16 Percutaneous Neuromodulation Therapy
How PNT Works
The transmission of pain signals throughout these pathways depends on the balance of neurotransmitters whose release depends, in turn, on a variety of factors, including the frequency of stimulation of the peripheral nerves. Rather than reviewing the various forms of gate theory, originally proposed by Melzack and Wall,1 we will focus here on the influence of different frequencies of electrostimulation on neurotransmitter release in the central nervous system because this neuromodulation underlies the therapeutic effectiveness of PNT. Much of this knowledge derives from electroacupuncture and neurosurgery research, as noted earlier.
Early work by Han and colleagues from Beijing Medical University demonstrated that analgesia induced by electroacupuncture of different frequencies is mediated by different opioid receptors.2 Analgesia induced by low-frequency (2 Hz) electroacupuncture resulted from activation of mu- and delta-opioid receptors, whereas analgesia induced by high-frequency (100 Hz) electroacupuncture derived from activation of kappa-opioid receptors. Intermediate frequency stimulation (2 to 15 Hz) activated all three types of receptors in the spinal cord of rats.
Further work by Han and colleagues3 showed that low-frequency (2 Hz) peripheral stimulation produces a significant increase in enkephalin release into the lumbar cerebrospinal fluid (CSF), whereas high-frequency (100 Hz) peripheral stimulation increases dynorphin release. This validated earlier work by Mayer and colleagues4 and Pomeranz and Chiu.5 Wang and associates went on to establish that a more potent analgesia could be established by asynchronous electroacupuncture stimulation—alternating between low-frequency (2 Hz) and high-frequency (100 Hz) stimulation—than could be attained by synchronous electroacupuncture stimulation, combining low and high-frequency stimulation at the same time.6 The clinical application of these findings will become evident later in this chapter.
The endorphins (enkephalin and dynorphin) are only part of an ever-elaborating story of pain neurotransmission. In the last few years, research has suggested a role in electroacupuncture (and, thus, PNT) for cocaine and amphetamine-regulated transcript (CART) peptide,7 arginine vasopressin,8 serotonin, catecholamine, and spinal Fos expression,9 interleukin-1-beta,10 corticotrophin-releasing hormone.11 No doubt more neurotransmitters and neurochemicals will play important parts in the transmission and perception of pain.
The PNT Technique
Location of Needles
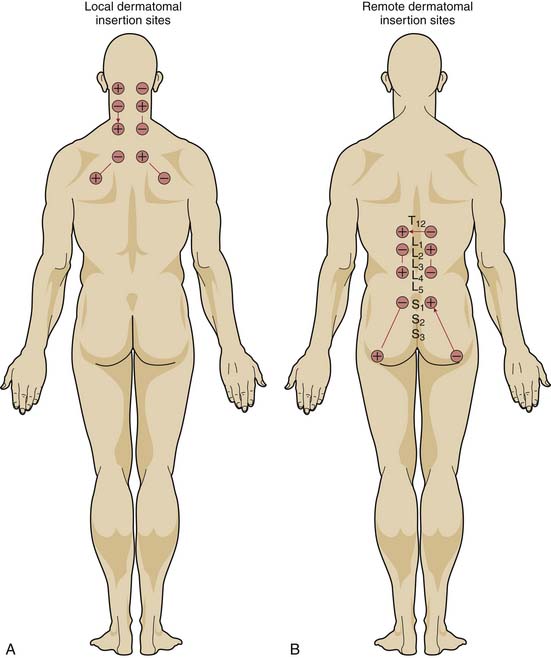
If the mechanism for analgesia relies primarily on increases in analgesic-like neurotransmitters within the central nervous system induced by peripheral nerve stimulation, varying the level of spinal stimulation should yield similar analgesic effects. Alternatively, if central neuromodulatory changes are primarily responsible for the analgesic effects, then stimulation of peripheral nerves in or near the affected area should prove more effective than stimulation of distant peripheral nerves. White, Craig, and others clarified the effect of the location of electrical stimulation on the acute analgesic response to PNT in a crossover study of 68 patients with nonradiating neck pain.12
When PNT was applied to needles located in the dermatomal distribution of the neck pain, visual analog pain scores decreased significantly more than when PNT was applied to needles located in the low back region or when needles were inserted without electrical stimulation. PNT applied to local needles also brought significant improvements in physical activity, quality of sleep, psychological well-being, and the need for oral analgesic medications, compared with PNT applied to distant needles or unstimulated needles. Moreover, unlike the other two treatments, PNT with the local needles showed a cumulative improvement over the course of therapy (Fig. 16-1).
