Chapter 28 Percutaneous Translaryngeal Jet Ventilation
I Introduction
Invasive airway management is relegated to the category of rescue techniques that one must know but hopes never to use. Students may be able to witness a surgical cricothyrotomy, and possibly to assist in the performance of an elective tracheostomy, but most anesthesiologists have successfully avoided incorporating these procedures into their practice. Although the modern airway armamentarium has made resorting to invasive procedures a rare event, the “cannot intubate, cannot ventilate” (CICV) scenario still occurs.1 By equipping the clinician with an alternative to routine upper airway management, the potential for rescuing a failed airway and preventing devastating adverse outcomes is substantially increased.
Multiple studies have indicated that the incidence of the CICV scenario is between 0.01 and 2.0 cases per 10,000 anesthesias.1–3 Although the root cause of this dire apneic or obstructive state may be fully or partially related to the patient’s disease, it often has an iatrogenic component, being recognized at the time of anesthetic induction or as a result of other interventions. The American Society of Anesthesiologists (ASA), The Difficult Airway Society, and The Advanced Trauma Life Support guidelines, as well as the opinions of other expert organizations, concur that, although invasive airway access is rarely practiced or prepared for, this capability must be at the fingertips of any operator who is charged with airway management.4–6 The conditions that commonly require the use of invasive airway access include facial fractures (32%), blood or vomit in the airway (32%), traumatic airway obstruction (7%), and failed intubation in the absence of other indications (11%).7 A completely obstructed airway state does not have to exist for a patient to be at jeopardy. Any time the clinician cannot ensure adequate, life-sustaining gas exchange by virtue of a problem within the conveyance portions of the respiratory tract, further actions (noninvasive or invasive) are mandatory.
Although surgical cricothyrotomy has long been a standard of emergency invasive airway rescue, its use has declined, in part because of advances in noninvasive airway devices such as supralaryngeal airways (SLAs) and video laryngoscopes, the adoption of pharmacologic agents for rapid-sequence intubation in the emergency department, and increased requirements for trainee supervision.8
There is widespread agreement in the literature that percutaneous translaryngeal jet ventilation (PTJV),5 using a small-bore catheter placed through the cricothyroid membrane (CTM), is a simple and effective treatment for the desperate CICV situation.8,9 Compared with surgical cricothyrotomy and tracheostomy, the establishment of PTJV is ordinarily quicker, simpler, and therefore more efficacious for most anesthesiologists, who may not be practiced in more formal surgical techniques.8,10 This may not hold true for the infant or small child, however. Although PTJV was faster to achieve than a surgical airway, it had an exceedingly high failure rate (83%) in a swine model of the juvenile population.11 This is likely due to the lack of circumferential support offered by the immature cricoid cartilage.
A A Note on Nomenclature
The literature is replete with descriptions of PTJV techniques and of a number of devices, both commercial and “operating room [OR] rigged.”12–16 As discussed here, many of these OR-rigged PTJV setups have not undergone objective testing; although they are easy to construct, they may not be practical, robust, or life-sustaining.
II Incorporating Percutaneous Translaryngeal Jet Ventilation into the Difficult Airway Plan
The 2003 ASA difficult airway (DA) algorithm lists three techniques that should be employed when circumstances are encountered in which one cannot intubate (i.e., via direct laryngoscopy) and cannot ventilate, either by bag-mask or with the use of a laryngeal mask airway (LMA; LMA North America, Inc., San Diego, CA). These techniques are insertion of an esophageal-tracheal Combitube (ETC; Ambu, Copenhagen, Denmark), use of PTJV, and provision of an emergency surgical airway. Because the LMA and ETC are familiar to most anesthesiologists, both of these noninvasive airways should be considered first in cases of supraglottic obstruction, unfavorable anatomy, or other CICV situations. Both have been demonstrated to be highly effective in this setting and are generally considered atraumatic.4,17,18 Though other SLAs are likely to be as applicable, they are not mentioned in the 2003 practice guidelines. Whether or not future guideline revisions call for other devices or refer to generic SLAs, operator experience and comfort should guide choice. The preferred device should be immediately available whenever the possibility of a CICV situation might arise.17
If insertion of an SLA does not affect gas exchange quickly, the ASA DA algorithm recommends that the clinician move to an invasive airway access maneuver without hesitation or delay. Of the maneuvers suggested, PTJV may be the most familiar to the anesthesiologist. A distinct advantage of PTJV over surgical cricothyrotomy is that it can be practiced in nonemergency clinical care. The anesthesiologist who is practicing elective, awake intubation may choose to make the percutaneous transtracheal injection of lidocaine a routine technique, thereby practicing laryngeal catheter placement, and some have advocated the placement of prophylactic transtracheal catheters in high-risk airway situations or for elective laryngeal surgery.19 It should be noted that severe subcutaneous emphysema is a rare complication of translaryngeal lidocaine injection, even in otherwise uncomplicated cases.20
III Incidence and Complications
Barotrauma with resultant pneumothorax and hemodynamic changes has been reported with both emergent and elective use of PTJV.8,19,21–31 In a retrospective chart review, Patel and associates described a 53-month experience with patients in acute respiratory failure in the intensive care unit.21 Of the 352 patients requiring endotracheal intubation, 29 emergent PTJV attempts were made. The procedure was performed by house staff (n = 5) and attending physicians (n = 24). Successful cannulation of the airway was achieved in 79% of patients. Subcutaneous emphysema occurred in 2 of these patients. Orotracheal intubation was subsequently required in 22 patients, with 1 requiring a surgical tracheostomy. PTJV failed in 6 patients. These failures were attributed to recent thyroid surgery (n = 1), obesity (n = 2), kinking of the PTJV cannula (n = 2), and misplacement of the cannula (n = 1). Subcutaneous emphysema was seen in only 1 patient for whom PTJV failed. The other patient who suffered subcutaneous emphysema experienced severe pneumomediastinum treated with bilateral chest tubes. Of the 6 patients with failed PTJV, 2 were subsequently orally intubated with the aid of a bougie, and the remaining 4 died.
Smith and colleagues reported a 29% incidence of complications in 28 patients managed with PTJV to provide an emergency airway.22 These complications included subcutaneous emphysema (7.1%), mediastinal emphysema (3.6%), exhalation difficulty (14.3%), and arterial perforation (3.6%), none of which were fatal. Other complications, such as esophageal puncture, bleeding, hematoma, and hemoptysis, have been also reported after PTJV.23
In a review of 265 elective PTJV and other forms of jet oxygenation/ventilation for otolaryngologic surgery, Jaquet and colleagues wrote that PTJV was associated with hemodynamic instability (8 patients), subcutaneous emphysema of the neck (3 patients), posterior tracheal mucosa tear (1 patient), catheter kinking (3 patients), severe mediastinal/cervical subcutaneous emphysema (1 patient), unilateral pneumothorax (1 patient), and bilateral pneumothorax (1 patient).19 The last 3 events were considered major complications, and all were associated with cough or laryngospasm during PTJV. Damage to the tracheal wall, witnessed by this group, was also demonstrated in a large-animal model of PTJV in which full-thickness erosion and hemorrhage of posterior tracheal mucosa was seen in all specimens.29 Subcutaneous emphysema has been associated with cannula shaft lacerations in the absence of frank cannula misplacement.30
In another series of elective PTJV cases, there was an overall minor complication rate of 3%.22 These complications (minor bleeding, subcutaneous emphysema) were typically seen when multiple attempts had to be made during cannula placement. The authors recommended that no more than two attempts should be made.
