonfused leaflet (Fig. 6-6). An acquired cause of a bicuspid valve is fusion of the commissures of the aortic leaflets in rheumatic heart disease.
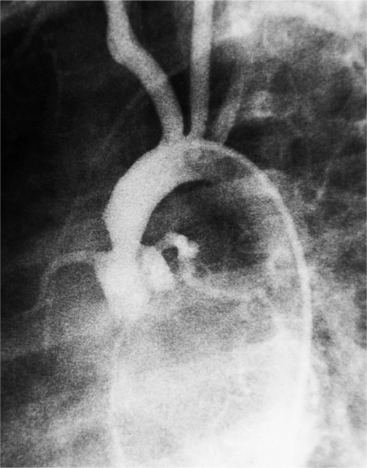
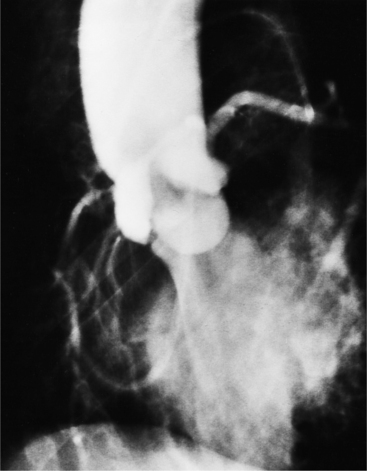
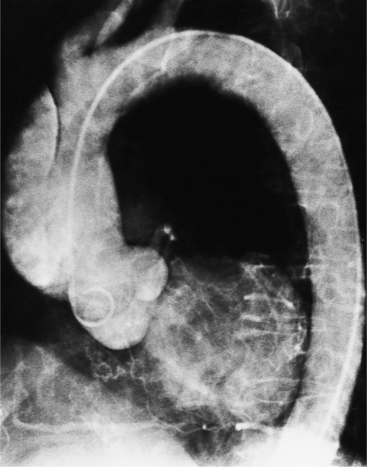
The angiographic appearance of a congenital bicuspid valve is two sinuses of Valsalva and two leaflets (Fig. 6-7). The valve orifice may be oriented in either an anteroposterior direction, which divides the leaflets into right and left cusps, or in a right-left direction, dividing the valve into anterior and posterior leaflets. If the cusps are on the right and left, there may be a raphe in the right cusp, and each sinus of Valsalva gives origin to a coronary artery. If the cusps are anterior and posterior, a raphe, if present, occurs in the anterior cusp, and both the coronary arteries arise from the anterior sinus of Valsalva. The normal aortic valve may acquire a bicuspid appearance when one commissure fuses as a result of rheumatic heart disease. In general, the congenital bicuspid valve will have one of the sinuses of Valsalva and its leaflet occupying half the circumference of the aortic annulus, whereas the acquired bicuspid valve with one fused commissure still demonstrates three sinuses of Valsalva of nearly equal size. In diastole, the congenital bicuspid valve may appear to have three sinuses of Valsalva because the raphe is visible and divides one of the two leaflets in half.
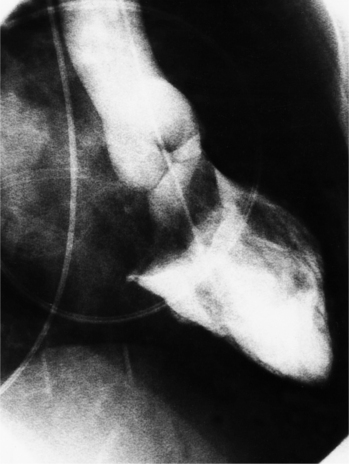
The congenital bicuspid aortic valve is recognizable most accurately during systole. The systolic appearance of a noncalcified congenital bicuspid valve typically includes domed and thickened leaflets (Fig. 6-8). The jet through the valve is usually slightly eccentric in direction but appears through the central part of the aortic annulus. In contrast, the jet through the rare unicommissural valve is quite eccentric.
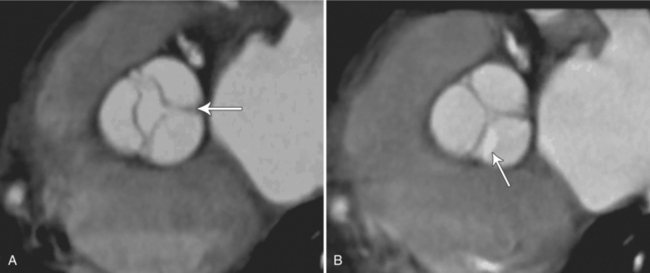
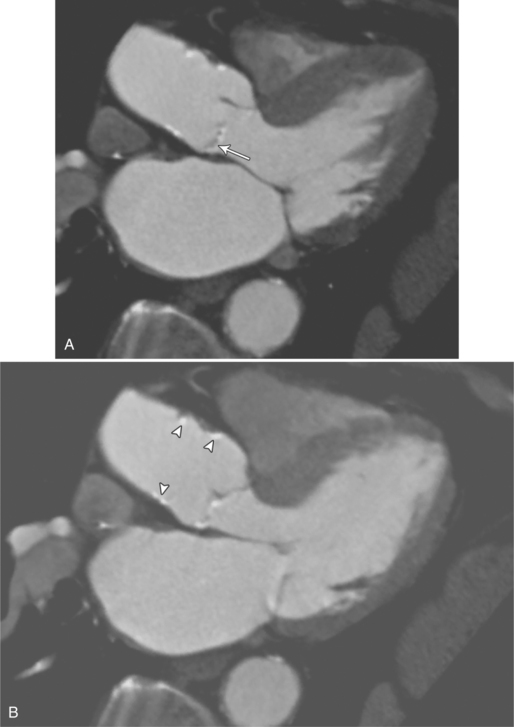
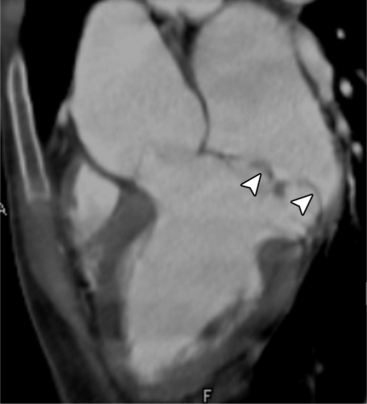
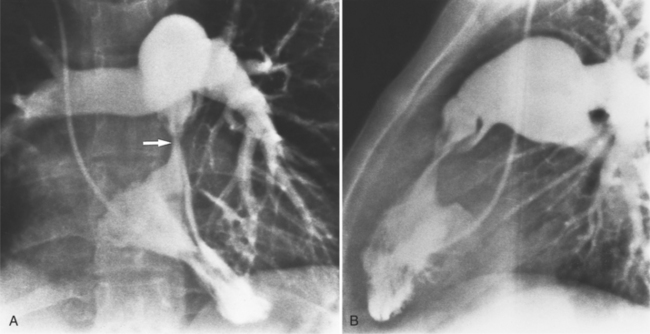
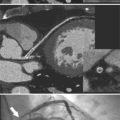
MDCT can be quite useful at characterizing congenital bicuspid aortic valves. Fig. 6-9 depicts a congenital bicuspid aortic valve in systole on MDCT in cross section and long axis, respectively. This patient has a normal systolic opening area with classic systolic dooming. Fig. 6-10 is a different case of a bicuspid aortic valve on MDCT. This valve demonstrates calcification of the cusps and diastolic prolapse. Cardiac MRI is more limited at depicting aortic valve leaflet morphology because the associated leaflet calcium causes profound signal void on MRI gradient pulse sequences, such as in Fig. 6-11. Black blood MRI pulse sequences may overcome this artifact.
Bicuspid aortic valves are associated with many types of congenital heart disease. Half of those with coarctation of the aorta have a bicuspid aortic valve (Fig. 6-12). More generally, any malformation of the aorta, such as aortic arch atresia or interruption or the hypoplastic left heart syndrome, has a high incidence of bicuspid aortic valve.
The congenital unicuspid aortic valve is intrinsically stenotic and may at times be incompetent. This valve may exist with no lateral attachments and appear as a diaphragm with a central opening. A second type of unicuspid valve has one lateral attachment to the aortic annulus with the commissure appearing as a raphe between the central point and the aortic wall. Both types of valve are rare. In systole, there is an eccentric jet against the posterior wall of the aorta with no leaflet tissue posteriorly. In diastole, a sinus of Valsalva may appear anteriorly but not posteriorly.
Acquired Valvular Aortic Stenosis
The aortic valve that is not stenotic at birth may become so in two ways:
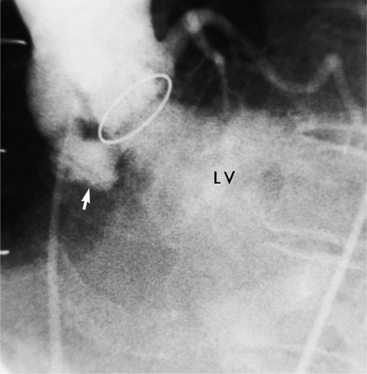
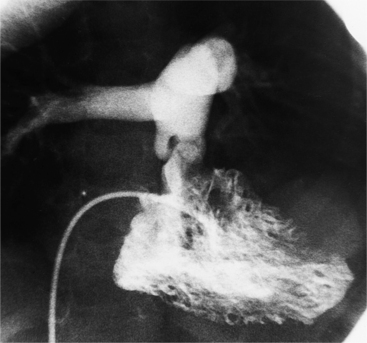
The end stage of any of these processes is a severely calcified valve, and it may be impossible to determine the initial process. In the extreme, the leaflets may be so thickened as to be akinetic. Unlike the congenital, noncalcified bicuspid aortic valve, these end-stage valves usually do not have a jet through them (Figure 6-13). The stream of contrast medium or blood through the distorted and curved linear orifice is turbulent. Doming of the leaflets is always seen in the noncalcified congenital aortic stenosis but is an inconstant feature in an end-stage aortic valve.