Stimulation Montage
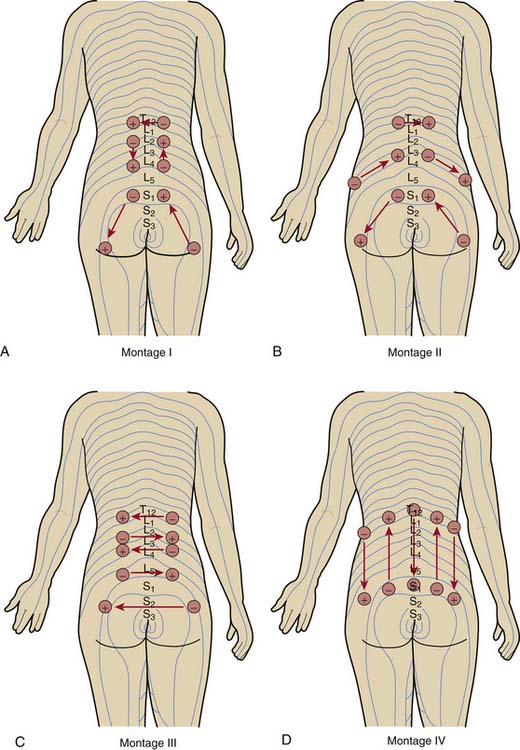
Having placed the needles into the appropriate dermatomes, does it matter how one connects the stimulator leads? In other words, does one specific pattern of electrical stimulation (i.e., montage) provide greater relief than another specific pattern? Work by White and associates demonstrates that montage matters.13
The investigators evaluated the effect of four different montages on the acute analgesic response to PNT when applied at the same dermatomal levels in a crossover study of 72 patients with low back pain (Fig. 16-2). Although all four montages provided significant improvements in pain visual analog scores and in the physical component summary (PCS) and the mental component summary (MCS) of the SF-36, one montage—in which the flow of electrical stimulation paralleled the dermatomes on both sides—produced significantly greater improvements. The cumulative effects over the course of 2 weeks of treatment were also superior with this montage than with the other patterns.
Stimulus Frequency
From the animal and human data already described, it would appear that stimulus with alternating frequencies should afford greater pain relief than stimulus with a single frequency. Ghoname and colleagues tested this hypothesis by comparing the effect of three different frequencies on the analgesic response to PNT therapy in 68 patients with low back pain resulting from degenerative lumbar disc disease. Patients were treated with PNT therapy at 4 Hz, alternating 3-second pulses of 15 Hz and 30 Hz, and 100 Hz, as well as sham-PNT (0 Hz), in random order, three times weekly for 2 weeks, with 1 week off between each sequence.
Duration of Electrical Stimulation
The needles should be placed within and around the affected dermatome, the stimulus should be applied pair-wise to needles within a single dermatome, and the stimulus should consist of two frequencies (one higher, one lower) used asynchronously. But how long should the electrical stimulation last?
Hamza and coworkers addressed this question in a crossover study of 75 patients with low back pain.14 All patients received electrical stimulation for 0, 15, 30, or 45 minutes in a randomized sequence over the course of an 11-week period (2 weeks of treatment with 1 week off between treatments). The researchers assessed pain scores, health status, sleep quality, and daily oral analgesic requirements.
Electrical stimulation for 15, 30, and 45 minutes significantly improved all measures of pain relief over results obtained with 0 minutes of electrical stimulation. After completion of the sixth treatment, mean improvements in the degree of pain, physical activity, and sleep quality and reductions in the need for oral analgesics were significantly greater with 30- and 45-minute treatment intervals than with the 15-minute treatment interval.