The site of PTJV access may also influence complications. In a swine tracheal model, Salah and coworkers studied trans-CTM versus transtracheal cannulation. With some percutaneous techniques, traumatic injury (including posterior wall tears or frank penetration and cartilaginous fractures) were more common when the PTJV cannula was inserted into the trachea.31
Complete or near-complete airway obstruction during PTJV may also contribute to hemodynamic instability.24 In a controlled trial employing graded airway obstruction in dogs undergoing PTJV with 45 psi inflation pressures through a 13-G catheter, intratracheal pressures of 24 cm H2O were associated with decreasing blood pressure and increased central venous pressure, even in the absence of pneumothorax. In complete upper airway obstruction simulated in dogs, intratracheal airway pressure rose precipitously as soon as the total lung capacity was exceeded. This was accompanied by a fall in systolic blood pressure and eventual rupture of the pulmonary system (pneumothoraces, pneumomediastinum, subcutaneous emphysema, cardiac fibrillation) when an intratracheal pressure of 250 cm H2O was achieved.25
In one canine study, methylene blue instilled into the oral cavity was less readily aspirated into the trachea during PTJV, compared with controls.26,27 The authors reasoned that the retrograde egress of gas from the larynx reduced oral content aspiration. A similar phenomenon has been noted in humans during O2 insufflation through an endotracheal tube (ETT) exchange catheter.28
A Mechanism of Barotrauma and Other Forms of Airway Trauma
Striving to verify the intraluminal position of a percutaneously placed cannula is mandatory during PTJV. Though cannula misadventures are responsible for many of the complications related to PTJV, complete upper airway obstruction during O2 insufflation is likely responsible for most episodes of barotrauma. During involuntary obstruction of the upper airway (e.g., due to laryngospasm) intraluminal pressures, generally remaining in the safe range of 18 to 25 cm H2O during PTJV, rise precipitously. In an artificial lung model, upper airway obstruction (airway resistance 200 cm H2O/L/sec) resulted in an intratracheal pressure of 60 cm H2O. Intraluminal pressures between insufflations did not rise above minimal levels (3 cm H2O) until maximal airway resistance was produced, at which time there was an equilibration to maximal pressure.32 In an adult sheep model, complete airway obstruction during constant tracheal insufflation of O2 at 15 L/min resulted in a pressure of 96 cm H2O in 12 seconds. When intermittent jet ventilation (JV) was used (inspiratory-to-expiratory [I : E] phase ratio, 3 : 5), a pressure of 108 cm H2O was reached in 33 seconds.25
IV Cannula Size and Oxygenation
A wide variety of cannula-over-needle devices have been described in the PTJV literature in both in vivo and mechanical model trials and in case reports. Trials of cannulas smaller than 3 mm in diameter (equivalent to 8 G) have typically demonstrated the need for a high-flow regulator/conveyance apparatus. Although flow through cannulas of various diameters and lengths has been measured during simulated PTJV, actual flow varies with the clinical situation and is affected by factors such as airway resistance and pulmonary compliance. Table 28-1 lists experimental data on flow rates through cannulas of various sizes with the use of a 50 psi gas supply.14,33,36
Neff and colleagues insufflated gas through cannulas of various diameters in a sheep model.25 Marginal oxygenation was achieved with a 1.4-mm-diameter (15-G) cannula using 15 L/min constant and 50-psi “pulsed” O2 flow. Use of low-pressure systems, such as resuscitator bags and anesthesia machine circuits, which provide 1 to 2 psi, was futile for catheters smaller than 3 mm in diameter. However, Zornow and colleagues were able to produce “delayed” reoxygenation in a hypoxic swine model with a 14-G cannula and “vigorous” ventilation with an anesthesia machine circuit.25,29 O2 resuscitation was significantly faster when 50-psi supplies were used (15 versus 120 seconds).25
Some authors have speculated that entrainment of room air via a Venturi effect produced by the high velocity of the gas injected into the larynx during PTJV might add as much as 40% or 50% to the volume of inspired gas.33–35 This hypothesis has been contradicted by the findings of arterial blood gas studies in both animals and healthy patients undergoing jet ventilation, which demonstrated O2 tensions consistent with the insufflation of 100% O2 (no entrainment of room air).36–38
Whereas complete upper airway obstruction has been associated with ineffective ventilation and barotrauma, partial obstruction may enhance both oxygenation and ventilation by driving gas flow toward the lower bronchial tree, facilitating tidal volume (VT) and improving alveolar oxygen tension (PaO2).24
V Ventilation
Effective ventilation during PTJV requires a pathway for gas egress. Fortunately, most patients who present with life-threatening airway failure (e.g., laryngeal edema, epiglottitis, laryngeal tumor) have isolated inspiratory obstruction with no or partial expiratory obstruction.39 This is true during spontaneous breathing as well as controlled ventilation through a face mask or SLA, and it is related both to the extrathoracic position of the upper airway and to the architecture of laryngeal and supralaryngeal structures. Complete obstruction to gas escape is a contraindication for PTJV and may be dependent on the devices used.
Because removal of CO2 is dependent on sufficient volumes of gas entering and exiting the lung, low-pressure systems (e.g., resuscitation bags) are generally inadequate for ventilation, as they are for oxygenation. Zornow and colleagues, using a 14-G transtracheal cannula in a swine model, was able to effectively ventilate with a 50 psi O2 source but could not reduce arterial CO2 despite “vigorous” ventilation with an anesthesia machine circuit.29 Whereas gas escaping from an in vivo ETT could be measured when PTJV was performed with the high-pressure source, no gas movement was appreciated with use of the low-pressure system.
Ward and coworkers examined no, partial, and complete upper airway obstruction during PTJV in dogs, using 13-G catheters and a high-flow regulator delivering 45 psi of pressure.40 With no or partial upper airway obstruction, CO2 levels decreased during PTJV. Partial airway obstruction improved CO2 removal, possibly by promoting improved alveolar expansion during the O2 injection phase of PTJV.
A Airway Rescue in Complete Upper Airway Obstruction
Although controversial and based on animal models, low-flow insufflation of O2 into the pulmonary circuit has been proposed as a mechanism for maintaining oxygenation, although not effective ventilation, in the patient with a completely obstructed airway.24,41,42 Several alternative techniques have been studied in the model of complete upper airway obstruction.