Calcific aortic stenosis in the adult results from rheumatic heart disease, occurs on a congenital bicuspid aortic valve, and occurs in the elderly. Although some overlap occurs, you can differentiate these three conditions by age and by the presence of other valvular lesions. The congenital bicuspid aortic valve begins to calcify in the fourth decade, whereas degenerative aortic stenosis affects those over 65 years of age. In developing countries, calcification in rheumatic aortic stenosis may appear in the late teens, but it is unusual in the United States until the fourth decade. With the aortic stenosis of rheumatic heart disease, mitral stenosis does not occur for at least 7 to 10 years after an episode of acute rheumatic fever, and aortic stenosis may develop about 7 years after that.
An important clue to the diagnosis of rheumatic disease in the aortic valve is the presence of mitral stenosis or regurgitation and calcification or thickening in the mitral leaflets in an otherwise functional valve. One characteristic of rheumatic aortic stenosis is the fusion of the commissures adjacent to the aortic wall. In general, these valves have either the usual appearance of a tricuspid valve or have one distorted sinus of Valsalva that occupies more than half the aortic annular circumference. When there is fusion of multiple commissures, the leaflets are usually so thickened and distorted that valve morphology is indistinguishable from other types of aortic stenosis.
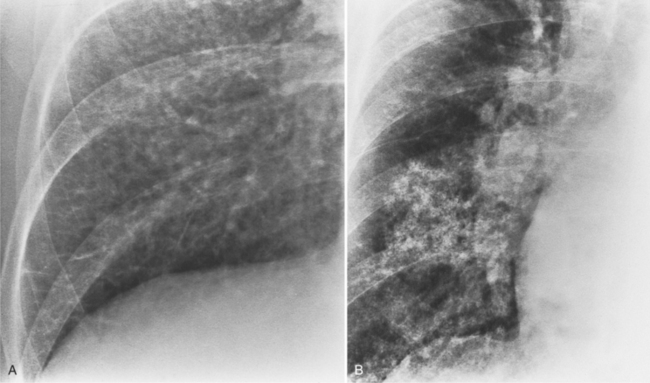
Aortic stenosis in the elderly results from degeneration of the valve leaflets with subsequent thickening and calcification. Some patients have coronary calcification and calcification in the mitral annulus and in the aortic arch. These aortic valves are tricuspid and have clumps of calcium within the webs of the leaflets. In contrast to rheumatic valves, the commissures are not fused (Fig. 6-14).
MDCT can image calcific degenerative aortic valve stenosis (Figures 6-15, (6-16). The valve area can be planimetered from the short-axis views.
SUBVALVULAR AORTIC STENOSIS
Pathologic Abnormalities
Subvalvular aortic stenosis, or subaortic stenosis, consists of a heterogeneous group of abnormalities, several of which are associated with other types of cardiac malformation. These obstructions include:
Angiographic Findings
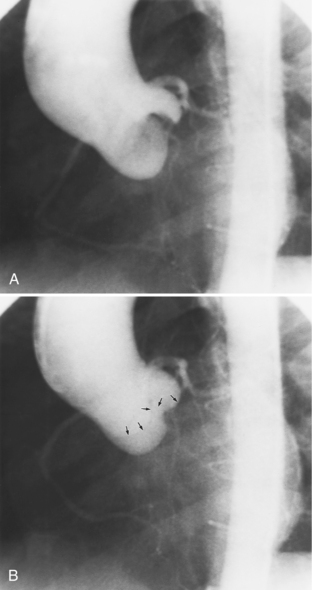
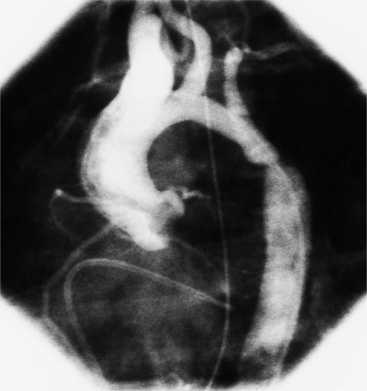
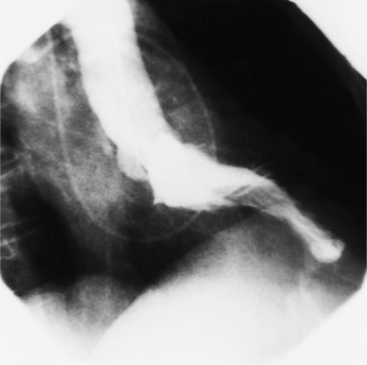
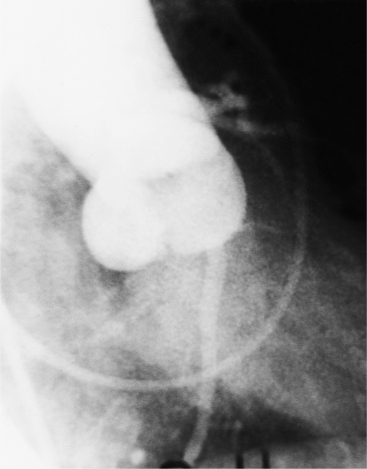
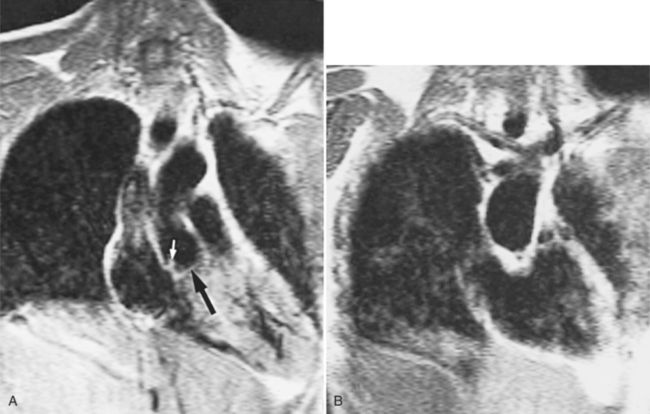
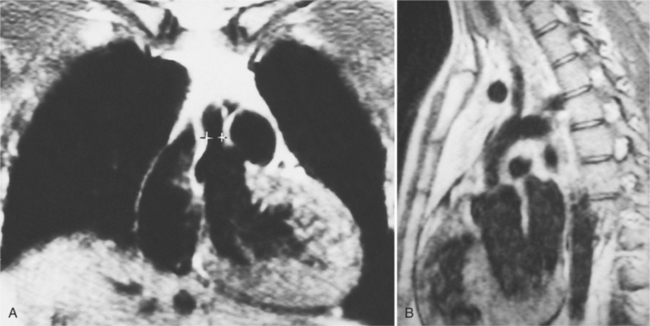
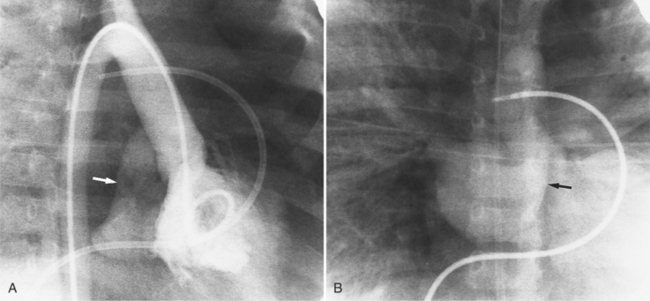
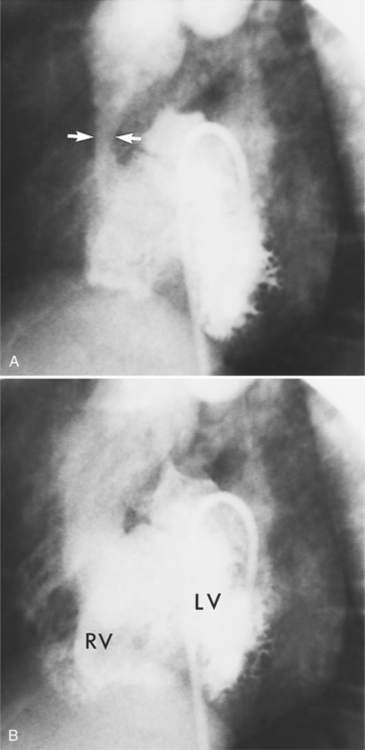
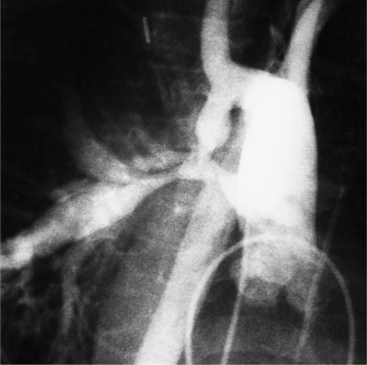
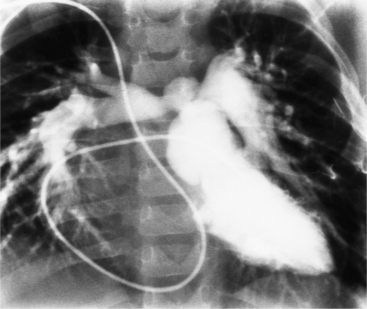
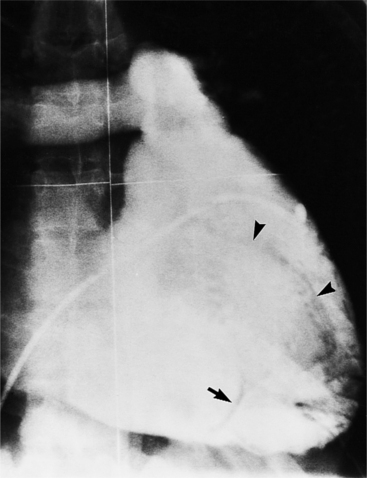
Optimal visualization of the angiographic features of most of these conditions is possible with biplane left ventriculography with cranial angulation in the left oblique projection. Because several of these malformations may be quite subtle (particularly discrete membranous subaortic stenosis), standard ventriculography may not demonstrate a lesion that is quite evident on pressure tracings. In these instances, an aortic root injection is useful because many of these valves are incompetent and the regurgitant stream outlines the subaortic chamber (Fig. 6-17). A magnetic resonance scan in planes aligned to the cardiac axis can sort out subaortic complexities with tomographic imaging (Fig. 6-18).