Clinical Applications of PNT
Back Pain
Back pain, especially low back pain, is one of the most common medical problems, with about 25% of Americans reporting at least one whole day of low back pain in any given 3-month period.15 Despite this high prevalence of back pain, analgesic therapy remains largely unsatisfactory, and controversy surrounds the effectiveness of such nonpharmacologic therapies as transcutaneous electrical nerve stimulation (TENS), acupuncture, spinal manipulation, and exercise therapy. At least five reports have demonstrated PNT to be safe and effective therapy for low back pain.
Ghoname and colleagues compared the effectiveness of PNT, sham-PNT (needle placement without electrical stimulation), TENS, and flexion-extension exercise therapies in a randomized, crossover study of 29 men and 31 women with at least 3 months of low back pain secondary to degenerative disc disease.16 Each treatment was administered for 30 minutes three times weekly for 3 weeks. Patients had 1 week without therapy between each of the treatments. PNT in this study used a uniform electrical stimulation frequency of 4 Hz. The principal outcome measures included pre- and posttreatment visual analog scale (VAS) scores for pain, physical activity, and quality of sleep; daily analgesic medication usage; a global patient assessment questionnaire; and Health Status Survey Short Form (SF-36).
Weiner and associates compared the efficacy of combined PNT and physical therapy (PT) with that of combined sham-PNT and PT for the treatment of chronic low back pain (defined as that occurring daily or almost every day for the previous 3 months) in a randomized, controlled trial of 34 community-dwelling adults aged 65 years and older.17 Patients received PNT or sham-PNT plus physical therapy twice weekly for 6 weeks. These investigators used a structured PNT protocol that allowed incremental stimulation frequencies based on the patient’s response to therapy. The primary outcome measures were pain intensity and pain-related disability, and secondary outcome measures included physical performance, psychosocial factors, and cognitive function.
Borg-Stein and associates extended the use of PNT to the management of subacute radiating low back pain.18 They investigated the efficacy and safety of 12 weeks of weekly PNT for 30 minutes in an open-label study of 59 patients who had moderate-to-severe radiating low back pain for 4 weeks to 6 months. According to their report, the pulse repetition frequency varied from 4 Hz to 10 Hz in a periodic, sweeping manner. Their outcome measures included VAS scores of radiating pain, low back pain, physical activity, and sleep, as well as the Oswestry Disability Questionnaire.
By the 5-week evaluation, 37 patients (63%) reported at least a 30% decrease in their lower extremity pain and 25 patients (42%) reported at least a 50% improvement. Among all 59 patients, mean leg/buttock pain decreased by 37%, mean low back pain decreased by 26%, activity levels improved by 38%, sleep improved by 27%, and Oswestry Low Back Pain Disability scores improved by 24%. These improvements were sustained between 5 and 12 weeks. By 24 weeks, 83% of 27 patients who granted phone interviews indicated either “very much benefit” or “much benefit,” and 59% said they were either “pain free” or “much better.”
Yokoyama and colleagues from Okayama University Medical School, Okayama City, Japan compared PNT with TENS for long-term pain relief in a study of 60 patients who reported having low back pain with intensity of at least 40/100 on VAS for more than 6 months.19 One group received PNT twice weekly for 8 weeks, one group received 4 weeks of twice-weekly PNT followed by 4 weeks of twice-weekly TENS, and one group received 8 weeks of twice-weekly TENS. In all cases, needles or TENS electrode pads were stimulated with alternating frequencies of 4 Hz and 30 Hz for 20 minutes. Pain level, degree of physical impairment, and daily intake of nonsteroidal antiinflammatory drugs (NSAIDs) were assessed 3 days after week 2, week 4, and week 8 treatments and again at 1 and 2 months after the sessions concluded.