Frame and associates, using a canine model (20 to 29 kg), found that with complete airway obstruction, O2 flows of 5 to 7 L/min through 10-G and 12-G catheters could maintain oxygenation with I:E phases of 1 : 4 seconds and provided reasonable ventilation (PaCO2 <75 mm Hg).41 When lower flow rates (3 L/min) were used, CO2 tension increased rapidly after 5 to 20 minutes. Although this study demonstrated the successful use of low-pressure O2 flow in complete airway obstruction (in an effort to avoid barotrauma), it must be cautioned that small animals and large insufflation catheters were used.
Low-flow translaryngeal rescue insufflation of oxygen (LF-TRIO) of as little as 2 L/min has been shown to maintain oxygenation in a large animal model (34-kg swine) for upwards of 60 minutes.5 LF-TRIO recovered the animals from O2 saturation nadirs of 50% in an average of 23 seconds. Cardiovascular parameters were stable in all animals for a minimum of 15 minutes. Although the animals experienced hypercapnia, the authors argued that this may be well tolerated if there are no specific comorbidities that may be aggravated by increasing CO2 (e.g., head trauma). Although imaging in this study was limited to a small number of animals, there was no evidence of microatelectasis after 1 hour of LF-TRIO. LF-TRIO was considered by these authors to be a short-term rescue option when definitive surgical airway control is anticipated.
More recently, the concept of an active expiratory phase has been applied to PTJV (Fig. 28-1). In a series of large-animal studies, Enk and Hamaekers described using the O2 flow from a standard flowmeter to create a negative-pressure, transcatheter expiratory phase.32 Bernoulli’s principle, application of which is familiar to anesthesiologists in the functioning of the Venturi valve, states that when a fluid in a vessel passes through a constriction, dynamic energy (speed of flow) is increased, whereas static pressure (potential energy) exerted on the vessel side-wall is diminished. This setup can be manipulated to produce a subatmospheric pressure during an expiratory phase of PTJV. In an initial bench model, a prototype expiratory ventilation assistance (EVA) device reduced by half the time required for a 1000-mL injected VT to be expired via a 2-mm ID cannula and also increased the effective minute ventilation by 33%.32 In another large-animal study by the same group that included a complete obstruction of the upper airway, EVA restored oxygenation within 10 seconds and limited hypercarbia over 15 minutes.42 However, EVA was less effective when the upper airway was unobstructed, possibly due to preferential entrainment of ambient air into the PTJV catheter via the lower-resistance upper airway path and, consequently, reduced removal of alveolar gases.43
B Percutaneous Translaryngeal Jet Ventilation in Relief of Upper Airway Obstruction
The introduction of PTJV may encourage partial relief of upper airway obstruction. In a controlled study, Okazaki and colleagues demonstrated that induced airway obstruction (by simple neck flexion) was partially relieved by the insufflation of 10 L/min O2, although not as well as by the chin lift–jaw thrust maneuver.44 This finding implies a potential utility of transtracheal insufflation in patients who are at high risk for airway obstruction during postanesthesia recovery.44,45
VI Elective Indications
A To Facilitate Operations of the Upper Airway
There have been several reports and various recommendations for the elective use of PTJV in surgical procedures to avoid tracheostomy, provide an unobstructed surgical field, or facilitate safe ETT. Singh and coworkers reported on 1500 cases of PTJV for diagnostic and surgical procedures of the upper airways, including bronchoscopy and esophagoscopy (n = 1257) and endolaryngeal surgery (n = 135).46 PTJV support was continued for 24 to 48 hours into the postoperative period in 108 cases. Spoerel and Greenway, describing ventilation techniques during endolaryngeal surgery, similarly found adequate pulmonary ventilation with excellent conditions for microscopic surgery.47 Smith and colleagues reported on elective transtracheal ventilation in children undergoing surgical procedures involving the head and neck.48 They described the use of this technique in two children, one undergoing an operation for laryngeal stenosis and one who became obstructed and cyanotic in the recovery room. PaCO2 measurement at the end of the procedures indicated that the patients were moderately hyperventilated. Similarly, because PTJV leaves the entire airway from the vocal cords to the face accessible for surgical manipulation, it is not surprising that its elective use has been described for a large variety of procedures on these structures in adults.49 In addition, Wagner and coauthors described a case in which high-frequency JV was used with success (thus avoiding tracheostomy) in a patient with a partial upper airway obstruction caused by a hypopharyngeal foreign body with sharp appendages.50
Dhara and colleagues reported on two cases in which a triple-lumen central venous catheter was placed through the CTM before induction of anesthesia.51 Making use of all three lumens, this unique approach allowed the clinicians to measure tracheal CO2 and intra-airway pressure, as well as providing jet oxygenation. In both cases, an upper airway egress for insufflated O2 was maximized by use of a nasopharyngeal airway (NPA) or tongue retraction.
Depierraz and associates investigated the elective use of PTJV in pediatric patients (aged from less than 1 to 12 years) with significant upper airway obstruction.52 They recited the major advantages of this technique: improved surgical exposure of the upper airway obstructive lesion, reduced manipulation of the lesion, reduced operative field movement during laser surgery, reduced danger of airway fire, and reduced risk of blowing particles into the distal airway.
B To Permit Safe Intubation of the Trachea
In situations in which there is a significant risk of airway management failure, PTJV has been used electively to preclude the CICV situation. One report described the use of prophylactic PTJV to provide oxygenation in a patient who was known to be difficult to intubate.53 PTJV enabled the patient to be anesthetized, muscle relaxed, and intubated in a safe, nonemergent manner. In another report of a 13-year-old girl with ankylosis of the temporomandibular joint resulting from a prior fracture, PTJV was electively instituted before the induction of anesthesia, and the trachea was subsequently intubated with the aid of fiberoptic bronchoscopy.54 In a third report, PTJV was used to supplement spontaneous ventilation via the natural airway in a patient with a massive upper tumor so that the airway could be secured in a nonemergent manner.55
C To Facilitate Training
Another benefit of the elective use of transtracheal techniques not to be ignored is training of students and anesthesiology residents as well as critical practice for attending physicians themselves. As discussed earlier, the 2003 ASA DA algorithm suggests four alternatives in the CICV situation: LMA, ETC, PTJV, and surgical airway.4 The first two techniques can be practiced electively (the cost of the single-use ETC may be decreased by reprocessing).56 Although a surgical airway can be electively practiced in appropriate patients, such as in a case of upper airway pathology when a tracheostomy is surgically planned and an otolaryngologist is available as a supervising physician, transtracheal catheter placement has definite clinical indications that can be employed for training purposes (e.g., awake intubation procedures, retrograde intubation). Many authors agree with this approach, especially because total airway obstruction may not be relieved by the SLA devices.22,57,58 However, elective use of translaryngeal needle placement is not without its complications, as reviewed earlier.20
VII Equipment
The literature is replete with manufactured and OR-rigged devices for delivering O2 to a translaryngeal catheter. The great advantage of using a preassembled, commercially made PTJV system is the quality assurance that is built into a commercial product and the freedom from having to assemble the system. In addition, because low-pressure systems (e.g., anesthesia breathing circuit, self-inflating resuscitation bag) are typically ineffective when coupled with percutaneous catheters and because the anesthesia machine manufacturers limit the utility of the common gas outlet (CGO) for PTJV, commercial systems offer specifically designed components and joints for use with high pressure.25 Many of the OR-rigged devices remain untested, and although they may resemble working systems, performance in a true clinical emergency is unknown.59 In addition, a working system should be prepared or made available before the need for it arises. In the CICV situation, small delays contribute to devastating effects. Not only can it take many seconds to minutes to assemble a device, not all anesthetizing or resuscitative locations contain uniform equipment. Basic characteristics of a PTJV system are listed in Box 28-1.