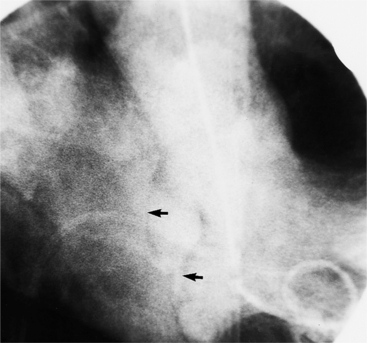
About 15% of patients with congenital obstruction to left ventricular outflow have discrete membranous subaortic stenosis. This consists of a 1- to 4-mm-thick membrane below the aortic valve. The membrane varies in position from just below the aortic valve to about 4 cm beneath it. The membrane may attach to the anterior leaflet of the mitral valve, and strands from this membrane may extend to the aortic cusps (Fig. 6-19). About one third to half of these patients have aortic insufficiency, and fewer than 5% have mild mitral insufficiency.
In tunnel subaortic stenosis, the obstruction extends 1 to 3 cm below the aortic annulus. One type is a fibromuscular bar extending near or on to the anterior leaflet of the mitral valve. Another type has a long conical narrowing in the subaortic region with little change between systole and diastole (Fig. 6-20). The left ventricle shows the usual signs of hypertrophy, but occasionally this overgrowth of muscle may produce bizarre shapes.
Idiopathic hypertrophic subaortic stenosis (discussed in Chapter 9) is a dynamic form of subaortic stenosis. The obstruction occurs in systole with the abnormal anterior systolic motion of the anterior leaflet of the mitral valve. During diastole, the stenosis disappears as the mitral leaflet resumes its normal position. A similar dynamic subvalvular stenosis may be seen in transposition of the great arteries. Here the mitral leaflet creates a subpulmonary stenosis as the left ventricle is connected to the pulmonary artery.
Subaortic Obstruction
Subaortic obstruction may rarely result from valve abnormalities or malalignment defects in complex congenital heart disease. Accessory mitral valve tissue, anomalous attachment of the mitral valve and its chordae tendineae, or a displaced annular insertion of the anterior leaflet result in outflow obstruction. The angiographic findings with each of these abnormalities varies; however, an asymmetric filling defect that moves into the subaortic region during systole and returns to a more posterior and inferior location during diastole is often visible during left ventriculography. Other complex congenital malformations have subaortic obstruction with conoventricular malalignment (e.g., aortic atresia and ventricular septal defect and in transposition with the aorta originating above an infundibular chamber).
SUPRAVALVULAR AORTIC STENOSIS
Classifications
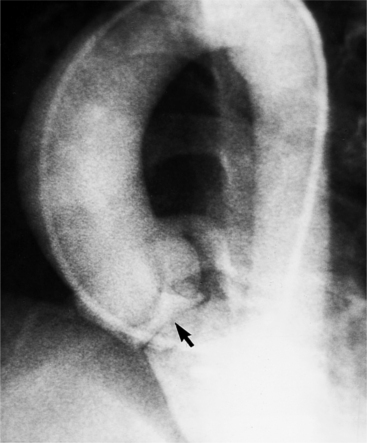
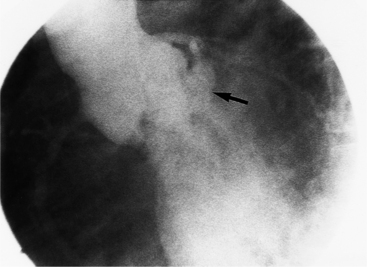
There are three types of supravalvular stenosis. The hourglass type consists of a narrow segment of the ascending aorta just distal to the origin of the coronary arteries (Fig. 6-21). The second type is a membrane or fibrous diaphragm at the sinotubular aortic junction. The least common is hypoplasia of the entire ascending aorta from the sinotubular junction to the origin of the brachiocephalic artery (Figure 6-22). In actual practice, these three forms overlap and are alternatively classified as discrete or diffuse stenoses, categories that correspond to the alternatives in surgical treatment.
AORTIC REGURGITATION
Aortic regurgitation results from a cusp abnormality, distortion of the aortic root, or dilatation of the aorta. Aortic valves that have stenosis generally also have some regurgitation. When the leaflets are abnormal, the regurgitation frequently is from rheumatic disease, infective endocarditis, or a bicuspid valve. When the ascending aorta is the cause of regurgitation, common causes are Marfan syndrome, aortic dissection, aortitis, ankylosing spondylitis, systemic hypertension, and syphilis (Box 6-2).
Chest Film Findings
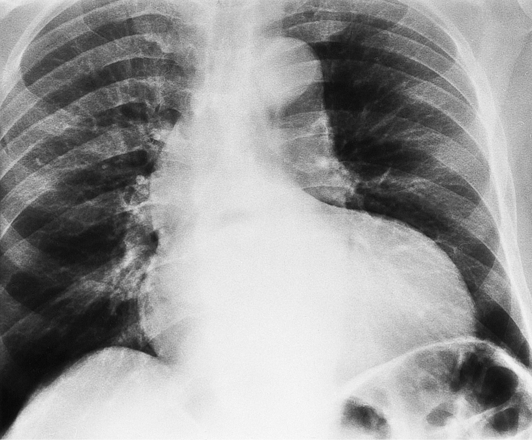
If the aortic regurgitation is both chronic and severe, the chest film hallmarks are left ventricular enlargement and dilatation of the entire aorta (Fig. 6-23). This pattern follows the principle that regurgitation of any of the heart valves enlarges structures on both sides of the insufficient valve. If the regurgitation is acute, signs of left ventricular failure are present—pulmonary edema and pleural effusions. Then, after several days, the left ventricle is visibly dilated.
Angiographic Technique
The typical examination is a biplane supravalvular aortogram performed in both oblique projections. The injection rate varies, depending on patient age, the size of the ascending aorta, and the clinical judgment of the severity of the runoff. For example, in the adult with suspected severe aortic regurgitation, an injection rate of 35 to 40 ml/sec over 2 seconds gives good visualization. Analysis of cuspal motion necessitates the cine technique.
Magnetic Resonance Imaging Technique
Cine MRI and color flow Doppler ultrasound can identify and quantify valvular regurgitation. On both techniques, the imaging plane is rotated to include the aortic root and the regurgitant jet. Cine MRI (gradient-refocused MRI), a “white blood” technique, is set up to provide repetitive loops of a cardiac cycle with both magnitude and phase reconstruction. Aortic regurgitation is identified as a discrete area of signal loss coming from the aortic valve into the left ventricle during diastole (Figure 6-24).