Sciatica
As many as 40% of people experience sciatica (radicular pain in the distribution of the sciatic nerve caused by herniation of one or more lumbar intervertebral discs) in their lifetime.20 Ghoname and coworkers compared PNT, sham-PNT, and TENS for the management of the radicular pain in a crossover study of 64 patients with sciatica due to radiologically-confirmed lumbar disc herniation. PNT and TENS (applied only to the affected leg) employed a stimulation frequency of 4 Hz during 30-minute sessions three times weekly for 3 weeks. All patients received all three modalities, and each set of treatments was separated by a 1-week period free of therapy. The primary outcome measures were the pain VAS and the SF-36. Secondary outcomes included physical activity, sleep quality, sense of well-being, and daily oral analgesic requirements.
Neuropathic Pain
Peripheral neuropathy may complicate the course of diabetes in as many as 47% of patients, and more than 26% of diabetics have reported experiencing pain or tingling.21 Various drug classes have been employed in an effort to ameliorate neuropathic pain, and nonpharmacologic approaches—TENS, acupuncture, and spinal cord stimulation—have afforded some relief.
Bone Pain
Ahmed and associates evaluated the use of PNT for the short-term management of pain associated with metastatic cancer in a pilot study of three patients whose symptoms were inadequately controlled with conventional opioid and nonopioid analgesics.22 The first patient, a 76-year-old Hispanic man with prostate cancer metastatic to the spine, received PNT with needles inserted into the periosteum (negative electrode) and soft tissue (positive electrode) bilaterally at the level of T10, T12, and S1. The needles were stimulated alternately at 15 Hz and 30 Hz for 30 minutes. Pain diminished from 7/10 to 2/10 (per visual analog pain scale) immediately after treatment but rebounded to 5.5/10 three days later. A second treatment reduced the pain to a level manageable by oral analgesic medication.
Other Possible Indications for PNT
Ghoname and colleagues used PNT to treat five patients who experienced migraine-like attacks associated with electroconvulsive therapy (ECT).23 The first patient obtained relief from a severe, bilateral, throbbing headache that developed after ECT after receiving 30 minutes of PNT delivered at 4 Hz through needle probes placed bilaterally in the temporalis muscles and the paraspinous muscles at dermatomal levels C1, C5, C7, and T4. Based on this result, the investigators administered PNT for 30 minutes prior to the induction of anesthesia for the patient’s next ECT treatment. The patient remained headache-free after this ECT session.
Antiviral drugs have been employed successfully to decrease the pain and enhance the resolution of acute herpes zoster lesions in immunocompetent patients, but their effects on postherpetic neuralgia (PHN) remain controversial. Ahmed and colleagues compared the effects of PNT with those of a standard antiviral regimen on pain, physical activity, sleep quality, cutaneous lesion resolution, and incidence and severity of PHN in 50 adult patients with the onset of herpes zoster lesions within the preceding 72 hours.24 The patients were randomized to receive either famciclovir 500 mg three times daily for 1 week or PNT for 30 minutes three times weekly for 2 weeks. The needles for PNT were inserted at dermatomes one level above and one level below the cutaneous lesions and were stimulated at frequencies ranging from 4 Hz to 100 Hz.
Although it is unlikely to supplant antiviral drugs as the first-line therapy for patients with acute herpes zoster, PNT may offer an important complement to antiviral therapy.25 More research is needed to elucidate the role of PNT in this painful condition.
Conclusion
Future studies should explore different montages and frequency combinations for different painful conditions. Although the duration of a treatment session has been studied, the frequency of treatment and the overall duration of a program of treatment demand further investigation. How long the beneficial effects of PNT can be expected to persist also requires additional study. In the meantime, PNT can be practiced safely and effectively for chronic pain using the parameters described here. PNT should be added to the therapeutic armamentarium of all practitioners who care for patients with chronic pain.
1. Melzack P., Wall P.D. Pain mechanisms: A new theory. Science. 1965;150:971-979.
2. Chen X.H., Han J.S. Analgesia induced by electroacupuncture of different frequencies is mediated by different types of opioid receptors: Another cross-tolerance study. Behav Brain Res. 1992;47:143-149.
3. Han J.S., Chen X.H., Sun S.L., et al. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain. 1991;47:295-298.
4. Mayer D.J., Price D.D., Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368-372.
5. Pomeranz B., Chiu D. Naloxone blocks acupuncture analgesia and causes hyperalgesia: Endorphin is implicated. Life Sci. 1976;19:1757-1762.