Box 28-1 Characteristics of an Ideal Transtracheal Jet Ventilation Device
Validated in clinical or laboratory studies
Low-compliance tubing, fixed component joints
Ability to connect to a high-pressure (50 psi) O2 source
Ability to connect to anesthesia circuit, self-inflating bag (if ≥3 mm ID)
EtCO2, End-tidal carbon dioxide concentration; ID, internal diameter.
A study testing four different OR-rigged devices arrived at the conclusion that only devices employing high pressure were capable of delivering adequate transcannula flows and maintaining oxygenation, although adequate ventilation was not consistently assured.15
Several of the described OR-rigged devices rely on intermittent occlusion of a port of a three-way stopcock with the intent of allowing diversion of the O2 supply flow through the unoccluded port during the expiration phase (expired gas is meant to be removed via the upper airway, if it is unobstructed).60–62 Hamaekers and colleagues showed that three-way stopcocks act as flow splitters.63 Unoccluding the open limb of the stopcock does not halt all flow into the transtracheal catheter; this results in continuous O2 flow into the trachea, possibly hindering CO2 removal and contributing to hemodynamic instability. In the case of total upper airway occlusion, continued flow from these devices can contribute to barotrauma.63
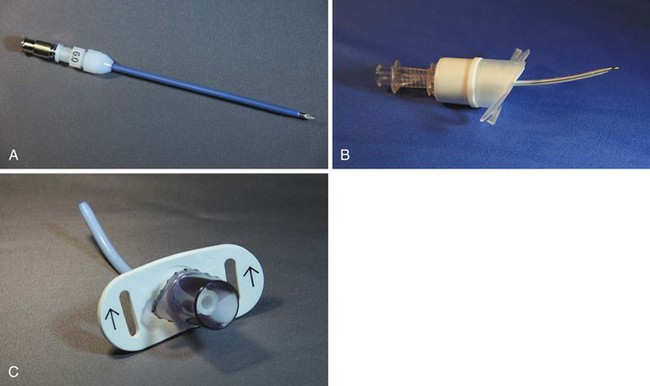
By comparison, a relatively simple manufactured device is the Enk Flow Modulator (Fig. 28-2; Cook Critical Care, Bloomington, IN). Unlike many OR-rigged devices, forward gas flow in the PTJV catheter is minimal when the five 4-mm holes of the Enk Flow Modulator are released. This reduces positive end-expiratory pressure (PEEP) and other consequences of continued flow during the expiratory phase.63,64 Some flow through the catheter during the expiratory phase may be desirable (when there is not complete upper airway occlusion). This flow may aid in elimination of CO2 from the unoccluded airway, as well as enhancing oxygenation.64
In two studies, investigators surveyed their colleagues to determine what OR-rigged devices were preferred. In Morley and Thorpe’s survey,14 three different configurations were described, and the survey by Ryder and colleagues15 described seven different configurations. In these studies, 5% and 10% of anesthesiologists, respectively, could not assemble any device, and only 5% and 40% of the devices, respectively, were considered adequate for PTJV. When clinicians were asked to assemble a device of their own choosing, they needed between 20 and 365 seconds (mean, 90 seconds) to produce a functional device, again raising doubts about the practicality of depending on unmanufactured systems.15 Industrial-grade valves, which may mimic the function of the devices manufactured specifically for JV, typically contain lubricants and should not be used for medical indications.
The most critical element in the system employed for PTJV may be the availability of pressurized O2, typically supplied at 45 to 60 psi. Central supply (“wall oxygen”) is ubiquitous in ORs, intensive care units, and other areas of hospitals. Portable O2 tanks are also fitted with regulators that are adjusted to deliver 50 psi. A variety of indexed systems may be used to prevent mismatching of gas sources and clinical devices (Fig. 28-3). When purchasing new equipment for PTJV use, the specific indexing system, as well as the male versus female configuration, must be specified to the vendor. Many anesthesia machine manufacturers provide an outlet that is tied to the high-pressure gas supply and is accessible by Diameter-Index Safety System (DISS) fittings (see Fig. 28-3A). Although the anesthesia machine CGO was previously employed as a source of high-pressure O2, its use is discouraged, and to this end, manufacturers have downregulated the available pressure when the machines’ O2 flush valve is activated (Fig. 28-4).33
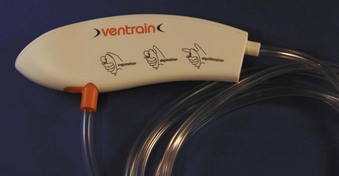
All systems used to convey O2 from high-pressure sources to the patient must have adjustable governors that limit static pressure or continuous flow in order to minimize barotrauma, and they must contain a mechanism for halting or diverting flow to produce an expiratory phase during PTJV. Two such systems are commonly used in PTJV: high-flow regulators (with manual triggers) and low-flow regulator-flowmeters (with flow diversion). High-flow regulator systems, such as the Manujet III (VBM Medizintechnik, Sulz, Germany) (Fig. 28-5), allow the user to preset the pressure delivered to the PTJV catheter (0 to 50 psi) during a no-flow state. Manual activation of a trigger starts the flow of O2 to the translaryngeal catheter; release of the trigger halts gas flow. A disadvantage of this system is that it is closed—there is no path for gas escape during the expiratory phase. This may increase the risk of barotrauma or encourage more hypercapnia. Although these systems are capable of delivering 50 psi to the translaryngeal catheter, intratracheal pressures do not rise above 25 cm H2O.
The second commonly studied system for O2 conveyance is the low-flow regulator, which limits continuous flow from 0 to 15 L/min (Fig. 28-6). Low-flow regulators are commonly available on small (E-cylinder) O2 transport tanks, on some anesthesia machines, and attached to central supply O2.34 Several studies have demonstrated the utility of these devices. When 15 L/min is delivered satisfactory VT with 16- and 14-G PTJV catheters may be achieved within the first 0.5 seconds.34 Low-flow regulators are ubiquitous in hospitals. Inexpensive systems have been developed to convey O2 from low-flow regulators to the patient.