Cardiac-Gated Multidetector Computed Tomography
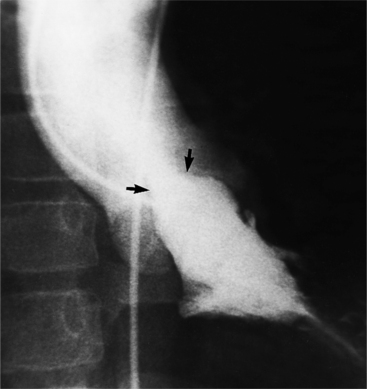
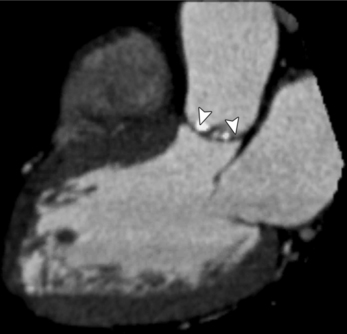
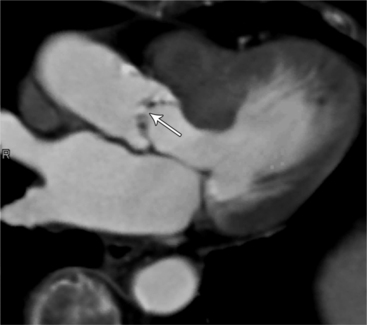
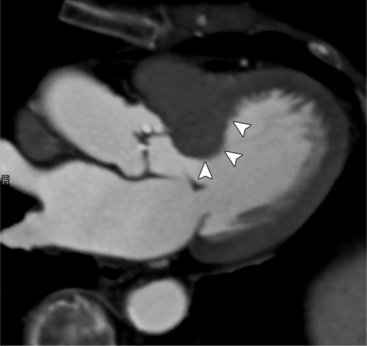
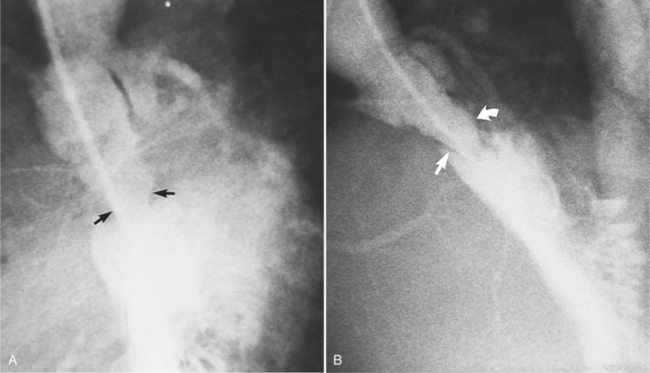
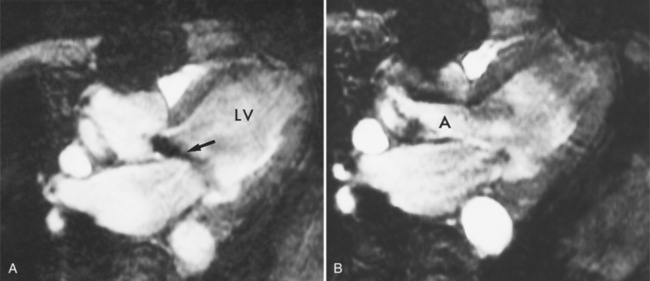
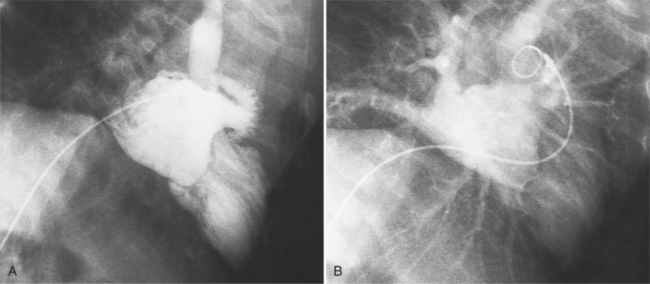
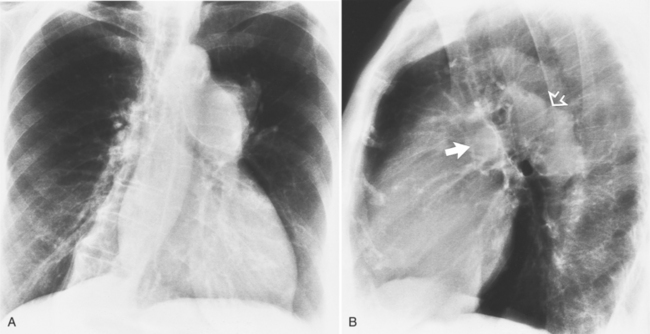
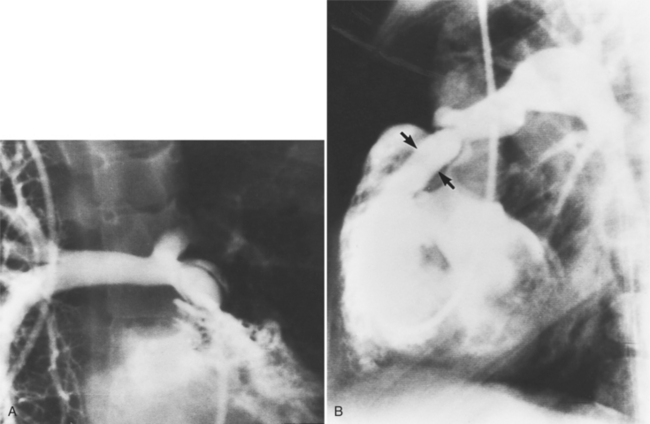
MDCT can detect the presence of aortic regurgitation by demonstrating in diastole the leaflet malcoaptation and the resultant regurgitant orifice. MDCT can demonstrate that the planimetered area of the central valvular leakage area correlates well to echocardiogram or MRI grades of aortic regurgitation severity. As with cases of aortic stenosis, MDCT has the added advantage of assessing for concurrent aortopathies. (Figures 6-25 and (6-26 demonstrate cases of mild and severe aortic regurgitation, respectively.
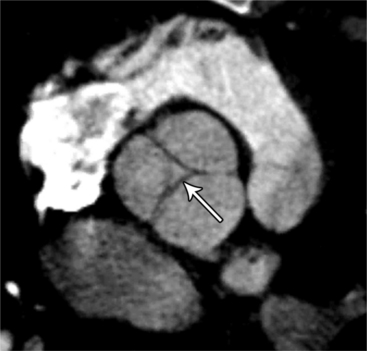
FIGURE 6-26 Severe aortic regurgitation. The aortic valve has a large central regurgitant area (arrow).
Specific Causes of Aortic Regurgitation
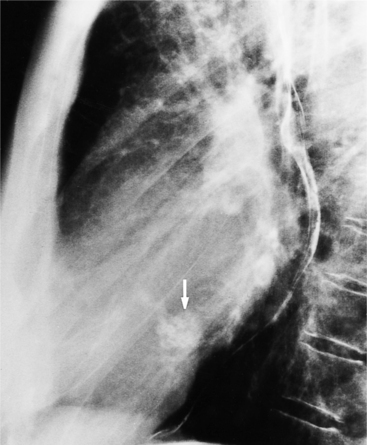
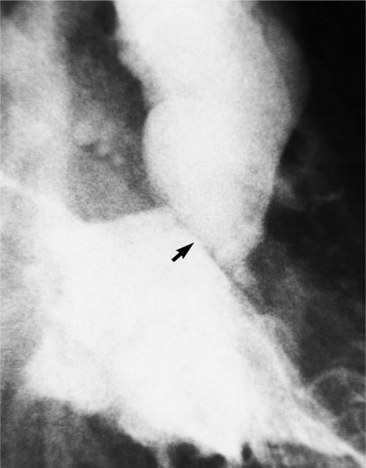
Fibrosis from the valvulitis in rheumatic heart disease is the most common aortic valve abnormality that causes regurgitation. These valves have thickened cusps that are shortened by the fibrotic process and may have some commissural fusion. Depending on the severity of the rheumatic process, the leaflets may have no visible calcium or, conversely, they may be reduced to irregular lumps with poor motion. The regurgitant jet is usually central unless the valve commissures are fused asymmetrically. In degenerative aortic valve disease, which occurs in persons over age 65, the cusps are thickened and immobile but without commissural fusion. Then regurgitation occurs over a broad front that is essentially the same diameter as the aortic annulus (Fig. 6-27). Fine fluttering of the anterior leaflet of the mitral valve is occasionally visible if the aortic regurgitation is severe.
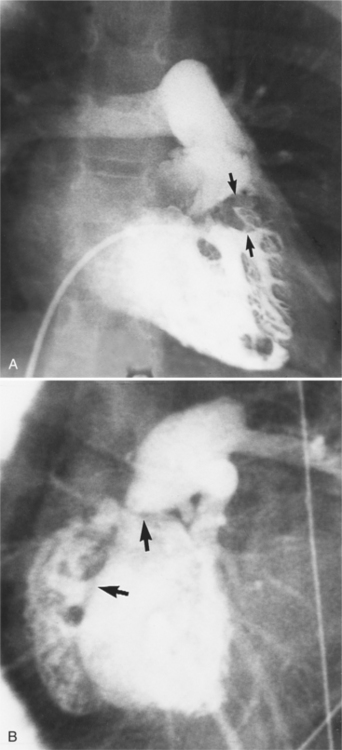
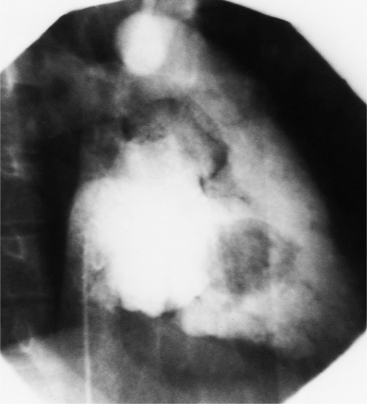
Infective endocarditis can lead to aortic regurgitation with either perforation of a cusp (Fig. 6-28) or prolapse of an aortic cusp from destruction of the adjacent annulus (Fig. 6-29).