6. Wang Y., Zhang Y., Wang W., et al. Effects of synchronous or asynchronous electroacupuncture stimulation with low versus high frequency on spinal opioid release and tail flick nociception. Exp Neurol. 2005;192:156-162.
7. Tian D.R., Li X.D., Wang F., et al. Up-regulation of the expression of cocaine and amphetamine-regulated transcript peptide by electroacupuncture in the arcuate nucleus of diet-induced obese rats. Neurosci Lett. 2005;383:17-21.
8. Yang J., Liu W.Y., Song C.Y., Lin B.C. Only arginine vasopressin, not oxytocin and endogenous opiate peptides, in hypothalamic paraventricular nucleus play a role in acupuncture analgesia in the rat. Brain Res Bull. 2006;68:453-458.
9. Li A., Wang Y., Xin J., et al. Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res. 2007;1186:171-179.
10. Zhang R.X., Li A., Liu B., et al. Electroacupuncture attenuates bone cancer pain and inhibits spinal interleukin-1-beta expression in a rat model. Anesth Analg. 2007;105:1482-1488.
11. Li A., Lao L., Wang Y., et al. Electroacupuncture activates corticotrophin-releasing hormone-containing neurons in the paraventricular nucleus of the hypothalamus to alleviate edema in a rat model of inflammation. BMC Complement Altern Med. 2008;8:20.
12. White P.F., Craig W.F., Vakharia A.S., et al. Percutaneous neuromodulation therapy: Does the location of electrical stimulation effect [sic] the acute analgesic response? Anesth Analg. 2000;91:949-954.
13. White P.F., Ghoname E.A., Ahmed H.E., et al. The effect of montage on the analgesic response to percutaneous neuromodulation therapy. Anesth Analg. 2001;92:483-487.
14. Hamza M.A., Ghoname E.A., White P.F., et al. Effect of the duration of electrical stimulation on the analgesic response in patients with low back pain. Anesthesiology. 1999;91:1622-1627.
15. Deyo R.A., Mirza S.K., Martin B.I. Back pain prevalence and visit rates: Estimates from U.S. national surveys, 2002. Spine. 2006;31:2724-2727.
16. Ghoname E.A., Craig W.F., White P.F., et al. Percutaneous electrical nerve stimulation for low back pain: A randomized crossover study. JAMA. 1999;281:818-823.
17. Weiner D.K., Rudy T.E., Glick R.M., et al. Efficacy of percutaneous electrical nerve stimulation for the treatment of chronic low back pain in older adults. J Am Geriatr Soc. 2003;51:599-608.
18. Borg-Stein J., Seroussi R.E., Gomba L., et al. Safety and efficacy of percutaneous neuromodulation therapy in the management of subacute radiating low back pain. Pain Practice. 2003;3:125-134.
19. Yokoyama M., Sun X., Oku S., et al. Comparison of percutaneous electrical nerve stimulation with transcutaneous electrical nerve stimulation for long-term pain relief in patients with chronic low back pain. Anesth Analg. 2004;98:1552-1556.
20. Stafford M.A., Peng P., Hill D.A. Sciatica: A review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br J Anaesth. 2007;99:461-473.
21. Barrett A.M., Lucero M.A., Le T., et al. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: A review. Pain Med. 2007;8S2:S50-S62.
22. Ahmed H.E., Craig W.F., White P.F., Huber P. Percutaneous electrical nerve stimulation (PENS): A complementary therapy for the management of pain secondary to bony metastasis. Clin J Pain. 1998;14:320-323.
23. Ghoname E.A., Craig W.F., White P.F. Use of percutaneous electrical nerve stimulation (PENS) for treating ECT-induced headaches. Headache. 1999;39:502-505.
24. Ahmed H.E., Craig W.F., White P.F., et al. Percutaneous electrical nerve stimulation: An alternative to antiviral drugs for acute herpes zoster. Anesth Analg. 1998;87:911-914.
25. Dworkin R.H. No alternative to antiviral drugs for acute herpes zoster [letter]. Anesth Analg. 1999;89:1585-1586.