A study by Preussler and colleagues compared the two aforementioned conveyance systems.65 This group compared a high-flow regulator (Manujet III) attached to a 50 psi O2 source and low-flow, 15 L/min regulator-flowmeter fitted with an Enk Oxygen Flow Modulator.66 Both systems were tested in anesthetized pigs using a specialized 15-G transtracheal cannula. The upper airway of the pigs was kept patent with an ETT and a 20-cm H2O PEEP valve in order to simulate a clinical partially obstructed airway. The investigators found that both device setups performed equally well, maintaining EtCO2, PCO2, and PaO2 in acceptable ranges. The Enk Flow Modulator includes a Luer-Lok syringe port for administration of drugs into the trachea without interruption of ventilation or oxygenation. The Manujet III has a variable-pressure regulator and pressure gauge. Other bench models have shown that close-circuit gas injectors, such as the Manujet III, achieve higher peak flows, greater VT (650 mL), and higher intra-airway pressures, compared with the Enk Flow Modulator (240 mL).64
During total expiratory pathway occlusion in a bench model, intra-airway pressure rises precipitously with closed-circuit gas injectors (e.g., Manujet III) that have no facility for gas release during the expiratory phase via the transtracheal catheter. Lenfant and colleagues showed that when 3-bar pressure was applied via the Manujet in a completely occluded airway, intra-airway pressures of 136 cm H2O were measured after two inspirations.64 In contrast, systems that use flow diversion by allowing gas escape during the expiration phase (e.g., Enk Flow Modulator with 15 L/min gas flow) generated significantly lower intra-airway pressures after multiple breaths. In the completely occluded airway scenario, these systems may have an advantage, providing a pressure release pathway. The authors caution, however, that aggressive insufflation with any device can be dangerous in the completely occluded airway. The Enk Flow Modulator is not immune to producing high pressures in this situation.64 In most cases of CICV, total airway occlusion does not occur. In situations in which high peak pressure is of concern, the driving pressure can be reduced. In the emergency clinical situation, close attention to chest rise (and fall, between insufflations) can guide adjustment.64
The last critical device in the PTJV setup is the translaryngeal cannula. Historically, a variety of intravenous catheter-over-needle devices have been employed.22,55,67,68 Others have attempted to measure expired CO2 and intratracheal pressure using alternative devices, such as triple-lumen central venous catheters.51 Garry and colleagues used a needle-in-needle catheter assembly (a 14 G or 16 G catheter fitted within a 3-mm ID catheter).69 O2 was insufflated in the central needle while suction and CO2 monitoring were applied to the annular space. Newer catheters constructed from kink-resistant materials have a preformed tip angulation, Luer-Lok connectors, 15-mm circuit adapters, or securing rings (Fig. 28-7).
VIII Technique of Percutaneous Translaryngeal Jet Ventilation
Emergent need for PTJV may be anticipated but is rarely expected. No clinician is likely to induce anesthesia when complete loss of airway control is a probable outcome. Conversely, most patient airways are managed without critical incident, and it is the job of the anesthesiologist to envisage the scenario in which invasive airway access could become necessary. Preparation for the use of PTJV may be the single most important aspect of its application. Table 28-2 lists the minimal materials and resources that must be available if PTJV is anticipated; my own opinion is that these should be accessible in any anesthetizing situation in a health care setting.
TABLE 28-2 Equipment and Resources for Percutaneous Translaryngeal Jet Ventilation
| Description | Example | |
|---|---|---|
| Minimal Resources | ||
| Oxygen source | Wall or regulated tank, 50 psi | Indexed central supply or regulated tank O2 |
| Low-flow O2 flowmeter, 15 L/min minimal flow | Wall outlet or tank flowmeter | |
| Needle catheter (specialized) | 13-G ID or greater diameter | Cook Transtracheal Catheter* Ravusin catheter† |
| Conveyance device | Regulated and adjustable, hand-triggered valve with indexed O2 source connection | Manujet III† |
| O2 flowmeter-powered, hand-regulated O2 outlet | Enk Flow Modulator* Ventrain‡ |
|
| Personal protective equipment | Protects operator from bloodborne acquired antigens | Gloves, face mask |
| Other | Syringe, Luer-Lok | 5 or 10 mL |
| Preferred Accessories | ||
| Skin-marking pen | To mark the location of the cricothyroid membrane | |
| Antiseptic solution, sterile drapes | For preparation of sterile field | |
| Scalpel | For small vertical skin incision | |
| Fluid | Helps to appreciate needle entrance into larynx | Saline, local anesthetic with epinephrine (1 : 200,000 dilution) |
| Fine-gauge needle | For local injection of anesthetic, epinephrine | 22-25 G |
* Cook Critical Care, Bloomington, IN.
† VBM, Medizintechnik, Sulz, Germany.
‡ Dolphys Medical BV, The Netherlands.