Common consequences of aortic valve infective endocarditis are:
Prolapse of one or more cusps appears in several acquired and congenital lesions. The aortic cusps in Marfan syndrome (rarely, in other diseases of elastic tissue) are large with deep sinuses of Valsalva and laxity in their supporting structures. Eversion of an aortic cusp into the left ventricle occurs in about 5% of ventricular septal defects in which the right or the noncoronary leaflet becomes adherent to the superior margin of the septal defect (Fig. 6-30). In a traumatic tear of the aortic root and in aortic dissection, the supporting points of the leaflets may become unhinged from the annulus and then prolapse. With more severe loss of support, the leaflets can be flail with coarse vibrations in the regurgitant stream.
Primary diseases of the ascending aorta that cause dilatation, such as ankylosing spondylitis and rheumatoid arthritis, may also enlarge the orifice of the aortic valve. Cystic medial necrosis of the aorta produces regurgitation by similar mechanisms, with or without annuloaortic ectasia and dissection (Fig. 6-31). In syphilitic aortitis, the cause of regurgitation may be purely the result of the rolled and thickened edges of the aortic valve or from aneurysms of the sinuses of Valsalva or other adjacent structures.
Prosthetic Aortic Valves
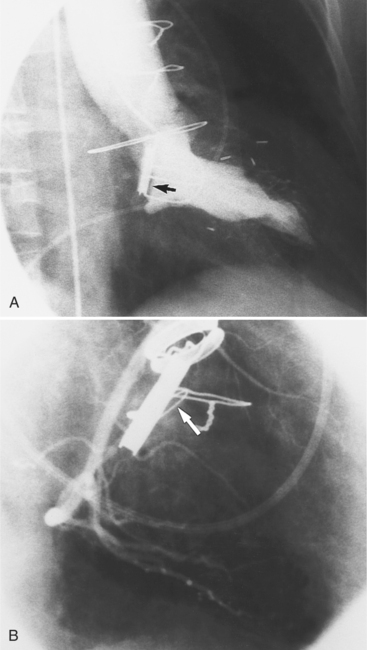
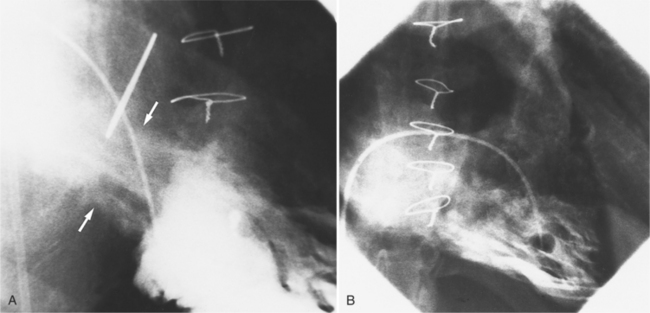
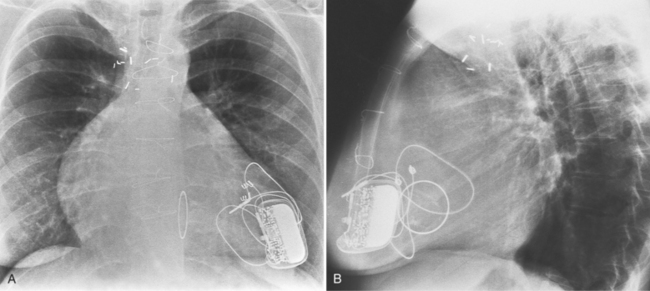
Regurgitation after cardiac valve replacement may be of two kinds: valvular or paravalvular. Growth of scar tissue into the valve may prevent the ball or disk from seating completely, thus causing regurgitation through the valve. In porcine valves, the leaflets may degenerate or perforate. The regurgitant jet occurs through the prosthesis with an eccentric jet. Paravalvular regurgitation is seen as contrast material beside the sewing ring. The usual cause of late paravalvular regurgitation, and in fact a possibility at any time after valve replacement, is prosthetic endocarditis. When this occurs, the angiographic findings are a fistula between the sinuses of Valsalva and the left ventricle and pseudoaneurysms adjacent to the prosthesis (Fig. 6-32). Vegetations may extend into the valve and cause decreased motion of the leaflets.
MITRAL STENOSIS
Early in the course of rheumatic mitral stenosis in the adult, the pulmonary blood flow redistributes to the upper lobes. Later, the pulmonary arteries enlarge as pulmonary arterial hypertension develops. Later still, the right ventricle fails, both from a pressure overload from pumping into hypertensive pulmonary arteries and from pulmonary regurgitation from a dilated annulus. In the late stage of rheumatic mitral stenosis, tricuspid regurgitation may develop from the dilated right ventricle or rarely from intrinsic rheumatic disease on the tricuspid valve.
The time course of mitral stenosis in the adult is:
Box 6-3 summarizes the causes of mitral stenosis.
Chest Film Findings
The chest film in mitral stenosis physiologically reflects the left atrial hypertension. There are signs of left atrial enlargement but the left ventricle is normal in size. In the child with a hypoplastic left heart syndrome and congenital mitral stenosis, there is an enlarged heart and pulmonary edema. In the adult with rheumatic heart disease, the onset of mitral thickening and chordal scarring and retraction occurs over such a long period that the lungs have made adaptive changes in the walls of the pulmonary arteries and veins. The lungs have an interstitial pattern that is probably part fibrosis and part edema. Pulmonary edema is visible as an interstitial pattern but not as an acinar pattern, unless there is a complication such as an infected or thrombosed valve. Patients with severe mitral stenosis may rarely have hemoptysis. The site of bleeding is probably in the engorged plexus of vessels around the middle to smaller bronchi. A late sequela of the bleeding is the development of hemosiderosis (Fig. 6-36). These deposits may ossify.
Calcification in the mitral valve is nodular and amorphous. The amount of calcium roughly correlates with the degree of mitral stenosis but, unlike the aortic valve, the mitral valve may be severely stenotic and have no radiologically visible calcification (Fig. 6-37). As a late sequela to the inflammatory carditis in acute rheumatic fever, the left atrium may calcify (Fig. 6-38). These patients have long-standing atrial fibrillation and are at risk for left atrial thrombus and emboli.
Imaging Approach to Mitral Stenosis
You will usually use echocardiography to evaluate abnormalities of the mitral valve. As discussed in Chapter 2, the area of the mitral orifice is measured by ultrasound, and the salient characteristics of mitral stenosis are graded: calcification and mobility in the valve, submitral scarring, and leaflet thickening. Left ventriculography (Fig. 6-39) is needed before percutaneous mitral valvuloplasty to grade mitral regurgitation because moderate to severe regurgitation precludes the procedure. Occasionally, pulmonary arteriogram or a left atrial injection through a patent foramen ovale helps evaluate suspected abnormalities on the left atrial side of the mitral valve. Of course, you should not use a left atrial injection from a transseptal approach if you suspect a myxoma or a thrombus because the procedure can dislodge emboli.
Rheumatic Mitral Stenosis
Retraction and scarring of the chordae tendineae may result in a subvalvular mass that mimics vegetations. The subvalvular scarring, particularly that involving the posteromedial papillary muscle, appears to pull this muscle toward the base of the left ventricle (Fig. 6-40). Subvalvular scarring itself can produce substantial mitral stenosis.
Cor Triatriatum
The chest film shows pulmonary venous hypertension and an enlarged heart. An angiogram with the catheter through the patent foramen ovale shows a distorted, small atrial chamber without visualization of the pulmonary veins or their connections with the heart (Fig. 6-41).
Congenital Mitral Stenosis—Hypoplastic Left Heart Syndrome
Congenital mitral stenosis is an uncommon malformation and comprises several anatomic types. Typical congenital mitral stenosis consists of obstruction by the leaflets and their chordal attachments. The leaflets are thickened and bulge into the left ventricular cavity during diastole (Fig. 6-42). There are two papillary muscles that have reduced interpapillary distance; the left ventricle is normal in size. Another type of mitral obstruction is a supramitral ring that originates on the left atrial side of the mitral valve as accessory tissue above and adjacent to the mitral orifice. In the parachute mitral valve, a single papillary muscle is present that receives all the chordae. This single papillary muscle is visible as a filling defect within the left ventricle on its inferior surface. The mitral leaflets are thickened and deformed and may show eccentric doming. Shone syndrome consists of the parachute mitral valve, supravalvular ring of the left atrium, subaortic stenosis, and coarctation of the aorta. A hypoplastic mitral valve associated with hypoplasia of the remainder of the left side of the heart characterizes the hypoplastic left heart syndrome. This can be distinguished from other congenital forms because this type has hypoplasia of all parts of the mitral valve: the leaflets, chordae, papillary muscles, and annulus.
MITRAL REGURGITATION
Box 6-4 summarizes the causes of mitral regurgitation.
Angiographic Evaluation
Mitral Valve Prolapse
There is a roughly 5% incidence of mitral valve prolapse in the general population. There may be mild prolapse in normal hearts, or mitral prolapse associated with severe mitral regurgitation, subacute bacterial endocarditis, chest pain, or rarely, sudden death. Intrinsic mitral prolapse is present from birth, such as that in Marfan syndrome in which both the mitral and tricuspid valves have redundant atrioventricular leaflets. The angiographic hallmark of mitral prolapse during left ventriculography is the passage of the mitral leaflets behind the plane of the mitral annulus into the left atrium. Geometric distortion of the left ventricle, such as right ventricular enlargement that bows the interventricular septum to the left in atrial septal defect, can create moderate prolapse (Fig. 6-43).
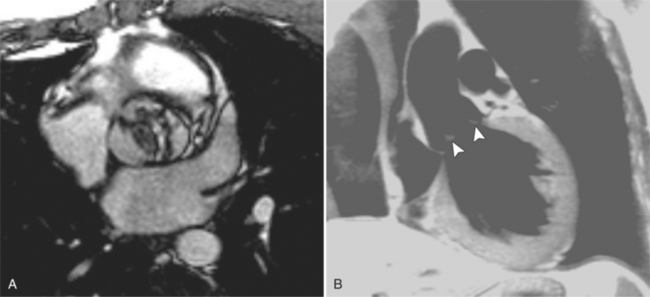
MDCT can also nicely demonstrate mitral valve prolapse (Fig. 6-44). Diagnostic criteria for mitral valve prolapse on cardiac-gated MDCT are the same as echocardiographic criteria, that is, that the mitral valve leaflets must prolapse beyond the annular plane into the left atrium by greater than 2 mm in the long-axis three-chamber view. The leaflet motion may begin in any scallop of the posterior leaflet or of the anterior leaflet. Then as systole continues, the prolapse proceeds as a posterior curling of the central and free edge of the leaflet. Occasionally, the chordae tendineae are visible as unusually long linear lucencies. The onset of prolapse occurs early in systole and becomes more severe toward the middle and end of systole.
Chordal Rupture
Mitral prolapse or flail leaflets occur in more than half of left ventriculograms performed in the clinical setting of chordae rupture (Fig. 6-45). Which leaflet prolapses depends on the location of the ruptured chordae, but in general the posterior leaflet, often the posteromedial scallop, is the more frequent location. A flail leaflet implies rupture of numerous chordae and suggests the possibility of detachment of the head of the papillary muscle. When a flail leaflet occurs, there is an erratic motion of one of the leaflets into the left atrium. The motion of the leaflet is different from beat to beat and frequently has a superimposed high-frequency whipping motion. There is always severe mitral regurgitation.
Imaging Complications of Mitral Valve Replacement
Prosthetic valve stenosis, regurgitation, and dysfunction are general indications for postoperative catheterization. Bleeding, tamponade, and myocardial infarction are usually diagnosed by clinical criteria. Prosthetic mitral stenosis is diagnosed by measuring the transvalvular gradient with a catheter in the left ventricle and another in the left atrium. The valve leaflet or disk may not have a full range of motion or may be stuck half-open (Fig. 6-46). Prosthetic mitral regurgitation, depending on the type of valve, may be through the valve or beneath it (Fig. 6-47).
PULMONARY STENOSIS AND REGURGITATION
(Boxes 6-5 and 6-6 summarize the causes of pulmonary stenosis and regurgitation.
Chest Film Findings
The chest film findings in pulmonary stenosis are variable and depend on the age of the patient and on associated abnormalities. In the infant, the large thymus may obscure the mediastinum so you can detect the disease only if there is decreased pulmonary flow. In the older child and adult, the classic film of valvular pulmonary stenosis shows mild enlargement of the right ventricle and moderate enlargement of the main and left pulmonary artery (Fig. 6-48). The right pulmonary artery has normal size (Fig. 6-49). In congenital pulmonary stenosis, particularly with hypoplasia of the main pulmonary artery, the pulmonary artery segment is concave. Unless the pulmonary obstruction is so severe as to reduce cardiac output, the overall heart size is normal. In pulmonary valve atresia, with or without an intact ventricular septum, the heart is enlarged, and the cardiac contour is distinctive with right atrial dilatation (Fig. 6-50). The chest film in supravalvular pulmonary stenosis typically shows a straight main pulmonary artery segment and small hilar structures.
Cardiac-Gated Multidetector Computed Tomography
The normal tricuspid and pulmonary cardiac valves are not well visualized on cardiac-gated MDCT. Typical scanning protocols optimize arterial phase enhancement and the right-sided chambers contain either saline or a mixture of saline and contrast at the time of image acquisition. Only infrequently are the thin pulmonic or tricuspid valve leaflets visualized.
Valvular Pulmonary Stenosis
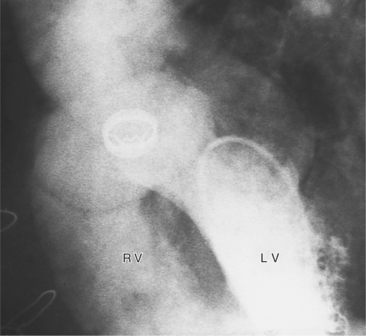
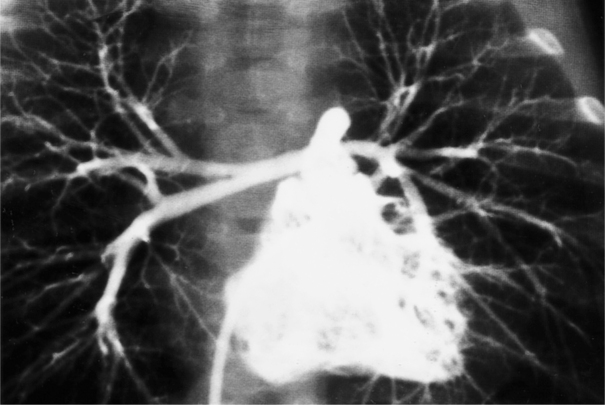
Angiographically, the pulmonary valve is evaluated with a right ventriculogram. The leaflets have a domed appearance in systole, yet form the normal sinuses of Valsalva in diastole (Fig. 6-51). The jet of contrast through the lesion is in the center of the valve and points toward the main and left pulmonary arteries, producing poststenotic dilatation of these structures and sparing the right pulmonary artery. Although there is usually poststenotic dilatation of the pulmonary artery, the degree of dilatation does not correlate well with the severity of the gradient. When other lesions are present, as in tetralogy of Fallot, the pulmonary artery segment is concave.
In some patients with valvular pulmonary stenosis, the infundibulum may be hyperkinetic with extreme narrowing in systole but a normal diastolic diameter (Fig. 6-52). The hyperkinetic systolic narrowing of the infundibulum occurs in its middle portion during contraction of the septal and parietal bands of the crista supraventricularis, giving an hourglass appearance to the infundibulum. After surgical or angioplasty repair of the stenotic valve, this subvalvular infundibular stenosis may be accentuated more because of the decreased afterload and, in rare instances, fatal right ventricular failure occurs because of complete occlusion of the hyperdynamic outflow tract: the suicidal right ventricle.
The dysplastic pulmonary valve and the bicuspid valve represent less common types of valvular stenosis. The dysplastic pulmonary valve, which occurs in 15% of hearts with isolated pulmonary stenosis, has three cusps, which are thickened with unfused commissures. The bases of the sinuses of Valsalva are deformed and small as a result of excess tissue at the base of the leaflets (Fig. 6-53). The dysplasia also includes the pulmonary annulus, which is hypoplastic, as is the main pulmonary artery adjacent to the sinuses. Because the leaflets are stiff and thick, there is little change in their position throughout the cardiac cycle. This motion is in contrast to that in typical valvular stenosis with fused commissures, which shows a flexible domed membrane in systole and rounded sinuses of Valsalva in diastole. The sinuses in the dysplastic pulmonary valve are irregular and narrow in diastole; the thick leaflets produce an asymmetrically oriented orifice in systole. The recognition of the dysplastic pulmonary valve is important because unlike other types of valvular stenosis, it responds poorly to percutaneous valvuloplasty.
The bicuspid pulmonary valve appears similar to the bicuspid aortic valve, namely, unequal sinuses of Valsalva with fusion of one of the leaflets with a raphe, and a sinus of Valsalva occupying about half the valve circumference. Bicuspid pulmonary valves are present in about 50% of patients with tetralogy of Fallot. As part of this malformation, the annulus is often small, and the supravalvular region of the main pulmonary artery is hypoplastic. This combination of abnormalities produces an angiographic appearance that has been likened to “an open clamshell engulfing the infundibulum” (Fig. 6-54). In addition to the other features of tetralogy of Fallot, the areas of obstruction include the infundibular and supravalvular narrowing. There is usually no poststenotic dilatation of the distal main pulmonary artery.
The annulus of the pulmonary valve may be hypoplastic. The leaflets of the valve and the adjacent main pulmonary artery are usually small and form part of the stenosis (Fig. 6-55).
Congenital absence of the pulmonary valve is usually associated with a ventricular septal defect, annular pulmonary stenosis, and aneurysmal dilatation of the main pulmonary artery and the central part of both the right and left pulmonary arteries. This lesion can occur as an isolated entity but is associated with tetralogy of Fallot. The syndrome may also include a right aortic arch and peripheral pulmonary artery stenoses. The hilar pulmonary arteries may compress the bronchi, leading to hyperinflated lungs.