Willingness to use PTJV is the next step of preparation. Most anesthesiologists, by the nature of their training, prefer noninvasive methods. The tendency (and trend) is to reduce the use of even well-established invasive procedures (e.g., adoption of ultrasound guided nerve block versus nerve stimulation techniques). Given the proliferation of SLA devices and endotracheal intubation techniques during the last 20 years, it is unsurprising that surgical and percutaneous airway rescue procedures, even when performed adequately, are often applied too late in the lost-airway scenario to effect a change in outcome and prevent death or brain death.70 Dogged perseverance with laryngoscopy and unsuccessful SLA ventilation must be avoided—evidence attests that this not only is futile but also contributes to a worsening outcome.70,71
A Insertion of the Transtracheal Catheter
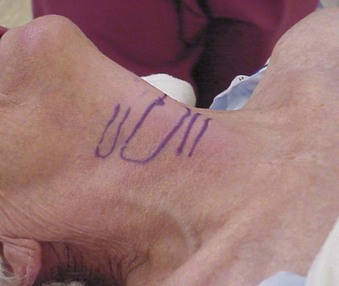
The right-hand–dominant operator stands on the left hand side of the patient. The CTM is palpated with the neck of the patient extended, unless an absolute contraindication exists (Fig. 28-8). The rate of misplacement of PTJV due to misidentification of the CTM is high, with one study showing that only 30% of clinicians were able to identify the overlying surface landmarks.72 The literature is replete with methods of identifying the CTM (Box 28-2). When airway management difficulty is anticipated, it is not unreasonable (and is often encouraged) for the clinician to identify the CTM before the start of any airway procedures such as anesthetic induction or preparation for awake management (see Fig. 28-8).4 The DA Taskforce of the ASA encouraged assessing the feasibility of invasive airway use in any patient whose airway is to be controlled. Preanesthesia palpation and marking draws attention to the potential futility of attempting invasive management (e.g., in the patient with super-obesity) and may lead to an early change in the anesthesia plan.1
The CTM is the accepted entrance point for PTJV.31 Insertion of a cannula-over-needle or surgical airway in the vicinity of the cricoid cartilage has several advantages. First, several landmarks have been described for CTM identification. Second, it is the only completely circumferential cartilage in the airway. The mature cricoid cartilage lends rigidity to the larynx, making it resilient to the anteroposterior pressure of an inserted cannula-over-needle, resulting in reduced trauma.31 Third, the posterior wall of the cricoid cartilage extends from the trachea inferiorly to the inferior border of the thyroid cartilage. This provides a solid “backstop” for the probing PTJV cannula-over-needle placed through the CTM. Several reports have described intentional and unintentional tracheal PTJV cannula placement when the CTM was difficult to identify. Salah and colleagues, in a swine cadaveric airway study, noted increased trauma to the tracheal anatomy with some, but not all, percutaneous techniques, emphasizing the preference for CTM access.9
The previously identified translaryngeal cannula-over-needle is readied with a 5- to 10-mL syringe, with or without fluid. If it is not already preshaped by the manufacturer, a small-angle bend (15 degrees) can be placed 2.5 cm from the distal end.73 This angulation helps prevent intratracheal cannula kinking or cephalad travel. The needle punctures the skin in the midline over the lower third of the surface landmarks of the CTM. The CTM is a relatively avascular structure, although blood is occasionally drawn into the syringe (this should not deter the intubator from completing this life-saving procedure). The cricothyroid artery and vein can be avoided by performing the puncture in the midline of the neck in the lower third of the CTM, just above the cricoid cartilage.74,75 Initially, the axis of the cannula-over-needle is oriented at a 90-degree angle to the imagined location of the cervical spine. As downward pressure is exerted, constant negative pressure is applied to the syringe plunger. It is practically mandatory that the intralaryngeal position be identified by aspiration of gas. Except in the case of drowning or pulmonary hemorrhage, the larynx and trachea should be gas filled. Under no circumstance should the intubator assume that the larynx or trachea has been entered based on surface landmarks or tactile sense alone. Not only is the injection of pressurized O2 into the subcutaneous tissue, the mediastinum, a large vessel, the pleural space, or other inappropriate location futile, but it may lead to rapid death and may obscure the anatomic planes needed to complete a surgical airway.
After reverification of the intra-airway cannula location, the available O2 conveyance system is attached via its Luer-Lok connections. Initial insufflations of O2 should be titrated to chest excursion. When a high-flow, hand-triggered regulator is used, a 1 second or less inspiratory time has been emphasized by several authors.34,64 Though 50 psi may be needed to cause chest excursion, commencing PTJV with more modest pressures (25 to 30 psi) may be prudent to preclude the devastating complications of cannula misplacement. The ability to designate precise insufflation pressures may not be available on all high-flow regulators, underscoring the recommendation that equipment be prepared or adjusted before the need arises. The maximum and midrange output settings on the available high-flow regulator should be determined during equipment preparation.
A variety of I : E phase ratios have been suggested without absolute advantages being established, in part because of the wide variety of artificial and animal models that have been studied. In general, low respiratory rates (e.g., 4 breaths/min) result in improved ventilation, especially if partial airway obstruction exists. Respiratory rates as high as 12 breaths/min (e.g., a phase ratio of 1:4) have been studied.64 Adhering to the principle of ensuring adequate chest wall recoil to guide the respiratory rate is of singular importance. Inadequate recoil may be caused by poor maintenance of upper airway patency, a shortened expiratory phase, or a fixed obstruction. Maneuvers to open the upper airway (e.g., oropharyngeal airways [OPAs], NPAs, SLAs, chin lift-jaw thrust) may be reassessed, and lengthening of the expiratory phase may be attempted. If the clinical history and course are consistent with a fixed upper airway obstruction, the strategies discussed earlier may be in order. As previously discussed, complete upper airway obstruction is a contraindication to the use of high-flow regulators.
When a low-flow regulator and conveyance system is in use (e.g., O2 flow meter, Enk Flow Modulator), VT will be reduced, yet the same oxygenation/ventilation strategies are applied.64 The low-flow regulator should be set to 15 L/min; inspiratory time should be based on observation of chest wall excursion, although 1 second may be used as the initial standard. The phase ratio should be based on observations of chest fall but has been studied in ranges similar to those noted earlier for high-flow regulators. The use of active expiratory valves (e.g., EVA) should improve ventilation by allowing faster respiratory rates and by reducing, but not eliminating, the dangers of barotrauma and hypoventilation during complete upper airway obstruction.
XI Clinical Pearls
• Translaryngeal insufflation of O2 requires a high-pressure O2 source unless catheters 3 mm or larger in diameter are used. Anesthesia circuits and air-mask-bag units cannot provide adequate pressure.
• Before contemplating translaryngeal insufflation of O2, the clinician should ensure that all materials and an O2 source are available. This requires planning prior to the event.
• Only medical-grade devices should be used for these life-salvaging techniques. Jerry-rigged devices should not be used.
• Dedicated translaryngeal catheters, 14 G or larger, and not angiocatheters should be used.
• Translaryngeal insufflation of O2 requires some degree of upper airway patency. If the airway is completely occluded, devices that allow exhalation of gas (passive or active) must be used. In any case, these patients demand a surgical airway as soon as possible.
• Whenever translaryngeal insufflation of O2 is undertaken, an oropharyngeal device (e.g., supralaryngeal airway [SLA], oropharyngeal airway [OPA], nasopharyngeal airway [NPA]) should be in place to promote gas escape.
• Apart from and earlier than recovering O2 saturation, chest recoil (not expansion) is the best monitor of well-performed translaryngeal insufflation.
All references can be found online at expertconsult.com.
8 Manoach S, Corinaldi C, Paladino L. Percutaneous transcricoid jet ventilation compared with surgical cricothyroidotomy in a sheep airway salvage model. Resuscitation. 2004;62:79–87.
9 Salah N, El Saigh I, Hayes N, et al. Comparison of four techniques of emergency transcricoid oxygenation in a manikin. Anesth Analg. 2010;110:1083–1085.
11 Johansen K, Holm-Knudsen RJ, Charabi B, et al. Cannot ventilate-cannot intubate an infant: Surgical tracheotomy or transtracheal cannula? Paediatr Anaesth. 2010;20:987–993.
19 Jaquet Y, Monnier P, Van Melle G, et al. Complications of different ventilation strategies in endoscopic laryngeal surgery: A 10 year review. Anesthesiology. 2006;104:52–59.
24 Carl ML, Rhee KJ, Schelegle ES, et al. Effects of graded upper-airway obstruction on pulmonary mechanics during transtracheal jet ventilation in dogs. Ann Emerg Med. 1994;24:1137–1143.
31 Salah N, El Saigh I, Hayes N, et al. Airway injury during emergency transcutaneous airway access: A comparison at cricothyroid and tracheal sites. Anesth Analg. 2009;109:1901–1907.
43 Hamaekers A, Theunissen M, Jansen J, et al: Emergency ventilation through a 2 mm ID transtracheal catheter in severe hypoxic pigs using expiratory ventilation assistance (EVA). Meeting Abstracts, September 2010, Society for Airway Management.
44 Okazaki J, Isono S, Atsuko T, et al. Usefulness of continuous oxygen insufflation into trachea for management of upper airway obstruction during anesthesia. Anesthesiology. 2000;93:62–68.
66 Enk D, Busse H, Meissner A, et al. A new device for oxygenation and drug administration by transtracheal jet ventilation. Anesth Analg. 1998;86:S203.
1 Heidegger T, Gerig HJ, Ulrich B, et al. Validation of a simple algorithm for tracheal intubation: Daily practice is the key to success in emergencies—An analysis of 13,248 intubations. Anesth Analg. 2001;92:517–522.
2 Rose DK, Cohen MM. The airway: Problems and predictions in 18,500 patients. Can J Anaesth. 1994;41:372–383.
3 Keenan RL, Boyan CP. Cardiac arrest due to anesthesia: A study of incidence and causes. JAMA. 1985;253:2373–2377.
4 American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for the management of the difficult airway. Anesthesiology. 2003;98:1269–1277.
5 Black IH, Janus SA, Grathwohl KW. Low-flow transtracheal rescue insufflation of oxygen after profound desaturation. J Trauma. 2005;59:344–349.
6 Henderson JJ, Popat MT, Latto IP, et al. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675–694.
7 Fortune JB, Judkins DG, Scanzaroli D, et al. Efficacy of prehospital surgical cricothyrotomy in trauma patients. J Trauma. 1997;42:832–836.
8 Manoach S, Corinaldi C, Paladino L. Percutaneous transcricoid jet ventilation compared with surgical cricothyroidotomy in a sheep airway salvage model. Resuscitation. 2004;62:79–87.
9 Salah N, El Saigh I, Hayes N, et al. Comparison of four techniques of emergency transcricoid oxygenation in a manikin. Anesth Analg. 2010;110:1083–1085.
10 Wong DT, Lai K, Chung FF, et al. Cannot intubate-cannot ventilate and difficult intubation strategies: Results of a Canadian national survey. Anesth Analg. 2005;100:1439–1446.
11 Johansen K, Holm-Knudsen RJ, Charabi B, et al. Cannot ventilate-cannot intubate an infant: Surgical tracheotomy or transtracheal cannula? Paediatr Anaesth. 2010;20:987–993.
12 DeLisser EA, Muravchick S. Emergency transtracheal ventilation. Anesthesiology. 1981;55:606–607.
13 Meyer PD. Emergency transtracheal jet ventilation system. Anesthesiology. 1990;73:787–788.
14 Morley D, Thorpe CM. Apparatus for emergency transtracheal ventilation. Anaesth Intensive Care. 1997;25:675–678.
15 Ryder IG, Paoloni CC, Harle CC. Emergency transtracheal ventilation: Assessment of breathing systems chosen by anaesthetists. Anaesthesia. 1996;51:764–768.
16 Sprague D. Transtracheal jet oxygenator from capnographic monitoring components. Anesthesiology. 1990;73:788.
17 Benumof JL. Laryngeal mask airway and the ASA difficult airway algorithm. Anesthesiology. 1996;84:686–699.
18 Parmet JL, Colonna-Romano P, Horrow JC, et al. The laryngeal mask airway reliably provides rescue ventilation in cases of unanticipated difficult tracheal intubation along with difficult mask ventilation. Anesth Analg. 1998;87:661–665.
19 Jaquet Y, Monnier P, Van Melle G, et al. Complications of different ventilation strategies in endoscopic laryngeal surgery: A 10 year review. Anesthesiology. 2006;104:52–59.
20 Wong DT, McGuire GP. Subcutaneous emphysema following trans-cricothyroid membrane injection of local anesthetic. Can J Anaesth. 2000;47:165–168.
21 Patel RG. Percutaneous transtracheal jet ventilation: A safe, quick, and temporary way to provide oxygenation and ventilation when conventional methods are unsuccessful. Chest. 1999;116:1689–1694.
22 Smith RB, Schaer WB, Pfaeffle H. Percutaneous transtracheal ventilation for anesthesia: A review and report of complications. Can J Anaesth. 1975;22:607.
23 Benumof JL, Scheller MS. The importance of transtracheal jet ventilation in the management of the difficult airway. Anesthesiology. 1989;71:769–778.
24 Carl ML, Rhee KJ, Schelegle ES, et al. Effects of graded upper-airway obstruction on pulmonary mechanics during transtracheal jet ventilation in dogs. Ann Emerg Med. 1994;24:1137–1143.
25 Neff CC, Pfister RC, Van Sonnenberg E. Percutaneous transtracheal ventilation: Experimental and practical aspects. J Trauma. 1983;23:84–90.
26 Bruno J, Cheung HK, Chong ZK, et al. Aspiration and tracheal jet ventilation with different pressures and depths and chest compressions. Crit Care Med. 1999;27:142–145.
27 Klain M, Keszler H, Stool S. Transtracheal high frequency jet ventilation prevents aspiration. Crit Care Med. 1983;11:170–172.
28 Loudermilk EP, Hartmannsgruber M, Stoltzfus DP, et al. A prospective study of the safety of tracheal extubation using a pediatric airway exchange catheter for patients with a known difficult airway. Chest. 1997;111:1660–1665.
29 Zornow MH, Thomas TC, Scheller MS. The efficacy of three different methods of transtracheal ventilation. Can J Anaesth. 1989;36:624–628.
30 Ames W, Venn P. Complication of the transtracheal catheter. Br J Anaesth. 1998;81:825.
31 Salah N, El Saigh I, Hayes N, et al. Airway injury during emergency transcutaneous airway access: A comparison at cricothyroid and tracheal sites. Anesth Analg. 2009;109:1901–1907.
32 Hamaekers AE, Götz T, Borg PA, Enk D. Achieving an adequate minute volume through a 2 mm transtracheal catheter in simulated upper airway obstruction using a modified industrial ejector. Br J Anaesth. 2010;104:382–386.
33 Gaughan SD, Benumof JL, Ozaki GT. Can an anesthesia machine flush valve provide for effective jet ventilation? Anesth Analg. 1993;76:800–808.
34 Gaughan SD, Ozaki GT, Benumof JL. Comparison in a lung model of low- and high-flow regulators for transtracheal jet ventilation. Anesthesiology. 1992;77:189–199.
35 Spoerel WE, Narayanan PS, Singh NP. Transtracheal ventilation. Br J Anaesth. 1971;43:932–939.
36 Klain M, Smith RB. High frequency percutaneous transtracheal jet ventilation. Crit Care Med. 1977;5:280–287.
37 Zornow M, Thomas T, Scheller MS. The efficacy of three different methods of transtracheal ventilation. Can J Anaesth. 1989;36:624–628.