Subvalvular Pulmonary Stenosis
Subpulmonary obstruction may occur either in the infundibulum or at the junction of the right ventricular body with the infundibulum. Primary infundibular obstruction is a rare malformation that may appear as either a fibrous band or a threadlike cavity through the infundibulum (Fig. 6-56). Discrete narrowing at the level of the crista supraventricularis divides the right ventricle into a main cavity and an infundibular chamber. In this situation, there is contraction of the infundibular chamber during systole, leading to more severe obstruction in addition to its fixed, discrete narrowing. The pulmonary valve may be normal but usually shows slight thickening, presumably because of the turbulence created by the obstruction.
Infundibular stenosis frequently coexists with valvular stenosis. This type is not seen until after the patient is several months old, when other signs of right ventricular hypertrophy also become visible. Dynamic infundibular stenosis during systole may appear with a ventricular septal defect. This hypertrophy in the outflow tract decreases the left-to-right flow across the ventricular septal defect (Gasul phenomenon) and may cause stenosis that increases with age (Fig. 6-57).
Obstructing muscular bands of the right ventricle, an abnormality that has also been called double-chambered right ventricle, may cause an obstruction either in the body of the right ventricle or higher in the infundibular region. These muscle bundles are seen as filling defects in the right ventricle, which may not contract. When the anomalous muscle occurs in the true right ventricular cavity, its location is variable; it may extend from the apex as a triangular mass on the posteroanterior projection or from the tricuspid valve to the junction of the infundibulum as a diagonal or wedge-shaped filling defect (Fig. 6-58).
Supravalvular Pulmonary Stenosis
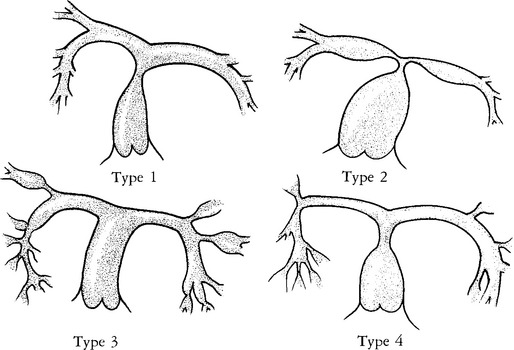
These stenoses have a wide spectrum of morphologic appearance, from a short, discrete area of narrowing to long, diffuse hypoplastic segments involving several branches. There may be poststenotic dilatation with variable caliber of the peripheral artery. Gay and colleagues have classified the stenoses for surgical therapy according to their location. Type 1 has a single stenosis in the main pulmonary artery. Type 2 occurs at the bifurcation of the main with the right and left pulmonary arteries. Type 3 has only peripheral or branch stenoses. Type 4 is a mixture of the other types (Fig. 6-59).
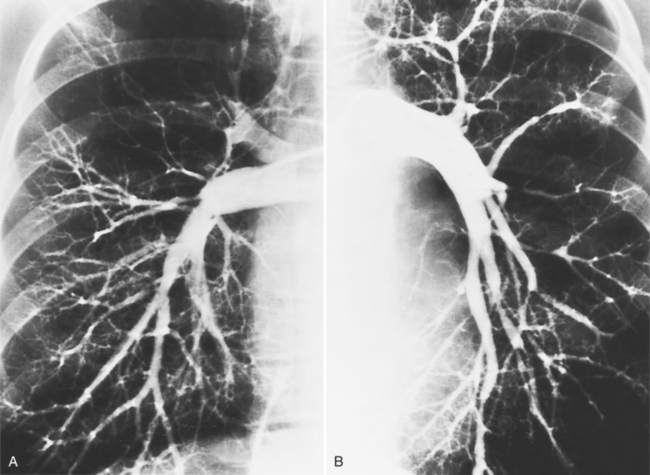
Diffuse pulmonary artery hypoplasia is a congenital malformation that may occur in isolation or with cardiac anomalies (Fig. 6-60). Centrally located segmental stenoses may be congenital (Fig. 6-61), whereas peripheral pulmonary arterial stenosis usually are secondary to other diseases (Fig. 6-62). Scarred lung from previous pneumonia, bullous emphysema, recanalized pulmonary emboli, and pulmonary arteritis from Takayasu disease are common causes.
Acquired supravalvular pulmonary stenosis in the form of pulmonary banding is created intentionally to reduce torrential pulmonary blood flow. The band is correctly positioned above the pulmonary annulus in the main pulmonary artery but may migrate distally causing unwanted branch stenosis (Fig. 6-63). A band placed too close to the pulmonary valve may cause leaflet thickening.
TRICUSPID VALVE DISEASE
Tricuspid Stenosis
The chest film in tricuspid valve disease is quite variable. The abnormalities in tricuspid stenosis follow the principle that the chamber behind a severe stenosis is large. Although right atrial enlargement is always seen, the right ventricle and the left side of the heart frequently are also big because tricuspid stenosis is a late sequela of rheumatic mitral stenosis. The superior vena cava and azygos vein are enlarged. In congenital Ebstein anomaly, the film may be strikingly specific with its right atrial and right ventricular enlargement. The right atrium at the junction with the superior vena cava has an unusual rounded appearance (Fig. 6-64). In other types of tricuspid valve disease, the plain film shows nonspecific cardiac enlargement, occasionally with dilatation of the superior and inferior vena cava. In rheumatic heart disease, the features of mitral stenosis predominate: left atrial enlargement and pulmonary artery enlargement. Tricuspid valve calcification rarely may be seen at fluoroscopy. Tricuspid annular calcification has the same causes as mitral annular calcification, namely, dystrophic degeneration from aging and from chronic severe right ventricular hypertension (Fig. 6-65).
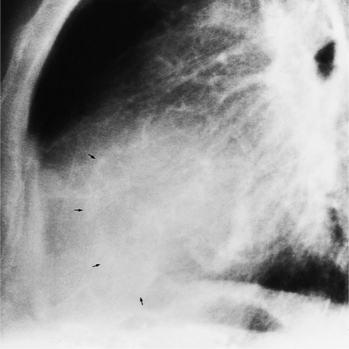
FIGURE 6-65 Tricuspid annular calcification. Arrows outline the tricuspid annulus. The mitral annulus is also calcified.
(Courtesy Robert E. Dinsmore, MD.)
Box 6-7 summarizes the causes of tricuspid stenosis.
Tricuspid Regurgitation
Similar to mitral regurgitation, tricuspid regurgitation occurs when there is an abnormality in one or several parts of the tricuspid apparatus: the annulus, leaflets, chordae, papillary muscles, and right ventricular wall. Dilatation of the right ventricle and the tricuspid annulus secondary to pulmonary artery hypertension from mitral valve disease is the most common cause of acquired regurgitation (Fig. 6-66). When the abnormality causing the right ventricular enlargement (i.e., mitral stenosis) is corrected, right ventricular size usually returns to normal and the regurgitation ceases.
The grading of tricuspid regurgitation is similar to that of mitral regurgitation:
Diseases of the leaflets include rheumatic heart disease, the carcinoid syndrome, infective endocarditis, and trauma. Endocarditis from Staphylococcus typically destroys the tricuspid leaflets or causes multiple perforations and small, irregular vegetations on the ventricular side of the leaflets. Infection from Candida can produce large fungal tumors that partially obstruct blood flow (Fig. 6-67).
Box 6-8 summarizes the causes of tricuspid regurgitation.
Ebstein Anomaly
The plain film of the chest in Ebstein anomaly shows a cardiac silhouette that varies from normal to strikingly enlarged. You should suspect this anomaly when you detect right atrial and right ventricular dilatation (Fig. 6-68). The contour of the large right atrium is round and occupies the right cardiac border. It is continuous with the superior vena cava and the right hemidiaphragm in the posteroanterior view. Dilatation of the right ventricle ordinarily is most visible on a lateral projection. However, if enlargement is massive, the right ventricle will contact the sternum and rotate the entire heart leftward. The result of this motion is that the frontal film will show a convexity in the upper cardiac border, which is not the left atrial appendage but rather the right ventricular outflow tract. Further dilatation of the right ventricle can cause it to become the entire left heart border. The size of the pulmonary vessels tends to reflect the amount of blood flowing through them. In those with atrial septal defects and moderate left-to-right shunts, the vascular markings are slightly large; in cyanotic patients with right-to-left flow across the interatrial shunt, the vessels tend to be small because of tricuspid stenosis.
You can make the angiographic diagnosis if the notch of the atrioventricular groove is separate from the displaced leaflet attachments (Fig. 6-69). This atrialized portion of the right ventricle is on the right atrial side of the tricuspid leaflets; it beats synchronously with the remainder of the ventricle because this segment contains contractile ventricular muscle. Occasionally, the wall of the atrialized portion of the ventricle may be only a fibrous sac and therefore be akinetic. The dilatation of the right atrium and right ventricle reflects the degree of tricuspid stenosis and regurgitation.
Patients with congenitally corrected transposition and a posteriorly placed right ventricle may have an associated Ebstein anomaly. Tricuspid regurgitation in congenitally corrected transposition should always raise the suspicion of a left-sided Ebstein anomaly; however, the tricuspid leaflet attachment in congenitally corrected transposition without Ebstein anomaly has slight apical displacement, so subtle degrees of the leaflet malformation may not be apparent.
Alkadhi H, Desbiolles L, Husmann L, et al. Aortic regurgitation: Assessment with 64-section CT. Radiology. 2007;245:111.
Alkadhi H, Wildermuth S, Bettex DA, et al. Mitral regurgitation: quantification with 16-detector row CT—initial experience. Radiology. 2006;238(2):454-463.
Alkadhi H, Bettex D, Wildermuth S, et al. Dynamic cine imaging of the mitral valve with 16-MDCT: a feasibility study. Am J Roentgenol. 2005;185(3):636-646.
Altrichter PM, Olson LJ, Edwards WD, et al. Surgical pathology of the pulmonary valve: a study of 116 cases spanning 15 years. Mayo Clin Proc. 1989;64(11):1352-1360.
Barlow JB, Pocok WA, Promund Obel IW. Mitral valve prolapse: primary, secondary, both or neither? Am Heart J. 1981;102:140.
Boughner DR, Thornton M, Dunmore-Buyze J, et al. The radiographic quantitation of aortic valve calcification: implications for assessing bioprosthetic valve calcification in vitro. Physiol Meas. 2000;21(3):409-416.
Boxt LM, Lipton MJ, Kwong RY, et al. Computed tomography for assessment of cardiac chambers, valves, myocardium and pericardium. Cardiol Clin. 2003;21:561-585.
Carabello BA. Mitral valve regurgitation. Curr Probl Cardiol. 1998;23(4):202-241.
Carey LS, Sellers RD, Shone JD. Radiology findings in the developmental complex of parachute mitral valve, supravalvular ring of left atrium, subaortic stenosis and coarctation of aorta. Radiology. 1964;82:1.
Carlsson E, Gross R, Holt RG. The radiological diagnosis of cardiac valvar insufficiencies. Circulation. 1977;55:921.
Castaneda-Zuniga WR, Formanek A, Amplatz K. Radiologic diagnosis of different types of pulmonary stenosis. Cardiovasc Radiol. 1978;1:45.
Cowell SJ, Newby DE, Burton J, et al. Aortic valve calcification on computed tomography predicts the severity of aortic stenosis. Clin Radiol. 2003;58(9):712-716.
Davachi F, Moller JH, Edwards JE. Diseases of the mitral valve in infancy: an anatomic analysis of 55 cases. Circulation. 1971;43:565.
Davies MJ. Pathology of cardiac valves. Boston: Butterworth, 1980.
Davies SW, Gershlick AH, Balcon R. Progression of valvar aortic stenosis: a long-term retrospective study. Eur Heart J. 1991;12(1):10-14.
Desilets DT, Marcano BA, Emmanoulides GC, et al. Severe pulmonary valve stenosis and atresia. Radiol Clin North Am. 1968;6:367.
Didier D. Assessment of valve disease: qualitative and quantitative. Magn Reson Imaging Clin N Am. 2003;11(1):115-134. vii
Didier D, Ratib O, Lerch R, et al. Detection and quantification of valvular heart disease with dynamic cardiac MR imaging. Radiographics. 2000;20:1279-1299.
Fisher CH, James AE, Humphries JO, et al. Radiographic findings in anomalous muscle bundle of the right ventricle: an analysis of 15 cases. Radiology. 1971;101:35-43.
Freedom RM, Culham JA G, Rowe RD. Angiocardiography of subaortic obstruction in infancy. Am J Roentgenol. 1977;129:813-824.
Gilkeson RC, Markowitz AH, Balgude A, et al. MDCT evaluation of aortic valvular disease. Am J Roentgenol. 2006;186(2):350-360.
Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56(1):56-64.
Kaden JJ, Freyer S, Weisser G, et al. Correlation of degree of aortic valve stenosis by Doppler echocardiogram to quantity of calcium in the valve by electron beam tomography. Am J Cardiol. 2002;90(5):554-557.
Kaminaga T, Naito H, Takamiya M, et al. Quantitative evaluation of mitral regurgitation with ultrafast CT. J Comput Assist Tomogr. 1994;18(2):239-242.
Kim JH, Wiseman A, Kisslo J, et al. Echocardiographic detection and clinical significance of left atrial vegetations in active infective endocarditis. Am J Cardiol. 1989;64(14):950-952.
Koos R, Kuhl HP, Muhlenbruch G, et al. Prevalence and clinical importance of aortic valve calcification detected incidentally on CT scans: comparison with echocardiography. Radiology. 2006;241(1):76-82.
Koos R, Mahnken AH, Sinha AM, et al. Aortic valve calcification as a marker for aortic stenosis severity: assessment on 16-MDCT. Am J Roentgenol. 2004;183(6):1813-1818.
Lembcke A, Borges AC, Dohmen PM, et al. Quantification of functional mitral valve regurgitation in patients with congestive heart failure: comparison of electron-beam computed tomography with cardiac catheterization. Invest Radiol. 2004;39(12):728-739.
Lembcke A, Wiese TH, Enzweiler CN, et al. Quantification of mitral valve regurgitation by left ventricular volume and flow measurements using electron beam computed tomography: comparison with magnetic resonance imaging. J Comput Assist Tomogr. 2003;27(3):385-391.
Liu F, Coursey CA, Grahame-Clarke C, et al. Aortic valve calcification as an incidental finding at CT of the elderly: severity and location as predictors of aortic stenosis. Am J Roentgenol. 2006;186(2):342-349.
Mahnken AH, Koos R, Wildberger JE, et al. [Value of cardiac multislice spiral CT for the assessment of degenerative aortic stenosis: comparison with echocardiography]. Rofo. 2004;176(11):1582-1588.
Marks AR, Choomg CY, Sanfilippo AJ, et al. Identification of high-risk and low-risk subgroups of patients with mitral-valve prolapse. N Engl J Med. 1989;330:1031-1036.
Messsika-Zeitoun D, Serfaty JM, Laissy JP, et al. Assessment of mitral valve area in patients with mitral stenosis by multislice computed tomography. J Am Coll Cardiol. 2006:411-413.
Miller SW, Dinsmore RE. Aortic root abscess resulting from endocarditis: spectrum of angiographic findings. Radiology. 1984;153:357-361.
Morgan-Hughes GJ, Owens PE, Roobottom CA, et al. Three dimensional volume quantification of aortic valve calcification using multislice computed tomography. Heart. 2003;89(10):1191-1194.
Muhlenbruch G, Wildberger JE, Koos R, et al. Calcium scoring of aortic valve calcification in aortic valve stenosis with a multislice computed tomography scanner: non-enhanced versus contrast-enhanced studies. Acta Radiol. 2005;46(6):561-566.
Nestico PF, Depace NL, Morganroth J, et al. Mitral annular calcification: clinical, pathophysiology, and echocardiographic review. Am Heart J. 1984;107:989-996.
Numan F, Islak C, Berkman T, et al. Behçet disease: pulmonary artery involvement in 15 cases. Radiology. 1994;192:465-468.
Pannu HK, Jacobs JE, Lai S, et al. Gated cardiac imaging of the aortic valve on 64-slice multidetector row computed tomography: preliminary observations. J Comput Assist Tomogr. 2006;30(3):443-446.
Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26(1):72-83.
Roberts WC. The congenitally bicuspid aortic valve: a study of 85 autopsy cases. Am J Cardiol. 1970;26:72-83.
Roberts WC. Valvular, subvalvular, and supravalvular aortic stenosis. Morphologic features. Cardiovasc Clin. 1973;5:97-126.
Roberts WC, Perloff JK. A clinicopathologic survey of the conditions causing the mitral valve to function abnormally. Ann Intern Med. 1972;77:939-975.
Rozenshtein A, Boxt LM. Computed tomography and magnetic resonance imaging of patients with valvular heart disease. J Thorac Imaging. 2000;15(4):252-264.
Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;2000(343):611-617.
Ryan R, Abbara S, Colen R, et al. Cardiac valve disease: Spectrum of findings on cardiac 64-MDCT. Am J Roentgenol. 2008;190:W294-W303.
Sechtem U, Pflugfelder PW, Cassidy MM, et al. Mitral or aortic regurgitation: quantitation of regurgitant volumes with cine MR imaging. Radiology. 1988;167:425-430.
Selzer A. Changing aspects of the natural history of valvular aortic stenosis. N Engl J Med. 1987;317:91-98.
Shavelle DM, Budoff MJ, Buljubasic N, et al. Usefulness of aortic valve calcium scores by electron beam computed tomography as a marker for aortic stenosis. Am J Cardiol. 2003;92(3):349-353.
Silver MD, Gotlieb AI, Schoen FJ. Cardiovascular pathology. New York: Churchill Livingstone, 2001.
Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol. 1997;29:630-634.
Vogel-Claussen J, Pannu H, Spevak PJ, et al. Cardiac valve assessment with MR imaging and 64-section multidetector row CT. Radiographics. 2006;26(6):1769-1784.
Wagner S, Selzer A. Patterns of progression of aortic stenosis: a longitudinal hemodynamic study. Circulation. 1982;65(4):709-712.
Waller BF. The operatively excised pulmonic valve—a forgotten entity. Mayo Clin Proc. 1989;64(11):1452-1454.
Willmann JK, Kobza R, Roos JE, et al. ECG-gated multi-detector row CT for assessment of mitral valve disease: initial experience. Eur Radiol. 2002;12(11):2662-2669.
Willmann JK, Weishaupt D, Lachat M, et al. Electrocardiographically gated multi-detector row CT for assessment of valvular morphology and calcification in aortic stenosis. Radiology. 2002;225(1):120-128.