38 Weymuller EA, Paugh D, Pavlin EG, et al. Management of the difficult airway problems with percutaneous transtracheal ventilation. Ann Otol Rhinol Laryngol. 1987;96:34–37.
39 Jacoby JJ, Reed JP, Hamelberg W, et al. A simple method of artificial respiration. Am J Physiol. 1951;167:789.
40 Ward KR, Menegazzi JJ, Yealy DM, et al. Translaryngeal jet ventilation and end-tidal PCO2 monitoring during varying degrees of upper airway obstruction. Ann Emerg Med. 1991;20:1193–1197.
41 Frame SB, Simon JM, Kerstine MD, et al. Percutaneous transtracheal catheter ventilation in complete airway obstruction: An animal model. J Trauma. 1989;29:774–781.
42 Frame SB, Timberlake GA, Kerstine MD, et al. Transtracheal needle catheter ventilation in complete airway obstruction: An animal model. Ann Emerg Med. 1989;18:127–133.
43 Hamaekers A, Theunissen M, Jansen J, et al: Emergency ventilation through a 2 mm ID transtracheal catheter in severe hypoxic pigs using expiratory ventilation assistance (EVA). Meeting abstracts, September 2010, Society for Airway Management.
44 Okazaki J, Isono S, Atsuko T, et al. Usefulness of continuous oxygen insufflation into trachea for management of upper airway obstruction during anesthesia. Anesthesiology. 2000;93:62–68.
45 Asai T, Koga K, Vaughan RS, et al. Respiratory complications associated with tracheal intubation and extubation. Br J Anaesth. 1998;80:767–775.
46 Singh NP, Agrawal AR, Dhawan R. Resuscitation centre for management of respiratory insufficiency cases. Indian J Chest Dis. 1971;13:99–113.
47 Spoerel WE, Greenway RE. Technique of ventilation during endolaryngeal surgery under general anesthesia. Can J Anaesth. 1973;20:369–377.
48 Smith RB, Myers EN, Sherman H. Transtracheal ventilation in pediatric patients. Br J Anaesth. 1974;46:313–314.
49 Layman PR. Transtracheal ventilation in oral surgery. Ann R Coll Surg Engl. 1983;65:318–320.
50 Wagner DJ, Coombs DW, Doyle SC. Percutaneous transtracheal ventilation for emergency dental appliance removal. Anesthesiology. 1985;62:664–666.
51 Dhara SS, Liu EH, Tan KH. Monitored transtracheal jet ventilation using a triple lumen central venous catheter. Anaesthesia. 2002;57:578–581.
52 Depierraz B, Ravussin P, Brossard E, et al. Percutaneous transtracheal jet ventilation for paediatric endoscopic laser treatment of laryngeal and subglottic lesions. Can J Anaesth. 1994;41:1200–1209.
53 McLellan I, Gordon P, Khawaja S, et al. Percutaneous transtracheal high frequency jet ventilation as an aid to difficult intubation. Can J Anaesth. 1988;35:404–405.
54 Baraka A. Transtracheal jet ventilation during fiberoptic intubation under general anesthesia. Anesth Analg. 1986;65:1091–1092.
55 Dallen LT, Wine R, Benumof JL. Spontaneous ventilation via transtracheal large-bore intravenous catheters is possible. Anesthesiology. 1991;75:531–533.
56 Lipp MD, Jaehnichen G, Golecki N, et al. Microbiological, microstructure, and material science examinations of reprocessed Combitubes after multiple reuse. Anesth Analg. 2000;91:693–697.
57 Patel SK, Whitten CW, Ivy R, et al. Failure of the laryngeal mask airway: An undiagnosed laryngeal carcinoma. Anesth Analg. 1998;86:438–439.
58 Warmington A, Hughes T, Walker J, et al. Cricothyroidotomy and transtracheal high frequency jet ventilation for elective laryngeal surgery: An audit of 90 cases. Anaesth Intensive Care. 2000;28:711.
59 Hamaekers A, Borg P, Enk D. Limitations of self-made jet devices. Paediatr Anaesth. 2008;18:984.
60 Raik Schaefer, Hueter L, Preussler NP, et al. Percutaneous transtracheal emergency ventilation with a self-made device in an animal model. Paediatr Anaesth. 2007;17:972–976.
61 Gal TJ. Airway management. In: Miller RD, ed. Miller’s anesthesia. ed 6. Philadelphia: Elsevier; 2005:1637–1639.
62 Bould MD, Bearfield P. Techniques for emergency ventilation through a needle cricothyroidotomy. Anaesthesia. 2008;63:535–539.
63 Hamaekers A, Borg P, Enk D. The importance of flow and pressure release in emergency jet ventilation devices. Paediatr Anaesth. 2009;19:452–457.
64 Lenfant F, Péan D, Brisard L, et al. Oxygen delivery during transtracheal oxygenation: A comparison of two manual devices. Anesth Analg. 2010;111:922–924.
65 Preussler NP, Schreiber T, Huter L, et al. Percutaneous transtracheal ventilation: Effects of a new oxygen flow modulator on oxygenation and ventilation in pigs compared with a hand triggered emergency jet injector. Resuscitation. 2003;56:329–333.
66 Enk D, Busse H, Meissner A, et al. A new device for oxygenation and drug administration by transtracheal jet ventilation. Anesth Analg. 1998;86:S203.
67 Metz S, Parmet JL, Levitt JD. Failed emergency transtracheal ventilation through a 14-gauge intravenous catheter. J Clin Anesth. 1996;8:58–62.
68 Fassl J, Jenny U, Nikiforov S, et al. Pressures available for transtracheal jet ventilation from anesthesia machines and wall-mounted oxygen flowmeters. Anesth Analg. 2010;110:94–100.
69 Garry B, Woo P, Perrault DF, Jr., et al. Jet ventilation in upper airway obstruction: Description and model lung testing of a new jetting device. Anesth Analg. 1998;87:915–920.
70 Peterson GN, Domino KB, Caplan RA, et al. Management of the difficult airway: Closed claims database. Anesthesiology. 2005;102:33–39.
71 Mort TC. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607–613.
72 Elliott DS, Baker PA, Scott MR, et al. Accuracy of surface landmark identification for cannula cricothyroidotomy. Anaesthesia. 2010;65:889–894.
73 Sdrales L, Benumof JL. Prevention of kinking of percutaneous transtracheal intravenous catheter. Anesthesiology. 1995;82:288–291.
74 Holst M, Hertegard S, Persson A. Vocal dysfunction following cricothyrotomy: A prospective study. Laryngoscope. 1990;100:749–755.
75 Little C, Parker M, Tarnopolsky R. The incidence of vasculature at risk during cricothyroidostomy. Ann Emerg Med. 1986;15:805–807.